Abstract
The aim of this study was to examine the numbers of CD4+CD25−forkhead box protein 3 (FoxP3)+, CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells in patients with new-onset systemic lupus erythematosus (SLE). The numbers of CD4+CD25−FoxP3+, CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells and the concentrations of serum interleukin (IL)-10 in 23 patients and 20 healthy controls (HC) were measured. The potential correlations between CD4+FoxP3+ T cells, serum IL-10 and clinical measures in SLE patients were analysed. In comparison with that in the HC, significantly reduced numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells, but increased numbers of CD4+CD25−FoxP3+ T cells, were detected, accompanied by significantly lower levels of serum IL-10 in the patients. Stratification analysis indicated the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells and serum IL-10 levels in the patients with seropositive anti-dsDNA were significantly less than that in those with seronegative anti-dsDNA. Treatment with the anti-SLE therapy, particularly with prednisone, leflunomide and methotrexate, significantly improved the imbalance of these types of FoxP3+ T cells and increased the concentrations of serum IL-10 in the drug-responding patients. The numbers of CD4+CD25+FoxP3+ T cells were correlated negatively with the values of SLE disease activity index (SLEDAI), whereas the numbers of CD4+CD25−FoxP3+ T cells were correlated positively with the values of SLEDAI, erythrocyte sedimentation rate (ESR) and serum C3. In addition, the concentrations of serum IL-10 were correlated positively with the numbers of CD4+CD25+FoxP3+ T cells, but negatively with the values of SLEDAI, serum C3, CRP and ESR in these patients. Our data indicate that the imbalance of different types of FoxP3+CD4+ T cells may contribute to the development of SLE in Chinese patients.
Keywords: CD25, FoxP3, IL-10, regulatory T cells, SLE
Introduction
Systemic lupus erythematosus (SLE) is a prototypic systemic autoimmune disease characterized by high levels of serum autoantibodies against different components of cells, leading to systemic tissue and organ damage [1]. However, the aetiology and pathogenic processes of SLE are still not fully understood. While genetic and environmental factors contribute to the development of SLE, immunological dysfunction is also important. Indeed, proinflammatory CD4+ T cells, particularly follicular helper T (Tfh) cells, can help in producing autoantibodies by activated B cells, but other anti-inflammatory CD4+ T cells may inhibit the pathogenesis of SLE directly and indirectly [2]. Therefore, further illustration of the precise role of anti-inflammatory CD4+ T cells will be of great significance.
Human regulatory T cells (Treg) are important regulators of immune tolerance and can actively suppress proinflammatory T cell responses [3,4]. Quantitative and/or qualitative deficiencies of Tregs have been associated with the development of autoimmune diseases [5,6]. Previous studies have shown that a deficiency in Tregs is associated with the development of lupus-like characteristics, including glomerulonephritis and the development of DNA-specific antibodies [7–9]. Human Tregs are CD4+CD25+CD127−, and their development and function depend on forkhead box protein 3 (FoxP3) expression [5,10,11]. Indeed, mutations in the FoxP3 gene result in the immune dysregulation, polyendocrinopathy, enteropathy and X-linked syndrome (IPEX) in humans [12]. A lower frequency of circulating CD4+CD25+ FoxP3+ T cells was detected in patients with SLE [13], and the numbers of CD4+CD25+ FoxP3+ T cells were associated negatively with the disease activity or anti-dsDNA antibody titres in SLE patients [14–16]. However, recent studies have shown that FoxP3 is also expressed in some CD4+CD25− T cells [17–19]. These CD4+CD25−FoxP3+ T cells are induced peripherally by environmental antigens presented by dendritic cells (DCs) [18,20]. The function of CD4+CD25−FoxP3+ T cells remains controversial. While some studies showed that CD4+CD25−FoxP3+ T cells inhibited inflammation, others indicated that CD4+CD25−FoxP3+ T cells lacked inhibitory function [16–19]. However, little is known about the frequency of CD4+CD25−FoxP3+ T cells in Chinese patients with new-onset SLE and whether or not these cells are associated with disease development.
In addition, recent studies have shown that some CD4+FoxP3+ T cells express CXCR5, a chemokine receptor for its ligand of CXCL13, and that the CD4+CXCR5+FoxP3+ T cells share phenotypical characteristics with Tfh cells [21–24]. Wollenberg et al. [21] found that these CD4+CXCR5+FoxP3+ T cells actively suppressed Tfh cell proliferation and antibody production. However, little is known about the frequency and potential association of CD4+CXCR5+FoxP3+ T cells with the development of SLE in Chinese patients.
Currently, patients with new-onset SLE are treated with anti-SLE therapy, which has been demonstrated to be effective in the control of disease activity. However, little is known about the impact of anti-SLE therapy on the frequency of circulating CD4+CD25+FoxP3+, CD4+CD25−FoxP3+ and CD4+CXCR5+FoxP3+ T cells in SLE patients.
In the current study, we characterized the numbers of circulating CD4+CD25+FoxP3+, CD4+CD25−FoxP3+ and CD4+CXCR5+FoxP3+ T cells, and examined the concentrations of serum interleukin (IL-10) in patients with new-onset SLE and healthy controls (HC), and determined the potential association of the numbers of these types of FoxP3+ T cells and the concentrations of serum IL-10 with clinical measures in these patients. Furthermore, we examined the dynamic changes in the numbers of those types of FoxP3+ T cells and the concentrations of serum IL-10 in these patients following the anti-SLE therapies.
Materials and methods
Subjects
A total of 23 patients with new-onset SLE were recruited from the in-patient service of the First Hospital of Jilin University, Changchun, China. Individual patients with SLE were diagnosed according to the criteria established by the American College of Rheumatology (ACR) [25]. The disease activity of individual patients was assessed using the SLE disease activity index (SLEDAI). Twenty gender-, age- and ethnicity matched healthy subjects were also recruited as HC. Individuals were excluded if she/he had a history of another autoimmune disease, recent infection or received immune suppressive or glucocorticoid therapies within the past 6 months. Written informed consent was obtained from individual participants. The experimental protocol was established according to the guidelines of the 1975 Declaration of Helsinki and approved by the Human Ethics Committee of Jilin University, China.
The demographic and clinical data, including age, sex and current medications, of individual subjects were collected. Their demographic and clinical characteristics are summarized in Table 1.
Table 1.
The demographic and clinical characteristics of subjects
| Parameters | HC | SLE (0W) | SLE (4W) | SLE (12W) |
|---|---|---|---|---|
| Number | 20 | 23 | 23 | 23 |
| Age (years) | 27 (22–48) | 26 (11–50) | 26 (11–50) | 26 (11–50) |
| Female/male (n) | 18/2 | 21/2 | 21/2 | 21/2 |
| SLEDAI | – | 14 (8–24) | 4 (2–16)† | 3 (2–12)† |
| Anti-dsDNA(+) | – | 15 (65·2%) | 17 (73·9%) | 10 (43·5%)† |
| Anti-Sm(+) | – | 20 (87%) | 17 (73·9%)† | 11 (47·5%)† |
| CRP (mg/l) | 3·5 (0–5) | 40 (20–120)* | 28 (3–78)† | 12 (2–42)† |
| ESR (mM/h) | 5 (3–21) | 44 (24–142)* | 37 (12–59) | 8 (5–39)† |
| C3 (units/ml) | – | 0·55 (0·16–0·82) | 0·54 (0·18–0·94) | 0·34 (0·08–0·55)† |
| C4 (units/ml) | – | 0·07 (0·02–0·17) | 0·05 (0·02–0·18) | 0·02 (0·01–0·09)† |
| WBC (×109/l) | 7·7 (3·6–9·4) | 4·2 (2·1–6·5)* | 7·1 (1·6–11·8)† | 8·7 (2·7–12·6)† |
| PBMCs (×109/l) | 2·7 (0·9–4·5) | 1·5 (0·6–3·1)* | 2·1 (0·5–4·72)† | 2·6 (0·8–4·9)† |
Data shown are median (range) of each group of subjects. SLEDAI: systemic lupus erythematosus (SLE) disease activity index; CRP: C-reactive protein; PBMCs: peripheral blood mononuclear cells; ESR: erythrocyte sedimentation rate (normal range: men, 0 ∼ 15 mM/h; women, 0 ∼ 20 mM/h); C3 and C4: complements 3 and 4 (normal ranges: C3, 0·9 ∼ 1·8E × 10−3units/ml; C4, 0·43 ∼ 0·64 × 10−3 units/ml); WBC: white blood cells (normal range: 4 ∼ 10 × 109/l); 0W: baseline; 4W and 8W: weeks after therapies; –: undetected.
P < 0·05 versus HC;
P < 0·05 versus baseline values.
Treatment
After being admitted, individual patients were treated with anti-SLE therapy. A total of 13 patients were treated orally with 5 mg prednisone (Wyeth, Suzhou, China) daily for 1 week and with 400 mg hydroxychloroquine (HCQ; Shanghai Xinyi Pharmacy, Shanghai, China) daily for 12 weeks; four patients orally with 10 mg prednisone, 20 mg leflunomide daily (Fujian Huitian Pharmacy, Fujian, China) and 10 mg methotrexate (MTX; Shanghai Xinyi Pharmacy, Shanghai, China) once per week for 12 weeks; and six patients with 10 mg prednisone daily, 10 mg MTX (Shanghai Xinyi Pharmacy) once per week and 150 mg cyclophosphamide (CTX; Boehringer Ingelheim, Shanghai, China) once per 3 weeks for 12 weeks, as described previously [25]. Individual patients with the values of SLEDAI < 6 post-therapy were defined as drug responders, while those with the values of SLEDAI > 6 post-therapy were considered as drug non-responders. After being discharged, these patients visited the out-patient service of our department for office visits and laboratory tests.
Clinical measurements
Peripheral venous blood samples were obtained from individual participants for laboratory tests before treatment and 4 and 12 weeks after the initial treatment. The routine laboratory investigations included full blood counts, the concentrations of serum C-reactive protein (CRP) and complement factors C3 and C4, which were determined by scattered turbidimetry on a Siemens special protein analyser (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany).
Flow cytometry analysis
Venous blood samples were collected from individual subjects, and peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Little Chalfont, UK). PBMCs at 5 × 105/tube were stained in duplicate with phycoerythrin-cyanin 7 (PE-Cy7)-anti-CD4/AlexaFluor647-anti-CXCR5, peridinin chlorophyll (PerCP)-anti-CD4/fluorescein isothiocyanate (FITC)-anti-CD25 or isotype-matched controls (BD PharMingen, San Diego, CA, USA) for 30 min, fixed and permeabilized using the permeabilization solution (BD Biosciences, San Jose, CA, USA), followed by intracellular staining with PE-anti-FoxP3 (BD PharMingen). After being washed with phosphate-buffered saline (PBS), the numbers of CD4+CXCR5+FoxP3+, CD4+CD25+FoxP3+ and CD4+CD25−FoxP3+ T cells were determined by flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of serum IL-10 in individual subjects were determined by enzyme-linked immunosorbent assay (ELISA) using a human IL-10 ELISA kit, according to the manufacturer's instructions (Roche Diagnostics, Lewes, UK). Briefly, individual sera at 1:4 dilutions were subjected to ELISA analysis and the concentrations of serum IL-10 in individual samples were calculated, according to a standard curve established using the recombinant IL-10 provided. The limitation of detection for IL-10 was 2·5 ng/l.
Statistical analysis
Data are expressed as median and range of each group unless specified otherwise. The difference between groups was analysed by the Kruskal–Wallis test or χ2 test using spss version 16·0 software. The relationship between variables was evaluated using Spearman's rank correlation test. A two-sided P-value < 0·05 was considered statistically significant.
Results
Clinical characteristics of SLE patients
To examine the frequency of different types of CD4+FoxP3+ T cells in patients with new-onset SLE, a total of 23 patients with new-onset SLE and 20 age- and gender-matched HC were recruited. As expected, there was no significant difference in the distribution of age and gender between the patients and HC (Table 1). Patients with new-onset SLE displayed varying degrees of disease severity, and the majority of patients were seropositive for anti-dsDNA and anti-Sm. Furthermore, significantly higher levels of serum CRP, C3 and C4, but lower values of erythrocyte sedimentation rate (ESR), white blood cells (WBC) and PBMCs, were detected in the patients compared with that in the HC in this population.
After treatment for 4 or 12 weeks, 16 and 20 patients, respectively, responded to the therapy. As a result, the values of SLEDAI in 16 or 20 of 23 patients at 4 or 12 weeks, respectively, post-treatment were reduced to <6. Similarly, the values of disease activity, the positivity of autoantibodies and the concentrations of serum CRP as well as ESR were reduced significantly compared with that before treatment. In addition, the levels of serum C3 and C4 decreased significantly, but the numbers of WBC and PBMCs increased significantly in the patients at 4 or 12 weeks post-treatment. Therefore, the anti-SLE treatment controlled systemic inflammation effectively in most patients with new-onset SLE.
Altered numbers of CD4+CD25−FoxP3+, CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells and changed levels of serum IL-10 in new-onset SLE patients
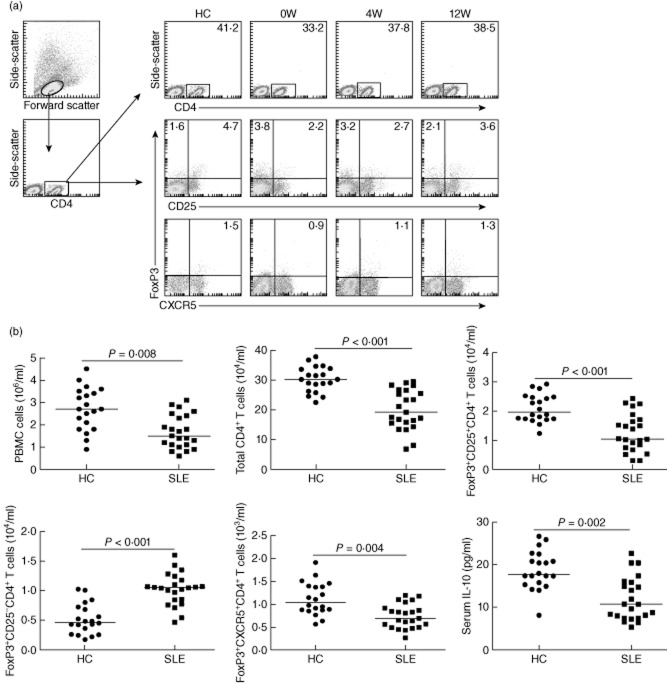
Tregs are crucial regulators of inflammation. Next, we examined the frequency of different types of CD4+FoxP3+ T cells by flow cytometry analysis (Fig. 1a). Because of the significant difference in the numbers of PBMCs between the patients and HC, we further calculated the numbers of different types of CD4+FoxP3+ T cells. The numbers of CD4+CD25−FoxP3+ T cells in the patients with new-onset SLE was significantly greater than those in the HC (Fig. 1b). In contrast, the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells in the SLE patients were significantly fewer than those in the HC. Further analysis indicated that the concentrations of serum IL-10 in the SLE patients were also significantly lower than that in the HC. In addition, the concentrations of serum IL-10 were correlated positively with the numbers of CD4+CD25+FoxP3+ T cells (r = 0·394, P = 0·005) and negatively with the numbers of CD4+CD25−FoxP3+ T cells (r = −0·479, P = 0·021), but not with the numbers of CD4+CXCR5+FoxP3+ in the patients. Therefore, the numbers of different types of CD4+FoxP3+ T cells were altered, accompanied by reduced levels of serum IL-10 in Chinese patients with new-onset SLE.
Fig. 1.
Fluorescence activated cell sorter (FACS) analysis of circulating CD4+forkhead box protein 3 (FoxP3)+ T cells. Peripheral blood mononuclear cells (PBMCs) from individual systemic lupus erythematosus (SLE) patients and healthy control (HC) subjects were stained with phycoerythrin (PE)-cyanin 7 (Cy7)-anti-CD4 and Alexafluor647-anti-CXCR5, peridinin chlorophyll (PerCP)-anti-CD4 and fluorescein isothiocyanate (FITC)-anti-CD25 or isotype controls, fixed and permeabilized, followed by intracellular staining with PE-anti-FoxP3. The frequency of total CD4+, CD4+CD25-FoxP3+, CD4+CD25+FoxP3+, and CD4+CXCR5+FoxP3+ T cells was determined by flow cytometry analysis. The cells were gated on living lymphocytes and then gated on CD4+ cells, and at least about 30 000 events were analysed for each sample. The numbers of each type of CD4+FoxP3+ T cells were calculated, according to the total numbers of PBMCs, the frequency of total CD4+ and different types of CD4+FoxP3+ T cells. The concentrations of serum interleukin (IL)-10 in individual subjects were determined by enzyme-linked immunosorbent assay (ELISA). (a) Representative charts of flow cytometry analysis. (b) Quantitative analysis. Data shown are representative FACS charts or the mean numbers of each type of cells per ml of peripheral blood and the mean levels of serum IL-10 in individual subjects from two separate experiments. The horizontal lines indicate the median values for each group.
Anti-dsDNA is commonly detected in some SLE patients. To analyse the relationship between the presence of serum anti-dsDNA and different types of CD4+FoxP3+ T cells, we stratified the patients according to individuals with seropositive and seronegative anti-dsDNA. We found that the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ in the anti-dsDNA+ patients were significantly fewer than those in the anti-dsDNA– patients, while the numbers of CD4+CD25−FoxP3+ in the anti-dsDNA+ patients were significantly greater than those in the anti-dsDNA− patients in this population. Furthermore, the levels of serum IL-10 in the anti-dsDNA+ patients were significantly lower than that in the anti-dsDNA− patients (Fig. 2). Apparently, the development of anti-dsDNA is associated with altered numbers of CD4+FoxP3+ T cells in Chinese patients with new-onset SLE.
Fig. 2.
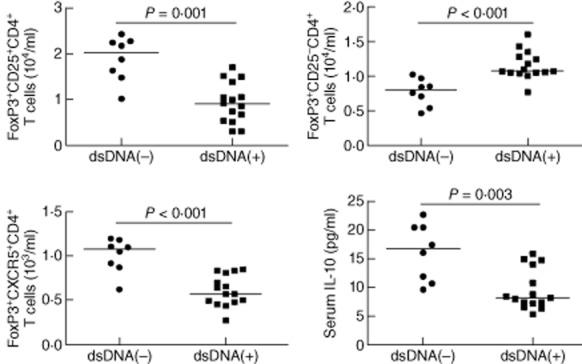
Stratification analysis of the numbers of different types of CD4+forkhead box protein 3 (FoxP3)+ T cells and the levels of serum interleukin (IL)-10 in seropositive and seronegative anti-ds-DNA systemic lupus erythematosus (SLE) patients. The patients were stratified into seropositive (15 cases) and seronegative (eight cases) anti-dsDNA groups, the numbers of CD4+CXCR5+FoxP3+, CD4+CD25+FoxP3+ and CD4+CD25−FoxP3+ T cells, and the levels of serum IL-10 in these two groups of patients were analysed. The horizontal lines indicate the median values for each group.
The relationship between the numbers of different types of CD4+FoxP3+ T cells and the values of clinical measures in patients with new-onset SLE
We further determined the potential association between the numbers of different types of CD4+FoxP3+ T cells and the clinical measures in these patients. We found that the numbers of CD4+CD25+FoxP3+ T cells were correlated negatively with the values of SLEDAI, whereas the numbers of CD4+CD25−FoxP3+ T cells were correlated positively with the values of SLEDAI, ESR and the levels of serum C3 in these patients (Fig. 3). However, there was no significant association between the numbers of CD4+CXCR5+FoxP3+ T cells and the other clinical measures tested in this population (data not shown). Furthermore, the concentrations of serum IL-10 were correlated negatively with the values of SLEDAI, serum C3, CRP and ESR in these patients (Fig. 3). These data suggest that the numbers of CD4+CD25+FoxP3+ and CD4+CD25−FoxP3+ T cells and the concentrations of serum IL-10 may be valuable markers for the evaluation of disease activity in patients with new-onset SLE.
Fig. 3.
Correlation analysis among the numbers of different types of CD4+forkhead box protein 3 (FoxP3)+ T cells, the levels of serum IL-10 and the values of clinical measures in systemic lupus erythematosus (SLE) patients. The potential correlations among the numbers of CD4+CXCR5+FoxP3+, CD4+CD25+FoxP3+ and CD4+CD25−FoxP3+ T cells, the levels of serum interleukin (IL)-10 and the values of clinical measures tested in these patients were analysed by Spearman's correlation tests. Data shown are the mean values of individual patients.
Treatment with anti-SLE therapy modulates significantly the numbers of different types of FoxP3+ regulatory T cells and concentrations of serum IL-10 in SLE patients
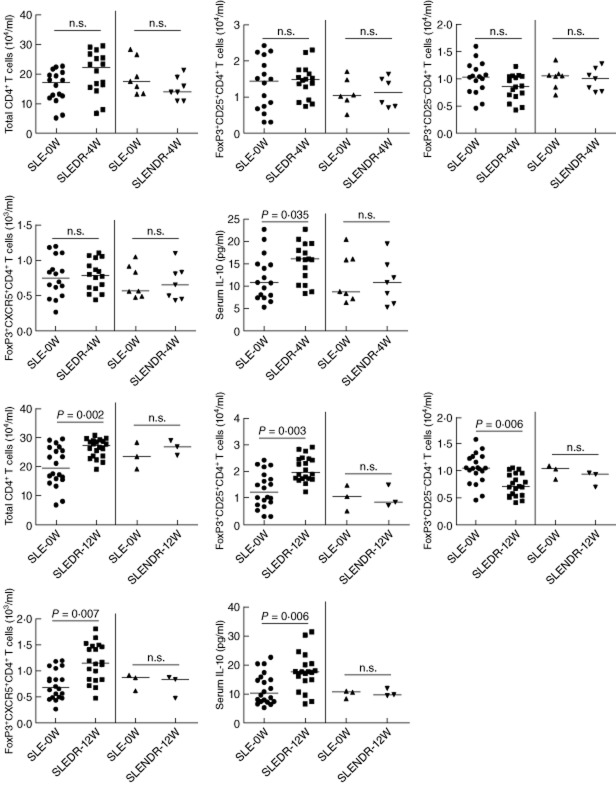
Finally, we tested how the treatment affected the numbers of different types of CD4+FoxP3+ T cells and the concentrations of serum IL-10 in these patients. After treatment for 4 or 12 weeks, 16 or 20 of 23 patients were classified into the drug-responders (SLEDAI < 6) and others were classified into the drug non-responders (SLEDAI > 6), respectively. We found that the concentrations of serum IL-10 increased significantly in the drug-responding patients at 4 and 12 weeks post-treatment compared with that before treatment (Fig. 4). However, the levels of serum IL-10 in the drug non-responding patients at 4 or 12 weeks post-treatment were not significantly different from that before the treatment. Furthermore, there was no significant change in the numbers of CD4+CD25+FoxP3+, CD4+CD25−FoxP3+ and CD4+CXCR5+FoxP3+ T cells in the drug non-responding patients between before and after treatment. Interestingly, following the treatment for 12 weeks, the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells in the drug-responding patients were greater than the baseline values, accompanied by significantly reduced numbers of CD4+CD25−FoxP3+ in these patients (Fig. 4). More importantly, the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ were correlated positively with the total numbers of CD4+ T cells in drug-responding patients (r = 0·643, P = 0·007; r = 0·583, P = 0·018 for 4 weeks; r = 0·609, P = 0·004; r = 0·688, P = 0·0018 for 12 weeks). However, there was no significant association of the numbers of CD4+CD25−FoxP3+ with the numbers of CD4+CD25+FoxP3+ or CD4+CXCR5+FoxP3+ T cells in this population.
Fig. 4.
Anti-systemic lupus erythematosus (SLE) therapy significantly improves the imbalance in the numbers of different types of CD4+forkhead box protein 3 (FoxP3)+ T cells and the levels of serum interleukin (IL)-10 in SLE patients. A total of 23 SLE patients were treated with the anti-SLE therapy for 4 or 12 weeks, and 16 or 20 patients responded (SLEDR) and others did not respond (SLENDR), respectively. The numbers of total CD4+ T and different types of CD4+FoxP3+ T cells and the levels of serum IL-10 in these patients were determined at the indicated time-points. Data are expressed as the mean numbers of different types of cells or the mean levels of serum IL-10 in individual patients. The horizontal lines indicate the median values for each group. SLE-0W: values before treatment; SLE-4W: values at 4 weeks post-treatment; SLE-12W: values at 12 weeks post-treatment; n.s.: not significant.
Next, to evaluate the effect of different anti-SLE drugs in modulating the imbalance between effector cells and Tregs, we stratified the drug-responding patients according to anti-SLE drugs in different subgroups of patients (Fig. 5). In comparison with that before the treatment, treatment with prednisone and HCQ (the group A, n = 7), prednisone, leflunomide and MTX (group B, n = 4) or prednisone, MTX and CTX (group C, n = 5) for 4 weeks did not change the numbers of CD4+, CD4+CD25+FoxP3+, CD4+CXCR5+FoxP3+ and CD4+CD25−FoxP3+ T cells significantly, but increased levels of serum IL-10 significantly in patient groups B and C (Fig. 5a). Furthermore, treatment with these drugs for 12 weeks increased significantly the numbers of CD4+ and CD4+CD25+FoxP3+ T cells in patient groups A–C, CD4+CXCR5+FoxP3+ in patient groups B and C, but reduced significantly the numbers of CD4+CD25−FoxP3+ T cells in patient groups A–C (Fig. 5b). Similarly, these treatments also elevated the levels of serum IL-10 in all drug responders. It notable that the patients who received prednisone, leflunomide and MTX had the greatest changes in the numbers of different subsets of FoxP3+ T cells and in the levels of serum IL-10 in this population. Collectively, these data indicated that the anti-SLE therapy improved significantly the imbalance of different subsets of CD4+FoxP3+ T cells and increased the concentrations of serum IL-10 in these patients; treatment with leflunomide and MTX may be more effective in modulating the imbalance between effector cells and Tregs.
Fig. 5.
The effects of different anti-systemic lupus erythematosus (SLE) therapies in modulating the imbalance between effector and regulator T cells (Tregs) in drug-responding patients. We stratified the drug-responding patients according to the therapeutic drugs and analysed the numbers of total CD4+, CD4+CD25+ forkhead box protein 3 (FoxP3)+, CD4+CD25−FoxP3+ and CD4+CXCR5+FoxP3+ T cells and the levels of serum IL-10 at 4 (a) and 12 (b) weeks post-treatment. Data shown are the mean values of individual drug-responding patients and horizontal lines indicate the median values of individual groups. Baseline: values before treatment; (a) patients received prednisone and hydroxychloroquine (n = 7 at 4 weeks; n = 10 at 12 weeks); (b) patients received prednisone, leflunomide and methotrexate (n = 4 at 4 and 12 weeks); (c) patients received prednisone, methotrexate and cyclophosphamide (n = 5 at 4 weeks, n = 6 at 12 weeks); n.s.: not significant.
Discussion
Tregs are important regulators of inflammation and autoimmune responses [26]. Previous reports of the numbers of peripheral blood Tregs in SLE patients are contradictory [27,28]. In this study, we examined the numbers of different types of circulating Tregs in patients with new-onset SLE. We found that the numbers of CD4+CD25−FoxP3+ T cells in SLE patients were significantly greater than those in the HC, whereas the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells were significantly fewer than those in the HC. Apparently, there was an imbalance of CD4+CD25+FoxP3+, CD4+CXCR5+FoxP3+ and CD4+CD25−FoxP3+ T cells in patients with new-onset SLE. The findings of significantly fewer numbers of circulating CD4+CD25+FoxP3+ T cells in patients with new-onset SLE were consistent with previous studies [29,30], but were different from another report [7]. The difference may stem from the definition of Treg cells because Zhang et al. [7] considered CD4+CD25+ T cells as Treg cells in their study, which included CD4+CD25+ effector cells. Indeed, we found that the levels of serum IL-10 in the SLE patients were significantly higher than those in the HC and correlated positively with the numbers of CD4+CD25+FoxP3+ T cells in these patients. More importantly, the numbers of CD4+CD25+FoxP3+ T cells were correlated negatively with the values of SLEDAI and the concentrations of serum IL-10 were correlated negatively with the values of SLEDAI, serum C3, CRP and ESR. IL-10 is an inhibitory cytokine, and lower levels of serum Il-10 are detected in mouse models of SLE [31–33]. Therefore, our findings support the notion that CD4+CD25+FoxP3+ T cells and serum IL-10 were negative regulators of the SLE process and suggest that the significantly reduced numbers of CD4+CD25+FoxP3+ T cells and decreased levels of serum IL-10 may contribute to the development of SLE in Chinese patients.
Recent studies have shown that CD4+FoxP3+ T cells express CXCR5 and CD4+CXCR5+FoxP3+ T cells share phenotypical characteristics with Tfh cells [21–24]. Wollenberg et al. [21] found that these CD4+CXCR5+FoxP3+ T cells actively suppressed Tfh cell proliferation and antibody production. In this study, we found that the numbers of circulating CD4+CXCR5+FoxP3+ T cells in the SLE patients were significantly fewer than those in the HC. Indeed, the numbers of circulating CD4+CXCR5+FoxP3+ T cells in the patients with seropositive anti-dsDNA were significantly fewer than in those with seronegative anti-dsDNA, suggesting that the numbers of CD4+CXCR5+FoxP3+ T cells might be associated negatively with autoantibody production in patients with new-onset SLE. Given that Tfh cells are important for positively regulating autoantibody production, it is possible that CD4+CXCR5+FoxP3+ T cells may regulate Tfh cells and autoantibody production negatively [21]. However, there was no significant association between the numbers of CD4+CXCR5+FoxP3+ T cells and clinical measures in this population. Hence, the precise roles of CD4+CXCR5+FoxP3+ T cells in the development of SLE need to be explored further.
Previous studies have shown that CD4+CD25− T cells can be induced for FoxP3 expression [17–20,34,35]. Functionally, while CD4+CD25−FoxP3+ T cells have been shown to inhibit inflammation in autoimmune inflammatory bowel disease, others indicate that CD4+CD25−FoxP3+ T cells have no inhibitory function [16–19]. In this study, we found that the numbers of CD4+CD25−FoxP3+ T cells in the SLE patients were significantly greater than those in the HC and were associated with the production of anti-dsDNA in SLE patients. More importantly, the numbers of CD4+CD25−FoxP3+ T cells were correlated positively with the values of SLEDAI, ESR and serum C3. These novel findings suggest that CD4+CD25−FoxP3+ T cells may be positive regulators of the SLE process in Chinese patients. Conceivably, CD4+CD25−FoxP3+ T cells may be a new target for the design of immunotherapies for the intervention of SLE. Interestingly, Anyfantakis et al. [36] reported an unusual fatal case of a woman with late-onset SLE; progressive polyneuropathy was misdiagnosed as discoid lupus due to unusual clinical features and negative serology. Our findings of the imbalance of different subsets of FoxP3+ T cells may help in the diagnosis of these untypical cases in the clinic.
The anti-SLE therapy has been demonstrated to control symptoms effectively in some patients with SLE in the clinic. We found that the therapy improved the imbalance of different types of CD4+FoxP3+ T cells and that treatment with the anti-SLE therapy for 4 or 12 weeks reduced significantly the numbers of CD4+CD25−FoxP3+ T cells, but increased the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells and the levels of serum IL-10 in the drug-responding patients, but not in the drug non-responding patients. It is possible that immunosuppressants affect mainly CD4+CD25−FoxP3+ T cells which, like effector cells, are prone to proliferation. Moreover, the numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells were correlated positively with the numbers of CD4+ T cells in the drug-responding patients, respectively. However, there was no significant association of the numbers of CD4+CD25−FoxP3+ with the numbers of CD4+CD25+FoxP3+ or CD4+CXCR5+FoxP3+ T cells in this population. The data suggest that the increased numbers of CD4+CD25+FoxP3+ and CD4+CXCR5+FoxP3+ T cells may be predominantly from an increase in the total number of CD4+ T cells in these drug-responding patients. Consequently, the increased numbers of CD4+CD25+FoxP3+ T cells may result in elevated levels of serum IL-10 in these patients, given that the numbers of CD4+CD25+FoxP3+ T cells were correlated positively with the levels of serum IL-10 in these patients. Thus, our findings may provide new insights into the pharmacological mechanisms by which treatment with the anti-SLE therapy effectively improved clinical symptoms in patients with SLE. Moreover, stratification analyses indicated that treatment with prednisone, leflunomide and MTX resulted in the greatest changes in the numbers of total CD4+, different subsets of FoxP3+ T cells and the levels of serum IL-10 compared with those receiving prednisone and HCQ or prednisone, MTX and CTX. These findings suggest that treatment with leflunomide and MTX may be more effective in modulating the imbalance between effector and Tregs in SLE patients.
In summary, our data indicate significantly increased numbers of circulating CD4+CD25−FoxP3+ T cells but decreased numbers of CD4+CD25+FoxP3+ T cells and CD4+CXCR5+FoxP3+ T cells, accompanied by decreased levels of serum IL-10 in Chinese patients with new-onset SLE. These novel findings suggest that CD4+CD25−FoxP3+ T cells may not be inhibitory Tregs, and that an imbalance of different types of CD4+FoxP3+ T cells may be associated with the early process of SLE in Chinese patients. The anti-SLE therapy not only effectively improved clinical symptoms, but also modulated the imbalance of different types of CD4+FoxP3+ T cells in SLE patients. Therefore, our findings may provide new insights into the pathogenesis of SLE and aid in the design of new immunotherapies for the intervention of SLE in the clinic. We recognize that our study has limitations, including a small sample size and the lack of functional studies of different types of CD4+FoxP3+ T cells. Thus, further studies in a bigger population are warranted.
Acknowledgments
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Funding source
This study was supported by grants from the National Natural Science Foundation of China (no. 30972610 and 81273240), Jilin Province Science and Technology Agency (no. 20110716), the Health Department Research Projects of Jilin Province (2009Z054) and Bethune B plan of Jilin University.
Disclosure
The authors declare no financial or commercial conflicts of interest.
References
- 1.Kleczynska W, Jakiela B, Plutecka H, Milewski M, Sanak M, Musial J. Imbalance between Th17 and regulatory T-cells in systemic lupus erythematosus. Folia Histochem Cytobiol. 2011;49:646–653. doi: 10.5603/fhc.2011.0088. [DOI] [PubMed] [Google Scholar]
- 2.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+T cells. Proc Natl Acad Sci USA. 2002;99:8832–8837. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2011;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 6.Bonelli M, Savitskaya A, von Dalwigk K, et al. Quantitative and qualitative deficiencies of regulatory T cells in patients with ystemic lupus erythematosus (SLE) Int Immunol. 2008;20:861–868. doi: 10.1093/intimm/dxn044. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Zhang X, Tang FL, Zhu LP, Liu Y, Lipsky PE. Clinical significance of increased CD4+CD25–Foxp3+ T cells in patients with new onset systemic lupus erythematosus. Ann Rheum Dis. 2008;67:1037–1040. doi: 10.1136/ard.2007.083543. [DOI] [PubMed] [Google Scholar]
- 8.Hahn BH, Anderson M, Le E, La Cava A. Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2488–2497. doi: 10.1002/art.23609. [DOI] [PubMed] [Google Scholar]
- 9.Chavele KM, Ehrenstein MR. Regulatory T-cells in systemic lupus erythematosus and rheumatoid arthritis. FEBS Lett. 2011;585:3603–3610. doi: 10.1016/j.febslet.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 12.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3(+) regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Bonelli M, von Dalwigk K, Savitskaya A, Smolen JS, Scheinecker C. Foxp3 expression in CD4+ T cells of patients with systemic lupus erythematosus: a comparative phenotypic analysis. Ann Rheum Dis. 2008;67:664–671. doi: 10.1136/ard.2007.074690. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Wang LC, Lin YT, Yang YH, Lin DT, Chiang BL. Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology. 2006;117:280–286. doi: 10.1111/j.1365-2567.2005.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellor-Pita S, Citores MJ, Castejon R, et al. Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:553–554. doi: 10.1136/ard.2005.044974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 19.Yang HX, Zhang W, Zhao LD, et al. Are CD4+CD25–Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res Ther. 2009;11:R153. doi: 10.1186/ar2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Wollenberg I, Agua-Doce A, Hernández A, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 22.Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;6:975–983. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;6:983–989. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang E, Cho WS, Cho ML, et al. Foxp3+ regulatory T cells control humoral autoimmunity by suppressing the development of long-lived plasma cells. J Immunol. 2011;186:1546–1553. doi: 10.4049/jimmunol.1002942. [DOI] [PubMed] [Google Scholar]
- 25.Chang NH, Tamara M, Gabriel B, et al. Expanded population of activated antigen-engaged cells within the naive B cell compartment of patients with systemic lupus erythematosus. J Immunol. 2012;180:1276–1284. doi: 10.4049/jimmunol.180.2.1276. [DOI] [PubMed] [Google Scholar]
- 26.Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human Foxp3+ regulatory T cells in systemic autoimmune disease. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Gerli R, Nocentini G, Alunno A, et al. Identification of regulatory T cells in systemic lupus erythematosus. Autoimmun Rev. 2009;8:426–430. doi: 10.1016/j.autrev.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Horwitz DA. Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis Res Ther. 2008;10:227–236. doi: 10.1186/ar2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crispin JC, Martínez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;3:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 30.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;4:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 31.Katsikis PD, Chu CQ, Brennan FM, Maini RN, Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto A, Fujio K, Okamura T, Yamamoto K. Regulatory T-cell-associated cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:1–9. doi: 10.1155/2011/463412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter NA, Vasconcellos R, Rosser EC, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 34.Ye ZJ, Zhou Q, Du RH, Li X, Huang B, Shi HZ. Imbalance of Th17 cells and regulatory T cells in tuberculous pleural effusion. Clin Vaccine Immunol. 2011;18:1608–1615. doi: 10.1128/CVI.05214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isomaki P, Clark JM, Panesar M, Cope AP. Pathways of T cell activation and terminal differentiation in chronic inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:287–293. doi: 10.2174/1568010054022042. [DOI] [PubMed] [Google Scholar]
- 36.Anyfantakis D, Symvoulakis EK, Barbounakis E, et al. A fatal case of seronegative, late-onset systemic lupus erythematosus presenting with motor sensory axonal polyneuropathy. Mod Rheumatol. 2013 doi: 10.3109/14397595.2013.874743. doi 10.1007/s10165-013-0842-y. [DOI] [PubMed] [Google Scholar]