Abstract
The extracellular matrix (ECM) of the human intervertebral disc is rich in molecules that interact with cells through integrin-mediated attachments. Porcine nucleus pulposus (NP) cells have been shown to interact with laminin (LM) isoforms LM-111 and LM-511 through select integrins that regulate biosynthesis and cell attachment. Since human NP cells lose many phenotypic characteristics with age, attachment and interaction with the ECM may be altered. Expression of LM-binding integrins was quantified for human NP cells using flow cytometry. The cell-ECM attachment mechanism was determined by quantifying cell attachment to LM-111, LM-511, or type II collagen after functionally blocking specific integrin subunits. Human NP cells express integrins β1, α3, and α5, with over 70% of cells positive for each subunit. Blocking subunit β1 inhibited NP cell attachment to all substrates. Blocking subunits α1, α2, α3 and α5 simultaneously, but not individually, inhibits NP cell attachment to laminins. While integrin α6β1 mediated porcine NP cell attachment to LM-111, we found integrins α3, α5, and β1 instead contributed to human NP cell attachment. These findings identify integrin subunits that may mediate interactions with the ECM for human NP cells and could be used to promote cell attachment, survival and biosynthesis in cell-based therapeutics.
Keywords: intervertebral disc, nucleus pulposus, integrin, extracellular matrix, human
Introduction
The intervertebral disc (IVD) consists of a dense extracellular matrix (ECM) rich in collagens, proteoglycans, and non-collagenous molecules (1; 2). The composition and structure of the ECM vary by region, with the central nucleus pulposus (NP) region containing a high concentration of water, non-collagenous proteins, and a diverse population of proteoglycans that contribute to a high interstitial swelling pressure (3). This ECM provides a host of mechanical and biochemical signals to the resident NP cells that may promote cell survival, ECM production, and regulate cell morphology and differentiation (1; 2). With aging, however, the human NP undergoes changes characterized by a decrease in cellularity, water content, and loss of proteoglycan content that contribute to loss of disc height and nucleus pressurization (4). Understanding the mechanisms that regulate cell interactions with the ECM is essential to understanding how these ECM changes affect cell survival, matrix synthesis, and metabolism, with consequences for understanding IVD function and regeneration with aging.
Integrins are a class of cell surface molecules that mediate cell interactions with the ECM, including adhesion and migration (5–8). Additionally, integrin-ECM interactions can regulate cell signaling, modulating cell functions such as cell survival, cell proliferation, and protein production (9). Structurally, integrins are heterodimers composed of α and β units that cooperate to interact with different ligands. Initial research has documented the presence of specific α and β subunits in rat intervertebral disc during development (10), including the integrin subunits α5 and β1 known to mediate interactions with collagens and fibronectin. Immunostaining of human intervertebral disc tissues has also documented the presence of these integrin subunits in NP or annulus fibrosus regions, and further identified the presence of α1, α3, α5, α6, αV, β1, β3, and β5 subunits (11; 12). Immunostaining has also confirmed detection of these same subunits in porcine NP tissues along with higher expression levels for the α6 and β4 integrin subunits in the porcine NP (11–13). While a functional role for these integrin subunits has been studied for cells of articular cartilage and fibrocartilage (14; 15), limited information is available about how these integrin subunits may regulate cell attachment, survival, function, and more in the human intervertebral disc.
For cells of the intervertebral disc, studies have shown the α5β1 integrin heterodimer regulates interactions with fibronectin (13; 16), and that these same integrin subunits are involved in the onset of cell pathobiology following exposure to degraded fragments of fibronectin (17). Studies of rat NP cells have shown that attachment to type II collagen is mediated by the α2 integrin subunit in a process that involves activation of ERK (18), while porcine NP cells were instead shown to use the α1 integrin subunit to attach to type II collagen (13). Studies by our group have focused on intervertebral disc cell interactions with laminin (LM) proteins, which may play a key role in immature NP tissue (11–13; 19; 20). Laminins are heterotrimeric proteins composed of α, β, and γ polypeptide chains that combine to form at least sixteen different isoforms (21). Porcine NP cells have been shown to interact with laminins LM-111 and LM-511 through integrin mediated mechanisms (12; 13). Specifically, porcine NP cells have been found to use integrins α6 and β1 to attach to LM-111 (13) with the finding that blocking integrin α6 selectively prevents attachment of larger, notochordal-like porcine NP cells to LM-111 (13). Together, these findings begin to reveal a role for specific integrin subunits in mediating NP cell-ECM interactions.
Mature human NP cells lose many phenotypic traits of immature NP cells, and NP cell-matrix interactions may be altered with maturity or aging (4). One study has shown that mechanotransduction of healthy adult NP cells was dependent on RGD-mediated interactions with ECM for cells embedded in alginate, although this interaction was lost for cells from degenerate NP tissue (22). Another study has reported elevated levels of α5 and β1 integrin subunits in tissues from herniated disc, with no evidence of differences in α1, α2 and αV and β3 subunits (23). Understanding which integrin subunits an adult human cell uses to attach to the ECM is critical to first understanding mechanisms used for survival and regulation of cell function, as well as their changes with degeneration and aging. The goal of this study is to evaluate the mechanism of cell attachment to the laminin proteins, LM-111 and LM-511, as well as type II collagen for adult human NP cells. This knowledge is prerequisite to understanding a role for these matrix molecules in disc cell biology.
Materials and Methods
Cell Isolation and Culture
Cells from the NP region of to-be-discarded degenerative disc surgical waste (ages 21–69) were isolated using a pronase-collagenase digestion (24). Cells were plated onto tissue culture plastic and expanded up to passage 2 at 5% CO2 and 37 °C in F-12 media (Invitrogen, Grand Island, NY) supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin (Sigma-Aldrich, St. Louis, MO). Following detachment from culture surfaces using 0.025% trypsin/EDTA, cells were used for both flow cytometry and integrin blocking protocols, as described below.
Flow Cytometry
Cells from monolayer culture (tissues aged 21–63, n=6) were allowed to recover following detachment in suspension with 10% FBS for 2 hours. NP cells (1–2 million cells/mL) were incubated for 30 minutes at 4 °C in solution with primary integrin monoclonal antibodies recognizing integrin subunits α1, α2, α3, α5, α6, β1, and β4, or negative isotype controls IgG1 and IgG2a (Table 1). After washing the cells twice in PBS, the cells were incubated for an additional 30 minutes at 4 °C with the appropriate secondary antibody (10 µg/mL Alexa 488 anti-mouse or anti-rat, Invitrogen). Cells were then rinsed twice with PBS and the mean fluorescence intensity (MFI) and percent of labeled cells was measured via flow cytometry (Accuri C6, BD Accuri Cytometers Inc., Ann Arbor, MI). Negative isotype controls were used to establish a gate containing 98% of the negative cells; the percent of cells above this gate were considered positively labeled. Results indicated that the β1 integrin MFI values were greater than any other condition. For this reason, MFI values were normalized by MFI for β1 integrin on a per sample basis to remove inter-subject variability. A minimum of 10,000 events were counted for each flow cytometry reading.
Table 1.
Monoclonal antibodies for human integrin subunits and IgG controls used in immunohistochemical staining, flow cytometry analysis, and function blocking experiments. All antibodies from Millipore (MA) unless otherwise indicated.
| Antibody | Working Concentration |
Isotype Control |
Clone/Part Number |
|---|---|---|---|
| α1 | 10µg/ml | Mouse IgG1 | FB12, MAB1973Z |
| α2 | 10µg/ml | Mouse IgG1 | P1E6, MAB1950Z |
| α3 | 10µg/ml | Mouse IgG1 | ASC-1, MAB2056 |
| α5 | 10µg/ml | Mouse IgG1 | P1D6, MAB1956 |
| α6 | 40µg/ml1 | Rat IgG2a | GoH3, MAB1378 |
| β1 | 10µg/ml | Mouse IgG1 | 4B4, 6603113 |
| β4 | 10µg/ml | Mouse IgG1 | ASC-3, MAB2058Z |
| IgG1 | 10µg/ml | - | DD7, CBL600 |
| IgG2a | 10µg/ml | - | DD13, CBL605 |
From Beckman Coulter (Brea, CA)
Integrin Blocking Assays
Ninety-six-well assay plates (half-well Costar plates, 3688, Corning, Corning, NY) were overlaid with 40 µl of either chicken type II collagen (40 µg/mL, Sigma-Aldrich), mouse LM-111 (20 µg/mL, Millipore, Billerica, MA), or mouse LM-511 (10 µg/mL, Sigma-Aldrich) and incubated overnight at 4 °C. The wells were blocked with 3.75% bovine serum albumin (BSA) for 3 hours at 37 °C, then rinsed with 1X PBS; wells without protein but blocked with BSA were negative controls to identify non-specific cell binding. NP cells (tissues aged 30–69, n = 4 or 5) were detached from tissue culture plastic as described above and incubated for 10–15 minutes in suspension with soybean trypsin inhibitor (T9777, Sigma-Aldrich). Cells were then incubated in serum free F-12 media (SFM) with function blocking integrin antibodies (Table 1) for 30 minutes at 37 °C.
Cell attachment to type II collagen was blocked with the following combinations of antibodies at the given concentrations recognizing integrin subunits: α1 (10 µg/mL), α2 (10 µg/mL), α1 + α2, β1 (10 µg/mL), or IgG1 (10 µg/mL). Cell attachment to LM-111 and LM-511 was blocked with the following combinations of antibodies recognizing integrin subunits: α1 + α2, α3 (10 µg/mL), α6 (40 µg/mL), α3 + α6, α5 (10 µg/mL), α3 + α5, α1 + α2 + α3 + α5, β1, β4 (10 µg/mL), IgG2a (10 µg/mL), or IgG1. Cells in solution with the antibodies were then placed in protein and BSA coated wells (4,000 cells/well), allowed to attach for 1 hour, and washed twice with serum-free media to remove unattached cells. Attached cells were then counted using the CellTiter-Glo luminescent cell viability assay (Promega, Madison, WI).
In separate experiments, the role of cations in cell attachment was determined by incubating NP cells (tissues aged 30–69, n=5) with 10 µM of the chelator ethylenediaminetetraacetic acid (EDTA) for 30 minutes at 37 °C. Cells were then similarly plated on substrates coated with LM-111, LM-511 or type II collagen and allowed to attach for 1 hour, washed twice with serum-free F-12 media, and finally counted using the CellTiter-Glo luminescent cell viability assay. Control NP cells that were not incubated with EDTA represented the maximum attachment values for each substrate.
The unblocked controls (IgG for antibody blocking) were used to determine the maximum cell attachment to each substrate for each sample. Cell attachment for each blocking condition was performed in triplicate for cells from each sample, averaged, and then normalized to the unblocked cell attachment value. Any decrease in cell attachment from the unblocked control was considered evidence of blocking.
Immunohistochemical Detection Integrins in IVD Tissue
Tissue samples (n=4–8, ages 30–74) were embedded (OCT compound, Tissue-Tek), and stored frozen at −80 °C until sectioning. Cryosections (7 µm) were fixed in 4% formaldehyde, incubated with blocking solution (5% goat serum + 3.75% BSA), and then incubated with antibodies against integrin subunits α1, α2, α3, α5, α6, or β1 (1:100 dilution, 1.5 hours) with appropriate IgG controls (Table 1). After primary antibody incubation, the slides were washed, incubated with a pre-diluted secondary antibody, and co-stained with propidium iodide (1 mg/mL, Sigma); anti-mouse AlexFluor 488 secondary antibody was used for α1, α2, α3, α5, β1, and IgG1 antibodies, and an anti-rat AlexFluor 488 secondary antibody was used for α6 and IgG2a antibodies. Stained sections were imaged using confocal laser scanning microscopy (Zeiss LSM 510, 63X water immersion objective, Carl Zeiss, Thornwood, NY, USA). For each tissue sample, visual fields across the entire tissue section were manually inspected for positive staining, and representative positive and negative images were acquired.
Statistical Analyses
One-way analysis of variance (ANOVA) with post-hoc Tukey’s Test was used to test for differences in both normalized MFI and the percent of positive cells amongst groups labeled with antibodies or IgG. Normalized cell attachment was tested for a difference amongst integrin subunits using a two-factor ANOVA (integrin subunit, substrate). The normalized cell attachment for EDTA was analyzed with a two-factor ANOVA (blocking condition, substrate). Post-hoc Tukey’s test was used to find differences amongst all blocking conditions.
Results
Flow Cytometry
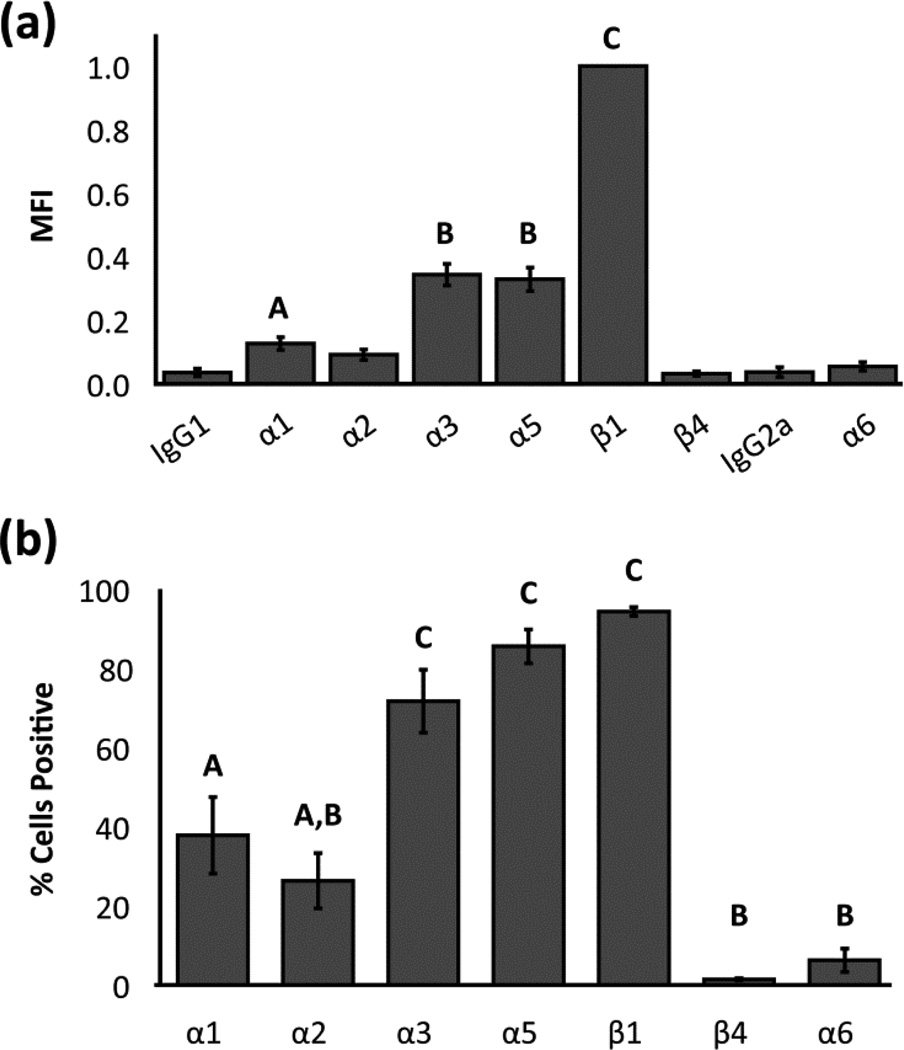
Human NP cells were found to express integrin subunits α1, α3, α5, and β1 with MFI values that were significantly higher than IgG1 controls (Figure 1a; ANOVA, p<0.05). On average, MFI values for integrin subunits α3 and α5 were 34% and 33% of β1 values, respectively. A majority of NP cells (72–94%) were positive for integrin subunits α3, α5, and β1 (Figure 1b), while only 38% and 26% of all NP cells were positively labeled for subunits α1 and α2, respectively.
Figure 1.
Flow cytometric analysis of integrin expression for human NP cells: (a) Mean +/− standard error of mean fluorescence intensity (MFI) averaged across all samples for each anti-integrin antibody. Data are normalized by values for β1 integrin expression on a per-sample basis; and (b) mean +/− standard error of the percentage of cells positive for each integrin subunit averaged across all samples for each anti-integrin antibody. Conditions labeled with different letters are significantly different from each other and from the IgG controls (ANOVA, p<0.05).
Integrin Blocking Assays
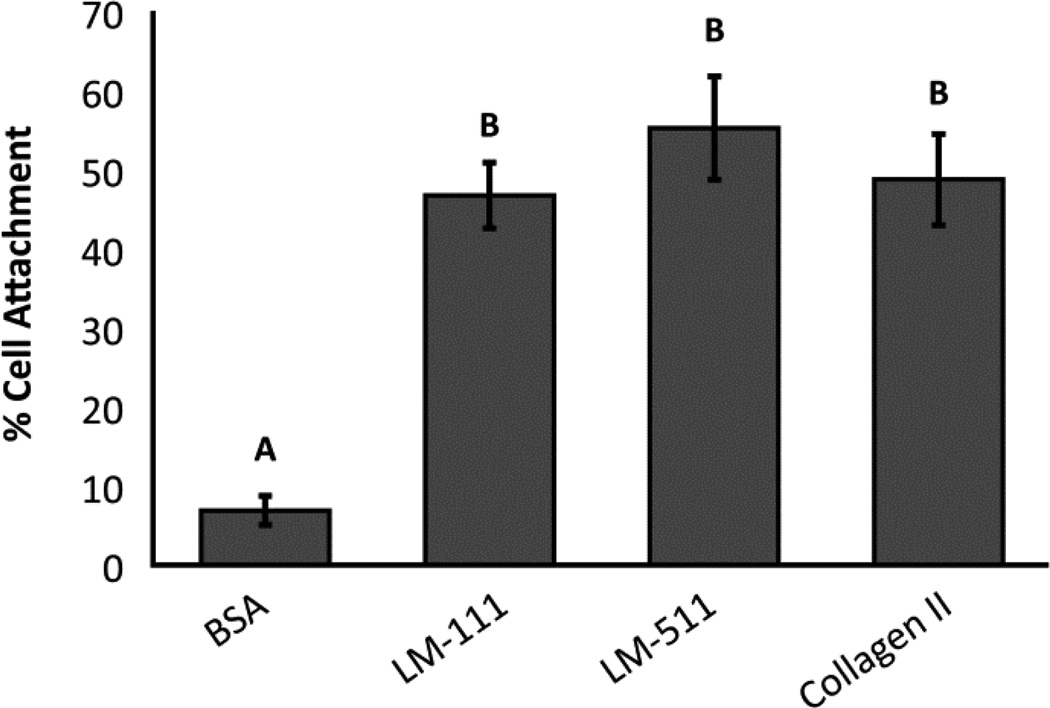
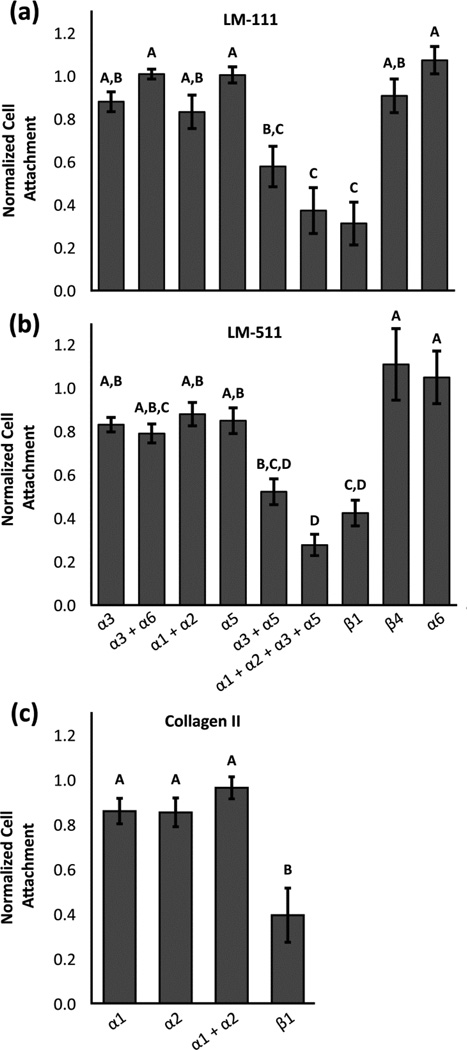
Human NP cells adhered to LM-111, LM-511, and type II collagen II in similar numbers (47%–55% of cells plated); in comparison, cell attachment to BSA control substrates was only 7% (Figure 2). On surfaces coated with LM-111 or LM-511, blocking integrin subunit β1 reduced the number of adherent NP cells to 31% and 42% of IgG control values, respectively (Figure 3a and 3b; ANOVA, p<0.05). There was no detectable difference in normalized cell attachment between the BSA control and the integrin β1 blocking condition (data not shown; ANOVA, p>0.05), suggesting that the integrin subunit β1 is necessary for all NP cell attachment to LM-111 and LM-511. Blocking integrin subunits α1 + α2, α3, or α5 did not significantly reduce cell attachment to LM-111 or LM-511 (Figure 3a and 3b; ANOVA, p>0.05). In contrast, blocking α3 and α5 integrin subunits together significantly reduced cell attachment to 58% and 52% of IgG control values for LM-111 and LM-511 respectively (Figure 3a and 3b; ANOVA, p<0.05). Blocking integrin subunits α1, α2, α3, and α5 simultaneously further reduced cell attachment to 37% and 28% of IgG control values for LM-111 and LM-511 respectively.
Figure 2.
Cell attachment to different protein coatings compared to the BSA control. Conditions labeled with different letters are significantly different from each other (ANOVA, p<0.05).
Figure 3.
The effect of integrin function-blocking antibodies on NP cell attachment to (a) LM-111, (b) LM-511, or (c) type II collagen normalized to the IgG control (mean +/− standard error). Conditions labeled with different letters are significantly different from each other (ANOVA, p<0.05).
There was no detectable decrease in NP cell attachment to type II collagen when integrin subunits α1 and/or α2 were blocked as compared to IgG control values (ANOVA, p>0.05). In contrast, blocking integrin subunit β1 reduced NP cell attachment to type II collagen to 39% of control values (Figure 3c; ANOVA, p<0.05).
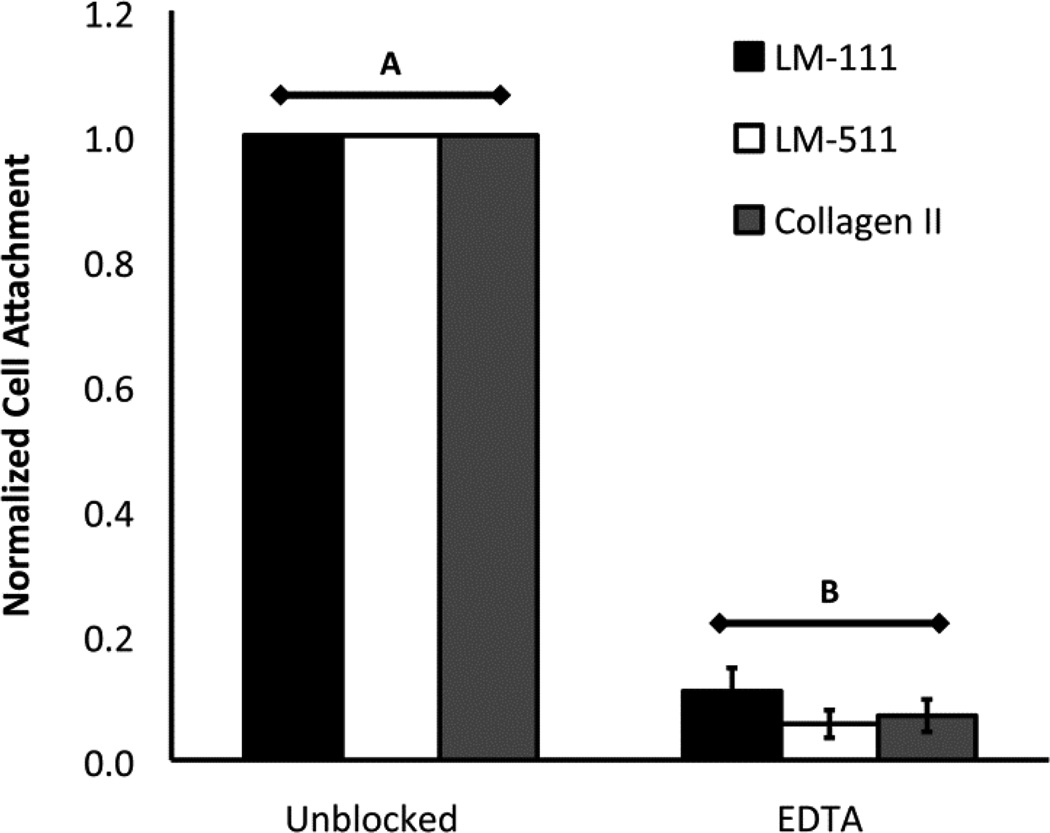
Pre-incubation of NP cells with EDTA reduced cell attachment to LM-111, LM-511, and type II collagen to 6%–11% of the unblocked control values, showing NP cell attachment is cation-dependent (Figure 4; ANOVA, p<0.05).
Figure 4.
The effect of 10 µM EDTA on cell attachment to LM-111, LM-511, or type II collagen (mean +/− standard error). Conditions labeled with different letters are significantly different from each other (2-way ANOVA, p<0.05).
Immunohistochemistry
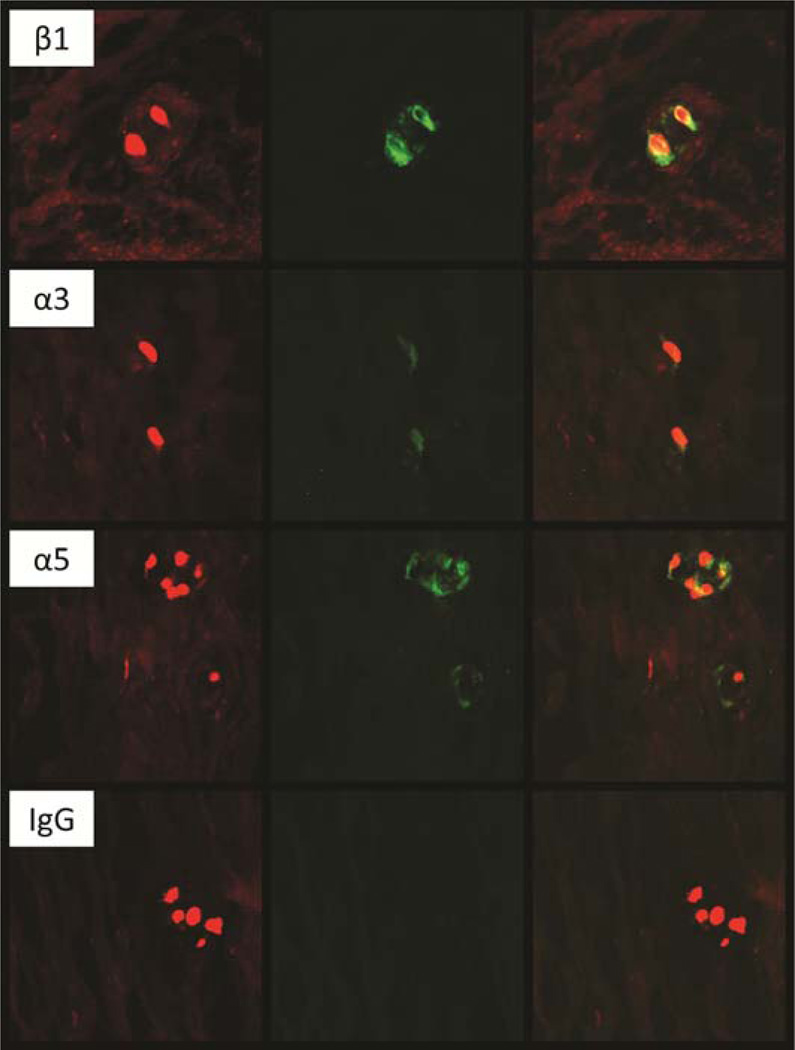
NP tissue from degenerate human IVDs exhibited positive and intense staining for integrin subunit β1 in every visual field examined (Figure 5). In contrast, immunopositivity of NP tissue for integrin subunits α3 and α5 was less frequently identified across tissue sections, and was often less intense compared to that observed for the integrin subunit β1. Over half of the tissue sections stained negative for integrin subunits α1, α2, and α6 in every visual field, while the remaining tissue sections stained slightly positive in only select visual fields. It should be noted that the adult human NP tissue generally had very low cellularity such that there were usually only a few cells in each visual field. These low cell numbers may decrease the power needed to detect infrequent positive staining via immunohistochemistry.
Figure 5.
Representative images of immunostaining adult human tissues showing the pattern for positive labeling with antibodies against integrin subunits β1, α3 and α5. Green = antibody reactivity and red = propidum iodide counterstain.
Discussion
The results of this study identify functional integrin subunits that mediate interactions between extracellular matrix (ECM) proteins of the intervertebral disc (IVD) and human nucleus pulposus (NP) cells. This study analyzed mature human NP cell attachment to the ECM proteins LM-111, LM-511, and type II collagen, with a focus on laminins due to their increased expression in the NP compared to the AF of immature IVD tissue (12; 13). Results of this study show that mature human NP cells express laminin-binding integrins α1, α2, α3, α5, and β1 and use integrin β1 to attach to LM-111, LM-511, and type II collagen. Attachment to LM-111 and LM-511 is additionally mediated by a combination of integrin subunits α3 and α5, in contrast to integrin subunit α6 for immature NP cells (13). Incubating NP cells with EDTA completely blocks cell adhesion to LM-111, LM-511, and type II collagen, suggesting cell attachment is cation dependent. That the integrin β1 function-blocking antibody and EDTA each block a majority of cell attachment to the tested substrates suggests that integrins are critical to NP cell attachment to these ECM proteins. The integrin subunit β1 mediates cell attachment to ligands using multiple α-subunit binding partners, including subunits α1, α2, α3, α4, α5, α6, α7, α8, α9, α10, α11, and αV (9). The results of the integrin blocking assays performed here show that integrin subunits α3 and α5, as well as α1 and α2, may contribute to NP cell attachment to the dominant laminin isoforms within the immature IVD, LM-111 and LM-511. Since these alpha subunits are only known to dimerize with subunit β1 (9), it can be concluded that adult human NP cells may use integrins α1β1, α2β1, α3β1 and α5β1 combined to interact with LM-111 and LM-511.
The results of flow cytometric analyses confirm NP cell expression of integrin subunits α1, α2, α3, α5, and β1. These results corroborate prior findings for protein expression via immunohistochemistry (11) and mRNA expression via PCR for α1, α2, α5, and β1, only (22). The findings in the current study of immunopositivity for integrin subunit α3 and no evidence of subunit β4, is consistent with our prior report for very young human NP tissue expression via immunohistochemistry and flow cytometry (12), but not that for younger adult NP tissue expression levels via immunohistochemistry (11). This difference could be due to differences in antibody reactivity, the sensitivity of different methods (histology vs. flow cytometry), the difference in tissue ages, or an alteration in integrin expression induced by cell isolation and a brief period of culture. In contrast to young human and porcine NP cells (12; 13), the current study demonstrates that adult human NP cells do not express the α6 integrin subunit or use it to attach to LM-111 or LM-511. This indicates integrin α6 expression and utilization may have a relationship with aging or degeneration of the NP.
The immunopositivity for integrin subunits α3, α5, and β1 via histochemical staining corroborates findings via flow cytometry and indicates that the subunits most highly expressed in vivo are also most highly expressed after cell culture through passage 2. Not every visual field examined was found to be positive for integrin subunits α3 and α5 using immunohistochemistry, most likely due to the low cellularity of human NP tissue combined with the lower incidence of α3 and α5 expression. This finding highlights a limitation of histological analyses when studying the largely hypocellular adult human NP tissue, and may provide an explanation for variances between this work and that reported previously for younger human NP tissues (11).
Despite demonstrating that integrins are critical for NP cell attachment to ECM proteins, no single integrin alpha subunit was found to completely block cell attachment to any of the tested substrates. Instead, we found evidence for multi-integrin alpha subunit cooperation by blocking multiple integrin subunits simultaneously. Blocking subunits α3 and α5 or α1, α2, α3, and α5 together significantly reduced cell attachment to laminins at levels similar to blocking subunit β1 alone. Despite a lack of statistical significance between blocking α3 + α5 and α1 + α2 + α3 + α5, it appears subunits α1 + α2 contribute to cell attachment, decreasing cell attachment an additional 36–47% compared to α3 + α5 alone. Thus it can be concluded that multiple alpha subunits demonstrate cooperativity to mediate NP cell attachment to LM-111 and LM-511 in studies as performed here. When one alpha subunit is blocked, another can compensate to prevent loss of cell attachment, in a process that deviates from a simple additive or subtractive effect.
Cell attachment to type II collagen has been previously reported to be mediated by integrin α1β1 for human chondrocytes and immature porcine NP cells (5; 13), and integrin α2β1 for rat NP cells (18). Loeser and co-workers found that human chondrocytes most highly expressed integrins α1β1 and α5β1, as compared to the findings reported here that human NP cells most highly express integrin subunits α3, α5, and β1. Human chondrocytes were reported to attach to type II collagen through the use of integrin subunits α1 and β1, blocking 38.3% and 98% of cell attachment, respectively (5). For human NP cells, only anti-integrin β1 antibody reduced attachment to type II collagen in a significant manner, inhibiting 61% of all NP cells attaching as compared to IgG controls. The absence of a significant effect of alpha integrin subunits regulating attachment to type II collagen for human NP cells, but not chondrocytes, was found despite using a similar cell attachment time (60 minutes versus 45 minutes), the same chick type II collagen from Sigma, a similar protein concentration (40 µg/mL versus 10 µg/mL), and obtaining a similar unblocked cell attachment efficiency (49% versus ~50%) across these studies (14). The data presented does not confirm that mature human NP cells attach to type II collagen through α1 or α2 integrin subunits. It must be concluded that human NP cells use a different set of integrin alpha subunits than chondrocytes and immature NP cells to attach to type II collagen, possibly the collagen-binding subunits α10β1 or α11β1 (25; 26).
In a study by Le Maitre and co-workers, NP cell attachment to the ECM binding domain, RGD, was found to be important in mechanosensing for healthy, but not degenerate, NP cells (22). Specifically, they found that blocking with an RGD peptide altered the ability of NP cells from non-degenerate discs to sense mechanical stimulation when embedded in an artificial matrix. RGD is a critical peptide for mediating cell binding to fibronectin, largely through an α5β1 integrin-mediated mechanism. Fibronectin was not studied here, as immature NP cells were not previously found to favor binding to fibronectin over laminins and collagens (20), which were the focus of the current study. Nevertheless, the reported change in mechanosensitivity suggests an altered cell-ECM interaction for degenerate IVD cells, supporting our findings identifying different functional integrin subunits in mature human NP cells compared to prior studies of immature NP cell binding (13).
This study identifies integrin subunits β1, α1, α2, α3 and α5 as functional units used by adult human NP cells to interact with the ECM, providing a foundation for developing bioconducive biomaterial interfaces that engage these cell receptors. Further work is needed to determine the phenotypic and biosynthetic impact that binding through each subunit may confer on mature and immature NP cells. Adult human NP cells express and utilize different integrin receptors as compared to porcine and rat NP cells, highlighting a significant limitation of animal models when designing therapies for the IVD. Human NP cell attachment information will enable an understanding of the mechanisms that regulate cell survival, cell phenotype, and mechanosensing, allowing for improved therapeutic interventions for the treatment of IVD disorders.
Acknowledgments
This research was supported by grants from the NIH (EB002263, AR047442, AR057410), the Howard Hughes Medical Institute, and the James McElhaney Duke BME Department Fellowship.
References
- 1.Andersson GBJ, An HS, Oegema TR, Setton LA. Intervertebral disc degeneration - Summary of an AAOS/NIH/ORS Workshop, September 2005. J Bone Joint Surg Am. 2006;88A:895–899. doi: 10.2106/JBJS.F.00028. [DOI] [PubMed] [Google Scholar]
- 2.Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachemson A. The lumbar spine: an orthopaedic challenge. Spine. 1976;1:59–69. [Google Scholar]
- 4.Lyons G, Eisenstein SM, Sweet MBE. Biochemical-Changes in Intervertebral-Disk Degeneration. Biochim Biophys Acta. 1981;673:443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
- 5.Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37:109–116. [PubMed] [Google Scholar]
- 6.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha 2 beta 1 and alpha 5 beta 1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis and rheumatism. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 7.Mobasheri A, Carter SD, Martin-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26:1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- 8.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–446. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 9.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. The Journal of pathology. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 10.Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Developmental dynamics : an official publication of the American Association of Anatomists. 1999;215:179–189. doi: 10.1002/(SICI)1097-0177(199907)215:3<179::AID-AJA1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Nettles DL, Richardson WJ, Setton LA. Integrin expression in cells of the intervertebral disc. J Anat. 2004;204:515–520. doi: 10.1111/j.0021-8782.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Jing LF, Gilchrist CL, et al. Expression of Laminin Isoforms, Receptors, and Binding Proteins Unique to Nucleus Pulposus Cells of Immature Intervertebral Disc. Connect Tissue Res. 2009;50:294–306. [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchrist CL, Chen J, Richardson WJ, et al. Functional integrin subunits regulating cellmatrix interactions in the intervertebral disc. J Orthop Res. 2007;25:829–840. doi: 10.1002/jor.20343. [DOI] [PubMed] [Google Scholar]
- 14.Loeser RF. Integrin-mediated attachment of articular chondrocytes to extracellular matrix proteins. Arthritis and rheumatism. 1993;36:1103–1110. doi: 10.1002/art.1780360811. [DOI] [PubMed] [Google Scholar]
- 15.Loeser RF, Carlson CS, McGee MP. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Experimental cell research. 1995;217:248–257. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- 16.Attia M, Santerre JP, Kandel RA. The response of annulus fibrosus cell to fibronectincoated nanofibrous polyurethane-anionic dihydroxyoligomer scaffolds. Biomaterials. 2011;32:450–460. doi: 10.1016/j.biomaterials.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Xia MS, Zhu Y. Fibronectin Fragment Activation of ERK Increasing Integrin alpha(5) and beta(1) Subunit Expression to Degenerate Nucleus Pulposus Cells. J Orthop Res. 2011;29:556–561. doi: 10.1002/jor.21273. [DOI] [PubMed] [Google Scholar]
- 18.Risbud MV, Guttapalli A, Albert TJ, Shapiro IM. Hypoxia activates MAPK activity in rat nucleus pulposus cells - Regulation of integrin expression and cell survival. Spine. 2005;30:2503–2509. doi: 10.1097/01.brs.0000186326.82747.13. [DOI] [PubMed] [Google Scholar]
- 19.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrist C, Francisco A, Plopper G, et al. Nucleus pulposus cell-matrix interactions with laminins. Eur Cell Mater. 2011;21:523–532. doi: 10.22203/ecm.v021a39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aumailley M, Bruckner-Tuderman L, Carter WG, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Le Maitre CL, Frain J, Millward-Sadler J, et al. Altered integrin mechanotransduction in human nucleus pulposus cells derived from degenerated discs. Arthritis and rheumatism. 2009;60:460–469. doi: 10.1002/art.24248. [DOI] [PubMed] [Google Scholar]
- 23.Xia M, Zhu Y. Expression of integrin subunits in the herniated intervertebral disc. Connect Tissue Res. 2008;49:464–469. doi: 10.1080/03008200802325425. [DOI] [PubMed] [Google Scholar]
- 24.Baer AE, Wang JY, Kraus VB, Setton LA. Collagen gene expression and mechanical properties of intervertebral disc cell-alginate cultures. J Orthop Res. 2001;19:2–10. doi: 10.1016/S0736-0266(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 25.Camper L, Hellman U, Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit alpha10, a beta1-associated collagen binding integrin expressed on chondrocytes. The Journal of biological chemistry. 1998;273:20383–20389. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- 26.Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D. cDNA cloning and chromosomal localization of human alpha(11) integrin. A collagen-binding, I domain-containing, beta(1)-associated integrin alpha-chain present in muscle tissues. The Journal of biological chemistry. 1999;274:25735–25742. doi: 10.1074/jbc.274.36.25735. [DOI] [PubMed] [Google Scholar]