Abstract
Rationale: Alveolar transforming growth factor (TGF)-β1 signaling and expression of TGF-β1 target genes are increased in patients with idiopathic pulmonary fibrosis (IPF) and in animal models of pulmonary fibrosis. Internalization and degradation of TGF-β receptor TβRI inhibits TGF-β signaling and could attenuate development of experimental lung fibrosis.
Objectives: To demonstrate that after experimental lung injury, human syndecan-2 confers antifibrotic effects by inhibiting TGF-β1 signaling in alveolar epithelial cells.
Methods: Microarray assays were performed to identify genes differentially expressed in alveolar macrophages of patients with IPF versus control subjects. Transgenic mice that constitutively overexpress human syndecan-2 in macrophages were developed to test the antifibrotic properties of syndecan-2. In vitro assays were performed to determine syndecan-2–dependent changes in epithelial cell TGF-β1 signaling, TGF-β1, and TβRI internalization and apoptosis. Wild-type mice were treated with recombinant human syndecan-2 during the fibrotic phase of bleomycin-induced lung injury.
Measurements and Main Results: We observed significant increases in alveolar macrophage syndecan-2 levels in patients with IPF. Macrophage-specific overexpression of human syndecan-2 in transgenic mice conferred antifibrotic effects after lung injury by inhibiting TGF-β1 signaling and downstream expression of TGF-β1 target genes, reducing extracellular matrix production and alveolar epithelial cell apoptosis. In vitro, syndecan-2 promoted caveolin-1–dependent internalization of TGF-β1 and TβRI in alveolar epithelial cells, which inhibited TGF-β1 signaling and epithelial cell apoptosis. Therapeutic administration of human syndecan-2 abrogated lung fibrosis in mice.
Conclusions: Alveolar macrophage syndecan-2 exerts antifibrotic effects by promoting caveolin-1–dependent TGF-β1 and TβRI internalization and inhibiting TGF-β1 signaling in alveolar epithelial cells. Hence, molecules that facilitate TβRI degradation via endocytosis represent potential therapies for pulmonary fibrosis.
Keywords: idiopathic pulmonary fibrosis, TGF-β1 signaling, syndecan-2, alveolar macrophage
At a Glance Commentary
Scientific Knowledge on the Subject
Transforming growth factor (TGF)-β1 plays a crucial role in alveolar tissue repair after injury. TGF-β1 signaling is increased in both patients with idiopathic pulmonary fibrosis (IPF) and animal models of pulmonary fibrosis. Syndecans, a family of transmembrane heparan-sulfate proteoglycans, have a protective role in fibrosis models in part by regulating the biologic activity of chemokines and growth factors.
What This Study Adds to the Field
The potential antifibrotic mechanisms of syndecan-2 remain unidentified. Here we demonstrate that alveolar macrophage syndecan-2 is overexpressed in patients with IPF and that syndecan-2 can attenuate experimental fibrosis by inhibiting TGF-β1 signaling.
Idiopathic pulmonary fibrosis (IPF), a debilitating chronic interstitial lung disease, affects approximately 0.05 to 0.1% of individuals aged 55 years or older, with average survival limited to 3 to 5 years (1). Lung transplantation in eligible individuals remains the only effective therapeutic option (2). The underlying mechanisms for the pathogenesis of IPF remain poorly understood, but involve aberrant fibrotic tissue repair responses, repetitive alveolar microinjury, and increased epithelial cell apoptosis, leading to ineffective reepithelialization of damaged alveolar walls (3, 4). Although epithelial–mesenchymal interactions likely represent the major pathogenic determinants of this disease, recent reports suggest that innate and adaptive immune responses can contribute to disease pathogenesis by modulating lung injury and repair processes (5–7).
Transforming growth factor-β1 (TGF-β1), a multifunctional cytokine, plays a crucial role in organ development, tissue maintenance, and tissue repair after injury (8, 9). Excessive TGF-β1 tissue expression may contribute to the pathogenesis of multiple fibrotic disorders, including IPF (8, 9). Several tissue- and cell-specific mechanisms tightly regulate TGF-β1 signaling and biological activity (10). These include activation of latent TGF-β1, modulation of intracellular signaling molecules, and regulation of cell membrane expression of TGF-β receptors (TβRI, TβRII, TβRIII) (11, 12). Regulation of TβRI and TβRII endocytosis and trafficking in turn regulates TGF-β intracellular signaling (13). Internalization of TGF-β receptors via lipid raft and/or caveolin-1–mediated endocytosis of TGF-β receptors may facilitate receptor degradation and inhibit cell signaling (14–16). Hence, strategies that promote TGF-β receptor internalization via caveolin-1–mediated endocytosis may lead to the development of new therapeutic approaches to inhibit TGF-β signaling in patients affected with fibrotic lung disorders (17, 18).
Syndecans, a family of four transmembrane heparan-sulfate proteoglycans, are expressed in multiple tissue and cell types (19, 20) and regulate diverse proteins affecting tissue injury and repair (20–24). Syndecan ectodomains, cleaved from cell membranes by proteases, bind to and modulate the activity of chemokines, growth factors, and cell surface receptors through highly glycosylated arms (13). Syndecan-1 can promote resolution of experimental lung injury through interactions with CXCL8 (20). Similarly, syndecan-4 reduces experimental lung fibrosis by inhibiting fibroblast migration via interactions with CXCL10 (22). These findings suggest a protective role for syndecans in the development of pulmonary fibrosis; however, the potential antifibrotic mechanisms of syndecan-2 remain unidentified. Here we demonstrate that alveolar macrophage syndecan-2 is overexpressed in patients with IPF and that syndecan-2 can attenuate experimental fibrosis by inhibiting TGF-β1 signaling.
Methods
For additional details regarding methods, see the online supplement.
Fiberoptic Bronchoscopy with Bronchoalveolar Lavage (Human)
Bronchoscopy with lavage as well as isolation of alveolar macrophages and peripheral blood mononuclear cells were performed as described (25).
Gene Expression Profiling and Metaanalysis
Gene expression profiles for the clinical groups were determined by using Affymetrix HG-U133A microarrays. A total of 84 arrays, including 45 normal volunteers, 16 unaffected relatives, 15 subjects with sporadic IPF, and 8 subjects with familial IPF, were analyzed. Gene expression data were submitted to the Gene Expression Omnibus (accession number GSE4907).
Generation of Human Syndecan-2 Transgenic Mouse
Syndecan-2 transgenic mice were generated using a transgene containing the human syndecan-2 (hsdc2) coding sequence under the control of the scavenger receptor A enhancer/promoter (SREP) (26–28), a kind gift from Dr. Jeanine D’Armiento (Columbia University, NY).
Hydroxyproline Assay
Left lungs were hydrolyzed in HCl 6N for 24 hours at 110°C, and hydroxyproline levels were quantified as described (29). Each sample was tested in triplicate. Data are expressed as micrograms of hydroxyproline per left lung.
Syndecan-2 Therapeutic Administration
Wild-type C57BL6 mice at 8 weeks of age were instilled by aspiration with 0.15 U/mouse of bleomycin (Tszchem, Framingham, MA) in 100 μl sterile saline at Day 0. Control animals were treated with an equal volume of sterile saline. In the treatment group, recombinant human syndecan-2 (R&D Systems) (5 μg in 100 μl of phosphate-buffered saline [PBS]) was administered at Days 14, 16, 18, and 20 post bleomycin or saline instillation. The control group was treated with an equal volume of sterile PBS. Mice were sacrificed 28 days after bleomycin or saline treatment.
Internalization of TGF-β1 and TGF-β Receptors
Method was modified from reported biochemical analysis of syndecan recycling (30). Human primary small airway epithelial cells (SAEC) were incubated with or without recombinant syndecan-2; biotinylated TGF-β1 was determined by Avidin precipitation and TGF-β1 immunoblotting. TGF-β1 and TβRI in membrane or cytoplasm were detected by immunoblotting.
Statistical Analyses
Statistical analyses were performed using a Student nonpaired t test (software Origin, version 8.5). A P value less than 0.05 was considered significant. Values are presented as means ± SD unless otherwise stated.
Results
Elevated Alveolar Macrophage and Shed Syndecan-2 Levels in IPF
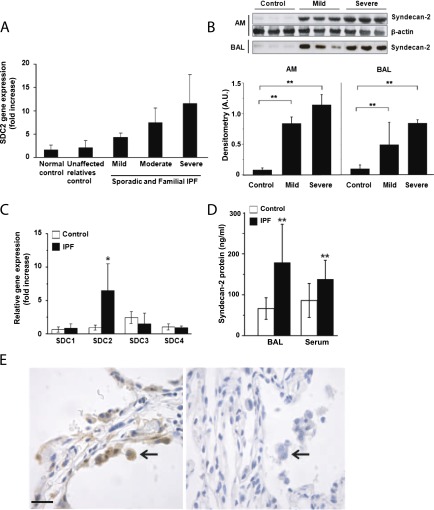
To identify genes associated with IPF, gene expression profiles for alveolar macrophages derived from subjects with sporadic and familial IPF were analyzed and compared with control subjects (see Table E1 in the online supplement). To select genes differentially expressed in human pulmonary fibrosis and not in other chronic lung diseases, we conducted a metaanalysis of alveolar macrophage gene expression profiles obtained from patients with IPF, asthma (31), and chronic obstructive lung disease (COPD) (32). Furthermore, we performed a cross-sectional analysis comparing lung function with expression levels of 84 differentially regulated genes in both sporadic IPF and familial pulmonary fibrosis (Figure E1). Syndecan-2 was up-regulated in patients with IPF, slightly increased in asthma, and not increased in COPD. Importantly, we found increases in syndecan-2 mRNA expression in subjects with mild, moderate, and severe lung diffusion abnormalities (Figure 1A). To validate the microarray findings, proteomic assays of alveolar macrophages and bronchoalveolar lavage fluid (BALF) from subjects with IPF or healthy control subjects were performed. Consistent with our gene expression data, we found significantly higher syndecan-2 protein levels in alveolar macrophages and BALF from subjects with IPF compared with control subjects (Figure 1B).
Figure 1.
Syndecan-2 up-regulation in idiopathic pulmonary fibrosis (IPF). (A) Syndecan-2 (SDC2) gene expression by microarray analysis of alveolar macrophage (AM) mRNA in sporadic and familial IPF. (B) Syndecan-2 protein levels in AMs and bronchoalveolar lavage (BAL) fluid were significantly increased in IPF compared with normal control subjects (n = 3 for each group; **P < 0.01). (C) Syndecan-2, and not syndecan-1, -3, or -4, gene expression by quantitative real-time polymerase chain reaction of alveolar macrophage mRNA was significantly increased in IPF (n = 5) compared with control subjects (n = 5) (*P < 0.05). (D) Syndecan-2 concentrations were significantly increased in BAL fluid and serum of subjects with (n = 38) compared with normal control subjects (n = 26) (**P < 0.01). (E) Syndecan-2 immunostaining of alveolar macrophages (arrows) was more intense in lung tissue sections from subjects with IPF (left panel) compared with those of control subjects (right panel). Scale bars = 20 μm.
To determine whether our findings were specific to syndecan-2, we performed quantitative real-time polymerase chain reaction assays to measure mRNA levels of syndecan-1, -2, -3, and -4 in alveolar macrophages. As expected, we found that mRNA levels of syndecan-2, but not syndecan-1, -3, or -4, were significantly increased in macrophages from patients with IPF compared with those of healthy control subjects (Figure 1C). Furthermore, we determined the syndecan-2 protein concentrations in BALF and serum from subjects with IPF using a modified human syndecan-2 ELISA. Syndecan-2 concentrations were significantly increased in BALF and serum of patients with IPF compared with normal control subjects (Figure 1D). Immunostaining of lung tissue sections revealed that syndecan-2 was highly expressed in alveolar macrophages in subjects with IPF compared with control subjects (Figure 1E). Moreover, we performed immunostaining of lung sections obtained from patients with IPF. We confirmed that syndecan-2 was robustly expressed in alveolar macrophages, in perivascular areas of CD31-positive endothelial cells, and to a lesser extent in fibroblasts and fibroblast foci of patients with IPF (Figure E2). These findings demonstrate that syndecan-2 is expressed in multiple cell types and strongly suggest that alveolar macrophages are the predominant source of this heparan-sulfate proteoglycan in patients with IPF.
Syndecan-2 is Up-regulated during Bleomycin-induced Lung Injury and Inhibits Fibrosis
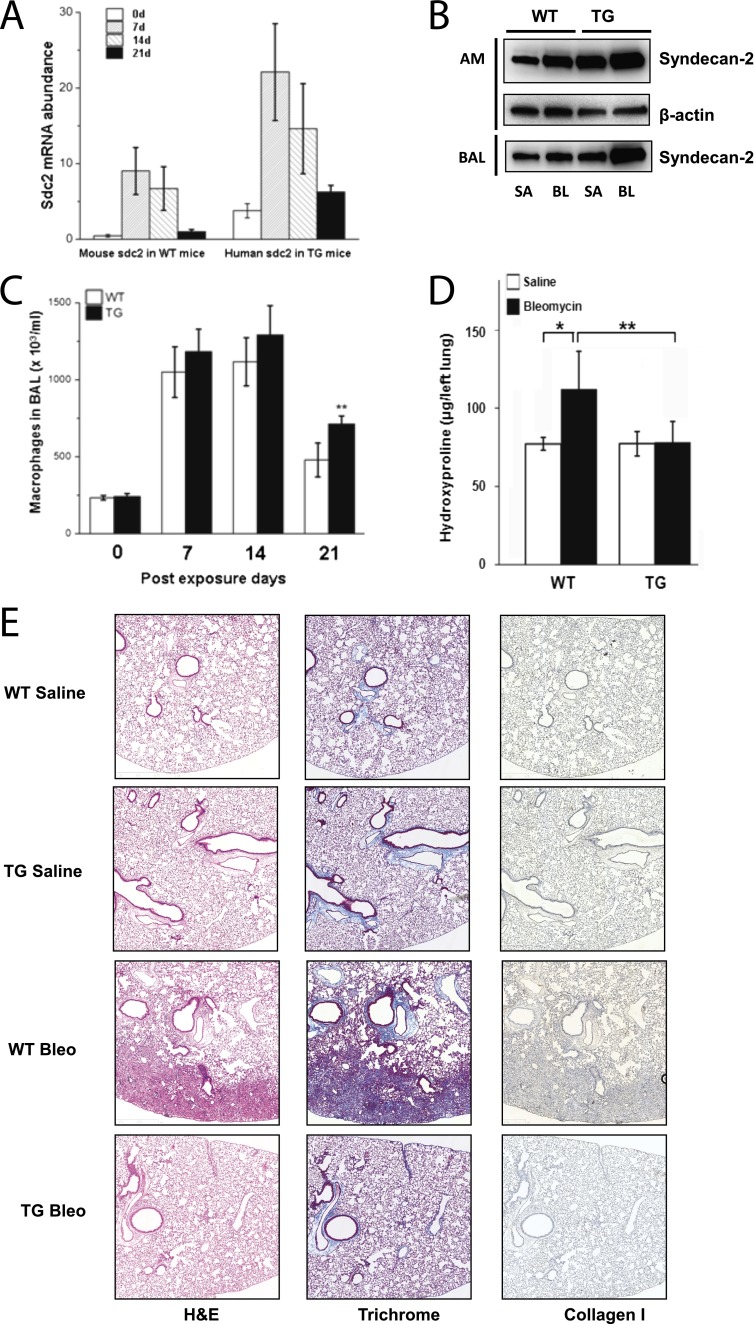
To investigate the role of syndecan-2 in pulmonary fibrosis, we generated a transgenic mouse (SREP-hsdc2) that specifically expresses human syndecan-2 in differentiated macrophages (Figure E3). Alveolar macrophages from SREP-hsdc2 transgenic or wild-type C57/BL6 mice were examined for syndecan-2 expression before and at weekly intervals after aspiration of either bleomycin or saline. We observed a significant increase in syndecan-2 mRNA levels in alveolar macrophages from transgenic and wild-type mice 7 days after bleomycin exposure (Figure 2A). The peak levels of syndecan-2 transcripts were followed by progressive decreases in syndecan-2 gene expression at Days 14 and 21. Immunoblots for total human and mouse syndecan-2 levels showed increased syndecan-2 in alveolar macrophages and BALF in wild-type and SREP-hsdc2 transgenic mice 21 days after instillation with bleomycin, when compared with saline (Figure 2B). Furthermore, to recapitulate experimental injury in vitro, bone marrow–derived macrophages were treated with TGF-β1 or bleomycin. Consistent with our findings in vivo, syndecan-2 gene expression was induced in transgenic and wild-type bone marrow–derived macrophages with either treatment (Figure E4). Taken together, these data suggest that syndecan-2 is up-regulated in alveolar macrophages in response to lung injury.
Figure 2.
Bleomycin-induced syndecan-2 expression and decreased pulmonary fibrosis in scavenger receptor A enhancer/promoter (SREP)–human syndecan-2 (hsdc2) transgenic mice. (A) Bleomycin or saline was administered to wild-type (WT) or SREP-hsdc2 transgenic (TG) mice by aspiration, and alveolar macrophages were harvested at 0, 7, 14, or 21 days post administration. Alveolar macrophage mouse syndecan-2 mRNA levels in WT mice and human syndecan-2 mRNA levels in TG mice were assessed by quantitative real-time polymerase chain reaction (n = 10). Syndecan-2 mRNA level was up-regulated by bleomycin-induced lung injury at all time points after bleomycin administration (*P < 0.05 and **P < 0.01 compared with saline control). (B) Representative Western blot analyses using anti–syndecan-2 antibody to detect total human and mouse syndecan-2 protein in alveolar macrophages (AM) and bronchoalveolar lavage (BAL) fluid showed increased syndecan-2 levels in WT and TG mice 21 days after administration of bleomycin (BL) compared with saline (SA). (C) Alveolar macrophage numbers in TG mice were significantly increased 21 days after bleomycin-induced lung injury compared with WT mice (**P < 0.01; n = 8–12). There were no significant changes in alveolar macrophages 7 and 14 days after bleomycin administration. (D) WT mice have significantly higher hydroxyproline lung levels following exposure to bleomycin compared with saline (*P = 0.02). Hydroxyproline content in the left lung 21 days after instillation of bleomycin, and not saline, was not increased in TG mice 21 days after instillation of bleomycin compared with saline and was significantly different from bleomycin-instilled WT mice (**P = 0.0016; n = 8–12). Data are representative of three independent experiments. (E) Lung sections of WT and TG mice 21 days after bleomycin administration were stained with hematoxylin and eosin (H&E), trichrome, and collagen-1a1 antibody. Pulmonary fibrosis was decreased in TG mice compared with WT control mice (scale bar = 200 μm).
To investigate the functional effect of syndecan-2, we studied in vivo responses to lung injury induced by bleomycin in SREP-hsdc2 transgenic mice. Alveolar macrophage counts in the BALF were significantly increased in SREP-hsdc2 mice compared with wild-type control mice at Day 21 after bleomycin injury, but not at Day 7 or 14 (Figure 2C). Total and differential cell counts showed similar neutrophil and lymphocyte numbers in lungs from transgenic and wild-type mice at the peak of cell influx (Day 7, Figure E5). There was a modest decrease in neutrophils but not lymphocytes at 14 days in the syndecan-2 transgenic mice, when infiltration starts to resolve. These data indicate that overexpression of syndecan-2 does not modulate inflammation in the early stages of bleomycin-induced lung injury. The increase in lung hydroxyproline content observed in wild-type mice 21 days after bleomycin instillation was completely suppressed in the SREP-hsdc2 transgenic mice (Figure 2D). Consistent with these results, lung collagen deposition and fibrosis were dramatically reduced in SREP-hsdc2 mice compared with wild-type mice after bleomycin injury, as demonstrated by trichrome and collagen I staining (Figure 2E). These data indicate that syndecan-2 decreases fibrogenesis after lung injury and that this protective effect is not due to inhibition of inflammatory cell infiltration, as the total number of both neutrophils and lymphocytes recruited during the 21-day period after injury were not significantly different between the wild-type and syndecan-2 transgenic mice.
Syndecan-2 Inhibits TGF-β Signaling and Protects Epithelial Cells from Apoptosis during Lung Injury in the Transgenic Mouse
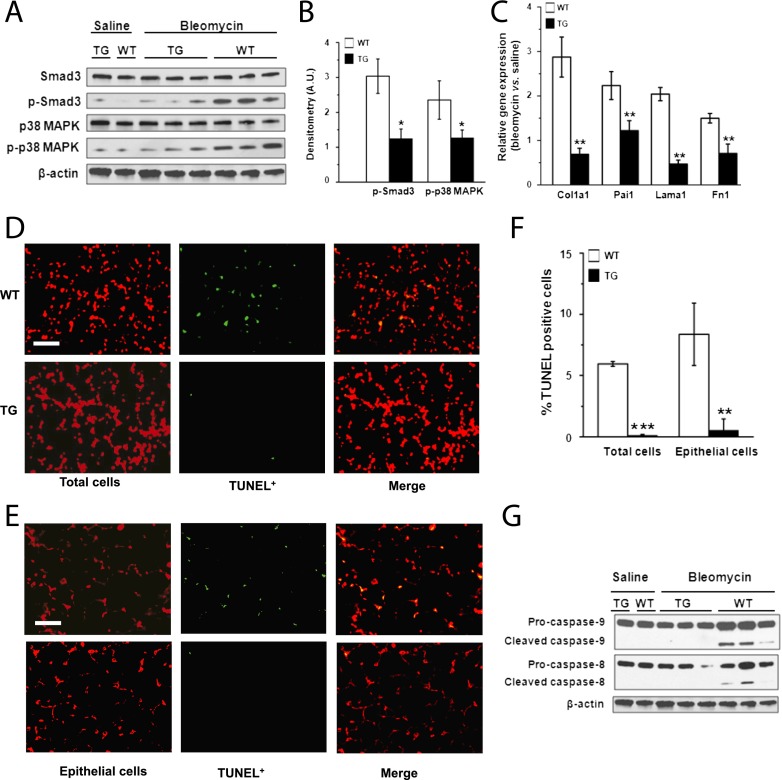
TGF-β1 contributes to the pathogenesis of lung fibrosis (2). Targeted transgenic overexpression of bioactive TGF-β1 in murine lung is sufficient to generate histopathologic findings resembling IPF (33). Thus, we examined whether TGF-β1 signaling was altered in lung tissue of SREP-hsdc2 mice compared with wild-type mice after bleomycin instillation. SMAD3 and p38 MAPK phosphorylation in the lungs from wild-type mice were significantly increased compared with the lungs of SREP-hsdc2 mice (Figures 3A and 3B). We also tested expression levels of known TGF-β1 target genes (i.e., Col1a1, Pai1, Lama1, Fn1) (34). As expected, these genes were up-regulated in wild-type mice after bleomycin injury compared with saline control mice (Figure 3C). However, the expression of these genes was significantly reduced in SREP-hsdc2 mice after bleomycin administration compared with wild-type mice. To determine if inhibition of apoptosis was associated with a relative increase in the expression of epithelial genes, EpCAM, podoplanin, and surfactant protein C (SP-C) expression was measured in transgenic and wild-type mice. After bleomycin administration, expression of Lama1, EpCAM, podoplanin, and SP-C were not significantly changed in SREP-hsdc2 mice, whereas significant changes were observed in wild-type mice (Figure E6).
Figure 3.
Inhibition of transforming growth factor (TGF)-β1 signaling and lung apoptosis in scavenger receptor A enhancer/promoter (SREP)–human syndecan-2 (hsdc2) transgenic mice. (A) Representative Western blot analyses showed reductions in phosphorylated SMAD3 and p38 MAPK in lungs of SREP-hsdc2 transgenic (TG) mice compared with wild-type (WT) mice 21 days after administration of bleomycin and not saline. (B) Densitometry shows significant reduction of phosphorylated SMAD3 and p38 MAPK in lungs from TG mice compared with WT control mice (*P = 0.02 and *P = 0.04, respectively; n = 3). (C) Gene expression measured by quantitative polymerase chain reaction of Col1a1, Pai1, Lama1, and Fn1, which are TGF-β1 target genes, was significantly down-regulated in lungs of TG mice compared with WT control mice 21 days after bleomycin administration (**P < 0.01; n = 3). (D) Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining of lung tissue 21 days after intratracheal instillation of bleomycin showed less apoptosis in TG mice compared with WT mice. Total cells were counterstained with propidium iodide (red). TUNEL-positive cells appear green or yellow (in merged images) (scale bar = 25 μm). (E) Epithelial cells in lung tissue sections were counterstained with an antibody to cytokeratin (red). Fewer TUNEL-positive cells (green or yellow [in merged images]) were found in TG mice compared with WT control mice 21 days after administration of bleomycin (scale bar = 25 μm). (F) Apoptotic cell index was calculated as the number of TUNEL-positive cells per 100 total or epithelial cells. The apoptotic cell index of total or epithelial cells was significantly reduced in lung tissue from TG mice compared with WT mice (***P < 0.001; **P = 0.002). (G) Consistent with these findings of decreased apoptosis in TG mice, Western immunoblot analyses showed reduced levels of cleaved caspase-8 and -9 in lungs from TG mice compared with WT control mice.
Given that our findings show inhibition of TGF-β1 signaling in SREP-hsdc2 transgenic mice, we hypothesized that apoptosis would be diminished in these mice. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays demonstrated significantly reduced apoptosis in total cells and epithelial cells in the SREP-hsdc2 transgenic mice compared with wild-type mice after bleomycin-induced lung injury (Figures 3D and 3F). Double labeling of cells using cytokeratin and TUNEL staining revealed that most of the apoptotic cells were epithelial cells (Figures 3E and 3F). Western immunoblot analyses of pro- and cleaved forms of caspase-8 and -9 revealed that apoptosis was activated after bleomycin instillation in the lungs of wild-type mice but not transgenic mice (Figure 3G). Taken together, these data indicate that overexpression of syndecan-2 protects epithelial cells from apoptosis induced by lung injury.
Syndecan-2 Protects Epithelial Cells from Apoptosis by Attenuating TGF-β1 Signaling
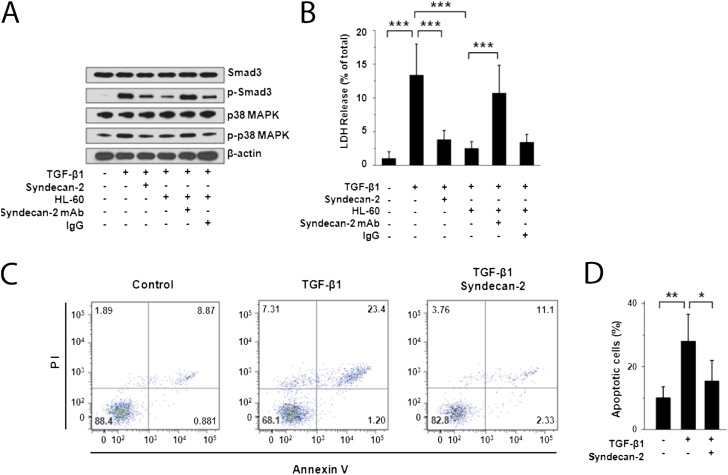
To further investigate the effects of syndecan-2 on TGF-β1 signaling and cell death in the lung epithelium, we studied human primary SAEC. We found that the TGF-β1 signaling proteins SMAD3 and p38 MAPK were expressed in SAEC stimulated with extracellular TGF-β1 (Figure 4A). However, the addition of recombinant syndecan-2 or a human monocytic cell line (i.e., HL-60 cells) down-regulated canonical TGF-β1–induced signaling. HL-60 cells were selected for coincubation, because we found that these human monocytic cells highly expressed syndecan-2 and released shed syndecan-2 after TGF-β1 stimulation (Figure E7). Coincubation with anti–syndecan-2 antibody, but not IgG control antibody, restored TGF-β1 signaling, indicating that this effect was specific to syndecan-2.
Figure 4.
Inhibition of cell death/apoptosis and transforming growth factor (TGF)-β1 signaling by recombinant syndecan-2 in small airway epithelial cells (SAEC). (A) Western immunoblot analyses of primary human SAEC stimulated with TGF-β1 (5 ng/ml) for 2 hours and incubated with recombinant human syndecan-2 demonstrated reduced phosphorylation of SMAD3 and p38 MAPK compared with SAEC that were not incubated with syndecan-2. Phosphorylation of SMAD3 and p38 MAPK was also reduced in SAEC stimulated with TGF-β1 and cocultured with HL-60 monocytic cells, which secrete syndecan-2. This inhibitory effect on SMAD3 and p38 MAPK phosphorylation was lost when these cells were incubated with anti–syndecan-2 antibody but not with an IgG control antibody. (B) Cell death measured by lactate dehydrogenase (LDH) release of SAEC stimulated with TGF-β1 (5 ng/ml) for 48 hours and incubated with recombinant syndecan-2 or HL-60 cells was significantly reduced compared with cells incubated without syndecan-2 or HL-60 cells (***P < 0.001). This inhibitory effect on cell death was reversed when these cells were incubated with anti–syndecan-2 antibody, but not with an IgG control antibody. LDH release assays are representative of at least three independent experiments performed in triplicate. (C) SAEC stimulated with TGF-β1 (5 ng/ml) were coincubated with or without recombinant syndecan-2 for 48 hours and stained with Annexin V-FITC/PI. Flow cytometry showed live cells in the lower left quadrant, early apoptotic cells in the lower right quadrant, late apoptotic cells in the upper right quadrant, and necrotic cells in the upper left quadrant. Data shown were representative of three independent experiments. (D) Percentage of apoptotic SAEC stimulated with TGF-β1 alone (28.1 ± 8.5%) was significantly higher than that of TGF-β1–stimulated cells treated with recombinant syndecan-2 (15.4 ± 6.5%; *P = 0.03) or unstimulated SAEC (10.2 ± 4.2%; **P = 0.009).
Given our findings that syndecan-2 has antiapoptotic activity in vivo, we assessed the effects of syndecan-2 on cell death in vitro by measuring lactate dehydrogenase (LDH) release by SAEC exposed to TGF-β1. We found that TGF-β1 stimulation induced death of SAEC and that coincubation of SAEC with recombinant syndecan-2 or with HL-60 cells conferred cytoprotection (Figure 4B). The addition of anti–syndecan-2 monoclonal antibody, but not an IgG control antibody, blocked the cytoprotective effect of HL-60 cell coculture. Furthermore, flow cytometry showed that annexin-V expression was significantly increased in SAEC incubated with TGF-β1 for 2 days compared with control subjects (Figures 4C and 4D). However, annexin-V expression was significantly reduced after coincubation with recombinant syndecan-2. These findings demonstrate that syndecan-2 inhibits TGF-β1 signaling and protects epithelial cells from TGF-β1–induced apoptosis and cell death.
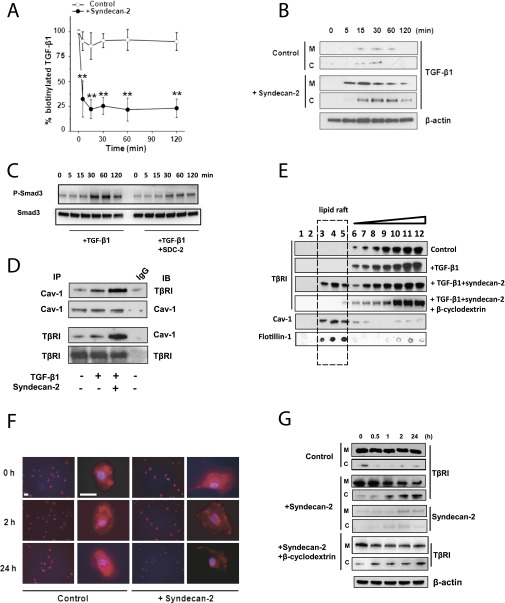
Syndecan-2 Blocks TGF-β1 Signaling by Enhancing Internalization and Degradation of TGF-β1 and TβRI via Caveolae
To evaluate the mechanism by which syndecan-2 inhibited TGF-β1 signaling, we analyzed potential protein interactions between syndecan-2 and TGF-β1 ligand or TGF-β receptors (i.e., TβRI, TβRII, TβRIII). The total amount of biotin-labeled TGF-β1 in cell culture medium rapidly and significantly decreased when SAEC were treated with syndecan-2 compared with untreated cells (Figure 5A). Immunoblotting for biotin-labeled TGF-β1 in the cell pellet demonstrated increased levels of TGF-β1 in the cytosolic fractions beginning 15 minutes after treatment with syndecan-2 (Figure 5B). These data suggest that internalization of TGF-β1 was enhanced by syndecan-2. Moreover, internalization of TGF-β1 ligand in the presence of recombinant syndecan-2 was associated with inhibition of SMAD3 phosphorylation, which occurs within minutes (Figure 5C).
Figure 5.
Syndecan-2 enhances transforming growth factor (TGF) β receptor (TβRI) and ligand’s internalization via caveolae pathway. (A) Small airway epithelial cells (SAEC) were cultured with and without syndecan-2 (Control); biotinylated TGF-β1 was added to the medium and measured at different time points. Approximately 25% of total labeled TGF-β1 remained in the medium after 15 minutes’ incubation with syndecan-2 compared with approximately 80% in control. (B) SAEC lysates of cells incubated with and without syndecan-2 were separated into membrane (M) and cytosolic (C) fractions, and biotinylated TGF-β1 was precipitated by Avidin beads. Immunoblots using anti–TGF-β1 antibody showed internalization of TGF-β1 after 15 minutes of incubation with syndecan-2. (C) SAEC were treated with TGF-β1 alone or TGF-β1 with syndecan-2 for 0 to 120 minutes, then phosphorylation of SMAD3 in cell lysates was measured by immunoblotting. (D) Coimmunoprecipitation showed increased interaction between Caveolin-1 (Cav-1) and TβRI when SAEC were incubated with recombinant syndecan-2 and TGF-β1 compared with cells incubated with TGF-β1 alone. (E) SAEC (Control) or SAEC incubated with TGF-β1 with or without syndecan-2 were solubilized, and the lipid raft/caveolae fraction was separated by sucrose gradient centrifugation. Western immunoblot analyses of TβRI, caveolin-1 (Cav-1), and flotillin-1 in 12 cell fractions from SAEC showed that syndecan-2 incubation caused a shift of TβRI from non–lipid raft fractions to the lipid raft fractions. Adding 10 mM β-cyclodextrin to disrupt lipid raft–caveolin internalization inhibited the shift of TβRI induced by syndecan-2. (F) SAEC incubated with or without syndecan-2 were stimulated with 10 ng/ml of TGF-β1 for 0, 2, or 24 hours. Cells were stained with anti-TβRI antibody (red) and DAPI (blue). Immunostaining of TβRI in SAEC was decreased after 2 or 24 hours in the presence of syndecan-2 (scale bar = 10 µm). (G) Immunoblot using anti-TβR1 antibody is shown of membrane (M) and cytosolic (C) fractions of SAEC stimulated with TGF-β1 with or without syndecan-2 (Control) for 0, 0.5, 1, 2, and 24 hours. TβRI in SAEC was expressed predominantly in the membrane (M) fraction. However, TβRI internalized from the membrane to cytosolic fraction in SAEC incubated for 1 hour with syndecan-2. Recombinant syndecan-2 was traced by biotinylation. In both membrane and cytosolic fractions, biotinylated syndecan-2 was precipitated by Avidin beads and immunoblotted with syndecan-2 antibody. The blot shows that syndecan-2 was also internalized from the membrane to the cytosolic fraction. The addition of 10 mM β-cyclodextrin inhibited the internalization of TβRI induced by syndecan-2.
Coimmunoprecipitation assays showed that TβRI, but not TβRII or TβRIII, strongly interacted with syndecan-2 in SAEC (Figure E8). TGF-β activity can be enhanced or inhibited by internalization of TGF-β receptors via clathrin-coated or lipid raft–mediated endocytosis of TGF-β receptors (16, 18, 35). We therefore studied the effects of syndecan-2 on the interaction between TβRI and caveolin-1. Binding between TβRI and caveolin-1 was robustly increased in SAEC stimulated with TGF-β1 and syndecan-2 compared with cells cultured without syndecan-2 (Figure 5D). Moreover, cell fractionation assays showed that TβRI localized to non–lipid-raft fractions in SAEC stimulated with TGF-β1. However, the distribution of TβRI shifted from non–lipid rafts to lipid-raft fractions when SAEC were coincubated with recombinant syndecan-2 (Figure 5E). β-Cyclodextrin, a lipid-raft chemical inhibitor, decreased syndecan-2–dependent receptor internalization in SAEC stimulated with TGF-β1, suggesting that TβRI is internalized via caveolin-rich lipid rafts. Furthermore, immunostaining for TβRI was decreased in SAEC stimulated with TGF-β1 and treated with recombinant syndecan-2 compared with untreated cells (Figure 5F). Western immunoblot analyses of serial samples of membrane and cytosolic fractions from SAEC stimulated with TGF-β1 and treated with syndecan-2 showed progressive increase in TβRI cytoplasmic levels and a corresponding decrease in membrane expression beginning within 1 hour of syndecan-2 exposure (Figure 5G). In contrast, TβRI localized mostly to the membrane in untreated SAEC stimulated with TGF-β1.
As described for TGF-β1 and TβRI SAEC internalization, there was a progressive increase in biotin-labeled syndecan-2 cytoplasmic levels and a corresponding decrease in membrane levels (Figure 5G). These findings suggest that syndecan-2 was internalized with both TGF-β ligand and receptor via caveolin-rich lipid rafts. Syndecan-2 did not lead to changes in TGF-β1 signaling or promote internalization of TGF-β1 and TβRI in human or mouse primary lung fibroblasts (Figure E9). These findings suggest that the syndecan-2 biological effects described above were cell specific.
Administration of Recombinant Human Syndecan-2 Rescues Bleomycin-induced Pulmonary Fibrosis
Overexpression of TGF-β1 in vivo leads to progressive fibrosis in the lung and other organs (8, 36, 37). Given the observed inhibitory effect of syndecan-2 on TGF-β1 signaling and on the development of lung fibrosis in syndecan-2 transgenic mice, we investigated whether treatment with exogenous syndecan-2 would have a therapeutic effect on established pulmonary fibrosis. We studied murine lung tissue specimens 14 days after instillation of bleomycin, because this stage of bleomycin-induced lung disease is characterized by pulmonary fibrosis. Four doses of recombinant syndecan-2 treatment were administered by aspiration into the lungs on Days 14, 16, 18, and 20 post-bleomycin exposure, and lung tissue was harvested for analysis on Day 28 (Figure 6A). We found that lung hydroxyproline content was significantly decreased in mice treated with syndecan-2 compared with untreated mice (Figure 6B). Moreover, the expression levels of three ECM remodeling genes, which were up-regulated in wild-type mice after bleomycin injury, were also significantly reduced in the syndecan-2–treated animals compared with untreated mice (Figure 6C). Consistently, trichrome and collagen I staining revealed dramatic reductions in lung collagen deposits in syndecan-2–treated mice compared with control animals (Figure 6C). Immunoblots and TUNEL assays confirmed that treatment with syndecan-2 significantly suppressed phosphorylation of SMAD3, cleavage of caspase-8, and apoptosis in the lung (Figure E10).
Figure 6.
Recombinant syndecan-2 attenuates bleomycin-induced pulmonary fibrosis in fibrotic phase. (A) After instillation of bleomycin, recombinant syndecan-2 was administered at Day 14, 16, 18, and 20 to wild-type mice by aspiration. (B) Hydroxyproline content in the right lung was measured at Day 28 post-bleomycin exposure (n = 8–10). The syndecan-2–treated group displayed significantly less fibrosis compared with the untreated group after bleomycin exposure (n = 8–10; *P < 0.05, ***P < 0.001). (C) Expression of transforming growth factor (TGF)-β1 target genes by quantitative polymerase chain reaction was significantly down-regulated in lungs from syndecan-2–treated mice after instillation of bleomycin compared with saline-treated mice (n = 5; **P < 0.01). (D) Representative lung sections from control (saline), untreated (bleomycin without syndecan-2), or treated group (bleomycin with syndecan-2) 28 days after bleomycin administration were stained with hematoxylin and eosin (H&E), Trichrome, and anti-collagen1a1 antibody. Pulmonary fibrosis was decreased in mice treated with syndecan-2 compared with untreated mice (scale bar = 200 μm).
Discussion
In the current study, we identified increased levels of alveolar macrophage syndecan-2 in patients with IPF and describe novel roles for syndecan-2 in inhibiting alveolar TGF-β1 signaling and promoting the resolution of fibrosis. We demonstrate that syndecan-2 is predominantly expressed in alveolar macrophages, although macrophage staining may represent uptake as well as production of syndecan-2. Expression of syndecan-2 is also observed in CD-31–positive perivascular areas and to a lesser extent in fibroblasts (38); hence, increases in syndecan-2 in BALF and peripheral blood of patients with IPF are likely derived from multiple cell sources. Using gain-of-function experiments, we demonstrate that syndecan-2 inhibits TGF-β1 signaling and abrogates experimental fibrosis. Importantly, our findings suggest that therapeutic administration of syndecan-2, or a small molecule mimetic that exerts similar biological actions, may represent a novel therapy to treat human pulmonary fibrosis. However, potential prooncogenic effects of syndecan-2 must be considered, as increased syndecan-2 expression has been reported in multiple solid organ cancers (39, 40).
Our findings demonstrate a cell surface interaction between TβRI and caveolin-1 that occurs in the presence of TGF-β1 ligand and is augmented by recombinant syndecan-2. Furthermore, we demonstrate that syndecan-2 promotes trafficking of the TβRI from a primarily plasma membrane nonraft localization, to the caveolin-1–rich lipid-raft fraction. Syndecan-2 increases caveolin-1–dependent TGF-β1 and TβRI internalization and degradation, inhibits TGF-β1 signaling, and reduces apoptosis in human primary small airway epithelial cells. Consistently, a prior report has shown that CD109 promotes localization of the TGF-β receptors to caveolae and facilitates TGF-β receptor degradation. Importantly, the authors found significant cell-dependent differences in the rate of receptor endocytosis (41). In contrast, a study in renal fibroblasts demonstrates that syndecan-2 interacts with TβRIll, resulting in increased signaling and TGF-β–mediated matrix deposition in vitro (42). These differences outline two potential diverging roles for syndecan-2 in modulating cell surface receptor function. Importantly, our findings also suggest that syndecan-2 inhibits TGF-β1 signaling by competing with binding of TGF-β1 ligand to its receptor. Hence, we propose that syndecan-2 exerts antifibrotic effects through several mechanisms. For instance, alveolar macrophages can promote resolution of lung injury by enhancing the clearance of apoptotic bodies (43), which suggests that recruitment of alveolar macrophages observed in syndecan-2 transgenic mice during the late phase of bleomycin-induced pulmonary fibrosis could enhance efferocytosis (44). Increased clearance of apoptotic cells by alveolar macrophages, and possibly by epithelial cells (efferocytosis), would favor alveolar repair over induction of fibrosis. Additional studies are required to define these and other alternative protective mechanisms and their individual contributions to the in vivo antifibrotic effects of syndecan-2.
We have shown that human monocyte/macrophage syndecan-2 expression in vitro is induced by TGF-β1; however, little is known regarding the regulation of human alveolar macrophage syndecan-2 expression. Bleomycin administration in vivo was followed by increases in syndecan-2 expression for both the endogenous mouse gene and the human syndecan-2 transgene. SREP used to drive transcription of the human transgene is stimulated by bleomycin (45); bleomycin administration or treatment with recombinant TGF-β1 in vitro increased syndecan-2 expression in human monocyte/macrophages. Although these findings suggest that increased alveolar TGF-β1 signaling up-regulates syndecan-2 expression, additional studies are required to confirm that human alveolar macrophage syndecan-2 is targeted, transcriptionally and/or post-transcriptionally, by TGF-β1. Our study demonstrates that shed syndecan-2 inhibits epithelial TGF-β1 signaling; however, the lung proteases that cleave syndecan-2 are unknown. We have previously shown that matrilysin (MMP7) levels are increased in lung, BALF, and peripheral blood of patients with IPF (46), and others have shown that MMP7 can cleave syndecan-1 and syndecan-2 ectodomains (21, 47, 48). Because many lung proteases are both expressed in IPF and regulated by TGF-β1 (49), it is plausible that proteolytic enzymes expressed in the lung of patients with IPF shed alveolar macrophage syndecan-2. These findings suggest that alveolar TGF-β1 signaling leads to overexpression and shedding of syndecan-2, which promotes caveolin-1–mediated endocytosis of TGF-β1 and TβRI that inhibits alveolar epithelial cell TGF-β1 signaling. Similar TGF-β1 autoregulatory loops that contribute to tissue homeostasis have been reported (50). Additional studies will define the transcriptional and post-translational regulation of syndecan-2 in the alveolar microenvironment and how this modulates TGF-β1 signaling and pulmonary fibrosis.
In conclusion, our findings suggest that in IPF, shed alveolar macrophage syndecan-2 exerts antifibrotic effects by inhibiting TGF-β1 signaling. Our preclinical findings suggest that inhibiting TGF-β1 signaling by promoting caveolin-1–dependent TGF-β1 and TβRI internalization and degradation may represent a novel therapeutic approach to treat human pulmonary fibrosis.
Acknowledgments
Acknowledgment
The authors thank Sandra MacDonald, Hai Ping Wu, Ping Ren, and Gustavo Pacheco for their contributions. They also thank Dr. Jeanine D’Armiento (University of Columbia) for kindly providing the alveolar macrophage–specific SREP promoter construct and Dr. Steven D. Nathan (INOVA Fairfax Hospital) for enabling the procurement of human IPF tissue samples. Finally, the authors thank Drs. Augustine M. K. Choi, Joel Moss, and William A. Gahl for scientific guidance and critical review of the manuscript. This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
Funded by National Heart, Lung, and Blood Institute grant K23HL087030 and Lovelace Respiratory Research Institute Fibrosis Program.
Author Contributions: All authors significantly contributed to this work. Yuanyuan Shi, B.R.G., and I.O.R. wrote the first draft of the manuscript. Ying Shi, B.R.G., G.Y., I.E.F., X.T., and I.O.R. designed, performed, and analyzed experimental assays. B.R.G., N.K., E.M.B., and I.O.R. designed, performed, and analyzed microarray assays. C.F.R., A.S.P., J.A.H., A.J.G., J.C.O., and D.M. assisted with development of cell-based assays and mouse experiments. S.W.R. and M.G.W provided technical support and assisted in manuscript preparation. I.O.R. had primary access to all data and supervised all study aspects.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201303-0434OC on August 7, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keating D, Levvey B, Kotsimbos T, Whitford H, Westall G, Williams T, Snell G. Lung transplantation in pulmonary fibrosis: challenging early outcomes counterbalanced by surprisingly good outcomes beyond 15 years. Transplant Proc. 2009;41:289–291. doi: 10.1016/j.transproceed.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 4.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontenot AP, Canavera SJ, Gharavi L, Newman LS, Kotzin BL. Target organ localization of memory CD4(+) T cells in patients with chronic beryllium disease. J Clin Invest. 2002;110:1473–1482. doi: 10.1172/JCI15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feghali-Bostwick CA, Tsai CG, Valentine VG, Kantrow S, Stoner MW, Pilewski JM, Gadgil A, George MP, Gibson KF, Choi AM, et al. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol. 2007;179:2592–2599. doi: 10.4049/jimmunol.179.4.2592. [DOI] [PubMed] [Google Scholar]
- 7.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, McCoy JP, Jr, May RM, Wu HP, Nguyen DM, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 10.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 11.Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Park JS, Kim JH, Jung SM, Lee JY, Kim SJ, Park SH. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling. Nat Commun. 2011;2:460. doi: 10.1038/ncomms1469. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, Li A, Lombardi V, Akbari O, Borok Z, et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 15.Schmierer BM, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Wang Q, Du J, Luo S, Xia J, Chen YG. PICK1 promotes caveolin-dependent degradation of TGF-β type I receptor. Cell Res. 2012;22:1467–1478. doi: 10.1038/cr.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CL, Huang SS, Huang JS. Cellular heparan sulfate negatively modulates transforming growth factor-beta1 (TGF-beta1) responsiveness in epithelial cells. J Biol Chem. 2006;281:11506–11514. doi: 10.1074/jbc.M512821200. [DOI] [PubMed] [Google Scholar]
- 18.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 19.Woods A. Syndecans: transmembrane modulators of adhesion and matrix assembly. J Clin Invest. 2001;107:935–941. doi: 10.1172/JCI12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 22.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J, Wang J, Li R, Dai Q, Yong Y, Zong B, Xu Y, Li E, Ferro A, Xu B. Syndecan-4 over-expression preserves cardiac function in a rat model of myocardial infarction. J Mol Cell Cardiol. 2012;53:250–258. doi: 10.1016/j.yjmcc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Ren P, Rosas IO, Macdonald SD, Wu HP, Billings EM, Gochuico BR. Impairment of alveolar macrophage transcription in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;175:1151–1157. doi: 10.1164/rccm.200607-958OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvai A, Palinski W, Wu H, Moulton KS, Kalla K, Glass CK. Scavenger receptor A gene regulatory elements target gene expression to macrophages and to foam cells of atherosclerotic lesions. Proc Natl Acad Sci USA. 1995;92:5391–5395. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemaître V, O’Byrne TK, Dalal SS, Tall AR, D’Armiento JM. Macrophage-specific expression of human collagenase (MMP-1) in transgenic mice. Ann N Y Acad Sci. 1999;878:736–739. doi: 10.1111/j.1749-6632.1999.tb07776.x. [DOI] [PubMed] [Google Scholar]
- 28.Lemaître V, O’Byrne TK, Borczuk AC, Okada Y, Tall AR, D’Armiento J. ApoE knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J Clin Invest. 2001;107:1227–1234. doi: 10.1172/JCI9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cisneros-Lira J, Gaxiola M, Ramos C, Selman M, Pardo A. Cigarette smoke exposure potentiates bleomycin-induced lung fibrosis in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2003;285:L949–L956. doi: 10.1152/ajplung.00074.2003. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N’Kuli F, Courtoy PJ, David G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9:377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Woodruff PG, Koth LL, Yang YH, Rodriguez MW, Favoreto S, Dolganov GM, Paquet AC, Erle DJ. A distinctive alveolar macrophage activation state induced by cigarette smoking. Am J Respir Crit Care Med. 2005;172:1383–1392. doi: 10.1164/rccm.200505-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaykhiev R, Krause A, Salit J, Strulovici-Barel Y, Harvey BG, O’Connor TP, Crystal RG. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J Immunol. 2009;183:2867–2883. doi: 10.4049/jimmunol.0900473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulichino AM, Wang IM, Caron A, Mortimer J, Auger A, Boie Y, Elias JA, Kartono A, Xu L, Menetski J, et al. Identification of transforming growth factor beta1-driven genetic programs of acute lung fibrosis. Am J Respir Cell Mol Biol. 2008;39:324–336. doi: 10.1165/rcmb.2007-0186OC. [DOI] [PubMed] [Google Scholar]
- 35.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 36.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest. 1997;100:2697–2713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz XD, Mlakar LR, Yamaguchi Y, Su Y, Larregina AT, Pilewski JM, Feghali-Bostwick CA. Syndecan-2 is a novel target of insulin-like growth factor binding protein-3 and is over-expressed in fibrosis. PLoS ONE. 2012;7:e43049. doi: 10.1371/journal.pone.0043049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popović A, Demirović A, Spajić B, Stimac G, Kruslin B, Tomas D. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:78–82. doi: 10.1038/pcan.2009.43. [DOI] [PubMed] [Google Scholar]
- 40.Park H, Kim Y, Lim Y, Han I, Oh ES. Syndecan-2 mediates adhesion and proliferation of colon carcinoma cells. J Biol Chem. 2002;277:29730–29736. doi: 10.1074/jbc.M202435200. [DOI] [PubMed] [Google Scholar]
- 41.Bizet AA, Liu K, Tran-Khanh N, Saksena A, Vorstenbosch J, Finnson KW, Buschmann MD, Philip A. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochim Biophys Acta. 2011;1813:742–753. doi: 10.1016/j.bbamcr.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Klass C, Woods A. Syndecan-2 regulates transforming growth factor-beta signaling. J Biol Chem. 2004;279:15715–15718. doi: 10.1074/jbc.C300430200. [DOI] [PubMed] [Google Scholar]
- 43.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol. 2003;81:289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 44.Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184:569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 45.Cabrera S, Gaxiola M, Arreola JL, Ramírez R, Jara P, D’Armiento J, Richards T, Selman M, Pardo A. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int J Biochem Cell Biol. 2007;39:2324–2338. doi: 10.1016/j.biocel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 46.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryu HY, Lee J, Yang S, Park H, Choi S, Jung KC, Lee ST, Seong JK, Han IO, Oh ES. Syndecan-2 functions as a docking receptor for pro-matrix metalloproteinase-7 in human colon cancer cells. J Biol Chem. 2009;284:35692–35701. doi: 10.1074/jbc.M109.054254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi S, Kim JY, Park JH, Lee ST, Han IO, Oh ES. The matrix metalloproteinase-7 regulates the extracellular shedding of syndecan-2 from colon cancer cells. Biochem Biophys Res Commun. 2012;417:1260–1264. doi: 10.1016/j.bbrc.2011.12.120. [DOI] [PubMed] [Google Scholar]
- 49.Pardo A, Selman M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc. 2006;3:383–388. doi: 10.1513/pats.200601-012TK. [DOI] [PubMed] [Google Scholar]
- 50.Briones-Orta MA, Tecalco-Cruz AC, Sosa-Garrocho M, Caligaris C, Macías-Silva M. Inhibitory Smad7: emerging roles in health and disease. Curr Mol Pharmacol. 2011;4:141–153. [PubMed] [Google Scholar]
- 51.Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 52.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]