Abstract
Angiogenesis plays a critical role in wound repair. Endothelial cells present CXC receptor 3 (CXCR3) for chemokines expressed late in wound regeneration. To understand the physiological role CXCR3 plays in regulating endothelial function, we analyzed the ability of a CXCR3 ligand, IP-10 (CXCL10), to influence endothelial cell tube formation. Treatment of endothelial cells with IP-10 in the presence of vascular endothelial growth factor (VEGF) inhibited tube formation on growth factor–reduced Matrigel and in a subcutaneous Matrigel plug. Furthermore, IP-10 significantly inhibited VEGF-induced endothelial motility, a response critical for angiogenesis. Previous work showed that CXCR3 ligandation initiates protein kinase A (PKA) phosphorylation-dependent inhibition of m-calpain, required for induced cell motility, in fibroblasts but not epithelial cells. Here we show that CXCR3 activation in endothelial cells induces an increase in cAMP and PKA activation. Treatment of endothelial cells with Rp-8-Br-cAMP, an inhibitor of PKA, or small interference RNA to PKA was able to reverse the inhibitory effects of IP-10 on VEGF-mediated tube formation and motility. Importantly, treatment of endothelial cells with VEGF induced the activation of m-calpain, but costimulation with IP-10 significantly decreased this activity. Using Rp-8-Br-cAMP, we show blocking PKA reversed the IP-10 inhibition of VEGF-induced m-calpain activity. These data indicate that the activation of CXCR3 inhibits endothelial tube formation through a PKA mediated inhibition of m-calpain. This provides a means by which late wound repair signals limit the angiogenesis driven early in the wound response process.
Keywords: CXCL10, CXCR3, angiogenesis, signal transduction, cAMP, receptor tyrosine kinase
Wound healing is a dynamic complex biological event.1 During the regenerative phase of wound healing angiogenesis stops, followed by involution during the remodeling phase. Key to both the initial proliferation of these vessels and the subsequent stasis and then involution of the vascular network is the response of the endothelial cells to signals from the surrounding tissues. At present it is not known whether the termination of angiogenesis occurs because of depletion of the proangiogenic or induction of antiangiogenic signals. Recently, we have found that during the regenerative phase of wound repair, chemokines are expressed to limit fibroblast immigration and motility.2 Thus, we asked whether the same signals stopped angiogenic ingrowth.
Two of the ELR (glutamic acid–leucine–arginine)-negative CXC chemokines appear late in the regenerative phase. IP-10 (interferon γ–inducible protein 10, CXCL10) is produced by endothelial cells themselves late in the regenerative phase3 and IP-9 (I-TAC, CXCL11) derives from redifferentiating keratinocytes.2 These ELR-negative chemokines are of particular interest, because they have been reported to be angiostatic.3–5 These chemokines bind to the common CXC chemokine receptor 3 (CXCR3),6 which has been found expressed on human endothelial cells.4,7
Endothelial cell chemotaxis is of significance as a rate-limiting event in neovascularization. CXCR3 ligands inhibit chemotaxis in fibroblasts8 and in endothelial cells.4 The ability of CXCR3 to induce chemotaxis in certain cells and inhibit chemotaxis in other cells, along with the recent finding of a variant CXCR3 isoform,6 suggests the existence of cell-specific signaling pathways mediated by the 2 CXCR3 isoforms. CXCR3 inhibition of fibroblast motility functions through a cAMP/protein kinase A (PKA)-mediated inhibition of m-calpain that abrogates rear retraction.9 However, the signaling pathway and cell responses mediated by CXCR3 in endothelial cells are unknown.
Herein we parse the motility promoting and inhibiting signaling pathways in human microvascular endothelial cells. We show that the ELR-negative chemokine IP-10 inhibits VEGF-induced endothelial cell tube formation concomitant with blocking motility. Furthermore, stimulation of CXCR3 by IP-10 induces an increase in cAMP production and activation of PKA. CXCR3 activation of PKA leads to the inhibition of VEGF-mediated m-calpain activation and thereby limits cell motility. We have identified a signaling pathway mediated by CXCR3 in microvascular endothelial cells that inhibits angiogenesis in vitro.
Materials and Methods
Tube Formation
Immortalized human adult dermal microvascular cells (dHMEC) (BioWhittaker) and human microvascular cell line (HMEC-1) (Centers for Disease Control and Prevention, Atlanta, Ga) were resuspended in serum-free media containing VEGF, IP-10, 8-Br-cAMP, Rp-8-Br-cAMP, CI-1, and calpain inhibitor IV as denoted on the figure then incubated on growth factor reduced Matrigel. The formed tubes were digitally imaged and analyzed using MetaMorph (Universal Imaging Corp).
Motility Assay
Cell migration was performed as previously described.10 In brief, a HMEC-1 monolayer was scraped making a 1-m wide denuded area then stimulated with VEGF, IP-10, 8-Br-cAMP, Rp-8-Br-cAMP, CI-1, and calpain inhibitor IV as denoted on the figures were taken at 0 and 24 hours, and the area unoccupied by the migrating cells was determined using MetaMorph and expressed as a percentage of control.
In Vivo Calpain Activity (Boc-LM-CMAC) Assay
In vivo calpain activity was determined by using the membrane permeable substrate t-BOC-LM-CMAC (BOC) as described previously.11 In brief, cells were incubated with BAPTA/AM, 8-Br-cAMP, Rp-8-Bromo-cAMP, CI-1, calpain inhibitor IV and/or forskolin before the addition of BOC. The cells were further incubated with VEGF, and/or IP-10, as indicated on the figure. The cleavage of BOC by calpain was visualized using a fluorescence microscope. An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
IP-10 Inhibits VEGF-Induced Endothelial Tube Formation
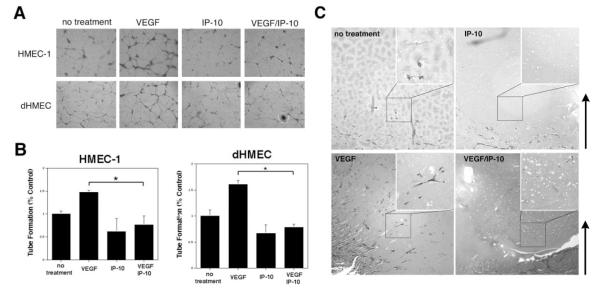
The central hypothesis posits that the ELR-negative chemokines limit vascularization late in wound healing. To examine the role the ELR-negative chemokines play in regulating the ability of endothelial cells to form vessel in vitro, both primary dHMEC and immortalized HMEC-1 cells were analyzed for their ability to form tubes on growth factor reduced Matrigel in the presence of VEGF and/or IP-10. dHMEC and HMEC-1 cells were able to form tubes, whereas addition of VEGF significantly enhanced the number of tubes (Figure 1A and 1B). When either dHMEC or HMEC-1 cells were incubated with IP-10, there was a significant reduction in tubes formed, down to 75% of control (Figure 1A and 1B). This reduction in the number of tubes formed by IP-10 treatment was not attributable to a loss of cells as a counting demonstrated that the number of cells did not differ between VEGF and IP-10 treatments (data not shown). Most importantly for our hypothesis, IP-10 was able to override the angiogenic signals from VEGF, and limit tube formation in the presence of VEGF to an extent similar to IP-10 alone. That this inhibitory action is a general property of ELR-negative CXC chemokines was shown as similar results were obtained when the endothelial cells were treated with IP-9 (CXCL11) and PF4 (CXCL4) (data not shown). These data strongly suggest that ELR-negative chemokines act directly on endothelial cells to inhibit tube formation, findings which correlate with published results.6
Figure 1.
IP-10 inhibits endothelial cell tube formation on Matrigel. A, HMEC-1 and dHMEC cells were detached and resuspended in serum-free media with VEGF (100 ng/mL), IP-10 (200 ng/mL), or H2O (no treatment). The cells (4.5×104 cells/well) were added to 24-well culture plates coated with growth factor reduced Matrigel and incubate for 24 hours. A magnification of ×4 was used to image the cells. B, Endothelial tube formation in A was analyzed using the MetaMorph program. The results are of N=4 (normalized average±SEM). Note, IP-10 inhibited both HMEC-1 and dHMEC tube formation in the presence of VEGF. *P<0.05. C, C57b/H6 mice were implanted subcutaneously with 750 μL of Matrigel containing VEGF (200 ng/mL) and/or IP-10 (400 ng/mL).22 Nine days post inoculation, the Matrigel plug was removed and stained with Massion’s trichrome for endothelial infiltration. VEGF induced the infiltration of endothelial cells, whereas IP-10 inhibited VEGF-mediated endothelial migration and vessel formation in the Matrigel plug. Arrows indicate orientation from integument (dermis) to Matrigel midline. Representative images are shown (×10) with 4-fold magnified insets.
Examining in vivo vessel formation, Matrigel containing VEGF and/or IP-10 was implanted subcutaneously into mice. After 9 days, the Matrigel plug was removed and Masson’s trichrome staining showed that VEGF induced invasion of endothelial cells into the Matrigel compared with no treatment (Figure 1C). IP-10 inhibited this angiogenesis even in VEGF-treated Matrigel (Figure 1C). The thin tube-like structures noted in untreated and VEGF-treated plugs stained for von Willebrand factor, validating that the invasion of endothelial cells (data not shown). These data provide further evidence that IP-10 inhibits VEGF-induced vessel formation.
IP-10 Inhibits Endothelial Cell Motility
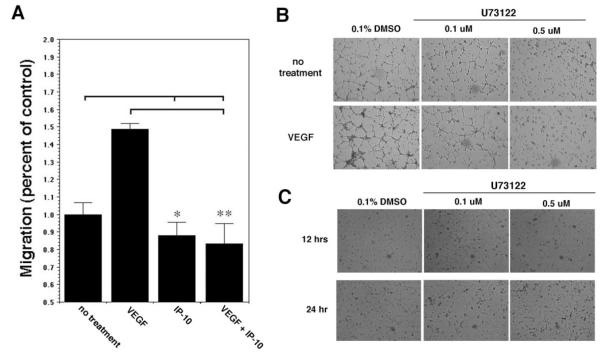
The above results show that IP-10 is able to inhibit endothelial cell tube formation, a process that requires endothelial cell motility. We have demonstrated that ELR-negative chemokines can inhibit fibroblast motility.2,8,9 To examine whether IP-10 inhibition of endothelial tube formation is due a similar inhibition of endothelial motility, HMEC-1 were analyzed for migration into a denuded area over 24 hours in the presence of IP-10. Treatment of HMEC-1 cells with VEGF increased cell motility compared with untreated cells, whereas IP-10 treatment significantly inhibited HMEC-1 cell migration compared with untreated cells (Figure 2A). When the cells were cotreated with VEGF and IP-10, IP-10 blocked VEGF-induced cell motility (Figure 2A). Similar migration in the presence of mitomycin C to prevent mitogenesis with or without VEGF or IP-10 stimulation (data not shown) suggests that VEGF-induced migration is not attributable to cell proliferation.
Figure 2.
IP-10 inhibits endothelial cell migration. A, HMEC-1 cells were analyzed for the ability to migrate into a denuded area using a 2D scratch assay.10 A 1-mm “scratch” was made in the monolayer. The cells were further incubated in 0.5% dialyzed FBS media alone (no treatment) or containing VEGF (100 ng/mL) and/or IP-10 (200 ng/mL) for 24 hours. The area unoccupied by the cells was determined and shown as a percentage of the control (no treatment). The results are of N=3 (average±SEM). P>0.05 for IP-10 and VEGF/IP-10 (*) and P>0.01 for VEGF/IP-10 (**). Note, IP-10 significantly inhibited VEGF-mediated HMEC-1 motility. B and C, HMEC-1 were resuspended in serum free media containing VEGF (100 ng/mL) or DMS0 in the presence of the PLC-inhibitor U73122. Tube formation (B) at 12 hours was inhibited, whereas cell viability, as ascertained by trypan blue exclusion (C), was unaffected even at 24 hours.
To discern whether motility per se plays an important role in angiogenesis, we found that inhibition of phospholipase C (PLC), a key regulatory protein involved in growth factor induced chemotaxis,12 inhibited HMEC-1 tube formation. HMEC-1 treated with U73122, a PLC inhibitor, was able to inhibit tube formation (Figure 2B). Inhibition of tube formation by U73122 is not a result of cell death, because U73122 did not affect HMEC-1 viability (Figure 2C). These data indicate that the ELR-negative chemokines inhibit endothelial cell migration, with the inference that loss of this process likely contributes to the noted inhibition of endothelial tube formation.
IP-10 Stimulates cAMP Production and PKA Activation in Endothelial Cells
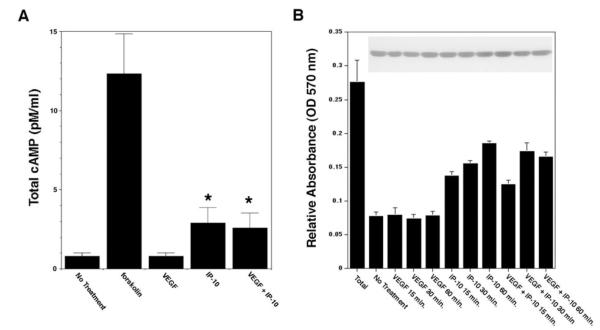
In fibroblasts, IP-9–induced activation of PKA is required for inhibition of motility,9 whereas little, if any, cAMP accumulates in response to IP-9 in keratinocytes in which IP-9 is motogenic.13 To determine whether stimulation of endothelial cells with IP-10 can induce an increase in cAMP, HMEC-1 cells were incubated with VEGF, and/or IP-10, and then assayed for total cAMP production. Treatment of HMEC-1 cells with IP-10 showed a 3-fold increase in cAMP production compared with untreated and VEGF-treated cells (Figure 3A), as previously reported.6 When the cells were stimulated with a combination of VEGF and IP-10, there was still a significant increase in cAMP production compared with VEGF alone (Figure 3A).
Figure 3.
PKA is activated by CXCR3 in endothelial cells. A, HMEC-1 cells were incubated with 0.1% DMSO (vehicle, no treatment), VEGF (100 ng/mL), and/or IP-10 (200 ng/mL). The cell lysate was analyzed for total cAMP using a cAMP assay kit (Amersham). The results are of N=3 (average±SEM) *P<0.05. Note that IP-10 treatment increased cAMP levels 3-fold more than the untreated or VEGF-treated cells. B, HMEC-1 were incubated with VEGF (100 ng/mL) and/or IP-10 (200 ng/mL). The cells were lysed in a hypotonic solution containing leupeptin and PMSF. The lysate was then analyzed for PKA activity using a PKA assay kit (Promega). The graph shows the amount of the synthetic substrate phosphorylated by PKA. Insert is a representative immunoblot for GAPDH to verify equal sample loading for the assay. N=3 (average±SEM).
PKA is an inhibitor of endothelial cell migration and tube formation.14,15 We sought to determine whether the increase in cAMP by IP-10 stimulation of CXCR3 mediates the activation of PKA. Incubation of HMEC-1 cells with IP-10 alone showed a significant increase in substrate phosphorylation by PKA compared with control and VEGF-treated cells (Figure 3B). When the endothelial cells were incubated with IP-10 in the presence of VEGF, there was a significant increase in PKA activation compared with VEGF treatment alone (Figure 3B). These results indicate that incubation of endothelial cells with IP-10 activates PKA. Thus, the signaling pathway activated by CXCR3 involves PKA activation.
PKA Regulates Endothelial Cell Tube Formation and Motility
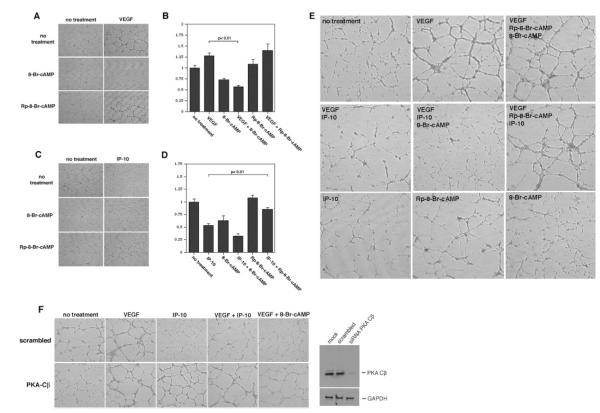
If PKA plays a significant role in endothelial cell tube formation and motility, then the cell permeable cAMP analogs 8-Br-cAMP, a PKA activator, and Rp-8-Br-cAMP, a PKA inhibitor, should regulate endothelial cell tube formation and motility. Incubation of HMEC-1 cells with 8-Br-cAMP inhibited the ability of the cells to form tubes compared with the untreated group (Figure 4A and 4B). 8-Br-cAMP also significantly inhibited VEGF-induced tube formation (Figure 4A and 4B). When the HMEC-1 cells were incubated with Rp-8-Br-cAMP in the presence of IP-10, Rp-8-Br-cAMP was able to reverse the inhibitory effects of IP-10 on tube formation (Figure 4C and 4D). To further show that IP-10 induced PKA activation plays a role in inhibiting endothelial tube formation on Matrigel, HMEC-1 cells were preincubated with Rp-8-Br-cAMP, then stimulated with VEGF, IP-10 and/or 8-Br-cAMP. As predicted, Rp-8-Br-cAMP diminished the inhibitory effects of IP-10 and 8-Br-cAMP on VEGF-induced HMEC-1 tube formation (Figure 4E). Next, RNA interference was used to ablate the catalytic subunit of PKA (PKA Cβ small interference RNA [siRNA]) in HMEC-1 cells. Ablation of PKA reversed the inhibitory effects of IP-10 and 8-Br-cAMP on tube formation; scrambled constructs had no effect on IP-10 and 8-Br-cAMP inhibition of VEGF-induced tube formation (Figure 4F). These results suggest that IP-10–mediated activation of PKA inhibits endothelial cell tube formation.
Figure 4.
IP-10–mediated PKA activation inhibits endothelial tube formation on Matrigel. A, HMEC-1 cells were treated with 8Br-cAMP (250 μmol/L) or Rp8Br-cAMP (50 μmol/L) in the absence (no treatment) or presence of VEGF (100 ng/mL). The cells were incubated on growth factor–reduced Matrigel for 24 hours and then imaged. Note, the PKA activator 8-Br-cAMP inhibited VEGF-mediated tube formation. B, Endothelial tube formation in A was analyzed using MetaMorph. The bar graph is the MetaMorph data expressed as a percentage of the control (no treatment). The results are of N=3 (average±SEM). C, HMEC-1 were treated as described in A but incubated with IP-10 (200 ng/mL) instead of VEGF. The PKA inhibitor Rp-8-Br-cAMP reversed the inhibitory effects of IP-10 on tube formation. D, Endothelial tube formation in C was analyzed using MetaMorph as described in B. As seen, Rp8Br-cAMP was able to reverse the inhibitory effects IP-10 had on endothelial cell tube formation. E, HMEC-1 were treated as described in A but incubated with Rp-8-Br-cAMP, 8-Br-cAMP, VEGF, and IP-10 together. Again, Rp-8-Br-cAMP is able to reverse the inhibitory effects that IP-10 and 8-Br-cAMP have on VEGF-mediated tube formation. N=3; 1 representative experiment shown. F, HMEC-1 cells were transfected with siRNA for the catalytic β subunit of PKA (AAGAGTTTCTAGCCAAAGCCA) or a scrambled (AACCGTCGATTTCACCGGG) oligo.23 The siRNA ablated the protein expression of PKA Cβ as seen in the Western blot. HMEC-1 transfected with PKA Cβ siRNA were resistant to the inhibitory effects of IP-10 and 8-Br-cAMP comparable to VEGF. Note that IP-10 and 8-Br-cAMP were able to inhibit VEGF-induced tube formation in HMEC-1 cells transfected with the scrambled siRNA.
HMEC-1 cells were analyzed for migration in the presence of 8-Br-cAMP and Rp-8-Br-cAMP. Incubation of HMEC-1 cells with 8-Br-cAMP significantly inhibited cell motility compared with untreated cells (Figure 5A). Also, 8-Br-cAMP significantly inhibited VEGF–induced cell motility to a level comparable to 8-Br-cAMP only (Figure 5A). When the cells were treated with Rp-8-Br-cAMP in the presence of IP-10, the Rp-8-Br-cAMP abrogated the inhibitory effects of IP-10 (Figure 5B). These results further indicate that PKA activation by CXCR3 plays a major role in inhibiting endothelial cell motility.
Figure 5.
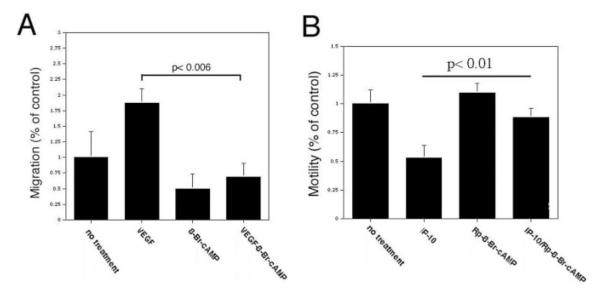
IP-10–mediated activation of PKA inhibits endothelial cell migration. A, HMEC-1 cells were analyzed for the ability to migrate into a denuded area using a 2D scratch assay. A 1-mm “scratch” was made in the HMEC-1 monolayer. The cells were then incubated in 0.5% dialyzed FBS media alone (no treatment) or containing VEGF (100 ng/mL) and/or 8-Br-cAMP (250 μmol/L) for 24 hours. The area unoccupied by the cells was determined and shown as a percentage of the control (no treatment) and results are of N=3 (average ± SEM). Note, 8-Br-cAMP is able to inhibit VEGF-induced endothelial migration. B, HMEC-1 cell migration was analyzed as described in A, except the cells were incubated with IP-10 (200 ng/mL) instead of VEGF. The graph is shown as a percentage of the control (no treatment), and results are of N=3 (average±SEM). Rp-8-Br-cAMP is able to reverse the inhibitory effects IP-10 has on endothelial cell migration.
In endothelial cells, PKA activation has been found to induce apoptosis.15 To verify that IP-10–mediated inhibition of tube formation was not caused by apoptosis, we analyzed the viability of cells treated with IP-10 by TUNEL assay. The treatment of HMEC-1 cells with IP-10 alone showed a <8% increase in the number of cells undergoing apoptosis, but no difference in the number of apoptotic cells was observed when IP-10 was incubated with VEGF compared with VEGF or nontreated cells (data not shown).
m-Calpain Activation Is Inhibited by IP-10 in Endothelial Cells
In fibroblasts, CXCR3-activation of PKA inhibits motility secondary to inhibitory phosphorylation of m-calpain.8,9 Using a membrane permeable synthetic calpain substrate, Boc-LM-CMAC (BOC), that can be cleaved by both m- and μ-calpain, we find that VEGF induces calpain activity in endothelial cells (Figure 6A). The increase in fluorescence attributable to BOC cleavage in VEGF-stimulated dHMEC cells was prevented by the pan-calpain inhibitor CI-1.
Figure 6.
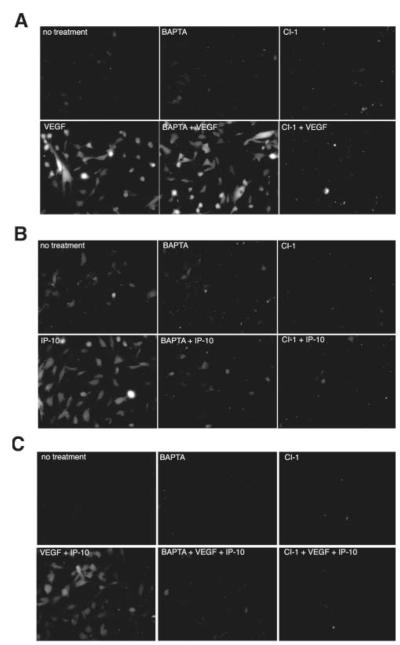
IP-10 inhibits VEGF-induced m-calpain activation. HMEC-1 cells were plated on gelatin coated glass chamber slides. The cells were treated with or without 50 μmol/L BAPTA/AM (BAPTA) or 10 μmol/L calpain inhibitor 1 (CI-1) before treatment with VEGF (100 ng/mL) in 0.5% dialyzed FBS media (A), IP-10 (200 ng/mL) (B), or in combination (C) in the presence of BOC (27 μmol/L), a synthetic calpain substrate that, when cleaved, fluoresces.11 Calpain activation was analyzed by fluorescence microscopy. Fluorescence within the cell indicates calpain activity. Note that IP-10 inhibits VEGF-induced m-calpain activity (C). In all parts, shown are representative of N=3, each performed in duplicate.
In cells, m-calpain is activated, at least in part, by ERK phosphorylation on serine 5016 and μ-calpain is activated secondary to a calcium flux.13 Therefore, we used BAPTA/AM, a membrane permeable calcium chelator, to distinguish between m and μ-calpain activation in cells as this blocks activation of μ- but not m-calpain.13 Preincubation of dHMEC cells with BAPTA/AM did not prevent VEGF-induced BOC cleavage (Figure 6A). Similar results were also observed in HMEC-1 (data not shown). Thus, VEGF activates m-calpain, as BAPTA/AM did not quench this cleavage. Finding that IP-10 itself led to BOC fluorescence complicated whether IP-10 blocked VEGF-induced calpain activity (Figure 6B). This was not surprising as CXCR3 ligandation can induce PLCβ-mediated calcium flux activation of μ-calpainin keratinocytes.13 In accord with these earlier findings, IP-10 induced μ-calpain activity as it was inhibited by BAPTA/AM (Figure 6B). When the cells were treated with both VEGF and IP-10, cleavage of BOC was observed (Figure 6C), but when the cells were preincubated with BAPTA/AM and then treated with VEGF and IP-10 there was almost no discernable BOC fluorescence (Figure 6C), indicating that IP-10 inhibits VEGF-mediated m-calpain activity.
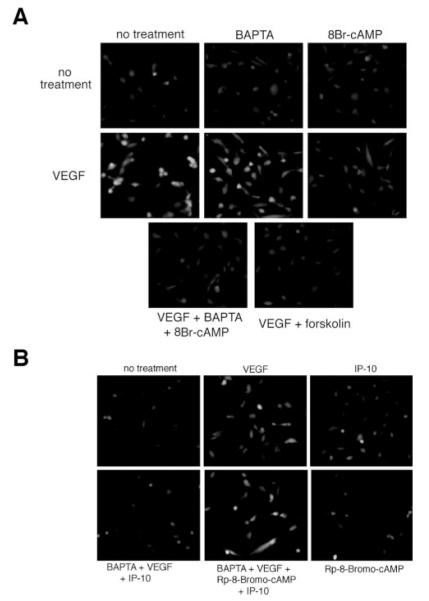
Importantly for the hypothesis being tested, growth factor activation of m-calpain is prevented by PKA-mediated phosphorylation of a specific sequence in domain III.9 Thus, we assessed the ability of 8-Br-cAMP and forskolin to inhibit VEGF-induced m-calpain activity. Cleavage of BOC was observed when the cells were incubated with VEGF alone or in the presence of BAPTA/AM (Figure 7A). When the cells were pretreated with either 8-Br-cAMP or forskolin before VEGF treatment BOC cleavage was significantly reduced (Figure 7A). These data suggest that the activation of PKA plays a role in inhibiting m-calpain activity. Next we wanted to determine whether Rp-8-Br-cAMP could reverse the inhibitory effects of IP-10 on VEGF-mediated m-calpain activation. HMEC-1 cells were pretreated with BAPTA/AM, so that only m-calpain activity would be noted, and then incubated with Rp-8-Br-cAMP before stimulating the cells with VEGF and IP-10. Treatment of the cells with BAPTA/AM, VEGF and IP-10 showed almost no cleavage of BOC (Figure 7B). When the cells were treated with BAPTA/AM, Rp-8- Br-cAMP, VEGF, and IP-10 cleavage of BOC was observed (Figure 7B). These data show that the PKA inhibitor Rp-8- Br-cAMP is able to reverse the inhibitory effect IP-10 has on VEGF-mediated m-calpain activation. Thus, these results provide sufficient evidence to conclude that CXCR3 activation of PKA inhibits m-calpain activity in endothelial cells.
Figure 7.
Activation of PKA inhibits VEGF-mediated m-calpain activity in endothelial cells. A, HMEC-1 cells were plated on gelatin-coated glass chamber slides. The cells were preincubated with or without BAPTA/AM (50 μmol/L), 8-Br-cAMP (250 μmol/L), or forskolin (25 μmol/L) before treatment with VEGF (100 ng/mL) in the presence of BOC (27 μmol/L) in 0.5% dialyzed FBS media. Calpain activation was analyzed by fluorescence microscopy. Fluorescence within the cell indicates calpain activity. Note that both 8-Br-cAMP and forskolin were able to inhibit VEGF-mediated m-calpain activation. B, HMEC-1 cells were treated similar to A. The cells were preincubated with Rp-8-Br-cAMP (50 μmol/L) and BAPTA/AM (50 μmol/L) and treated with VEGF (100 ng/mL) and/or IP-10 (200 ng/mL) in the presence of BOC (27 μmol/L). Note that incubation of Rp-8-Br-cAMP is able to reverse the inhibitory effects of IP-10 on VEGF-mediated m-calpain activity. In both parts, shown are representative of N=3 performed in duplicate.
m-Calpain Activation Is Required for Endothelial Tube Formation and Motility
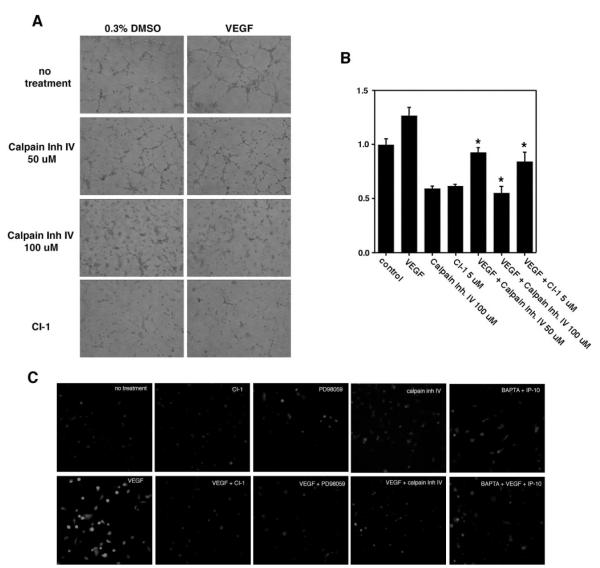
PKA is a signaling molecule in a variety of inhibitory pathways and has been shown in other endothelial cell lines to inhibit Raf activation.14 Thus, to determine whether m-calpain inhibition is critical to IP-10 blockade of angiogenesis, rather than a distracting epiphenomenon, HMEC-1 cells were pretreated with the nonspecific calpain inhibitor, CI-1, or a m-calpain selective inhibitor, calpain inhibitor IV, and analyzed for tube formation or migration. Treatment of HMEC-1 cells with calpain inhibitor IV or CI-1 almost completely abolished the ability of HMEC-1 cells to form tubes or to locomote into a denuded area (Figure 8A and 8B). We then verified that these calpain inhibitors did indeed inhibit m-calpain. Analysis of the cleavage of BOC verifies that calpain inhibitor IV does inhibit VEGF-mediated m-calpain activation (Figure 8C). In addition, this pathway appears similar to those from other receptors with tyrosine kinase activity, as the MEK inhibitor, PD98059, was also able to inhibit m-calpain activation by VEGF (Figure 8C). These results provide sufficient evidence that m-calpain activation is required for VEGF-mediated endothelial motility and, thereby, tube formation.
Figure 8.
Inhibition of m-calpain inhibits endothelial tube formation and migration. A, HMEC-1 cells were resuspended in serum-free media. The cells were treated with the m-calpain–specific inhibitor calpain inhibitor IV or CI-1 (5 μmol/L), in the absence (no treatment) or presence of VEGF (100 ng/mL). The cells were incubated on Matrigel for 12 hours then imaged. The images are representative of 4 experiments. Calpain inhibitor IV at the 100 μmol/L concentration almost completely abolished VEGF-mediated tube formation. B, HMEC-1 cells were analyzed for the ability to migrate into a denuded area using a 2D scratch assay. A 1-mm “scratch” was made in the HMEC-1 monolayer. The cells were incubated in serum-depleted media alone (no treatment) or containing VEGF (100 ng/mL) and/or calpain inhibitor IV (50 or 100 μmol/L) or CI-1 (5 μmol/L) for 24 hours. The area unoccupied by the cells was determined and shown as a percentage of the control (no treatment) and results are of N=3 (average±SEM). *P>0.01. Inhibition of m-calpain significantly reduced VEGF-mediated cell migration. C, HMEC-1 cells were grown on gelatin coated glass chamber slides. The cells were preincubated with either CI-1 (10 μmol/L), calpain inhibitor IV (50 μmol/L), PD98059 (2.0 μmol/L), or BAPTA/AM (50 μmol/L) before incubation with BOC (27 μmol/L); concentrations were determined empirically.13 The cells were stimulated with either VEGF (100 ng/mL) or IP-10 (200 ng/mL) for 1 hour. Calpain activation was analyzed by fluorescence microscopy. Fluorescence within the cell indicates calpain activity. Note that both CI-1 and calpain inhibitor IV inhibited VEGF-mediated m-calpain activity. Also, the inhibition of calpain activity by the MEK inhibitor (PD98059) suggests that m-calpain is activated by the MAPK pathway, a known pathway activated by VEGF. Images are representative of N=3.
Discussion
The ELR-negative CXC chemokines, including IP-10 and IP-9, have antiangiogenic properties,3,5 but the mechanism by which these chemokines inhibit angiogenesis has not been determined. Only recently has the CXC receptor 3, the only defined receptor for all ELR-negative CXC chemokines, been shown to be expressed on endothelial cells.4,7 In this study, we provide evidence that the ELR-negative chemokines inhibit angiogenesis through a PKA-mediated pathway via its effects on calpain. The evidence shows that IP-10 stimulation of microvascular endothelial cells increases cAMP levels, promoting the activation of PKA and thereby inhibiting endothelial cell migration. Herein we show that VEGF-mediated m-calpain activation is necessary for in vitro endothelial motility and tube formation and IP-10 inhibits VEGF-mediated m-calpain activation. To verify that this is PKA mediated, we show that Rp-8-Br-cAMP, a PKA inhibitor, andthe ablation of PKA using siRNA reversed the IP-10 inhibition of VEGF-mediated m-calpain activation. These results demonstrate that IP-10 inhibits endothelial cell migration through a PKA-mediated inhibition of m-calpain.
The data provide herein suggest a novel pathway for the regulation of angiogenesis. In endothelial cells, CXCR3 activation by its ligands mediates an increase in cAMP, thus activating PKA. This then inhibits m-calpain activity; m-calpain has numerous cellular functions, with a key function being to control locomotion by enabling rear detachment during haptokinetic motility.9 Thus, these results suggest that the inhibitory mechanism of the ELR-negative chemokines is by limiting the ability of endothelial cell migration through inhibition of rear-cell detachment.
An issue arises during wound healing of how these ELR-negative CXC chemokines regulate angiogenic drive. One such chemokine, PF4 (CXCL4) is released at high levels by the platelet clot. This early PF4 release and angiostasis would act to limit vascularization of the nascent clot and aid in hemostasis and prevention of fluid loss. During the inflammatory phase, in which the provisional matrix starts to take shape, the levels of PF4 drop and vascularization is noted. Only later during the resolution and remodeling phase do CXCR3 ligands reappear. This timing is consistent with our finding that the angiostatic effects of the ELR-negative CXC chemokines are concentration dependent and thus reversible (data not shown). Thus, there appears to be a proangiogenic state of low CXCR3 activation during the later inflammatory and earlier regenerative phases of wound healing wherein vascularization is noted.
The implications for CXCR3-mediated angiogenesis likely extend beyond wound repair. The regulation of angiogenesis is thought to be a coordinate regulation of angiogenic and angiostatic factors, thus a dysregulation of either of these factors can lead to pathological conditions such as idiopathic pulmonary fibrosis,17 endometriosis,18 and diabetic retinopathy.19 The downregulation of the angiostatic chemokine IP-10 has been found to be a factor in the development of idiopathic pulmonary fibrosis17 and endometriosis.18 These studies provide further evidence that understanding the signaling pathways mediated by the ELR-negative chemokines may provide new therapies in the treatment of such pathological conditions. Tumor growth, on the other hand, is not necessarily attributable to a dysregulation of the angiogenic/ angiostatic signaling pathways but a recruitment of new vessels by the tumor cells themselves. IP-10 or Mig have been presented to successfully inhibit non–small cell lung cancer20 and Burkitt’s lymphoma.21 These studies demonstrate the importance of the endogenous expression of angiostatic factors in the regulation of tumor growth. IP-10, in tumor studies, has been found to cause tumor necrosis and vascular damage at the tumor site while not eliciting an appreciable inflammatory response.20
The results of this study provide a signaling mechanism for the inhibition of angiogenesis by the ELR-negative chemokines and, thus, provides a possible mechanism by which to regulate physiological and pathological angiogenesis. Further characterization of the signaling mechanism mediated by CXCR3 may provide new insights into the development of new therapeutic strategies to promote or inhibit blood vessel formation. Furthermore, understanding the regulation of CXCR3 expression may be another method by which the regulation of angiogenesis maybe controlled.
Acknowledgments
This study was supported by funds from National Heart, Lung, and Blood Institute and National Institute of General Medical Sciences. We thank Latha Satish, Sourabh Kharait, and Clayton Yates for technical support.
References
- 1.Babu M, Wells A. Dermal-epidermal communication in wound healing. Wounds. 2001;13:183–189. [Google Scholar]
- 2.Satish L, Yager D, Wells A. Glu-Leu-Arg-negative CXC chemokine interferon gamma inducible protein-9 as a mediator of epidermal-dermal communication during wound repair. J Invest Dermatol. 2003;120:1110–1117. doi: 10.1046/j.1523-1747.2003.12230.x. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210:51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 6.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Powell D, Wasserman K, Oppenheim JJ. Differential expression and responsiveness of chemokine receptors (CXCR1–3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055–2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- 8.Shiraha H, Glading A, Gupta K, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243–254. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Gupta K, Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol. 1994;124:547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glading A, Chang P, Lauffenburger DA, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Xie H, Sekar MC, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satish L, Blair HC, Glading A, Wells A. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol Cell Biol. 2005;25:1922–1941. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Angelo G, Lee H, Weiner RI. cAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking Raf activation. J Cell Biochem. 1997;67:353–366. [PubMed] [Google Scholar]
- 15.Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest. 2002;110:933–941. doi: 10.1172/JCI14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keane MP, Arenberg DA, Lynch JP, 3rd, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- 18.Yoshino O, Osuga Y, Koga K, Hirota Y, Tsutsumi O, Yano T, Morita Y, Momoeda M, Fujiwara T, Kugu K, Taketani Y. Concentrations of interferon-gamma-induced protein-10 (IP-10), an antiangiogenic substance, are decreased in peritoneal fluid of women with advanced endometriosis. Am J Reprod Immunol. 2003;50:60–65. doi: 10.1034/j.1600-0897.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 19.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR, Yao L, Gupta G, Kanegane C, Tosato G. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood. 1997;89:2635–2643. [PubMed] [Google Scholar]
- 22.Malinda K. In vivo Matrigel migration and angiogenesis assay. In: Murray JC, editor. Angiogenesis Protocols. Humana Press Inc; Totowa, NJ: 2001. pp. 47–52. [Google Scholar]
- 23.Dumaz N, Marais R. Protein kinase A blocks Raf-1 activity by stimulating 14–3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem. 2003;278:29819–29823. doi: 10.1074/jbc.C300182200. [DOI] [PubMed] [Google Scholar]