Abstract
Although increasing efforts are being made to restore tropical forests, little information is available regarding the time scales required for carbon and plant biodiversity to recover to the values associated with undisturbed forests. To address this knowledge gap, we carried out a meta-analysis comparing data from more than 600 secondary tropical forest sites with nearby undisturbed reference forests. Above-ground biomass approached equivalence to reference values within 80 years since last disturbance, whereas below-ground biomass took longer to recover. Soil carbon content showed little relationship with time since disturbance. Tree species richness recovered after about 50 years. By contrast, epiphyte richness did not reach equivalence to undisturbed forests. The proportion of undisturbed forest trees and epiphyte species found in secondary forests was low and changed little over time. Our results indicate that carbon pools and biodiversity show different recovery rates under passive, secondary succession and that colonization by undisturbed forest plant species is slow. Initiatives such as the Convention on Biological Diversity and REDD+ should therefore encourage active management to help to achieve their aims of restoring both carbon and biodiversity in tropical forests.
Keywords: secondary forest, forest restoration, biomass, carbon, plant biodiversity, meta-analysis
1. Introduction
Tropical forests contain between half and two-thirds of terrestrial global biodiversity [1], and approximately 37% of the global terrestrial carbon pool [2]. These forests also provide vital ecosystem services at local, regional and global scales [3,4]. Despite these benefits, tropical forests are undergoing widespread loss, largely as a result of agricultural expansion [5]. These losses have led to increased carbon emissions, species extinctions and structural alteration of the majority of tropical forests worldwide [3,4].
To combat these ongoing losses, many projects have been implemented in different countries over the past two decades with the aim of restoring millions of hectares of tropical forest [6,7]. The need for tropical forest restoration is recognized in international policy through the Convention on Biological Diversity (CBD) and REDD+ initiatives [8,9]. The 2020 targets of the CBD aim to enhance biodiversity and carbon stocks by restoring 15% of the world's degraded ecosystems [9]. In addition, REDD+ aims to enhance carbon stocks partly through forest restoration, using funding from carbon credits [8]. However, despite the perceived importance of restoring tropical forests for both carbon storage and biodiversity, information is lacking on their patterns and rates of recovery following disturbance.
To determine the relative value of recovering forests as carbon pools and for biodiversity conservation, comparison with a reference forest is required (e.g. a site that is relatively free of human disturbance). Previous studies of carbon accumulation in tropical secondary forests [10,11] have not undertaken comparisons against such reference systems. As such, these syntheses provide limited information about the recovery of carbon pools in tropical forests, but rather examine the factors explaining differences in biomass and soil carbon among tropical secondary forest sites, with climate emerging as a major driver [11–13].
As biomass recovers following disturbance, it is to be expected that forest ecosystems should accumulate carbon pools with time [10,14]. In the case of secondary tropical forests, little information is available regarding the time period required for recovery of these carbon pools to the values of undisturbed forests. The most studied of these pools is that associated with above-ground biomass, for which recovery appears to become asymptotic over time [15–18]. However, the time required for this pool to recover completely has been hypothesized to be anywhere between 50 and 200 years [15,17]. Below-ground biomass has been studied less frequently, but may require similar periods for complete recovery, with Saldarriaga et al. [16] suggesting an interval of over 80 years.
Changes in soil carbon in secondary forests are less well documented than biomass recovery. A transition from agricultural use to secondary forest generally results in an increase in soil carbon content [19], but the evidence for soil carbon accumulation during secondary succession is conflicting. Recovery of soil carbon in secondary tropical forests to values similar to those in undisturbed forest can take 20–100 years [20,21], but some secondary forests have higher soil carbon than undisturbed forests [22].
In contrast to studies of carbon pools, there have been a number of syntheses of biodiversity recovery in secondary tropical forests. These suggest that faunal species richness recovers relatively quickly during succession [23], but more than 150 years may be required for community composition to reach equivalence to undisturbed forests [24]. However, relatively little is known about changes in plant communities during secondary succession in tropical forests. The only previous synthesis—albeit of only eight locations across Central and South America—of plant biodiversity in secondary forests suggests that they may take longer to become equivalent to undisturbed forest than faunal communities, with only 40% of undisturbed forest species having colonized secondary forests after 80 years of recovery [25].
No integrated meta-analysis of the recovery of both carbon pools and plant biodiversity in tropical forests has been undertaken previously. Such information is urgently required to inform policy and management practice. To address this knowledge gap, we address the following questions by conducting a meta-analysis based on systematic review:
— At what age following forest clearance do carbon pools in secondary tropical forests reach equivalent values to those of undisturbed forests?
— At what age following forest clearance do plant species richness and the proportion of undisturbed forest species in secondary tropical forests reach equivalent values to those of undisturbed forests?
— How do the rates of recovery of biodiversity and carbon pools compare, and what are the consequences for tropical forest restoration policy?
2. Material and methods
(a). Systematic review
We defined a tropical secondary forest as a previously forested area undergoing secondary succession following total or near-total removal of trees [26], located between the latitudes 40° N and 40° S [27]. To collate relevant studies, a systematic review was carried out using standard methodologies [28], outlined in the electronic supplementary material, appendix S1. Studies were retained if they included: (i) at least one measurement of above-ground biomass, below-ground biomass, soil carbon content, plant species richness and/or plant species community composition in both a secondary tropical forest and a reference undisturbed forest (following [29]); (ii) the time since last disturbance for secondary forests; and (iii) definition of the type of disturbance prior to secondary succession, which included conversion to pasture, cropland or small-scale shifting agriculture. In addition, we extracted data on forest type determined by Holdridge life zone [30] (hereafter referred to as forest type), and geographical location. Although methodologies differed among studies, measurements in secondary and undisturbed forests within a study were carried out using the same methods and the same plot sizes.
Almost all of the data we collated came from chronosequence studies where secondary forest stands of different ages were used to infer successional dynamics. One of the assumptions of chronosequences is that all sites have been subjected to the same environmental conditions, though in practice this condition is rarely met [31]. For the purposes of our study, we also assumed that undisturbed forests had stable carbon pools and species composition. This assumption is again unlikely to be met as many undisturbed forests are known to be increasing in biomass [32] and undergoing changes in biodiversity, but we consider these changes to be less dramatic than those caused by secondary succession. As such, our study is reflective of the wider secondary forest literature, which tends to make similar assumptions about chronosequences.
(b). Statistical analysis
We calculated secondary forest carbon pool and species richness recovery using the equation
where 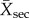 is the mean of a measurement in a secondary forest and
is the mean of a measurement in a secondary forest and 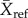 is the mean of the same measurement in the corresponding undisturbed reference site. This is a logit transformation of the proportional difference between secondary and undisturbed forests that conforms to the assumptions of linear models. Following model fitting, predicted values were converted to proportions relative to reference forests by calculating the inverse logit and multiplying by two.
is the mean of the same measurement in the corresponding undisturbed reference site. This is a logit transformation of the proportional difference between secondary and undisturbed forests that conforms to the assumptions of linear models. Following model fitting, predicted values were converted to proportions relative to reference forests by calculating the inverse logit and multiplying by two.
As most studies did not provide estimates of variation along with measurements of carbon pools or species richness, an unweighted analysis was used. Although this technique gives equal weight to studies that may differ in quality and accuracy, it has been used frequently in the ecological literature [33–35], where data reporting standards are very variable. A linear mixed model was constructed for each variable of interest using time since last disturbance, disturbance type and forest type as explanatory variables. We included quadratic or log relationships with time since disturbance where our hypotheses suggested there may be nonlinear changes during succession. A random factor was included to group secondary forests that shared an undisturbed forest reference site, eliminating the problems of pseudo-replication at the study scale [36]. In addition, random variables were included to account for differences in study methods, such as in measurement depth for soil carbon and whether allometric equations for calculation of biomass were locally derived or represented general multi-species allometries [37]. Random variables accounting for the difference in minimum diameter at breast height (DBH) of trees included in assessments of species richness were also considered, but were found to add little explanatory value, and thus were excluded from models (see the electronic supplementary material, table S15 for details of different minimum DBH used in studies). The proportion of the undisturbed forest plant species found in secondary forests was used as a metric of changes in community composition [25,38] and was analysed using a binomial generalized linear mixed model with logit link. While there are techniques that are better suited to determining whether species are undisturbed forest specialists [39], they require detailed data for each study, to which we did not have access.
All possible additive models were computed using restricted maximum-likelihood methods. Model comparison was based on AICc, excluding all models with ΔAICc ≥ 7 [40]. We estimated the goodness of fit of each model by calculating the marginal R2 using the equations developed by Nakagawa & Schielzeth [41]. Coefficients were derived from the weighted mean of all models with ΔAICc ≤ 7. The importance of variables in explaining recovery of carbon pools and plant biodiversity was assessed by summing up the weight of all models that included the variable [40]. Analyses were performed in R v. 2.15.3 [42], with model averaging using the MuMIn package [43], and all graphs were produced using the ggplot2 package [44].
3. Results
The systematic review yielded data for 607 secondary forest sites from 74 studies describing above-ground biomass, below-ground biomass, soil carbon, plant species richness or plant species composition, with comparable data for a reference undisturbed forest (further details in the electronic supplementary material, table S1). The majority of these sites were relatively young, with mean ages between 20 and 30 years for each variable of interest (see electronic supplementary material, figure S1). Thus, biomass and carbon recovery were measured for forests up to 85 years old. Biodiversity data were available for forests up to a little over 150 years old, although virtually all sites were under 100 years old. Most sites were in Central or South America (see electronic supplementary material, figure S2), with a few sites in Africa or Asia.
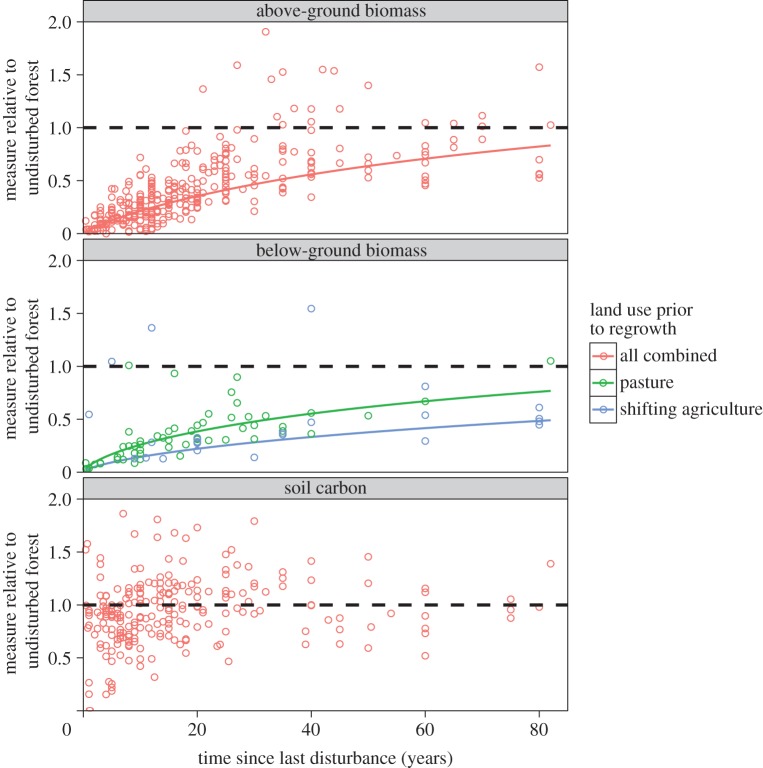
Model selection suggested that the best model describing above-ground biomass recovery in secondary forests included only a log relationship with time since disturbance. This model predicted recovery of above-ground biomass to slow over time and to be about 83% of that of undisturbed forests after 85 years (figure 1). This model had an AICc weight of 0.57 and a marginal R2 of 0.56 (see electronic supplementary material, table S1). The relationship between relative biomass recovery and age was much more important than those of forest type and prior land use (see electronic supplementary material, table S14).
Figure 1.
Recovery of above-ground biomass (n = 326), below-ground biomass (n = 76) and soil carbon (n = 185) in secondary tropical forests, relative to undisturbed reference forests. Solid lines represent model predictions, with different colours representing different disturbance types. Parameters included in figures have AICc importance values greater than 0.5. The horizontal dashed line represents no difference between secondary and undisturbed forests. (Online version in colour.)
Below-ground biomass increased more slowly than above-ground biomass as a function of forest age. As with above-ground biomass, there was a log relationship with time since disturbance; after 80 years stocks in sites previously subjected to shifting agriculture were still only about 50% of those in reference forests (figure 1). Forests established on pastures appeared to recover below-ground biomass more rapidly than those following shifting agriculture, with recovery to 76% of reference levels in approximately 80 years. Forest type was not important in explaining differences between undisturbed and secondary forests (importance value = 0; electronic supplementary material, table S14). Models with ΔAICc ≤ 7 had marginal R2 values of 0.60–0.64 (see electronic supplementary material, table S3).
Soil carbon stocks showed very weak relationships with all variables; an intercept-only model had the most support (AICc weight = 0.43; electronic supplementary material, table S3). However, models predicting slight increases in soil carbon with time since disturbance were also supported, although these had extremely small marginal R2 of less than or equal to 0.01 (see electronic supplementary material, table S4).
Plant species richness increased with time since last disturbance—again following log relationships—with epiphyte richness showing slower recovery than tree richness (figure 2). Tree species richness was predicted to recover after approximately 50 years, whereas epiphyte richness was predicted to take longer than 100 years. Model fits of tree species richness were also much better than those for epiphytes, with marginal R2 of 0.24–0.26 and 0–0.08, respectively (see electronic supplementary material, tables S5 and S6). By contrast, a relationship between time since last disturbance and proportion of species associated with undisturbed forest was relatively poorly supported (importance value = 0.35). The proportion of species associated with undisturbed forest was generally low, with a mean of 26% of species also being found in secondary forest (upper CI = 67%, lower CI = 6%; figure 3; electronic supplementary material, tables S7 and S13).
Figure 2.
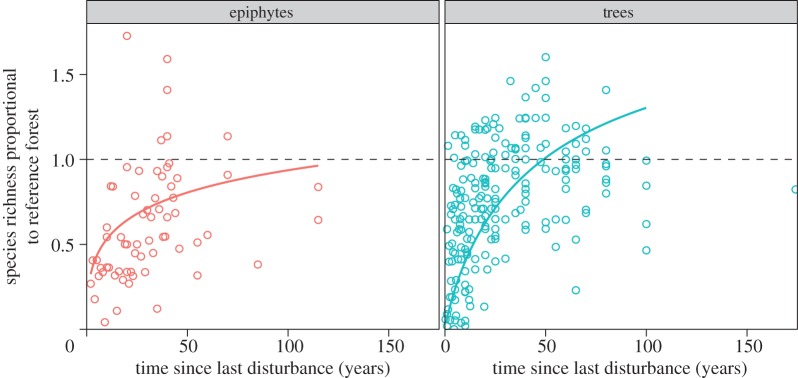
Recovery of epiphyte (n = 65) and tree (n = 204) species richness in secondary tropical forests, relative to undisturbed reference forests. Solid lines represent model predictions, with different colours representing different disturbance types. Parameters included in figures have AICc importance values greater than 0.5. The horizontal dashed line represents no difference between secondary and undisturbed forests. (Online version in colour.)
Figure 3.
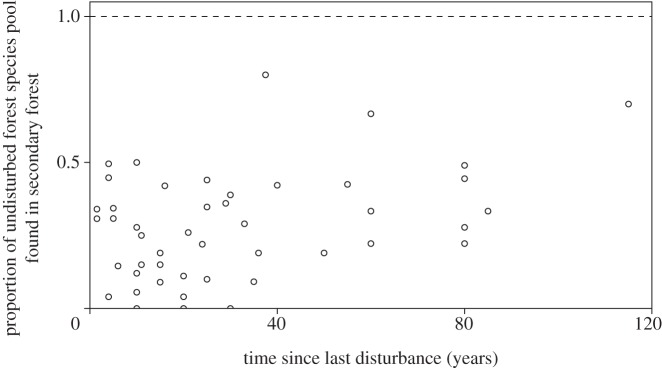
Recovery of species associated with undisturbed tropical forest in secondary forest (n = 50). The horizontal dashed line represents no difference between secondary and undisturbed forests.
4. Discussion
This study is the first to assess the recovery of both carbon pools and plant biodiversity across a large number of secondary tropical forest sites. Our results indicate that the various carbon pools and measures of biodiversity recover at different rates. Above-ground biomass approaches recovery 85 years after the last disturbance. Below-ground biomass also increases over time, with former pastures recovering 75% of below-ground biomass after about 80 years, whereas areas affected by shifting agriculture take longer to recover. Soil carbon remained largely unchanged over time. In terms of biodiversity, tree species richness reached equivalence to reference forests after approximately 50 years and epiphyte richness only approached recovery after 100 years, while the recovery of undisturbed forest species in secondary forests was limited and showed little relationship with time.
(a). Recovery of carbon pools
Although previous work has suggested that rates of biomass accumulation differ between dry, moist and wet tropical forests [45], as well as among disturbance types [10], our study indicates that these factors are largely unimportant in determining the rate of recovery towards the state of undisturbed forests. Our estimated time required for above-ground biomass to reach approximately 85% of undisturbed forest levels is similar to suggested rates for basal area recovery in the neotropics [46]. While our results and previous observations [46] suggest that forest biomass approaches that of undisturbed forest within a century, full recovery may take substantially longer. This is because secondary forests are often composed of relatively small-stemmed trees and lack the very large trees characteristic of old-growth forest, which can have very high biomass [47]. However, without more data from older secondary forests it is difficult to determine how long the full recovery takes. One important caveat regarding above-ground biomass recovery is that allometric equations used for its estimation are usually derived from undisturbed forest plots [48]. As a result of this, measurements in secondary forests, which are often dominated by trees with low DBH, may overestimate their biomass [48], possibly because of differences in secondary forest height–diameter relationships [49]. This is a potential bias in all the individual studies we used, and we suggest that further research should aim to develop and test allometries designed for use in secondary forests to characterize recovery more accurately.
Below-ground biomass represents an average of 19% of total biomass in tropical forests [50], although root : stem ratios tend to be higher in younger forests [51,52]. Thus, we would expect below-ground biomass to recover more rapidly than those of above-ground biomass, and it is surprising that we found the opposite pattern. However, this effect may be an artefact because those sites for which we had below-ground biomass data had lower above-ground biomass than other forests of similar age (see electronic supplementary material, figures S3 and S4).
We found that secondary tropical forests have soil carbon contents similar to undisturbed forests, contradicting a recent meta-analysis [53], which suggested lower soil carbon in secondary forests. The differences between our study and that of Don et al. [53] result from differing definitions of secondary forest, which they considered to be forests affected by any human disturbance. That definition conflates different types of disturbance, and covers human-impacted forests and plantations as well as those undergoing secondary succession. As such we believe that our study more accurately represents soil carbon content in secondary forests as it covers more than usually defined—those that are recovering from near total removal of tree cover [26]. Our findings do, however, support those of Marín-Spiotta & Sharma [11], who also found similar soil carbon pools in secondary and undisturbed tropical forests. These results indicate either that soil carbon in tropical forests is resilient to moderate, short-term land-use change or that carbon is accumulated rapidly following abandonment of farmland. However, as with below-ground biomass, further research is required to explain the drivers of differences in soil carbon between sites. Given that the world's soils contain two to three times the carbon stored in above-ground biomass [54], such research should be considered a priority.
Former land use had an inconsistent effect on recovery of carbon pools in our study: there was no effect on above-ground biomass or soil carbon, but below-ground biomass recovered faster in former pastures than following shifting agriculture. The intensity and length of time under previous land-use influence factors, for example soil nutrient content, undoubtedly play important roles in biomass recovery [55]. For example, research has suggested that above-ground biomass is lower in secondary tropical forests that have experienced multiple cycles of conversion for shifting agriculture [56,57]. However, such detailed data were not collected for the majority of studies we analysed, and future studies should do so to aid our understanding of the factors that control carbon stocks in secondary forests.
Overall, these findings suggest that when attempting to restore carbon pools on tropical forest sites cleared for agriculture, the greatest gains are likely to be made in plant biomass, as soil carbon appears to be relatively insensitive to moderate land-use change. Independent of forest type, carbon pools in secondary forest sites could be expected to be 77–81% of those of undisturbed forests approximately 80 years after disturbance, given that above-ground biomass has been estimated as five times that of below-ground biomass in tropical forests [50].
(b). Recovery of species richness and community composition
We found that tree species richness recovered within 50 years, compared with more than 100 years for epiphyte richness. We have less confidence in the prediction of a continuing increase after 50 years, which is likely to be an artefact of the steep increase in younger forests and the relatively few data for older forests, meaning that the shape of the log relationship was constrained. Indeed, the data suggest relatively little increase after 50 years, and our model tends to overpredict tree richness in older forests. In addition to differing recovery rates, our model of tree species richness change also showed a much better fit than that of epiphyte richness. These differences in recovery and our ability to explain changes in richness are likely to be driven by contrasting dispersal traits and requirements for establishment. Secondary tropical forest tree communities are initially dominated by short-lived pioneer tree species, and these are sequentially replaced by longer-lived species [46]. Some secondary forests may be isolated from seed sources, leading to an impeded recovery of richness, but our results, and the observations of others [46], suggest that this is relatively rare. By contrast, epiphyte dispersal is largely local and propagation is often restricted to individual trees [58]. In addition, epiphytes seem to occur more commonly on large trees [59]. These factors may lead to relatively poor recovery of epiphyte species because many secondary forests are fragmented and tend to consist of smaller-stemmed trees [46]. An important caveat of our analysis is that few estimates of species richness were rarefied by either number of individuals or area sampled. It is possible that, as secondary forests almost always have higher stem densities, our analysis overestimates species richness recovery. However, from a conservation perspective, given that plot size was equal for the secondary and undisturbed plots in all pairwise comparisons, our estimation of species per unit area remains valid.
Although tree species richness recovers relatively well in secondary forests, there was little or no accumulation of species associated with the reference undisturbed forests. This contrasts with the more rapid colonization rates of animal species, communities of which may attain similarity to those of undisturbed forests within 150 years [24]. The poor recovery of plant community composition is likely to be the result of a number of interacting mechanisms. First, small secondary forest patches are likely to be subjected to greater edge effects than larger undisturbed patches, making them less likely to be colonized by species adapted to old-growth forest conditions [60]. Second, patches of secondary forest can be distant from undisturbed forests [61], and thus receive few seeds from them. Finally, the extent of degradation of the landscape surrounding secondary forests will also influence seed dispersal processes, such as the behaviour of frugivorous birds [55].
In addition to these ecological mechanisms, which might explain differences in the responses of species richness and community composition in secondary tropical forests, our study is subjected to some of the limitations of the literature we used in our analyses. The most important factor is likely to be associated with distance-decay in community similarity [62]. Sites used in this study are likely to vary in their distance from undisturbed reference sites, and thus the proportion of species shared with undisturbed forests would be expected to vary, even without any human disturbance [62,63]. Unfortunately, very few studies give details of distances between secondary and reference sites. We hope that future studies might record such landscape metrics. Despite this, our findings suggest that natural colonization alone may not be sufficient to restore tropical forest plant biodiversity effectively in less a century.
(c). Comparative rates of carbon and biodiversity recovery
Our results indicate that carbon pools and tree species richness recover more quickly than epiphyte species richness, whereas undisturbed forest plant species do not accumulate over time in secondary forests. Analyses of the carbon and biodiversity benefits of avoided deforestation have often suggested synergistic relationships between these goals owing to overlap of priority areas for biodiversity conservation and carbon storage [64,65]. By contrast, reforestation schemes that have the primary aim of carbon sequestration have often been criticized as they may support relatively little forest biodiversity [66]. Our study suggests a more nuanced relationship between biodiversity and carbon in secondary tropical forests: while both carbon storage and conservation value increase as secondary forests age, the trajectories of these increases differ. As a result of this, tropical forests recovering from agricultural conversion are likely to have greater value for carbon storage and sequestration than for biodiversity, especially during the first 100 years of development. These differing rates of recovery should be acknowledged by policies targeting the recovery of biodiversity and carbon in tropical forests.
The failure of species associated with undisturbed forest to colonize secondary forests effectively is worrying for those aiming to conserve biodiversity in tropical forest landscapes subjected to human disturbance. These species are likely to be adapted to old-growth conditions, and thus are likely to be sensitive to human disturbance and have small ranges and populations [67], and, as a result, they are likely to face greater threats of extinction [68]. This result clearly indicates that old-growth forests are vital for the conservation of some specialist species, but also that if goals to conserve species in human disturbed ecosystems are to be achieved we require novel solutions and further research.
5. Conclusion
This study is the first integrated meta-analysis of both plant biodiversity and carbon pool recovery in tropical secondary forests. We have shown that the recovery periods for the two differ markedly. This has important implications for policies that target recovery of both carbon and biodiversity, such as the CBD and REDD+. Carbon pools may take approximately 80 years to recover following disturbance, faunal biodiversity 150 years [24] and plant biodiversity well over 100 years. Thus, initiatives aiming to support recovery of both biodiversity and carbon should not assume that the two are closely coupled. Enhancement of carbon stocks to the values associated with local undisturbed forests appears possible through passive restoration. However, in many situations active restoration involving human interventions (e.g. planting trees) or other strategies, such as increasing seed dispersal across the non-forest matrix by creating woodland islets [69], may be required to enable long-term recovery of plant species community composition. In addition, further research into active restoration of tropical forests is required to identify novel solutions to this problem.
Acknowledgements
We thank all authors who contributed data to this project, without whom this work would not have been possible. Particular thanks are due to Erika Marín-Spiotta and Alfredo Cascante Marín, who provided us with unpublished data. We also thank Robin Chazdon and an anonymous reviewer, whose comments improved the paper, along with Sara Fuentes Perez, Catherine Sayer and Becks Spake, who made improvements to an earlier version of the manuscript. Thanks for statistical advice are due to Nick Golding, Louise Barwell and Gary Powney. The data used in this study are available on request from P.A.M.
Funding statement
P.A.M. thanks NERC for funding.
References
- 1.Gardner TA, Barlow J, Sodhi NS, Peres CA. 2010. A multi-region assessment of tropical forest biodiversity in a human-modified world. Biol. Conserv. 143, 2293–2300 (doi:10.1016/j.biocon.2010.05.017) [Google Scholar]
- 2.Dixon RK, Solomon AM, Brown S, Houghton RA, Trexier MC, Wisniewski J. 1994. Carbon pools and flux of global forest ecosystems. Science 263, 185–190 (doi:10.1126/science.263.5144.185) [DOI] [PubMed] [Google Scholar]
- 3.Gardner TA, Barlow J, Chazdon R, Ewers RM, Harvey CA, Peres CA, Sodhi NS. 2009. Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 12, 561–582 (doi:10.1111/j.1461-0248.2009.01294.x) [DOI] [PubMed] [Google Scholar]
- 4.Foley JA, et al. 2007. Amazonia revealed: forest degradation and loss of ecosystem goods and services in the Amazon Basin. Front. Ecol. Environ. 5, 25–32 (doi:10.1890/1540-9295(2007)5[25:ARFDAL]2.0.CO;2) [Google Scholar]
- 5.Gibbs HK, Ruesch AS, Achard F, Clayton MK, Holmgren P, Ramankutty N, Foley JA. 2010. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl Acad. Sci. USA 107, 16 732–16 737 (doi:10.1073/pnas.0910275107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayer J, Chokkalingam U, Poulsen J. 2004. The restoration of forest biodiversity and ecological values. Forest Ecol. Manag. 201, 3–11 (doi:10.1016/j.foreco.2004.06.008) [Google Scholar]
- 7.Calmon M, Brancalion PHS, Paese A, Aronson J, Castro P, da Silva SC, Rodrigues RR. 2011. Emerging threats and opportunities for large-scale ecological restoration in the Atlantic forest of Brazil. Restor. Ecol. 19, 154–158 (doi:10.1111/j.1526-100X.2011.00772.x) [Google Scholar]
- 8.Alexander S, et al. 2011. Opportunities and challenges for ecological restoration within REDD+. Restor. Ecol. 19, 683–689 (doi:10.1111/j.1526-100X.2011.00822.x) [Google Scholar]
- 9.CBD. 2010. Decision adopted by the Conference of the Parties to the Convention on Biological Diversity at its Tenth Meeting: the Strategic Plan for Biodiversity 2011–2020 and the Aichi Biodiversity Targets. See http://www.cbd.int/decisions/cop .
- 10.Silver WL, Ostertag R, Lugo AE. 2000. The potential for carbon sequestration through reforestation of abandoned tropical agricultural and pasture lands. Restor. Ecol. 8, 394–407 (doi:10.1046/j.1526-100x.2000.80054.x) [Google Scholar]
- 11.Marín-Spiotta E, Sharma S. 2012. Carbon storage in successional and plantation forest soils: a tropical analysis. Glob. Ecol. Biogeogr. 22, 105–117 (doi:10.1111/j.1466-8238.2012.00788.x) [Google Scholar]
- 12.Anderson KJ, Allen AP, Gillooly JF, Brown JH. 2006. Temperature-dependence of biomass accumulation rates during secondary succession. Ecol. Lett. 9, 673–682 (doi:10.1111/j.1461-0248.2006.00914.x) [DOI] [PubMed] [Google Scholar]
- 13.Johnson CM, Zarin DJ, Johnson AH. 2000. Post-disturbance aboveground biomass accumulation in global secondary forests. Ecology 81, 1395–1401 (doi:10.1890/0012-9658(2000)081[1395:pdabai]2.0.co;2) [Google Scholar]
- 14.Brown S, Lugo AE. 1990. Tropical secondary forests. J. Trop. Ecol. 6, 1–32 (doi:10.1017/S0266467400003989) [Google Scholar]
- 15.Hughes RF, Kauffman JB, Jaramillo VJ. 1999. Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of México. Ecology 80, 1892–1907 (doi:10.2307/176667) [Google Scholar]
- 16.Saldarriaga JG, West DC, Tharp M, Uhl C. 1988. Long-term chronosequence of forest succession in the upper Rio Negro of Colombia and Venezuela. J. Ecol. 76, 938–958 (doi:10.2307/2260625) [Google Scholar]
- 17.Cifuentes-Jara M. 2008. Aboveground biomass and ecosystem carbon pools in tropical secondary forests growing in six life zones of Costa Rica. Ann Arbor, MI: ProQuest [Google Scholar]
- 18.Read L, Lawrence D. 2003. Recovery of biomass following shifting cultivation in dry tropical forests of the Yucatan. Ecol. Appl. 13, 85–97 (doi:10.1890/1051-0761(2003)013[0085:ROBFSC]2.0.CO;2) [Google Scholar]
- 19.Guo LB, Gifford RM. 2002. Soil carbon stocks and land use change: a meta analysis. Glob. Change Biol. 8, 345–360 (doi:10.1046/j.1354-1013.2002.00486.x) [Google Scholar]
- 20.Rhoades CC, Eckert GE, Coleman DC. 2000. Soil carbon differences among forest, agriculture, and secondary vegetation in Lower Montane Ecuador. Ecol. Appl. 10, 497–505 (doi:10.1890/1051-0761(2000)010[0497:SCDAFA]2.0.CO;2) [Google Scholar]
- 21.Neumann-Cosel L, Zimmermann B, Hall JS, van Breugel M, Elsenbeer H. 2011. Soil carbon dynamics under young tropical secondary forests on former pastures: a case study from Panama. Forest Ecol. Manag. 261, 1625–1633 (doi:10.1016/j.foreco.2010.07.023) [Google Scholar]
- 22.Saynes V, Hidalgo C, Etchevers JD, Campo JE. 2005. Soil C and N dynamics in primary and secondary seasonally dry tropical forests in Mexico. Appl. Soil Ecol. 29, 282–289 (doi:10.1016/j.apsoil.2004.11.007) [Google Scholar]
- 23.Dunn RR. 2004. Recovery of faunal communities during tropical forest regeneration. Conserv. Biol. 18, 302–309 (doi:10.1111/j.1523-1739.2004.00151.x) [Google Scholar]
- 24.Dent DH, Wright SJ. 2009. The future of tropical species in secondary forests: a quantitative review. Biol. Conserv. 142, 2833–2843 (doi:10.1016/j.biocon.2009.05.035) [Google Scholar]
- 25.Chazdon RL, Peres CA, Dent D, Sheil D, Lugo AE, Lamb D, Stork NE, Miller SE. 2009. The potential for species conservation in tropical secondary forests. Conserv. Biol. 23, 1406–1417 (doi:10.1111/j.1523-1739.2009.01338.x) [DOI] [PubMed] [Google Scholar]
- 26.Corlett RT. 1994. What is secondary forest? J. Trop. Ecol. 10, 445–447 (doi:10.1017/S0266467400008129) [Google Scholar]
- 27.Newbold T, Scharlemann JPW, Butchart SHM, Şekercioğlu ÇH, Alkemade R, Booth H, Purves DW. 2013. Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc. R. Soc. B 280, 20122131 (doi:10.1098/rspb.2012.2131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pullin AS, Stewart GB. 2006. Guidelines for systematic review in conservation and environmental management. Conserv. Biol. 20, 1647–1656 (doi:10.1111/j.1523-1739.2006.00485.x) [DOI] [PubMed] [Google Scholar]
- 29.Gibson L, et al. 2011. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (doi:10.1038/nature10425) [DOI] [PubMed] [Google Scholar]
- 30.Holdridge LR. 1967. Life zone ecology. San Jose, CA: Tropical Science Center [Google Scholar]
- 31.Johnson EA, Miyanishi K. 2008. Testing the assumptions of chronosequences in succession. Ecol. Lett. 11, 419–431 (doi:10.1111/j.1461-0248.2008.01173.x) [DOI] [PubMed] [Google Scholar]
- 32.Baker TR, et al. 2004. Increasing biomass in Amazonian forest plots. Phil. Trans. R. Soc. Lond. B 359, 353–365 (doi:10.1098/rstb.2003.1422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rey Benayas JM, Newton AC, Diaz A, Bullock JM. 2009. Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science 325, 1121–1124 (doi:10.1126/science.1172460) [DOI] [PubMed] [Google Scholar]
- 34.Moreno-Mateos D, Power ME, Comín FA, Yockteng R. 2012. Structural and functional loss in restored wetland ecosystems. PLoS Biol. 10, e1001247 (doi:10.1371/journal.pbio.1001247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putz FE, et al. 2012. Sustaining conservation values in selectively logged tropical forests: the attained and the attainable. Conserv. Lett. 5, 296–303 (doi:10.1111/j.1755-263X.2012.00242.x) [Google Scholar]
- 36.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 37.Chave J, et al. 2005. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 (doi:10.1007/s00442-005-0100-x) [DOI] [PubMed] [Google Scholar]
- 38.Barlow J, et al. 2007. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl Acad. Sci. USA 104, 18 555–18 560 (doi:10.1073/pnas.0703333104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chazdon RL, et al. 2011. A novel statistical method for classifying habitat generalists and specialists. Ecology 92, 1332–1343 (doi:10.1890/10-1345.1) [DOI] [PubMed] [Google Scholar]
- 40.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35 (doi:10.1007/s00265-010-1029-6) [Google Scholar]
- 41.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (doi:10.1111/j.2041-210x.2012.00261.x) [Google Scholar]
- 42.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 43.Barton K.2013. MuMIn: multi-model inference (1.9.5 ed). See http://cran.r-project.org/web/packages/MuMIn/index.html .
- 44.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer [Google Scholar]
- 45.Saatchi SS, et al. 2011. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl Acad. Sci. USA 108, 9899–9904 (doi:10.1073/pnas.1019576108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guariguata MR, Ostertag R. 2001. Neotropical secondary forest succession: changes in structural and functional characteristics. Forest Ecol. Manag. 148, 185–206 (doi:10.1016/S0378-1127(00)00535-1) [Google Scholar]
- 47.Lindenmayer DB, et al. In press New policies for old trees: averting a global crisis in a keystone ecological structure. Conserv. Lett. (doi:10.1111/conl.12013) [Google Scholar]
- 48.van Breugel M, Ransijn J, Craven D, Bongers F, Hall JS. 2011. Estimating carbon stock in secondary forests: decisions and uncertainties associated with allometric biomass models. Forest Ecol. Manag. 262, 1648–1657 (doi:10.1016/j.foreco.2011.07.018) [Google Scholar]
- 49.Montgomery RA, Chazdon RL. 2001. Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 82, 2707–2718 (doi:10.2307/2679955) [Google Scholar]
- 50.Cairns MA, Brown S, Helmer EH, Baumgardner GA. 1997. Root biomass allocation in the world's upland forests. Oecologia 111, 1–11 (doi:10.1007/s004420050201) [DOI] [PubMed] [Google Scholar]
- 51.Fearnside PM, Guimarães WM. 1996. Carbon uptake by secondary forests in Brazilian Amazonia. Forest Ecol. Manag. 80, 35–46 (doi:10.1016/0378-1127(95)03648-2) [Google Scholar]
- 52.Mokany K, Raison R, Prokushkin AS. 2006. Critical analysis of root: shoot ratios in terrestrial biomes. Glob. Change Biol. 12, 84–96 (doi:10.1111/j.1365-2486.2005.001043.x) [Google Scholar]
- 53.Don A, Schumacher J, Freibauer A. 2011. Impact of tropical land-use change on soil organic carbon stocks: a meta-analysis. Glob. Change Biol. 17, 1658–1670 (doi:10.1111/j.1365-2486.2010.02336.x). [Google Scholar]
- 54.Marin-Spiotta E, Silver WL, Swanston CW, Ostertag R. 2009. Soil organic matter dynamics during 80 years of reforestation of tropical pastures. Glob. Change Biol. 15, 1584–1597 (doi:10.1111/j.1365-2486.2008.01805.x) [Google Scholar]
- 55.Chazdon RL, Letcher SG, van Breugel M, Martínez-Ramos M, Bongers F, Finegan B. 2007. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Phil. Trans. R. Soc. B 362, 273–289 (doi:10.1098/rstb.2006.1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eaton JM, Lawrence D. 2009. Loss of carbon sequestration potential after several decades of shifting cultivation in the Southern Yucatán. Forest Ecol. Manag. 258, 949–958 (doi:10.1016/j.foreco.2008.10.019) [Google Scholar]
- 57.Lawrence D. 2005. Biomass accumulation after 10–200 years of shifting cultivation in Bornean rain forest. Ecology 86, 26–33 (doi:10.1890/03-0564) [Google Scholar]
- 58.KöSter N, Friedrich K, Nieder J, Barthlott W. 2009. Conservation of epiphyte diversity in an andean landscape transformed by human land use Conservación de la Diversidad de Epífitas en una Paisaje Andino Transformado por Uso de Suelo Humano. Conserv. Biol. 23, 911–919 (doi:10.1111/j.1523-1739.2008.01164.x) [DOI] [PubMed] [Google Scholar]
- 59.Woods CL, DeWalt SJ. 2013. The conservation value of secondary forests for vascular epiphytes in Central Panama. Biotropica 45, 119–127 (doi:10.1111/j.1744-7429.2012.00883.x) [Google Scholar]
- 60.Benitez-Malvido J. 1998. Impact of forest fragmentation on seedling abundance in a tropical rain forest. Conserv. Biol. 12, 380–389 (doi:10.1111/j.1523-1739.1998.96295.x) [Google Scholar]
- 61.Turner IM, Corlett TR. 1996. The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol. Evol. 11, 330–333 (doi:10.1016/0169-5347(96)10046-X) [DOI] [PubMed] [Google Scholar]
- 62.Condit R, et al. 2002. Beta-diversity in tropical forest trees. Science 295, 666–669 (doi:10.1126/science.1066854) [DOI] [PubMed] [Google Scholar]
- 63.Ramage BS, et al. 2012. Pseudoreplication in tropical forests and the resulting effects on biodiversity conservation. Conserv. Biol. 27, 364–372 (doi:10.1111/cobi.12004) [DOI] [PubMed] [Google Scholar]
- 64.Ebeling J, Yasué M. 2008. Generating carbon finance through avoided deforestation and its potential to create climatic, conservation and human development benefits. Phil. Trans. R. Soc. B 363, 1917–1924 (doi:10.1098/rstb.2007.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venter O, Meijaard E, Possingham H, Dennis R, Sheil D, Wich S, Hovani L, Wilson K. 2009. Carbon payments as a safeguard for threatened tropical mammals. Conserv. Lett. 2, 123–129 (doi:10.1111/j.1755-263X.2009.00059.x) [Google Scholar]
- 66.Bekessy SA, Wintle BA. 2008. Using carbon investment to grow the biodiversity bank. Conserv. Biol. 22, 510–513 (doi:10.1111/j.1523-1739.2008.00943.x) [DOI] [PubMed] [Google Scholar]
- 67.Gardner TA, Barlow J, Parry LW, Peres CA. 2007. Predicting the uncertain future of tropical forest species in a data vacuum. Biotropica 39, 25–30 (doi:10.1111/j.1744-7429.2006.00228.x) [Google Scholar]
- 68.IUCN SSC. 2001. IUCN Red List categories and criteria: v 3.1. Prepared by the IUCN Species Survival Commission; See http://www.iucnredlist.org/technical-documents/categories-and-criteria/2001-categories-criteria. [Google Scholar]
- 69.Benayas JMR, Bullock JM, Newton AC. 2008. Creating woodland islets to reconcile ecological restoration, conservation, and agricultural land use. Front. Ecol. Environ. 6, 329–336 (doi:10.1890/070057) [Google Scholar]