Abstract
Cuticular hydrocarbons (CHCs) play an essential role in mate recognition in insects but the form and intensity of sexual selection on CHCs has only been evaluated in a handful of studies, and never in a natural population. We quantified sexual selection operating on CHCs in a wild population of sagebrush crickets, a species in which nuptial feeding by females imposes an unambiguous phenotypic marker on males. Multivariate selection analysis revealed a saddle-shaped fitness surface, suggesting a complex interplay between the total abundance of CHCs and specific CHC combinations in their influence on female choice. The fitness surface resulting from two axes of disruptive selection reflected a trade-off between short- and long-chained CHCs, suggesting that males may be sacrificing some level of desiccation resistance in favour of increased attractiveness. There was a significant correlation between male body size and total CHC abundance, suggesting that male CHCs provide females with a reliable cue for maximizing benefits obtained from males. Notwithstanding the conspicuousness of males’ acoustic signals, our results suggest that selection imposed on males via female mating preferences may be far more complex than previously appreciated and operating in multiple sensory modalities.
Keywords: chemical communication, Cyphoderris strepitans, fitness surface, mate choice, multivariate selection analysis
1. Introduction
The importance of visual and acoustic traits in mate choice has been widely addressed in a variety of animal taxa, but the role of chemical cues and signals remains relatively unexplored [1,2]. Although chemical signalling is the most widespread form of communication, our understanding of how sexual selection shapes the evolution of pheromones is poorly developed [1–4]. Cuticular lipids, which primarily function as an evaporation barrier and are virtually ubiquitous in terrestrial arthropods, have been recognized to play an important role in species and mate recognition in insects [5–7]. More recently, there has been a growing appreciation that the evolution of these compounds may be shaped by sexual selection arising through female mating preferences. The form and intensity of sexual selection on cuticular hydrocarbons (CHCs) have been studied extensively but, with the exception of a single study of field crickets [8], only in Drosophila [9,10] and never in the wild. Little is known about the role of sexual selection in shaping CHC profiles in natural populations.
In this study, we assess the form and intensity of sexual selection acting on CHCs of free-living male sagebrush crickets, Cyphoderris strepitans, mating in the field under natural conditions. Measuring the mating success of insects in nature is normally a daunting task [11]. The genus Cyphoderris, however, offers an ideal model system in this regard because mating imposes an unambiguous phenotypic marker on males that results from an unusual form of nuptial feeding by females. The sagebrush cricket, C. strepitans (Orthoptera: Haglidae), is one of only three extant species of hump-winged grigs in North America, relatively obscure ensiferans that are restricted to mountainous areas of western North America [12]. Cyphoderris strepitans occur in high-elevation sagebrush meadows nestled within coniferous forests in Wyoming and Colorado [12]. In Grand Teton National Park, where the majority of field studies of C. strepitans have been conducted [13–16], sexual activity commences in mid-May, an arduous time of year at the higher elevations when patches of snow remain scattered on the ground and night-time temperatures frequently fall below freezing [17]. Each night of the breeding season, males emerge from the ground cover to secure a calling perch in sagebrush or lodgepole pine, where they sing to attract sexually receptive females [18,19].
Once a calling male has attracted a female, the female mounts the male dorsally to initiate a 3–5 min mating that ends with the transfer of a spermatophore to the female [20,21]. During the time that the female remains mounted on the male, she feeds on the tips of the male’s fleshy hind wings and ingests haemolymph seeping from the open wounds that result from nuptial feeding. Thus, it is possible to ascertain whether or not a male has mated merely by inspecting his hind wings for the wounds inflicted by the female while mating. Males exhibit differential mating success based on their previous mating experience: virgin males have a higher probability of obtaining a mating than do non-virgin males of securing an additional mating, a pattern that has been termed the virgin-male mating advantage [13,22]. The decreased likelihood of non-virgin mating apparently arises from the loss of haemolymph and costly immune responses that ensue as a result of wing wounding during copulation [15,16]. Although much of our previous work has focused on establishing the proximate basis of the virgin-male mating advantage, it has overshadowed a more fundamental question: what factors influence the success of a male in obtaining a mate? Measurements of lifetime mating success in male C. strepitans have revealed that the median mating frequency is one, with many males failing to secure a mate at all and a small minority obtaining between two and four mates [22]. Because calling is required for mate attraction, it seems likely that certain features of males’ calls influence variation in male mating success. Indeed, previous studies of acoustic Orthoptera have shown that the acoustical properties of a male's song can influence his attractiveness to females [23,24], and more recent work on C. strepitans has revealed significant multivariate nonlinear sexual selection acting on male song traits [25].
However, males successful at attracting females through acoustic signalling are not assured of mating unless they succeed in inducing females to mount and to remain mounted sufficiently long to ensure successful transfer of the spermatophore. Numerous laboratory studies have shown that even when closely confined with singing males, female sagebrush crickets will often forgo mating, suggesting that some males are perceived as more attractive than others [14,21,26]. Although male song may play a role in a female's decision to mount a male, it seems likely that other sensory modalities come into play in the close-range interactions that ensue after the female locates the male, including tactile and chemical signals. Indeed, CHCs are known to facilitate species recognition, kin recognition and sex recognition in a variety of cricket species [27–29]. Thomas & Simmons [8] have recently shown that although male song is vital to a male's ability to attract females, the CHC profile of male field crickets (Teleogryllus oceanicus) has a significant influence on female mating decisions resulting in significant sexual selection on male CHCs.
Here, we estimate the strength and form of sexual selection on male CHCs in a natural population of sagebrush crickets, C. strepitans. We take advantage of an important feature of the mating behaviour of this species, nuptial feeding by females on the hind wings of males, to accurately assign mating success to males collected in the field. We apply formal multivariate selection analysis to our field data to estimate the standardized linear and nonlinear selection gradients and conduct a canonical analysis of the matrix of standardized nonlinear selection gradients (γ) to provide a best quadratic approximation of the individual fitness surface for male CHCs [30]. This work is, to our knowledge, the first study examining sexual selection on male CHCs in a natural population of an insect.
2. Material and methods
(a). Experimental protocol
The study was conducted in 2010 on a population of sagebrush crickets in Grand Teton National Park, WY, USA located in an area of approximately 3 ha in sagebrush meadow habitat adjacent to the Snake River at Deadman's Bar (43°45'33.91″ N, 110°37'25.12″ W). We began monitoring the population on a nightly basis (weather permitting) beginning 20 May continuing into June, which spans the breeding season of C. strepitans at this locality. Males were found at night by orienting to their calls and using head lamps to determine their location within a sagebrush bush. The mating status of males was determined by examining their hind wings for evidence of wing wounding by females. Virgin males were identified by their intact wings, whereas mated males were identified by visibly wet wounds with no discoloration, indicating that the male had mated on the night of capture, or dry, melanized wounds, indicating that the male had mated at least one night previous to the night of capture (see fig. 1 in [16]). We continued to monitor the population until it attained a ratio of approximately 1 : 1 virgin to mated males, at which time we collected a total of 224 males at random from the population over two successive nights. This protocol ensured that females were given ample opportunity to mate with the most attractive males in the population. Males were held individually in collecting vials and transported to the University of Wyoming-National Park Service Research Station, less than 30 km away, for processing.
(b). Chemical analysis of cuticular hydrocarbons
Upon their transport to the field station, males were frozen overnight and thawed the following day, after which their pronotum width was measured to the nearest 0.01 mm using digital callipers (Fowler, Newton, MA, USA). Male size was measured because increased body size in crickets often is favoured by female mating preferences [31,32] and we wished to determine whether any male CHCs were associated with this trait. Male CHCs were extracted by whole-body immersion in 2.5 ml of hexane (Fisher H303–4) for 10 min. Samples were analysed on an Agilent Technologies gas chromatography-mass spectrometer (GC-MS) (Agilent 7890 GC coupled with an Agilent 5975 mass spectrometer) fitted with a DB1-MS column (30 m × 0.25 mm ID × 0.25 μm film thickness; see the electronic supplementary material, methods S1).
Prior to analysis, the area under each chromatograph peak was divided by the area of the internal standard (pentadecane) to control for among-cricket variance in CHC extraction efficiency. This proportion was then log10 transformed to ensure normality. Owing to the large number of CHCs examined (table 1), we extracted principal components (PCs) based on the correlation matrix and retained PCs with eigenvalues exceeding 1 for further analysis [33]. In total, 10 PCs were retained for our selection analysis based on this criterion. We interpret factor loadings that exceed |0.25| as biologically important [33].
Table 1.
The vector of standardized linear selection gradients (β) and the matrix of standardized quadratic and correlational selection gradients (γ) for male CHCs in C. strepitans. (Significant values were determined by randomization tests. *p < 0.05, **p < 0.01, ***p < 0.001.)
| γ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 | |
| PC1 | −0.055 | −0.003 | |||||||||
| PC2 | −0.404*** | −0.018 | 0.109* | ||||||||
| PC3 | 0.010 | 0.038 | −0.076 | 0.011 | |||||||
| PC4 | 0.011 | −0.161* | −0.137 | −0.090 | 0.095 | ||||||
| PC5 | 0.040 | 0.343** | 0.010 | −0.052 | 0.144 | −0.133* | |||||
| PC6 | 0.062 | −0.212* | 0.021 | 0.114 | −0.084 | −0.065 | 0.019 | ||||
| PC7 | 0.167** | −0.087 | 0.090 | 0.118 | 0.094 | −0.024 | 0.170* | 0.022 | |||
| PC8 | 0.182** | −0.145 | −0.067 | 0.118 | 0.099 | 0.054 | −0.058 | −0.089 | 0.030 | ||
| PC9 | −0.002 | −0.214* | 0.002 | −0.078 | 0.085 | −0.152* | 0.248** | 0.032 | 0.014 | 0.035 | |
| PC10 | 0.114 | 0.073 | −0.023 | −0.033 | 0.016 | −0.080 | −0.067 | 0.037 | 0.062 | −0.041 | 0.049 |
(c). Multivariate selection analysis
We used a standard multivariate selection analysis to estimate the strength and form of linear and nonlinear sexual selection acting on male CHCs [34]. We assigned an absolute fitness score of 0 to virgin males and 1 to males that had mated based on patterns of wing wounds. As recommended by Lande & Arnold [34], this absolute fitness score was transformed to relative fitness by dividing by the mean absolute fitness of the population. We then fitted a linear regression model including the PCs describing CHC composition as the predictor variables and relative fitness as the response variable to estimate the vector of standardized linear selection gradients (β). A quadratic regression model including all the linear, quadratic and cross-product terms was then used to estimate the matrix of standardized nonlinear selection gradients (γ). Quadratic regression coefficients are known to underestimate the stabilizing and disruptive selection gradients by a factor of 0.5, and we therefore doubled these gradients following the recommendation of Stinchcombe et al. [35].
Interpreting the size and significance of individual γ terms is likely to underestimate the strength of nonlinear selection [36]. We therefore explored the extent of nonlinear sexual selection on male CHCs by conducting a canonical analysis to locate the major eigenvectors of the fitness surface [37]. The strength of linear selection along each of the eigenvectors is given by theta (θi) and the strength of nonlinear selection is given by their eigenvalues (λi). We estimated θi and λi for each eigenvector using the double regression method of Bisgaard & Ankenman [38].
Relative fitness was not normally distributed and although this does not influence the sign or magnitude of selection gradients [34], it does present problems with testing the significance of these gradients. Therefore, to assess the significance of our linear and nonlinear selection gradients we used a resampling procedure in which we randomly shuffled relative fitness across individual males in our dataset to obtain a null distribution for each selection gradient where there is no relationship between CHCs and fitness. Probabilities are the number of times (out of 9999 iterations) in which the gradient pseudo-estimate was equal to or less than the original estimated gradient (see [23] for an application of this approach). We conducted separate randomization tests for the multiple regression models for linear selection and for the full quadratic model. The same resampling procedure was used to assess the significance of θi and λi for each eigenvector after the canonical rotation of γ.
We used thin-plate splines [39] to visualize the major eigenvectors of the fitness surface extracted from the canonical rotation of γ. We used the Tps function of the FIELDS package in R (v. 2.13.0, www.r-project.org) to fit the thin-plate splines and to visualize them in both the perspective and contour-map views. We used the value of the smoothing parameter (λ) that minimized the generalized cross-validation score when fitting the thin-plate splines [39].
3. Results
GC-MS analysis of CHCs in male C. strepitans revealed 48 individual CHCs ranging in length from C25 to C35 and consisting of a mixture of straight-chained alkanes, mono-methylalkanes, dimethylalkanes and trimethylalkanes (see the electronic supplementary material, table S1 and figure S1).
PC analysis of these 48 individual CHC peaks yielded 10 PCs with eigenvalues exceeding 1, which collectively explain 76.04% of the total variation in CHC expression (see the electronic supplementary material, table S2). PC1 accounts for 33.07% of the variance in male CHC expression and is positively loaded to each CHC peak (see the electronic supplementary material, table S2). Consequently, this vector describes the absolute amount of CHCs possessed by males. PC2 explains a further 12.30% of the variance in male CHCs and is positively loaded to longer chained CHCs (peak 32 and over) and negatively loaded to shorter chained CHCs (peak 23 and below). PC3 explains a further 6.90% of the variance in male CHCs and is also positively loaded to longer chained CHCs (peak 17 and over) and negatively loaded to shorter chained CHCs (peak 16 and under). Thus, although both PC2 and PC3 describe the trade-off between short- and long-chained CHCs, this trade-off is centred around longer chained CHCs for PC2. PC4–PC10 each describes the trade-off between specific CHCs and there is no obvious relationship to chain length. PC4 explains a further 5.64% of the variance in male CHCs and is positively loaded to seven peaks (peaks 1, 6, 25, 32, 36, 37 and 45) and negatively loaded to seven peaks (peaks 13, 21, 26, 30, 33, 34 and 40). PC5 explains a further 4.13% and is positively loaded to six peaks (peaks 18, 33, 38, 43, 46 and 48) and negatively loaded to four peaks (peaks 26, 35, 39 and 41), whereas PC6 explains a further 3.51% and is positively loaded to six peaks (peaks 26, 33, 39, 41, 42 and 48) and negatively to four peaks (peaks 24, 25, 30 and 32). PC7 explains a further 3.39% and is positively loaded to seven peaks (peaks 12, 23, 26, 39, 44 and 47) and negatively loaded to a single peak (peak 3). Therefore, with the exception of unidentified peaks, PC5–PC7 reflect trade-offs between mono, di- and trimethylalkanes (see the electronic supplementary material, table S1). PC8 explains a further 2.70% of the variance in male CHCs and is positively loaded to six peaks (peaks 7, 12, 13, 21, 23 and 48) and negatively to a single peak (peak 20). PC9 and PC10 each represents a trade-off between three different CHC peaks. PC9 explains a further 2.33% of the variance in male CHCs and is positively loaded to peak 3 and negatively loaded to two unidentified peaks (peaks 5 and 8), all of which are relatively short chained. PC10 explains a final 2.09% of the variance in male CHCs and is also positively loaded to peak 3 and negatively loaded to two peaks (peaks 7 and 27). Male pronotum width was positively correlated with PC1 (r = 0.22, n = 223, p = 0.001), but was not correlated with any of the other PCs (all p > 0.05).
Standardized linear, quadratic and correlational selection gradients are presented in table 1. There was significant linear sexual selection favouring lower values for PC2 and higher values for PC7 and PC8. There was also significant disruptive sexual selection operating on PC2 and stabilizing sexual selection operating on PC5. There was significant positive correlational sexual selection operating on the covariance between PC1 and PC5, PC6 and PC7, and PC6 and PC9. There was also significant negative correlational sexual selection operating on the covariance between PC1 and PC4, PC1 and PC6, PC1 and PC9, and PC5 and PC9.
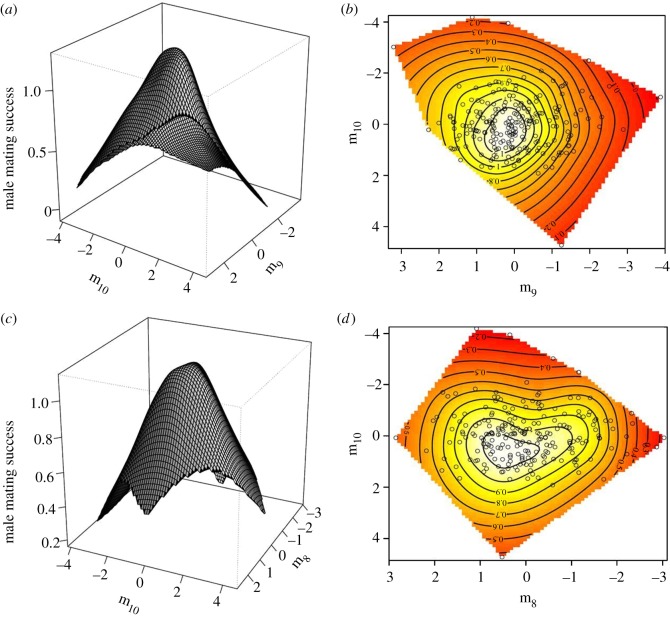
Canonical analysis of the γ matrix resulted in five eigenvectors (m1, m2, m8, m9 and m10) with significant nonlinear sexual selection (table 2). There was significant stabilizing selection acting along three of these five eigenvectors (m8, m9 and m10). Visualization of the fitness surfaces of the strongest axis of stabilizing selection, m10, against each of the minor axes, m8 and m9, reveals well-defined, narrow cones (figure 1). The eigenvector of the strongest stabilizing selection, m10, was heavily weighted by a positive contribution from PC5 and a negative contribution from PC1 (table 2). The remaining two eigenvectors of significant stabilizing selection (m8 and m9) also experienced significant linear selection (table 2 and figure 1). The m9 eigenvector was heavily weighted by a positive contribution from PC7 and negative contribution from PC4 and PC6 and linear selection favoured higher values of this eigenvector (i.e. an increase in PC7 and decreases in PC4 and PC6) (table 2). The m8 eigenvector was heavily weighted by a positive contribution from PC6 and negative contributions from PC3 and PC9 and linear selection favoured higher values of this eigenvector (i.e. an increase in PC6 and decreases in PC3 and PC9) (table 2).
Table 2.
The M matrix of eigenvectors from the canonical analysis of γ. (The linear (θi) and quadratic (λi) gradients of selection operating along each eigenvector are provided in the last two columns. The quadratic selection gradient (λi) of each eigenvector (mi) is equivalent to the eigenvalue. Significant values were determined by randomization tests. *p < 0.05, **p < 0.01, ***p < 0.001.)
| M |
selection |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 | θi | λi | |
| m1 | 0.552 | −0.041 | −0.055 | −0.139 | 0.296 | −0.488 | −0.271 | −0.050 | −0.509 | 0.101 | −0.077 | 0.343*** |
| m2 | 0.142 | 0.549 | 0.070 | −0.654 | −0.093 | 0.226 | 0.133 | −0.391 | −0.044 | −0.115 | −0.290*** | 0.225*** |
| m3 | −0.054 | 0.496 | −0.674 | 0.388 | 0.110 | −0.206 | −0.024 | −0.255 | 0.160 | −0.036 | −0.261*** | 0.122 |
| m4 | 0.145 | 0.198 | 0.296 | 0.386 | 0.206 | 0.058 | 0.726 | −0.070 | −0.266 | 0.233 | 0.067 | 0.082 |
| m5 | −0.177 | 0.305 | −0.045 | −0.179 | −0.267 | −0.162 | −0.062 | 0.379 | −0.014 | 0.772 | 0.012 | 0.071 |
| m6 | −0.159 | 0.499 | 0.239 | 0.065 | 0.148 | −0.107 | −0.116 | 0.600 | −0.159 | −0.482 | −0.153* | 0.044 |
| m7 | 0.502 | 0.098 | 0.015 | 0.006 | 0.453 | 0.375 | −0.138 | 0.260 | 0.521 | 0.184 | 0.020 | −0.051 |
| m8 | −0.154 | −0.137 | −0.516 | −0.146 | 0.228 | 0.545 | 0.076 | 0.238 | −0.508 | 0.028 | 0.158* | −0.126* |
| m9 | 0.107 | −0.200 | −0.321 | −0.376 | 0.014 | −0.389 | 0.582 | 0.325 | 0.273 | −0.187 | 0.177** | −0.145* |
| m10 | −0.554 | −0.049 | 0.147 | −0.232 | 0.706 | −0.198 | −0.024 | −0.203 | 0.113 | 0.150 | 0.043 | −0.309*** |
Figure 1.
Thin-plate spline visualizations of the major axis of stabilizing selection (m10) and each of two other axes of stabilizing selection (m8 and m9) operating on CHCs of males captured in the wild. m9 versus m10: (a) perspective-view and (b) contour map. m8 versus m10: (c) perspective-view and (d) contour map. Each point on the contour plots represents an individual male.
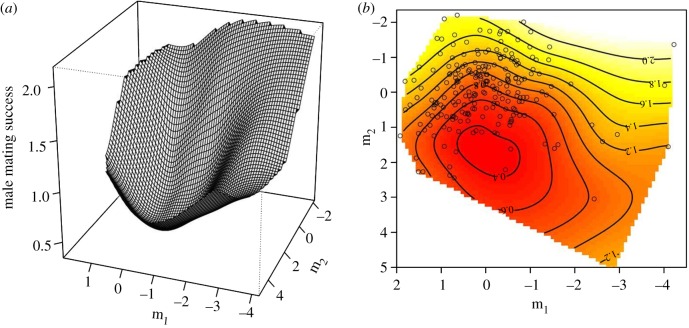
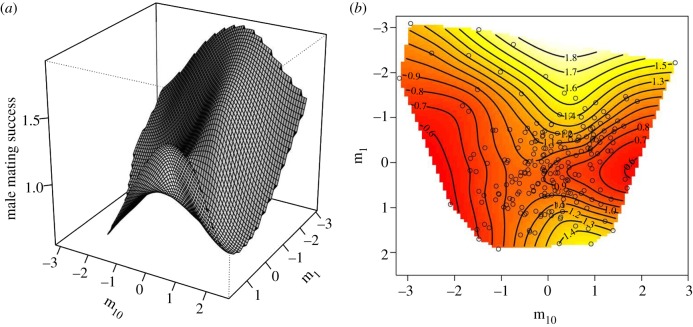
There was significant disruptive selection operating along the remaining two eigenvectors of significant nonlinear selection, m1 and m2 (table 2 and figure 2). The dominant eigenvector of nonlinear sexual selection (m1) was heavily weighted by a positive contribution from PC1 and negative contributions from PC6 and PC9 (table 2). The second eigenvector of significant disruptive selection (m2) was heavily weighted by a positive contribution from PC2 and negative contributions from PC4 and PC8. This eigenvector also experienced significant linear selection favouring lower values of m2 (i.e. increases in PC4 and PC8 and a decrease in PC2) (table 2). The combination of positive (m1 and m2) and negative (m8, m9 and m10) eigenvalues (table 2) formally indicates that the fitness surface is best described as a multivariate saddle and can be visualized along the two most dominant eigenvectors (m1 and m10) of the fitness surface (figure 3).
Figure 2.
Thin-plate spline (a) perspective-view and (b) contour-map visualization of the major axes of disruptive selection (m1 and m2) operating on CHCs of males captured in the wild.
Figure 3.
Thin-plate spline (a) perspective-view and (b) contour-map visualization of the two major axes of nonlinear selection (m1 and m10) operating on CHCs of males captured in the wild. Each point on the contour plot represents an individual male.
There was significant linear selection along two eigenvectors, m3 and m6, that did not experience significant nonlinear selection. In both instances, linear selection favoured lower values for these eigenvectors. For m3, this corresponds to an increase in PC3 and decreases in PC2 and PC4, whereas for m6 this corresponds to an increase in PC10 and decreases in PC2 and PC8 (table 2).
4. Discussion
Although male sagebrush crickets must call to attract sexually receptive females [18], our results suggest that a female's decision to mate does not end upon successful phonotaxis. Instead, chemical signals in the form of CHCs appear to influence a female decision to consummate a mating once pair formation has occurred. Although sexual selection on a number of male CHC traits was significant, we cannot be certain that male CHCs were the target of female mating preferences and not simply correlated with some other trait that influenced female mate choice. Nevertheless, previous studies have revealed that chemical signals are essential to successful mating in crickets, playing a critical role in species, sex and mate recognition [8,27–29]. Physical or chemical ablation of female antennae profoundly diminishes female receptivity [29,40], and the use of hexane solvents to strip females of their CHCs, followed by reapplication of male- or female-derived CHCs, has revealed significant effects on the likelihood of male courtship [29]. Cricket CHCs have also been shown to be the target of female mate choice in trials staged in the laboratory [8,41]; for example, female crickets prefer CHCs of dominant males over those derived from subordinate males [41]. Thus, it seems likely that the sexual selection on male CHCs documented in this study is mediated, at least in part, by female mating preferences.
Our analyses revealed both significant linear and nonlinear sexual selection on CHCs of male sagebrush crickets captured from wild populations. The complex pattern of multivariate nonlinear selection that emerged was characterized primarily by strong stabilizing and disruptive selection on male CHCs, resulting in a saddle-shaped fitness surface. Such a fitness surface seems characteristic of the majority of laboratory studies measuring multivariate sexual selection in male crickets, including male song traits [24], cuticular hydrocarbons [8], morphological characters [42] and the chemical composition of males’ nuptial food gifts [43], and a similar pattern has emerged in studies of other insects [44,45]. Many of these sexual signals are known to impose significant energy costs on males, and thus may serve as honest indicators of male quality [46]. Whether CHCs of male sagebrush crickets constitute an honest signal remains to be determined, but it would be advantageous to females if some aspect of male CHCs was reliably correlated with the provision of a greater volume of haemolymph during hind-wing nuptial feeding. Hinting at this possibility was the significant correlation between male body size and PC1. In male ground crickets, Allonemobius socius, a species in which males also provide females with a haemolymph gift that females obtain during copulation by chewing on a specialized spur on the male's hind tibia, larger males provide larger gifts [47]. Similarly, in decorated crickets, Gryllodes sigillatus, larger males provide females with a larger spermatophylax at mating, a gelatinous mass forming part of the male's spermatophore and consumed by the female after mating [48,49]. We do not know whether larger male C. strepitans also provide a greater volume of haemolymph to females during mating, but if they do, the link between male CHCs and male body size could conceivably provide females with a valuable cue by which they could maximize the direct benefits they obtain from prospective mates.
Our selection analysis assumes that males in the virgin and mated groups had equal opportunities to mate, an assumption that may be violated if males eclose at different times during the breeding season. If mated males eclose earlier in the season than virgin males and thus have more time to obtain matings, any differences in the CHC profiles of virgin and mated males could be attributed as much to age-related changes in CHC composition as to any differences in intrinsic male attractiveness. However, a previous study of sagebrush crickets emerging within a field enclosure erected at the study site suggests that males become sexually active within a few days of each other [22]. A more recent mark–recapture study conducted in a free-living population revealed no significant effect of date of capture on time to mating; the difference in the time of initial capture of virgin and mated males was less than half a day [25]. We conclude, therefore, that observed differences in the CHCs of virgin and mated males are likely to stem from their effect on male attractiveness and not from any age-related effects.
There appears to be a complex interplay between the total abundance of CHCs and specific CHC combinations in their influence on female choice, as suggested by the saddle-shaped selection surface arising from the two major axes of nonlinear selection, m1 and m10 (figure 3). There was significant disruptive selection acting on m1 resulting in two fitness peaks, one occurring at low values of m1 and a smaller peak at high values of m1. Examination of the PC loadings on this eigenvector suggests that disruptive selection acts most strongly on the total amount of CHCs present on the surface of the male's cuticle (PC1), coupled with selection for specific CHC combinations, including both di- and trimethylalkanes and mono- and dimethyalkanes (PC6 and PC9). The dominant axis of stabilizing selection, m10, was also heavily weighted by a negative contribution from total CHC abundance (PC1) and a positive contribution from PC5, which appears to reflect a trade-off between specific mono-, di- and trimethylalkanes.
Our results also suggest that female choice and desiccation resistance may impose contrasting selection patterns on male CHCs. There was both strong linear (table 1) and nonlinear selection (figure 2) acting on PC2, which contrasts relatively shorter and longer CHCs. A trade-off between short- and long-chained CHCs is often related to desiccation tolerance in insects, with a greater relative increase in long-chained CHCs providing greater desiccation resistance [50,51]. More long-chained CHCs are produced at higher temperatures in Drosophila [52,53], and more long-chained CHCs are produced when flies are selected for desiccation resistance [51,54]. Thus, male C. strepitans may be sacrificing some level of desiccation resistance in favour of increased attractiveness to females because shorter chained, and thus, more volatile CHCs have been shown to increase male attractiveness in several Drosophila species [9,55]. Such a trade-off is not unique to C. strepitans, as it has also been documented in male decorated crickets, Gryllodes sigillatus, where it is influenced by a significant genotype × environment interaction [56]. It must be acknowledged, however, that there was no comparable linear selection on PC3, which also describes a trade-off between short- and long-chained CHCs. Although this trade-off was centred around shorter chained CHCs compared with PC2, the evidence in favour of a trade-off between attractiveness and desiccation resistance must be considered equivocal.
Undoubtedly because of its conspicuousness as a sexual signal, the overwhelming majority of studies on sexual selection in crickets have focused on male calling song as the primary target of female mating preferences [23–25]. However, a growing body of evidence suggests that male chemical signals in the form of CHCs can have a profound influence on female mating preferences even after pair formation has occurred [7,8,57,58]. Most evidence to date has been based on laboratory observation and the dearth of studies in wild populations is almost certainly a consequence of the difficulty in measuring male mating success under natural conditions. This study, which involved measurement of male mating success in a wild population based on a phenotypic marker of mating, revealed a complex pattern of multivariate linear and nonlinear selection characterized primarily by strong stabilizing and disruptive selection on male CHCs. Hence, selection imposed on male sexual traits via female mating preferences may be far more nuanced than previously appreciated and operating in multiple sensory modalities. What remains to be seen is the extent to which selection on male song traits is opposed or reinforced by selection on male chemical signals, or whether these selection regimes operate independently. Thus, future studies will be focused on investigating the genetic linkages between male acoustic and chemical traits.
Data accessibility
Data have been deposited in the Dryad repository.
Funding statement
This work was supported by grants from the National Science Foundation to S.K.S. (IOS-0718140 and IOS-1118160). Further support to S.K.S. was provided by a visiting professorship through a grant from The Leverhulme Trust to J.H. S.S. was supported by a Feodor Lynen Fellowship from the Alexander von Humboldt Foundation and the Office of Research and Sponsored Programs at Illinois State University. J.H. was supported by the Natural Environment Research Council, a University Royal Society Fellowship, and a Royal Society Equipment grant.
References
- 1.Symonds MRE, Elgar MA. 2008. The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220 (doi:10.1016/j.tree.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 2.Steiger S, Schmitt T, Schaefer HM. 2011. The origin and dynamic evolution of chemical information transfer. Proc. R. Soc. B 278, 970–979 (doi:10.1098/rspb.2010.2285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt T. 2003. Pheromones and animal behaviour: communication by smell and taste. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Johansson BG, Jones TM. 2007. The role of chemical communication in mate choice. Biol. Rev. 82, 265–289 (doi:10.1111/j.1469-185X.2007.00009.x) [DOI] [PubMed] [Google Scholar]
- 5.Howard RW, Blomquist GJ. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Ann. Rev. Entomol. 50, 371–393 (doi:10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- 6.Steiger S, Franz R, Eggert A-K, Müller JK. 2008. The Coolidge effect, individual recognition and selection for distinctive cuticular signatures in a burying beetle. Proc. R. Soc. B 275, 1831–1838 (doi:10.1098/rspb.2008.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weddle CB, Hunt J, Sakaluk SK. 2013. Self-referent phenotype matching and its role in female mate choice in arthropods. Curr. Zool. 59, 239–248 [Google Scholar]
- 8.Thomas ML, Simmons LW. 2009. Sexual selection on cuticular hydrocarbons in the Australian field cricket, Teleogryllus oceanicus. BMC Evol. Biol. 9, 162 (doi:10.1186/1471-2148-9-162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenoweth SF, Blows MW. 2005. Contrasting mutual sexual selection on homologous signal traits in Drosophila serrata. Am. Nat. 165, 281–289 (doi:10.1086/427271) [DOI] [PubMed] [Google Scholar]
- 10.Howard RW, Jackson LL, Banse H, Blows MW. 2003. Cuticular hydrocarbons of Drosophila birchii and D. serrata: identification and role in mate choice in D. serrata. J. Chem. Ecol. 29, 961–976 (doi:10.1023/A:1022992002239) [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Muñoz R, Bretman A, Slate J, Walling CA, Tregenza T. 2010. Natural and sexual selection in a wild insect population. Science 328, 1269–1272 (doi:10.1126/science.1188102) [DOI] [PubMed] [Google Scholar]
- 12.Morris GK, Gwynne DT. 1978. Geographical distribution and biological observations of Cyphoderris (Orthoptera: Haglidae) with a description of a new species. Psyche 85, 147–167 (doi:10.1155/1978/84389) [Google Scholar]
- 13.Morris GK, Gwynne DT, Klimas DE, Sakaluk SK. 1989. Virgin male mating advantage in a primitive acoustic insect (Orthoptera: Haglidae). J. Insect Behav. 2, 173–185 (doi:10.1007/BF01053290) [Google Scholar]
- 14.Johnson JC, Ivy TM, Sakaluk SK. 1999. Female remating propensity contingent on sexual cannibalism in sagebrush crickets, Cyphoderris strepitans: a mechanism of cryptic female choice. Behav. Ecol. 10, 227–233 (doi:10.1093/beheco/10.3.227) [Google Scholar]
- 15.Sakaluk SK, Campbell MTH, Clark AP, Johnson JC, Keorpes PA. 2004. Hemolymph loss during nuptial feeding constrains male mating success in sagebrush crickets. Behav. Ecol. 15, 845–849 (doi:10.1093/beheco/arh113) [Google Scholar]
- 16.Leman JC, Weddle CB, Gershman SN, Kerr AM, Ower GD, St John JM, Vogel LA, Sakaluk SK. 2009. Lovesick: immunological costs of mating to male sagebrush crickets. J. Evol. Biol. 22, 163–171 (doi:10.1111/j.1420-9101.2008.01636.x) [DOI] [PubMed] [Google Scholar]
- 17.Sakaluk SK, Eggert A-K. 2009. Coping with the cold: temperature and mating activity of male sagebrush crickets Cyphoderris strepitans (Orthoptera: Haglidae). Physiol. Entomol. 34, 251–255 (doi:10.1111/j.1365-3032.2009.00683.x) [Google Scholar]
- 18.Snedden WA, Sakaluk SK. 1992. Acoustic signaling and its relation to male mating success in sagebrush crickets. Anim. Behav. 44, 633–639 (doi:10.1016/S0003-3472(05)80291-X) [Google Scholar]
- 19.Snedden WA, Irazuzta S. 1994. Attraction of female sagebrush crickets to male song: the importance of field bioassays. J. Insect Behav. 7, 233–236 (doi:10.1007/BF01990083) [Google Scholar]
- 20.Dodson G, Morris GK, Gwynne DT. 1983. Mating behavior in the primitive orthopteran genus Cyphoderris (Haglidae). In Orthopteran mating systems: sexual competition in a diverse group of insects (eds Gwynne DT, Morris GK.), pp. 305–318 Boulder, CO: Westview Press [Google Scholar]
- 21.Eggert A-K, Sakaluk SK. 1994. Sexual cannibalism and its relation to male mating success in sagebrush crickets, Cyphoderris strepitans (Orthoptera: Haglidae). Anim. Behav. 47, 1171–1177 (doi:10.1006/anbe.1994.1155) [Google Scholar]
- 22.Snedden WA. 1996. Lifetime mating success in male sagebrush crickets: sexual selection constrained by a virgin male mating advantage. Anim. Behav. 51, 1119–1125 (doi:10.1006/anbe.1996.0113) [Google Scholar]
- 23.Brooks R, Hunt J, Blows MW, Smith MJ, Bussière LF, Jennions MD. 2005. Experimental evidence for multivariate stabilizing sexual selection. Evolution 59, 871–880 [PubMed] [Google Scholar]
- 24.Bentsen CL, Hunt J, Jennions MD, Brooks R. 2006. Complex multivariate sexual selection on male signaling in a wild population of Teleogryllus commodus. Am. Nat. 167, E102–E116 (doi:10.1086/501376) [DOI] [PubMed] [Google Scholar]
- 25.Ower GD, Judge KA, Steiger S, Caron KJ, Smith RA, Hunt J, Sakaluk SK. 2013. Multivariate sexual selection on male song structure in wild populations of sagebrush crickets, Cyphoderris strepitans (Orthoptera: Haglidae). Ecol. Evol. 3, 3590–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weddle CB, Sakaluk SK. 2003. Ingestion of male hemolymph and mating propensity of female sagebrush crickets: no evidence of a male-derived anti-aphrodisiac. Anim. Behav. 65, 83–88 (doi:10.1006/anbe.2002.2060) [Google Scholar]
- 27.Tregenza T, Wedell N. 1997. Definitive evidence for cuticular pheromones in a cricket. Anim. Behav. 54, 979–984 (doi:10.1006/anbe.1997.0500) [DOI] [PubMed] [Google Scholar]
- 28.Nagamoto J, Aonuma H, Hisada M. 2005. Discrimination of conspecific individuals via cuticular pheromones by males of the cricket Gryllus bimaculatus. Zool. Sci. 22, 1079–1088 (doi:10.2108/zsj.22.1079) [DOI] [PubMed] [Google Scholar]
- 29.Ryan KM, Sakaluk SK. 2009. Dulling the senses: the role of the antennae in mate recognition, copulation and mate guarding in decorated crickets. Anim. Behav. 77, 1345–1350 (doi:10.1016/j.anbehav.2009.02.011) [Google Scholar]
- 30.Chenoweth SF, Hunt J, Rundle HD. 2012. Analyzing and comparing the geometry of individual fitness surfaces. In The adaptive landscape in evolutionary biology (eds Svensson EI, Calsbeek R.), pp. 126–149 Oxford, UK: Oxford University Press [Google Scholar]
- 31.Bateman PW, Gilson LN, Ferguson JWH. 2001. Male size and sequential mate preference in the cricket Gryllus bimaculatus. Anim. Behav. 61, 631–637 (doi:10.1006/anbe.2000.1617) [Google Scholar]
- 32.Gray DA. 1997. Female house crickets, Acheta domesticus, prefer the chirps of large males. Anim. Behav. 54, 1553–1562 (doi:10.1006/anbe.1997.0584) [DOI] [PubMed] [Google Scholar]
- 33.Tabachnick B, Fidell L. 2006. Using multivariate statistics, 5th edn Boston, MA: Allyn and Bacon [Google Scholar]
- 34.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 35.Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. 2008. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution 62, 2435–2440 (doi:10.1111/j.1558-5646.2008.00449.x) [DOI] [PubMed] [Google Scholar]
- 36.Blows MW, Brooks R. 2003. Measuring nonlinear selection. Am. Nat. 162, 815–820 (doi:10.1086/378905) [DOI] [PubMed] [Google Scholar]
- 37.Phillips PC, Arnold SJ. 1989. Visualizing multivariate selection. Evolution 43, 1209–1222 (doi:10.2307/2409357) [DOI] [PubMed] [Google Scholar]
- 38.Bisgaard S, Ankenman B. 1996. Standard errors for the eigenvalues in second-order response surface models. Technometrics 38, 238–246 (doi:10.1080/00401706.1996.10484503) [Google Scholar]
- 39.Green PJ, Silverman BW. 1994. Nonparametric regression and generalised linear models. London, UK: Chapman and Hall [Google Scholar]
- 40.Adamo SA, Hoy R. 1994. Mating behaviour of the field cricket Gryllus bimaculatus and its dependence on social and environmental cues. Anim. Behav. 47, 857–868 (doi:10.1006/anbe.1994.1117) [Google Scholar]
- 41.Hedrick AV, Kortet R. 2012. Effects of body size on selectivity for mating cues in different sensory modalities. Biol. J. Linnean Soc. 105, 160–168 (doi:10.1111/j.1095-8312.2011.01786.x) [Google Scholar]
- 42.Judge KA. 2010. Female social experience affects the shape of sexual selection on males. Evol. Ecol. Res. 12, 389–402 [Google Scholar]
- 43.Gershman SN, Mitchell C, Sakaluk SK, Hunt J. 2012. Biting off more than you can chew: sexual selection on the free amino acid composition of the spermatophylax in decorated crickets. Proc. R. Soc. B 279, 2531–2538 (doi:10.1098/rspb.2011.2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bussière LF, Gwynne DT, Brooks R. 2008. Contrasting sexual selection on males and females in a role-reversed swarming dance fly, Rhamphomyia longicauda (Diptera: Empididae). J. Evol. Biol. 21, 1683–1691 (doi:10.1111/j.1420-9101.2008.01580.x) [DOI] [PubMed] [Google Scholar]
- 45.Punzalan D, Rodd FH, Rowe L. 2008. Contemporary sexual selection on sexually dimorphic traits in the ambush bug Phymata americana. Behav. Ecol. 19, 860–870 (doi:10.1093/beheco/arn042) [Google Scholar]
- 46.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 47.Fedorka KM, Mousseau TA. 2002. Tibial spur feeding in ground crickets: larger males contribute larger gifts (Orthoptera: Gryllidae). Florida Entomol. 85, 317–323 (doi:10.1653/0015-4040(2002)085[0317:TSFIGC]2.0.CO;2) [Google Scholar]
- 48.Sakaluk SK. 1984. Male crickets feed females to ensure complete sperm transfer. Science 223, 609–610 (doi:10.1126/science.223.4636.609) [DOI] [PubMed] [Google Scholar]
- 49.Sakaluk SK. 1985. Spermatophore size and its role in the reproductive behaviour of the cricket, Gryllodes supplicans (Orthoptera: Gryllidae). Can. J. Zool. 63, 1652–1656 (doi:10.1139/z85-245) [Google Scholar]
- 50.Gibbs AG, Chippindale AK, Rose MR. 1997. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 200, 1821–1832 [DOI] [PubMed] [Google Scholar]
- 51.Foley BR, Telonis-Scott M. 2011. Quantitative genetic analysis suggests causal association between cuticular hydrocarbon composition and desiccation survival in Drosophila melanogaster. Heredity 106, 68–77 (doi:10.1038/hdy.2010.40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibbs AG, Louie AK, Ayala JA. 1998. Effects of temperature on cuticular lipids and water balance in a desert Drosophila: is thermal acclimation beneficial? J. Exp. Biol. 201, 71–80 [DOI] [PubMed] [Google Scholar]
- 53.Ingleby FC, Hosken DJ, Flowers K, Hawkes MF, Lane SM, Rapkin J, Dworkin I, Hunt J. 2013. Genotype-by-environment interactions for cuticular hydrocarbon expression in Drosophila simulans. J. Evol. Biol. 26, 94–107 (doi:10.1111/jeb.12030) [DOI] [PubMed] [Google Scholar]
- 54.Kwan L, Rundle HD. 2009. Adaptation to desiccation fails to generate pre- and postmating isolation in replicate Drosophila melanogaster laboratory populations. Evolution 64, 710–723 (doi:10.1111/j.1558-5646.2009.00864.x) [DOI] [PubMed] [Google Scholar]
- 55.Ferveur JF, Cobb M. 2010. Behavioral and evolutionary roles of cuticular hydrocarbons in Diptera. In Insect hydrocarbons (eds Blomquist GJ, Bagnères AG.), pp. 325–343 New York, NY: Cambridge University Press [Google Scholar]
- 56.Weddle CB, Mitchell C, Bay SK, Sakaluk SK, Hunt J. 2012. Sex-specific genotype-by-environment interactions for cuticular hydrocarbon expression in decorated crickets, Gryllodes sigillatus: implications for the evolution of signal reliability. J. Evol. Biol. 25, 2112–2125 (doi:10.1111/j.1420-9101.2012.02593.x) [DOI] [PubMed] [Google Scholar]
- 57.Weddle CB, Steiger S, Hamaker CG, Ower GD, Mitchell C, Sakaluk SK, Hunt J. 2013. Cuticular hydrocarbons as a basis for chemosensory self-referencing in crickets: a potentially universal mechanism facilitating polyandry in insects. Ecol. Lett. 16, 346–353 (doi:10.1111/ele.12046) [DOI] [PubMed] [Google Scholar]
- 58.Ivy TM, Weddle CB, Sakaluk SK. 2005. Females use self-referent cues to avoid mating with previous mates. Proc. R. Soc. B 272, 2475–2478 (doi:10.1098/rspb.2005.3222) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data have been deposited in the Dryad repository.