Abstract
Homogeneous Pd catalysts have been identified for the conversion of cyclohexenone and tetralone O-pivaloyl oximes to the corresponding primary anilines and 1-aminonaphthalenes. This method is inspired by the Semmler-Wolff reaction, a classic method that exhibits limited synthetic utility owing to its forcing conditions, narrow scope and low product yields. The oxime N–O bond undergoes oxidative addition to Pd0(PCy3)2, and the product of this step has been characterized by X-ray crystallography and shown to undergo dehydrogenation to afford the aniline product.
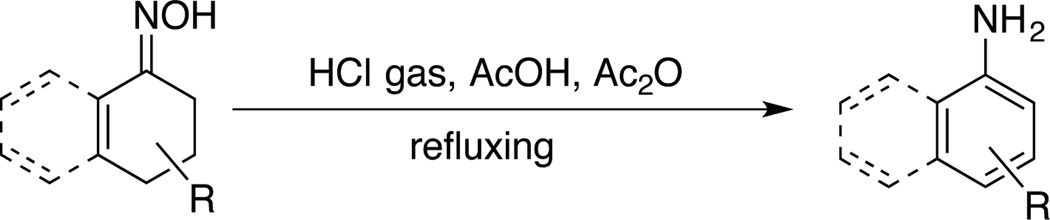
Aromatic amines are prevalent in pharmaceuticals, agrochemicals and functional organic materials.1 Among such structures, primary anilines (ArNH2) are among the most difficult to access via metal-catalyzed C–N coupling with aryl halides or arylboronic acid derivatives (e.g., Ullmann-Goldberg, Buchwald-Hartwig, Chan-Lam). Strategies to address this challenge include the use of ammonia surrogates, such as benzyl amine and tert-butyl carbamate,2 which can undergo C–N coupling and yield the parent aniline upon deprotection. Recent efforts have identified catalytic methods compatible with ammonia as the nitrogen nucleophile,3 as well as uncatalyzed reactions of aryl boron reagents with methoxyor aryloxyamines.4 The Semmler-Wolff reaction, is a classic but rarely used method for the synthesis of aromatic primary amines involving dehydration of cyclohexenone- and tetralone-derived oximes. This reaction is typically carried out under harsh conditions with anhydrous HCl gas in refluxing AcOH/Ac2O (Scheme 1),5–8 it exhibits limited functional group compatibility, and it typically affords products in moderate-to-low (≤ 60%) yields, with the Beckmann rearrangement as a common side-reaction. Here, we show that homogeneous Pd catalysts overcome many of these limitations in the conversion of cyclohexenone and tetralone O-acyl oximes to the corresponding primary aromatic amines.
Scheme 1.
Semmler-Wolff Reaction.
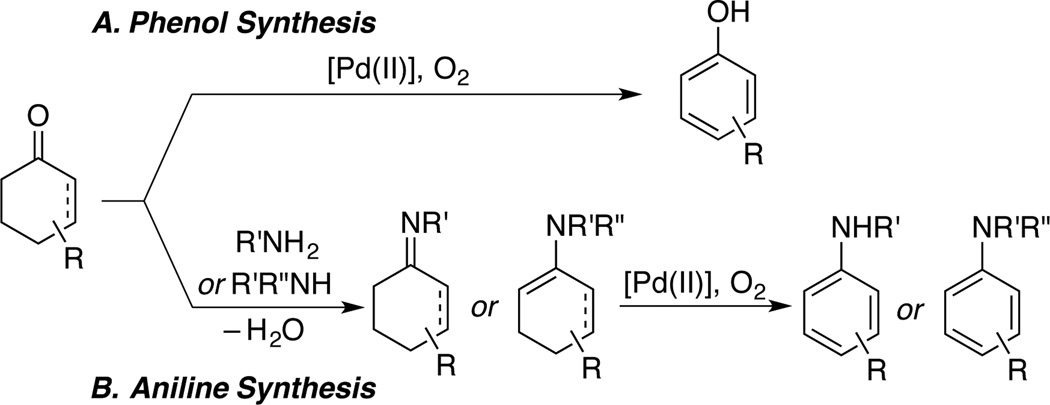
We recently reported Pd-catalyzed methods for aerobic dehydrogenation of diverse substituted cyclohexanones and cyclohexenones to phenols (Scheme 2A).9,10 These reactions complement metal-catalyzed cross-coupling routes to such molecules, and we envisioned that analogous methods could be used to prepare anilines. Dehydrogenation of pre-formed cyclohexanone-derived enamines has been used to prepare tertiary anilines, but these reactions exhibit relatively narrow scope and often use undesirable stoichiometric oxidants.11,12 Our preliminary efforts focused on in situ condensation of an amine with the ketone, followed by dehydrogenation of the imine or enamine intermediate (Scheme 2B). These reactions were complicated by parallel dehydrogenation of the ketone and imine/enamine and led to mixtures of phenol and aniline products. Independently, however, the groups of Yoshikai and Li identified suitable Pd catalysts and substrate partners for this transformation, enabling good yields of various secondary and tertiary aryl amines via this strategy.13 The condensation of ammonia with ketones is not strongly favored, and it is unlikely that primary anilines could be prepared by a similar approach. Oximes are quite stable, however, and the Semmler-Wolff precedent raised the prospect of identifying Pd catalysts to enhance the synthetic utility of this transformation. Oxidative addition of N–O bonds has been exploited in various Pd0/II-catalyzed reactions, 14 and the oxime could therefore serve as an internal oxidant to promote Pd-mediated dehydrogenation of the substrate.
Scheme 2.
Known Pd-catalyzed Aerobic Dehydrogenation Routes to Substituted Phenols and Anilines.
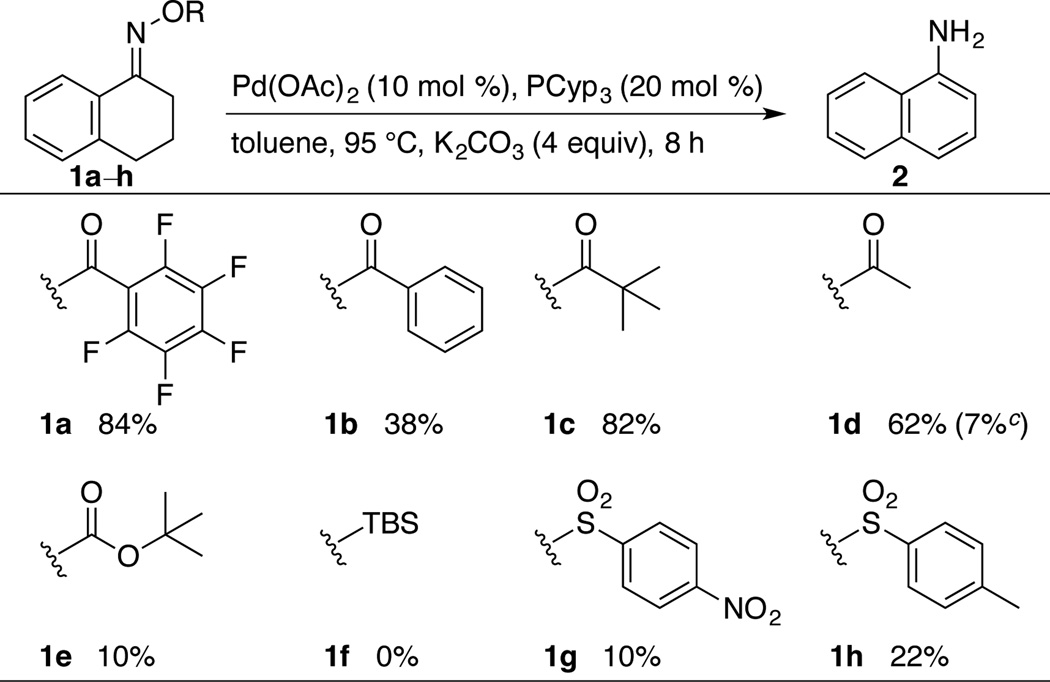
A number of different 1-tetralone oxime derivatives (1a–h) were synthesized and tested under a variety of catalytic conditions (Chart 1, Table 1). The O-pentafluorobenzoyl and O-pivaloyl derivatives 1a and 1c led to the highest yield of 1-aminonapthalene 2. Lower yields were obtained with the benzoyl and acetyl derivatives, and very low yields were obtained with the carbonate, siloxy, and sulfonate derivatives 1e–h. The reaction with the O-acetyl derivative (1d) led to a 7% yield of N-acetyl-1-aminonaphthalene as a side product. On the basis of these observations, we opted to proceed with pivaloyl oxime derivatives rather than the more expensive pentafluorobenzoyl derivatives.
Chart 1.
Comparison of O-Substituents in the Conversion of Tetralone Oximes to 1-Aminonaphthalene (2)a
a Reaction conditions: 1a–h (0.2 mmol), Pd(OAc)2 (0.02 mmol), tricyclopentylphosphine (0.04 mmol), K2CO3 (0.8 mmol), toluene (2.0 mL). b 1H NMR yield; internal standard = tetrachloroethane. c Yield of N-acetyl-1-aminonaphthalene.
Table 1.
Reaction Optimization for Conversion of Tetralone Pivaloyl Oxime to 1-Aminonaphthalene (2)a
 | ||||
|---|---|---|---|---|
| Entry | palladium | ligand | additive | % conv/ % yieldb |
| 1 | 10% Pd(P(o-tol)3)2 | 50/ 24 | ||
| 2 | 5% Pd2(dba)3 | 56/ 23 | ||
| 3 | 10% Pd(PPh3)4 | 96/ 70 | ||
| 4 | 5% Pd2(dba)3 | 20% PPh3 | 99/ 63 | |
| 5 | 5% Pd2(dba)3 | 20% PCy3 | 99/ 68 | |
| 6 | 10% Pd(OAc)2 | 20% PCyp3 | 97/ 82 | |
| 7 | 10% Pd(OAc)2 | 20% PCy3 | 97/ 80 | |
| 8 | 10% Pd(OAc)2 | 20% PPh3 | 74/ 37 | |
| 9 | 10% Pd(OAc)2 | 20% dppe | 73/ 62 | |
| 10 | 10% Pd(OAc)2 | 20% dppf | 78/ 44 | |
| 11 | 10% Pd(OAc)2 | 20% PCyp3 | 30% PivOH | 99/ 87 |
Reaction condition of Entry 11: 1c (0.2 mmol), Pd(OAc)2 (0.02 mmol), tricyclopentylphosphine (0.04 mmol), PivOH (0.06 mmol), K2CO3 (0.8 mmol), toluene (2.2 mL).
1H NMR yield; internal standard = tetrachloroethane.
Representative catalyst screening data for the conversion of of 1-tetralone pivaloyl oxime to 1-aminonaphthalene is summarized in Table 1 (see supporting information for additional data).15 Pd(PPh3)4 gave full conversion and 70% yield (entry 3), while other Pd0 sources, including Pd(P(o-tol)3)2 or Pd2(dba)3, were less effective (entries 1 and 2). Good yields could be obtained by combining Pd2(dba)3 with an appropriate phosphine ligand (entries 4 and 5), and even better results were obtained by using Pd(OAc)2 with 2 equiv of a phosphine ligand (entries 6-11). Further screening studies revealed that the optimal result could be obtained with a Pd(OAc)2/tricyclopentylphosphine (PCyp3) catalyst system, containing pivalic acid (30 mol %; entry 11 - 87% yield of 2). The idea of testing pivalic acid as an additive was inspired by precedents in which pivalate/pivalic acid serves as a proton shuttle in C–H activation.16
Upon the identification of suitable reaction conditions, a variety of substituted tetralone-derived oximes were tested and shown to react in good yield (Table 2). The formation of 5 is noteworthy because of the presence of a sensitive nitro group, which is not tolerated under Semmler-Wolff aromatization conditions.7 Formation of 7 was successfully reproduced on 2.2 g scale. Several aminonaphthalene products (9, 10, and 11) represent key intermediates in the synthesis of pharmaceutically active compounds. A previously reported synthesis of the 3α-hydroxysteroid dehydrogenase inhibitor HKI0231B prepared aminonaphthalene 9 in 50% yield via aromatization of the tetralone tosyl oxime in the presence of strong base (KOH, refluxing MeOH).6d The Pd-catalyzed method described here generates 9 in 80% isolated yield. Aminonapthalene 10 is the central intermediate in the preparation of benzo[c]phenanthridine alkaloids such as chelerythrine, fagaridine and decarine. The yield of 10 is only 58% under the present conditions, but this result is significantly better than the 28% yield obtained under classical Semmler-Wolff conditions.17 Compound 11 is an intermediate in the synthesis of the anti-tumor/anti-viral alkaloid, norallonitidine.18 Efficient access to 11 via this Pd-catalyzed method (79% yield) may be compared to a previously reported route (Scheme 3), in which classical electrophilic nitration affords a mixture of difficult-to-separate nitro-naphthalene products,19,20 and only 17% of this mixture consists of the nitronaphthalene precursor to 11. These results highlight the regioselectivity advantage of (catalytic) Semmler-Wolff methods relative to nitration/reduction routes to substituted anilines. Complications were encountered in the reaction of 6-bromo-1-tetralone; unsuccessful reaction of this substrate (not shown) suggests that competing oxidative addition of Ar–Br to Pd0 can initiate unwanted side reactions. Efforts to engage 2-tetralone pivaloyl oxime in this reaction resulted in only low yield (32%).
Table 2.
Aromatization of Substituted Tetralone Pivaloyl Oximesa
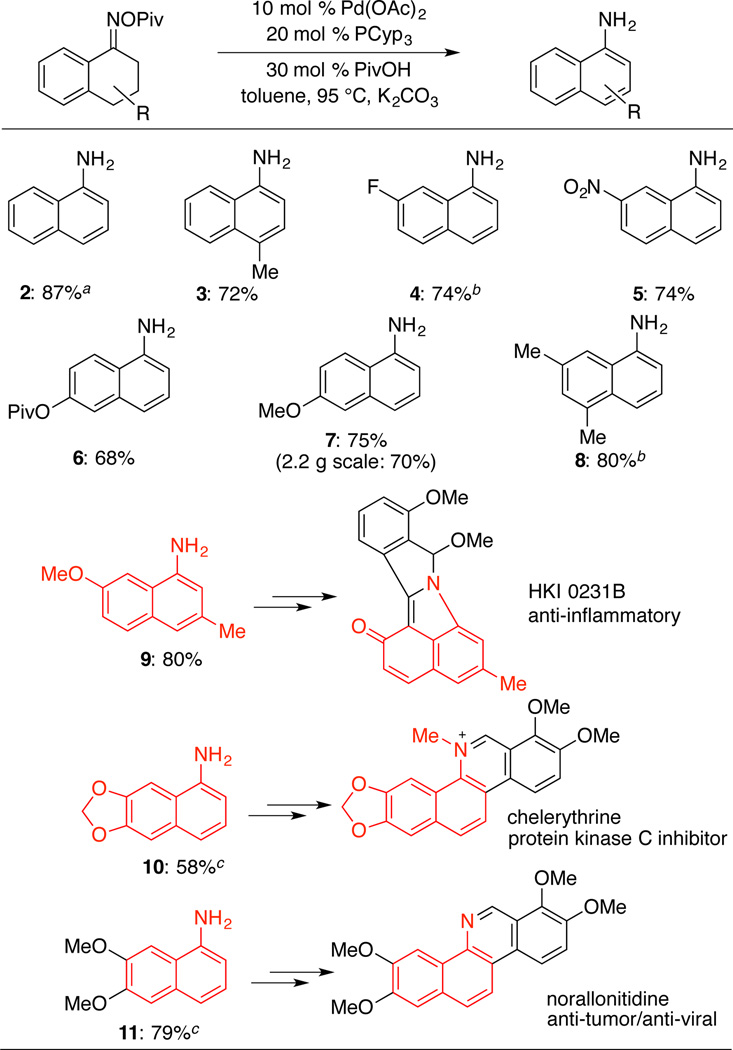 |
Reaction conditions: pivaloyl oxime (0.2 mmol), Pd(OAc)2 (0.02 mmol), tricyclopentylphosphine (0.04 mmol), PivOH (0.06 mmol), toluene (2.2 mL).
Structural data were analyzed after acetylation.
reaction temperature: 120 °C.
Scheme 3.
Previously Reported Route to 1119
The catalytic conditions also proved to be successful for the aromatization of cyclohexenone-derived pivaloyl oximes (Table 3). An array of primary anilines was obtained, including products with cyano, trifluoromethyl and chloro substituents. The more sterically hindered 2-phenyl- and 6-phenylcyclohexenone oximes proceed effectively to 2-aminobiphenyl (20) in 70% yield, and even the 2,5,6-triphenyl derivative affords the corresponding aniline 21 in 46% yield. A silyl ether group (in 22) was retained under the catalytic reaction conditions, unlike traditional Semmler-Wolff conditions, which result in a product with both N- and O-acetyl groups.6a Cyclohexenone oximes bearing sulfone substituents were compatible with the reaction conditions, and the corresponding sulfone-substituted anilines 23 and 24 were obtained in good yield. Reactions of 4-alkoxycarbonylcyclohexenone derivatives afforded the corresponding anilines 25 and 26.21
Table 3.
Aromatization of Substituted Cyclohexenone Pivaloyl Oximesa
 |
Reaction conditions: pivaloyl oxime (0.2 mmol), Pd(OAc)2 (0.02 mmol), tricyclopentylphosphine (0.04 mmol), PivOH (0.06 mmol), K2CO3 (0.8 mmol), toluene (2.2 mL).
Structural data were analyzed after acetylation.
derived from 2-phenylcyclohexenone.
derived from 6-phenylcyclohexenone.
reaction temp. = 120 °C.
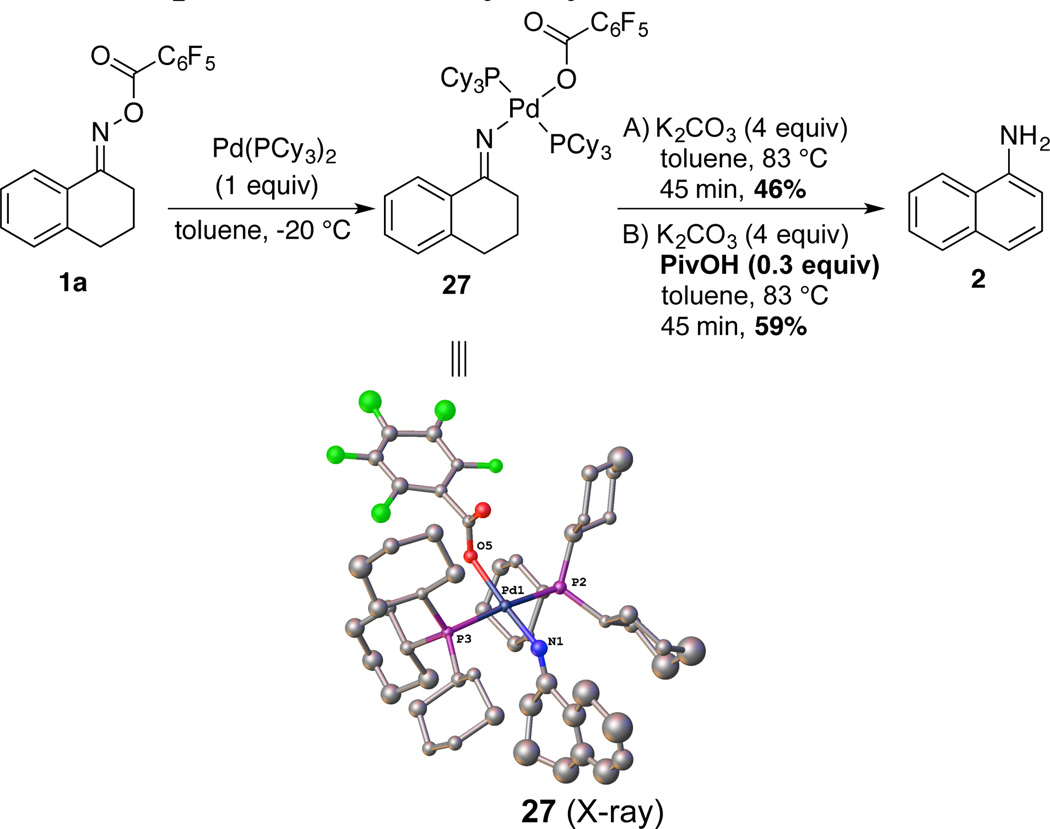
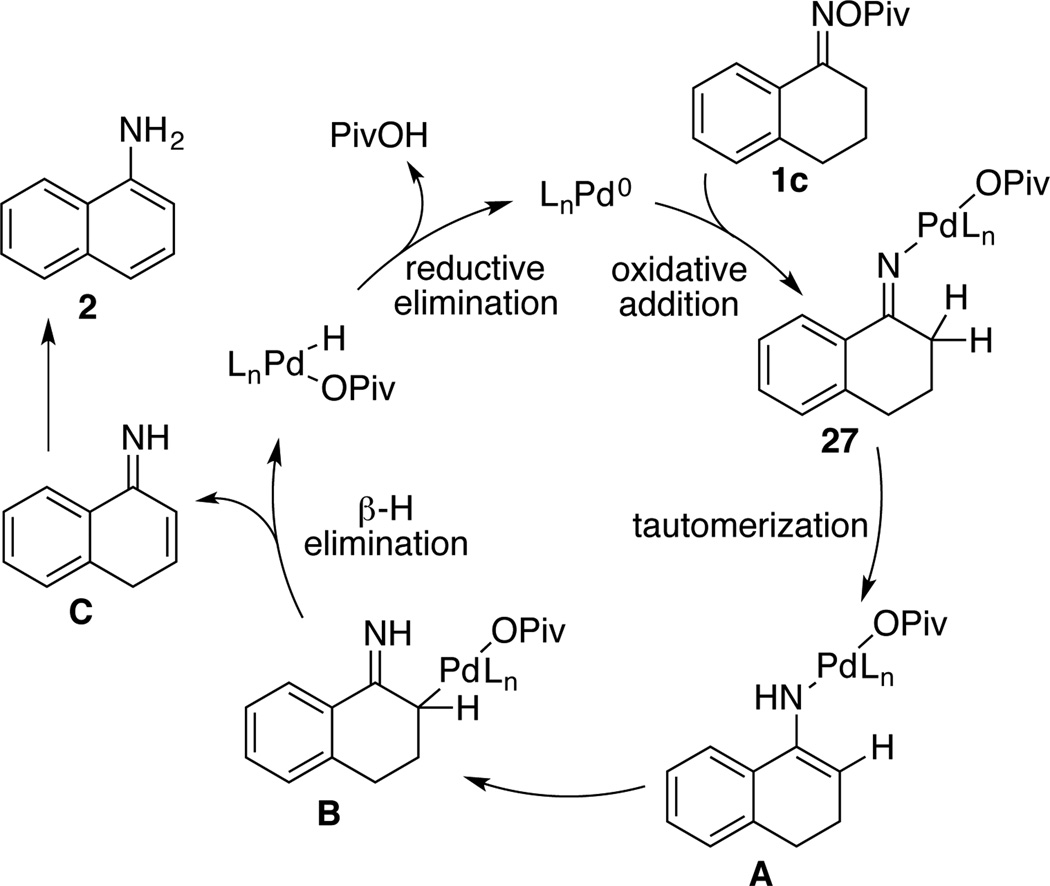
Oxidative addition of oxime N–O bonds to Pd0 has been utilized in a number of catalytic transformations, including aza-Heck coupling and directed C–H activation/C–N reductive elimination reactions.14 Several different oxime and LnPd0 derivatives were evaluated in an effort to probe the viability of this fundamental step as a prelude to aniline formation in the present catalytic reactions.22 The reaction of tetralone pivaloyl oxime with a stoichiometric amount of Pd(PPh3)4 led to a complex product mixture, but the combination of tetralone pentafluorobenzoyl oxime 1a and Pd(PCy3)2 afforded the N–O oxidative addition product 27, which was characterized by NMR spectroscopy and X-ray crystallography (Scheme 4). Upon heating in toluene, 27 reacts to afford 1-aminonaphthalene in 46% yield. The reaction proceeds with somewhat faster rate and in higher yield (59%) if it is carried out in the presence of pivalic acid and K2CO3, which are valuable additives in the catalytic reaction.
Scheme 4.
Stoichiometric Oxidative Addition/Aromatization Sequence and X-Ray Crystal Structure of 27
On the basis of these observations we propose the mechanism shown in Scheme 5. Oxidative addition of the tetralone oxime 1c affords intermediate 27, which can undergo tautomerization to afford the enamine-derived amido-PdII species A. Further isomerization of this species results in coversion of this N-bound enamido–PdII species to its C-bound isomer B. β-Hydride elimination from C generates the primary enimine, which can tautomerize to its more stable isomer, 1-aminonaphthaline (2). We speculate that the beneficial effect of the pivalic acid under catalytic and stoichiometric conditions (Table 1 and Scheme 5, respectively) reflects its ability to facilitate proton transfer reactions involved in the tautomerization step. The base (K2CO3) is heterogeneous under the reaction conditions, but it could play a similar role, in addition to promoting the reductive elimination step, which forms carboxylic acid as a stoichiometric by product.
Scheme 5.
Proposed Catalytic Cycle
In summary, these results show that homogeneous Pd catalysts promote efficient Semmler-Wolff-type reactions for conversion of readily accessible tetralone and cyclohexenone pivaloyl oxime derivatives to the corresponding primary anilines. These reactions provide an important demonstration of the complementarity of dehydrogenation and cross-coupling methods to access valuable substituted aromatic molecules.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to Dr. Ilia A. Guzei and Brian Dolinar for X-ray crystallographic analysis of 27, and we thank the NIH for financial support of this work (R01-GM100143). NMR spectroscopy facilities were funded by NSF (CHE-1048642 and CHE-0342998).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Additional catalyst screening data, experimental procedures, full compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCE
- 1.(a) Rappoport Z, editor. The Chemistry of Anilines, Parts 1 and 2. New York: John Wiley & Sons; 2007. [Google Scholar]; (b) Negwer M. Organic Chemical Drugs and their Synonyms. 7th ed. Verlag GmbH: Berlin: Akademie; 1994. [Google Scholar]
- 2.For leading references, see: Wolfe JP, Åhman J, Sadighi JP, Singer RA, Buchwald SL. Tetrahedron Lett. 1997;38:6367. Hartwig JF, Kawatsura M, Hauck SI, Shaughnessy KH, Alcazar-Roman LM. J. Org. Chem. 1999;64:5575. doi: 10.1021/jo990408i. Shen QL, Ogata T, Hartwig JF. J. Am. Chem. Soc. 2008;130:6586. doi: 10.1021/ja077074w.
- 3.For leading references, see: Shen Q, Hartwig JF. J. Am. Chem. Soc. 2006;128:10028. doi: 10.1021/ja064005t. Xia N, Taillefer M. Angew. Chem. Int. Ed. 2009;48:337. doi: 10.1002/anie.200802569. Vo GD, Hartwig JF. J. Am. Chem. Soc. 2009;131:11049. doi: 10.1021/ja903049z. Lundgren RJ, Peters BD, Alsabeh PG, Stradiotto M. Angew. Chem. Int. Ed. 2010;49:4071. doi: 10.1002/anie.201000526. Surry DS, Buchwald SL. Chem. Sci. 2011;2:27. doi: 10.1039/C0SC00331J. Ji PJ, Atherton JH, Page MI. J. Org. Chem. 2012;77:7471. doi: 10.1021/jo301204t.
- 4.(a) Mlynarski SN, Karns AS, Morken JP. J. Am. Chem. Soc. 2012;134:16449. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu C, Li G, Ess DH, Falck JR, Kürti L. J. Am. Chem. Soc. 2012;134:18253. doi: 10.1021/ja309637r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Semmler W. Ber. Dtsch. Chem. Ges. 1892;25:3352. [Google Scholar]; (b) Wolff L. Justus Liebigs Ann. Chem. 1902;322:351. [Google Scholar]
- 6.Modified conditions have been investigated for leading references, see: Newman MS, Hung WM. J. Org. Chem. 1973;38:4073. Tamura Y, Yoshimoto Y, Sakai K, Kita Y. Synthesis. 1980:483. Tamura Y, Yoshimoto Y, Sakai K, Haruta J, Kita Y. Synthesis. 1980:887. Scopton A, Kelly TR. J. Org. Chem. 2005;70:10004. doi: 10.1021/jo051762l.
- 7.Traditional Semmler-Wolff reactions often lead to mixtures of mono- and diacetylated anilines, which are treated with aqueous base to afford the primary aniline; see: Kelly TR, Chandrakumar NS, Saha JK. J. Org. Chem. 1989;54:980.
- 8.Heterogeneous Pd catalysts (Pd/C) can promote the Semmler- Wolff reaction; however, very high temperatures (e.g. 200 °C) are required. Horning EC, Horning MG. J. Am. Chem. Soc. 1947;27:1907. Matsumoto M, Tomizuka J, Suzuki M. Synth. Commun. 1994;24:1441. Matsumoto M, Tomizuka J. ITE Letters. 2000;1:127.
- 9.(a) Izawa Y, Pun D, Stahl SS. Science. 2011;333:209. doi: 10.1126/science.1204183. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Izawa Y, Zheng C, Stahl SS. Angew. Chem. Int. Ed. 2013;52:3672. doi: 10.1002/anie.201209457. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pun D, Diao T, Stahl SS. J. Am. Chem. Soc. 2013;135:8213. doi: 10.1021/ja403165u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For related transformations, see: Diao T, Stahl SS. J. Am. Chem. Soc. 2011;133:14566. doi: 10.1021/ja206575j. Simon MO, Girard SA, Li CJ. Angew. Chem. Int. Ed. 2012;51:7537. doi: 10.1002/anie.201200698. Gao W, He Z, Qian Y, Zhao J, Huang Y. Chem. Sci. 2012;3:883. Diao T, Wadzinski TJ, Stahl SS. Chem. Sci. 2012;3:887. doi: 10.1039/C1SC00724F. Imahori T, Tokuda T, Taguchi T, Takahata H. Org. Lett. 2012;14:1172. doi: 10.1021/ol300145g. Sutter M, Sotto N, Raoul Y, Métay E, Lemaire M. Green Chem. 2013;15:347. Xie Y, Liu S, Liu Y, Wen Y, Deng G-J. Org. Lett. 2012;14:1692. doi: 10.1021/ol3002442. Kim D, Min M, Hong S. Chem. Commun. 2013;49:4021. doi: 10.1039/c3cc41296b.
- 11.(a) Ishikawa T, Uedo E, Tani R, Saito S. J. Org. Chem. 2001;66:186. doi: 10.1021/jo001331x. [DOI] [PubMed] [Google Scholar]; (b) Cossy J, Belotti D. Org. Lett. 2002;4:2557. doi: 10.1021/ol026184z. [DOI] [PubMed] [Google Scholar]; (c) Horillo-Martinez P, Virolleaud MA, Jaekel C. ChemCatChem. 2010;2:175. [Google Scholar]; (d) Bigdelli MA, Rahmati A, Abbasi-Ghadim H, Mahdavinia GH. Tetrahedron Lett. 2007;48:4575. [Google Scholar]
- 12.Primary anilines have been accessed via dehydrogenation of amino cyclohexene derivatives obtained via Diels-Alder cycloaddition of Cbz-protected dienamines and activated dienophiles, such as N-methyl maleimide: Neumann H, von Wangelin AJ, Klaus S, Strübing D, Gördes D, Beller M. Angew. Chem. Int. Ed. 2003;42:4503. doi: 10.1002/anie.200351484.
- 13.(a) Hajra A, Wei Y, Yoshikai N. Org. Lett. 2012;14:5488. doi: 10.1021/ol302568b. [DOI] [PubMed] [Google Scholar]; (b) Girard SA, Hu X, Knauber T, Zhou F, Simon MO, Deng GJ, Li CJ. Org. Lett. 2012;14:5606. doi: 10.1021/ol3027279. [DOI] [PubMed] [Google Scholar]; (c) Wei Y, Deb I, Yoshikai N. J. Am. Chem. Soc. 2012;134:9098. doi: 10.1021/ja3030824. [DOI] [PubMed] [Google Scholar]
- 14.For leading references, see: Tsutsui H, Narasaka K. Chem. Lett. 1999:45. Kitamura M, Narasaka K. Chem. Rec. 2002;2:268. doi: 10.1002/tcr.10030. Narasaka K. Pure. Appl. Chem. 2003;75:19. Zaman S, Kitamura M, Narasaka K. Bull. Chem. Soc. Jpn. 2003;76:1055. Narasaka K, Kitamura M. Eur. J. Org. Chem. 2005:4505. Zaman S, Mitsuru K, Abell AD. Org. Lett. 2005;7:609. doi: 10.1021/ol047628p. Tan Y, Hartwig JF. J. Am. Chem. Soc. 2010;132:3676. doi: 10.1021/ja100676r. Faulkner A, Bower JF. Angew. Chem. Int. Ed. 2012;51:1675. doi: 10.1002/anie.201107511.
- 15.The reaction conditions in Table 1 often produced a dimeric compound (up to 6% yield) arising from the condensation of 1- aminonaphthalene with pivaloyl oxime 1c.
- 16.Lafrance M, Fagnou K. J. Am. Chem. Soc. 2006;128:16496. doi: 10.1021/ja067144j. [DOI] [PubMed] [Google Scholar]
- 17.Kessar SV, Gupta YP, Balakrishnan P, Sawal KK, Mohammad T, Dutt M. J. Org. Chem. 1988;53:1708. [Google Scholar]
- 18.Geen GR, Mann IS, Mullane MV, McKillop A. J. Chem. Soc. Perkin Trans. 1. 1996:1647. [Google Scholar]
- 19.The distribution of products is 5-nitro-2,3-dimethoxynaphthalene (17%), 6-nitro-2,3-dimethoxynaphthalene (36%) and 4-nitro-2,3- dimethoxynaphthalene (47%).
- 20.Stermitz FR, Gillespie JP, Amoros LG, Romero R, Stermitz TA, Larson KA, Earl S, Ogg JE. J. Med. Chem. 1975;18:708. doi: 10.1021/jm00241a014. [DOI] [PubMed] [Google Scholar]
- 21.Attempted aromatization of the parent oxime of 25 using modified Semmler-Wolff conditions (ref. 7) led to hydrolysis of the ester followed by decarboxylation, with no formation of the desired aniline.
- 22.Characterization of oxime N–O oxidative addition was recently reported in the context of intramolecular C–H amination reactions. See ref. 14g.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.