Abstract
Rationale: The origin of cells that make pathologic fibrillar collagen matrix in lung disease has been controversial. Recent studies suggest mesenchymal cells may contribute directly to fibrosis.
Objectives: To characterize discrete populations of mesenchymal cells in the normal mouse lung and to map their fate after bleomycin-induced lung injury.
Methods: We mapped the fate of Foxd1-expressing embryonic progenitors and their progeny during lung development, adult homeostasis, and after fibrosing injury in Foxd1-Cre; Rs26-tdTomato-R mice. We studied collagen-I(α)1–producing cells in normal and diseased lungs using Coll-GFPTg mice.
Measurements and Main Results: Foxd1-expressing embryonic progenitors enter lung buds before 13.5 days post-conception, expand, and form an extensive lineage of mesenchymal cells that have characteristics of pericytes. A collagen-I(α)1–expressing mesenchymal population of distinct lineage is also found in adult lung, with features of a resident fibroblast. In contrast to resident fibroblasts, Foxd1 progenitor–derived pericytes are enriched in transcripts for innate immunity, vascular development, WNT signaling pathway, and cell migration. Foxd1 progenitor–derived pericytes expand after bleomycin lung injury, and activate expression of collagen-I(α)1 and the myofibroblast marker αSMA in fibrotic foci. In addition, our studies suggest a distinct lineage of collagen-I(α)1–expressing resident fibroblasts that also expands after lung injury is a second major source of myofibroblasts.
Conclusions: We conclude that the lung contains an extensive population of Foxd1 progenitor–derived pericytes that are an important lung myofibroblast precursor population.
Keywords: pericyte, myofibroblast, lung injury, fibrosis, Foxd1
At a Glance Commentary
Scientific Knowledge on the Subject
The origin of lung myofibroblasts remains controversial. Recent studies suggest epithelial cells and bone marrow–derived circulating fibroblast progenitors (fibrocytes) do not directly contribute to fibrosis in vivo. Rather, they contribute to fibrosis by indirect means. Mesenchymal cells, however, likely play a major role directly in fibrogenesis.
What This Study Adds to the Field
We used genetic tools to map the fate of a population of embryonic lung mesenchymal progenitors that express the Foxd1 transcription factor. Their progeny differentiate into adult lung cells with features of pericytes. We show that these cells are a major pool of myofibroblast precursors in bleomycin-induced lung injury. These findings establish the novel concept that pericytes are key effectors and potential therapeutic targets in pulmonary fibrosis.
Idiopathic pulmonary fibrosis is a fatal illness characterized by proliferation of activated myofibroblasts and abnormal deposition of extracellular matrix (1–3). To date, no single therapy has proved to be effective in the treatment of idiopathic pulmonary fibrosis (4). One reason for the lack of therapies is an incomplete understanding of its pathogenesis. One area of controversy is the origin of the lung myofibroblasts. Proposed sources of lung myofibroblasts include epithelial-derived myofibroblasts, circulating fibrocytes, resident fibroblasts, and pericytes (5, 6). New studies in lung, kidney, and liver provide strong evidence that epithelial cells and circulating fibrocytes are not significant sources of myofibroblasts. Rather, increasing evidence suggests that these cells contribute to fibrogenesis by indirect means (7–10).
Recent studies have proposed that multiple mesenchymal cell subpopulations contribute to lung fibrosis (6). One mesenchymal cell type, the pericyte, may play a previously unrecognized role in fibrosis. Pericytes are specialized mesenchymal cells that share a common basement membrane with endothelial cells (11, 12). Although there is heterogeneity across species and tissues and developmental age, pericytes frequently express markers including PDGFRβ, αSMA, Angiopoietin1, P75 NGFR, CD146, and GFAP. In organs including brain, eye, and kidney, they regulate angiogenesis, vascular permeability, stability, and regional blood flow. Investigations in other organs have demonstrated a central role for pericytes as myofibroblast precursors and therefore fibrogenic cells (9, 13–19). Several studies suggest that when pericytes become myofibroblasts they lose pericyte functions, leaving an unstable endothelium prone to leak, ineffective angiogenesis, and ultimately destruction (20). Although little is currently known about lung pericytes and their contribution to lung injury, their function suggests they may play an important role in pathogenic features of lung injury, such as vascular leak and fibrogenesis.
Studies in the kidney identified progenitor cells in the embryonic metanephric mesenchyme that activate the forkhead transcription factor Foxd1 only in development (15, 21). Their progeny become vascular smooth muscle, mesangial cells, adventitial fibroblasts, and pericytes of the peritubular capillaries in the kidney. Deletion of Foxd1 is lethal because of failure of nephrogenesis. We have identified a similar population of Foxd1 progenitors that enter lung buds early in embryogenesis and rapidly silence Foxd1 after differentiating into lung mesenchyme. Using genetic fate mapping tools, we examined the fate of this mesenchymal progenitor lineage in adult lungs. Some of the results have been previously reported in the form of abstracts (22, 23).
Methods
Mouse Models
This study was approved by the University of Washington Institutional Animal Care and Use Committee. Coll-GFPTg transgenic mice were generated, validated, and genotyped as previously described on the C57BL/6 background (9, 24, 25). Foxd1+/GFPCre (Foxd1-Cre) and Foxd1+/GFPCreER (Foxd1-CreER) mice were generated as previously described (14, 15). Foxd1+/GFPCre mice were crossed with the universal Cre dependent reporter mice Rosa26-CAGGS fl-(S)-fl-tdTomato-Reporter (Rs26-tdTomatoR or TdT-R) or Rosa26 fl-(S)-fl-lacz-Reporter (Rs26R), to generate bitransgenic mice (Foxd1-Cre; Rs-TdT-R or Foxd1-Cre; Rs26R) that have the red fluorescent reporter or the lacz reporter permanently expressed only in cells and their daughters that previously activated the Foxd1 transcription factor during lung development. The Foxd1-Cre and Rs-tdT-R alleles were bred onto the Coll-GFPTg mice to obtain the triple transgenic Foxd1-Cre; Rs-tdT-R; Coll-GFPTg mice that identify cells of Foxd1 lineage and cells that express collagen-I(α)1 protein in vivo.
Microarray Experiments
Total RNA was isolated from four populations of fluorescence-activated cell sorter lung cells using three Foxd1-Cre; tdT-R; Coll-GFPTg mice for a total of 12 samples. We applied pair-wise comparisons to identify differential gene expression changes between: (1) Foxd1 progenitor–derived+/Coll-GFP– pericytes versus Foxd1 progenitor–derived+/Coll-GFP+ pericytes; (2) Foxd1 progenitor–derived+/Coll-GFP– pericytes versus resident fibroblasts (Foxd1-derived–/Coll-GFP+); and (3) Foxd1 progenitor–derived+/Coll-GFP– pericytes versus other lung cell populations (comprising a mixture of epithelial, endothelial, and immune cells). Functional enrichment analysis was performed for differentially expressed genes in each comparison group. Additional details are available in the online supplement. Cell sorting (9) and additional methods are described in the online supplement.
Results
Normal Lung Has an Extensive Population of Foxd1 Progenitor–derived Mesenchymal Cells
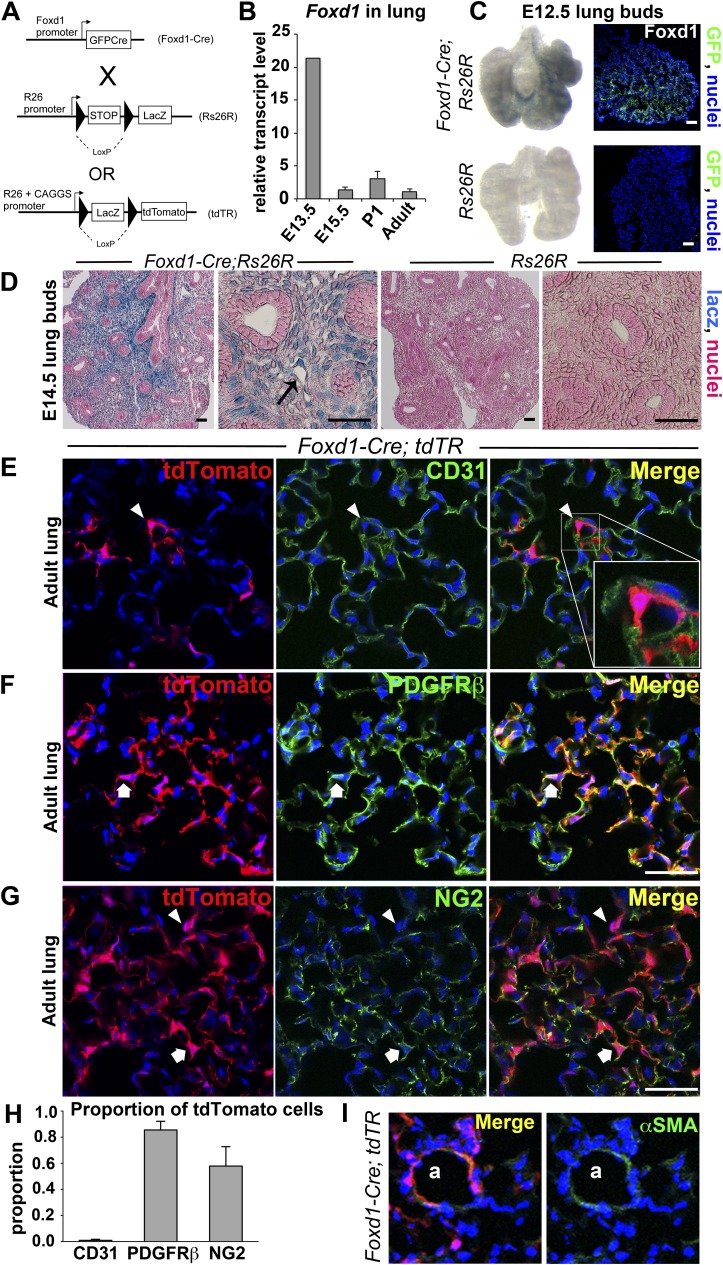
We generated mice whose Foxd1 gene locus was targeted such that GFPCre (GC) recombinase fusion protein was expressed under the endogenous regulation of the Foxd1 locus (Figure 1A). By quantitative reverse-transcriptase polymerase chain reaction, we found Foxd1 expression was increased by E13.5 but not significantly detected by E15.5 (Figure 1B). To map the fate of these progenitors, we expressed the Foxd1 gene targeted locus in mice that expressed either lacz enzyme or tdTomato fluorophore in all progeny of cells that have expressed Foxd1 (Figure 1A). At E12.5, Foxd1 lineage progenitors were detected in early lung buds (Figure 1C), and permanently tagged cells of the Foxd1 progenitor lineage could be seen by lacz staining of lung buds. By E14.5, an extensive population of Foxd1 progenitor–derived cells was readily apparent in developing lung buds, some of which were attached to developing blood vessels (Figure 1D). To define entry of Foxd1 progenitors into the developing lung buds we used tamoxifen-inducible Foxd-CreER; Rs26R mice and administered tamoxifen to pregnant dames at E10.5, but no blue-stained progeny of Foxd1 progenitors was detected in the lung (data not shown). Similarly, tamoxifen administration later in development and in neonates did not label any further progeny of Foxd1 progenitors. In combination, these findings suggest that Foxd1-expressing progenitors enter the lung between E11.5 and E12.5 and Foxd1 expression is down-regulated by E15.5.
Figure 1.
Foxd1-expressing progenitors enter early lung buds and differentiate into lung mesenchyme, which matures to form mural cells of the adult lung. (A) Bigenic Foxd1-Cre; Rs26R mice or Foxd1-Cre; tdTR mice activate GFPCre fusion protein expression in lung progenitor cells present in early lung buds and differentiate into a population of lung mesenchyme. The GFPCre recombinase results in removal of the loxP-STOP-loxP sequence in genomic DNA of these mesenchymal cells, leading to permanent, heritable expression of lacz or tdTomato in Foxd1 progenitor–derived cells. (B) Real-time polymerase chain reaction analysis of Foxd1 mRNA expression during lung development. Data were normalized to hypoxanthine-guanine phosphoribosyltransferase expression. Y-axis represents fold increase compared with adult. Mean value ± SD is indicated. n = 3–4 per time point. (C) Whole-mount heart and lung buds from Foxd1-Cre; Rs26R mice show presence of blue-stained mesenchymal cells derived from Foxd1 progenitors by E12.5. Specificity of this blue stain is shown by lack of lacz expression in control lung (left). Foxd1-expressing progenitors are seen predominantly in the posterior portion of the whole lung bud by detection of CreGFP expression under regulation of the Foxd1 locus (right). (D) E14.5 lung buds from Foxd1-Cre; Rs26R mice show large numbers of blue-stained mesenchymal cells derived from Foxd1 progenitors (left). Arrow illustrating a lacz+ mesenchymal cell adjacent to a developing blood vessel. Specificity of this blue stain is shown by lack of expression of lacz in control lung buds (right). (E–G) Confocal images of normal adult Foxd1-Cre; tdTR lung showing heritable labeling with tdTomato fluorophore of progeny of Foxd1 progenitors. tdTomato cells lie in close apposition to alveolar endothelium labeled with CD31 (arrowhead) (E), but do not overlap with this endothelium (inset). By contrast, almost all tdTomato cells coexpress PDGFRβ (arrow) (F), and many show expression of NG2 (arrow), whereas a minority do not express this protein (arrowhead) (G). (H) Graph quantifying the proportion of tdTomato cells coexpressing the indicated markers. (I) Images showing the presence of αSMA+ vascular smooth muscle in an arteriole (a) coexpressing tdTomato. Bar = 50 μm. Mean ± SEM. n = 3 per group.
Next we mapped Foxd1 progenitor–derived cells in adult lung using the tdTomato fluorophore. We found an extensive population of Foxd1-derived mesenchymal cells, comprising 13.5% (±4% SD) of nucleated cells (Figures 1E–1G, left column panels). The Foxd1 progenitor–derived cells were in close apposition to endothelial cells but did not coexpress the endothelial marker CD31 (Figure 1E). Likewise, Foxd1-derived cells were physically distinct from the epithelium and did not express epithelial markers (AquaporinV, EpCAM) or hematopoietic marker (CD45) (see Figures E2C–E2E in the online supplement). However, they expressed typical pericyte markers including PDGFRβ and NG2 (Figures 1F and 1G) and a subpopulation expressed PDGFRα (see Figure E2A). In normal lung, Foxd1 progenitor–derived cells did not express αSMA (we excluded large vessel and airway smooth muscle cells) (see Figure E2B). Taken together, the localization of these cells and cell surface marker expression (Foxd1 progenitor–derived, PDGFRβ+, NG2+, αSMA−, AqaporinV− CD31−, CD45−) (Figure 1D; see Figure E2F) are consistent with pericytes or pericyte-like cells. This cell lineage was also identified in vascular smooth muscle of arterioles (Figure 1I), in addition to the pericyte network in the lung.
Collagen-I(α)1+, PDGFRα+ Resident Fibroblasts Are Readily Identified in Normal Lung of Coll-GFPTg Reporter Mice
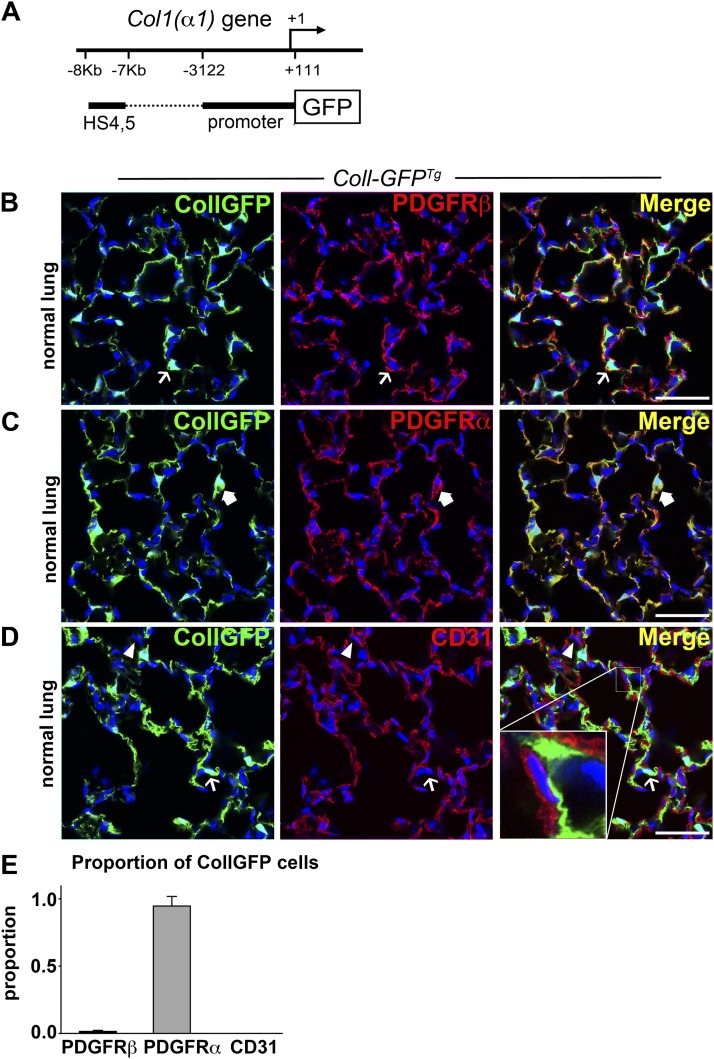
Using a mouse that reports active expression of collagen-I(α)1 transcripts (Figure 2A), and is a sensitive marker of collagen-I(α)1 production (abbreviated to Coll-GFP) (24), we found that the normal lungs have a network of Coll-GFP+ cells comprising 11.2% (±2.6% SD) of nucleated cells (Figures 2B–2D, left panel). Whereas in the kidney there is complete overlap of Foxd1-derived and Coll-GFP+ cells (9, 14, 15, 26), in the lung Coll-GFP+ cells formed a population of mesenchymal cells that was largely distinct from the Foxd1 progenitor–derived cells, with limited overlap. In contrast to Foxd1-derived cells, relatively few Coll-GFP+ cells expressed the pericyte marker PDGFRβ (Figure 2B). Characterization of Coll-GFP+ cells showed they were PDGFRα+, PDGFRβ−, αSMA−, CD31−, CD45−, EpCam−, and AquaporinV− (Figures 2B–2E; see Figure E3). In addition, Coll-GFP+ cells were not in direct apposition to endothelium (Figure 2D, inset), but many showed a close relationship to AquaporinV-expressing epithelium (see Figure E3C) and type II alveolar epithelial cells (see Figure E3D).
Figure 2.
Collagen-Iα1 GFP transgene–expressing cells coexpress PDGFRα in normal lung. (A) Schema showing the Coll-GFP transgene with a 3.2-kb fragment of the Col1a1 promoter and a 1-kb enhancer fused to GFP. (B–D) Confocal images showing the expression of GFP in the adult lung of Coll-GFPTg mice and colabeling with (B) PDGFRβ, (C) PDGFRα, and (D) CD31. Example of Coll-GFP+ cell (green) lacking the marker is indicated by a thin arrow. GFP− cell expressing the indicated marker (red) is indicated by an arrowhead. Coll-GFP+ cell coexpressing the indicated marker (yellow plasma membrane in merged image) is indicated by a thick arrow. Inset in D shows a space separating Coll-GFP+ cell from the endothelium. (E) Graph showing the proportion of Coll-GFP cells coexpressing the indicated markers. Bar = 50 μm. Mean ± SEM. n = 3 mice per group.
At Least Three Populations of Mesenchymal Cells Are Distinguishable in Normal Lung
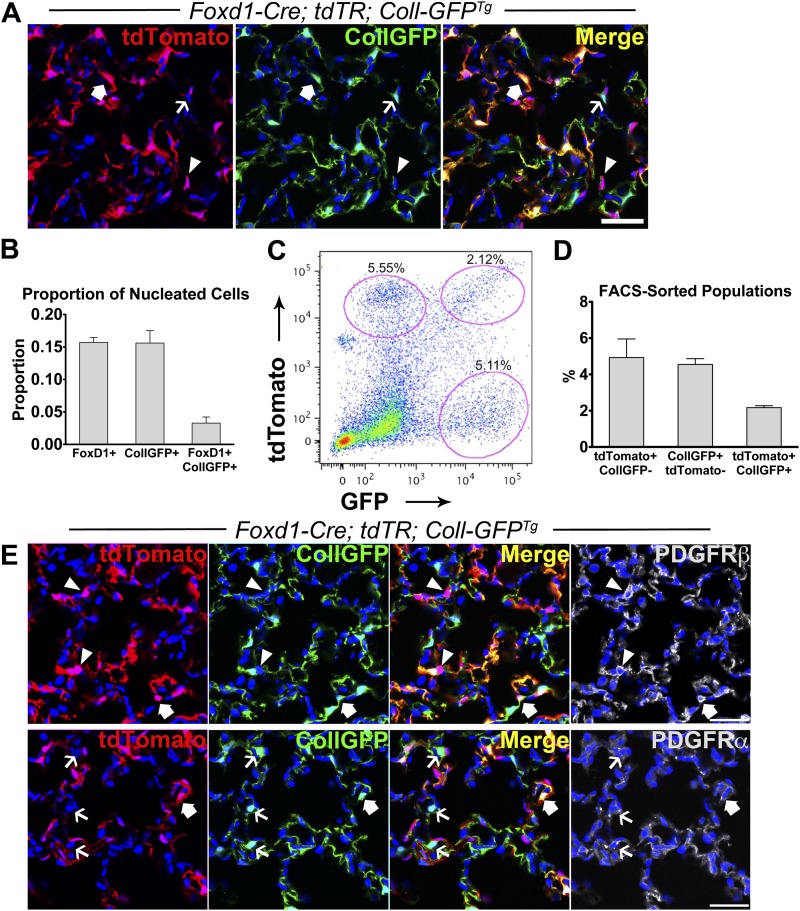
To clarify whether Foxd1-derived pericytes overlapped with cells that produced collagen-I(α)1, we generated Foxd1-Cre; tdT-R; Coll-GFPTg mice. In normal lung, we identified three distinct mesenchymal populations: (1) Foxd1 progenitor–derived/Coll-GFP−, (2) Coll-GFP+ only, and (3) a smaller population of Foxd1 progenitor–derived/Coll-GFP+ that represented 15–20% of total Foxd1 progenitor–derived pericytes (Figures 3A and 3B). We confirmed the presence of these populations by flow cytometry of lung digests (Figures 3C and 3D). The Foxd1 progenitor–derived/Coll-GFP− pericytes expressed PDGFRβ but not PDFGRα, whereas the Coll-GFP+ cells only expressed PDGFRα but not PDGFRβ (Figure 3E). Interestingly, the subset of Foxd1 progenitor–derived cells that expressed Coll-GFP expressed both PDGFRα and PDGFRβ (Figure 3E). In addition, this subset of cells expressed NG2 (see Figure E3F).
Figure 3.
Three populations of mesenchymal cells in normal lung are identified in Foxd1-Cre; tdTR; Coll-GFPTg mice. (A) Confocal image of normal lung showing three distinct populations of mesenchymal cells: arrowheads indicate tdTomato+ cells (red), thin arrows indicate Coll-GFP+ cells (green), and block arrows indicate tdTomato+ cells that also express Coll-GFP transgene (yellow in merged image). (B) Quantification of nucleated cells in lung parenchyma that are tdTomato+, Coll-GFP+, and tdTomato+ Coll-GFP+. (C) Fluorescence-activated cell sorter plot of single-cell preparation of normal lung from Foxd1-Cre; tdTR; Coll-GFPTg mice showing three distinct populations of lung mesenchymal cells. (D) Data showing the three populations: (1) tdTomato+ CollGFP−; (2) CollGFP+ tdTomato−; and (3) tdTomato+ CollGFP+ as a percentage of total counted events by fluorescence-activated cell sorter. (E) Foxd1-Cre; tdTR; Coll-GFPTg mouse lung colabeled with PDGFRβ or PDGFRα (white). Arrowheads indicate tdTomato cells (red) colabeled with PDGFRβ (white), whereas thin arrows indicate Coll-GFP+ cells (green) colabeled with PDGFRα (white). Block arrows indicate tdTomato/Coll-GFP+ cells colabeled with PDGFRα or β (white). Bars = 50 μm. Mean ± SEM. n = 3 mice.
Transcriptional Analysis of Foxd1 Progenitor–derived Lung Pericytes Identifies Distinct Signatures among Pericyte Subpopulations
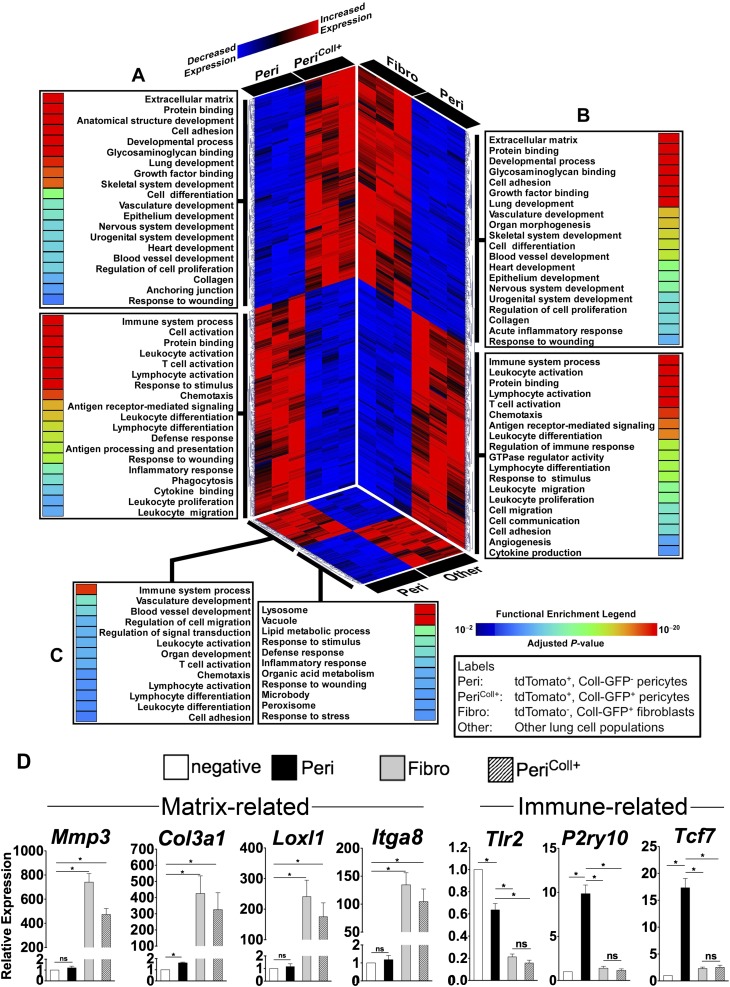
To explore the functional diversity of lung pericytes, we profiled the transcriptome of fluorescence-activated cell sorted cells from lungs of Foxd1-Cre; Rs26-TdT-R; Coll-GFPTg mice (Figures 4A–4C). Four populations were compared: (1) Foxd1 progenitor–derived/Coll-GFP− pericytes, (2) Foxd1 progenitor–derived/Coll-GFP+ pericytes, (3) Coll-GFP+ resident fibroblasts, and (4) other lung cells. Compared with other lung cells, Foxd1 progenitor–derived/GFP− pericytes (Peri in Figure 4) demonstrated significantly higher levels of transcripts involved in immune pathways, vascular development, and cell migration, processes consistent with known biology of pericytes (Figure 4C). Furthermore, these pericytes were characterized by a distinct transcriptional signature when compared with Coll-GFP+ resident fibroblasts (fibro in Figure 4), with more than 2,600 differentially expressed genes between these two populations (Figure 4B). Importantly, we observed remarkable differences in gene expression between the two subpopulations of Foxd1 progenitor–derived pericytes: Coll-GFP− and Coll-GFP+ (Figure 4A). Although both subsets share the Foxd1 lineage, the Coll-GFP+ pericytes also express the collagen-I(α)1 gene. The Foxd1 progenitor–derived/Coll-GFP+ pericytes were significantly enriched in processes involved in matrix remodeling, organ development, and wound repair. In contrast, the Foxd1 progenitor–derived/Coll-GFP− pericytes were overrepresented in immune-related pathways.
Figure 4.
Gene expression profiling of Foxd1 progenitor–derived pericytes identifies two subpopulations with distinct transcriptional and functional characteristics. This composite figure summarizes the results of three separate pair-wise comparisons. (A) Foxd1 progenitor–derived/CollGFP− pericytes (Peri) versus Foxd1 progenitor–derived CollGFP+ pericytes (PeriColl+). (B) Foxd1 progenitor–derived/CollGFP− pericytes (Peri) versus resident fibroblasts (non-Foxd1/CollGFP+) (Fibro). (C) Foxd1 progenitor–derived/CollGFP- pericytes (Peri) versus other lung cell populations (Foxd1−/CollGFP−) (Other). For each panel, differentially expressed genes are displayed using a heatmap (red, up-regulated; blue, down-regulated) and each expression pattern is linked to its corresponding functional categories (adjusted enrichment P values are shown using a rainbow scale). Note the profound transcriptional and functional differences between the two Foxd1-derived pericyte populations (A). When compared with Foxd1 progenitor–derived/CollGFP− pericytes, both the collagen-producing Foxd1 progenitor–derived/CollGFP+ pericytes and non-Foxd1/CollGFP+ fibroblasts are enriched in similar processes involved in matrix remodeling, development, and wound repair, whereas Foxd1-derived/CollGFP− pericytes are characterized by up-regulated genes mapping to immune pathways (A and B). Compared with other lung cell populations (non-Foxd1/CollGFP−), Foxd1 progenitor–derived/CollGFP− pericytes are enriched in immune processes, vasculature development, and cell migration (C). (D) Validation of microarray data by quantitative reverse-transcriptase polymerase chain reaction. Several matrix-associated and immune-related genes identified in microarray assay were evaluated. Expression is shown relative to the tdTomato− CollGFP− population (Other). Mean ± SEM. n = 3.
To validate the microarray findings, we evaluated gene expression of several matrix- and immune-related genes using quantitative reverse-transcriptase polymerase chain reaction (Figure 4D). Consistent with the microarray profile, Foxd1 progenitor–derived/Coll-GFP− pericytes expressed higher levels of typical innate immune receptors, such as Toll-like receptor 2, and the purinoceptor P2RY10. In addition, WNT signaling pathway genes were enriched in lung pericytes, exemplified by the WNT transcription factor target TCF-7. In contrast, Coll-GFP+ resident fibroblasts expressed high levels of matrix-related genes including MMP3, collagen III, Loxl1, and α8 integrin, differences borne out at the protein expression level (see Figure E5). Foxd1 progenitor–derived/Coll-GFP+ pericytes showed transcript levels more similar to Coll-GFP+ fibroblasts.
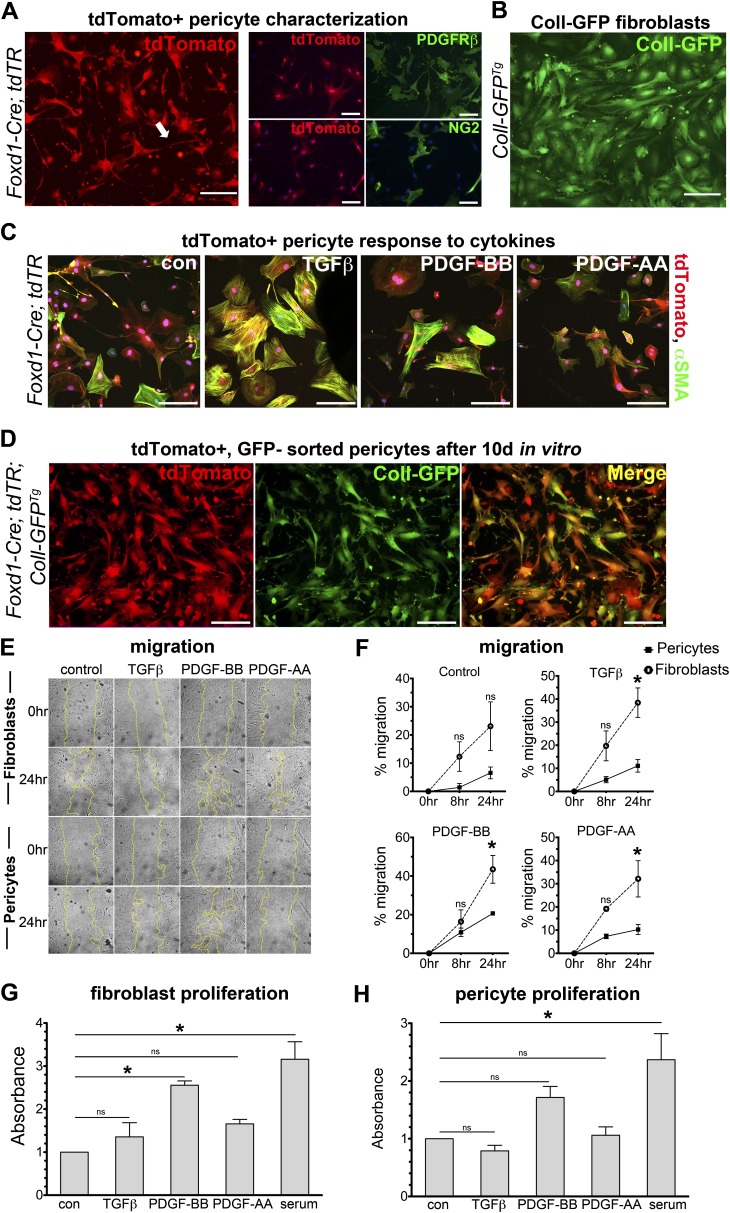
Foxd1 Progenitor–derived Pericytes and Collagen-I(α)1+ Resident Fibroblasts Show Distinct Functional Responses to Cytokines In Vitro
To determine whether differences in the transcriptional profiles translated to differences in behaviors, we examined morphology, migration, and proliferation of Foxd1 progenitor–derived cells and Coll-GFP+ cells cultured in vitro in response to TGFβ1, PDGF-BB, and PDGF-AA. Foxd1 progenitor–derived pericytes and Coll-GFP+ fibroblasts exhibited distinct morphology (Figures 5A and 5B). Foxd1 progenitor–derived pericytes had long cell processes, often more than the length of 10 cell bodies (Figure 5A), a typical feature of pericytes (14, 27–29). In contrast, Coll-GFP+ fibroblasts had broader cell shape without formation of long processes. Cell shape analysis found that Foxd1-derived cells were significantly more stellate than Coll-GFP+ fibroblasts (Cell Shape Index of 0.16 in pericytes vs. 0.37 in fibroblasts; P < 0.05). Foxd1-derived pericytes expressed PDGFRβ, NG2 (Figure 5A), and CD146 (not shown) in culture, typical markers of mural cells. TGFβ treatment caused marked shape change, formation of stress fibers, and up-regulation of αSMA expression within 24 hours (Figure 5C). By contrast, PDGFs did not stimulate a phenotypic change. We also examined whether pericytes activated expression of collagen-I(α)1 in culture. Primary pericyte cultures were generated from Foxd1-Cre; tdTR; Coll-GFPTg mice taking the tdTomato+, GFP− fraction of cells from lung (Figure 3C). After the first passage, we examined activation of the Coll-GFP transgene, indicative of collagen-I(α)1 protein production (Figure 5D). These findings indicate that all Foxd1 progenitor–derived lung pericytes have the capacity to express the myofibroblast marker αSMA and collagen-I(α)1. Next, cells were evaluated for migration (Figures 5E and 5F). Coll-GFP+ cells showed significantly higher migration rates at 24 hours in response to TGFβ1, PDGF-BB, and PDGF-AA compared with pericytes. Nevertheless, pericytes migrated significantly to PDGF-BB. Coll-GFP+ cells also showed significant increases in proliferation in response to PDGF-BB, whereas Foxd1 progenitor–derived cells did not have a significant increase in proliferation (Figures 5G and 5H).
Figure 5.
Foxd1 progenitor–derived pericytes and Coll-GFP+ resident fibroblasts exhibit morphologic and behavioral differences in two-dimensional culture. (A) Fluorescence images of primary cultures of the Foxd1 progenitor–derived cells (pericytes) permanently expressing tdTomato, isolated from Foxd1-Cre; tdTR mice (left). Foxd1 progenitor–derived cells exhibit long foot processes characteristic of pericytes in vitro (arrow). Immunostaining of tdTomato+ cells in vitro demonstrates colabeling of PDGFRβ in tdTomato+ cells, whereas only some tdTomato+ cells colabel with NG2 (right). (B) Coll-GFP+ fibroblasts from Coll-GFPTg mice at passage 2 show a distinctly different morphology from Foxd1 progenitor–derived pericytes. (C) tdTomato+ pericytes isolated from Foxd1-Cre;tdTR mice activate αSMA (green) protein expression and stress fiber formation in response to TGFβ stimulation in vitro after 24 hours. (D) Foxd1 progenitor–derived pericytes (tdTomato+, Coll-GFP−) isolated from Foxd1-Cre; tdTR; Coll-GFPTg mice shown after two passages in culture. Foxd1-derived pericytes show persistent expression of red fate marker (left, red) in vitro. Nearly all Foxd1 progenitor–derived pericytes activate high levels of Coll-GFP (middle, green) in vitro (bars = 50 μm). (E and F) Coll-GFP+ fibroblasts show increased migration compared with pericytes in response to TGFβ (1 ng/ml), PDGF-BB (50 ng/ml), and PDGF-AA (30 ng/ml) at 24 hours control: serum free (mean ± SEM, n = 3, *P < 0.05 fibroblast vs. pericyte). (G and H) PDGF-BB treatment increased proliferation in Coll-GFP+ fibroblasts compared with control (serum-free) at 72 hours, but not in pericytes. Proliferation measured by BrdU ELISA reported as absorbance relative to control. (mean ± SEM, n = 3, *P < 0.05 vs. control).
Foxd1 Progenitor–derived Lung Pericytes Are a Major Source of Myofibroblasts after Lung Injury
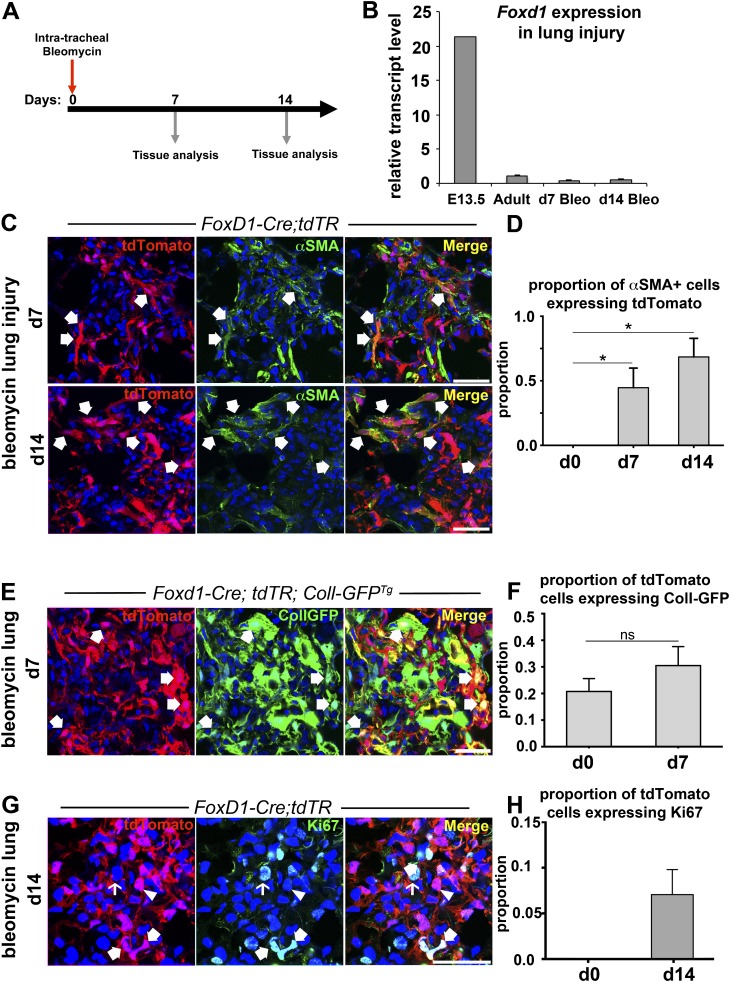
To evaluate whether Foxd1 progenitor–derived pericytes contributed to myofibroblasts, we used Foxd1-Cre; tdTR mice to map the fate of pericytes after bleomycin-induced lung injury (Figure 6C). There was no evidence of Foxd1 reactivation in injured mouse lungs at Days 7 and 14 by quantitative polymerase chain reaction (Figure 6B), suggesting that Foxd1-Cre; tdTR mice faithfully map the fate of pericytes after lung injury. On Day 7, many tdTomato cells, indicative of Foxd1 lineage, were present in fibroblastic foci and 45% of αSMA+ myofibroblasts expressed tdTomato (Figures 6C and 6D). By Day 14, 68% of αSMA+ myofibroblasts expressed tdTomato (Figures 6C and 6D). To explore the extent to which Foxd1-derived pericytes activated collagen-Iα1 production, the lung injury response to bleomycin was evaluated in Foxd1-Cre; tdT-R; Coll-GFPTg mice after 7 days. More than 30% of tdTomato-expressing cells also expressed GFP transgene (Figures 6E and 6F) indicating that many Foxd1 progenitor–derived pericytes were producing collagen protein in these lesions. Moreover, 47% of Coll-GFP+ cells were also tdTomato+ (data not shown), consistent with our finding that 45% of αSMA+ myofibroblasts were tdTomato+ in the Foxd1-Cre; tdTR bleomycin model at Day 7. Nevertheless, 53% of cells producing collagen in the fibroblastic foci were not derived from Foxd1 progenitors. To determine whether pericytes proliferated in fibroblastic foci or migrated from other areas of the lung, the proportion of Ki67+ tdTomato cells in fibrotic foci at Day 14 was nearly 10% of pericytes suggesting local proliferation in these lesions (Figures 6G and 6H). Although Foxd1 progenitor–derived pericytes made up a significant proportion of αSMA+ myofibroblasts after injury, non–Foxd1-derived cell populations also contributed to myofibroblasts. We evaluated collagen-I(α)1–producing cells in fibroblastic foci further in CollGFPTg mice (see Figure E4). Although many expressed αSMA after injury, none of these cells expressed the leukocyte marker CD45 or the epithelial marker EpCam (see Figure E4).
Figure 6.
Fate mapping of Foxd1 progenitor–derived pericytes identifies them as a major source of myofibroblast precursors in lung injury. (A) Schema of bleomycin lung injury experiments. Lungs were harvested at Days 7 or 14 after intratracheal bleomycin administration. (B) Quantitative reverse-transcriptase polymerase chain reaction of Foxd1 in injured mouse lungs at Days 7 and 14 post-bleomycin injury shows no activation of Foxd1 expression compared with uninjured adult mouse lung (mean ± SD; n = 3–7 per group). (C) Confocal images showing fibrotic foci on Days 7 and 14 after bleomycin lung injury in Foxd1-Cre; tdTR mice stained for the myofibroblast marker αSMA (green).Coexpression of myofibroblast marker αSMA and the tdTomato fate marker of Foxd1-derived pericytes indicated by arrows. (D) Graph indicating proportion of tdTomato+ cells coexpressing αSMA+ in fibroblastic foci at indicated time points after bleomycin injury (mean ± SEM; n = 3). (E and F) Confocal images showing fibrotic foci on Day 7 after bleomycin lung injury in triple transgenic mouse Foxd1-Cre; tdTR; Coll-GFPTg lungs, and graph summarizing proportion of tdTomato cells coexpressing Coll-GFP in fibrotic foci (mean ± SEM; n = 3). (G and H) Confocal images and graph showing the proportion of tdTomato+ cells in fibroblastic foci in cell cycle (Ki67+) at Day 7 (mean ± SEM; n = 3). Bar = 50 μm.
Discussion
In these studies we demonstrate the existence of an unappreciated population of lung mesenchymal cells that derive from Foxd1-expressing progenitors that enter the lung buds early in embryonic development. These cells silence Foxd1 expression on differentiation; mature to form an extensive network of cells with close relationship to the microvasculature; and express markers typical for mural cells, or pericytes including PDGFRβ, NG2, and CD146. This lineage of cells also forms the vascular smooth muscle of some arterioles. Foxd1 progenitor–derived cells did not express markers of leukocytes, epithelial cells, or endothelial cells. Transcriptional analysis of adult lung showed that this population was enriched in genes related to immune regulation, migration, angiogenesis, and the WNT pathway. Within this Foxd1 lineage, two subsets were identified in adult lungs. The major population lacked expression of collagen-I(α)1 protein but a minor population produced this protein in lung. This minor subpopulation expressed PDGFRβ and NG2, but was transcriptionally distinct from the larger population with a signature more similar to resident fibroblasts. In studies of the kidney, Foxd1 progenitors give rise to all the mesenchymal cells that lead to vascular smooth muscle and pericytes in adult kidney (15). Foxd1 activity is critical because deletion of this transcription factor is lethal due to failure of nephrogenesis. Similarly, Foxd1 deletion results in abnormal lung development (not shown), emphasizing the critical role of this gene and its lineage during lung development. Although little is known about the progenitors that form the lung microvasculature, a population of WNT-expressing progenitors has been proposed to develop into vascular smooth muscle by migrating from the foregut into the early lung buds at E9.5 (30, 31). It is possible that Foxd1 progenitors overlap with WNT expressing progenitors but further studies are required.
In addition to Foxd1 progenitor–derived cells, we identified a discrete but extensive mesenchymal cell population that derived from a separate lineage. These cells express collagen-I(α)1 protein in normal lung. This population did not express NG2 or PDGFRβ but all expressed PDGFRα. Transcriptionally and morphologically, these cells resembled classic resident fibroblasts.
Using fate-mapping strategies, we showed that the Foxd1-derived pericytes were major progenitors of fibrogenic myofibroblasts in the lung in response to bleomycin lung injury. Our studies are robust because Foxd1 is not reactivated in injured adult lungs; therefore, the appearance of myofibroblasts bearing the lineage marker tdTomato reflects transdifferentiation from the tdTomato pericytes in normal lung. Our studies also identified a distinct population of collagen-I(α)1+, PDGFRα+ resident fibroblasts in normal lung. Because these cells produce fibrillar collagens basally in the lung, express αSMA when cultured, and migrate and proliferate in response to TGFβ and PDGF in culture (Figure 5), it is likely that these are a major second progenitor population for myofibroblasts in bleomycin-induced lung injury, although definitive fate mapping studies are required. Although we cannot exclude the possibility that cells within the arteriolar smooth muscle wall could be a source of Foxd1-derived myofibroblasts, it is likely that numerically they represent a minority of the Foxd1-derived myofibroblasts. The finding that Foxd1 progenitor–derived pericytes are myofibroblast precursors in lung is consistent with recent studies in kidney, skin, muscle, and central nervous system demonstrating pericytes as important progenitors of myofibroblasts with injury (9, 14–16, 19, 22, 28, 29, 32–36). In those reports, pericytes detached from capillaries in response to injury, activated, migrated, proliferated, and became myofibroblasts. Recent investigations by Rock and colleagues (6) demonstrated that multiple stromal cells in the lung contribute to myofibroblasts. Although they detected significant expansion of pericytes (defined as NG2+ cells in fibrogenic foci), they did not find evidence of pericyte-derived myofibroblasts (defined by αSMA expression). Our study demonstrated up to 68% of αSMA+ cells in fibrosis are pericyte derived. There are several explanations to reconcile the studies. We defined pericyte-like cells as fate-mapped from Foxd1 progenitors. Our mouse reporter system labeled an extensive population of these cells: as many as 15% of all cells in normal lung. Rock and colleagues (6) examined mice in which NG2-expressing cells recombined in the presence of tamoxifen. However, only a small percentage of cells underwent recombination, thus potentially underestimating the contribution of these cells to myofibroblasts. Of significance, we found only about 60% of Foxd1 progenitor–derived cells expressed NG2 in healthy lung, suggesting that additional subpopulations of pericytes may exist in the lung that contributes to myofibroblasts or to disease (16, 29). It is important to acknowledge that no single marker exists that uniquely identifies all pericytes. The cell surface marker and transcriptional profile of Foxd1 progenitor–derived cells in the lung are consistent with known biology of pericytes; thus, the Foxd1-derived mesenchymal cells seem to be enriched with pericytes. Nonetheless, a population of pericytes derived from other progenitors may exist, just as nonpericytes are likely present in the Foxd1 progenitor–derived population. In our injury model, nonpericyte Foxd1 lineage cells may also expand and contribute to myofibroblasts. Thus, understanding the contributions of different fate-mapped lung mesenchymal cells to fibrosis is critical to sort out the complex mesenchymal cell heterogeneity.
Because Foxd1 lineage pericytes account for only 45% of Day 7 postbleomycin myofibroblasts, we estimate that as many as 55% of myofibroblasts derive from collagen-Iα1+ PDGFRα resident fibroblasts that are not of the Foxd1 lineage. This could be lower depending on whether there are other sources of myofibroblasts (i.e., epithelial-to-mesenchymal transition or fibrocytes). However, recent data cast into doubt whether epithelial cells become myofibroblasts in vivo (6, 7, 14, 15). Our studies did not identify other cell types that coexpress collagen-I(α)1 protein in vivo, suggesting that epithelium and fibrocytes contribute little to cells that make pathogenic fibrillar collagen matrix. Further confirmation of these findings await lineage mapping of collagen-I(α)1–expressing cells.
An unexpected finding in these studies was that Foxd1-derived pericytes that do not express collagen-I(α)1 protein in normal lung were highly enriched in innate immune genes. This finding suggests these cells may have an unappreciated role in immune regulation in the lung. Interestingly, studies of kidney, brain, and muscle pericytes suggest critical roles of pericytes as sentinels of the innate immune response (37–41). Further studies are required to understand lung pericyte functions during injury or infection. Another unexpected finding is that a subpopulation of Foxd1 progenitor–derived cells expressing collagen-I(α)1 has a transcriptional profile most similar to collagen-I(α)1+ PDGFRα+ resident fibroblasts, yet continue to express PDGFRβ and NG2. This suggests that a subpopulation of Foxd1 progeny may activate a second transcriptional program later in development to support lung growth and matrix production. Alternatively, Foxd1 progenitor–derived pericytes may exhibit plasticity in the adult lung. Because pericytes have been proposed to share overlapping features with mesenchymal stem cells (42–44), this characteristic might not be surprising. Thus, our data suggest that Foxd1 progenitor–derived pericytes possess multifaceted roles in the lung: a capacity to perform innate immune tasks and to undergo profound transcriptional changes that render them matrix-synthesizing cells that coordinate reparative responses. To evaluate the functional roles of Foxd1-derived cells in the lung, future studies directed at ablation of Foxd1-derived cells with lung injury are needed.
Our present study builds on an evolving appreciation for the heterogeneity of mesenchymal populations in the lung and their importance in fibrogenesis. We show for the first time that one mesenchymal population with distinctive transcriptional signatures and marker expression can be lineage-traced by previous Foxd1 transcription factor expression in early development. Importantly, we demonstrate that Foxd1-derived cells are one of the fibrogenic precursors in lung fibrosis, consistent with recent studies suggesting multiple mesenchymal populations contribute to lung myofibroblasts. Future studies elucidating the role of different mesenchymal populations, including Foxd1-derived pericytes and collagen-I(α)1+ PDGFRα resident fibroblasts, in myofibroblast differentiation and their functions are critical to understanding the pathogenesis of inflammation and fibrosis, and to future development of antifibrotic therapies.
Acknowledgments
Acknowledgment
The authors thank Dr. William Stallcup (Burnham Institute) for development and use of antibodies; Dr. Jeff Delrow and Crissa Bennett (FHCRC core, University of Washington) for their expertise with microarray analysis; Jeff Boyd (FACS Core SLU, University of Washington) for technical support with flow sorting; the Lynn and Mike Garvey Cell Imaging Laboratory at the University of Washington Institute for Stem Cell and Regenerative Medicine for their support in confocal microscopy; and Dr. Shuyu Ren, Allie Roach (University of Washington), and Natalie Naiman (Brigham and Women’s Hospital) for assistance with experiments.
Footnotes
Supported by NIH grants (DK84077, DK87389, DK93493, DK94768, NCATS TR000504) and AHA grant 12040023 to the Duffield laboratory. The Schnapp Laboratory is funded by NIH K24 HL068796, AHA Grant-in-Aid, and Grant #2496490 from the Washington State Life Sciences Discovery Fund to the Center for Intracellular Drug Delivery. The Kobayashi Laboratory is supported by NIH DK094933.
Author Contributions: Conception and design, C.H., W.A.A., S.A.G., L.M.S., and J.S.D. Analysis and interpretation, C.H., G.L., Y.-H.C., K.M., W.A.A., S.A.G., L.M.S., and J.S.D. Drafting the manuscript for important intellectual content, C.H., L.M.S., and J.S.D.
Originally Published in Press as DOI: 10.1164/rccm.201212-2297OC on August 7, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 2.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 4.Luppi F, Spagnolo P, Cerri S, Richeldi L. The big clinical trials in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18:428–432. doi: 10.1097/MCP.0b013e3283567ff9. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380:680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 6.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine D, Rockey DC, Milner TA, Breuss JM, Fallon JT, Schnapp LM. Expression of the integrin alpha8beta1 during pulmonary and hepatic fibrosis. Am J Pathol. 2000;156:1927–1935. doi: 10.1016/s0002-9440(10)65066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ, et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 17.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sponheim J, Pollheimer J, Olsen T, Balogh J, Hammarström C, Loos T, Kasprzycka M, Sørensen DR, Nilsen HR, Küchler AM, et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177:2804–2815. doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Palmer R, Gao X, Kreidberg J, Gerald W, Hsiao L, Jensen RV, Gullans SR, Haber DA. Transcriptional activation of placental growth factor by the forkhead/winged helix transcription factor FoxD1. Curr Biol. 2003;13:1625–1629. doi: 10.1016/j.cub.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 22.Hung CF, Linn GR, Chow Y, Kobayashi A, Duffield JS, Schnapp LM. Pericyte-like cells and resident fibroblasts are sources of myofibroblasts in acute lung injury. Am J Respir Crit Care Med. 2012;185:A6805. [Google Scholar]
- 23.Hung CF, Chow Y, Gharib SA, Duffield JS, Schnapp LM. Lung stromal cell diversity identified by transcriptional profiling. Am J Respir Crit Care Med. 2013;187:A3827. [Google Scholar]
- 24.Krempen K, Grotkopp D, Hall K, Bache A, Gillan A, Rippe RA, Brenner DA, Breindl M. Far upstream regulatory elements enhance position-independent and uterus-specific expression of the murine alpha1(I) collagen promoter in transgenic mice. Gene Expr. 1999;8:151–163. [PMC free article] [PubMed] [Google Scholar]
- 25.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 26.Lin S-L, Chang F-C, Schrimpf C, Chen Y-T, Wu C-F, Wu V-C, Chiang W-C, Kuhnert F, Cuo CJ, Chen Y-M, et al. Targeting endothelium-pericyte crosstalk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction & fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 28.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 29.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113:147–154. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MF, Cohen ED, Baggs JE, Lu MM, Hogenesch JB, Morrisey EE. Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc Natl Acad Sci USA. 2012;109:15348–15353. doi: 10.1073/pnas.1201583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 33.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, Wu TH, Linn GR, Ling H, Wu KD, et al. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013;182:118–131. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffield JS. The elusive source of myofibroblasts: problem solved? Nat Med. 2012;18:1178–1180. doi: 10.1038/nm.2867. [DOI] [PubMed] [Google Scholar]
- 35.Campanholle G, Ligresti G, Gharib SA, Duffield JS. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol. 2013;304:C591–C603. doi: 10.1152/ajpcell.00414.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kida Y, Duffield JS. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol. 2011;38:467–473. doi: 10.1111/j.1440-1681.2011.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson LE, Soriano P. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev Cell. 2011;20:815–826. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voisin MB, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun. 2013;5:336–347. doi: 10.1159/000346659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alon R, Nourshargh S. Learning in motion: pericytes instruct migrating innate leukocytes. Nat Immunol. 2013;14:14–15. doi: 10.1038/ni.2489. [DOI] [PubMed] [Google Scholar]
- 40.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campanholle G, Chen CL, Mittelsteadt K, Kobayashi A, Lin S-L, Gharib SA, Heinecke J, Hamerman JA, Altemeier WA, Duffield JS.Trem-1, tlr-2 and tlr-4 are dispensable in kidney macrophages PLoS ONEIn press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerlach JC, Over P, Turner ME, Thompson RL, Foka HG, Chen WC, Péault B, Gridelli B, Schmelzer E. Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev. 2012;21:3258–3269. doi: 10.1089/scd.2012.0296. [DOI] [PubMed] [Google Scholar]
- 43.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blocki A, Wang Y, Koch M, Peh P, Beyer S, Law P, Hui J, Raghunath M. Not all MSCS can act as pericytes: Functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev. 2013;22:2347–2355. doi: 10.1089/scd.2012.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]