Abstract
The extracellular matrix (ECM) plays an important role in determining cell and organ function: (1) it is an organizing substrate that provides tissue tensile strength; (2) it anchors cells and influences cell morphology and function via interaction with cell surface receptors; and (3) it is a reservoir for growth factors. Alterations in the content and the composition of the ECM determine its physical and biological properties, including strength and susceptibility to degradation. The ECM components themselves also harbor cryptic matrikines, which when exposed by conformational change or proteolysis have potent effects on cell function, including stimulating the production of cytokines and matrix metalloproteinases (MMPs). Collectively, these properties of the ECM reflect a dynamic tissue component that influences both tissue form and function. This review illustrates how defects in ECM synthesis and metabolism and the physiological process of ECM turnover contribute to changes in the fetal membranes that precede normal parturition and contribute to the pathological events leading to preterm premature rupture of membranes (PPROM).
Keywords: extracellular matrix, PPROM, matrix metalloproteinase, collagens, fibronectin, amnion, chorion
Introduction
Reproduction requires dramatic remodeling of tissues associated with critical events in procreation including ovulation, menstruation, implantation, placentation, parturition, uterine, and cervical repair following delivery. The remodeling and repair processes affect both cells and the surrounding extracellular matrix (ECM). Dynamic changes in the ECM result in alterations in the composition of the stroma, which in turn can influence cellular function mediated through membrane receptors that recognize specific ECM components, or through the sequestration or release of growth factors, particularly members of the transforming growth factor (TGF) family, which are known to play key roles in reproduction.1–3 The unique attributes of major ECM proteins also influence the stability of the ECM, and therefore can be a factor leading to adverse events including abnormal placentation, preterm premature rupture of membranes (PPROM), cervical insufficiency, uterine rupture, and pelvic organ prolapse.4–10 Extracellular matrix metabolism also plays an important role in other pathological conditions of the reproductive tract including uterine fibroids, endometriosis, and gynecologic malignancies.2,3,11–13
The synthesis and catabolism of ECM are tightly controlled through growth factors and cytokines, as well as through the expression of chaperone proteins, and activators and inhibitors of proteolytic enzymes.14–17 When turnover of ECM occurs, besides removal of specific components, enzymes that catabolize ECM expose or release bioactive fragments that have actions that are distinct from the parent proteins, and influence cells via different signaling pathways than those used by the parent molecule.15,17 This review describes recent advances in the understanding of ECM structure and metabolism that are relevant to the process of normal and abnormal parturition, focusing on fetal membrane rupture, which is a leading cause of preterm birth.5
Collagen Composition and Structure
There are 28 different types of collagen that share common features in that they are trimeric molecules composed of α chains encoded by distinct genes.15,18,19 At least 1 trimer region of the α chains consists of a triple helical structure, resulting from repeating triplets in the α chains containing glycine at every third residue. Triple helical regions of the collagen molecules are flanked by noncollagenous (NC) domains that lack the glycine-containing tripeptides. The NC domains of several collagens contain “cryptic” signaling molecules that are released upon proteolysis. The investigation of the different types of collagens present in reproductive tissues, particularly those which undergo extensive remodeling, and in which tissue integrity is important for normal reproduction, has been relatively limited.
The fibrillar collagens (types I, II, III, V, XXIV, and XXVII) differ with respect to their susceptibility to cleavage by matrix metalloproteinases (MMPs).15,18,19 For example, type III collagen is more efficiently cleaved by MMP-1 than type I collagen. Type V collagen is resistant to most collagenases, except the gelatinases MMP-2 and MMP-9 and a limited number of other enzymes.
The trimeric collagen molecules can be composed of 3 identical α chains as in the case of type II collagen, or they can be heterotrimeric as in the case of types I and V collagens. The composition of the α chains has a profound influence on the properties of the collagen molecules. An example of how α chain composition influences collagen stability is found in type I collagen, which is composed of 2 different α chains, α1(I) and α2(I). Homotrimers of α1(I) have been identified in animal fetal tissue, fibrotic tissue, and cancers.20This form of type I collagen is resistant to degradation by MMP-1 compared to the type I collagen α1(I)/α2(I) heterotrimer. The relative resistance to MMP-1 degradation appears to be due to greater stability and less efficient unwinding of the homotrimer triple helix.
The fibrillar collagen molecules are assembled into fibrils, which are responsible for the tensile strength of tissues.15,18,19 The size of the fibrils is determined by the actions of type V collagen, which copolymerizes with types I and III collagens and proteoglycan molecules. Other collagens associate with these fibrils (types IX, XII, and XIV collagens), and others form networks (types IV, VI, VII, VIII, and X collagens) in basement membranes. Some collagens are transmembrane molecules (types XIII, XVII, XXIII, and XXV collagens), which can be shed from the cell surface as soluble forms.
Extracellular matrix proteins influence cell morphology behavior by signaling through cell surface receptors. “Matrikine” subdomains in these proteins function in the native ECM molecules or they are exposed (cryptic matrikines or matricryptins) by a conformational change or released as a result of proteolytic processing, mainly by MMPs.15,17 The prototypic cryptic matrikines are derived from the NC domains (NC1 domains) of collagen IV α1(IV)/α2(IV)/α3(IV) chains (arresten, canstatin, and tumstatin, respectively), collagen XV (restin), and collagen XVIII (endostatin).
ECM Structure and Metabolism in Fetal Membranes
It has been hypothesized that fetal membrane rupture involves a sequence of events that starts with distension and loss of elasticity, separation of the chorion and amnion, disruption of the chorion, distension and herniation of the amnion, and finally amnion rupture.21 This proposed sequence of events appears to be the result of structural alterations in ECM with resulting biomechanical changes in the membranes, primarily the amnion, which is the strongest component of the fetal membranes.5,22Since cervical insufficiency is often associated with PPROM, it is likely that preterm cervical changes facilitate the unscheduled rupture of membranes in PPROM.
Composition of the Fetal Membrane ECM
The human fetal membranes are one of the better studied reproductive tissues with respect to ECM composition. The content of the ECM molecules in the membranes is determined by the rates of synthesis and deposition and rates of degradation. Despite the large number of ECM molecules that are known, existing studies have focused mainly on the common fibrillar and basement membrane collagens (types I, II, III, IV, and V) and well-known ECM molecules such as fibronectin, hyaluronan, biglycan, and decorin. Other components important to ECM structure, like tenascin-c, the matrilins, and osteonectin/SPARC, have been sparingly studied.
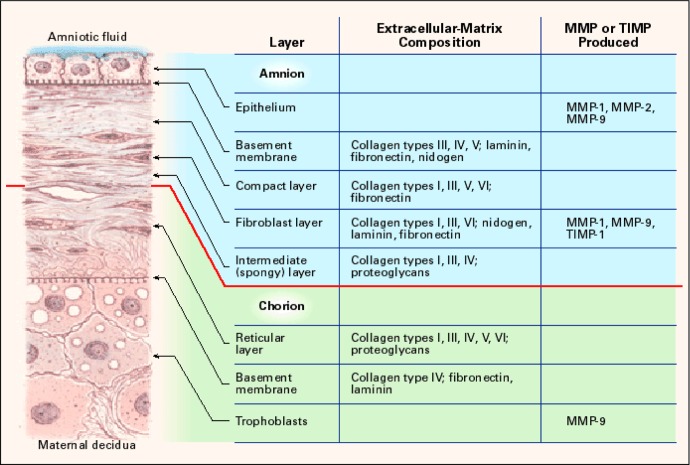
The fetal membranes are a viscoelastic avascular tissue comprising several morphologically distinct layers (Figure 1).5 The layer nearest the fetus is composed of amnion epithelial cells which rest on a basement membrane containing IV collagen and NC glycoproteins. Beneath the basement membrane lies the compact layer composed of types I, III, and V collagens secreted by the mesenchymal cells in the fibroblast layer. The spongy layer lies below the fibroblast layer, consisting of proteoglycans and glycoproteins and type III collagen. It separates the amnion from the chorion and allows the amnion to slide on the underlying chorion. The chorion layer contains cytotrophoblasts embedded in a matrix of types IV and V collagens. It firmly adheres to the uterine decidual tissue.
Figure 1.
Structure of the human fetal membranes and localization of ECM components and matrix degrading enzymes (MMP) and inhibitors (TIMP). ECM indicates extracellular matrix; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase. Reprinted from Parry and Strauss.5
The biomechanical properties of the fetal membranes have been characterized, providing an opportunity to relate biochemical composition to function.23–27 The biomechanical parameters examined include testing of tensile strength by pulling the membranes apart while monitoring forces and strains; characterizing burst, by applying increasing pressure to a piece of membranes until the tissue fails; and puncture testing, in which a probe displaces the central portion of a membrane, while force is quantified.
The distribution of ECM components, including the collagen types I, III, IV, V, and VI, in term human fetal membranes has been examined by immunohistochemistry techniques.28,29 Types I and III collagens were found in most of the layers of fetal membranes, except in the chorion trophoblast layer. Fibronectin, laminin, and types I and IV collagens are located in a network of ECM which encapsulates cells of the cytotrophoblasts in the chorion. Type V collagen was found in the reticular and in the trophoblast layers as well. Type VI collagen was mainly found in the amnion and the reticular layer. Fibulins 1, 3, and 5 were found in the amnion, and their abundance is reduced in amnion weak zone relative to other regions.30
Mesenchymal cells are the site of collagen synthesis and processing in the amnion. Procollagen α1(I), α2(I), and α1(III) messenger RNA (mRNA) abundance is greatest in amnion mesenchymal cells, with negligible amounts found in amnion epithelial cells.31,32 Synthesis of types I and III collagens is also minimal in amnion epithelial cells, whereas large amounts are produced by mesenchymal cells. Levels of procollagen α1(I), α2(I), and α1(III) subunit mRNAs, and the specific activities of the enzymes prolyl 4-hydroxylase and lysyl hydroxylase, which are required for collagen synthesis, are greatest in early gestation amnion, decreasing after the 12th to 14th weeks of gestation to a nadir persisting until term. The density of mesenchymal cells in human amnion declines after the first trimester of pregnancy. Therefore, the increase in the ratio of epithelial to mesenchymal cells as a function of gestational age may explain the decline in the levels of collagen mRNAs and the specific activities of lysyl and prolyl hydroxylases in the amnion.
Proteoglycans are important NC components of the fetal membranes. Biglycan, a small leucine-rich proteoglycan, binds to fibrillar collagen and several growth factors. Decorin, another proteoglycan with 55% homology to biglycan, associates with a number of matrix molecules and TGF-β1. Decorin is involved in collagen fibrilogenesis, whereas biglycan and hyaluronan disrupt collagen.33 Large amounts of hyaluronan, a high-molecular-weight nonsulfated glycosaminoglycan composed of polymeric disaccharides of d-glucuronic acid and N-acetyl-d-glucosamine, are found in the amnion and in the decidua in association with parturition.34
As discussed below, alterations in the ECM content and composition of the fetal membrane are mediated in part by the action of matrix degrading enzymes, particularly members of the MMP family, zinc-containing metalloenzymes that act on specific substrates, including the fibrillar and basement membrane collagens (Table 1).35
Table 1.
Matrix metalloproteinase substrates.
| MMP | 1 | 2 | 3 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 16 | 18 | 19 | 26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aggrecan | + | + | + | + | + | + | + | + | + | + | |||||
| Collagen I | + | + | − | + | + | − | + | + | + | + | + | − | |||
| Collagen II | + | + | − | + | + | − | |||||||||
| Collagen III | + | + | + | − | + | − | + | + | + | + | |||||
| Collagen IV | − | + | + | + | − | + | + | − | + | − | + | + | |||
| Collagen V | − | + | + | − | − | + | + | − | |||||||
| Collagen VI | − | − | − | − | + | ||||||||||
| Collagen VII | + | + | + | ||||||||||||
| Collagen VIII | + | ||||||||||||||
| Collagen IX | − | − | + | + | |||||||||||
| Collagen X | + | + | + | − | + | ||||||||||
| Collagen XI | + | + | + | + | − | ||||||||||
| Collagen XIV | − | − | − | + | + | ||||||||||
| Decorin | − | + | + | + | + | ||||||||||
| Elastin | − | + | + | + | + | + | + | − | |||||||
| Entactin/Nidogen | + | + | + | + | + | + | |||||||||
| Fibrillin | + | + | + | + | + | + | |||||||||
| Fibronectin | + | + | + | + | − | − | + | + | + | + | + | + | + | ||
| Fibulins | + | + | |||||||||||||
| Gelatin I | + | + | + | + | + | + | + | + | + | + | + | ||||
| Laminin | + | + | + | + | + | − | + | + | − | ||||||
| Link Protein | + | + | + | + | + | + | |||||||||
| Osteonectin | + | + | + | + | + | ||||||||||
| Tenascin | + | + | + | + | − | + | |||||||||
| Vitronectin | + | + | + | + | + | + | + |
aThe symbols indicate whether the indicated protein is a known (+) or is not (−) digested by the indicated MMP. Modified from Sternlicht and Werb, Annu Rev Cell Dev Biol. 2001; 17: 463–516.35
Changes in ECM Content and Composition During Pregnancy
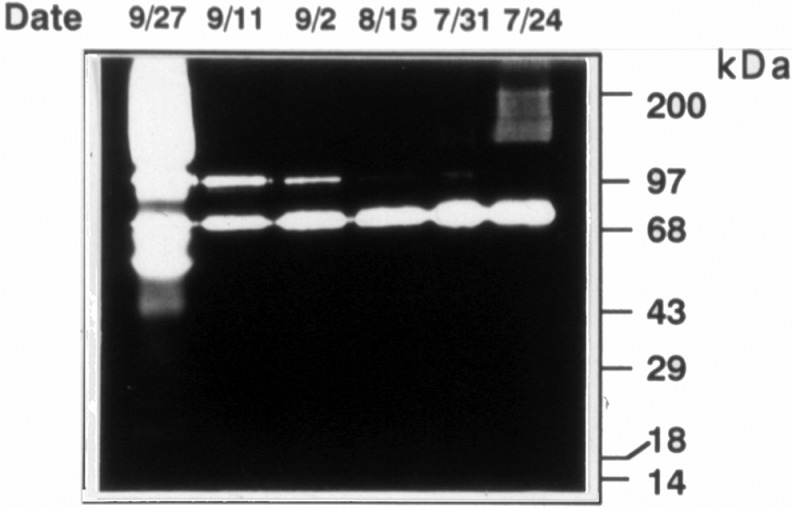
Biochemical and mechanical properties of fetal membranes from 23 to 41 weeks of gestation, and membranes that rupture prematurely, have been characterized.23–27,36,37 In some studies, collagen content is reported to be decreased in PPROM.36 In PPROM samples, the ratios of type III/type I, type III/type V, and type III/total collagen were significantly lower than in non-PPROM samples. The ratios of type I/type V, type I/total collagen, and type V/total collagen showed no change in gestations with and without PPROM. These alterations in fetal membrane ECM reflect changes in component synthesis and turnover. Evidence that the latter is important has been derived from animal studies, which revealed induction of MMPs in the fetal membranes or increases in levels in amniotic fluid associated with normal parturition (Figure 2).
Figure 2.
Zymogram demonstrating amniotic fluid levels of gelatinases MMP-2 (72 kDa) and MMP-9 (97 kDa) from a pregnant baboon. Sampling dates are noted above each lane. The female delivered on 9/28. MMP indicates matrix metalloproteinase.
Membrane strength, reflected by rupture force and work to rupture, declines with increasing gestational age in both weak and strong regions of the membranes. The decline in strength is most dramatic after 38 weeks of gestation. The amnion and chorion are also thinner in the weak areas compared to the strong areas.38
In membranes prior to labor, the biglycan concentration in amnion overlying the cervix is 40% lower than in the mid-zone amnion. After delivery the cervical amnion shows a 2-fold increase in biglycan and a 50% decrease in decorin concentrations.34 A recent report described preterm birth in mice lacking both decorin and biglycan, indicating that deficiencies in these matrix components can contribute to prematurity.39
The amnion and choriodecidua show varying degrees of separation. Increased separation and decreased adherence are seen both with increasing gestational age and with labor. This separation of the membranes is associated with the formation of a hyaluronan-rich gelatinous substance.21,34,40
It has been assumed that common biochemical mechanisms are involved in the rupture of the fetal membranes at term and pathological preterm rupture. However, these biochemical changes are likely triggered by different events in the setting of normal parturition versus unscheduled rupture associated with preterm birth, although a final common pathway of matrix degradation involving MMPs, apoptosis, and structural weakening of the membranes may be involved.
It has become evident that the biochemical and structural changes take place in a restricted area of the membranes, generally in the portion overlying the cervix. This may reflect the consequences of several different independent factors. Among these are physical forces (ie, distension or stretch) resulting in biochemical responses.41 This is consistent with the observations that stretch affects the expression of ECM genes and MMPs in other tissues. Microbes ascending the reproductive tract can trigger inflammatory responses resulting in the production of endogenous matrix degrading enzymes, or the microbes may produce their own matrix degrading proteases. Theoretically, a regional “clock” in the fetal membranes may entrain a biochemical sequence, or the process of rupture may be initiated by factors (eg, hormones or cytokines) derived from the fetus, placenta, or lower uterine segment (cervix).
Focal Changes in Fetal Membrane Composition, Structure, and Cell Function
A zone of extremely altered morphology, characterized by swelling and disruption of the connective tissue, thinning of the trophoblast layer, probably related to a regional increase in trophoblast apoptosis, and thinning or absence of decidua, has been identified in the fetal membranes at the rupture site in term pregnancies and in PPROM.42–49 The morphological features of the zone of extremely altered morphology correlate with structural weakness. These features are seen in fetal membranes at term in the region overlying the cervix. Because of the focal nature of these alterations, care must be taken in the interpretation of studies that do not carefully define the regions of the fetal membranes analyzed.
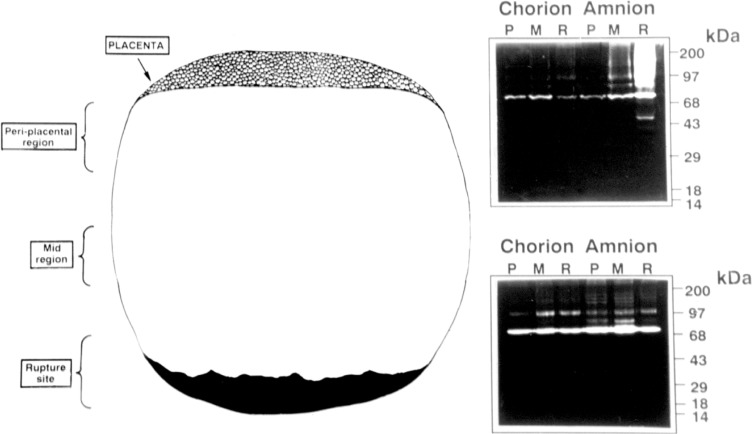
The mean rupture strength within these zones is reported to be 60% of the remaining membranes. A loss of lattice-like arrangement in the organization of collagen is seen near the rupture site.50 There is an increase in fibril spacing and a 50% decrease in fibrillar organization. The weak zones overlying the cervix also have increased MMP-9 (Figure 3), increased levels of certain transcription factors, and transcriptional pathways including NF-kB, Fox03, and Fox04, which regulate genes involved in inflammation, ECM remodeling and apoptosis, increased poly (ADP-ribose) polymerase I cleavage (a marker of apoptosis), decreased tissue inhibitor of metalloproteinase 3 (TIMP-3), and histological features consistent with remodeling and cellular apoptosis.47–49, 51–60
Figure 3.
Zymogram demonstrating regional expression of gelatinases, MMP-2 (72 kDa), and MMP-9 (97 kDa) in the portion of the human amnion lying over the cervix in labored membranes (top panel) and the relative lack of regional induction in membranes collected from a nonlaboring participant (bottom panel). A indicates amnion; C, chorion; P, periplacental; M, mid-region; R, putative rupture site; MMP, matrix metalloproteinase.
Apoptotic cell death is seen in both the amnion and chorion in normal term membrane rupture as well as in PPROM. The increase in MMP-9 levels associated with amnion cell apoptosis mirrors what is seen in the rat amnion as labor approaches.51–62 It is likely that ECM catabolism initiates the apoptotic process, since forced expression of MMP-9 provokes apoptosis in organ cultures of rat amnion and inhibitors of MMPs prevent apoptotic cell death in the presence of MMP-9 expression.60 In addition, apoptotic cell death and the subsequent release of cellular “danger” signals (eg, the heat shock proteins Hsp60, Hsp70, and Hsp90 and high-mobility group box 1 [HMGB1]) that activate toll-like receptors could induce MMP expression and activation reinforcing the catabolism of ECM.63 Additionally, hormones may modulate cell survival since progesterone inhibits fetal membrane cell apoptosis triggered by tumor necrosis factor-α (TNF-α).64
Genetic Factors
Synthesis of ECM
The risk of preterm birth is influenced by a variety of factors including genetics, environment, and social context. The strongest evidence for a genetic contribution to preterm birth associated with PPROM derives from the phenotype of pregnancies associated with genetic defects in collagen synthesis that affect the structure and function of fibrillar collagens and other key proteins involved in matrix metabolism.65 Ehlers-Danlos syndrome (EDS) is a heterogeneous group of diseases in which collagen synthesis is affected. The prevalence for all types of EDS is estimated to be 1 in 5000, and all racial/ethnic group backgrounds are affected equally.
Autosomal dominant forms of the disorder, which includes EDS types I and II (classical type) and EDS type IV (vascular type), impair production of fibrillar collagens. Ehlers-Danlos syndrome types I and II involve mutations in COL5A1 (9q34-q34.3) and COL5A2 (2q14-q32), which encode α chains in type V collagen, and EDS type IV is caused by mutations in COL3A1 (2q31). When the fetus is affected by these mutations there is a 40% to 58% risk of PPROM, far exceeding the risk in the normal population.
Mutations resulting in a nonfunctional COL5A1 allele, leading to haploinsufficieny of type V collagen, or mutations that result in a structural alteration in the type V collagen proteins are among the more common causes of classical EDS. Mutations in the COL3A1 gene causing type IV (vascular) EDS encompass multiple exon deletion, skipping of a single exon or a point mutation resulting in the substitution of a glycine by another amino acid. Mutations in the COL1A1 and COL1A2 genes that cause the rare forms of EDS, type VIIA and B and type II osteogenesis imperfecta, are associated with preterm birth and PPROM.65
Interestingly, other forms of osteogenesis imperfecta caused by mutations in the type I collagen gene are not associated with the markedly increased risk of PPROM as those disorders in which COL3A and COL5A genes are mutated. This may reflect the impact of specific mutations on the organization of the ECM, the impact on cellular function, including the production of matrix degrading enzymes, and the susceptibility of the ECM components to degradation.
Restrictive dermopathy, a rare and lethal genetic disorder inherited in an autosomal recessive pattern, has clinical features that include intrauterine growth retardation, shiny, tight, and rigid skin with prominent vessels, enlarged fontanels, multiple joint contractures, and characteristic facial features.65 Affected pregnancies generally end in preterm deliveries as a result of PPROM. Polyhydramnios is reported as a complication as well as chorioamniotic membrane separation, both of which could contribute to PPROM.
The precise cause of premature rupture of membranes in restrictive dermopathy is uncertain, but abnormal collagen structure and absence of or reduction in elastic fibers in the fetal membranes may be contributing factors. Mutations in the ZMPSTE24 gene, which encodes a zinc metalloproteinase essential for lamin A maturation, have recently been identified in some cases of restrictive dermopathy. Accumulation of prelamin A in the nuclear envelope results in abnormalities in nuclear architecture, which may affect gene expression.
Polymorphisms in genes encoding ECM proteins have been associated with PPROM.66 Haplotypes of COL4A3 single-nucleotide polymorphisms (SNPs) in the mother were associated with PPROM. A multilocus analysis of the same population identified a 3-locus model, which included maternal SNPs in COL1A2 and the genes encoding defensin-α 5 and endothelin 1. Interestingly, Hermanns-Lê et al. reported changes in the dermal collagen architecture similar to those found in patients with EDS and suggested a link between recurrent PPROM and connective tissue abnormalities.67
SERPINH1 encodes heat-shock protein 47, a chaperone essential for the proper folding of collagen molecules and prevention of their aggregation in the endoplasmic reticulum.68,69 The essentiality of this chaperone for collagen synthesis is reflected in embryonic lethality in mice lacking the protein, and rare cases of SERPINH1 mutations causing a recessive form of osteogenesis imperfecta.70 The promoter of the SERPINH1 gene contains a number of polymorphisms, some of which are functional. The −656 promoter SNP minor T allele has a greater frequency in African populations and African Americans than in European Americans.71 The −656 T allele also displays significantly reduced promoter activity compared to the major −656 C allele when studied in the context of amnion fibroblasts but not adult skin fibroblasts or uterine smooth muscle cells where the promoter activity is increased. This suggests a unique transcriptional control of the SERPINH1 gene in extraembryonic fetal tissues. An initial case–control study demonstrated that the SERPINH1 -656 T allele is significantly more frequent in African American neonates born from pregnancies complicated by PPROM compared with controls. A follow-up case–control study gave similar results. The combined case–control findings showed a highly significant association between the −656 T allele and PPROM. The SERPINH1 −656 T allele represents an example of an ancestry-selective marker associated with preterm birth in African Americans.
A 12-bp deletion NT_033927.7: g.5495364_5495375del in the SERPINH1 gene promoter that increases promoter activity was also discovered.72 The 12-bp deletion is in linkage disequilibrium with the minor "T" allele of the −656 C/T SNP that reduces the promoter activity in amnion fibroblast cells and is associated with a significantly increased risk of preterm birth as a result of PPROM. In a case–control study, fetal carriage of the 12-bp deletion was found to protect against PPROM, apparently overcoming the influence of the SERPINH1 −656 minor T allele.
Degradation of ECM
There is substantial evidence for the expression of matrix degrading enzymes and factors that inhibit matrix degrading enzyme activity in human fetal membranes, and an expression pattern that suggests their involvement in normal and pathologic membrane rupture.58–61,73–81 Most attention has been focused on gelatinases, MMP-2, and MMP-9. These enzymes degrade type V collagen as well as basement membrane collagens (type IV), which may be important for the maintenance of viability of the amnion epithelial cells. Increased expressions of these enzymes, but mainly increases in MMP-9, have been found by numerous authors to be associated with normal term rupture as well as PPROM. Matrix metalloproteinase 8, which is expressed by chorion trophoblast cells, and MMP-1, expressed by amnion mesenchymal cells, have also been implicated in fetal membrane rupture. These enzymes represent appealing candidate genes for PPROM, and the analysis of genetic variation that influences either the level of expression or activity of these enzymes has yielded positive associations with PPROM risk.
The variants associated with PPROM described to date are all promoter variants that are associated with increased promoter function. These have been found in genes that degrade fibrillar collagens including MMP1 (types I, II, and III collagens), MMP8 (types I, II, and III collagens), and MMP9 (type V collagen).82–84
Matrix metalloproteinase 1 catalyzes the initial step in the degradation of fibrillar collagens. A polymorphism at −1607 in the MMP1 promoter (an insertion of a guanine [G]) creates a core Ets-binding site and increases the promoter activity. The 2G promoter has >2-fold greater activity than the 1G allele in amnion mesenchymal cells. Phorbol 12-myristate 13-acetate (PMA) treatment increases MMP-1 mRNA expression to a significantly greater extent in amnion mesenchymal cells with a 1G/2G or 2G/2G genotype compared with cells homozygous for the 1G allele. When treated with PMA, the 1G/2G and 2G/2G cells produced greater amounts of MMP-1 protein than 1G/1G cells. A significant association was found between fetal carriage of a 2G allele and PPROM, indicating that the 2G allele, which has stronger promoter activity in amnion mesenchymal cells, confers increased responsiveness of amnion cells to stimuli that induce MMP-1, and that this polymorphism contributes to the risk of PPROM. Interestingly, the Ets-1 transcription factor is upregulated in fetal membranes at rupture (both term and preterm), which would reinforce the impact of the 2G variant on MMP1 expression.85
Matrix metalloproteinase 8, an enzyme that also degrades fibrillar collagens imparting strength to the fetal membranes, is expressed by leukocytes and chorionic cytotrophoblast cells. Single nucleotide polymorphisms at −799C/T, −381A/G, and +17C/G from the major transcription start site have functional significance.83 The minor alleles +17 (G) and −381 (G) are in complete linkage disequilibrium. A promoter fragment containing the 3 minor alleles has a 3-fold greater activity in chorion-like trophoblast cells (BeWo, JEG-3, and HTR-8/SVneo) compared with the major allele promoter construct. A case–control study of African American neonates using allele-specific primers revealed a statistically significant association between the 3 minor allele haplotype, which displays the highest MMP8 promoter activity in trophoblast cells, with PPROM. None of the minor alleles were individually associated with PPROM.
Fetal membrane rupture is associated with increased expression of MMP-9. The functional significance of variable number of tandem repeats in the MMP9 gene on promoter activity and association with PPROM has been reported.84 The 14 CA-repeat allele was a stronger promoter than the 20 CA-repeat allele in amnion epithelial cells. An SNP at −1562 did not significantly affect promoter activity. A case–control study of African American neonates revealed that the 14 CA-repeat allele was more common in newborns delivered after PPROM than in those delivered at term.
Proteins that inhibit the activity of MMPs have also been implicated in preterm birth resulting from PPROM. A maternal SNP in TIMP2, which encodes an endogenous inhibitor of MMPs, was significantly associated with PPROM.66
Although the studies reviewed above suggest roles for specific genes in the risk of PPROM, they suffer in most instances from a lack of replication, and critical assessment of the potential confounding effects of population stratification, a major issue when heterogeneous populations like African Americans are studied. Moreover, as discussed below, environmental factors are quite important in the pathophysiology of preterm birth and there have been few studies in which candidate gene–environment interactions have been assessed.
Environmental Factors
Interactions between genes and the environment are important to the pathophysiology of preterm birth.86 Among the many environmental factors that are thought to play a role in prematurity related to PPROM are nutrition, smoking, and infection.5
Nutrition
Vitamins play an essential role in ECM metabolism. Vitamin C is a cofactor for lysyl hydroxylase, a key enzyme involved in collagen synthesis, and vitamin C deficiency affects ECM production. Women whose pregnancies are affected by PPROM have reduced intake of vitamin C or plasma vitamin C levels in some studies, and vitamin C supplementation in a high-risk population reduces PPROM.87,88 Although these findings suggest that nutritional status can influence the risk of PPROM, epidemiological studies and nutritional intervention did not establish that vitamin C influenced the risk of PPROM as a direct result of the ECM content of the fetal membranes. However, study of PROM membranes revealed a reduced ascorbic acid and collagen concentrations in prelabor ruptured membrane compared to term membranes. Resistance to proteolysis in vitro was reduced in the prelabor ruptured membranes as well.89
Two sodium-dependent membrane transporters encoded by SLC23A1 and SLC23A2 play key roles in human vitamin C metabolism, including dietary uptake, reabsorption, and tissue distribution. An analysis of maternal haplotypes in SLC23A1 and individual SNPs in SLC23A2 revealed an association with the increased risk of preterm birth, including women with premature rupture of membranes. The strongest association was with an intron 2 variant in SLC23A2, with heterozygotes and homozygotes for this variant having a 1.7-fold and 2.7-fold increased risk of spontaneous preterm birth, respectively.90
While the studies noted above suggest a role of maternal vitamin C status and PPROM, other reports demonstrate that the relationship is complex and may only be evident in states of vitamin C deficiency.
Maternal supplementation with vitamins C and E beginning at 9 to 16 weeks of gestation in nulliparous women at low risk does not reduce spontaneous preterm births. The rates of PROM and PPROM were increased in women receiving vitamin C (1 g/d) and vitamin E (400 IU/d) in a secondary analysis of a preeclampsia prevention trial. The antioxidant vitamins did not modify fetal membrane rupture strength, work to rupture, or MMP-9 protein or activity either within or outside the term fetal membrane physiological weak zone.91 ,92
Collectively, the existing literature leaves open the possibility that vitamin C deficiency increases PPROM risk, but that supplemental vitamin C does not have benefit in populations that have adequate vitamin C intake.
Other dietary compounds may influence the risk of PPROM. α-lipoic acid, an antioxidant found in the human diet, inhibits proinflammatory cytokine-induced and thrombin-induced weakening of fetal membrane preparations in an in vitro system.93,94 Tumor necrosis factor-α–induced MMP-9 expression and production of prostaglandin E2 were also prevented by α-lipoic acid treatment.
Smoking
Cigarette smoking is associated with PPROM.95 It has been hypothesized that exposure of fetal membranes to cigarette smoke components induces oxidative stress and apoptotic cell death. When fetal membrane explants collected at term were stimulated with cigarette smoke extract F2-isoprostane, a biomarker of oxidative stress, was increased.96 There was also a dose-dependent decrease in the expression of the anti-apoptotic protein, Bcl2, and increases in the death effectors, active caspase 3, in association with nuclear DNA fragmentation in both amnion and chorion cells compared to control preparations. It was concluded that the smoke extract-induced pathway also increases proteolysis and results in membrane weakening.
Infection/Inflammation
Inflammation, particularly inflammation induced by microbial infection, is commonly associated with preterm birth and PPROM.5,7,34,97–99 Alterations in the fetal ECM structure and integrity are influenced by the endogenous host response, which includes the elaboration of proinflammatory cytokines, like TNF-α and interleukin 1β (IL-1β) that induce the production of endogenous ECM degrading MMPs. In addition, the invading microbes may produce their own matrix degrading enzymes, including collagenases, that act on the host ECM proteins.100 Endogenous matrix degrading enzymes and those derived from microbes can expose or release cryptic matrikines that reinforce the inflammatory process.
Epigenetic Factors and Non-Coding RNAs
Gene expression is regulated by alterations in chromatin structure resulting from modification of DNA by methylation of cytosines or from modification of histone proteins (acetylation, methylation, phosphorylation). Genetic variation in the MMP1 promoter is associated with PPROM. Inhibition of DNA methylation with 5-aza-2'-deoxycytidine in amnion fibroblasts resulted in significantly increased MMP1 gene transcription, and an associated significant increase in MMP1 production.101 These effects were correlated with reduced DNA methylation at a particular site (−1538) in the MMP1 promoter. DNA methylation at this site in the amnion was reduced in a larger percentage of fetal membranes that ruptured prematurely. A T>C SNP (AF007878.1 [MMP1]: g.3447T>C) in the MMP1 promoter was also identified. The minor C allele was always methylated in vivo, and when methylated, resulted in increased affinity for a nuclear protein in amnion fibroblasts. The minor C allele had reduced promoter activity as assessed by plasmid transfection studies and chromatin immunoprecipitation assays using amnion fibroblasts heterozygous for the T>C SNP. In a case–control study, the minor C allele was found to be protective against PPROM, consistent with its reduced promoter function. These observations suggest that in addition to genetic variation, DNA methylation plays a role in controlling MMP1 expression and risk of an adverse obstetrical outcome.
Noncoding RNAs (microRNAs) could regulate ECM metabolism in the fetal membranes. MicroRNA control of matrix synthesis and metabolism have not been explored in the amnion and chorion, although microRNA profiles have been reported.102
Matrikines
Collagen-Derived Matrikines
As noted above, biologically active domains are exposed or released by proteolysis from collagens, including molecules involved in angiogenesis and tumor growth.15,17 The role of the collagen-derived factors (eg, endostatin) in reproduction has not been extensively investigated, specifically their roles in preterm birth and PPROM.
Fibronectin-Derived Matrikines
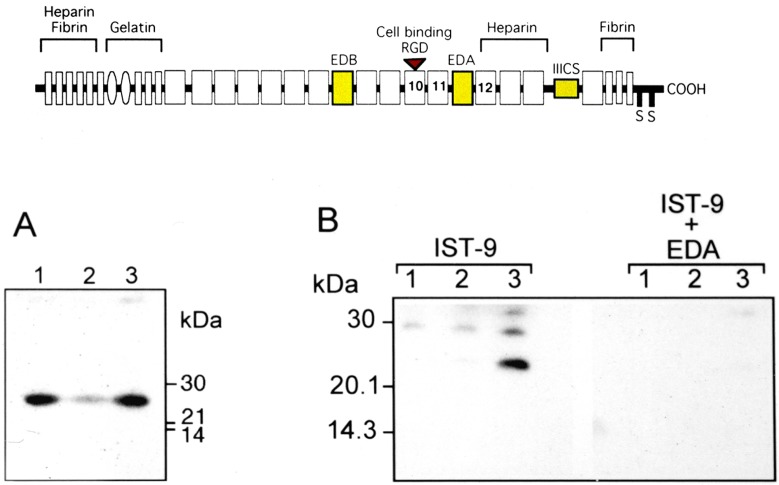
Fibronectin is a dimeric glycoprotein found in the basement membrane.103 It binds to collagen and proteoglycans. There are up to 20 different isoforms of fibronectin that are the result of alternative RNA splicing of exons encoding 2 extra domains (A and B; EDA and EDB, respectively) and a connecting segment (IIICS; Figure 4A). The predominant circulating form of fibronectin, mainly derived from the liver, lacks 3 domains. Fibronectin molecules containing these domains are referred to as “oncofetal fibronectins” since they are present during fetal development but also produced in response to inflammation and by cancer cells.103 ,104 Increased levels of oncofetal fibronectin in cervical fluid are associated with preterm birth, possibly a reflection of inflammation, and oncofetal fibronectin has been used as a clinical biomarker for preterm birth.105 In addition to intact oncofetal fibronectin, which is a dimmer of monomers of 250 000 molecular weight, analysis of cervical fluid in Western blotting using a monoclonal antibody to EDA demonstrates that smaller fragments of oncofetal fibronectin containing the EDA domain are present in vivo (Figure 4B).
Figure 4.
Schematic of the domain structure of fibronectin splice variants (upper panel) showing oncofetal fibronectin exons in yellow, and the presence of EDA-containing fibronectin fragments detected by Western blotting using monoclonal antibody IST-9 (recognizing EDA) in cervical vaginal fluid from women in preterm labor (lower panel A), and neutralization of the antibody with recombinant EDA (lower panel B). Note that oncofetal fibronectin monomers have a molecular weight of 250 000 and are not shown in the blots. EDA indicates extra domain A.
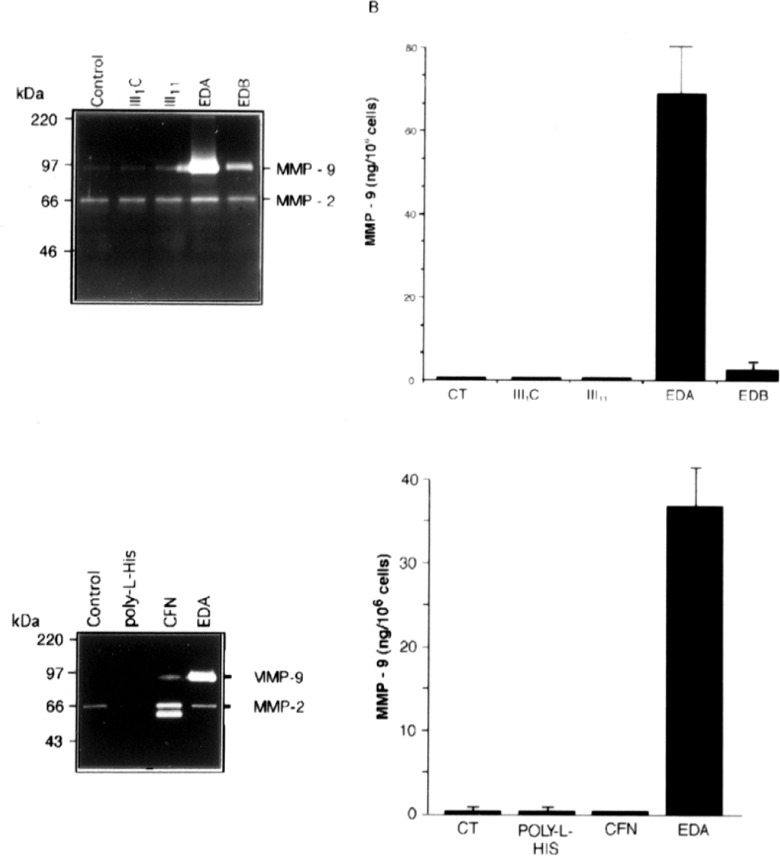
Fibronectin influences cell morphology by binding to a number of different integrins.101However, there is emerging evidence that the EDA domain of fetal fibronectin can activate signaling cascades that are different from those mediated by integrins, including the toll-like receptors (TLRs) that mediate cellular responses to infection and tissue damage. Recombinant EDA, but not intact oncofetal fibronectin, was shown to stimulate MMP-9 production by THP-1 cells, a human acute monocytic leukemia cell line (Figure 5).106 Recombinant fibronectin domains III1C and III11 did not activate THP-1 cell MMP-9 production, and recombinant EDB had only a modest stimulatory effect, revealing the specificity of the response to recombinant EDA. Moreover, the effects of recombinant EDA were not blocked by polymixin B, an antibiotic that binds bacterial lipopolysaccharide (LPS) and prevents it from activating TLR 4 (TLR-4) or the LPS antagonist, E5331. Recombinant EDA, but not other recombinant fibronectin domains, activated human TLR-4 expressed in HEK 293 cells. Recombinant EDA stimulation of TLR-4 was dependent upon the coexpression of MD-2, a TLR-4 accessory protein. Unlike LPS, the activity of EDA was heat sensitive and persisted in the presence of polymyxin B, and a potent LPS antagonist, E5564, which completely suppressed LPS activation of TLR-4. Finally, EDA was not able to activate splenocytes from mice lacking TLR-4 (C3H/HeJ mice) but could in the companion TLR-4-expressing mice (C3H/HeN).
Figure 5.
Induction of gelatinase expression in cultured THP-1 cells exposed to the indicated recombinant His-tagged fibronectin domains. The THP-1 cells (5 × 105 cells/well) were either untreated (control) or treated with 1 μM of different recombinant fibronectin type III repeats, poly-l-histidine, or cellular fibronectin (CFN) for 48 hours. A, Gelatinase activities released from THP-1 cells analyzed using gelatin zymography. B, MMP-9 in conditioned medium measured by ELISA and expressed as the mean ± SD from triplicate cultures. MMP indicates matrix metalloproteinase; ELISA, enzyme-linked immunosorbent assay; SD, standard deviation. Reprinted from Okamura et al.10 5
Subsequent studies demonstrated that the EDA domain stimulates mast cells and polymorphonuclear leukocytes and monocyte suspensions via activation of TLR-4.107,108 In mice producing fibronectin lacking the EDA domain, it was found that fibrotic resolution of lung injury through TGF-β activation and responsiveness was impaired, suggesting that EDA-containing fibronectin plays a critical role in tissue fibrogenesis.109 Collectively, these findings suggest that the EDA domain of fibronectin is a cryptic matrikine, released by proteolysis, that is perceived as an endogenous “danger” signal an alarmin in the setting of PPROM, or part of a choreographed sequence of biochemical events in normal term pregnancy.
Glycosaminoglycan-Derived Matrikines
Like fetal fibronectin, the ECM glycosaminoglycan, hyaluronan, is produced after tissue injury and levels of hyaluronan are increased in the fetal membranes at rupture.34,110 Hyaluronan is a high-molecular-weight polymer of ≥1000 kDa. Fragments of hyaluronan (<500 kDa) are typically produced at sites of inflammation. In vitro studies demonstrated that these fragments can activate cells via TLRs, resulting in an upregulation of expression of matrix metalloproteases (eg, MMP-9, MMP-10, and MMP-13) and cytokines (eg, IL-1β and TNF-α).111,112 In some systems, both TLR-4 and TLR-2 are required for in vitro and in vivo responses to hyaluronan fragments.
Cryptic Matrikines and a Feed-Forward Mechanism for Parturition
The demonstration that 2 ECM components of the fetal membranes, fetal fibronectin and hyaluronan, harbor cryptic matrikines is consistent with the role of the innate immune system in monitoring both exogenous and endogenous “danger” signals. Both TLR-2 and TLR-4 are expressed in the fetal membranes and placenta where they could respond to the matrikines released from fetal fibronectin and hyaluronan. While the danger theory can be invoked in pathological situations, it can also have an important role in physiological events. The process of fetal membrane rupture and the ripening of the cervix in preparation for parturition both entail significant remodeling of the ECM and apoptotic cell death, mediated in part by proteolytic enzymes and the production of proinflammatory cytokines. The re-enforcement of tissue remodeling through induction of MMPs and proinflammatory cytokines by once fetal fibronectin fragments containing EDA and small hyaluronan fragments through TLRs may facilitate normal human parturition. The same signaling pathways, when activated by exogenous molecules like bacterial LPS, could result in unscheduled fetal membrane rupture or cervical changes leading to preterm birth. This feed-forward mechanism could also be at work in the cervix and lower uterine segment and consequently be a general process in parturition.
Acknowledgments
Dr Sergey Leiken, National Institutes of Health, provided valuable comments during the preparation of this manuscript. The data presented in Figure 3 were derived from preliminary studies conducted in collaboration with the late Dr Laird Wilson, University of Illinois at Chicago.
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by National Institutes of Health grants R01 HD034612 and P60 MD002256.
References
- 1. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FaEBS J. 2011;278(1):28–45 [DOI] [PubMed] [Google Scholar]
- 4. Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31(6):465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338(10):663–670 [DOI] [PubMed] [Google Scholar]
- 6. Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010;21(6):353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park JS, Park CW, Lockwood CJ, Norwitz ER. Role of cytokines in preterm labor and birth. Minerva Ginecol. 2005;57(4):349–366 [PubMed] [Google Scholar]
- 8. Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci. 2007;14(7):629–645 [DOI] [PubMed] [Google Scholar]
- 9. Klipple GL, Riordan KK. Rare inflammatory and hereditary connective tissue diseases. Rheum Dis Clin North Am. 1989;15(2):383–398 [PubMed] [Google Scholar]
- 10. Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144(suppl 1):S146–S158 [DOI] [PubMed] [Google Scholar]
- 11. Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78(1):1–12 [DOI] [PubMed] [Google Scholar]
- 12. Pitsos M, Kanakas N. The role of matrix metalloproteinases in the pathogenesis of endometriosis. Reprod Sci. 2009;16(8):717–726 [DOI] [PubMed] [Google Scholar]
- 13. Helleman J, Jansen MP, Burger C, van der Burg ME, Berns EM. Integrated genomics of chemotherapy resistant ovarian cancer: a role for extracellular matrix, TGFbeta and regulating microRNAs. Int J Biochem Cell Biol. 2010;42(1):25–30 [DOI] [PubMed] [Google Scholar]
- 14. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aszódi A, Legate KR, Nakchbandi I, Fässler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621 [DOI] [PubMed] [Google Scholar]
- 16. Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40(6-7):1362–1378 [DOI] [PubMed] [Google Scholar]
- 17. Ricard-Blum S, Ballut L. Matricryptins derived from collagens and proteoglycans. Front Biosci. 2011;16:674–97 [DOI] [PubMed] [Google Scholar]
- 18. Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339(1):247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han S, Makareeva E, Kuznetsova NV, et al. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J Biol Chem. 2010;285(29):22276–22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arikat S, Novince RW, Mercer BM, et al. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol. 2006;194(1):211–217 [DOI] [PubMed] [Google Scholar]
- 22. Oyen ML, Calvin SE, Landers DV. Premature rupture of the fetal membranes: is the amnion the major determinant? Am J Obstet Gynecol. 2006;195(2):510–515 [DOI] [PubMed] [Google Scholar]
- 23. Joyce EM, Moore JJ, Sacks MS. Biomechanics of the fetal membrane prior to mechanical failure: review and implications. Eur J Obstet Gynecol Reprod Biol. 2009;144(suppl 1):S121–S127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Helmig R, Oxlund H, Petersen LK, Uldbjerg N. Different biomechanical properties of human fetal membranes obtained before and after delivery. Eur J Obstet Gynecol Reprod Biol. 1993;48(3):183–189 [DOI] [PubMed] [Google Scholar]
- 25. Jabareen M, Mallik AS, Bilic G, Zisch AH, Mazza E. Relation between mechanical properties and microstructure of human fetal membranes: an attempt towards a quantitative analysis. Eur J Obstet Gynecol Reprod Biol. 2009;144(suppl 1):S134–S141 [DOI] [PubMed] [Google Scholar]
- 26. Pressman EK, Cavanaugh JL, Woods JR. Physical properties of the chorioamnion throughout gestation. Am J Obstet Gynecol. 2002;187(3):672–675 [DOI] [PubMed] [Google Scholar]
- 27. Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27(11-12):1037–1051 [DOI] [PubMed] [Google Scholar]
- 28. Ockleford C, Malak T, Hubbard A, et al. Confocal and conventional immunofluorescence and ultrastructural localisation of intracellular strength-giving components of human amniochorion. J Anat. 1993;183(pt 3):483–505 [PMC free article] [PubMed] [Google Scholar]
- 29. Malak TM, Ockleford CD, Bell SC, Dalgleish R, Bright N, Macvicar J. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta. 1993;14(4):385–406 [DOI] [PubMed] [Google Scholar]
- 30. Moore RM, Redline RW, Kumar D. et al. Differential expression of fibulin family proteins in the para-cervical weak zone and other areas of human fetal membranes. Placenta. 2009;30(4):335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Casey ML, MacDonald PC. Lysyl oxidase (ras recision gene) expression in human amnion: ontogeny and cellular localization. J Clin Endocrinol Metab. 1997;82(1):167–172 [DOI] [PubMed] [Google Scholar]
- 32. Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol Reprod. 1996;55(6):1253–1260 [DOI] [PubMed] [Google Scholar]
- 33. Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29(4):248–253 [DOI] [PubMed] [Google Scholar]
- 34. Meinert M, Malmström A, Tufvesson E. et al. Labour induces increased concentrations of biglycan and hyaluronan in human fetal membranes. Placenta. 2007;28(5-6):482–486 [DOI] [PubMed] [Google Scholar]
- 35.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Dev Biol 2001;17:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hampson V, Liu D, Billett E, Kirk S. Amniotic membrane collagen content and type distribution in women with preterm premature rupture of the membranes in pregnancy. Br J Obstet Gynaecol. 1997;104(9):1087–1091 [DOI] [PubMed] [Google Scholar]
- 37. Meirowitz NB, Smulian JC, Hahn RA. et al. Collagen messenger RNA expression in the human amniochorion in premature rupture of membranes. Am J Obstet Gynecol. 2002;187(6):1679–1685 [DOI] [PubMed] [Google Scholar]
- 38. Frigo P, Lang C, Sator M, Ulrich R, Husslein P. Membrane thickness and PROM−high-frequency ultrasound measurements. Prenat Diagn. 1998;18(4):333–337 [PubMed] [Google Scholar]
- 39. Calmus ML, Macksoud EE, Tucker R, Iozzo RV, Lechner BE. A mouse model of spontaneous preterm birth based on the genetic ablation of biglycan and decorin. Reproduction. 2011;142(1):183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strohl A, Kumar D, Novince R. et al. Decreased adherence and spontaneous separation of fetal membrane layers−amnion and choriodecidua−a possible part of the normal weakening process. Placenta. 2010;31(1):18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pandey V, Jaremko K, Moore RM. et al. The force required to rupture fetal membranes paradoxically increases with acute in vitro repeated stretching. Am J Obstet Gynecol. 2007;196(2):165.e1–e7 [DOI] [PubMed] [Google Scholar]
- 42. Malak TM, Bell SC. Structural characteristics of term human fetal membranes: a novel zone of extreme morphological alteration within the rupture site. Br J Obstet Gynaecol. 1994;101(5):375–386 [DOI] [PubMed] [Google Scholar]
- 43. McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14(1):237–241 [DOI] [PubMed] [Google Scholar]
- 44. El Khwad M, Stetzer B, Moore RM. et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72(3):720–726 [DOI] [PubMed] [Google Scholar]
- 45. El Khwad M, Pandey V, Stetzer B. et al. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13(3):191–195 [DOI] [PubMed] [Google Scholar]
- 46. McParland PC, Taylor DJ, Bell SC. Mapping of zones of altered morphology and chorionic connective tissue cellular phenotype in human fetal membranes (amniochorion and decidua) overlying the lower uterine pole and cervix before labor at term. Am J Obstet Gynecol. 2003;189(5):1481–1488 [DOI] [PubMed] [Google Scholar]
- 47. Lappas M, Odumetse TL, Riley C. et al. Pre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signalling pathways. Placenta. 2008;29(12):995–1002 [DOI] [PubMed] [Google Scholar]
- 48. Lappas M, Riley C, Rice GE, Permezel M. Increased expression of ac-FoxO1 protein in prelabor fetal membranes overlying the cervix: possible role in human fetal membrane rupture. Reprod Sci. 2009;16(7):635–641 [DOI] [PubMed] [Google Scholar]
- 49. Lappas M, Lim R, Riley C, Menon R, Permezel M. Expression and localisation of FoxO3 and FoxO4 in human placenta and fetal membranes. Placenta. 2010;31(12):1043–1050 [DOI] [PubMed] [Google Scholar]
- 50. Connon CJ, Nakamura T, Hopkinson A. et al. The biomechanics of amnion rupture: an X-ray diffraction study. PLoS One. 2007;2(11):e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen ZY, Li EM, Lu SQ. et al. Autophagic and apoptotic cell death in amniotic epithelial cells. Placenta. 2008;29(11):956–961 [DOI] [PubMed] [Google Scholar]
- 52. George RB, Kalich J, Yonish B, Murtha AP. Apoptosis in the chorion of fetal membranes in preterm premature rupture of membranes. Am J Perinatol. 2008;25(1):29–32 [DOI] [PubMed] [Google Scholar]
- 53. Reti NG, Lappas M, Riley C, et al. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. Am J Obstet Gynecol. 2007;196(5):484.e1–e10 [DOI] [PubMed] [Google Scholar]
- 54. McLaren J, Taylor DJ, Bell SC. Increased incidence of apoptosis in non-labour-affected cytotrophoblast cells in term fetal membranes overlying the cervix. Hum Reprod. 1999;14(11):2895–2900 [DOI] [PubMed] [Google Scholar]
- 55. Kumagai K, Otsuki Y, Ito Y, Shibata MA, Abe H, Ueki M. Apoptosis in the normal human amnion at term, independent of Bcl-2 regulation and onset of labour. Mol Hum Reprod. 2001;7(7):681–689 [DOI] [PubMed] [Google Scholar]
- 56. Sağol S, Sağol O, Ozkal S, Asena U. Role of apoptosis, bcl-2 and bax protein expression in premature rupture of fetal membranes. J Reprod Med. 2002;47(10):809–815 [PubMed] [Google Scholar]
- 57. Kataoka S, Furuta I, Yamada H. et al. Increased apoptosis of human fetal membranes in rupture of the membranes and chorioamnionitis. Placenta. 2002;23(2-3):224–231 [DOI] [PubMed] [Google Scholar]
- 58. McLaren J, Taylor DJ, Bell SC. Increased concentration of pro-matrix metalloproteinase 9 in term fetal membranes overlying the cervix before labor: implications for membrane remodeling and rupture. Am J Obstet Gynecol. 2000;182(2):409–416 [DOI] [PubMed] [Google Scholar]
- 59. Arechavaleta-Velasco F, Mayon-Gonzalez J, Gonzalez-Jimenez M, Hernandez-Guerrero C, Vadillo-Ortega F. Association of type II apoptosis and 92-kDa type IV collagenase expression in human amniochorion in prematurely ruptured membranes with tumor necrosis factor receptor-1 expression. J Soc Gynecol Investig. 2002;9(2):60–67 [DOI] [PubMed] [Google Scholar]
- 60. Fortunato SJ, Menon R, Bryant C, Lombardi SJ. Programmed cell death (apoptosis) as a possible pathway to metalloproteinase activation and fetal membrane degradation in premature rupture of membranes. Am J Obstet Gynecol. 2000;182(6):1468–1476 [DOI] [PubMed] [Google Scholar]
- 61. Athayde N, Edwin SS, Romero R. et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179(5):1248–1253 [DOI] [PubMed] [Google Scholar]
- 62. Lei H, Furth EE, Kalluri R. et al. A program of cell death and extracellular matrix degradation is activated in the amnion before the onset of labor. J Clin Invest. 1996;98(9):1971–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lei H, Kalluri R, Furth EE, Baker AH, Strauss JF., 3rd Rat amnion type IV collagen composition and metabolism: implications for membrane breakdown. Biol Reprod. 1999;60(1):176–182 [DOI] [PubMed] [Google Scholar]
- 64. Luo G, Abrahams VM, Tadesse S, et al. Progesterone inhibits basal and TNF-alpha-induced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17(6):532–539 [DOI] [PubMed] [Google Scholar]
- 65. Anum EA, Hill LD, Pandya A, Strauss JF., 3rd Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity. Placenta. 2009;30(3):207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Romero R, Friel LA, Velez Edwards DR, et al. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). Am J Obstet Gynecol. 2010;203(4):361.e1–361e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hermanns-Lê T, Piérard G, Quatresooz P. Ehlers-Danlos-like dermal abnormalities in women with recurrent preterm premature rupture of fetal membranes. Am J Dermatopathol. 2005;27(5):407–410 [DOI] [PubMed] [Google Scholar]
- 68. Ishida Y, Kubota H, Yamamoto A, Kitamura A, Bächinger HP, Nagata K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol Biol Cell. 2006;17(5):2346–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nagai N, Hosokawa M, Itohara S, et al. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol. 2000;150(6):1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Christiansen HE, Schwarze U, Pyott SM, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86(3):389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang H, Parry S, Macones G, et al. A functional SNP in the promoter of the SERPINH1 gene increases risk of preterm premature rupture of membranes in African Americans. Proc Natl Acad Sci U S A. 2006;103(36):13463–13467 Erratum in: Proc Natl Acad Sci U S A 2006;103(50):19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang H, Sammel MD, Tromp G, et al. 12 bp Wang SERPINH1A 12-bp deletion in the 5'-flanking region of the SERPINH1 gene affects promoter activity and protects against preterm premature rupture of membranes in African Americans. Hum Mutat. 2008;29(2):332. [DOI] [PubMed] [Google Scholar]
- 73. Vadillo-Ortega F, González-Avila G, Furth EE, et al. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1995;146(1):148–156 [PMC free article] [PubMed] [Google Scholar]
- 74. Nishihara S, Someya A, Yonemoto H, et al. Evaluation of the expression and enzyme activity of matrix metalloproteinase-7 in fetal membranes during premature rupture of membranes at term in humans. Reprod Sci. 2008;15(2):156–165 [DOI] [PubMed] [Google Scholar]
- 75. Yonemoto H, Young CB, Ross JT, Guilbert LL, Fairclough RJ, Olson DM. Changes in matrix metalloproteinase (MMP)-2 and MMP-9 in the fetal amnion and chorion during gestation and at term and preterm labor. Placenta. 2006;27(6-7):669–677 [DOI] [PubMed] [Google Scholar]
- 76. Ota A, Yonemoto H, Someya A, Itoh S, Kinoshita K, Nagaoka I. Changes in matrix metalloproteinase 2 activities in amniochorions during premature rupture of membranes. J Soc Gynecol Investig. 2006;13(8):592–597 [DOI] [PubMed] [Google Scholar]
- 77. Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002;87(3):1353–1361 [DOI] [PubMed] [Google Scholar]
- 78. Tromp G, Kuivaniemi H, Romero R, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. 2004;191(4):1331–1338 [DOI] [PubMed] [Google Scholar]
- 79. Li W, Alfaidy N, Challis JR. Expression of extracellular matrix metalloproteinase inducer in human placenta and fetal membranes at term labor. J Clin Endocrinol Metab. 2004;89(6):2897–2904 [DOI] [PubMed] [Google Scholar]
- 80. Fortunato SJ, Menon R. Screening of novel matrix metalloproteinases (MMPs) in human fetal membranes. J Assist Reprod Genet. 2002;19(10):483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fortunato SJ, Menon R, Lombardi SJ. Presence of four tissue inhibitors of matrix metalloproteinases (TIMP-1, -2, -3 and -4) in human fetal membranes. Am J Reprod Immunol. 1998;40(6):395–400 [DOI] [PubMed] [Google Scholar]
- 82. Fujimoto T, Parry S, Urbanek M, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277(8):6296–6302 [DOI] [PubMed] [Google Scholar]
- 83. Wang Wang H, Parry S, Macones G, et al. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet. 2004;13(21):2659–2669 [DOI] [PubMed] [Google Scholar]
- 84. Ferrand PE, Parry S, Sammel M, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8(5):494–501 [DOI] [PubMed] [Google Scholar]
- 85. Michaelis SA, Okuducu AF, Sarioglu NM, von Deimling A, Dudenhausen JW. The transcription factor Ets-1 is expressed in human amniochorionic membranes and is up-regulated in term and preterm premature rupture of membranes. J Perinat Med. 2005;33(4):314–319 [DOI] [PubMed] [Google Scholar]
- 86. York TP, Strauss JF, 3rd, , Neale MC, Eaves LJ. Racial differences in genetic and environmental risk to preterm birth. PLoS One. 2010;5(8):e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Siega-Riz AM, Promislow JH, Savitz DA, et al. Vitamin C intake and the risk of preterm delivery. Am J Obstet Gynecol. 2003;189(2):519-525. [DOI] [PubMed] [Google Scholar]
- 88. Casanueva E, Ripoll C, Tolentino M, et al. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: a randomized trial. Am J Clin Nutr. 2005;81(4):859–863 [DOI] [PubMed] [Google Scholar]
- 89. Stuart EL, Evans GS, Lin YS, Powers HJ. Reduced collagen and ascorbic acid concentrations and increased proteolytic susceptibility with prelabor fetal membrane rupture in women. Biol Reprod. 2005;72(1):230–235 [DOI] [PubMed] [Google Scholar]
- 90. Erichsen HC, Engel SA, Eck PK, et al. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol. 2006;163(3):245–254 [DOI] [PubMed] [Google Scholar]
- 91. Spinnato JA 2nd, Freire S, Pinto e Silva JL, et al. Antioxidant supplementation and premature rupture of the membranes: a planned secondary analysis. Am J Obstet Gynecol. 2008;199(4):433.e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mercer BM, Abdelrahim A, Moore RM, et al. The impact of vitamin C supplementation in pregnancy and in vitro upon fetal membrane strength and remodeling. Reprod Sci. 2010;17(7):685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moore RM, Novak JB, Kumar D, Mansour JM, Mercer BM, Moore JJ. Alpha-lipoic acid inhibits tumor necrosis factor-induced remodeling and weakening of human fetal membranes. Biol Reprod. 2009;80(4):781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Moore RM, Schatz F, Kumar D, et al. Alpha-lipoic acid inhibits thrombin-induced fetal membrane weakening in vitro. Placenta. 2010;31(10):886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Savitz DA, Dole N, Terry JW, Jr, Zhou H, Thorp JM., Jr Smoking and pregnancy outcome among African-American and white women in central North Carolina. Epidemiology. 2001;12(6):636–642 [DOI] [PubMed] [Google Scholar]
- 96. Menon R, Fortunato SJ, Yu J, et al. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta. 2011;32(4):317–322 [DOI] [PubMed] [Google Scholar]
- 97. Jones HE, Harris KA, Azizia M, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4(12):e8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gomez-Lopez N, Laresgoiti-Servitje E, Olson DM, Estrada-Gutiérrez G, Vadillo-Ortega F. The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol Reprod. 2010;82(5):809–814 [DOI] [PubMed] [Google Scholar]
- 99. Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191(4):1346–1355 [DOI] [PubMed] [Google Scholar]
- 100. McGregor JA, Lawellin D, Franco-Buff A, Todd JK, Makowski EL. Protease production by microorganisms associated with reproductive tract infection. Am J Obstet Gynecol. 1986;154(1):109–114 [DOI] [PubMed] [Google Scholar]
- 101. Wang H, Ogawa M, Wood JR, et al. Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17(8):1087–1096 [DOI] [PubMed] [Google Scholar]
- 102. Montenegro D, Romero R, Pineles BL, et al. Differential expression of microRNAs with progression of gestation and inflammation in the human chorioamniotic membranes. Am J Obstet Gynecol. 2007;197(3):289.e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. White ES, Muro AF. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life. 2011;63(7):538–546 [DOI] [PubMed] [Google Scholar]
- 104. McFadden JP, Basketter DA, Dearman RJ, Kimber IR. Extra domain A-positive fibronectin-positive feedback loops and their association with cutaneous inflammatory disease. Clin Dermatol. 2011;29(3):257–265 [DOI] [PubMed] [Google Scholar]
- 105. Conde-Agudelo A, Romero R. Cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in multiple pregnancies: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2010;23(12):1365–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276(13):10229–10233 [DOI] [PubMed] [Google Scholar]
- 107. Gondokaryono SP, Ushio H, Niyonsaba F, et al. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J Leukoc Biol. 2007;82(3):657–665 [DOI] [PubMed] [Google Scholar]
- 108. Lefebvre JS, Lévesque T, Picard S, et al. Extra domain a of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of Toll-like receptor 4. Arthritis Rheum. 2011;63(6):1527–1533 [DOI] [PubMed] [Google Scholar]
- 109. Muro AF, Moretti FA, Moore BB, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(6):638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461 [DOI] [PubMed] [Google Scholar]
- 111. Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279(17):17079–17084 [DOI] [PubMed] [Google Scholar]
- 112. Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–1179 [DOI] [PubMed] [Google Scholar]