Abstract
Plasmacytoid dendritic cells (PDC) are involved in innate immunity by interferon (IFN)-α production, and in adaptive immunity by stimulating T cells and inducing generation of regulatory T cells (Treg). In this study we studied the effects of mammalian target of rapamycin (mTOR) inhibition by rapamycin, a commonly used immunosuppressive and anti-cancer drug, on innate and adaptive immune functions of human PDC. A clinically relevant concentration of rapamycin inhibited Toll-like receptor (TLR)-7-induced IFN-α secretion potently (−64%) but TLR-9-induced IFN-α secretion only slightly (−20%), while the same concentration suppressed proinflammatory cytokine production by TLR-7-activated and TLR-9-activated PDC with similar efficacy. Rapamycin inhibited the ability of both TLR-7-activated and TLR-9-activated PDC to stimulate production of IFN-γ and interleukin (IL)-10 by allogeneic T cells. Surprisingly, mTOR-inhibition enhanced the capacity of TLR-7-activated PDC to stimulate naive and memory T helper cell proliferation, which was caused by rapamycin-induced up-regulation of CD80 expression on PDC. Finally, rapamycin treatment of TLR-7-activated PDC enhanced their capacity to induce CD4+forkhead box protein 3 (FoxP3)+ regulatory T cells, but did not affect the generation of suppressive CD8+CD38+lymphocyte activation gene (LAG)-3+ Treg. In general, rapamycin inhibits innate and adaptive immune functions of TLR-stimulated human PDC, but enhances the ability of TLR-7-stimulated PDC to stimulate CD4+ T cell proliferation and induce CD4+FoxP3+ regulatory T cell generation.
Keywords: IFN-α, mTOR, Toll-like receptor
Introduction
Plasmacytoid dendritic cells (PDC) have important functions in innate and adaptive immunity. They are unique in rapidly producing massive amounts of type I interferon upon recognition of viral nucleotides or self-DNA-protein complexes by their Toll-like receptors (TLR). In addition, after maturation in response to TLR-ligation or CD40-engagement they acquire dendritic cell morphology and capacity to present antigens to T cells. Mature PDC can activate as well as inhibit T cell responses. On one hand, mature PDC can prime productive CD4+ and CD8+ T cell responses [1], and on the other hand they possess a capacity to induce generation of CD4+ and CD8+ regulatory T cells (Treg) from naive CD4+ or CD8+ T cells, respectively [2–7]. Recently, we showed that human PDC preferentially induce generation of a unique type of CD8+ Treg, but not CD4+forkhead box protein 3 (FoxP3)+ Treg, when both CD4+ and CD8+ T cells are present [8]. Importantly, these CD8+CD38+lymphocyte activation gene (LAG)-3+ CTLA-4+ Treg were not only able to inhibit naive T cells, but also memory T cell responses. Indeed, in vivo, depending on the experimental animal model, PDC either induce effective T cell immunity [9–11] or inhibit T cell responses by driving differentiation of Treg in vivo [12–14]. A recent study in which PDC were eliminated selectively from mice showed that PDC can simultaneously suppress and stimulate T cell responses in vivo [15].
Recently, it has been shown that the selective mammalian target of rapamycin (mTOR)-inhibitor rapamycin inhibits production of interferon (IFN)-α and proinflammatory cytokines by TLR-activated mouse PDC, and reduces their capacity to stimulate CD4+ T cells. Rapamycin was found to block the interaction of TLR with myeloid differentiation primary response gene 88 (MyD88), resulting in reduced interferon regulatory factor-7 (IRF-7) phosphorylation [16]. However, important questions regarding the effects of rapamycin on PDC functions have still be to be resolved. First, the effect of rapamycin on the ability of PDC to generate Treg has not been studied. Secondly, Cao et al. studied mouse PDC and, whereas they recapitulated the inhibitory effect of rapamycin on IFN-α secretion on human PDC, it remains to be established whether and how rapamycin affects the T cell stimulatory capacity of human PDC.
These questions are clinically highly relevant, because the indications for rapamycin treatment are expanding. Used originally as an immunosuppressive drug in transplant recipients, rapamycin and rapamycin analogues are now increasingly being evaluated as an anti-proliferative drug in cancer treatment [17]. Moreover, studies have been initiated to determine its efficacy in autoimmune diseases such as systemic lupus erythematosus (SLE) [18], which are caused mainly by overproduction of IFN-α by PDC [19,20].
Therefore, the aims of the present study were to determine systematically the effects of a clinically relevant concentration of rapamycin on cytokine production, T cell stimulatory capacity and CD8+ Treg-generating capacity of human PDC.
Materials and methods
Reagents
Interleukin (IL)-3, anti-blood dendritic cell antigen (BDCA)2-fluorescein isothiocyanate (FITC), anti-BDCA4-phycoerythrin (PE), anti-BDCA1-PE monoclonal antibody (mAb), CD15 microbeads, CD235 microbeads, CD14 microbeads, anti-PE microbeads, MS, LS and LD columns were obtained from Miltenyi Biotec (Bergisch Gladbach, Germany). 7-Aminoactinomycin D (7-AAD), anti-human leucocyte antigen DR (HLA-DR)-allophycocyanin (APC), CD3-peridinin chlorophyll (PerCP), CD4-PerCP, CD45RO-APC, CD56-FITC, p-S6-Pacific blue, CD3-horizon V500, CD8-Pacific blue, CD25-PE and CD14-PE mAb were obtained from BD Biosciences (Erembodegem, Belgium). CD19-PE, CD45RA-FITC, CD38-FITC, CD45-FITC, CD80-FITC and CD123-PE mAb were purchased from Beckman Coulter (Immunotech, Marseille, France) and CD40-APC, CD45RA-PE, immunoglobulin (Ig)G1-FITC, IgG2a-FITC, CD8-APC, anti-IFN-γ-PECy7, IL-17-PE, CD4-APC-eFluor780, anti-FoxP3-APC (clone: 236A/E7), functional grade IgG2a isotype control mAb and IFN-α, IL-6, IL-10 and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were obtained from eBiosciences (Vienna, Austria). CD86-APC, anti-HLA-ABC-FITC, anti-IL-10-APC and IgG1-APC were obtained from Biolegend (London, UK). cytosine–phosphate–dinucleotide (CpG) A oligodeoxynucleotide (ODN) 2336 and loxoribine (LOX) were purchased from InVivogen (San Diego, CA, USA). Anti-LAG3-PE and IL-17 ELISA kit were purchased from R&D Systems (Abingdon, UK). IFN-γ, IL-4 and CXCL-10 (IP-10) ELISA kits and 5,6 carboxy-succinimidyl-fluorescein ester (CFSE) were purchased from Life Technologies (Bleiswijk, the Netherlands). Neutralizing IFN-αReceptor2 mAb was obtained from Merck Millipore (Amsterdam, the Netherlands). Rabbit anti-phosphorylated S6 antibody was from Cell Signaling Technology (Danvers, MA, USA) and mouse-anti-β-actin antibody from SantaCruz Technology (Heidelberg, Germany). Granulocyte–macrophage colony-stimulating factor (GM-CSF) was a kind gift of Schering-Plough (Kenilworth, NJ, USA) and neutralizing CD80 mAb B7-24 [21] was a kind gift of M. de Boer (Tanox Pharma BV, Amsterdam, the Netherlands), phytohaemagglutinin (PHA) was obtained from Murex (Paris, France). Rapamycin was purchased from Merck (Schiphol-Rijk, the Netherlands) and phosphatase and tensin homologue (PTEN)-inhibitor VO-OHpic trihydrate, PMA, ionomycin and brefeldin A from Sigma-Aldrich (St Louis, MO, USA). The Fix&perm cell permeabilization kit was obtained from An der Grub (Vienna, Austria).
Purification of PDC, T cells and monocytes from human blood and generation of monocyte-derived DC
PBMC were isolated from buffy coats of healthy blood-bank donors by Ficoll density centrifugation. For isolation of PDC, PBMC were incubated with anti-BDCA4-PE mAb, washed and incubated with anti-PE microbeads. After a second wash, PDC were isolated in two rounds of separation over MS columns. Alternatively, BDCA-4-labelled PDC were isolated by enrichment over an LS-column, followed by flow cytometric sorting on a FacsAria II cellsorter. The purity of isolated PDC, as determined by staining with anti-BDCA2-FITC and flow cytometry, was > 94%. T cells were purified from PBMC by negative selection upon labelling with PE-conjugated antibodies against BDCA1, CD14, CD19, CD56 and CD123 as well as CD15 and CD235 microbeads followed by incubation with anti-PE microbeads. T cells were isolated over an LD column. The purity of the T cells analysed after labelling with CD3-PerCP and CD45-FITC was > 97%. In selected experiments, the isolated T cells were labelled with CD45RA-FITC and CD45RO-APC to isolate naive and memory T cells by flow cytometric sorting. Monocytes were isolated using CD14 microbeads over an MS column. Purity was > 98%. For the generation of monocyte-derived DC (MoDC) monocytes (1 × 106/ml) were cultured in RPMI supplemented with 10% fetal calf serum (FCS), 50 ng/ml GM-CSF and 200 U/ml IL-4 for 7 days. On day 6, 500 ng/ml lipopolysaccharide (LPS) was added to stimulate MoDC maturation.
Analysis of PDC immunophenotype and cytokine production
Purified PDC (2 × 104/200 μl RPMI supplemented with 10% FCS) were stimulated in round-bottomed wells with 5 μg/ml CpG A ODN2336 or 400 μM loxoribine in the absence or presence of 20 ng/ml rapamycin. In all conditions 10 U/ml IL-3 was added as a survival factor. Rapamycin, or dimethylsulphoxide (DMSO) vehicle in the case of non-stimulated cells, were added 1 h before addition of the stimuli. After 18 h supernatants were collected for quantification of cytokines, and the PDC immunophenotype was analysed. The following combinations of antibodies were used: CD80-FITC, anti-BDCA4-PE and CD86-APC, anti-BDCA4-PE and CD40-APC, and anti-HLA-ABC-FITC, anti-BDCA4-PE and anti-HLA-DR-APC. Dead cells were excluded with 7-AAD. Cells were analysed on a Canto II flow cytometer using Diva version 6·0 software (Becton Dickinson) or a Calibur flow cytometer with CellQuest Pro version 5·2 software. Isotype-matched irrelevant mAb labelling was used to analyse expression of these molecules appropriately.
Western blot analysis
PDC were stimulated with CpG-A or loxoribine as described, and thereafter lysed in Laemmli buffer. The lysates were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on Immobilon-FL transfer membrane (Millipore, Billerica, MA, USA). The membranes were incubated with the appropriate antibodies, and for detection anti-rabbit or anti-mouse IRDye-conjugated secondary antibodies (Li-cor Biosciences, Lincoln, NE, USA) were used according to the manufacturer's directions. The blots were scanned by Odyssey infrared imaging (LI-COR Biosciences). Results were visualized with Odyssey version 3·0 software.
Stimulation of T cells with allogeneic PDC
After stimulation of purified PDC (2 × 104/200 μl) for 18 h with CpG A ODN 2336 or loxoribine in the absence or presence of 20 ng/ml rapamycin, rapamycin was carefully washed away, and allogeneic CD3+ T cells were added (1 × 105/200 μl RPMI supplemented with 10% FCS). The cells were cultured at 37°C with 5% CO2. Proliferation was determined after 5 days of culture by measurement of incorporation of 0·5 μCi/well [3H]-thymidine (Radiochemical Centre, Amersham, Little Chalfont, UK) during the last 18 h of the culture. In all cultures, T cells stimulated with PHA (5 μg/ml) served as a positive control to assess their proliferative capacity. Alternatively, after 7 days cell-free supernatant was collected for cytokine analysis, total numbers of viable cells were counted using trypan blue exclusion and proportions of CD3+CD4+, CD3+CD8+ and CD8+CD38+LAG-3+ T cells were analysed. In another set of experiments, CFSE-labelled allogeneic naive and memory CD3+ T cells were added to PDC, and T cell proliferation was determined by flow cytometric measurement of CFSE dilution.
Cytokine measurement
The supernatants of the stimulated PDC were analysed for IFN-α, interleukin-6 and TNF-α concentrations by standard ELISA, according to the manufacturer's instructions. The IFN-α ELISA detects the main subtypes IFN-α2a, IFN-α2b and IFN-α2c. The supernatants of T cells co-cultured with allogeneic PDC were analysed for IFN-γ, IL-10, IL-4, IL-17 and CXCL-10 also by standard ELISA, according to the manufacturer's instructions. In other cases the cytokine production of LOX-PDC stimulated T cells was assessed by restimulating the T cells with PMA (40 ng/ml) and ionomycin (1 ug/ml) for 6 h. During the last 5 h of restimulation brefeldin A (5 ug/ml) was added to inhibit protein transport processes. Intracellular IFN-γ, IL-17 and IL-10 expression was determined by using Fix&perm cell permeabilization kit, according to the manufacturer's instructions.
Suppression assay
To assess the suppressive capacity of CD8+CD38+LAG3+ regulatory T cells generated during co-cultures with allogeneic PDC, CD8+CD38+LAG3+ T cells were purified from cultured cells by flow cytometric sorting using a FacsAria Cell Sorter (Becton Dickinson), and added in graded doses to cultures of CD3+ T cells (1 × 105/200 μl) that were stimulated with allogeneic irradiated (3000 rad) donor-specific MoDC (1·5 × 104) in round-bottomed wells. In these experiments Mo-DC and PDC were derived from the same donor. After 5 days, proliferation was assessed by determination of [3H]-thymidine incorporation for 18 h.
Statistical analysis
All experiments were performed n times, as indicated in the figure legends, with cells from different individuals, and mean values ± standard error of the mean (s.e.m.) were calculated. Significance of differences between paired observations was tested in the paired t-test using Microsoft Excel 2003 software. A P-value of less than 0·05 was considered significant.
Results
Effects of rapamycin on cytokine secretion by human PDC
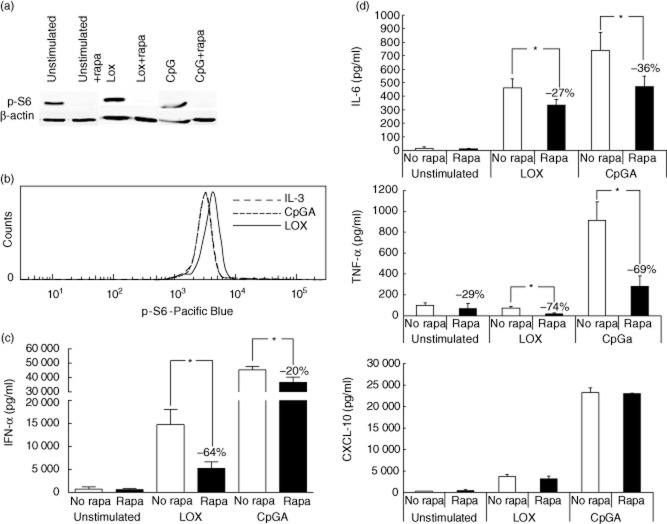
The effects of rapamycin were studied using purified human PDC stimulated with TLR-9 ligand CpG-A-ODN 2336 or TLR-7 ligand loxoribine, in the presence of IL-3 as essential survival factor. To determine whether a clinically relevant concentration of 20 ng/ml rapamycin, which is similar to the blood peak level reached during rapamycin treatment (Rapamune summary of product characteristics; Wyeth-Ayerst Pharmaceuticals Inc., Philadelphia, PA, USA), inhibits mTOR-signalling in PDC, we measured phosphorylation of the 40S ribosomal protein S6, which is a downstream phosphorylation target of mTOR [22]. Figure 1a shows that S6 is phosphorylated in both non-stimulated and stimulated PDC, and that 20 ng/ml rapamycin inhibits S6-phosphorylation completely in all conditions, indicating that this concentration of rapamcyin suppresses mTOR-signalling in PDC effectively.
Fig. 1.
Rapamycin inhibits mammalian target of rapamycin (mTOR) signalling and cytokine production in human plasmacytoid dendritic cells (PDC). (a). Immunoblot analysis of phosphorylated S6 protein in lysates of purified unstimulated PDC, PDC stimulated for 15 min with 400 μM loxoribine (LOX) or with 5 μg/ml cytosine–phosphate–dinucleotide (CpG) A oligodeoxynucleotide (ODN) 2336, in the absence or presence of 20 ng/ml rapamycin. In all conditions 10 IU/ml interleukin (IL)-3 was added as a survival factor. Rapamycin, or dimethylsulphoxide (DMSO) vehicle in the case of non-stimulated cells, were added 1 h before addition of the stimuli. As a reference protein, β-actin expression was determined. (b) Histogram shows intracellular p-S6 expression in PDC after the same stimulations as in (a). PDC stimulated with IL-3 (dotted line), with CpGA (dashed line) and loxoribine (solid line). Note that the lines for IL-3 and CpGA overlap each other. (c,d) Two × 104 human PDC in 200 μl were stimulated with 400 μM loxoribine, or 5 μg/ml CpG A ODN 2336, or left non-stimulated, in the absence or presence of 20 ng/ml rapamycin. IL-3 was added as a survival factor. Rapamycin, or DMSO vehicle in the case of non-stimulated cells, were added 1 h before addition of the stimuli. After 18 h cell-free media were harvested, and (c) interferon (IFN)-α and (d) IL-6, tumour necrosis factor (TNF)-α and CXCL10 were determined. Data depicted are means ± standard error of the mean of six independent experiments. *P < 0·03.
To determine if the stimuli enhanced the S6 phosphorylation, PDC were stimulated with CpGA or loxoribine in the presence of IL-3 and intracellular p-S6 expression was determined with flow cytometric staining (Fig. 1b). CpGA stimulation resulted in the same fluorescence intensity as IL-3 treatment alone, while loxoribine stimulation slightly increased the p-S6 expression.
CpG-A was a more effective stimulus than loxoribine to induce IFN-α secretion (Fig. 1c). While 20 ng/ml rapamycin inhibited loxoribine-induced IFN-α secretion by 64%, it inhibited CpG-A-induced IFN-α secretion by only 20%, despite almost complete suppression of mTOR-signalling. In contrast, secretion of the proinflammatory cytokines IL-6 and TNF-α was inhibited by rapamycin with similar efficacy in both stimulation conditions (Fig. 1d). The observed inhibitory effects of rapamycin were not due to general impairment of PDC function, because no inhibition of CXCL-10 secretion was observed (Fig. 1d) and rapamycin did not induce apoptosis, as demonstrated by the absence of active caspase-3 (data not shown).
As mTOR inhibition decreased cytokine secretion by PDC, we reasoned that mTOR stimulation might increase cytokine production. Therefore we added 10 nM VO-OHpic trihydrate, a specific inhibitor of PTEN, during PDC activation. The upstream signalling pathway that activates mTOR is initiated by phosphatidylinositol 3-kinase (PI3K), which generates 3-phosphorylated inositol lipids (PIP3) [23]. PTEN is a negative regulator of PIP3K-signalling because it dephosphorylates PIP3 [24], and therefore inhibition of PTEN can abrogate negative regulation of mTOR phosphorylation. The addition of VO-OHpic trihydrate to TLR-activated PDC in a concentration that increased generation of PDC from human CD34+ progenitor cells [25] did not, however, affect p-S6 expression and cytokine production by PDC (data not shown), suggesting that PI3K-mTOR signalling is not limited by PTEN in human PDC. Together, these data show that a clinically relevant concentration of rapamycin inhibits proinflammatory cytokine production by TLR-7-activated PDC and TLR-9-activated PDC, while it suppresses IFN-α secretion in TLR-7-activated PDC but almost not in TLR-9-engaged PDC.
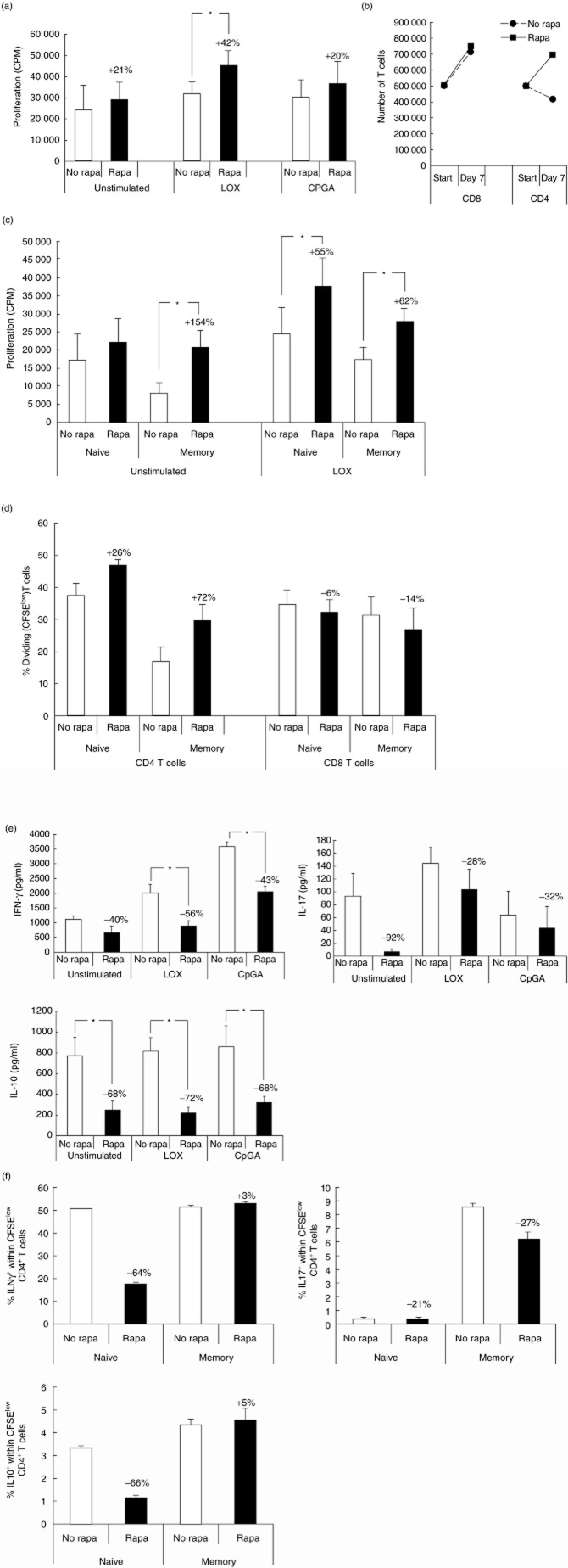
Rapamycin promotes the capacity of TLR-7-activated PDC to stimulate CD4+ T cell proliferation by enhancing CD80 expression
To study the effects of mTOR inhibition on the T cell stimulatory capacity of PDC, we activated PDC with TLR ligands for 18 h and then added allogeneic CD3+ T cells. After activation in the presence or absence of rapamycin, PDC were washed carefully to remove rapamycin before T cells were added. Activation of PDC via TLR-7 in the presence of rapamycin increased their capacity to stimulate T cell proliferation, while the addition of rapamycin during TLR-9 activation did not (Fig. 2a). The increased proliferation of T cells upon mTOR inhibition in TLR-7-activated PDC was confined to enhanced expansion of the CD4 compartment (Fig. 2b), and was observed in both memory (CD45RO+) and naive (CD45RA+) T cells (Fig. 2c). Experiments wherein CFSE-stained naive and memory CD3+ T cells were activated by TLR-7-stimulated PDC that were treated or not treated with rapamycin confirmed that proliferation of naive and memory CD4+ T cells, but not CD8+ T cells, was enhanced upon rapamycin treatment of PDC (Fig. 2d). Higher concentrations of rapamycin (up to 100 ng/ml) did not further enhance T cell proliferation after TLR-7 ligation of PDC. T cells stimulated by PDC secreted proinflammatory (IFN-γ, IL-17) and anti-inflammatory (IL-10) cytokines (Fig. 2e), but no T helper type 2 (Th2) cytokines (data not shown). Treatment of PDC with rapamycin suppressed the capacity of PDC to stimulate IFN-γ and IL-10 secretion by T cells irrespective of the mode of PDC-activation. Because rapamycin enhances the capacity of TLR-7 activated PDC to stimulate CD4+ T cells, we determined whether these CD4+ cells acquired a different cytokine production profile. CFSE-stained naive and memory T cells were stimulated by TLR-7 activated PDC that were treated or not treated with rapamycin. After 7 days these T cells were restimulated with PMA/ionomycin and intracellular IFN-γ, IL-17 and IL-10 accumulation was determined. Figure 2f shows that rapamycin treatment of PDC reduced the generation of IFN-γ-producing and IL-10-producing naive Th cells, while leaving IFN-γ and IL-10 production in the memory Th cell compartment unaffected. IL-17 was not induced in naive Th cells by TLR-7 PDC (< 1%), but rapamycin treatment of PDC slightly reduced the numbers of IL-17-producing memory Th cells. To find an explanation for the observed increase in T cell proliferation induced by rapamycin-treated TLR7-activated PDC, we determined the effects of rapamycin on the expression of major histocompatibility complex (MHC) and co-stimulatory molecules on PDC. Rapamycin did not affect expression of MHC class I and II molecules on PDC under any of the stimulation conditions (data not shown). CD40 expression on PDC was suppressed by rapamcyin in both stimulation conditions, while CD86 expression was not affected. Interestingly, rapamycin enhanced up-regulation of CD80 significantly on TLR-7-ligated PDC, but not on TLR-9-activated PDC (Fig. 3a). In the absence of rapamycin a subpopulation of TLR-7-stimulated PDC did not express CD80, while in the presence of rapamycin all PDC up-regulated CD80 expression. To determine whether the increased CD80 expression might be responsible for the increased ability of rapamycin-treated TLR-7-activated PDC to stimulate T cell proliferation, a neutralizing antibody against CD80 was added to co-cultures of TLR-7-stimulated PDC and allogeneic T cells. As rapamycin inhibits IFN-α production by TLR-7-activated PDC and IFN-α has an inhibitory effect on T cell proliferation [26,27], we also determined the effect of a neutralizing IFN-α-R2 antibody on the T cell stimulatory capacity of TLR-7-activated PDC. Addition of the anti-IFN-αR2 antibody did not abolish the difference in T cell stimulatory ability between PDC that were treated or not treated with rapamycin (Fig. 3b). However, the blocking of CD80 on TLR-7-activated PDC reduced their capacity to stimulate T cell proliferation by ±15% and completely abrogated the increase in T cell stimulatory ability of rapamycin-treated TLR-7-activated PDC, indicating that this is caused by the enhanced CD80 expression. Blockade of IFN-αR2 did not abrogate the difference in ability between rapamycin-treated and non-rapamycin-treated PDC to stimulate cytokine secretion by T cells, indicating that this was not due to reduced IFN-α production by rapamycin-treated PDC.
Fig. 2.
Rapamycin promotes the CD4+ T cell stimulatory capacity of Toll-like receptor (TLR)-7-stimulated plasmacytoid dendritic cells (PDC). PDC were stimulated with loxoribine (LOX) or cytosine–phosphate–dinucleotide (CpG) A oligodeoxynucleotide (ODN)2336, in the absence or presence of 20 ng/ml rapamycin, similar to that described in the legend for Fig. 1. After 18 h PDC were washed to remove all additions and 1 × 105 allogeneic CD3+ T cells were added. (a) After 5 days [3H]-thymidine incorporation was determined. Data are means ± standard error of the mean (s.e.m.) of eight independent experiments. *P = 0·002 (b). After 7 days cell-free supernatants and cells were harvested. Numbers of CD3+CD4+ T cells and CD3+CD8+ T cells at the start and at the end of cultures with LOX-PDC were calculated from numbers of viable cells counted using trypan-blue exclusion, and percentages of T cells determined by flow cytometry. Data are means ± s.e.m. of four independent experiments. (c). 105 purified naive CD3+CD45RA+ or memory CD3+CD45RO+ T cells were added to the PDC, and after 5 days [3H]-thymidine incorporation was determined. Data are means ± s.e.m. of six independent experiments (P < 0·007). (d). Purified naive CD3+CD45RA+ or memory CD3+CD45RO+ T cells were stained with 5,6 carboxy-succinimidyl-fluorescein ester (CFSE) and added to loxoribine-stimulated PDC that had either been treated or not with rapamycin. After 7 days the T cells were stained with CD3, CD4 and CD8 antibodies and proliferation was assessed (n = 3). (e). Cytokine production in 7-day co-cultures of CD3+ T cells with allogeneic PDC. Data are means ± s.e.m. of five independent experiments (*P < 0·03). (f) Purified naive CD3+CD45RA+ or memory CD3+CD45RO+ T cells were stained with CFSE and added to loxorbine-stimulated PDC that were either treated with rapamycin or not. After 7 days the T cells were restimulated with phorbol myristate acetate (PMA) and ionomycin for 6 h. For the last 5 h brefeldin A was added. Expression of interferon (IFN)-γ, interleukin (IL)-17 and IL-10 was determined on CD4+ T cells (n = 3).
Fig. 3.
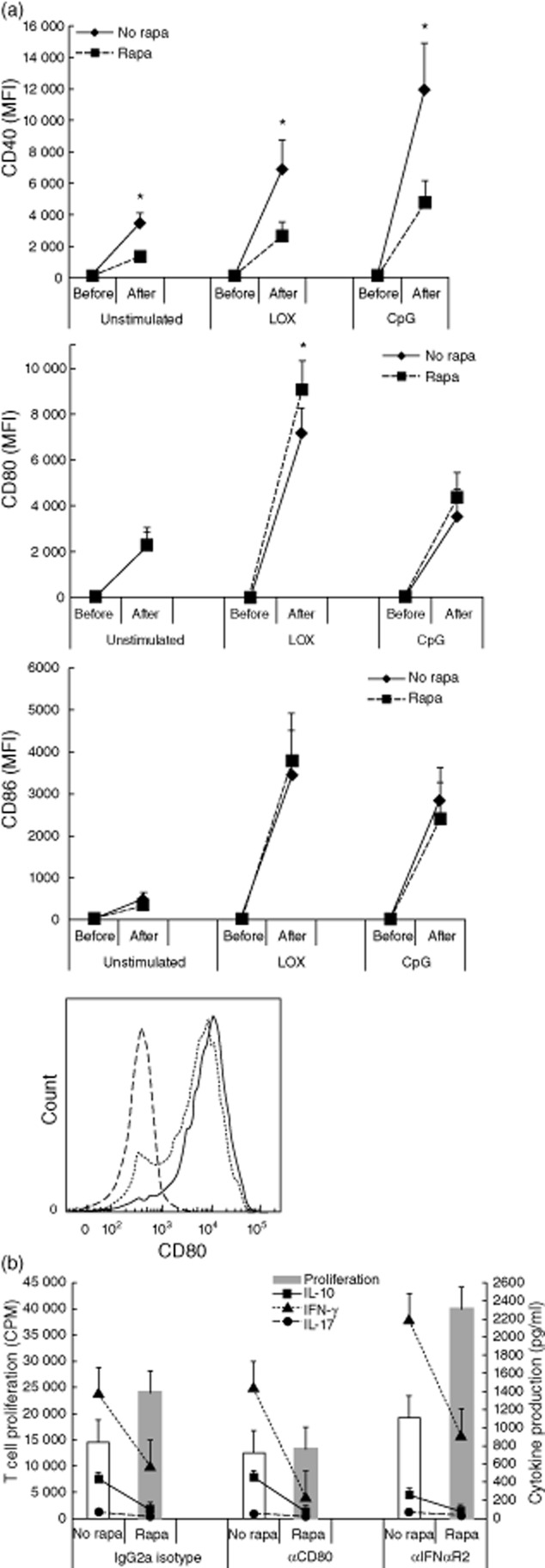
The enhanced T cell stimulatory capacity of rapamycin-treated Toll-like receptor (TLR)-7-activated plasmacytoid dendritic cells (PDC) is caused by enhanced CD80-expression. (a). Effect of rapamycin on the expression of co-stimulatory molecules on TLR-activated PDC. Two × 104 human PDC in 200 μl were stimulated with loxoribine (LOX) or cytosine–phosphate–dinucleotide (CpG) A oligodeoxynucleotide (ODN) 2336, in the absence or presence of 20 ng/ml rapamycin, similar to that described in the legend to Fig. 1. After 18 h PDC were harvested and expression of CD80, CD86, and CD40 before and after culture was measured by flow cytometry. Graphs show means of geometric means of fluorescence (MFI) ± standard error of the mean (s.e.m.) of eight independent experiments. *P < 0·05. Histogram shows CD80 expression on PDC stimulated with loxoribine (dotted line), with loxoribine and rapamycin (solid line) and isotype control monoclonal antibody (mAb) (dashed line). (b). PDC were stimulated with loxoribine in the absence or presence of 20 ng/ml rapamycin, similar to that described in the legend for Fig. 1. After 18 h PDC were washed to remove all additions and 1 × 105 allogeneic CD3+ T cells were added together with neutralizing mAb against CD80 or IFN-αReceptor2, or an isotype-matched irrelevant mAb. After 5 days part of the supernatants was collected for interleukin (IL)-10, interferon (IFN)-γ and IL-17 measurement and [3H]-thymidine incorporation was determined. Data are means ± s.e.m. of four independent experiments.
Together, these data show that, on one hand, rapamycin promotes the ability of TLR-7-activated PDC, but not of TLR-9-activated PDC, to stimulate CD4+ memory T cell and CD4+ naive T cell proliferation by increasing their expression of CD80, but on the other hand inhibits the capacity of PDC to stimulate cytokine production by mainly naive T cells.
Rapamycin enhances the capacity of TLR-7-activated PDC to generate CD4+ Treg but does not affect their ability to induce CD8+ Treg
Activated human PDC can stimulate the generation of CD4+FoxP3+ Treg from naive CD4+ T cells [3,6,7]. Previously, we have shown that human PDC induce the generation of alloantigen-specific CD8+CD38+LAG-3+CTLA-4+ Treg from allogeneic CD3+ T cells, and that activation of PDC by TLR ligation enhances their ability to generate CD8+ Treg [8]. Here, we determined whether or not rapamycin affects the ability of TLR-7-activated PDC to generate CD4+ and CD8+ Treg. Seven-day co-cultures of CFSE-stained naive or memory CD3+ T cells with TLR-7 activated allogeneic PDC resulted in CD4+ T cells with high FoxP3 expression within the proliferating (CFSE-low) cells. Treatment of PDC with rapamycin enhanced their capacity to induce CD4+FoxP3+ Treg in the proliferating cells in the naive Th compartment (Fig. 4a,b). Because, after culture, many CD4+FoxP3– cells expressed CD25 (Fig. 4a) and CD127 expression was up-regulated on CD4+FoxP3+ T cells generated during these cultures (data not shown), it was not possible to purify CD4+FoxP3+ Treg after culture in order to determine their suppressive function.
Fig. 4.
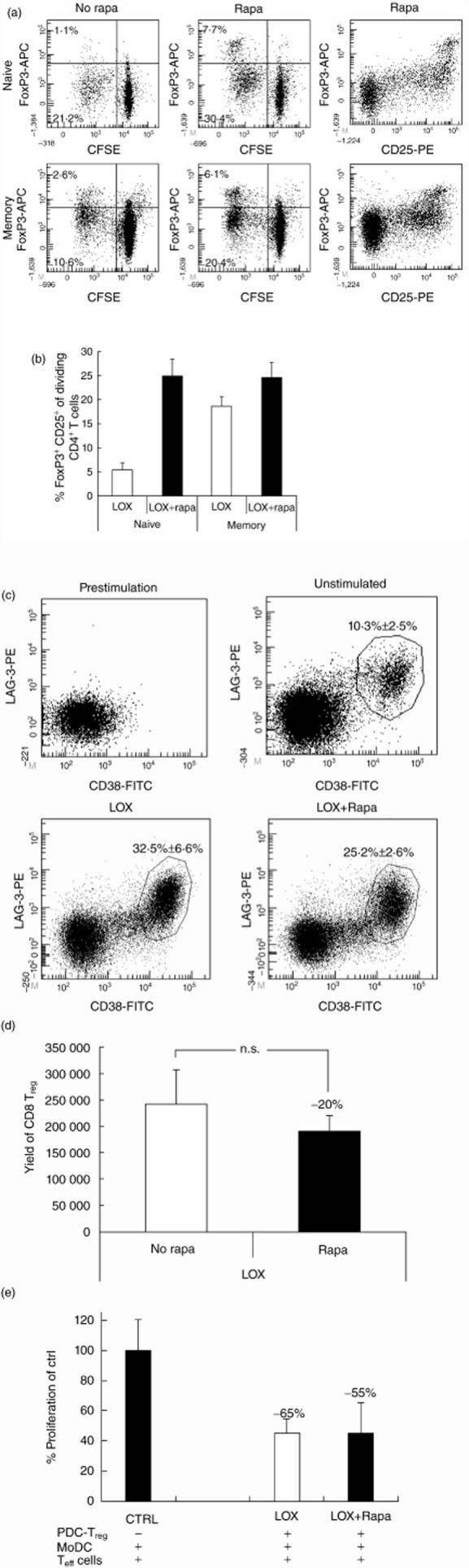
Rapamycin enhances generation of CD4+FoxP3+regulatory T cells (Treg) by Toll-like receptor (TLR-)7-stimulated plasmacytoid dendritic cells (PDC)(a). Purified naive CD3+CD45RA+ or memory CD3+CD45RO+ T cells were stained with 5,6 carboxy-succinimidyl-fluorescein ester (CFSE) and added to loxorbine (LOX)-stimulated PDC that were either treated or not with rapamycin. After 7 days the T cells were stained with CD25, forkhead box protein 3 (FoxP3) and CD4. Dot-plots are gated on CD3 and CD4. (b). Graph shows the mean ± standard error of the mean (s.e.m.) of (a) depicted as percentage FoxP3+CD25+ of dividing (CFSElow) CD4+T cells (n = 3). (c–e) Two × 104 human PDC in 200 μl were stimulated with loxoribine in the absence or presence of 20 ng/ml rapamycin, similarly to that described in the legend for Fig. 1. After 18 h PDC were washed to remove all additions and 1 × 105 allogeneic CD3+ T cells were added. After 7 days of co-culture, cells from several wells were pooled and viable cells were counted. Percentages of CD8+CD38+lymphocyte activation gene (LAG)-3+ Treg were measured by flow cytometry. (c). Representative flow cytometry dot-plots showing the absence of CD8+CD38+LAG-3+ Treg before co-culture with PDC and their presence after co-culture with TLR-7-activated PDC or PDC with interleukin (IL)-3 alone treated or not treated with rapamycin. Figures in the dot-plots show the average percentages (± s.e.m.) of CD38+LAG-3+ cells within CD3+CD8+ cells at the end of the cultures in four independent experiments. (d). The calculated yields (± s.e.m.) of CD8+CD38+LAG-3+ Treg yielded in four independent experiments normalized to an input of 1 × 106 CD3+ T cells. (e). To determine the suppressive capacity of CD8+ Treg generated in the cultures, CD8+CD38+LAG-3+ Treg were purified from the cultures by flow cytometric sorting and added in a 1:2 ratio to autologous CD3+ T cells stimulated by monocyte-derived DC (moDC) from the same donor as the PDC. [3H]-thymidine incorporation was measured after 5 days. Data depicted are means ± s.e.m. of three independent experiments.
Seven-day co-cultures of CD3+ T cells with loxoribine-stimulated PDC resulted in 32 ± 7% of CD8+ T cells showing the regulatory CD38+LAG3+ phenotype, while co-cultures with rapamyin-treated loxoribine-stimulated PDC generated 25 ± 3% CD38+LAG3+ Treg within total CD8 T cells (Fig. 4c). In absolute numbers, the addition of rapamycin to PDC during their activation with loxoribine did not significantly affect the yield of CD8+CD38+LAG3+ Treg at the end of the cultures (Fig. 4d). In addition, the suppressive function of the CD8+ Treg was not affected by rapamycin (Fig. 4e). Thus, rapamycin treatment of TLR-7-stimulated PDC enhances their capacity to induce CD4+FoxP3+ Treg, but does not affect their capacity to generate CD8+CD38+LAG3+ Treg.
Discussion
Rapamycin is an immunosuppressive and anti-proliferative drug, used as maintenance medication to prevent transplant rejection, currently being evaluated for treatment of cancer and autoimmune diseases. We found that a clinically relevant concentration of rapamycin inhibits innate as well as adaptive immune functions of TLR-activated human PDC, but with two exceptions: (1) it enhances the ability of TLR-7-stimulated PDC to stimulate CD4+ T cell proliferation by enhancing CD80 expression; and (2) it enhances the ability of TLR-7-stimulated PDC to induce CD4+FoxP3+ Treg, while it leaves their capacity to generate functional CD8+ Treg unaffected.
Rapamycin inhibited IFN-α secretion by PDC effectively in the case of TLR-7 stimulation, but only a minor inhibitory effect was observed upon TLR-9 stimulation despite effective suppression of mTOR-signalling in TLR-9-stimulated PDC. This observation is of critical importance for emerging studies on rapamycin treatment of autoimmune diseases caused by chronic stimulation of IFN-α production by PDC, such as SLE and psoriasis [18,28]. In these diseases, PDC are stimulated continuously by immune complexes comprising self-DNA and RNA. While RNA complexes are sensed by TLR-7, DNA complexes are sensed by TLR-9 in the early endosomes, such as CpG-A[29]. Our results predict that rapamycin treatment can ameliorate overproduction of IFN which is induced by self-RNA complexes, but not self-DNA-driven IFN production. Similarly, our findings suggest that rapamycin treatment may abrogate the early IFN-α response to RNA viruses which are sensed by TLR-7, such as influenza virus, respiratory syncytial virus (RSV) and hepatitis C virus (HCV), thereby enhancing susceptibility to these viruses, but not to DNA-viruses sensed by TLR-9.
Cao et al. [16] also reported that rapamycin, in the same concentration as we used in the present study, inhibits CpG-A ODN 2336-induced IFN-α production by human PDC less efficiently compared to loxoribine-induced IFN-α production. Nevertheless, these authors reported a twofold inhibition, while we observed only 20% inhibition of CpG-A ODN 2336-induced IFN-α secretion. One explanation for this difference may be related to the use of different IFN-α ELISA kits with different sensitivities for IFN-α subtypes. The ELISA that we used detects the main subtypes IFN-α2a, IFN-α2b and IFN-α2c.
In addition to its well-known immunosuppressive effects, recent studies revealed immunostimulatory effects of rapamycin, such as stimulation of proinflammatory cytokine production in myeloid cells [30] and promotion of CD8+ memory T cell differentiation [31,32]. The data presented here add to the emerging contrasting effects of rapamycin on the immune system. Immunogenic functions of PDC that are inhibited by rapamycin include: proinflammatory cytokine production, IFN-α secretion induced by TRL-7 ligation and the capacity to stimulate proinflammatory cytokine production in allogeneic T cells. Conversely, rapamycin enhances the capacity of TLR-7-activated PDC to stimulate CD4+ T cell expansion, and inhibits the ability of TLR-engaged PDC to stimulate IL-10 secretion by T cells.
While rapamycin increased the ability of TLR-7-activated PDC to stimulate CD4+ T cell proliferation and induce CD4+FoxP3+ Treg, it did not modulate their capacity to stimulate CD8+ T cell proliferation and generate CD8+ Treg. Rapamycin enhanced the T cell stimulatory capacity of TLR-7-activated PDC by stimulating the up-regulation of the co-stimulatory molecule CD80. Apparently, CD80 is less important in stimulating expansion of CD8+ T cells and generation of CD8+ Treg. Rapamycin also enhanced CD252 (OX40-ligand; ligand for the secondary co-stimulatory molecule CD134) and CCR7 expression on TLR-7-activated PDC (data not shown). We do not know why mTOR-inhibition has opposite effects on CD40 and CD80/CD252/CCR7 expression. PDC maturation, resulting in up-regulation of co-stimulatory molecules, is thought to be mediated by nuclear factor kappa B (NFκB) signalling [33], which is inhibited in PDC by rapamycin [16]. PDC utilize an autocrine IFN-α feedback loop that further enhances INF-α production [34] after stimulation with CpG or loxoribine. We tested if mTOR inhibition is involved in this autocrine IFN-α feedback loop to explain the reduced IFN-α production of the PDC after rapamycin treatment. This was performed by blocking the IFN-α-receptor2 with neutralizing antibodies during TLR-9 or TLR-7 activation. Blocking the IFN-α-receptor reduced IFN-α production by PDC, but did not influence the effects of rapamycin on IFN-α production, nor on IL-6 production. In addition, blocking of the IFN-α-receptor had no effect on CD40, CD80 and CCR7 expression on PDC (data not shown). These data indicate that rapamycin does not affect the autocrine IFN-α feedback loop in PDC, and that this loop is not involved in the differential regulation of CD40 and CD80/CD252/CCR7 expression.
While rapamycin enhanced the capacity of loxoribine-activated PDC to stimulate CD4+ T cell proliferation, we found no effect of rapamycin on the T cell stimulatory capacity of CpG-A-stimulated PDC. Accordingly, rapamycin did not up-regulate CD80 expression on TRL-9-activated PDC. In contrast, Cao et al. [16] reported that rapamycin suppresses the capacity of CpG-A-stimulated mouse PDC to stimulate antigen-specific proliferation by CD4+ T cells. Apart from the species difference, it should be realized that Cao et al. used a more artificial system by adding T cells which expressed a transgenic T cell receptor specific for an ovalbumin peptide to the PDC, while we used primary T cells.
Currently, we do not know how rapamycin inhibits the capacity of TLR-activated PDC to stimulate cytokine production by T cells. Neither blocking of CD80 nor blocking of IFN-αR2 abrogated the difference in cytokine production of T cells that were stimulated by PDC-activated loxoribine in the presence or absence of rapamycin.
Previously, we have reported that corticosteroids induce apoptosis of resting human PDC and suppress the functions of activated PDC [35]. In the present study we show that rapamycin inhibits innate and most adaptive immune functions of human PDC, but can enhance their capacity to stimulate CD4+ T cell proliferation, inhibit their capacity to stimulate IL-10 production by T cells and enhance their ability to induce CD4+FoxP3+ Treg. Therefore, together with other recent studies [31,32], these observations may help to understand why rapamycin monotherapy is not very effective in preventing graft rejection, and is sometimes even accompanied by inflammatory side effects, including pneumonitis and glomerulonephritis [36].
Acknowledgments
The authors would like to thank Dr Gwenny M. Fuhler for advice on immunoblotting.
Disclosures
The authors declare no financial or commercial conflicts of interest.
References
- 1.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura K, Kadowaki N, Kitawaki T, Uchiyama T. Virus-stimulated plasmacytoid dendritic cells induce CD4+ cytotoxic regulatory T cells. Blood. 2006;107:1031–1038. doi: 10.1182/blood-2005-04-1737. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Yang M, Wang YH, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boor PP, Metselaar HJ, Jonge S, Mancham S, van der Laan LJ, Kwekkeboom J. Human plasmacytoid dendritic cells induce CD8 LAG-3 Foxp3 CTLA-4 regulatory T cells that suppress allo-reactive memory T cells. Eur J Immunol. 2011;41:1663–1674. doi: 10.1002/eji.201041229. [DOI] [PubMed] [Google Scholar]
- 9.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouries J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama M, Hashimoto D, Aoyama K, et al. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 12.de Heer HJ, Hammad H, Soullie T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 14.Irla M, Kupfer N, Suter T, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takagi H, Fukaya T, Eizumi K, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Manicassamy S, Tang H, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K–mTOR–p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez D, Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med. 2010;9:173–178. [PMC free article] [PubMed] [Google Scholar]
- 19.Guiducci C, Coffman RL, Barrat FJ. Signalling pathways leading to IFN-alpha production in human plasmacytoid dendritic cell and the possible use of agonists or antagonists of TLR7 and TLR9 in clinical indications. J Intern Med. 2009;265:43–57. doi: 10.1111/j.1365-2796.2008.02050.x. [DOI] [PubMed] [Google Scholar]
- 20.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer M, Conroy L, Min HY, Kwekkeboom J. Generation of monoclonal antibodies to human lymphocyte cell surface antigens using insect cells expressing recombinant proteins. J Immunol Methods. 1992;152:15–23. doi: 10.1016/0022-1759(92)90084-7. [DOI] [PubMed] [Google Scholar]
- 22.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 24.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 25.van de Laar L, van den Bosch A, Boonstra A, et al. PI3K–PKB hyperactivation augments human plasmacytoid dendritic cell development and function. Blood. 2012;120:4982–4991. doi: 10.1182/blood-2012-02-413229. [DOI] [PubMed] [Google Scholar]
- 26.Krishnaswamy G, Smith JK, Srikanth S, Chi DS, Kalbfleisch JH, Huang SK. Lymphoblastoid interferon-alpha inhibits T cell proliferation and expression of eosinophil-activating cytokines. J Interferon Cytokine Res. 1996;16:819–827. doi: 10.1089/jir.1996.16.819. [DOI] [PubMed] [Google Scholar]
- 27.Belardelli F, Gresser I. The neglected role of type I interferon in the T cell response: implications for its clinical use. Immunol Today. 1996;17:369–372. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 28.Perl A. Emerging new pathways of pathogenesis and targets for treatment in systemic lupus erythematosus and Sjogren's syndrome. Curr Opin Rheumatol. 2009;21:443–447. doi: 10.1097/BOR.0b013e32832efe6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 30.Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Keeffe M, Grumont RJ, Hochrein H, et al. Distinct roles for the NF-kappaB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood. 2005;106:3457–3464. doi: 10.1182/blood-2004-12-4965. [DOI] [PubMed] [Google Scholar]
- 34.Kerkmann M, Rothenfusser S, Hornung V, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170:4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 35.Boor PP, Metselaar HJ, Mancham S, Tilanus HW, Kusters JG, Kwekkeboom J. Prednisolone suppresses the function and promotes apoptosis of plasmacytoid dendritic cells. Am J Transplant. 2006;6:2332–2341. doi: 10.1111/j.1600-6143.2006.01476.x. [DOI] [PubMed] [Google Scholar]
- 36.Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]