Abstract
Numerous reports have shown that a diet containing large amounts of trans fatty acids (TFAs) is a major risk factor for metabolic disorders. Although recent studies have shown that TFAs promote intestinal inflammation, the underlying mechanisms are unknown. In this study, we examined the effects of dietary fat containing TFAs on dextran sodium sulphate (DSS)-induced colitis. C57 BL/6 mice were fed a diet containing 1·3% TFAs (mainly C16:1, C18:1, C18:2, C20:1, C20:2 and C22:1), and then colitis was induced with 1·5% DSS. Colonic damage was assessed, and the mRNA levels of proinflammatory cytokines and major regulators of T cell differentiation were measured. The TFA diet reduced survival and exacerbated histological damage in mice administered DSS compared with those fed a TFA-free diet. The TFA diet significantly elevated interleukin (IL)-6, IL-12p40, IL-23p19 and retinoic acid-related orphan receptor (ROR)γt mRNA levels in the colons of DSS-treated animals. Moreover, IL-17A mRNA levels were elevated significantly by the TFA diet, with or without DSS treatment. We also examined the expression of proinflammatory cytokines in lipopolysaccharide (LPS)-stimulated RAW264.7 cells and peritoneal macrophages. These cells were exposed to TFAs (linoelaidic acid or elaidic acid) with or without LPS and the mRNA levels of various cytokines were measured. IL-23p19 mRNA levels were increased significantly by TFAs in the absence of LPS. Cytokine expression was also higher in LPS-stimulated cells exposed to TFAs than in unexposed LPS-stimulated cells. Collectively, our results suggest that TFAs exacerbate colonic inflammation by promoting Th17 polarization and by up-regulating the expression of proinflammatory cytokines in the inflamed colonic mucosa.
Keywords: inflammatory bowel disease, macrophages, proinflammatory cytokines, trans fatty acids
Introduction
Fatty acids in our daily diet are broadly classified as cis and trans fatty acids (TFAs). TFAs are steric isomers of the common cis unsaturated fatty acids that contain at least one double bond in the trans configuration. TFAs are not found in most natural vegetable oils, but they are formed during the manufacturing of hydrogenated vegetable oils such as margarine, fat spread and shortening. Modern diets, especially those in developed countries, including the Western-style diet, which includes baked goods, deep-fried products, frozen foods and packaged snacks, commonly include large quantities of margarine, which contains TFAs [1,2]. Many recent studies have shown that TFA intake is a risk factor for metabolic diseases such as diabetes mellitus and coronary heart disease. These diseases are associated with systemic or localized inflammation and increased plasma levels of several proinflammatory cytokines, C-reactive protein, triglycerides and low-density lipoprotein (LDL) cholesterol [2,3].
Although the aetiology of inflammatory bowel disease (IBD) remains unclear, a great deal of clinical and experimental data indicate that dietary fat may play important roles in the pathogenesis and clinical course of IBD [4–6]. However, little is known about TFA intake as a risk factor for IBD. TFA intake appears to be related to the chronic inflammation associated with disorders such as diabetes and cardiovascular disease, and it has been reported that dietary TFAs influence the function of multiple cell types, including hepatocytes, adipocytes, endothelial cells and macrophages [2,7,8]. Recently, the role of macrophages in the pathology of intestinal inflammation has received increased attention. Compared to the amount of interleukin (IL-23) produced by lamina propria macrophages in healthy individuals, these macrophages in patients with IBD produce large amounts of IL-23, which is consistent with dysregulated tolerance [9]. Macrophage function is modulated by the binding of fatty acids to Toll-like receptor (TLR)-2 and TLR-4 located on the cell surface, which stimulates and/or suppresses the production of several proinflammatory cytokines [10–12]. It is known that the proinflammatory cytokines IL-12 and IL-23 induce the differentiation of T helper type 1 (Th1) and Th17 cells. In addition to these cytokines, T box expressed in T cells (T-bet) and retinoic acid-related orphan receptor (RORγt), a transcription factor, are required for the induction and survival of Th1 and Th17 cells, respectively. Recent studies have shown that Th1 and Th17 cells play important roles in the regulation of mucosal immunity, which is linked to the development of IBD [13]. However, the direct anti/proinflammatory effects of TFAs and the mechanisms by which they act upon immune cells such as macrophages have not been clarified.
In this study, to investigate whether a diet containing TFAs exacerbates intestinal inflammation, we used a mouse model of colitis induced by dextran sodium sulphate (DSS). A relatively low concentration (1·5%) of DSS was used, which allowed almost all mice to survive for at least 2 weeks. The mice were divided into two groups; one group was fed a TFA-rich diet (a fat-free diet plus margarine) and the other was fed a TFA-free diet (a fat-free diet plus soya bean oil). We also examined whether two distinct types of TFAs (linoelaidic acid and elaidic acid) and their cis forms, linoleic acid and oleic acid, differentially modulate the function of LPS-stimulated macrophages in vitro. For this, we used a macrophage cell line (RAW264.7) and primary peritoneal macrophages isolated from 1·5% DSS-treated mice, and we focused on the induction of several cytokines. Linoelaidic acid and elaidic acid were chosen because they are the predominant isomers in partially hydrogenated vegetable oils such as margarine [7,14,15].
Materials and methods
Animal treatment and diet
Specific pathogen-free (SPF), 8-week-old female C57BL/6NCrj mice were purchased from Charles River Japan, Inc. (Yokohama, Japan). The mice were housed in specific pathogen-free conditions with free access to food and water, and their care and use were in accordance with the guidelines of the National Defense Medical College. The study protocol was approved by the Animal Ethics Committee of the National Defense Medical College (no. 09033). Experimental diets were made from a fat-free AIN-76 diet (Japan Clea Co., Tokyo, Japan) supplemented with either salt-free margarine (TFA diet) or natural soya bean oil (normal diet). The total fat content in each experimental diet was 6%. Salt-free margarine was purchased from NOF Corporation (Tokyo, Japan). Natural soya bean oil was purchased from Carolina Soy Products, Inc. (Vernon, CA, USA). The composition of the experimental diet is shown in Table 1. A total of 13 g/kg of TFAs was included in the TFA diet. These TFAs were not detectable in either the natural soya bean oil or the normal diet. The concentrations of the various TFAs were measured with a gas chromatograph (G-5000; Hitachi, Tokyo, Japan). The diets were prepared every week and stored with individual degassing packs at 4°C. The packs were changed every day to avoid oxidation. The TFA diet or the normal (TFA-free) diet was provided for 7 days, along with additive-free drinking water. Thereafter, 1·5% DSS (MW 40 000; ICN Biochemicals, Cleveland, OH, USA), dissolved in drinking water, was administered for 7 consecutive days to induce colitis (for a total experimental period of 14 days).
Table 1.
Experimental diet composition
| Normal diet | TFA diet | |
|---|---|---|
| Protein (%) | 18 | ← |
| Carbohydrate (%) | 61·2 | ← |
| Fat (%) | 6·0 | ← |
| Trans fatty acid composition (g/kg) | n.d. | 13† |
| Fibre (%) | 4·8 | ← |
| Ash (%) | 2·8 | ← |
| Moisture (%) | <10 | ← |
| Energy (Kcal/g) | 3·628 | 3·568 |
| C16:1 | 1·0 | |
| C18:1 | 6·0 | |
| C18:2 | 1·0 | |
| C20:1 | 2·0 | |
| C20:2 | 1·0 | |
| C22:1 | 2·0 | |
| Total | 13·0 |
n.d.: not detected, †trans fatty acids (TFA) composition of the TFA diets (g/kg).
Mice were killed on day 14 and their colons were removed. Their mesenteric lymph nodes (MLNs) were also removed and dissociated into single-cell suspensions. The cell suspensions were then applied to a Percoll (GE Healthcare Japan, Tokyo, Japan) gradient (40% Percoll on the top, 80% Percoll on the bottom) to determine the ratio of lymphocytes by centrifugation at 800 g for 20 min at 25°C [16]. The cells at the interface were collected and purified using anti-CD4+ magnetic bead-based cell separation (Miltenyi Biotec K.K., Tokyo, Japan), before isolating total RNA according to the manufacturer's instructions. After the experimental period, the survival rates of each group were calculated. The four experimental groups were as follows: TFA-free diet and drinking water alone (control group); TFA-free diet with DSS (DSS group); TFA diet and drinking water alone (TFA group); and TFA diet with DSS (DSS with TFA group). Each of these experimental groups consisted of 10 mice.
Assessment of colonic damage
Body weight was recorded daily. Mice were killed using pentobarbital anaesthesia. A segment of the proximal colon was fixed in 10% buffered formalin and embedded in paraffin, and then 4-μm longitudinal sections were stained with haematoxylin and eosin (H&E). Histological damage was assessed by the crypt scoring method described by Cooper et al. [17]: grade 0, intact crypt; grade 1, loss of the basal one-third of the crypt; grade 2, loss of the basal two-thirds of the crypt; grade 3, loss of the entire crypt with the surface epithelium remaining intact; and grade 4, loss of both the entire crypt and the surface epithelium. Assessments of histological damage were performed in a blinded manner.
In-vitro experiments
Stock solutions (50 mM) of TFAs [omega-6 (linoelaidic acid); omega-9 (elaidic acid)] and their cis forms [omega-6 (linoleic acid); omega-9 (oleic acid)] were prepared by mixing the fatty acids with fatty acid-free bovine serum albumin (BSA). The molar ratio of fatty acid to BSA was 7·5:1. BSA, TFAs and their cis forms were purchased from Sigma-Aldrich (St Louis, MO, USA) [18].
The mouse macrophage cell line RAW264.7 was purchased from the European Collection of Cell Cultures (ECACC, Salisbury, UK) and was seeded at 1 × 106/ml in a 6-well culture plate. Primary peritoneal macrophages were isolated from 1·5% DSS-treated mice. Briefly, the peritoneum was flushed with ice-cold PBS, red blood cells were lysed, and the remaining cells were seeded at 1·0 × 106/ml in a six-well culture plate. After 2 h, non-adherent cells were removed. RAW264.7 cells and peritoneal macrophages were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Sigma) and 4 mM l-glutamine at 37°C in 5% CO2. For the experiments, cells were cultured for 24 h and then stimulated with lipopolysaccharide (LPS) (1 μg/ml, serotype B55:O5 Escherichia coli; Sigma-Aldrich) for 16 h. In some experiments, the cells were exposed to TFAs or their cis forms with or without LPS for 16 h [for quantitative real-time polymerase chain reaction (PCR)] or 24 h [for enzyme-linked immunosorbent assay (ELISA)]. The concentrations of TFAs and their cis forms (0·1 and 1·0 mM) used in this study, as well as that of LPS (1 μg/ml), were based on a previous in-vitro study [7]. The viability of RAW264.7 cells and peritoneal macrophages in experiments involving LPS and fatty acid (cis and trans) exposure was greater than 90% as determined by the trypan blue exclusion test. After incubation, cells were harvested, and the mRNA levels of cytokines were determined by quantitative real-time PCR. The culture supernatant was collected, and cytokine protein levels were quantified by ELISA.
Quantitative real-time PCR and ELISA
Colonic tissues, RAW264.7, primary peritoneal macrophages cells or CD4+ T cells were homogenized in the lysis buffer included in the RNeasy Mini kit (Qiagen, Hilden, Germany). After isolation, total RNA (1 μg) was used for reverse transcription (RT) using a QuantiTect reverse transcription kit (Qiagen). TaqMan PCR reactions were performed on cDNA samples using TaqMan Universal PCR Master Mix and an ABI PRISM 7900HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). TaqMan probes and primers for IL-1β, IL-6, IL-10, IL-12p40, IL-17A, IL-23p19, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, T-bet, retinoic acid-related orphan receptor gamma T (RORγt) and 18S rRNA developed for TaqMan gene expression assays were purchased from Applied Biosystems. Target mRNA levels were determined using the comparative cycle threshold method for relative quantification. The calibrator sample was isolated from healthy C57/BL6 mice using 18S rRNA as the internal control.
The concentrations of murine IL-1β, IL-6, IL-10, IL-12p70, IL-23 and TNF-α in culture supernatants were measured using commercially available ELISA kits (eBioscience, San Diego, CA, USA), according to the manufacturer's instructions. All experimental samples were assayed in duplicate.
Statistical analysis
Statistical analyses were performed using one-way analysis of variance followed by Tukey–Kramer test at the 5% significance level. All results are expressed as the means ± standard error of the mean of 10 animals or five experiments.
Results
DSS-induced colitis was exacerbated by dietary TFAs
After day 13, the survival rate in the DSS with TFA group was significantly lower than that in the DSS group (Fig. 1a). Although the TFA diet by itself did not induce any significant change in body weight during the experiment, TFA-containing diets induced significant body weight loss in mice with DSS-induced colitis (DSS with TFA group). Moreover, the degree of body weight loss in the DSS with TFA group was significantly greater than that in the DSS group (Fig. 1b). DSS induced a characteristic colonic shortening compared to the control group, and significant aggravation of colonic shortening in the intestine was observed in the DSS with TFA group relative to the DSS group (Fig. 1d). However, the TFA diet by itself did not induce any significant morphological changes.
Fig. 1.
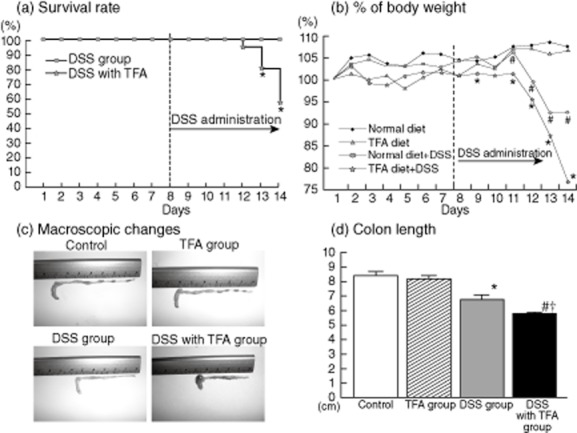
(a) Effect of dietary trans fatty acids (TFAs) on the survival (Kaplan–Meier) of 1·5% dextran sodium sulphate (DSS)-treated mice; *P < 0·05 versus the DSS group. (b) Effect of dietary TFAs on the body weight of 1·5% DSS-treated mice; *P < 0·05 versus the DSS group; #P < 0·05 versus the control. (c) Macroscopic changes in the colon on day 7 after administration of 1·5% DSS. In the control and TFA groups, there were no macroscopic changes in the tissue (upper panels). In the DSS group, stool softening and colon shortening were observed (lower left panel). The DSS with TFA group had enhanced colon shortening and haemorrhaging (lower right panel). (d) Colon length measured on day 7 after administration of 1·5% DSS; *P < 0·05 versus the control; #P < 0·05 versus the TFA group; †P < 0·05 versus the DSS group. All results are expressed as the mean (± standard error of the mean) (n = 10 mice/group).
As shown in Fig. 2a, only mild crypt damage was observed in the DSS group, with moderate inflammatory cell infiltration in the colonic mucosal layer. In contrast, depletion of goblet cells, muscle layer thickening and prominent inflammatory infiltration were observed in the colonic tissue of the DSS with TFA group. Based on the crypt scoring system, the damage scores in the DSS with TFA group were significantly higher than those in the DSS group (Fig. 2b). Again, the TFA diet by itself did not induce any significant histological changes.
Fig. 2.
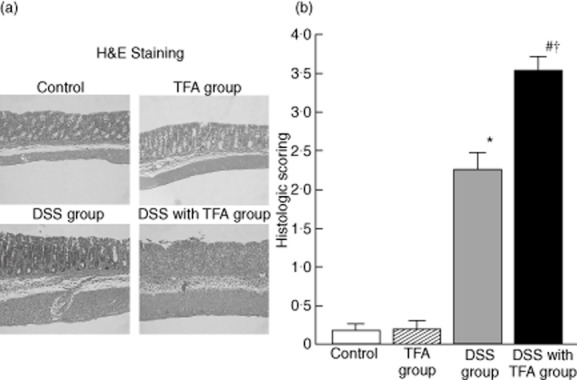
(a) Effect of dietary trans fatty acids (TFAs) on the histology of 1·5% dextran sodium sulphate (DSS)-induced colitis (haematoxylin and eosin staining, ×100). In the control and TFA groups, no histological changes were observed (upper panels). In the DSS group, there was a small amount of inflammatory cell infiltration in the colonic mucosal layer (lower left panel). In the DSS with TFA group, there was strong inflammation with goblet cell depletion, marked cell infiltration and muscle layer thickening (lower right panel). (b) Effect of dietary TFAs on histological damage score in 1·5% DSS-induced colitis. Histological damage scores were determined in paraffin sections of the proximal colon. The crypt scoring system (0–4 scale) was used to assess histological damage in haematoxylin and eosin-stained sections (grade 0, intact crypt; grade 1, loss of the basal one-third of the crypt; grade 2, loss of the basal two-thirds of the crypt; grade 3, loss of the entire crypt with intact surface epithelium; grade 4, loss of both the entire crypt and the surface epithelium); *P < 0·05 versus control; #P < 0·05 versus TFA group; †P < 0·05 versus DSS group. All results are expressed as the mean (± standard error of the mean) (n = 10 mice/group).
Effect of dietary TFAs on cytokine mRNA expression in DSS-induced colitis
The mRNA levels of IL-1β, IL-6 and TNF-α in the DSS with TFA group were significantly higher than in the DSS group (Fig. 3a). IL-12p40 mRNA levels were also significantly higher (by a factor of approximately 1·7) in the DSS with TFA group than in the DSS group. IL-23p19 mRNA levels in the DSS with TFA group were more than 38-fold higher than in the control group. However, the mRNA levels of proinflammatory cytokines, including IL-1β, IL-6, TNF-α and IL-23, were not affected by the TFA diet alone (i.e. without DSS) (Fig. 3b). The increase in IL-10 mRNA levels induced by DSS was independent of TFA intake (Fig. 3c).
Fig. 3.
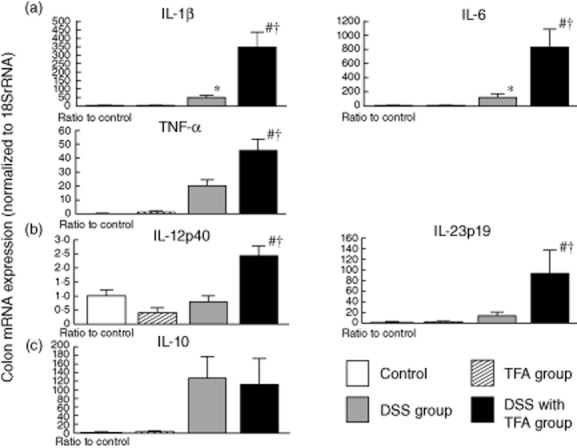
Expression of the mRNA of various cytokines in the proximal colon in 1·5% dextran sodium sulphate (DSS)-induced colitis and the exacerbating effect of trans fatty acids (TFAs). The mRNA levels of (a) interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α; (b) IL-12p40 and IL-23p19; and (c) IL-10 were determined by quantitative real-time polymerase chain reaction (PCR). Columns represent the average ratio of each cytokine to 18S rRNA from 10 mice; *P < 0·05 versus control; #P < 0·05 versus TFA group; †P < 0·05 versus DSS group. All results are expressed as the mean (± standard error of the mean) (n = 10 mice/group).
Effect of dietary TFAs on the Th1 and Th17 balance in DSS-induced colitis
As shown in Fig. 4, mRNA levels of T-bet and IFN-γ were increased significantly by DSS treatment, as shown by the levels in DSS-treated mice compared to those in untreated mice, but were not elevated further by the TFA diet. In contrast, RORγt mRNA levels were not increased significantly, and IL-17A mRNA levels were only slightly increased by DSS treatment. However, the levels of these mRNAs were robustly increased by the combination of the TFA diet and DSS treatment. Interestingly, RORγt mRNA levels tended to increase in the TFA group, even without DSS administration, compared with the control group. IL-17A mRNA levels in the TFA group were also significantly higher than in the control group under non-inflamed conditions (i.e. without DSS). These results suggest that TFAs induce RORγt and IL-17A expression in the colonic mucosa more robustly than DSS.
Fig. 4.
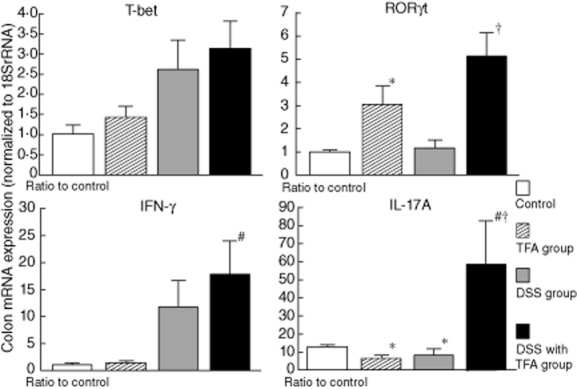
The expression of interferon (IFN)-γ and interleukin (IL)-17A mRNA and their master transcription factors (T-bet and retinoic acid-related orphan receptor (ROR)γt, respectively) in the proximal colon in 1·5% dextran sodium sulphate (DSS)-induced colitis. The mRNA levels were determined by quantitative real-time polymerase chain reaction (PCR). Columns represent the average ratio of each cytokine to 18S rRNA from 10 mice; *P < 0·05 versus control; #P < 0·05 versus trans fatty acid group; †P < 0·05 versus DSS group. All results are expressed as the mean (± standard error of the mean) (n = 10 mice/group).
The Th1 and Th17 balance in CD4+ T cells isolated from the MLNs was also determined by measuring the mRNA levels of RORγt and IL-17A (Th17 markers) and T-bet and IFN-γ (Th1 markers) using quantitative real-time PCR (Fig. 5). RORγt and IL-17A mRNA levels in the TFA with DSS group were significantly higher than in the control and DSS groups. In contrast, the mRNA levels of T-bet and IFN-γ, which were increased by DSS treatment, were not affected by the TFA diet.
Fig. 5.
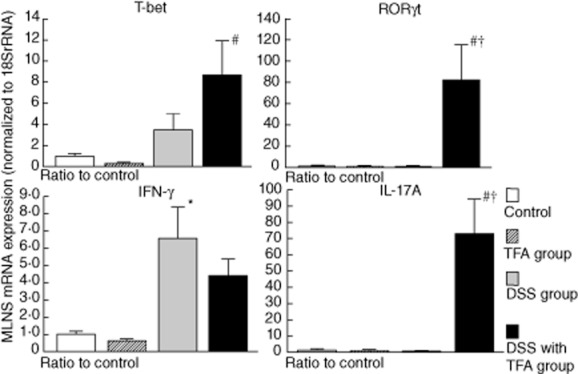
The expression of interferon (IFN)-γ and interleukin (IL)-17A mRNA and their master transcription factors [T-bet and retinoic acid-related orphan receptor (ROR)γt, respectively] in CD4+ T cells isolated from the mesenteric lymph nodes (MLNs) in mice with 1·5% dextran sodium sulphate (DSS)-induced colitis. CD4+ T cells were purified from MLNs with anti-CD4-coated magnetic beads. The mRNA levels were determined by quantitative real-time polymerase chain reaction (PCR). The columns represent the average ratio of each cytokine to 18S rRNA from 10 mice; *P < 0·05 versus control; #P < 0·05 versus trans fatty acid (TFA) group; †P < 0·05 versus DSS group. All results are expressed as the mean (± standard error of the mean) (n = 10 mice/group).
Comparison of the effects of the trans and cis forms of fatty acids on cytokine mRNA expression in RAW264.7 cells and peritoneal macrophages
As shown in Fig. 6, the results of the in-vitro study using RAW264.7 cells demonstrated that administration of 1·0 mM fatty acids alone (the higher concentration) induced a significant increase in many cytokine mRNAs even in LPS-free conditions, whereas at 0·1 mM these fatty acids did not induce such changes (data not shown).
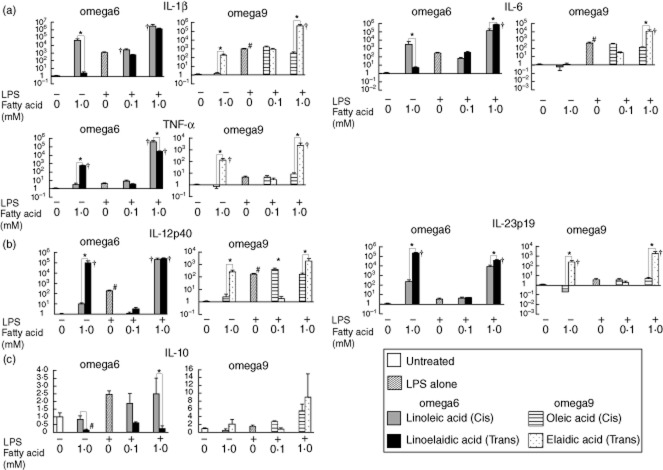
Fig. 6.
The expression of various cytokine mRNAs in RAW264.7 cells after exposure to lipopolysaccharide (LPS), and the effects of trans fatty acids (TFAs) and their cis forms. Linoelaidic acid (omega-6) and elaidic acid (omega-9) are the TFAs that were used, and linoleic acid (omega-6) and oleic acid (omega-9) are the cis forms that were used. RAW264.7 cells were exposed to 0·1 mM or 1·0 mM fatty acids in conditioned media with or without LPS (1 μg/ml) for 16 h. The mRNA levels of (a) interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α; (b) IL-12p40 and IL-23p19; and (c) IL-10 were determined by quantitative real-time polymerase chain reaction (PCR). Columns represent the average ratio of each cytokine to 18S rRNA from five experiments; #P < 0·05 versus negative control (unstimulated RAW264.7 cells without LPS or fatty acids); *P < 0·05 versus corresponding trans or cis forms of the fatty acids; †P < 0·05 versus LPS alone. All results are expressed as the mean (± standard error of the mean) (n = 5).
In the absence of LPS, treatment of RAW264.7 cells with the cis form of the omega-6 fatty acid (linoleic acid) increased IL-1β and IL-6 mRNA expression. In contrast, treatment with the omega-6 and omega-9 TFAs linoelaidic acid and elaidic acid significantly increased TNF-α, IL-12p40 and IL-23p19 mRNA levels compared with the levels in the presence of their cis forms. We also obtained an intriguing result. Exposure to 1·0 mM omega-6 or omega-9 TFAs without LPS increased the levels of IL-12p40 and Il-23p19 mRNA in RAW264.7 cells more than 102–105-fold compared to untreated cells, and these levels were similar to those induced by a combination of TFAs and LPS. In contrast, treatment of peritoneal macrophages with TFAs or their cis forms in LPS-free conditions did not significantly affect the mRNA levels of most cytokines compared to the levels in untreated cells. However, IL-23p19 mRNA was increased significantly by exposure to 1·0 mM omega-6 or omega-9 TFAs (Fig. 7).
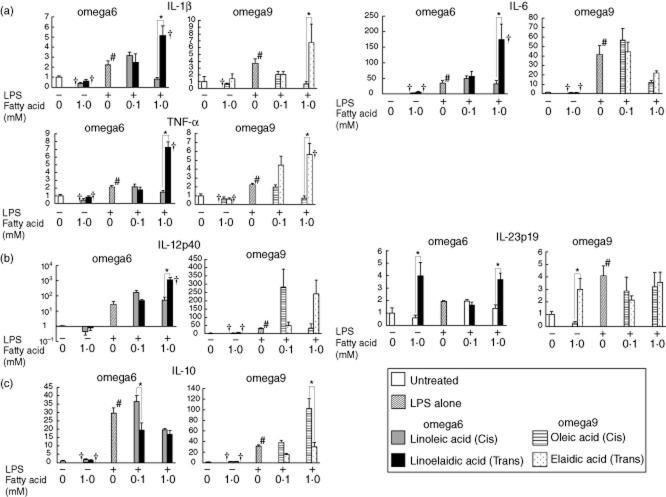
Fig. 7.
The mRNA expression of various cytokines in macrophages collected from the peritoneal fluid of 1·5% dextran sodium sulphate (DSS)-treated mice after exposure to lipopolysaccharide (LPS), and the effects of trans fatty acids (TFAs) and their cis forms. Linoelaidic acid (omega-6) and elaidic acid (omega-9) are the TFAs that were used, and linoleic acid (omega-6) and oleic acid (omega-9) are the cis forms that were used. Mononuclear cells were exposed to 0·1 and 1·0 mM fatty acids in conditioned media with or without LPS (1 μg/ml) for 16 h. The mRNA levels of (a) interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α; (b) IL-12p40 and IL-23p19; and (c) IL-10 were determined by quantitative real-time PCR. The columns represent the average ratio of each cytokine to 18S rRNA from five experiments. #P < 0·05 versus negative control (unstimulated mononuclear cells without LPS or fatty acids); *P < 0·05 versus corresponding trans or cis forms of the fatty acids. †P < 0·05 versus LPS alone. All results are expressed as the mean (± standard error of the mean) (n = 5).
There was a significant increase in the mRNA levels of all proinflammatory cytokines (IL-1β, TNF-α, L-12p40, IL-6 and IL-23p19) when a high dose (1 mM) of TFAs (either omega-6 or omega-9) was combined with LPS treatment. It should be noted that omega-9 TFA (elaidic acid), when combined with LPS, elevated significantly the expression of all proinflammatory cytokines tested in RAW264.7 cells beyond that induced by LPS alone, while the cis form (oleic acid) did not exhibit such an additive effect. This increase in proinflammatory cytokine expression was also observed in peritoneal macrophages, particularly when omega-6 TFA was combined with LPS. In RAW264.7 cells, a significant additive effect on proinflammatory cytokine expression was observed with the combination of LPS and the cis form of omega-6 fatty acid (linoleic acid); however, there was a slight, but significant, difference between the effects of omega-6 TFA and its cis form on IL-6 and IL-23 mRNA levels. Conversely, no significant additive effect of the cis form of omega-6 fatty acid (linoleic acid) on cytokine levels was observed in peritoneal macrophages.
A significant decrease was observed in the mRNA levels of the anti-inflammatory cytokine IL-10 in both RAW264.7 cells and peritoneal macrophages treated with linoelaidic acid (omega-6 TFA) in combination with LPS, compared to cells treated with the cis form. In peritoneal macrophages, omega-9 TFA (elaidic acid) also induced a significant decrease in IL-10 mRNA levels when combined with LPS (Figs 6c and 7c).
Comparison of the effects of the trans and cis forms of fatty acids on proinflammatory cytokine secretion in RAW264.7 cells and peritoneal macrophages
After fatty acid exposure, proinflammatory cytokine secretion from macrophages was measured by ELISA (Figs 8 and 9). Even in the absence of LPS, TNF-α secretion into the culture supernatant of RAW264.7 cells following treatment with the omega-6 and omega-9 TFAs was significantly higher than the levels secreted following treatment with their cis forms. There were no significant differences in IL-1β and IL-6 secretion from both RAW264.7 cells and peritoneal macrophages between omega-6 and omega-9 TFAs and their cis forms. In contrast, the concentration of IL-23 in both RAW264.7 cells and peritoneal macrophages treated with either omega-6 or omega-9 TFA was significantly higher than in cells treated with their cis forms.
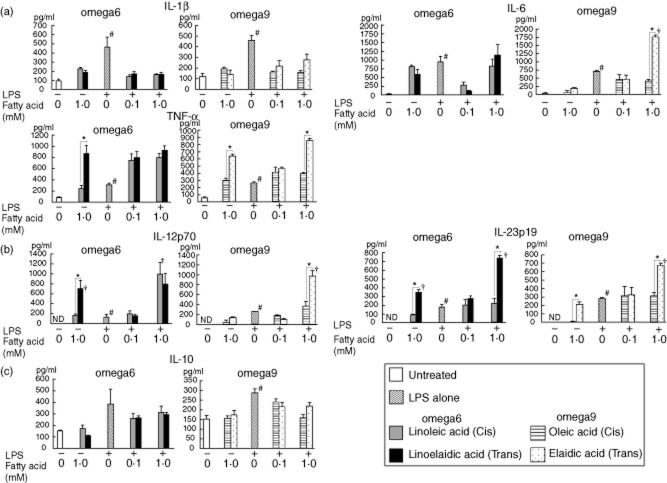
Fig. 8.
The concentrations of various cytokines in RAW264.7 cells after exposure to lipopolysaccharide (LPS), and the effects of trans fatty acids (TFAs) and their cis forms. Linoelaidic acid (omega-6) and elaidic acid (omega-9) are the TFAs that were used, and linoleic acid (omega-6) and oleic acid (omega-9) are the cis forms that were used. Cell culture supernatant samples were obtained after 24 h of treatment. These samples were pooled, and then the levels of various cytokines [interleukin (IL)-1β, IL-6, IL-10, IL-12p70, IL-23 and tumour necrosis factor (TNF)-α] were determined by enzyme-linked immunosorbent assay (ELISA). *P < 0·05 versus the corresponding trans or cis forms of the fatty acids. †P < 0·05 versus LPS alone; n.d.: not detected. All results are expressed as the mean (± standard error of the mean) (n = 5).
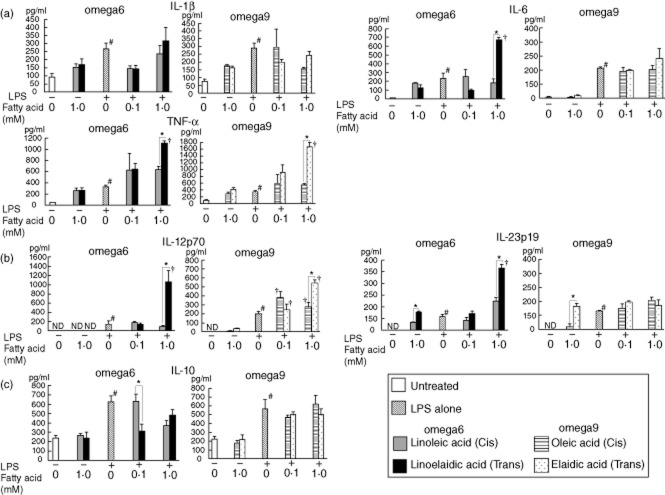
Fig. 9.
The concentrations of various cytokines in macrophages collected from the peritoneal fluid of 1·5% dextran sodium sulphate (DSS)-treated mice after exposure to lipopolysaccharide (LPS) and the effects of trans fatty acids (TFAs) and their cis forms. Linoelaidic acid (omega-6) and elaidic acid (omega-9) are the TFAs that were used, and linoleic acid (omega-6) and oleic acid (omega-9) are the cis fatty acids that were used. Cell culture supernatant samples were obtained after 24 h. These samples were pooled, and then the levels of various cytokines [interleukin (IL)-1β, IL-6, IL-10, IL-12p70, IL-23 and tumour necrosis factor (TNF)-α] were determined by enzyme-linked immunosorbent assay (ELISA). *P < 0·05 versus the corresponding trans or cis forms of the fatty acids. †P < 0·05 versus LPS alone; n.d. not detected. All results are expressed as the mean (± standard error of the mean) (n = 5).
In the presence of LPS, IL-23 secretion was higher from cells treated with a high dose (1 mM) of TFA (both omega-6 and omega-9 TFAs for RAW264.7 cells, and omega-6 TFAs for peritoneal macrophages) than from cells treated with LPS alone or with LPS and their cis forms. The concentrations of IL-6 and IL-12p70 secreted from RAW264.7 cells were also increased by omega-9 TFA treatment and LPS, whereas IL-6 and IL-12p70 secretion from peritoneal macrophages was increased by omega-6 TFA treatment and LPS.
There were no significant differences in the secretion of the anti-inflammatory cytokine IL-10 from RAW264.7 cells treated with omega-6 or omega-9 TFAs and their cis forms both with and without LPS compared to untreated cells. In contrast, a significant decrease was observed in IL-10 secretion from peritoneal macrophages treated with linoelaidic acid (omega-6 TFA) in combination with LPS, compared to secretion from cells treated with the cis form.
In general, there was good agreement between the mRNA expression and their corresponding secreted protein levels.
Discussion
In this study, we demonstrated clearly that colitis induced by low-dose DSS (1·5% w/v) treatment is aggravated by a diet containing TFAs, and this aggravated colitis is accompanied by a marked decrease in body weight and a significant reduction in colonic length (Fig. 1b,d). Lethality, not usually observed in the DSS group, was observed in the DSS with TFA group (Fig. 1a). The survival rate of mice in the DSS with TFA group was nearly identical to that of mice with colitis induced by a higher dose of DSS (2% w/v), observed in our previous study [19]. Although numerous studies have shown that TFA intake is correlated positively with biomarkers of systemic inflammation in humans [2,3,20–22], there are only a few reports on TFA-induced local inflammatory reactions in the colonic mucosa. Here we show experimentally that TFA intake promotes inflammatory cell infiltration and tissue damage significantly, and increases the production of proinflammatory cytokines such as IL-1β, IL-6 and TNF-α in animals administered DSS (Fig. 3). Recently, Laroui et al. showed that DSS must be internalized to exert its action and that DSS–lipid complexes are required for fusion with cellular membranes, which is followed by release of DSS into the cytoplasm [23]. The possibility that TFAs and their cis forms have different capacities to form nano-lipocomplexes with DSS should be considered in future studies, in addition to TFA accumulation in intestinal tissue.
Several epidemiological studies have shown that margarine intake is associated positively and significantly with colonic inflammation in ulcerative colitis [24–26]. Although the TFA content in margarine was not determined carefully in these studies, many types of margarine contain 6·8–41% TFA [8,27]. In addition, a pilot study showed that the levels of the TFAs octadecenoate and hexadecenoate in subcutaneous adipose tissue were significantly higher in patients with Crohn's disease than in healthy individuals [28]. Numerous studies have reported that the composition of total fatty acids in the diet is an important factor influencing intestinal inflammation in IBD [4–6,29–32]. Our data suggest that patients with IBD should also be concerned with the proportion of the trans forms of fatty acids in their diet, because it is probably a significant risk factor for the progression or relapse of colitis.
We have demonstrated that the TFA diet alone was incapable of inducing colitis (Figs 1 and 2). The mRNA levels of IL-1β, IL-6 and TNF-α in the colonic mucosa were also not up-regulated by the TFA diet alone (Fig. 3a). Several studies have shown that a high-TFA diet induces inflammatory cytokine production without overt inflammation, even in healthy subjects [3,21,27]. In our present study, the TFA-containing diet induced slight, but significant, increases in IL-17A mRNA levels in the colonic mucosa of mice, even in mice not treated with DSS. We also showed in our in-vitro experiment that even under LPS-free conditions, TNF-α, IL-12p40 and IL-23p19 mRNA levels were elevated significantly in RAW264.7 cells after administration of omega-6 (linoelaidic acid) or omega-9 (elaidic acid) TFAs, compared with control untreated cells or cells treated with the cis forms of these fatty acids. Moreover, both omega-6 and omega-9 TFAs enhanced expression of IL-23p19 mRNA in LPS-untreated primary peritoneal macrophages isolated from DSS-treated mice compared with the expression in macrophages treated with the cis forms. Collectively, our results suggest that TFAs can modulate the immune system independently. In particular, they may influence IL-17 signalling pathways.
The process underlying TFA-dependent proinflammatory cytokine production, especially IL-17, in these cells was not elucidated in this study. However, incorporation of TFAs into the cell membrane might affect cytokine expression. Noris et al. showed that omega-3 fatty acids (cis forms) could incorporate into the membranes of RAW264.7 cells [33]. It is also well known that TFAs can modify cellular functions by interacting with the hydrophobic regions of membrane proteins [34]. Furthermore, recent studies reported that TFAs might alter cell membrane permeability, eicosanoid production and gene expression [35–37]. Several interesting studies have reported on receptors for fatty acids. Some studies have shown that the saturated cis-forms of fatty acids in particular function through TLR-2 or TLR-4 [10–12]. In contrast, several recent studies have reported that G protein regulator (GPR) family proteins such as GPR120 act as receptors for fatty acids [38,39]. Further studies are needed to determine whether certain cell membrane receptors are actually involved in the TFA-induced up-regulation of proinflammatory cytokines associated with in intestinal inflammation.
The IL-17 family of cytokines mainly induces the production of neutrophils and macrophages, which are potent inflammatory mediators [13,40]. Several studies have shown that IL-17A is crucial for sustaining colitis in human IBD [41] and in experimental animal models [42,43]. Our results demonstrate that the mRNA levels of IL-17A and RORγt, a master regulator of Th17 cell differentiation [44,45], in colonic tissue were elevated significantly by the TFA diet in mice with DSS-induced colitis (Fig. 4). Moreover, RORγt and IL-17A mRNA levels in CD4 T cells isolated from the MLNs were increased significantly by the TFA diet in mice with DSS-induced colitis (Fig. 5). These data suggest that consumption of the TFA-containing diet by individuals with an inflammatory condition may enhance not only the Th17 response, but also the migration of Th17 cells to the local inflammatory site. Differentiation of Th17 cells could be also induced by IL-6 [44,45], which was robustly increased by TFAs when combined with DSS (Fig. 3a). We speculate that the TFA intake-mediated increase in IL-17A expression during inflammation may disrupt normal intestinal immunity, thereby inducing hypersensitivity and further exacerbating inflammation. Sakai et al. demonstrated that the levels of IFN-γ, which is produced primarily by Th1 cells, are notably higher in mice fed TFAs than in control mice [46], suggesting a role for Th1 cells in TFA-induced aggravation of DSS colitis. However, in our study, the mRNA levels of IFN-γ, which is produced primarily by Th1 cells, and the mRNA levels of T-bet, a key Th1-inducing factor, in both the colonic mucosa and the MLNs, did not differ between DSS-treated mice fed the TFA diet and mice fed the TFA-free diet, suggesting few roles for Th1 cells in the aggravation of DSS colitis.
We show that the mRNA levels of IL-23p19 in animals treated with DSS were greatly enhanced by the TFA diet compared to levels in mice fed the TFA-free diet. IL-23 is known to be required for the accumulation and function of Th17 cells, and is a key inflammatory factor involved in the pathogenesis of colitis [45,47,48]. Several studies have shown that IL-23 is produced preferentially by the cells of the innate immune system, such as macrophages and dendritic cells [9,13,45]. Han et al. reported that IL-6 and TNF-α production was higher in LPS-stimulated peripheral blood mononuclear cells (PBMCs) isolated from human subjects who consumed a stick margarine diet containing 6·7% TFA (% energy) than in cells from subjects who consumed a soya bean oil diet containing 0·7% TFA [49].
The effects of TFAs on cytokine expression by macrophages were quantified in RAW264.7 cells and peritoneal macrophages that were treated with LPS to mimic inflammatory conditions. We also compared the effects of two different forms of TFAs (omega-6 and omega-9), in addition to their cis forms. LPS treatment significantly increased the mRNA and protein secretion levels of various inflammatory cytokines in RAW264.7 cells and peritoneal macrophages, and their expression was enhanced further by concomitant exposure to TFA. Although previous studies reported that treatment of macrophages with cis-fatty acids and/or LPS stimulated increased expression of IL-10 levels [50,51], IL-10 mRNA and protein levels were not increased significantly after TFA treatment. This suggests that the anti-inflammatory function of macrophages is decreased under TFA exposure. However, different responses to LPS and fatty acids were observed between RAW264.7 cells and peritoneal macrophages. In general, the response to LPS and fatty acids is stronger in RAW264.7 cells than in peritoneal macrophages, possibly because RAW264.7 cells are a transformed cell line established from the ascites of a tumour induced in a male mouse by intraperitoneal injection of Abelson leukaemia virus (A-MuLV). For example, peritoneal macrophages only responded to TFAs and LPS, whereas RAW264.7 cells also responded to cis fatty acids and LPS. TNF-α mRNA, IL-12p40 mRNA and IL-12p70 protein levels increased in response to fatty acid and LPS treatment. We also observed slightly different responses to omega-6 and omega-9 TFAs. In general, the induction of proinflammatory cytokines (not IL-10) in response to LPS and omega-9 fatty acid exposure was lower than that induced by omega-6 fatty acid exposure. In fact, there was no significant additive effect of omega-9 TFAs on the mRNA and protein levels of IL-6, IL-12 (at 0·1 mM), or IL-23 in peritoneal macrophages compared with the levels induced by LPS alone. In contrast, although the effect of omega 9 and LPS on IL-6, IL-12p70 and TNF-α protein levels in RAW264.7 cells was synergistic, such a synergistic effect was not observed with omega 6 and LPS. The exact reason for the observed differences between the two types of macrophages and the two types of TFAs observed in this study is unknown. Therefore, further investigations of the differences in the cell surface receptor responses and cell signalling pathways activated after stimulation with LPS and fatty acids are needed to address this issue. It is particularly interesting that the expression of IL-12p40 and IL-23p19, which are associated with IL-17 polarization and maintenance, was enhanced significantly after exposure to TFAs and LPS, as other intriguing data have been reported recently. Bassett et al. reported that consumption of a diet rich in vaccenic acid, a TFA and regioisomer of elaidic acid, protects against atherosclerosis in an animal model, whereas an elaidic acid-rich diet stimulates atherosclerosis [52]. These data suggest that vaccenic acid and elaidic acid may also have quite different effects on intestinal inflammation. Further studies should be conducted to determine the role of ruminant TFAs in intestinal inflammation. However, it is important to note that these in-vitro data obtained using LPS do not agree completely with the results and underlying proposed mechanisms obtained from the DSS-colitis experiments due to the various systemic immune modifications in vivo.
In summary, our results indicate that dietary TFAs exacerbate DSS-induced colitis by increasing the levels of macrophage-derived proinflammatory cytokines involved in the polarization and maintenance of Th17 cells. Excessive intake of TFAs through the consumption of TFA-rich foods such as margarine exacerbates inflammation in the colonic mucosa by altering the profile of cytokines produced by immune cells such as macrophages. Therefore, TFA intake should be reduced to a minimum, particularly in patients with colitis, including those with IBD.
Acknowledgments
This research was supported by grants from the National Defense Medical College and Food Science Institute Foundation of Japan.
Author contributions
Yoshikiyo Okada, Yoshikazu Tsuzuki and Soichiro Miura designed the study. Yoshikiyo Okada, Yoshikazu Tsuzuki and Ryota Hokari analysed the data. Yoshikiyo Okada, Hideaki Hozumi, Shingo Sato, Chie Kurihara, Chikako Watanabe, Kengo Tomita, Shunsuke Komoto, Atsushi Kawaguchi and Shigeaki Nagao collected data. Yoshikiyo Okada wrote the first draft of the paper. Yoshikiyo Okada, Yoshikazu Tsuzuki, Ryota Hokari and Soichiro Miurac contributed to the writing of the paper.
Disclosure
The authors do not have any conflicts of interest or any other disclosures.
References
- 1.Stender S, Dyerberg J, Bysted A, Leth T, Astrup A. A trans world journey. Atheroscler Suppl. 2006;7:47–52. doi: 10.1016/j.atherosclerosissup.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol. 2009;5:335–344. doi: 10.1038/nrendo.2009.79. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Garcia E, Schulze MB, Meigs JB, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–566. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 4.Miura S, Tsuzuki Y, Hokari R, Ishii H. Modulation of intestinal immune system by dietary fat intake: relevance to Crohn's disease. J Gastroenterol Hepatol. 1998;13:1183–1190. [PubMed] [Google Scholar]
- 5.MacLean CH, Mojica WA, Newberry SJ, et al. Systematic review of the effects of n-3 fatty acids in inflammatory bowel disease. Am J Clin Nutr. 2005;82:611–619. doi: 10.1093/ajcn.82.3.611. [DOI] [PubMed] [Google Scholar]
- 6.Matsunaga H, Hokari R, Kurihara C, et al. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis. 2008;14:1348–1357. doi: 10.1002/ibd.20491. [DOI] [PubMed] [Google Scholar]
- 7.Zapolska-Downar D, Kosmider A, Naruszewicz M. Trans fatty acids induce apoptosis in human endothelial cells. J Physiol Pharmacol. 2005;56:611–625. [PubMed] [Google Scholar]
- 8.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 9.Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn's disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins de Lima T, Gorjao R, Hatanaka E, et al. Mechanisms by which fatty acids regulate leucocyte function. Clin Sci (Lond) 2007;113:65–77. doi: 10.1042/CS20070006. [DOI] [PubMed] [Google Scholar]
- 11.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 12.Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20:379–385. doi: 10.1097/MOL.0b013e32832fa5c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res. 2010;35:940–946. doi: 10.1007/s11064-009-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tjonneland A, Overvad K, Bergmann MM, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case–control study within a European prospective cohort study. Gut. 2009;58:1606–1611. doi: 10.1136/gut.2008.169078. [DOI] [PubMed] [Google Scholar]
- 15.Craig-Schmidt MC. World-wide consumption of trans fatty acids. Atheroscler Suppl. 2006;7:1–4. doi: 10.1016/j.atherosclerosissup.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Jeon SG, Kayama H, Uead Y, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLOS Pathog. 2012;8:e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 18.van Greevenbroek MM, Voorhout WF, Erkelens DW, van Meer G, de Bruin TW. Palmitic acid and linoleic acid metabolism in Caco-2 cells: different triglyceride synthesis and lipoprotein secretion. J Lipid Res. 1995;36:13–24. [PubMed] [Google Scholar]
- 19.Okada Y, Tsuzuki Y, Miyazaki J, et al. Propionibacterium freudenreichii component 1.4-dihydroxy-2-naphthoic acid (DHNA) attenuates dextran sodium sulphate induced colitis by modulation of bacterial flora and lymphocyte homing. Gut. 2006;55:681–688. doi: 10.1136/gut.2005.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–973. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Pischon T, Hankinson SE, et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–1525. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laroui H, Ingersoll SA, Liu HC, et al. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLOS One. 2012;7:e32084. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A case–control study of ulcerative colitis in relation to dietary and other factors in Japan. The Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J Gastroenterol. 1995;30(Suppl):9–12. [PubMed] [Google Scholar]
- 25.Hart AR, Luben R, Olsen A, et al. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion. 2008;77:57–64. doi: 10.1159/000121412. [DOI] [PubMed] [Google Scholar]
- 26.Maconi G, Ardizzone S, Cucino C, Bezzio C, Russo AG, Bianchi Porro G. Pre-illness changes in dietary habits and diet as a risk factor for inflammatory bowel disease: a case–control study. World J Gastroenterol. 2010;16:4297–4304. doi: 10.3748/wjg.v16.i34.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enig MG, Atal S, Keeney M, Sampugna J. Isomeric trans fatty acids in the U.S. diet. J Am Coll Nutr. 1990;9:471–486. doi: 10.1080/07315724.1990.10720404. [DOI] [PubMed] [Google Scholar]
- 28.Heckers H, Melcher FW, Kamenisch W, Henneking K. [Chemically prepared fats and Crohn's disease. A pilot study of the occurrence of trans-fatty acids in the subcutaneous tissue of Crohn's patients in comparison with healthy controls as a parameter of long-term fat intake] Z Gastroenterol. 1988;26:259–264. [PubMed] [Google Scholar]
- 29.Lorenz-Meyer H, Bauer P, Nicolay C, et al. Omega-3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Study Group Members (German Crohn's Disease Study Group) Scand J Gastroenterol. 1996;31:778–785. doi: 10.3109/00365529609010352. [DOI] [PubMed] [Google Scholar]
- 30.Matsunaga H, Hokari R, Kurihara C, et al. Omega-3 polyunsaturated fatty acids ameliorate the severity of ileitis in the senescence accelerated mice (SAM)P1/Yit mice model. Clin Exp Immunol. 2009;158:325–333. doi: 10.1111/j.1365-2249.2009.04020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741–745. doi: 10.1093/ajcn/63.5.741. [DOI] [PubMed] [Google Scholar]
- 32.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 33.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci USA. 2012;109:8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz AM. Should trans fatty acids be viewed as membrane-active drugs? Atheroscler Suppl. 2006;7:41–42. doi: 10.1016/j.atherosclerosissup.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Niu SL, Mitchell DC, Litman BJ. Trans fatty acid derived phospholipids show increased membrane cholesterol and reduced receptor activation as compared to their cis analogs. Biochemistry. 2005;44:4458–4465. doi: 10.1021/bi048319+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassagno N, Palos-Pinto A, Costet P, Breilh D, Darmon M, Berard AM. Low amounts of trans 18:1 fatty acids elevate plasma triacylglycerols but not cholesterol and alter the cellular defence to oxidative stress in mice. Br J Nutr. 2005;94:346–352. doi: 10.1079/bjn20051512. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim A, Natrajan S, Ghafoorunissa R. Dietary trans-fatty acids alter adipocyte plasma membrane fatty acid composition and insulin sensitivity in rats. Metabolism. 2005;54:240–246. doi: 10.1016/j.metabol.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirasawa A, Tusmaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 40.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito R, Kita M, Shin-Ya M, et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12–16. doi: 10.1016/j.bbrc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Takedatsu H, Michelsen KS, Wei B, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 46.Sakai T, Ire AV, Matoba T, Yamamoto S. Dietary trans fatty acids suppress the development of spontaneous atopic-like dermatitis in NC/Nga mice. J Nutr Sci Vitaminol (Tokyo) 2009;55:412–416. doi: 10.3177/jnsv.55.412. [DOI] [PubMed] [Google Scholar]
- 47.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 49.Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. 2002;43:445–452. [PubMed] [Google Scholar]
- 50.Skuladottir IH, Petursdottir DH, Hardardottir I. The effects of omega-3 polyunsaturated fatty acids on TNF-alpha and IL-10 secretion by murine peritoneal cells in vitro. Lipids. 2007;42:699–706. doi: 10.1007/s11745-007-3081-1. [DOI] [PubMed] [Google Scholar]
- 51.Walloschke B, Fuhrmann H, Schumann J. Enrichment of RAW264.7 macrophages with essential 18-carbon fatty acids affects both respiratory burst and production of immune modulating cytokines. J Nutr Biochem. 2010;21:556–560. doi: 10.1016/j.jnutbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Bassett CM, Edel AL, Patenaude AF, et al. Dietary vaccenic acid has antiatherogenic effects in LDLr2/2 mice. J Nutr. 2010;140:18–24. doi: 10.3945/jn.109.105163. [DOI] [PubMed] [Google Scholar]