Abstract
Objective
Bipolar disorder may be characterized by a hypersensitivity to reward-relevant stimuli, potentially underlying the emotional lability and dysregulation that characterizes the illness. In parallel, research highlights the predominant role of striatal and orbitofrontal cortical (OFC) regions in reward-processing and approach-related affect. We aimed to examine whether bipolar disorder, relative to healthy, participants displayed elevated activity in these regions during reward processing.
Methods
Twenty-one euthymic bipolar I disorder and 20 healthy control participants with no lifetime history of psychiatric disorder underwent functional magnetic resonance imaging (fMRI) scanning during a card-guessing paradigm designed to examine reward-related brain function to anticipation and receipt of monetary reward and loss. Data were collected using a 3T Siemens Trio scanner.
Results
Region-of-interest analyses revealed that bipolar disorder participants displayed greater ventral striatal and right-sided orbitofrontal [Brodmann area (BA) 11] activity during anticipation, but not outcome, of monetary reward, relative to healthy controls (p < 0.05, corrected). Wholebrain analyses indicated that bipolar disorder, relative to healthy, participants also displayed elevated left-lateral OFC activity (BA 47) activity during reward anticipation (p < 0.05, corrected).
Conclusions
Elevated ventral striatal and OFC activity during reward anticipation may represent a neural mechanism for predisposition to expansive mood and hypo/mania in response to reward-relevant cues that characterizes bipolar disorder. Our findings contrast with research reporting blunted activity in the ventral striatum during reward processing in unipolar depressed individuals, relative to healthy controls. Examination of reward-related neural activity in bipolar disorder is a promising research focus to facilitate identification of biological markers of the illness.
Keywords: bipolar disorder, fMRI, orbitofrontal cortex, reward, ventral striatum
Bipolar disorder is a severe and recurrent illness involving significant impairment, including erratic work performance, high rates of divorce and suicide, and high rates of alcohol and substance abuse (1, 2). Yet, bipolar disorder is often diagnosed late in illness course, or misdiagnosed as other illnesses such as unipolar depression (3). Examination of underlying pathophysiological processes of bipolar disorder with neuroimaging techniques such as fMRI is a step forward not only toward understanding the neural basis of bipolar disorder, but also toward identifying biological markers to facilitate earlier and more accurate diagnosis and treatment of the illness (4).
Research suggests that bipolar disorder may be characterized by a hypersensitivity to reward-relevant stimuli which may be a key component of the emotional lability and dysregulation that characterize the illness (5). A hypersensitivity to potential future rewards may lead to an excessive increase in approach or goal-related affect in the presence of reward-relevant life events, which may be reflected in a vulnerability to hypo/manic symptoms (5). Support for this perspective comes from psychosocial research indicating that, compared to relevant control groups, individuals with bipolar disorder display elevated scores on self-report measures of sensitivity to reward-relevant stimuli (6, 7), and among bipolar disorder individuals, a heightened sensitivity to reward-relevant stimuli is associated with a more severe course (8). Furthermore, both reward-striving and reward-attainment-relevant life events have been demonstrated to trigger hypo/manic episodes among individuals with bipolar disorder (9, 10).
The ventral striatum is a core component of the neural circuitry of reward processing and is involved in processing both primary (pleasant tastes/smells/sights) and secondary (monetary) rewards (11–14). The ventral striatum is part of a larger cortico-limbic circuit subserving reward-related processing, and a subregion of the prefrontal cortex, specifically the orbitofrontal cortex (OFC), has also been implicated in reward processing (14, 15). Bermpohl and colleagues (16) recently reported that bipolar manic patients displayed increased lateral OFC activity (BA11, BA47) during anticipation of reward-relevant cues, as compared to healthy controls. Using electroencephalography (EEG), we also have reported that individuals with bipolar disorder displayed abnormally elevated left-lateral prefrontal activity during reward anticipation, supporting a reward hypersensitivity model of bipolar disorder (17).
No neuroimaging studies have yet examined neural activity during reward processing in bipolar euthymic individuals. Accordingly, the goal of the present study was to examine neural activity during reward processing in bipolar euthymic, relative to age and gender-ratio-matched healthy control, participants using a well validated card-guessing paradigm designed to examine neural activity during both anticipation and receipt of monetary reward and loss (12, 18, 19). We focused on examination of euthymic bipolar disorder adults to examine whether abnormalities in reward-related brain function may represent a persistent, rather than mood state-dependent, feature of bipolar disorder. Using region-of-interest analyses, we hypothesized that bipolar euthymic, relative to healthy control, participants would show elevated ventral striatal and OFC activity to reward but not loss-relevant cues. Previous research indicating that (i) the ventral striatum is involved in monitoring the anticipation of reward (15, 20), and (ii) individuals with bipolar disorder are particularly affected by anticipatory or goal-striving based life events/stimuli (10, 21), also allowed us to hypothesize that bipolar disorder, relative to healthy control, participants would show elevated reward-related brain activity during anticipation, rather than receipt, of reward. Exploratory wholebrain analyses were also conducted to examine group differences in other reward-related brain regions.
Materials and methods
Participants
Twenty-one remitted adults with bipolar I disorder [mean age = 31.53, standard deviation (SD) = 8.66; male/female: 9/12] participated in the study. Bipolar disorder was diagnosed according to DSM-IV criteria using the Structured Clinical Interview for DSM-IV-research version (SCID-P) (22). All bipolar disorder participants were in remission, as determined by SCID-P criteria for at least two months at the time of scanning. Current mood state was confirmed by having a 25-item Hamilton Rating Scale for Depression (HRSD-25) score ≤ 7 and a Young Mania Rating Scale (YMRS) score ≤ 10 on the day of the scan. Bipolar disorder participants were in remission for an average of 24 months and 85% had a depressive episode as their last episode. Sixteen bipolar disorder participants had at least one lifetime comorbid psychiatric disorder. This rate of lifetime comorbidity is consistent with existing epidemiological research on lifetime comorbidity rates in bipolar disorder (23, 24). Importantly, participants with bipolar disorder were free from alcohol/substance abuse or dependence for a minimum of seven months (range: 7 to 269 months). Comorbid diagnoses, including alcohol/substance abuse or dependence, were diagnosed according to DSM-IV criteria using the SCID-P. Twenty bipolar disorder participants were taking at least one psychotropic medication, representative of the bipolar population, where most bipolar disorder adults require psychotropic medication (25) (Table 1).
Table 1.
Demographic and clinical variables
| Bipolar disorder (n = 21) Mean or proportion |
SD | Healthy controls (n = 20) Mean or proportion |
SD | Statistic | p-value | |
|---|---|---|---|---|---|---|
| Age at scan | 31.53 | 8.66 | 31.56 | 6.87 | t(39)= −0.01 | 0.99 |
| Females | 12/21 | 12/20 | χ2(1) = 0.03 | 0.85 | ||
| Daily nicotine consumption | 7/21 | 2/20 | χ2(1) = 2.03 | 0.15 | ||
| Daily caffeine consumption | 16/21 | 7/20 | χ2(1) = 5.48 | 0.02 | ||
| Age of illness onset | 18.14 | 6.33 | ||||
| Illness duration | 13.39 | 8.07 | ||||
| HRSD-25 | 6.43 | 4.20 | ||||
| YMRS | 2.29 | 2.51 | ||||
| No. of psychotropic drugs | 2.14 | 1.01 | ||||
| Total-medication load | 3.00 | 1.64 | ||||
| Antipsychotic-medication load (chlorpromazine equivalent) | 1.57 | 0.51 | ||||
| Mood stabilizers | 15/21 | |||||
| Antipsychotics | 12/21 | |||||
| Antidepressants | 8/21 | |||||
| Benzodiazepines | 3/21 | |||||
| Dopaminergic antidepressants (buproprion) | 3/21 | |||||
| Lifetime presence of anxiety disorder | 9/21 | |||||
| Lifetime presence of eating disorder | 0/21 | |||||
| Lifetime presence of somatoform disorder | 0/21 | |||||
| Prior history of alcohol/drug abuse or dependence | 13/21a |
SD = standard deviation; HRSD-25 = Hamilton Rating Scale for Depression (25-item); YMRS = Young Mania Rating Scale.
Bipolar disorder participants were free from alcohol/drug abuse or dependence for a minimum of seven months prior to the present study (range: 7 to 269 months).
Twenty healthy adult control participants (mean age = 31.56, SD = 6.87; male/female: 8/12) with no previous personal or family history of psychiatric illness in first-degree relatives participated in the study. We used the Family History Questionnaire (Nimgaonkar, personal communication) to assess psychiatric illness of first-degree relatives of participants. Healthy control participants were gender-ratio-matched with bipolar disorder participants [χ2(1) = 0.03, p = 0.85] and age-matched [t(39) = −0.01, p = 0.99). All participants were right-handed and native English speaking.
Exclusion criteria for all participants included: history of head injury (from medical records and participant report), systemic medical illness, cognitive impairment [score < 24 Mini-Mental State Examination (26)], premorbid IQ estimate < 85 National Adult Reading Test (27), Axis-II borderline personality disorder, and general exclusion criteria for MRI. Further exclusion criteria for bipolar disorder participants included rapid cycling disorder, and, for control participants, previous or current alcohol/illicit substance abuse (determined by SCID-P, saliva, and urine screen).
The participant population reflected the demographics of Pittsburgh and the surrounding area. The study protocol was approved by the University of Pittsburgh Institutional Review Board. After complete description of the study to the participants, written informed consent was obtained.
Paradigm
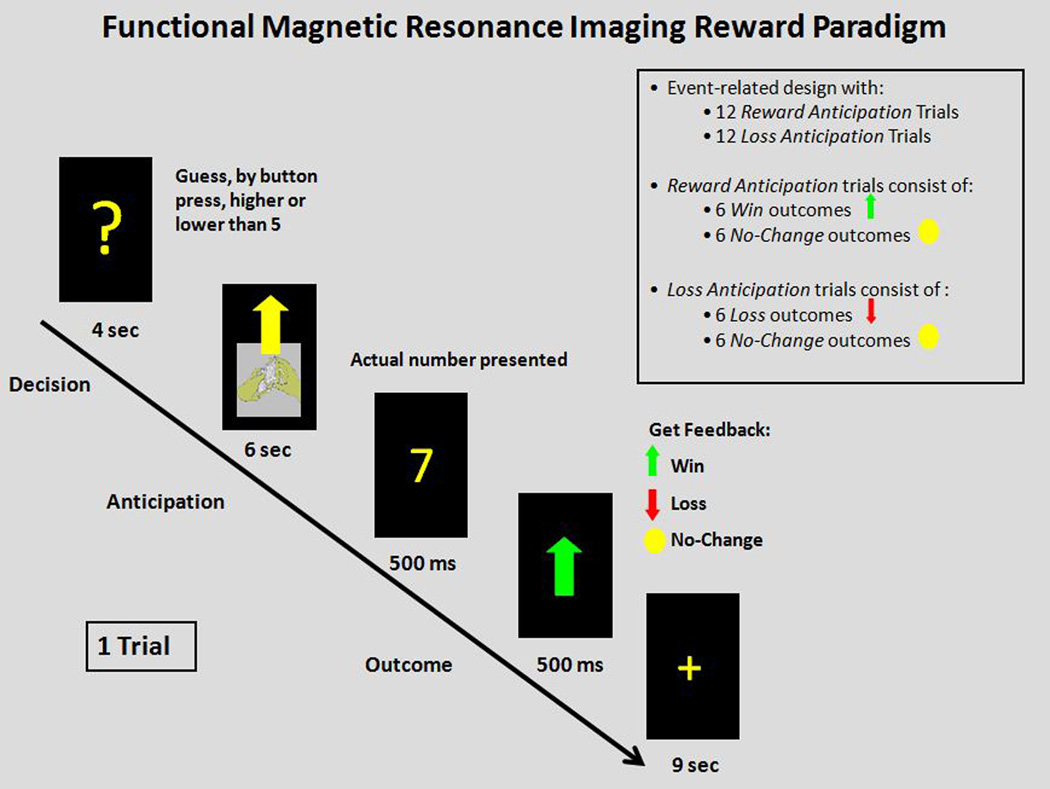
We employed a slow event-related fMRI card-guessing paradigm (Fig. 1) designed to examine reward-related brain function to anticipation and receipt of monetary reward and loss. Each trial included an anticipation period and outcome period, where participants received win, loss, or no-change feedback for each trial.
Figure 1.
Slow event-related card-guessing paradigm with monetary reward.
Trials were presented in a pseudorandom order with predetermined outcomes. During each 20-sec trial, participants had four secs to guess, via button press, whether the value of a visually presented card with a possible value of 1–9 was higher or lower than 5. After a choice was made, the trial type was presented visually for six secs indicating whether the trial was a reward-anticipation (presentation of an upward arrow) or loss-anticipation (presentation of a downward arrow) type. In reward-anticipation trials participants would win money if their guess was correct and there would be no-change in earnings if their guess was incorrect. In loss-anticipation trials participants would lose money if their guess was incorrect and there would be no-change in earnings if their guess was correct. The anticipation period was immediately followed by the outcome period, where participants were presented with the actual numerical value of the card (500 msec) and received outcome feedback (additional 500 msed): a green upward-facing arrow for win, a red downward-facing arrow for loss, or a yellow circle for no-change feedback. A crosshair was then presented for nine secs [inter-stimulus interval (ISI)]. The outcome period was defined as a 7-sec period starting at the point when participants were presented with the actual numerical value of the card, and the baseline period comprised the last three seconds of the ISI. Twenty-four trials were presented in one run with 12 reward-anticipation and 12 loss-anticipation trials. Within reward-anticipation trials there were a balanced number of six win-outcome-after-reward-anticipation trials and six no-change-outcome-after-reward-anticipation trials. Within the loss-anticipation trials there were a balanced number of six loss-outcome-after-loss-anticipation trials and six no-change-outcome-after-loss-anticipation trials. Previous findings indicate that one run of twenty-four trials (12 trials for each of the two possible anticipation trial types and six trials for each of the four possible outcome trial types) is effective for assessing reward-related brain function and minimizing fatigue and habituation (18, 19).
Participants were told they would receive $1 for each win, lose 50 cents for each loss, and obtain no earnings change for no-change-outcomes. Outcome probabilities were, in fact, fixed such that each participant received $3 in earnings. Participants were unaware of the fixed outcome probabilities in the paradigm, and were led to believe their performance would determine net monetary gain.
The version of the card-guessing task employed in the present study was designed to examine reward-related brain activity while equating behavior across groups. In line with existing research that utilized the current version of the card-guessing task (18, 19), we predicted no differences in reaction time between individuals with bipolar disorder and healthy control participants.
Medication
To examine possible effects of psychotropic medication on neuroimaging measures in bipolar disorder participants, we computed: (i) medication load, an index that reflects the number and dose of different medications, as in our previous neuroimaging studies on bipolar disorder (28, 29) (see Supplementary materials); (ii) total antipsychotic medication load (in chlorpromazine equivalents); (iii) total-number of psychotropic medications; and (iv) identified medication status (taking versus not taking each of five main psychotropic medication subclasses: mood stabilizers, antipsychotics, antidepressant, anxiolytics, dopaminergic-antidepressants, e.g., bupropion). Given the possible role that dopamine plays in ventral striatal-centered reward processing (14), we also re-ran ventral striatal ROI analyses after excluding three bipolar disorder participants taking dopaminergic antidepressants.
Neuroimaging data acquisition
Neuroimaging data were collected using a 3.0 Tesla Siemens Trio MRI scanner at the University of Pittsburgh. Structural 3-D axial MPRAGE images were acquired in the same session [echo time (TE) = 3.29 msec; repetition time (TR) = 2200 msec; flip angle = 9°; field of view (FOV) = 256 × 192 mm; slice thickness = 1 mm; matrix = 256 × 256; 192 continuous slices]. Mean blood-oxygen-level-dependent (BOLD) images were then acquired with a gradient echo EPI sequence during 13 min covering 39 axial slices (3.1 mm thick; TR/TE = 2000/28 msec; FOV = 205 × 205mm; matrix 64 × 64; flip angle = 90°).
Neuroimaging data analysis
Data were preprocessed and analyzed with Statistical Parametric Mapping software, version 5 [(SPM5); http://www.fil.ion.ucl.ac.uk/spm]. Data for each participant were realigned to the first volume in the time series to correct for head motion. Realigned images were then coregistered with the subject’s anatomical image, segmented, normalized to standard Montreal Neurological Institute (MNI) template, and spatially smoothed with a Gaussian kernel of 8 mm full-width at half-maximum (FWHW).
A first-level fixed-effect model was constructed for each participant and scan and predetermined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for six contrasts: reward-anticipation-minus-baseline, loss-anticipation-minus-baseline, win-outcome-after-reward-anticipation-minus-baseline, no-change-outcome-after-reward-anticipation-minus-baseline, loss-outcome-after-loss-anticipation-minus-baseline, no-change-outcome-after-loss-anticipation-minus-baseline. Movement parameters from the realignment stage were entered as covariates of no interest to control for participant movement. No participant displayed greater than 4mm of movement. Trials were modeled with the Canonical Hemodynamic Response Function.
Two general linear models (GLMs) were conducted on the t-contrast images generated in the previous single-subject analyses to examine BOLD signal during the anticipation-period and during the outcome period, as there were different numbers of variables for anticipation (2: reward versus loss-anticipation) and outcome (4: win-outcome-after-reward-anticipation, no-change-outcome-after-reward-anticipation, loss-outcome-after-loss-anticipation, no-change-outcome-after-loss-anticipation). In the GLM for the anticipation-period, a second-level random-effects within-group and between-group analysis was therefore conducted as a 2 (diagnostic group) × 2 (anticipation type) repeated-measures analysis of variance (ANOVA). In the GLM for the outcome period, a second-level random-effects within-group and between-group analysis was conducted as a 2 (diagnostic group) × 4 (outcome type) repeated-measures ANOVA. Both GLMs were conducted on an a priori bilateral ventral striatal region-of-interest (ROI) mask, and an a priori bilateral OFC ROI, and additionally on wholebrain data. The bilateral ventral striatal ROI was defined as two 8 mm spheres based on MNI coordinates (right: x = 9, y = 9, z = −8; left: x = −9, y = 9, z = −8) from previous meta-analyses (30, 31). The OFC ROI was defined as bilateral BA11 and BA47 [Wake Forest Toolbox PickAtlas Talairach Daemon template (32)], given Bermpohl and colleagues’ (16) finding that manic bipolar disorder patients displayed elevated activity in these OFC regions during reward expectation. To control for multiple statistical testing in ROI analyses we maintained a family wise error (FWE) rate at p < 0.05. Given the conservative nature of the FWE correction for wholebrain analyses, we used the AlphaSim method to control for multiple voxelwise statistical testing in wholebrain analyses. This provided an empirically driven clusterwise threshold of p < 0.05, across the whole brain. For wholebrain analyses, we therefore used a voxelwise threshold of p < 0.005, and a cluster (k) extent, determined by Monte Carlo simulations at the wholebrain level implemented in AlphaSim, of 58 voxels. This accounted for spatial correlations between BOLD signal changes in neighboring voxels. Post-hoc analyses with pairwise and independent t-tests were performed within SPM in ROI and wholebrain clusters showing a significant (corrected) group × condition interaction in each ANOVA. Beta values were extracted for graphical purposes only.
To control for multiple post-hoc tests we used a Bonferroni-corrected voxel-wise threshold to correct for the four a priori post-hoc comparisons in the anticipation-period GLM: (i) bipolar-reward-anticipation vs. control-reward-anticipation; (ii) bipolar-loss-anticipation vs. control-loss-anticipation; (iii) bipolar-reward-anticipation vs. bipolar-loss-anticipation; (iv) control-reward-anticipation vs. control-loss anticipation; p < 0.05/4 = 0.013. We similarly controlled for four main a priori post-hoc comparisons of interest focusing on actual win or actual loss in the outcome period GLM: (i) bipolar-win-outcome-after-reward-anticipation vs. control-win-outcome-after-reward-anticipation; (ii) bipolar-loss-outcome-after-loss-anticipation vs. control-loss-outcome-after-loss-anticipation; (iii) bipolar-win-outcome-after-reward-anticipation vs. bipolar-loss-outcome-after-loss-anticipation; (iv) control-win-outcome-after-reward-anticipation vs. control-loss-outcome-after-loss-anticipation; p < 0.05/4 = 0.013.
Exploratory analyses
We explored possible relationships between activity in regions from ROI and wholebrain analyses showing a significant main effect of group or a group × condition interaction and demographic, clinical, daily caffeine and nicotine consumption, and medication variables, as well as medication load (total and antipsychotic), total number of psychotropic medications, and taking versus not taking each of five main psychotropic medication subclasses: mood stabilizers, antipsychotics, antidepressants, anxiolytics, dopaminergic-antidepressants (bupropion).
Results
Behavioral analyses
In line with prediction and existing research that employed the current version of the card-guessing task (18, 19), bipolar disorder and healthy control participants did not differ in reaction time during the card-guessing task, t(35) = 1.10, p = 0.29.
Ventral striatal activity during anticipation period
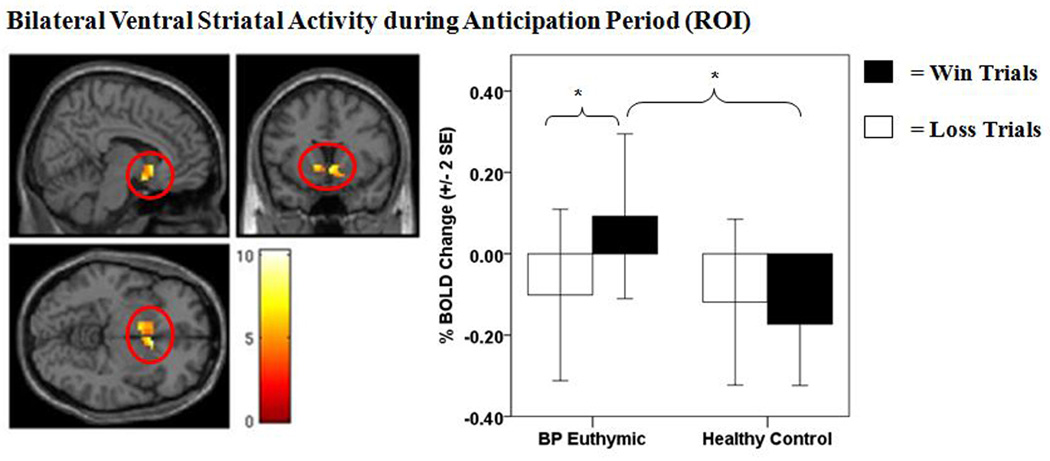
There was a main effect of anticipation-condition on activity in both the right and left ventral striatal ROI mask (p < 0.05, FWE corrected), such that all participants had greater bilateral ventral striatal activity during reward, as opposed to loss, anticipation trials (Table 2). There was a significant group × anticipation condition interaction on activity in both the right and left ventral striatum (p < 0.05, FWE corrected) (Table 2, Fig. 2). This effect was maintained after removing three bipolar disorder participants taking dopaminergic antidepressants (p < 0.05, FWE corrected). Post-hoc analyses revealed that bipolar disorder participants displayed significantly greater right, but not left, ventral striatal activity during reward anticipation relative to healthy controls (p < 0.007, Bonferroni corrected) (Table 2). No difference in ventral striatal activity was observed between individuals with bipolar disorder and healthy control participants during loss-anticipation. Post-hoc analyses also indicated that bipolar disorder, but not healthy control, participants showed significantly greater bilateral ventral striatal activity during reward anticipation than loss anticipation (p < 0.001, Bonferroni corrected) (Table 2).
Table 2.
ROI analyses on ventral striatal and orbitofrontal cortical BOLD signal during anticipation period
| Condition | Hemisphere | Cluster size |
F/t | df | Corrected for multipletests |
|---|---|---|---|---|---|
| Main effect of anticipation type | Right VS | 48 | 11.51a | 39 | Yes (p < 0.05, FWE) |
| (reward > loss) | Left VS | 66 | 18.49a | 39 | Yes (p < 0.05, FWE) |
| Right OFC | 15 | 7.59a | 39 | No | |
| Left OFC | 4 | 11.68a | 39 | No | |
| Main effect of group | Right VS | 2 | 4.68a | 39 | No |
| Left VS | 0 | 2.17a | 39 | No | |
| Right OFC | 3 | 5.01a | 39 | No | |
| Left OFC | 4 | 5.26a | 39 | No | |
| Group × anticipation interaction | Right VS | 42 | 10.91a | 39 | Yes (p < 0.05, FWE) |
| Left VS | 28 | 8.34a | 39 | Yes (p < 0.05, FWE) | |
| Right OFC | 41 | 15.21a | 39 | Yes (p < 0.05, FWE) | |
| Left OFC | 92 | 10.22a | 39 | No | |
| Between-group post-hoc effects | |||||
| BD > HC: reward anticipation | Right VS | 36 | 2.50b | 39 | Yes (p < 0.013, Bonferroni) |
| Left VS | 4 | 1.88b | 39 | No | |
| Right OFC | 27 | 2.75b | 39 | Yes (p < 0.013, Bonferroni) | |
| BD > HC: loss anticipation | Right VS | 1 | 1.67b | 39 | No |
| Left VS | 0 | 0.64b | 39 | No | |
| Right OFC | 1 | 1.83b | 39 | No | |
| Within-group post-Hoc effects | |||||
| BD reward anticipation > | Right VS | 80 | 4.14b | 20 | Yes (p < 0.013, Bonferroni) |
| BD loss anticipation | Left VS | 78 | 5.15b | 20 | Yes (p < 0.013, Bonferroni) |
| Right OFC | 56 | 3.14b | 20 | Yes (p < 0.013, Bonferroni) | |
| HC reward anticipation > | Right VS | 0 | 1.33b | 19 | No |
| HC loss anticipation | Left VS | 3 | 1.80b | 19 | No |
| Right OFC | 2 | 1.42b | 19 | No |
Analyses were conducted on an a priori bilateral ventral striatal region of interest (ROI) defined as two 8 mm spheres based on Montreal Neurological Institute (MNI) coordinates (right: x = 9, y = 9, z = −8; left: x = −9, y = 9, z = −8) from previous meta-analyses [Di Martino et al. 2008 (30); Postuma and Dagher 2006 (31)] and an a priori bilateral OFC ROI (BA11, BA47). Main effect, interaction, and post-hoc analyses were conducted on the blood-oxygen-level-dependent (BOLD) signal for anticipation period minus baseline period. To control for multiple statistical tests for ROI main effect and interaction analyses, we maintained a family wise error (FWE) rate at p < 0.05. We used a Bonferroni-corrected voxel wise cut-off to correct for four multiple a priori post-hoc comparisons (p < 0.05/4 = 0.013). BD = bipolar disorder; HC = healthy controls; VS = ventral striatum; OFC = orbitofrontal cortex.
F-value;
t-value.
Figure 2.
Bilateral ventral striatal activity during anticipation period [region of interest (ROI)]. The left panel displays the anatomical location of the significant group × anticipation condition interaction on the bilateral ventral striatal ROI mask, defined as 8 mm spheres based on the Montreal Neurologic Institute coordinates system (right: x = 9, y = 9, z = −8; left: x = −9, y = 9, z = −8) from previous meta-analytic research [Di Martino et al. 2008 (30); Postuma and Dagher 2006 (31)]. [Right: F(1,39) = 10.91, p < 0.001, k = 42 voxels, p < 0.05, family wise error (FEW) corrected; Left: F(1,39) = 8.34, p < 0.005, k = 28 voxels, p < 0.05, FEW corrected]. The right panel displays a histogram of the mean bilateral ventral striatal activity depicting the group × anticipation condition interaction. Color bars reflect beta values and significant clusters were overlaid on sagittal, coronal, and axial brain slices. Statistical tests were performed within SPM and beta values were extracted for graphical purposes only. BOLD = blood-oxygen-level-dependent; SE = standard error; BP euthymic = bipolar disorder patients in a euthymic state (n = 21); healthy controls (n = 20). *significant post-hoc comparison at p < 0.013 (Bonferroni corrected).
Orbitofrontal cortical activity during anticipation period
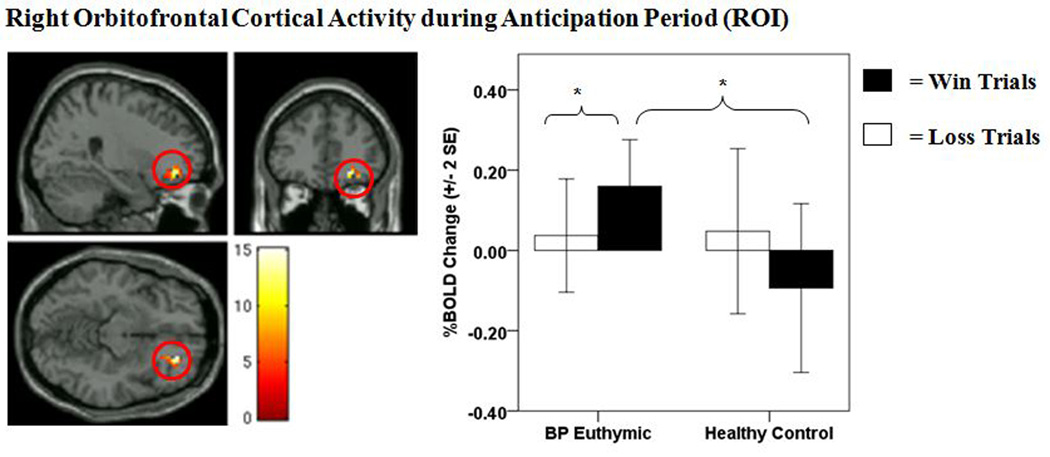
There was a significant group × anticipation condition interaction on activity in the right OFC [(BA11) p < 0.05, FWE corrected] (Table 2, Fig. 3). Post-hoc analyses revealed that bipolar disorder participants displayed significantly greater right-sided OFC activity during reward anticipation relative to healthy controls (p < 0.004, Bonferroni corrected) (Table 2). Bipolar disorder participants also displayed significantly greater right OFC activity during reward versus loss anticipation (p < 0.001, Bonferroni corrected) (Table 2). No difference in right OFC activity was observed between bipolar disorder and healthy control participants during loss-anticipation, or in healthy control participants between reward and loss anticipation. The group × anticipation condition interaction on activity in the left lateral OFC (BA47) (Table 2) failed to meet FWE correction. Exploratory post-hoc analyses, however, revealed that left-lateral OFC activity during the anticipation period displayed a similar pattern as right-sided OFC activity (see Supplementary materials).
Figure 3.
Right orbitofrontal cortical (OFC) activity during anticipation period [region of interest (ROI)]. The left panel displays the anatomical location of the significant group × anticipation condition interaction on right-sided OFC activity (BA11) from the bilateral OFC ROI mask [(1,39) = 15.21, p = < 0.001, k = 41 voxels, p < 0.05, family wise error (FEW) corrected]. The right panel displays a histogram of mean right-sided OFC activity depicting the group × anticipation condition interaction. Color bars reflect beta values and significant clusters were overlaid on sagittal, coronal, and axial brain slices. Statistical tests were performed within SPM and beta values were extracted for graphical purposes only. BOLD = blood-oxygen-level-dependent; SE = standard error; BP euthymic = bipolar disorder patients in a euthymic state (n = 21); healthy controls (n = 20). *significant post-hoc comparison at p < 0.013 (Bonferroni corrected).
Wholebrain activity during anticipation period
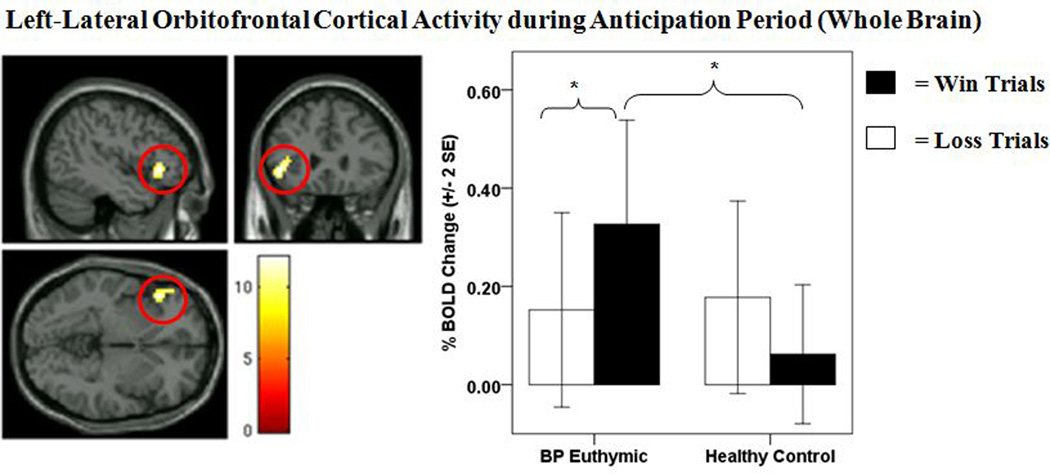
There was a main effect of anticipation condition on activity in the right middle occipital gyrus and in a number of neural regions supporting reward processing and affect that surpassed our cluster extent threshold of 58 voxels. In all cases, these regions were characterized by greater activity during reward, as opposed to loss, anticipation trials. There was a significant group × anticipation condition interaction on wholebrain activity in the left-lateral OFC (BA47) that surpassed our AlphaSim-corrected cluster extent threshold of 58 voxels (Table 3, Fig. 4). Post-hoc analyses on left-lateral OFC revealed that bipolar disorder, relative to healthy control, participants displayed significantly greater left-lateral OFC activity during reward anticipation (p = 0.007, Bonferroni corrected) (Table 3). No difference in left-lateral OFC activity was observed between bipolar disorder and control participants during loss-anticipation. Post-hoc analyses also indicated that bipolar disorder, but not healthy control, participants showed significantly greater left-lateral OFC activity during reward anticipation than loss anticipation (p < 0.001, Bonferroni corrected) (Table 3).
Table 3.
Wholebrain analyses for the anticipation period
| Group × anticipation period interaction on wholebrain BOLD signal | |||||||
|---|---|---|---|---|---|---|---|
| Brodman | Cluster | Talairach coordinates | |||||
| area | size | x | y | z | F-value | Corrected for multiple tests | |
| Left-lateral orbitofrontal cortex | 47 | 60 | −45 | 27 | −3 | 12.10 | Yes (AlphaSim) |
| Post-hoc effects on left-lateral orbitofrontal cortex (BA47) BOLD signal during anticipation period | |||||||
| Cluster | |||||||
| Condition | Region | size | t-value | df | Corrected for multiple tests | ||
| Between-group post-hoc effects | |||||||
| BD > HC: reward anticipation | Left OFC | 24 | 2.53 | 39 | Yes (p < 0.013, Bonferroni) | ||
| BD > HC: loss anticipation | Left OFC | 0 | 0.95 | 39 | No | ||
| Within-group post-hoc effects | |||||||
| BD reward anticipation > | Left OFC | 60 | 3.45 | 20 | Yes (p < 0.013, Bonferroni) | ||
| BD loss anticipation | |||||||
| HC reward anticipation > | Left OFC | 0 | −0.84 | 19 | No | ||
| HC loss anticipation | |||||||
To control for multiple statistical testing for wholebrain analyses, we used a cluster (k) extent determined by Monte Carlo simulations at the wholebrain level implemented in AlphaSim of 58 voxels. Post-hoc analyses were conducted on the cluster in the left-lateral orbitofrontal cortex [Montreal Neurological Institute (MNI) coordinates: x = −45, y = 27, z = −3] that was significant in the group × anticipation-period interaction. Main effect, interaction, and post-hoc analyses were conducted on the blood-oxygen-level-dependent (BOLD) signal for anticipation period minus baseline period. To control for multiple post-hoc tests, we used a Bonferroni-corrected voxel wise cut-off to correct for four multiple a priori post-hoc comparisons (p < 0.05/4 = 0.013). BD = bipolar disorder; HC = healthy controls; OFC = orbitofrontal cortex.
Figure 4.
Left-lateral orbitofrontal cortical (OFC) activity during anticipation period (wholebrain). The left panel displays the anatomical location of the significant group × anticipation condition interaction on left-lateral OFC activity (BA47) from wholebrain analyses [(1,39) = 12.10, p = 0.001, k = 60 voxels, AlphaSim corrected]. The right panel displays a histogram of mean left-lateral OFC activity from wholebrain analyses depicting the group × anticipation condition interaction. Color bars reflect beta values and significant clusters were overlaid on sagittal, coronal, and axial brain slices. Statistical tests were performed within SPM and beta values were extracted for graphical purposes only. BOLD = blood-oxygen-level-dependent; SE = standard error; BP euthymic = bipolar disorder patients in a euthymic state (n = 21); healthy controls (n = 20). *significant post-hoc comparison at p < 0.013 (Bonferroni corrected).
Outcome period
There was no significant main effect of group, or group × condition interaction, within the ventral striatal or OFC ROI mask (Supplementary Table 1), or for wholebrain activity, for the four contrasts of interest in the outcome period.
Relationships between ventral striatal and OFC activity during reward processing and demographic, clinical, and medication variables
There were no relationships between ventral striatal or OFC activity during reward processing and demographic variables, comorbid anxiety diagnosis, daily caffeine and nicotine consumption, total medication load, medication load for antipsychotics, total number of psychotropic medications, and taking versus not taking each of the five main psychotropic medication subclasses: mood stabilizers, antipsychotics, antidepressant, anxiolytics, and dopaminergic antidepressants (Supplementary Table 2). There was also no relationship between ventral striatal and OFC activity during reward processing and a lifetime history of alcohol/substance abuse or dependence (Supplementary Table 2) [as noted, participants with bipolar disorder were free from alcohol/substance abuse or dependence for a minimum of seven-months (range: 7 to 269 months)].
Discussion
The present study is the first to examine reward-related brain activity in bipolar euthymic participants and healthy controls during reward processing. Consistent with hypotheses, ROI analyses indicated that bipolar disorder participants showed greater ventral striatal activity and right-sided-OFC activity during anticipation, but not outcome, of monetary reward, relative to healthy controls. Wholebrain analyses indicated elevated left-lateral OFC activity among bipolar disorder participants during reward anticipation, relative to healthy controls. No difference in ventral striatal activity and OFC activity was observed between bipolar disorder and healthy control participants during loss-anticipation. All main findings were specific to the anticipation-period.
The ventral striatum is implicated in reward processing (11–14) and there is growing evidence that dopamine plays an important role in ventral striatal-centered reward processing (14). Dopaminergic abnormalities may therefore serve as the neurochemical basis for elevated ventral striatal activity in bipolar disorder. Elevated OFC activity has also been linked to reward processing, and Bermpohl and colleagues (16) recently reported that bipolar manic patients displayed greater OFC activity during reward anticipation relative to healthy controls. We now show abnormally elevated OFC and ventral striatal activity during reward anticipation in bipolar disorder euthymic adults, and suggest that this may represent a neural mechanism for the elevated self-report and neurophysiological indices of reward sensitivity (6–8, 17) and approach-related behavior (5) underlying vulnerability for hypo/mania in bipolar illness.
While control participants did not recruit the ventral striatum, they did recruit the left-lateral OFC during both reward and loss anticipation. Rodent studies suggest that the ventral striatum may encode both the value, or magnitude, and identity, or quality, of rewarding stimuli, while the OFC may be necessary for encoding the identity, but not the value, of reward (33). This suggests that bipolar disorder and control participants may have employed different strategies during encoding of potential future reward during the anticipation-period, and that, unlike participants with bipolar disorder, control participants may have focused on encoding the identity rather than value of potential future reward. Accordingly, previous and present data suggest that ventral striatal hypersensitivity, together with elevated OFC activity to anticipation of reward-relevant cues, may be a key biological marker of bipolar disorder, potentially reflecting an underlying neural mechanism for abnormal processing of potential future reward, that in turn may predispose a person to hypo/mania. These patterns of abnormal neural activity may be a useful future biological target for novel interventions to help individuals with bipolar disorder develop strategies for effectively regulating their behavior in response to reward-relevant environmental events (34).
Neuroimaging studies in unipolar depression reported abnormally reduced, as opposed to elevated, ventral striatal activity, versus healthy controls, during reward-related laboratory tasks (18, 35, 36) and to positive emotional stimuli (37, 38). Together with our present findings, these findings suggest that unipolar depression and bipolar disorder may be characterized by differential patterns of abnormal ventral striatal activity during reward anticipation and receipt. It is important to note, however, that research on ventral striatal activity to reward cues in unipolar depression has typically examined participants in a depressive episode at the time of fMRI scanning. Future research is needed directly comparing euthymic bipolar and euthymic unipolar depressed individuals to determine whether ventral striatal activity during reward processing may yield state-independent biological markers to help distinguish the two disorders.
Rates of lifetime comorbidity reported in the present study were consistent with existing epidemiological research on lifetime comorbidity rates in bipolar disorder (23, 24). Importantly, bipolar disorder participants were free from alcohol/substance abuse or dependence for a minimum of seven months (range: 7 to 269 months), and we did not observe any significant relationships between a prior history of substance or alcohol abuse and ventral striatal or OFC activity during reward anticipation in participants with bipolar disorder. Further, individuals with substance use disorders have previously been shown to display decreased, rather than increased, ventral striatal activity during anticipation of non-drug related cues, such as monetary reward (39). Thus, the elevated ventral striatal and OFC activity observed in the present study among bipolar disorder participants during reward anticipation is likely not attributable to alcohol/substance abuse or dependence.
There were limitations to the present study. Future studies are needed to examine mood state-independent versus mood state-dependent components of abnormally elevated neural activity during reward anticipation and receipt in bipolar disorder by comparing euthymic bipolar disorder participants with manic and/or depressed bipolar disorder. It will be important for this research to employ fMRI reward paradigms examining both the anticipation and receipt of reward, as well as omission of reward. This is particularly relevant given research suggesting that bipolar manic patients fail to show the previously reported pattern of decreased ventral striatal activation when an expected reward is omitted (40). Second, future research is needed to examine whether elevated neural activity to reward cues is specific to bipolar disorder or indicative of more general motivational and/or regulatory deficits observed across multiple psychiatric disorders. Third, bipolar disorder participants were medicated at the time of study. We did not, however, observe any significant relationships between psychotropic or antipsychotic medication load, total number of psychotropic medications, or between any specific class of psychotropic medication, including dopaminergic-antidepressants or antipsychotic medications, and neural activity during reward anticipation in participants with bipolar disorder. Furthermore, removing individuals on dopaminergic-antidepressants did not alter the critical interaction in the ventral striatal ROI. Given that 12 of 21 bipolar disorder participants were taking antipsychotic medications at fMRI scanning, we did not have the statistical power to examine our a priori hypotheses excluding participants taking antipsychotic medications. Future studies, may wish to examine this issue. Lastly, we used one run of twenty-four trials for the fMRI reward paradigm given previous research indicating that this configuration is effective for assessing reward-related brain function and minimizing fatigue and habituation (18–19). However, we cannot rule out the possibility that a larger number of trials could have produced group differences to other conditions or in other neural regions. Future research may wish to address this issue.
Abnormally elevated ventral striatal and OFC activity during reward anticipation is a potential neural basis for the observed hypersensitivity to reward-relevant stimuli in bipolar disorder. The possible dopaminergic basis of elevated ventral striatal activity in bipolar disorder has important implications for treatment choices and new treatment development for the illness. Future studies should aim to replicate our findings and examine the extent to which abnormally elevated ventral striatal and OFC activity during reward processing may serve as a potential biological marker of bipolar disorder. It will be important for this subsequent research to examine reward-related brain activity in individuals at heightened risk for bipolar disorder, but who have not yet developed the disorder. This will help determine whether abnormally elevated ventral striatal and OFC activity during reward processing represents a pre-existing vulnerability for bipolar disorder, or a consequence of having a bipolar episode.
Supplementary Material
Acknowledgements
All work was carried out within the Department of Psychiatry, University of Pittsburgh, and neuroimaging data were collected at the Magnetic Resonance Research Center (MRRC), University of Pittsburgh, Pittsburgh, PA, USA. We thank Dr. Fernando Boada and his staff for their help acquiring neuroimaging data.
This research was supported by National Institute of Mental Health (NIMH) grant R01 MH076971. JRCA’s, AV’s, EJL’s, and MLP’s contribution to this work was supported by grant R01 MH076971. MLP also received support from a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award, and AV and EEF received support from a NARSAD Young Investigator Award. EEF is also supported by NIMH grants K01 74769 and RO1 DA026222. RN’s contribution was supported by NIMH grant T32 MH016804.
Footnotes
This paper was presented as part of a symposium at the 65th Annual Meeting of the Society of Biological Psychiatry, May 20–22, 2010, New Orleans, LA, USA.
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK, Jamison KR. Manic-depressive illness: Bipolar disorders and recurrent depression. 2nd edition. New York: Oxford University Press; 2007. [Google Scholar]
- 3.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64:161–174. [PubMed] [Google Scholar]
- 4.Phillips ML, Frank E. Redefining bipolar disorder: toward DSM-V. Am J Psychiatry. 2006;163:1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- 5.Urosevic S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clin Psychol Rev. 2008;28:1188–1205. doi: 10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. J Psychopathol Behavior Assessment. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. J Psychopathol Behavior Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alloy LB, Abramson LY, Walshaw PD, et al. Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson SL, Sandrow D, Meyer B, et al. Increases in manic symptoms after life events involving goal attainment. J Abnorm Psychol. 2000;109:721–727. doi: 10.1037//0021-843x.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. A goal-striving life event and the onset of hypomanic and depressive episodes and symptoms: perspective from the behavioral approach system (BAS) dysregulation theory. J Abnorm Psychol. 2007;116:105–115. doi: 10.1037/0021-843X.116.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 12.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 13.Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 14.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 16.Bermpohl F, Kahnt T, Dalanay U, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp. 2010;31:958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmon-Jones E, Abramson LY, Nusslock R, et al. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biol Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J Adolesc Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 21.Urosević S, Abramson LY, Alloy LB, et al. Increased rates of events that activate or deactivate the behavioral approach system, but not events related to goal attainment, in bipolar spectrum disorders. J Abnorm Psychol. 2010;119:610–615. doi: 10.1037/a0019533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 23.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- 24.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 25.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein M, Folstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 28.Hassel S, Almeida JR, Kerr N, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 31.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 32.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 33.McDannald MA, Lucantonio F, Burke KA, Niv Y, Schoenbaum G. Ventral striatum and orbitofrontal cortex are both required for model-based, but not model-free, reinforcement learning. J Neurosci. 2011;31:2700–2705. doi: 10.1523/JNEUROSCI.5499-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Coan JA. Psychosocial interventions for bipolar disorder: perspective from the Behavioral Approach System (BAS) dysregulation theory. Clin Psychol. 2009;16:449–469. doi: 10.1111/j.1468-2850.2009.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiol. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 37.Epstein J, Pan H, Kocsis JH, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 38.Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Beck A, Schlagenhauf F, Wustenberg T, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 40.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacol. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.