Abstract
Carbon and oxygen stable isotopes within modern and fossil tooth enamel record the aspects of an animal's diet and habitat use. This investigation reports the first isotopic analyses of enamel from a large chimpanzee community and associated fauna, thus providing a means of comparing fossil ape and early hominin palaeoecologies with those of a modern ape. Within Kibale National Park forest, oxygen isotopes differentiate primate niches, allowing for the first isotopic reconstructions of degree of frugivory versus folivory as well as use of arboreal versus terrestrial resources. In a comparison of modern and fossil community isotopic profiles, results indicate that Sivapithecus, a Miocene ape from Pakistan, fed in the forest canopy, as do chimpanzees, but inhabited a forest with less continuous canopy or fed more on leaves. Ardipithecus, an early hominin from Ethiopia, fed both arboreally and terrestrially in a more open habitat than inhabited by chimpanzees.
Keywords: stable isotopes, chimpanzees, Sivapithecus, Ardipithecus
1. Introduction
Great apes have larger bodies, relatively larger brains and slower life histories than other primates. Given that larger bodies and brains are both metabolically expensive [1,2], and longer periods of lactation require greater calories per reproductive event for mothers [3], modern apes require a high-quality diet, and most meet this demand with ripe fruit in rainforests. Chimpanzees feed more on ripe fruits than do frugivorous (fruit-eating) monkeys sharing the same forest, and their diets are of higher quality than those of frugivorous monkeys when measured for carbohydrates, fibre and antifeedants [4,5]. Gorillas are an exception among apes in subsisting on lower-quality foods because their extremely large guts allow for more fibrous diets. A few chimpanzee populations live in drier regions than in rainforests, but these populations live at very low densities, and whereas their home ranges are extensive during the wet season, during dry seasons, they are limited to riverine forests similar to typical ape habitats [6,7].
Chimpanzees are relevant as models for early hominin evolution, because molecular evidence indicates they are humans' closest living relatives, they appear little changed from the African ape ancestor, and early hominins have chimpanzee-like features [8]. Furthermore, many fossil apes as well as early hominins were similar to chimpanzees in body size, brain size and maturation patterns. Given the energetic demands of these traits, two particularly interesting questions in palaeoanthropology suggest themselves: how did fossil apes that lived throughout Africa, Asia and Europe differ in their habitat requirements from their modern equatorial rainforest-bound ripe-fruit-specialist cousins? And how did early hominins differ from their ape ancestors?
To address these questions, tooth enamel isotopic values from chimpanzees and other mammals from Kibale National Park are compared with isotopic values of the Miocene ape Sivapithecus and its fauna from the Siwalik Group of Pakistan both before and after Sivapithecus become extinct [9]. The purpose of this study is to understand whether Sivapithecus had habitat requirements similar to those of modern apes, and to document changes in habitat leading to Sivapithecus’s extinction. Kibale fauna are also compared with early hominin Ardipithecus fauna from Aramis to determine how Ardipithecus differed from its ape ancestors [10].
Isotopic values of enamel reflect the isotopic values of an animal's diet and drinking habits during tooth formation [11–14]. Tooth carbon (δ13C) values in the range of −20 to −8‰ reflect feeding on C3 plants, or all trees and shrubs, whereas values of +1 to +4‰ reflect a diet of pure grazing on C4 grasses or sedges in environments with hot, wet growing seasons. Carbon and oxygen (δ18O) isotopic values are also affected by the degree of water and light stress plants undergo. Plants under irradiance stress must become water-use-efficient to function at lower respiration rates but still absorb CO2 for plant growth. This process leads to enrichment of 13C in plants in evaporative habitats [15]. Plants in evaporative habitats are also enriched in 18O, because the lighter oxygen isotope evaporates more readily [16]. Therefore, the lowest δ13C and δ18O values represent forest floors where plants are shaded and under less evaporative stress, whereas the highest values represent open habitats such as savannahs or forest upper canopies where light and evaporative stresses are high. Furthermore, in forests with canopies so dense that air below the canopy cannot exchange CO2 with air above, carbon values are extremely depleted [17]. Tooth enamel records the carbon signal from vegetation eaten, with an enrichment in δ13C owing to fractionation from metabolic processes. The enrichment factor for species in this study is assumed to be 14‰, as found in an extensive analysis of ungulates [18]. It should be noted, however, that these ungulates were all ruminant foregut fermenters, and suids and primates may have slightly lower enrichment factors owing to different digestive systems, but this remains to be determined.
2. Material and methods
Fifty-seven individuals representing 11 species were sampled from Kanyawara and Ngogo within Kibale National Park, Uganda (latitude 0.433′ N, longitude 30.4′ E). Taxa include six primates—Pan troglodytes (chimpanzee), Papio anubis (olive baboon), Colobus guereza (black-and-white colobus), Procolobus badius (red colobus), Cercopithecus ascanius (redtail) and Cercopithecus l'hoesti (L'Hoest's monkey); three suids—Hylochoerus meinertzhageni (giant forest hog), Potamochoerus porcus (red river hog or bushpig) and Phacochoerus aethiopicus (warthog); and two bovids—Tragelaphus spekii (sitatunga) and Cephalophus callipygus (red duiker). Diets and habitat use are known for these species from behavioural, scat and stomach content studies.
Kanyawara is located at 1500 m altitude and comprises moist evergreen forest (60–70%) with canopy heights of 20–30 m, tall grassland, swamps and softwood plantations [19,20]. Ngogo is 10 km from Kanyawara at 1350 m elevation [21] and remains primary forest, while logging has occurred within Kanyawara. Kanyawara and Ngogo fauna were collected in the 1990s when carcasses were found in the forest. Ngogo faunal remains were collected from crowned-hawk eagle nests [22].
Enamel was removed by high-speed drill. For most Kibale individuals, samples were taken from premolars and molars with the average value per individual reported. Samples were washed with 3% hydrogen peroxide for 15 min and rinsed, followed by 0.1 M acetic acid for 15 min and rinsed. Samples were reacted at 77°C ± 1°C with anhydrous phosphoric acid for 17 min in a Finnigan MAT Kiel IV device coupled to a Finnigan MAT 253 isotope ratio mass spectrometer. Isotopic ratios are presented in the per mil (‰) notation δ13C (or 18O) = (Rsample/RPDB − 1) × 1000, where Rsample and RPDB are the ratios 13C/12C (or 18O/16O) in the sample and standard, respectively, and the isotope reference standard is PDB.
Kibale samples were compared with 101 fossil teeth from 17 taxa from Siwaliks, Pakistan from two time-intervals: 9.3–9.2 Ma, when Sivapithecus was present, and 8.1–8.0 Ma, shortly after Sivapithecus went extinct [9]. Taxa from the ape interval comprise five Sivapithecus, 23 suoids (including 12 Propotamochoerus hysudricus), four anthracotheres, 10 proboscidians, nine giraffes, five rhinocerotids, a chalicothere, nine bovids, 14 tragulids and 10 equids. Kibale fauna were also compared with 4.4 Ma Ardipithecus fauna from Aramis, Ethiopia, comprising seven Ardipithecus, monkeys (represented by 20 Kuseracolobus and 14 Pliopapio), 22 suids (including seven Kolpochoerus), 40 bovids, 13 carnivores, 15 proboscidians, eight giraffes, nine hippopotamids, 18 rhinocerotids and 11 equids [10].
3. Results and discussion
(a). Kibale fauna isotopic results
The δ13C results for Kibale fauna fall between −18.3 and −2.4‰, representing Kibale's spectrum of habitats from rainforest to grasses, with an average value of −15.0‰ (see electronic supplementary material, table S1; figure 1). L'Hoest's monkeys and sitatunga represent the most depleted values (averaging −17.8‰ and −17.3‰), indicating feeding in the most closed or wettest parts of the forest. A grazing warthog has the highest δ13C value (−2.4‰). When compared with isotopic analyses from the Ituri forest in the Democratic Republic of Congo [23], both datasets have in common a chimpanzee, olive baboons, red colobus, sitatunga, bushpigs and giant forest hogs. For these species, the Kibale individuals are on average 2.5‰ higher in δ13C values than those of the Ituri, compatible with the Ituri Forest having a more closed canopy. Even with a less continuous canopy than the Ituri, the Kibale forest upper crown receives almost twice as much light as the lower crown [24], but despite the height gradient in irradiance stress, carbon values of Kibale tooth enamel do not reflect differences in canopy feeding height. Within primates, both chimpanzees and black-and-white colobus feed primarily below 13 m, whereas redtails and red colobus feed primarily above 13 m, with red colobus spending more time above 25 m than any of the other monkeys [25–28]. If irradiance stress, and hence canopy height, were the sole determining isotopic factor, chimpanzees and black-and-white colobus should have the lowest δ13C values. Instead, chimpanzees and black-and-white colobus yield the highest δ13C values for primates (−14.5‰ and −14.7‰), with chimpanzees significantly higher than redtails (−16.5‰) and red colobus (−15.3‰; Mann–Whitney Z = −4.29, p < 0.001; Z = −2.21, p = 0.027). Nor do the carbon isotopic differences correspond strictly to degree of frugivory (fruit-eating) and folivory (leaf-eating). Frugivorous redtail carbon values are significantly lower than those of folivorous colobus monkeys (Mann–Whitney with black-and-white colobus Z = −2.63, p = 0.009; with red colobus Z = −3.34, p = 0.001), but chimpanzees are not. Species differences in carbon isotopic values are likely to be driven by multiple factors, including food selection, use of central forest versus forest fringe and possible differences in fractionation factors between different digestive systems. While it is possible some variability is introduced by differences in juvenile foraging strategies as they mature, because tooth enamel forms early in life, data from primates suggest that juvenile and adult diets greatly overlap. Juveniles and mothers are usually in close proximity to each other, often arriving at food patches together, and isotopic investigations of diet at different life stages yield homogeneous results [29–31].
Figure 1.
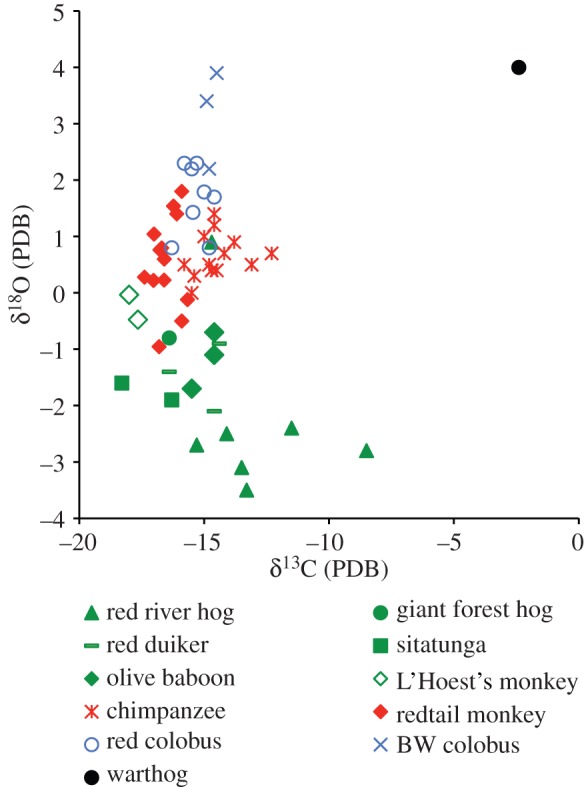
Carbon and oxygen isotopic values for Kibale fauna. Individuals cluster within a species, indicating niche separation. Oxygen values distinguish between forest floor and canopy feeders; within the canopy, oxygen distinguishes between frugivores and folivores. BW, black-and-white. (Online version in colour.)
Kibale δ18O values, however, suggest a clear separation of species based on forest floor versus arboreal feeding and degree of frugivory versus folivory within the canopy. Kibale values fall between −3.5‰ and +4‰ (see electronic supplementary material, table S1 and figure 1). Warthogs yield the highest δ18O values, as they did with carbon isotopes, again indicating feeding in the most open areas. When species are grouped into terrestrial feeders (L'Hoest's monkeys, olive baboons, sitatunga, red duiker, bushpigs, giant forest hogs and warthog), canopy frugivores (chimpanzees and redtail monkeys) and canopy folivores (red colobus and black-and-white colobus), there are significant differences in oxygen between all groups (floor versus canopy frugivore Mann–Whitney Z = −2.89, p = 0.004; floor versus canopy folivore Z = −2.73, p = 0.006; canopy frugivore versus canopy folivore Z = −4.01, p < 0.001). Forest floor species have on average the lowest δ18O values (−1.3‰), with canopy frugivores having higher values (average 0.6‰), and canopy folivores possessing the highest average value (2.1‰). Thus, there is a height effect from floor to canopy, but within the canopy, δ18O differences reflect amount of fruit versus leaves. Given the role of leaves in transpiration as well as a higher moisture content in fruits, fruits are likely to have lower values than leaves, but this has not been tested. Even within the canopy folivore group, black-and-white colobus monkeys have higher δ18O values (3.2‰) than red colobus (1.7‰; Mann–Whitney Z = −1.95, p = 0.051), probably owing to greater fruit consumption by red colobus [28,32,33].
The Kibale fauna demonstrate clear distinctions in carbon and oxygen isotopes between species associated with different diets and feeding positions within the forest. The combination of oxygen and carbon isotopic values allows for distinctions between forest floor (low δ13C and δ18O), forest canopy (low δ13C and high δ18O) and open habitat (high δ13C and δ18O), and possible affinities for open aquatic habitats not represented here could include hippopotamuses (high δ13C and low δ18O). The Kibale data provide ecological inferences about fossil primates. Here, Kibale fauna are first compared with fauna from two time-intervals in the Miocene Siwalik Group of Pakistan—when the ape Sivapithecus is present and shortly after Sivapithecus went extinct [9]. Kibale species are then compared with Pliocene fauna at Aramis, Ethiopia, including the early hominin Ardipithecus [10]. The goal is to determine how similar the fossil ape and hominin fauna were to modern chimpanzees in forest use, arboreality and frugivory. To make the comparison, fossil δ13C values were adjusted to correct for shifts in atmospheric carbon isotope ratios over geologic time. For Siwalik samples, 2.5‰ was subtracted, and for Aramis samples, 1.5‰ was subtracted to reflect changes measured in deep ocean carbonates over the past 20 million years [34,35].
(b). Kibale–Siwalik comparisons
When Kibale fauna are compared with Sivapithecus fauna, there is overlap in δ13C values between the datasets, indicating the presence of some Miocene forests isotopically similar to those inhabited by chimpanzees (figure 2). Despite the overlap, Siwalik fauna have higher δ13C values than Kibale fauna (average −13.8 versus −15.0; Mann–Whitney Z = −4.86, p < 0.001). Even when the Siwalik faunal list is limited to primates, suids and bovids as a comparison with those Kibale taxonomic groups, the Siwalik fauna has significantly higher δ13C values (average −14.4 versus −15.0; Z = −2.88, p = 0.004). Propotamochoerus, a Miocene member of the suid tribe of giant forest hogs and bushpigs, exhibits dental microwear similar to that of modern bushpigs [36]. It does not significantly differ from the Kibale bushpigs for carbon isotopes (average −14.3‰ versus −13‰). Sivapithecus has lower carbon values than do chimpanzees (−15.5 versus 14.5; Z = −2.12, p = 0.034), suggesting that it possibly relied on more central parts of the forest than do chimpanzees.
Figure 2.
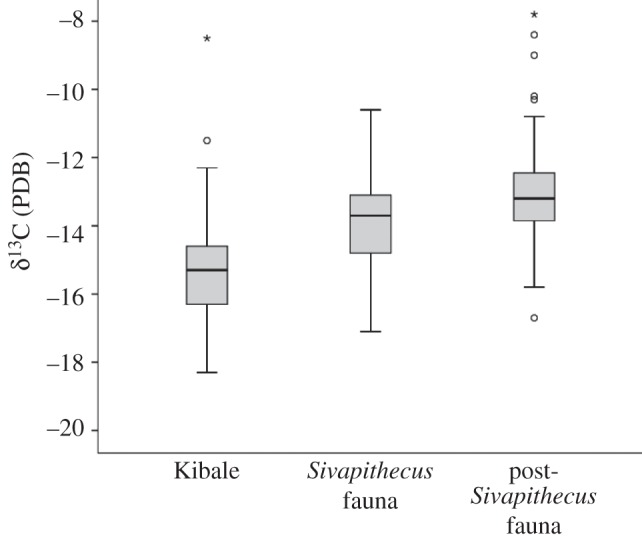
Distribution of carbon values for Kibale, Sivapithecus faunas, and post-Sivapithecus faunas. The Sivapithecus fauna overlap with Kibale fauna but overall exhibit higher carbon values, indicative of more woodland habitat. The post-Sivapithecus fauna indicate a shift to even more open habitat and less overlap with Kibale. For each faunal set, the middle line represents the median. The bottom of the box is the 25th percentile, while the top of the box is the 75th percentile. T-bars extend to 1.5 times the height of the box or to the minimum and maximum values. Points are outliers, with asterisks being extreme outliers more than three times the height of the box. For Kibale, warthogs are included in the analysis but not shown on graph, given their extreme value of −2.4‰.
In both the Kibale and Siwalik faunas, bushpigs exhibit the lowest δ18O values. Isotopic studies of modern bushpigs suggest that they are not sensitive to water deficits or evaporation [37], and the lowest δ18O value for Siwalik bushpigs (−9.2‰) is close to the lowest palaeosol δ18O value during the same time-interval (−9.9‰) [9], also suggesting Siwalik bushpigs were evaporation-insensitive. Therefore, among both faunas, bushpigs probably best reflect forest drinking water δ18O values. While the δ13C offsets between ape and bushpig are comparable between faunal datasets (Sivapithecus on average 1.5‰ lower than Propotamochoerus, chimpanzees 1.2‰ lower than Potamochoerus), Sivapithecus has δ18O values 4.7‰ higher on average than its contemporaneous bushpig [9], whereas chimpanzees differ by only 3.0‰ (figure 1). The 4.7‰ difference for Sivapithecus falls between the 4.0‰ red colobus–bushpig gap and the 5.7‰ black-and-white colobus–bushpig gap. Sivapithecus δ18O values suggest that it fed arboreally, but it fed on foods under greater evaporative stress than those of chimpanzees, perhaps owing to feeding higher in the canopy, feeding in a less continuous canopy or relying more on leaves.
When the post-Sivapithecus extinction time period of the Siwaliks is compared with Kibale fauna, there is less overlap, with the Siwaliks significantly higher in δ13C values (average −13.1, Mann–Whitney Z = −6.33, p < 0.001; figure 3). When the two datasets are limited to bovids and suids, the taxonomic groups they have in common, this difference remains marginally significant (average −13.3 versus −13.6; Z = −1.95, p = 0.051). The decrease in taxa exhibiting low carbon values indicates a loss of forest that coincides with the extinction of Sivapithecus. The post-Sivapithecus level has significantly higher δ13C and δ18O values than the Sivapithecus level, indicating a shift to more open habitat, such as woodlands as opposed to forest [9].
Figure 3.
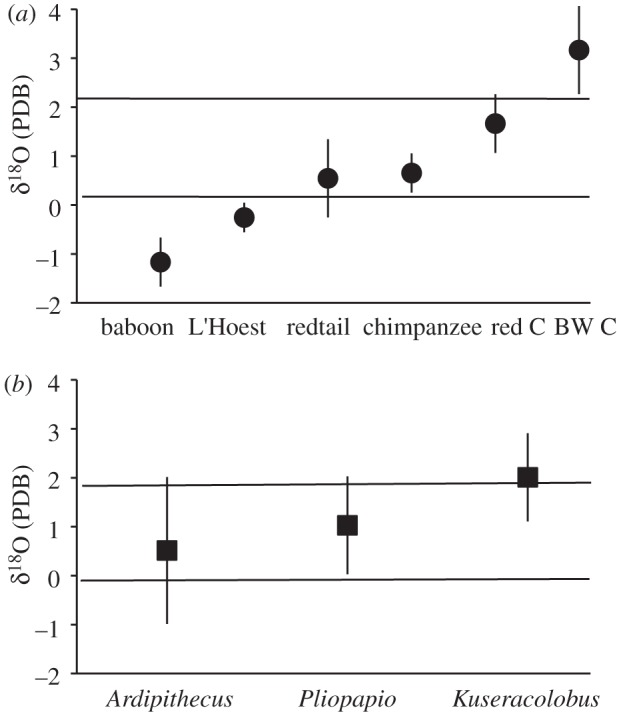
(a) When Kibale primate δ18O values are divided into thirds, terrestrial feeders fall into the lower third, arboreal frugivores fall into the middle third, and arboreal folivores fall at the top of the middle third and into the upper third. (b) Within Aramis primates, Ardipithecus falls into the lower and middle thirds, indicative of feeding both terrestrially and on arboreal fruits. Pliopapio falls within the middle third, comparable with Kibale arboreal frugivores. Kuseracolobus falls into the upper third, comparable with Kibale arboreal folivores. Each species is represented by its average and standard deviation. BW, black-and-white; C, colobus.
(c). Kibale–Aramis comparisons
When the Kibale fauna are compared with Ardipithecus and its associated fauna from Aramis [10], disparities between species composition as well as δ13C values (average −7.2‰; Z = −9.91, p < 0.001) indicate a more open habitat for Ardipithecus than chimpanzees. However, some species within the Aramis dataset share taxonomic affinities with species from Kibale, suggesting that the two ecosystems may have similar microhabitats (in particular forests) in common. Ardipithecus is described as a hominin temporally close to the divergence of hominins from chimpanzees, Kuseracolobus is a large colobus monkey, Pliopapio is similar to a small baboon [10], and Kolpochoerus is a member of the suid tribe of giant forest hogs and bushpigs [38,39]. When related taxa are compared, the Ardipithecus fauna have significantly higher δ13C values, averaging −12.1‰ versus −14.6‰ (Mann–Whitney Z = −4.57, p < 0.001). All Aramis species except the colobus monkey have significantly higher δ13C values than the Kibale counterpart (Ardipithecus–chimpanzee, Z = −3.49, p < 0.001; Pliopapio–baboon, Z = −2.67, p = 0.008; Kolpochoerus–bushpig, Z = −2.75, p = 0.006), suggesting that the forest component at Aramis was unlike rainforest and was associated with more woodland. Primates from Kibale and Aramis share similar ranges of δ18O values (Kibale −1.7 to 3.9‰, Aramis −1.8 to 3.6‰). Within the Kibale primate range, terrestrial primates fall into the lower third, whereas frugivores fall into the middle third (figure 3). Red colobus, the most omnivorous colobus monkey, falls at the upper middle third, and black-and-white colobus, the most folivorous colobus monkey, falls into the top third. Within the Aramis primate range, Kuseracolobus, such as the Kibale colobus monkeys, falls within the top third and upper middle third of δ18O values, consistent with post-cranial evidence suggesting arboreality [10] and a primarily folivorous diet, but not as specialized as black-and-white colobus. Pliopapio falls within the middle third, comparable with arboreal frugivores in the Kibale dataset. This isotopic evidence for arboreality corroborates arboreal post-cranial features [10]. Ardipithecus exhibits a wide range of δ18O values (3.9‰ versus range of 1.4‰ for chimpanzees) and falls within both the lower and middle third of the Aramis primate range, suggesting feeding both terrestrially and arboreally. Alternatively, the wide range might reflect feeding in both wooded and more open environments, but if so, individuals with the highest δ18O values should also have the highest δ13C values, and they do not. Another alternative is that while the Kibale specimens were collected over a few years, the Aramis locality could represent fossil accumulation over thousands of years, and the wide range of δ18O values might reflect changes in vegetation or climate regime through time. However, Kibale black-and-white colobus and Aramis colobus do not differ in the standard deviation of their δ18O values (figure 3), suggesting the Aramis colobus at least were not affected by temporal mixing.
4. Conclusion
This comparison of fossil ape and hominin habitats with a modern chimpanzee site yields new insights into reconstructing palaeoecologies by combining carbon and oxygen isotopic signals to distinguish feeding habits within a forest. δ18O values were especially useful for distinguishing between terrestrial feeding, arboreal fruit-eating and arboreal leaf-eating. Isotopic analyses suggest that Sivapithecus was an arboreal feeder, whereas Ardipithecus fed both terrestrially and on arboreal fruits.
The isotopic distinction between arboreal versus terrestrial feeding could be particularly useful for reconstructions of cattarhine origins, Miocene hominoids and early hominins lacking post-cranial evidence. For example, there has been much debate on whether the earliest monkeys were arboreal or semi-terrestrial, and folivorous or frugivorous. This question has implications for understanding the divergence of Old World monkeys and apes as well as the evolution of Old World monkey bilophodont molars, a tooth morphology associated with shearing leaves in modern animals. Central to this argument is Victoriapithecus, one of the oldest Old World monkeys. Its frugivorous dentition and semi-terrestrial post-cranial adaptations cast doubt on hypotheses that reconstruct the earliest monkeys as arboreal folivores [40]. The Kibale results suggest a new means of exploring the ecological divergences of cercopithecines, colobines and hominoids.
With respect to ape evolution, most Miocene apes do not exhibit the suite of suspensory (below-branch hanging) adaptations shared by extant apes, and some are believed to have been terrestrial. Griphopithecus, a middle Miocene ape, was likely to have included some terrestrial activity in its repertoire based upon phalanx morphology [41]. One Griphopithecus specimen and its contemporaneous fauna from Pasalar, Turkey, were among the first Miocene sites sampled for carbon and oxygen isotopes [16]. In the light of the Kibale faunal isotopic analyses, the Griphopithecus specimen falls with terrestrial contemporaneous fauna. Unlike Sivapithecus, it does not yield the combination of low δ13C and high δ18O values of arboreal primates and contemporaneous giraffids. Terrestrial activity has been suggested for other Miocene hominoids based upon phalanges, body size and dental morphology, such as Nakalipithecus and Ouranopithecus [42], and the Kibale results suggest an isotopic means of testing these hypotheses.
Within the hominin lineage, australopithecine anatomy reflecting both arboreal and terrestrial locomotion is replaced by obligate bipedalism in Homo erectus. Little is known of locomotion for hominins falling chronologically in-between, however, given a dearth of post-cranial for H. habilis and H. rudolfensis. The Kibale results could provide us an isotopic method of establishing the early Homo locomotor repertoires in greater detail using the abundant dental material for these hominins as well as their surrounding fauna.
Acknowledgements
Uganda Wildlife Authority and Makerere University Biological Field Station sponsor long-term research in Kibale National Park. This manuscript greatly benefited from suggestions of two anonymous reviewers.
Funding statement
R. Wrangham and M. Muller provided Kanyawara specimens collected under funding from NSF, Leakey Foundation, the National Geographic Society and Wenner-Gren. J. Mitani provided Ngogo specimens collected under grants NSF Presidential Faculty Fellowship Award SBR-9253590 and NSF IOB-0516644. R. Kityo provided assistance in faunal identification. This research was supported by the American School of Prehistoric Research.
References
- 1.McNab B. 1990. The physiological significance of body size. In Body size in mammalian paleobiology (eds Damuth J, MacFadden B.), pp. 11–23 Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Aiello L, Wheeler P. 1995. The expensive-tissue hypothesis. Curr. Anthropol. 36, 199–221 (doi:10.1086/204350) [Google Scholar]
- 3.Aiello L, Key C. 2002. Energetic consequences of being a Homo erectus female. Am. J. Hum. Biol. 14, 551–565 (doi:10.1002/ajhb.10069) [DOI] [PubMed] [Google Scholar]
- 4.Conklin-Brittain N, Wrangham R, Hunt K. 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. II. Macronutrients. Int. J. Primatol. 19, 971–998 (doi:10.1023/A:1020370119096) [Google Scholar]
- 5.Wrangham R, Conklin-Brittain N, Hunt K. 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance I. Antifeedants. Int. J. Primatol. 19, 949–969 (doi:10.1023/A:1020318102257) [Google Scholar]
- 6.McGrew W, Baldwin P, Tutin C. 1981. Chimpanzees in a hot, dry open habitat: Mt. Asserik, Senegal, West Africa. J. Hum. Evol. 10, 227–244 (doi:10.1016/S0047-2484(81)80061-9) [Google Scholar]
- 7.Baldwin P, McGrew W, Tutin C. 1982. Wide-ranging chimpanzees at Mt. Asserik, Senegal. Int. J. Primatol. 3, 367–385 (doi:10.1007/BF02693739) [Google Scholar]
- 8.Wrangham R, Pilbeam D. 1991. African apes as time machines. In All apes great and small (eds Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J.), pp. 5–17 New York, NY: Kluwer Academic Publishers [Google Scholar]
- 9.Nelson S. 2007. Isotopic reconstructions of habitat change surrounding the extinction of Sivapithecus, a Miocene hominoid, in the Siwalik Group of Pakistan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 243, 204–222 (doi:10.1016/j.palaeo.2006.07.017) [Google Scholar]
- 10.White T, et al. 2009. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science 326, 67–93 (doi:10.1126/science.1175822) [PubMed] [Google Scholar]
- 11.Van der Merwe NJ. 1982. Carbon isotopes, photosynthesis, and archaeology. Am. Sci. 70, 596–606 [Google Scholar]
- 12.Luz B, Kolodny Y. 1985. Oxygen isotope variations in phosphates of biogenic apatites, IV: mammal teeth and bones. Earth Planet. Sci. Lett. 75, 29–36 (doi:10.1016/0012-821X(85)90047-0) [Google Scholar]
- 13.Quade J, Cerling TE, Barry JC, Morgan ME, Pilbeam DR, Chivas AR, Leethorp JA, Van der Merwe NJ. 1992. A 16-Ma record of paleodiet using carbon and oxygen isotopes in fossil teeth from Pakistan. Chem. Geol. 94, 183–192 (doi:10.1016/S0009-2541(10)80003-8) [Google Scholar]
- 14.Wang Y, Cerling TE. 1994. A model of fossil tooth and bone diagenesis: implications for paleodiet reconstruction from stable isotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 281–289 (doi:10.1016/0031-0182(94)90100-7) [Google Scholar]
- 15.Ehleringer JR, Field CB, Lin ZF, Kuo CY. 1986. Leaf carbon isotope and mineral-composition in subtropical plants along an irradiance cline. Oecologia 70, 520–526 (doi:10.1007/BF00379898) [DOI] [PubMed] [Google Scholar]
- 16.Quade J, Cerling TE, Andrews P, Alpagut B. 1995. Paleodietary reconstruction of Miocene faunas from Pasalar, Turkey using stable carbon and oxygen isotopes of fossil tooth enamel. J. Hum. Evol. 28, 373–384 (doi:10.1006/jhev.1995.1029) [Google Scholar]
- 17.Cerling TE, Harris JM, Ambrose SH, Leakey MG, Solounias N. 1997. Dietary and environmental reconstruction with stable isotope analyses of herbivore tooth enamel from the Miocene locality of Fort Ternan, Kenya. J. Hum. Evol. 33, 635–650 (doi:10.1006/jhev.1997.0151) [DOI] [PubMed] [Google Scholar]
- 18.Cerling TE, Harris JM. 1999. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120, 347–363 (doi:10.1007/s004420050868) [DOI] [PubMed] [Google Scholar]
- 19.Struhsaker T. 1997. Ecology of an African rain forest: logging in Kibale and the conflict between conservation and exploitation. Gainesville, FL: University Press of Florida [Google Scholar]
- 20.Wrangham R, Rogers M, Basuta G. 1993. Ape food density in the ground layer in Kibale Forest, Uganda. Afr. J. Ecol. 31, 49–57 (doi:10.1111/j.1365-2028.1993.tb00517.x) [Google Scholar]
- 21.Potts K, Chapman C, Lwanga J. 2009. Floristic heterogeneity between forested sites in Kibale National Park, Uganda: insights into the fine-scale determinants of density in a large-bodied frugiovorous primate. J. Anim. Ecol. 78, 1269–1277 (doi:10.1111/j.1365-2656.2009.01578.x) [DOI] [PubMed] [Google Scholar]
- 22.Mitani J, Sanders W, Lwanga J, Windfelder T. 2001. Predatory behavior of crowned hawk-eagles (Stephanoaetus coronatus) in Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 49, 187–195 (doi:10.1007/s002650000283) [Google Scholar]
- 23.Cerling T, Hart J, Hart T. 2004. Stable isotope ecology in the Ituri forest. Oecologia 138, 5–12 (doi:10.1007/s00442-003-1375-4) [DOI] [PubMed] [Google Scholar]
- 24.Houle A, Chapman C, Vickery W. 2007. Intratree variation in fruit production and implications for primate foraging. Int. J. Primatol. 28, 1197–1217 (doi:10.1007/s10764-007-9214-9) [Google Scholar]
- 25.Kahlenburg S.2006. Female–female competition and male sexual coersion in Kanyawara chimpanzees. PhD thesis, Harvard University, Cambridge, MA.
- 26.Oates J. 1994. The natural history of African colobines. In Colobine monkeys (eds Davies A, Oates J.), pp. 75–128 Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Struhsaker T. 1975. The red colobus monkey. Chicago, IL: University of Chicago Press [Google Scholar]
- 28.Struhsaker T. 1980. Comparison of the behaviour and ecology of red colobus and redtail monkeys in the Kibale Forest, Uganda. Afr. J. Ecol. 18, 35–51 (doi:10.1111/j.1365-2028.1980.tb00269.x) [Google Scholar]
- 29.Pusey A. 1983. Mother–offspring relationships in chimpanzees after weaning. Anim. Behav. 31, 363–377 (doi:10.1016/S0003-3472(83)80055-4) [Google Scholar]
- 30.Janson C, van Shaik C. 1993. Ecological risk aversion in juvenile primates: slow and steady wins the race. In Juvenile primates life history, development, and behavior (eds Pereira M, Fairbanks L.), pp. 57–74 New York, NY: Oxford University Press [Google Scholar]
- 31.Smith C, Morgan M, Pilbeam D. 2010. Isotopic ecology and dietary profiles of Liberian chimpanzees. J. Hum. Evol. 58, 43–55 (doi:10.1016/j.jhevol.2009.08.001) [DOI] [PubMed] [Google Scholar]
- 32.Danish L, Chapman C, Hall M, Rode K, Worman C. 2006. The role of sugar in diet selection in redtail and red colobus monkeys. In Feeding ecology in apes and other primates. ecological, physical and behavioral aspects (eds Hohmann G, Robbins M, Boesch C.), pp. 471–485 Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Chapman C, Chapman L, Gillespie T. 2002. Scale issues in the study of primate foraging: red colobus of Kibale National Park. Am. J. Phys. Anthropol. 117, 349–363 (doi:10.1002/ajpa.10053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (doi:10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 35.Cerling T, Harris J, Leakey M, Passey B, Levin N. 2010. Stable carbon and oxygen isotopes in East African mammals: modern and fossil. In Cenozoic mammals of Africa (eds Werdelin L, Sanders W.), pp. 941–952 Berkeley, CA: University of California Press [Google Scholar]
- 36.Nelson S. 2003. The extinction of Sivapithecus: faunal and environmental changes in the Siwaliks of Pakistan. American School of Prehistoric Research Monographs Boston, MA: Brill Academic Publishers [Google Scholar]
- 37.Levin N, Cerling T, Passey B, Harris J, Ehleringer J. 2006. A stable isotope aridity index for terrestrial environments. Proc. Natl Acad. Sci. USA 103, 11 201–11 205 (doi:10.1073/pnas.0604719103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooke H. 1997. The status of the african fossil suids Kolpochoerus limnetes (Hopwood, 1920), K. phacochoeroides (Thomas, 1884), and ‘K.’ afarensis (Cooke, 1978). Geobios 30, 121–126 (doi:10.1016/S0016-6995(97)80262-8) [Google Scholar]
- 39.Geraads D. 1993. Kolpochoerus phacochoeroides (Thomas, 1884) (Suidae, Mammalia) du Pliocene superieur de Ahl al Oughlam (Casablanca, Maroc). Geobios 26, 731–743 (doi:10.1016/S0016-6995(93)80056-W) [Google Scholar]
- 40.Benefit B. 1999. Victoriapithecus: the key to Old World monkey and catarrhine origins. Evol. Anthropol. 7, 155–174 (doi:10.1002/(SICI)1520-6505(1999)7:5<155::AID-EVAN2>3.0.CO;2-D) [Google Scholar]
- 41.Ersoy A, Kelley J, Andrews P, Alpagut B. 2008. Hominoid phalanges from the middle Miocene site of Pasalar, Turkey. J. Hum. Evol. 54, 518–529 (doi:10.1016/j.jhevol.2007.08.004) [DOI] [PubMed] [Google Scholar]
- 42.Bernor RL. 2007. New apes fill the gap. Proc. Natl Acad. Sci. USA 104, 19 661–19 662 (doi:10.1073/pnas.0710109105) [DOI] [PMC free article] [PubMed] [Google Scholar]


