Abstract
Viral pathogens present many challenges to organisms, driving the evolution of a myriad of antiviral strategies to combat infections. A wide variety of viruses infect invertebrates, including both natural pathogens that are insect-restricted, and viruses that are transmitted to vertebrates. Studies using the powerful tools available in the model organism Drosophila have expanded our understanding of antiviral defenses against diverse viruses. In this review, we will cover three major areas. First, we will describe the tools used to study viruses in Drosophila. Second, we will survey the major viruses that have been studied in Drosophila. And lastly, we will discuss the well-characterized mechanisms that are active against these diverse pathogens, focusing on non-RNAi mediated antiviral mechanisms. Antiviral RNAi is discussed in another paper in this issue.
1. Introduction
Viral pathogens are a common cause of morbidity and mortality worldwide, including recently emerging and re-emerging pathogens (Geisbert and Jahrling, 2004; Weaver and Barrett, 2004; Weaver and Reisen, 2010). Since viruses are obligate intracellular pathogens with a limited coding capacity, they must hijack cellular pathways to successfully replicate as well as evade the host immune system. Some viruses infect only a limited number of hosts, while others have a large host range and must be able to survive within organisms with diverse lifestyles. Arthropod-borne viruses (arboviruses) are one such group of viruses. They have complex life cycles dependent on arthropods, often mosquitoes, and vertebrates. They are transmitted via the bite of an infected hematophagous insect vector to vertebrate hosts. The cycle continues when an uninfected insect vector further feeds on a viremic vertebrate, becoming infected and continuing the cycle during subsequent bloodmeals (Beaty, 1996; Weaver and Barrett, 2004). Arboviruses can cause a broad range of symptoms in infected humans and livestock (Denizot et al., 2012; Weaver and Barrett, 2004; Weaver and Reisen, 2010). Interestingly, arboviruses are largely non-pathogenic in the insect host, which is attributed, at least in part, to the robust innate immune system of insects. Indeed, insects with compromised immune systems can succumb to typically non-pathogenic infections (Carré-Mlouka et al., 2007; Costa et al., 2009a; Sabin et al., 2009; van Mierlo et al., 2012; Zambon et al., 2005), suggesting that insect immunity is active and protective during these infections. We are just beginning to decipher the innate mechanisms by which arthropods, including vectors of human diseases and commercially important species (e.g., honeybees, silkworms, and shrimp), can combat viral pathogens (Evans and Spivak, 2010; Flegel, 2009; Johnson et al., 2008; Sabin and Cherry, 2013). This lack of knowledge is due, in part to the difficult nature of performing molecular and genetic studies in pertinent arthropod species, many of which cannot be reared in the laboratory. These challenges have led to the use of Drosophila melanogaster as a model insect, which has historically been a useful model system for deciphering the molecular mechanisms involved in fundamental biological processes, including development, innate immunity, and cancer. Furthermore, population genetic studies in Drosophila have also contributed to our understanding of the evolution of antiviral resistance, a process that is difficult to dissect in other systems. And importantly, many discoveries made in flies have been extended to mammalian systems, which expand the utility of this system. In this review, we will survey the technologies available in Drosophila to study viral infections, focusing on the genetic tools that have been used. Next, we will review the viruses that have been studied in Drosophila, including both insect-restricted viruses and arthropod-borne mammalian viruses. Finally, we will review the major host antiviral immune mechanisms that are active against these pathogens in Drosophila, with a focus on non-RNAi mediated antiviral pathways.
2. Drosophila melanogaster as a model organism for antiviral innate immunity
Drosophila offers several advantages as a model organism to identify and study antiviral mechanisms. Since its beginnings with Thomas Hunt Morgan in the early twentieth century (Avery et al., 1959; Kohler, 1994), the fruit fly has been an important animal model in laboratory research (Bellen et al., 2010; Brookes, 2001; Grumbling and Strelets, 2006). The practical nature of Drosophila as a model system has greatly contributed to its success as a tool for understanding complex developmental processes and behaviors (Beckingham et al., 2005; Rubin and Lewis, 2000). Fruit flies are easy to maintain, inexpensive, produce numerous progeny, and have short generation times (Avery et al., 1959; Roberts, 1998). Drosophila also shares a high degree of conservation with other arthropods, exhibiting similar metamorphic life cycles and genetic pathways (Behura et al., 2011; Merkling and van Rij, 2012; Schneider, 2000). In the field of innate immunity, this has allowed researchers to take advantage of powerful genetic tools in Drosophila to make fundamental discoveries (Lemaitre and Hoffmann, 2007; Sabin et al., 2010), including the discovery of Toll as antimicrobial (Lemaitre et al., 1996). Many viral restriction pathways have also been characterized using Drosophila, including RNA silencing (Chotkowski et al., 2008a; Galiana-Arnoux et al., 2006a; Li et al., 2002; Sabin et al., 2009; van Rij et al., 2006; Wang et al., 2006; Zambon et al., 2006) and JAK/STAT signaling (Dostert et al., 2005), which have been found to be conserved in other insect vectors of human disease (Christophides et al., 2002; Kingsolver and Hardy, 2012; Merkling and van Rij, 2012).
Drosophila also has conserved developmental and cellular processes with vertebrate hosts, further extending its value as an experimental model (Beckingham et al., 2005; Bergstralh and St Johnston, 2012; Bier, 2005; Reiter and Bier, 2002; Reiter et al., 2001). For example, many of the classic signal transduction systems were first identified in Drosophila using forward genetic screens and were subsequently found to be conserved in mammalian hosts (Nüsslein-Volhard and Wieschaus, 1980; Ribeiro et al., 2003; Simon, 1994; St Johnston, 2002). Furthermore, seventy-five percent of known human genetic disease genes have orthologs in the fruit fly (Chien et al., 2002). As illustrated by studies on Toll, Drosophila has proven to be a useful model system to identify shared innate immune recognition mechanisms with vertebrates (Lemaitre et al., 1996; Medzhitov et al., 1997; Poltorak et al., 1998). With its extensive conservation in both insect vectors and mammalian hosts, Drosophila provides us with a uniquely valuable opportunity for understanding anciently evolved and conserved mechanisms of defense.
Lastly, more than twenty diverse groups of viruses can infect insects, many of which also infect humans or serve as models for human-related pathogens (Huszar and Imler, 2008; Weaver and Barrett, 2004; Weaver and Reisen, 2010). Many diverse human arboviruses can infect Drosophila, including Rift Valley Fever Virus (RFV), Sindbis virus (SINV), Dengue virus (DEN), and West Nile virus (WNV) (Chotkowski et al., 2008a; Kemp et al., 2013; Mukherjee and Hanley, 2010; Sabin et al., 2009; Shelly et al., 2009; Xu et al., 2012). These pathogens have diverse replication strategies, tropism, and pathogenicity. Some of the same innate restriction mechanisms identified in Drosophila also play similar roles in insect vectors of human disease (Campbell et al., 2008; Léger et al., 2013; Xi et al., 2008). Thus, characterizing the spectrum of unique and common antiviral restriction mechanisms is likely to aid in the development of targeted therapies and control strategies against these diseases.
3. Tools to identify novel antiviral mechanisms in vitro
Drosophila is a genetic model system that has powerful tools for performing genetic screens and analyses. Its value has been further complemented by the completion of the Drosophila genome sequence in 2000 (Adams et al., 2000; Myers et al., 2000) and by the subsequent expansion of post-genomic technologies, including RNA interference (RNAi), transcriptional profiling, and proteomics (Carmena, 2009; Mohr et al., 2010). Given that biological systems operate through various classes of molecules (DNA, RNA, proteins, metabolites), “omics” approaches that query each class of molecules reveal a global view. Since networks of interactions can be highly dynamic and interconnected, parallel approaches are essential to discover the underlying biology. Furthermore, computational integration of these disparate data types can reveal important functional classes and mechanisms that may have been otherwise overlooked, providing a more comprehensive view of cellular processes (Kint et al., 2010).
Genome-wide screening for virus-host interactions
The development and integration of high-throughput technologies, including systems-biology approaches using genome-wide RNAi libraries in Drosophila, have dramatically altered how genetic screens can be performed. RNAi produces a gene-specific loss-of-function phenotype by taking advantage of specific targeting and destruction of cognate mRNAs by the RNA-induced silencing (RISC) complex, depleting transcripts for any gene of interest (Siomi and Siomi, 2009). Since the Drosophila genome is compact (~14,000 genes) and has low redundancy, single gene mutants are more likely to reveal phenotypes of interest (Mohr et al., 2010; Mohr and Perrimon, 2012). This is in contrast to mammals, where redundant gene families can make similar analyses less robust (Ramadan et al., 2007). RNAi in Drosophila cells is also relatively efficient and straightforward, and has been extensively used to study many aspects of biology that can be modeled in cells in vitro (Mohr and Perrimon, 2012; Panda and Cherry, 2012). Drosophila cell lines have been derived from various cell types (e.g., embryonic, larval, hemocyte, and neuronal), allowing for the study of cell type specific responses (Cherry, 2008; Mohr and Perrimon, 2012). For example, embryonic hemocyte-derived cells such as Schneider Line 2 (S2) cells and Kc167 cells are responsive to Pattern Associated Molecular Patterns (PAMPs) and can be used to elucidate this aspect of immune defense (Baum and Cherbas, 2008). Furthermore, the highly phagocytic nature of these cells can probe mechanisms of pathogen uptake and clearance (Cherry, 2008).
Cell culture-based high-throughput RNAi screening has been widely used to identify genes involved in viral infection (Ulvila et al., 2011). Different gene sets can be queried, ranging from focused gene sets (e.g., kinome) to genome-wide libraries (Panda and Cherry, 2012). Knockdown of genes involved in antiviral defense leads to increases in viral infection. An important challenge using genome wide RNAi screens is the implementation of a robust pipeline to filter large amounts of data and identify true hits (König et al., 2007; Mohr et al., 2010). Statistical analysis coupled with secondary assays lead to the identification of validated gene sets (Moser et al., 2010; Panda and Cherry, 2012). In addition meta-analyses that integrate this data with other ‘omic studies can reveal pathways or players with increased confidence if they are identified across multiple data sets (Bushman et al., 2009; Hao et al., 2008).
Altogether, this RNAi screening has been successfully applied to the study of host factors that impact viral infection, leading to the discovery of both virus-specific and broadly acting antiviral factors. For example, RNAi screening against disparate RNA viruses led to the discovery of the novel antiviral factor Ars2, an RNA binding protein that restricts diverse RNA viruses (Panda and Cherry, 2012; Sabin et al., 2009). In addition to antiviral factors, genes required for infection can also be identified. A genome-wide RNAi screen against Sindbis virus (SINV) led to the discovery that the transmembrane iron transporter NRAMP is an essential entry receptor in both insects and mammals (Rose et al., 2011a).
Transcriptional profiling
In mammals, it is well established that antiviral innate immunity relies heavily on the interferon response. However, insects do not appear to encode such a pathway and their transcriptional responses to viral infection are not fully characterized. Recent advances in transcriptional profiling and deep sequencing technologies have created opportunities to further explore these responses to viral infection, including the global analysis of transcriptional changes during disparate infections in Drosophila (Castorena et al., 2007; Dostert et al., 2005; Mudiganti et al., 2010b; Roxström-Lindquist et al., 2004; Tsai et al., 2008a; Zambon et al., 2005). Studies have been performed in a variety of conditions, both in adult flies and in cell culture, querying various time points and cell/tissue types (Castorena et al., 2007; Dostert et al., 2005; Mudiganti et al., 2010b; Roxström-Lindquist et al., 2004; Tsai et al., 2008a; Xu et al., 2012; Zambon et al., 2005). These studies have revealed large numbers of virus-induced genes triggered by natural Drosophila pathogens and medically relevant human arboviruses (Castorena et al., 2007; Dostert et al., 2005; Mudiganti et al., 2010b; Roxström-Lindquist et al., 2004; Tsai et al., 2008a; Zambon et al., 2005).
In addition, new technologies such as next-generation sequencing are creating new avenues of research by sequencing the total transcriptome, including small RNAs and long-coding RNAs. For instance, deep sequencing of virus-derived small interfering RNAs (vsiRNAs) by several groups have revealed distinct patterns and biases towards specific regions of viral genomes in Drosophila (Mueller et al., 2010; Sabin et al., 2013), which can further elucidate antiviral mechanisms of RNA silencing.
Proteomics
Complementary to RNAi screening, changes in the proteome upon viral infection provide additional insight into the host-pathogen relationship. Studies that monitor changes in protein abundance, post-translational modifications and protein-protein interaction networks are essential (Carmena, 2009; Veraksa, 2010). Identification of novel pathways, network “hubs” (i.e., highly connected nodes), and “bottlenecks” (i.e., key connector proteins that are central to multiple sub-networks within the larger network) can extend our understanding of innate immune biology (Carmena, 2009; Veraksa, 2010). This has been made possible by advances in genome annotation, protein fractionation, and mass spectrometry instrumentation (Veraksa, 2010). Large scale efforts to map and catalog the Drosophila proteome (Ahrens et al., 2007; Brunner et al., 2007), high-throughput as well as targeted approaches applied to analyze protein–protein interactions (Friedman et al., 2011; Giot et al., 2003; Murali et al., 2011), and systematic analysis of post-translational modifications (Goetze et al., 2009; Koles et al., 2007; Schwientek et al., 2007; ten Hagen et al., 2009). Furthermore, stable isotope labeling of flies and other applications of quantitative proteomics have also opened up new possibilities for functional analyses (Gouw et al., 2010). Identification of host factors degraded during infection as well as altered complexes driven by viral infection reveal new insights. Use of virus mutants, or cells with defects in innate pathways, coupled with proteomic analysis can reveal new insights into virus-host interactions. For instance, a a proteomic study of Drosophila cells infected with flock house virus (FHV) found 150 proteins that were increased and 66 proteins that were decreased in abundance upon infection (Go et al., 2006). Analyses of virus-induced Drosophila immune proteins characterized Drosophila C virus-induced proteins in the hemolymph and identified, a pheromone- and odor-binding protein, pherokine-2 (Sabatier et al., 2003). Integrating the innate-immune interactome with the virus-host protein-protein interface would provide insight into the interconnection of viral proteins and host innate immune components.
4. Tools to dissect antiviral mechanisms in vivo
Drosophila’s rapid life cycle combined with a wide array of genetic tools makes the investigation of virus-host interactions in vivo facile. The wealth of genetic mutants readily obtainable from stock centers (e.g., multiple genome-wide in vivo RNAi transgenic libraries available, insertional mutations in >40% of genes) allows for systematic investigation of antiviral factors at the organismal level (Cherry and Silverman, 2006; Eleftherianos and Schneider, 2011; Venken and Bellen, 2005). Genetic manipulations using conditional drivers allow for detailed virus-host interaction studies in vivo (Beckingham et al., 2005; Venken and Bellen, 2005). For example, the Gal4/UAS system can be used to drive the expression of UAS transgenes encoding cDNAs to ectopically express genes-of-interest or long hairpin dsRNAs to target endogenous transcripts by RNAi in vivo (Perrimon et al., 2010). To bypass developmental requirements, one can take advantage of conditional drivers (e.g. heat shock inducible) and tissue-specific promoters (e.g. hemocyte) to manipulate factors of interest precisely in both time and space (Dietzl et al., 2007; Perrimon et al., 2010). Even more fine-scale genetic approaches, including lineage tracing and clonal analysis, are facile in Drosophila (Lee, 2009).
Importantly (and perhaps surprisingly), adult Drosophila can be infected by a wide variety of viruses, including both insect pathogens and members of each major family of human arboviruses (Chotkowski et al., 2008a; Huszar and Imler, 2008; Kemp et al., 2013; Mukherjee and Hanley, 2010; Sabin et al., 2009; Shelly et al., 2009; Xu et al., 2012). Most studies inoculate adult flies by injection into the open circulatory system, which allows for reproducible and robust infection (Hoffmann, 2003; Merkling and van Rij, 2012; Sabin et al., 2010). A variety of assays have been used to monitor viral replication and pathogenesis in vivo, including survival studies as well as molecular assays to monitor viral protein, viral nucleic acid, or infectious particles that are produced during the course of an infection (Cherry and Silverman, 2006; Lemaitre and Hoffmann, 2007; Merkling and van Rij, 2012). Microscopy can also be used to monitor the infection of individual cells in disparate tissues, since dissection of adult flies is a simple task (Cherry and Silverman, 2006; Lemaitre and Hoffmann, 2007; Merkling and van Rij, 2012). By comparing the dependencies across viral pathogens, both general and virus-specific innate restriction mechanisms can be identified.
5. Viruses
A subset of viruses naturally infects invertebrates, and a fraction of those infect Drosophila. There are a handful of viruses that have been described as natural Drosophila melanogaster pathogens (Sabin et al., 2010; van Rij et al., 2006). The best characterized are Drosophila C virus (DCV), Nora virus, and Sigma virus (Habayeb et al., 2006; Iconomidis and L'Hertier, 1961; Jousset et al., 1977; Sabin et al., 2010; van Rij et al., 2006). These viruses vary in infection strategies and pathogenesis, but are thought to have a narrow host range (Gomariz-Zilber et al., 1995; Habayeb et al., 2006; Kapun et al., 2010; Tsai et al., 2008b). In contrast, other insect viruses have a broad host range and can infect a wide variety of insects, including Drosophila, and the best studied are Flock House virus (FHV) and Cricket Paralysis virus (CrPV) (Dasgupta et al., 2007; Plus et al., 1978; Reinganum, 1975). Furthermore, Drosophila has emerged as a model for mammalian arboviruses that are a common cause of morbidity and mortality worldwide (Weaver and Reisen, 2010). These viruses do not normally infect Drosophila; but cycle in nature between a hematophagous arthropod host, usually a mosquito or tick, and an animal reservoir (Weaver and Reisen, 2010). Arboviruses are (re)emerging and are extending beyond their traditional geographical boundaries (Beaty, 1996; Griffin, 2007; Weaver and Reisen, 2010). Most arboviruses impacting public health fall into three viral families: Alphaviridae, Flaviviridae, and Bunyaviridae. Viruses from each of these families can infect Drosophila experimentally (Chotkowski et al., 2008a; Filone et al., 2010; Mueller et al., 2010; Xu et al., 2012), which has expanded the use of Drosophila to explore virus-host interactions of these medically important pathogens. Importantly, flies deficient in innate immune pathways can carry higher viral loads and/or succumb more rapidly to infection. Therefore, increasing effort has been employed to discover the full spectrum of genes and pathways involved in antiviral defense. We will review the basics of the viruses most commonly studied in Drosophila (reviewed in Table 1).
Table 1.
Summary of viruses that have been studied in Drosophila
| Virus | Host Range |
Family | Genus | Genome | Segments | Pathogenesis in Drosophila | Tissue Penetrance in Drosophila |
Type of Infection in Drosophila Cell lines |
Host Anti-Viral Mechanisms |
Restricted by Wolbachia |
Suppressor of Silencing? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drosophila C Virus (DCV) | Insects | Dicistroviridae | Cripavirus | (+)ssRNA | one | Potentially Lethal | Fat body, periovarial sheath, tracheae, muscles and digestive tract | Cytopathic | JAK/STAT, RNAi, Autophagy, Transcriptional pausing | Yes | Yes |
| Cricket Paralysis Virus (CrPV) | Insects | Dicistroviridae | Cripavirus | (+)ssRNA | one | Potentially Lethal | Undetermined (Alimentary canal, epidermis, and nerve ganglia in crickets) | Cytopathic | Imd, RNAi | Yes | Yes |
| Flock House Virus (FHV) | Insects | Nodaviridae | Alphanodavirus | (+)ssRNA | two | Potentially Lethal | Trachea, cardiac muscles, fat body, egg chamber | Cytopathic | JAK/STAT, RNAi | Yes | Yes |
| Nora virus | Insects | Unclassified | Unclassified | (+)ssRNA | one | Asymptomatic, Persistent | Digestive tract | Undetermined | RNAi | Yes | Yes |
| Sigma virus | Insects | Rhabdoviridae | Sigmavirus | (−)ssRNA | one | Asymptomatic, CO2 sensitivity | Female reproductive tract, Nervous system, all tissues tested | Noncytopathic, Persistent | ref(2)p, CHKov 1, CHKov2 | Undetermined | Yes |
| Drosophila X Virus (DXV) | Insects | Birnaviridae | Entomobirnavirus | dsRNA | two | Asymptomatic, CO2 sensitivity | Digestive tract, trachea, muscles, ovaries, fat body | Cytopathic | Toll, RNAi | Undetermined | unknown |
| Invertebrate iridescent virus 6 (IIV-6) | Insects | Iridoviridae | Iridovirus | dsDNA | one | Modest lethality | Undetermined | Noncytopathic, Persistent | RNAi | No | unknown |
| Bluetongue virus (BTV-1) | Arbovirus | Reoviridae | Orbivirus | dsRNA | ten | Asymptomatic | Proventriculus, salivary glands, fat body | Noncytopathic, Persistent | Undetermined | unknown | |
| Vesicular Stomatitis Virus (VSV) | Arbovirus | Rhabdoviridae | Vesiculovirus | (−)ssRNA | one | Asymptomatic | Fat Body | Noncytopathic, Persistent | Autophagy, RNAi, Transcriptional Pausing | Undetermined | unknown |
| Sindbis Virus (SINV) | Arbovirus | Togaviridae | Alphavirus | (+)ssRNA | one | Asymptomatic | Fat Body, Brain | Noncytopathic, Persistent | Imd, RNAi, Transcriptional Pausing | Undetermined | unknown |
| Chikungunya virus (CHIKV) | Arbovirus | Togaviridae | Alphavirus | (+)ssRNA | one | Asymptomatic | Undetermined | Noncytopathic, Persistent | Modestly Affected | unknown | |
| Venezuelan Equine Encephalitis virus (VEE) | Arbovirus | Togaviridae | Alphavirus | (+)ssRNA | one | Asymptomatic | Undetermined | Noncytopathic, Persistent | Undetermined | unknown | |
| Rift Valley Fever Virus (RFV) | Arbovirus | Bunyavirus | Phleboviridae | (−)ssRNA | three | Undetermined | fat body, visceral muscles | Noncytopathic, Persistent | Transcriptional Pausing, RNAi | Undetermined | unknown |
| La Crosse Virus (LACV) | Arbovirus | Bunyavirus | Orthobunyavirus | (−) ssRNA | three | Asymptomatic | Undetermined | Noncytopathic, Persistent | RNAi | Modestly Affected | unknown |
| Tahyna virus | Arbovirus | Bunyavirus | Orthobunyavirus | (−)ssRNA | three | Undetermined | Undetermined | Noncytopathic, Persistent | Undetermined | unknown | |
| West Nile Virus (WNV) | Arbovirus | Flaviviridae | Flavivirus | (+)ssRNA | one | Asymptomatic | Undetermined | Noncytopathic, Persistent | Transcriptional Pausing, RNAi | Yes | Yes |
| Dengue Virus (DEN) | Arbovirus | Flaviviridae | Flavivirus | (+)ssRNA | one | Asymptomatic | Undetermined | Noncytopathic, Persistent | Toll, JAK/STAT, RNAi | Undetermined | Yes |
Insect-restricted viruses
Drosophila C virus
Drosophila C virus (DCV) is one of the best-studied natural pathogens of flies. DCV belongs to the genus Cripavirus and was previously thought to be a member of the virus family Picornaviridae (Jousset et al., 1977; King, 1988; Murphy, 1995; Scotti et al., 1981). This was due to similarities in size, sedimentary coefficient, buoyant density, the number and size of their structural proteins, and genomic structure, which is a polyadenylated genomic RNA with a small protein (VPg) attached to the 5' end of the sequence (Murphy, 1995; Scotti et al., 1981). However, detailed analysis of the complete ~9kb genome shows that it contains two large open reading frames (ORFs) separated by 191 nucleotides which encodes an Internal Ribosome Entry Site (IRES) (Johnson and Christian, 1998; Sasaki and Nakashima, 2000; Wilson et al., 2000a). In contrast, picornavirus genomes contain only a single open reading frame (ORF) that is translated into a single polyprotein via an IRES (Johnson and Christian, 1998; Sasaki and Nakashima, 2000; Wilson et al., 2000a). Also, the capsid proteins are encoded at the 3' end of the DCV genome, opposite of that for picornaviruses (Johnson and Christian, 1998). These crucial differences led to the formation of the Family Dicistroviridae by the International Committee on Taxonomy of Viruses (ICTV) (Bonning and Miller, 2010; Christian, 2008; Johnson and Christian, 1998). Insects harbor many small picorna-like viruses (Gordon and Waterhouse, 2006; Moore and Tinsley, 1982), most of which belong to the virus family Dicistroviridae; members include cricket paralysis virus (CrPV) (described below), Taura syndrome virus (TSV),an economically important shrimp pathogen, and Israeli acute paralysis virus (IAPV), a honeybee virus (Bonami et al., 1997; Maori et al., 2007; Reinganum, 1975).
DCV virions are composed of three major capsids along with the single stranded RNA genome (Johnson and Christian, 1998). The virus enters the cell by clathrin-mediated endocytosis in a low pH compartment (Cherry et al., 2005; Cherry and Perrimon, 2004). Once uncoated, the viral RNA serves as a messenger RNA and accesses host ribosomes for translation of the two ORFs (Cherry et al., 2005; Cherry and Perrimon, 2004). These dicistroviruses use two IRESs to initiate cap-independent translation of each ORF (Cherry et al., 2005; Cherry and Perrimon, 2004). These ORFs encode polyproteins, which are consequently processed and cleaved into the structural and non-structural proteins (Le Gall et al., 2008; Racaniello, 2006). Once the non-structural proteins are made, they remodel cellular membranes for RNA replication in the cytoplasm, which is dependent on de novo lipid biosynthesis and the COPI coatamer (Cherry et al., 2006). The 5’ end of ORF1 encodes a suppressor of RNA silencing important for immune suppression (van Rij et al., 2006). Finally, mature virions assemble into paracrystalline arrays, and cytolysis frees these virions for subsequent infections.
DCV was first identified in healthy wild Drosophila melanogaster populations in France in the 1970’s and was subsequently found in populations worldwide (Gomariz-Zilber et al., 1995; Jousset and Plus, 1975). While DCV can infect other strains of Drosophila, in general, it has a narrow host range (Kapun et al., 2010). The virus is thought to be transmitted orally and has only modest effects on survival, although genetic polymorphisms may play an important role (Gomariz-Zilber et al., 1995; Jousset et al., 1972; Lautié-Harivel and Thomas-Orillard, 1990; Thomas-Orillard et al., 1995). Drosophila that are infected with DCV develop more quickly and the females produce more eggs in comparison to uninfected flies (Gomariz-Zilber et al., 1995). Experimentally, the virus is highly pathogenic when injected into adult flies, replicating to high titers and causing mortality (Cherry and Perrimon, 2004). DCV infects the fat body, periovarial sheath, tracheae, muscles, and the digestive tract of injected flies (Cherry and Perrimon, 2004; Lautié-Harivel and Thomas-Orillard, 1990; Sabatier et al., 2003). There is no evidence for germline infections or vertical transmission (Gomariz-Zilber et al., 1995; Jousset and Plus, 1975). It remains unclear how infection leads to mortality. However, the innate immune system appears to play an important role since flies deficient in innate immune pathways can carry higher viral loads and succumb more rapidly to infection.
Cricket Paralysis virus
Cricket Paralysis Virus (CrPV) was initially discovered in field crickets in Australia (Reinganum, 1975, 1970). The paralytic disease spread rapidly through a breeding colony, as well as through a laboratory population causing significant mortality (Reinganum, 1975). CrPV has been detected in at least five different orders of the class Insecta in both natural and laboratory populations, thus possessing one of the widest host ranges of any insect virus (Reinganum, 1975; Scotti et al., 1981). In crickets, CrPV particles can be found in the cytoplasm of cells of the alimentary canal, epidermis, and nerve ganglia (Moore N, 1991; Reinganum, 1975). CrPV has been shown to replicate in cultured cell lines and adult flies, where it leads to cytopathic effects and mortality (Scotti et al., 1996; van Rij et al., 2006; Wang et al., 2006). While the tissue tropism in flies has not been well-characterized, infection is associated with the depletion of hemocytes in the absence of a canonical humoral immune response (Costa et al., 2009a).
CrPV, like DCV, was previously thought to be a member of the virus family Picornaviridae. The crystal structure of CrPV showed that the conformation of its capsid proteins closely resembled those of picornaviruses (Tate et al., 1999). However, CrPV closely resembles DCV in sequence and replication strategies and has since been classified as belonging to the Dicistroviridae (Christian and Scotti, 1994; Tate et al., 1999). Interestingly, CrPV infection of Drosophila cells leads to a rapid shutoff of host protein synthesis, concomitant with phosphorylation of eIF2α (Garrey et al., 2010; Khong and Jan, 2011; Wilson et al., 2000b). The CrPV IRES has been used to dissect and understand many facets of IRES-dependent initiation factor-independent translation (Bushell and Sarnow, 2002; Cevallos and Sarnow, 2005; Garrey et al., 2010; Hellen, 2009; Masoumi et al., 2003; Schüler et al., 2006; Spahn et al., 2004). More recently, it was found that RpS25 has an essential role in the translation of the second ORF from the internal IRES of CrPV (Landry et al., 2009). As in DCV, CrPV encodes a suppressor of silencing at the 5’ end of ORF1, but interestingly, it functions by inhibiting a distinct step in the silencing pathway (Nayak et al., 2010).
Flock House virus
Flock House virus (FHV) is the prototypical virus of the Nodaviridae family of insect viruses (Scotti et al., 1983). These are small, non-enveloped bi-segmented positive sense RNA viruses (Dasgupta et al., 1994; Schneemann et al., 1998). FHV virions contain RNA1 (3.1 kb) and RNA2 (1.4 kb), which encode protein A, the viral RNA-dependent RNA polymerase, and the structural capsid protein precursor (Dasgupta et al., 1994; Schneemann et al., 1998). The Nodavirus family is genetically, biochemically and structurally well characterized in part due to the simple reverse genetic system that has revealed important insights into fundamental aspects of virology and virus-host interactions (Schneemann et al., 1998; Venter and Schneemann, 2008). FHV is a robust model to study how non-enveloped viruses penetrate cellular membranes during cell entry (Maia et al., 2006; Odegard et al., 2010; Odegard et al., 2009; Walukiewicz et al., 2006). Once uncoated, FHV produces a subgenomic RNA3 (0.4 kb) during active genome replication, which encodes the RNA silencing inhibitor protein B2 (Galiana-Arnoux et al., 2006b; Li et al., 2002; Lu et al., 2005). The FHV life cycle shares many common features with other positive-sense RNA viruses, including the membrane-specific targeting and assembly of functional RNA replication complexes (Miller and Ahlquist, 2002; Miller et al., 2001; Miller et al., 2003), the exploitation of various cellular processes and host factors for viral replication, including a helicase (Castorena et al., 2007; Kampmueller and Miller, 2005; Kovalev et al., 2012; Sharma et al., 2011; Weeks and Miller, 2008), and the induction of large-scale membrane rearrangements (Kopek et al., 2007; Lanman et al., 2008; Miller et al., 2001; Miller et al., 2003). Glycerophospholipid metabolism (in particular phosphatidylcholine biosynthesis) is a critical regulator of FHV RNA replication (Castorena et al., 2010). Mitochondrion-enriched anionic phospholipids facilitate FHV RNA polymerase membrane association (Stapleford et al., 2009). And these RNA replication complexes form on outer mitochondrial membranes inside virus-induced invaginations (Miller and Ahlquist, 2002; Miller et al., 2001; Venter and Schneemann, 2008). This novel compartment seems to form a protective niche for the viral dsRNA intermediate. Lastly, genome packaging and assembly is functionally coupled to replication (Seo et al., 2012).
FHV was isolated from a coleopteran (Scotti et al., 1983) and is not a natural pathogen of Drosophila. It can infect a wide variety of animals and can replicate in cells from yeast to humans (Dasgupta et al., 2007; Johnson and Ball, 1997; Price et al., 1996; Selling et al., 1990). As expected, FHV can infect Drosophila cells and adult flies, and causes high lethality after intrathoracic injection (Dasgupta et al., 2007; Dasgupta et al., 1994; Galiana-Arnoux et al., 2006b; Johnson and Ball, 1999). The fat body is heavily infected along with the tracheae and muscles including the cardiac muscle (Eleftherianos et al., 2011; Galiana-Arnoux et al., 2006a). It is interesting to note that ATP-sensitive potassium channels mediate resistance to FHV in the heart (Croker et al., 2007; Eleftherianos et al., 2011). FHV can also be detected in most if not all cell types and developmental stages of the egg chamber including the germarium (Thomson et al., 2012). FHV infection results in decreased TOR activity in FHV-infected flies and ovaries and leads to the downregulation of Drosophila inhibitor of apoptosis 1 (DIAP1), rendering cells susceptible to apoptotic death (Liu et al., 2013; Settles and Friesen, 2008). Rapid induction of apoptosis prevents the expression of viral genes blocking infection (Liu et al., 2013). The recent development of a transgenic model for FHV allows for analysis of individual aspects of FHV replication in flies (Galiana-Arnoux et al., 2006a).
Nora virus
Nora virus is a small, non-enveloped virus with a single-stranded, positive-sense RNA genome. It has a unique genome organization (and capsid organization) in that the 12 kb genome encodes four open reading frames (Ekström et al., 2011; Habayeb et al., 2006). ORF1-3 are partially overlapping, suggesting frame-shift events during their translation. An 88-nucleotide untranslated region precedes ORF4, indicating independent translational control of this open reading frame that encodes the capsid proteins. ORF2 encodes a typical picorna-like replicative cassette, with a helicase, a protease, and an RNA-dependent RNA polymerase, while the proteins encoded in the remaining ORFs are not closely related to any described protein sequences (Habayeb et al., 2006).
Nora virus was first identified in laboratory fly stocks, where it can persist for years without causing any overt pathological effects (Habayeb et al., 2006). Nora virus infects the intestine of flies, and is excreted in the feces, resulting in horizontal transmission (Habayeb et al., 2006). There is no evidence that the virus can be vertically transmitted. Little is known about the replication cycle of Nora virus. Unlike other RNA viruses in Drosophila, mutants in the RNAi pathway do not support higher levels of infection. However, recent studies demonstrate that Nora virus carries a suppressor of RNA silencing as has been observed with many RNA viruses that are natural pathogens of insects (Kemp et al., 2013; Sabin et al., 2010; van Mierlo et al., 2012). In addition, Nora virus-like sequences have been identified more broadly, including in a beetle, a mollusk and a plant virus. For instance, Nasonia virus (NvitV-3) is similar to Nora virus and infects the wasp Nasonia (Oliveira et al., 2010). This suggests that Nora virus may represent the first member of a widespread family of viruses.
Sigma virus
Rhabdoviruses are enveloped, non-segmented negative-sense RNA viruses. More than 160 species of rhabdoviruses have been described, many of which are a threat to human, animal, or plant health (Ammar et al., 2009; Fu, 2005; Hogenhout et al., 2003; Walker P.J., 2000). All plant-infecting and many vertebrate-infecting rhabdoviruses are transmitted by insect vectors (Brun and Plus, 1980; Fleuriet, 1988; L'Heritier and Teissier, 1937). The Drosophila-infecting rhabdovirus Sigma virus occurs naturally in several Drosophila species and is maintained in fly populations through vertical transmission via germ cells (Longdon and Jiggins, 2012). Sigma virus infection has been studied for over seventy years, following the observation that Sigma virus-infected flies remain irreversibly paralyzed and die after CO2 anesthetization, whereas uninfected flies recover their motility shortly following their return to fresh air (L'Heritier and Teissier, 1937). Sigma virus infection of wild Drosophila is common; typically about 0–30% of the population is infected (Carpenter et al., 2007; Wayne et al., 2011; Wilfert and Jiggins, 2010). The sensitivity to CO2 is correlated with Sigma virus titers in flies, and is associated with infection of the thoracic ganglia, although Sigma virus infects almost all Drosophila tissues (Bussereau, 1970a, b; L'Heritier, 1948).
However, a lethal dose of CO2 is seldom encountered in nature, making it unclear whether infected flies are at any disadvantage (Longdon and Jiggins, 2012). Sigma-like viruses are probably common endosymbionts of insects, if not arthropods as a whole, since they have been isolated from additional species of Drosophila and one species of Muscid fly (Longdon and Jiggins, 2012; Longdon et al., 2010; Longdon et al., 2011). These viruses form a new genus in the family Rhabdoviridae. Sigma virus-like genes are present in Drosophila genomes suggesting a long co-evolution (Ballinger et al., 2012). CO2 sensitivity was also observed when Drosophila were injected with various vesiculoviruses, and temporary CO2 sensitivity was reported in aphid vectors infected with some plant rhabdoviruses, making this a common feature of rhabdovirus pathogenesis in insects (Busserea and Contamine, 1980; Rosen, 1980; Shroyer and Rosen, 1983; Sylvester, 1992).
In wild Drosophila populations, Sigma virus infection does not appear to affect the fertility, longevity, or sexual selection of Drosophila, but laboratory reared animals have reduced egg viability (Fleuriet, 1981; Seecof, 1964). As expected, Sigma virus causes low virulence (Fine, 1975), but if the flies are placed under higher density conditions, the virus shows a decline in frequency. This suggests the infected flies are outcompeted by their uninfected counterparts (Yampolsky et al., 1999). Sigma virus strains selected for high replication rates adversely affect the fertility and egg viability of Drosophila under laboratory conditions (Fleuriet, 1981; Seecof, 1964). Therefore, Drosophila must limit Sigma virus infection, since unrestrained replication leading to high titers has negative effects on fitness. The interaction between fly populations and Sigma virus is a good model system for investigating the molecular aspects of rhabdovirus pathogenesis (Longdon and Jiggins, 2012). Genetic variation in the susceptibility of flies to infection by Sigma virus has been mapped, leading to the discovery of at least six polymorphic regions that confer resistance in natural populations (Bangham et al., 2008; Gay, 1978). One of these loci, ref(2)P, the Drosophila homolog of p62, is necessary for male fertility. Furthermore, ref(2)P complexes with the viral protein P, that is necessary for viral replication. It is likely that the involvement of ref(2)P in the autophagy pathway protects Drosophila against Sigma virus infection. The second locus that has been cloned, ref(3)D, encodes two paralogous genes: CHKov1 and CHKov2 (Bangham et al., 2008; Dru et al., 1993; Gay, 1978; Magwire et al., 2011). Successive changes in this genomic region dramatically altered resistance to Sigma virus. The truncated allele of CHKov1 increases the resistance to both Sigma virus and insecticides. Since this allele pre-dates insecticide use, it is possible that these changes pre-adapted flies to insecticides (Bangham et al., 2008; Dru et al., 1993; Gay, 1978; Magwire et al., 2011). Further studies are needed to uncover the mechanisms by which these resistance loci mechanistically impact Sigma virus infection.
Drosophila X virus
Drosophila X virus (DXV) is the prototype species of the monospecific genus Entomobirnavirus within the family Birnaviridae (Delmas et al., 2011). Birnaviruses are icosahedral, non-enveloped viruses that contain a nucleocapsid and bisegmented dsRNA genome which a large number infect fish and birds. RNA segment A encodes a polyprotein (N-VP2-VP4-VP3-C) and segment B encodes one viral polypeptide 1 (VP1) (Dobos, 1995; Dobos et al., 1979; Nagy and Dobos, 1984). Little is known about the replication cycle of DXV.
DXV was isolated as a contaminant during infection studies with Sigma virus in Drosophila cell lines. Infection of flies with DXV, like Sigma virus, cause sensitivity to CO2 and increased mortality (Dobos et al., 1979; Teninges et al., 1979) (Teninges et al., 1979). DXV replicates in the cytoplasm of cells of the digestive tract, trachea, muscles, ovaries and fat body (Teninges et al., 1979; Zambon et al., 2005). However, it is unclear which tissue is responsible for anoxia sensitivity (Teninges et al., 1979). DXV has never been found as a natural infection in Drosophila but has been detected in natural populations of Culicoides sp (Bonami and Adams, 1991). While the true origin of DXV in flies is unknown, it is hypothesized that the virus originated as a contaminant from fetal calf serum used in the original infection studies (Teninges et al., 1979).
Reoviruses constitute another group of dsRNA viruses. Nearly all insect viruses in the family Reoviridae are cytoplasmic polyhedrosis viruses (Patton, 2008; Shaw et al., 2012). These share structural and biochemical similarities with other members of the Reoviridae (Patton, 2008; Shaw et al., 2012). Insect members of this family can be distinguished by host range, morphology of the capsid (lack of a double capsid) sequence homology, and a major virally-encoded polyhedron protein that forms the cytoplasmic inclusion bodies in insects (Patton, 2008; Shaw et al., 2012). None of these viruses have been shown to infect Drosophila. However, it was recently found that Bluetongue virus (BTV), a member of Reoviridae, can experimentally infect Drosophila. BTV is an arbovirus transmitted between ruminant hosts and biting midges. It can cause bluetongue, a hemorrhagic disease of ruminants with high levels of morbidity and mortality (Maclachlan, 2011; Oura, 2011; Roy, 1989). The current lack of genomic resources and genetic tools for Culicoides restricts any detailed study of the mechanisms involved in the virus-insect interactions. Using reverse genetics, a mCherry-expressing BTV was not only replication competent, but also retained many characteristics of the wild-type virus in flies (Shaw et al., 2012).
Invertebrate Iridescent Virus 6
Invertebrate iridescent viruses (IVs) belong to the family Iridoviridae, which are icosahedral viruses with a large genome of double-stranded DNA and represent the largest family of non-occluded insect viruses (Eaton et al., 2007; Jakob et al., 2001). The name is derived from the characteristic iridescence observed in the infected insect. Insect Iridescent Virus 6 (IIV-6) (also named Chilo iridescent virus (CIV)) is a large, complex virus with a dsDNA genome of 212,482 bp that encodes 211 putative ORFs distributed along the two strands of the viral genome, and the termini have complex repetitive elements (Eaton et al., 2007; Jakob et al., 2001).
IIV-6 was first isolated from a lepidopteran, but can infect a large number of different insects and cultured insect cells (Constantino et al., 2001; Jakob et al., 2002). Infection is thought to be horizontal and not vertical. IIV-6 was the first DNA virus shown to undergo a complete replication cycle in Drosophila melanogaster where it can infect and replicate in cells (Constantino et al., 2001) and causes adult flies to die upon injection (Teixeira et al., 2008). This has opened the door for the exploration of unique interactions between DNA viruses and Drosophila, and led to the surprising finding that IIV-6 is restricted by the RNAi pathway in adult flies (Bronkhorst et al., 2012; Kemp et al., 2013; Teixeira et al., 2008).
Vertebrate viruses
Vesicular stomatitis virus
Vesicular stomatitis virus (VSV) is the prototypic member of the genus Vesiculovirus of the family Rhabdoviridae, and one of the most well-characterized viruses for biomedical research (Griffin, 2007). VSV can infect all cells and organisms tested. This extensive host range, the simplicity of this virus, and the existing array of molecular tools, including the reverse-genetic system, make VSV a very useful tool for studying virus-host interactions. VSV is a zoonotic virus that is transmitted by sandflies and other biting insects (Letchworth et al., 1999; Mead et al., 2000). Symptoms of VSV infection resembles foot-and-mouth disease in many mammals, including humans, and can be fatal to infected cattle (Griffin, 2007; Letchworth et al., 1999; Mead et al., 2000). While Rhabdoviridae is a much less clinically significant arboviral family, important members include Chandipura virus and Piry virus (Busscereau, 1975; Walker P.J., 2000).
Once Sigma virus was found to be in the rhabdovirus family, flies were challenged with mammalian rhabdoviruses, including VSV (Brun, 1991; Busscereau, 1975; Wyers et al., 1980). It was found that like Sigma virus infection, VSV infection presents with no mortality under normal conditions but causes flies to become CO2 sensitive (Rosen, 1980). This finding was also extended to both Chandipura and Piry viruses, where flies infected with these vertebrate rhabdoviruses also displayed CO2 sensitivity (Busscereau, 1975). However, unlike Sigma virus infection of flies, these mammalian rhabdoviruses could not be vertically transmitted (Fine, 1975; Ohanessian, 1989). VSV has a wide tissue tropism in the fly; the fat body is heavily infected. The relative fitness of VSV infected flies appears to be due to immune control. Flies deficient in innate immune pathways, including RNAi and autophagy, present with increased titers and mortality (Mueller et al., 2010; Sabin et al., 2009; Shelly et al., 2009).
The VSV genome is a single stranded, negative-sense RNA of ~11kb that encodes five proteins: G protein (G), large protein (L), phosphoprotein (P), matrix protein (M) and nucleoprotein (N) (Griffin, 2007; Rose and Schubert, 1987). The virion binds to an unknown cellular receptor, traffics via clathrin-mediated endocytosis to a low pH compartment where fusion occurs (Cureton et al., 2009; Griffin, 2007; Rose and Schubert, 1987). Once uncoated the viral RNA dependent RNA polymerase (L) binds the encapsidated genome at the leader region, then sequentially transcribes each gene by recognizing start and stop signals flanking viral genes (Ball and White, 1976; Griffin, 2007; Rose and Schubert, 1987). mRNAs are capped and polyadenylated by the L protein during synthesis and are translated by the cell (Ball and White, 1976; Griffin, 2007; Rose and Schubert, 1987). Unlike vertebrate infection, during infection of invertebrate cells there is no inhibition of host macromolecular synthesis, and the infection is non-cytopathic and persistent (Dezélée et al., 1987; Wyers et al., 1980). The mechanisms involved in persistent non-cytocidal infections are unknown, but it has been found that posttranslational maturation of some VSV proteins is altered in insect cells, including the observation that the M protein is hyperphosphorylated and that the G protein is synthesized in reduced amounts with an altered glycosylation pattern (Dezélée et al., 1987; Laurent and Lavay, 1983; Rose and Schubert, 1987; Wyers et al., 1980).
Sindbis Virus and other Alphaviruses
Sindbis virus (SINV) is the prototypical member of the Togaviridae family in the alphavirus subfamily. The virus was first isolated in 1952 in Cairo, Egypt (Taylor et al., 1955). SINV is arthropod-borne and is maintained in nature by transmission between vertebrate (bird) hosts and invertebrate (mosquito) vectors (Strauss and Strauss, 1994). In humans, SINV infection is widespread in the Old World and can cause chronic polyarthritis and Pogosta disease (Kurkela et al., 2005; Sane et al., 2010). Many additional alphaviruses infect humans and can cause severe illnesses and are thus considered serious human pathogens, including Chikungunya virus, Ross River virus, and Venezuelan Equine Encephalitis virus (Griffin, 2007; Weaver and Barrett, 2004).
Alphaviruses are enveloped viruses with a single-stranded, positive-sense ~12 kb RNA genome with a 5' cap and 3' polyadenylated tail (Hefti et al., 1975; Strauss and Strauss, 1994). Therefore, genomic RNA serves directly as an mRNA for translation (Strauss and Strauss, 1994). The genome encodes two ORFs with four non-structural proteins in the 5' ORF and the structural proteins (capsid and two envelope proteins) at the 3' end (Lemm and Rice, 1993; Lemm et al., 1994; Strauss and Strauss, 1994). To initiate infection these viruses bind to a cell surface receptor and traffic via clathrin-mediated endocytosis into an acidified compartment where this triggers fusion and uncoating releasing the genome into the cytoplasm (Rose et al., 2011b; Strauss and Strauss, 1994; Strauss et al., 1994). NRAMP was recently identified as the plasma membrane entry receptor for SINV in Drosophila (Rose et al., 2011b). Interestingly, SINV uses the mammalian ortholog, NRAMP2, for entry into vertebrate cells (Rose et al., 2011b). In the cytoplasm, the first ORF is translated to produce the non-structural proteins, which are involved in genome replication, the production of new genomic RNA, and a shorter sub-genomic RNA strand (Griffin, 2007; Lemm and Rice, 1993). This subgenomic strand contains the second ORF and is used for translation of the structural proteins (Lemm and Rice, 1993). SINV replication activates the PI3K-Akt-TOR pathway, causing the phosphorylation of 4E-BP1 and increasing the formation of eukaryotic initiation factor 4F (eIF4F), which promotes cap-dependent translation of viral mRNA (Patel and Hardy, 2012). The viruses assemble at the host cell membrane and acquire their envelope with an icosohedral capsid via budding (Sanchez-San Martin et al., 2009; Sylvester, 1992).
First used in fly experiments in 1960’s and in insect cell culture in the 1970’s, it was observed that SINV causes no lethality or cytopathic effects (Bras-Herreng, 1973, 1975, 1976). SINV, like VSV, replicates in most cultured cell lines tested, and Drosophila cells become persistently infected (Mudiganti et al., 2010a). This promiscuous and useful expression system has been adapted to efficiently transduce many cells and organisms, including vector mosquitoes (Avadhanula et al., 2009a; Collins et al., 1982; Olson et al., 2000). Indeed, the reverse genetics are facile (easier than VSV) and have been used to create a large number of transgenic insects, including vector mosquitoes, to introduce genes-of-interest and to study viral replication. Moreover, a transgenic fly system expressing a self-replicating viral RNA genome has been used to study viral infection in vivo (Phillips et al., 2010). As observed in mosquitoes, intrathoracic challenge of SINV fails to kill the flies although a large number of tissues are infected, including the fat body and intestinal visceral muscles (Galiana-Arnoux et al., 2006a). Again, it appears that the relative fitness of SINV-infected flies is due to immune control; flies deficient in innate immunity, including RNAi, display increased titers and mortality (Avadhanula et al., 2009b; Phillips et al., 2010).
Studies have shown that additional alphaviruses can infect and replicate in flies. Chikungunya virus (CHIKV) is a mosquito-borne human pathogen that is emerging globally. CHIKV proliferates robustly after inoculation into flies making this a potentially useful model (Avadhanula et al., 2009b; Phillips et al., 2010). We have also found that Venezuelan Equine Encephalitis virus infects Drosophila cells (unpublished). This suggests that Drosophila may be used to model a larger array of medically important alphaviruses.
Rift Valley fever virus and other Bunyaviruses
The Bunyaviridae is comprised of a large number of enveloped, tri-segmented negative-sense RNA viruses that infect mammals, insects, and plants and fall into five families, four of which are transmitted by arthropods (Walter and Barr, 2011; Zeller and Bouloy, 2000). Rift Valley Fever virus (RVFV) is a bunyavirus in the Phlebovirus group (Boshra et al., 2011). Rift Valley Fever was first reported among livestock in Kenya around 1915, but the virus was not isolated until 1931 (Daubney et al., 1931). RVFV is a USDA and Human Health and Services select agent (Boshra et al., 2011; Hartley et al., 2011; Métras et al., 2011), because it can cause fatal hemorrhagic disease in infected humans and has a high mortality rate among livestock (Boshra et al., 2011; Hartley et al., 2011). This mosquito-transmitted virus is endemic to Africa, but has recently spread to the Arabian Peninsula (Métras et al., 2011; Niu et al., 2012). In other locations, including the US, endemic mosquito species can carry and transmit RVFV (Konrad and Miller, 2012; Métras et al., 2011; Niu et al., 2012).
Bunyaviruses have an outer lipid envelope with two glycoproteins, G(N) and G(C), which encapsidate the three viral genomic strands along with the nucleoprotein N and the viral polymerase L (Griffin, 2007; Walter and Barr, 2011). Some bunyaviruses encode nonstructural proteins NSs and NSm (Walter and Barr, 2011). Some bunyaviruses, including RVFV, use an ambisense strategy to encode NSs (Flick and Bouloy, 2005). Bunyaviruses traffic within infected cells by largely unknown routes to a low pH compartment where they fuse and deliver their genome into the host cell cytoplasm where they replicate (Griffin, 2007). As with other negative sense viruses, the viral polymerase catalyzes both transcription and replication of the viral genome. Interestingly, bunyaviruses cleave host mRNAs at the 5’ end and use this capped primer for transcription initiation (Bishop et al., 1983; Bouloy, 1991; Bouloy and Hannoun, 1976; Bouloy et al., 1990). In contrast, RNA replication copies the entire template and does not depend on any primer for initiation. Viral glycoproteins, in contrast to the other viral proteins, are translated on the rough ER, traffic to the Golgi for glycosylation and processing of envelope proteins (Rusu et al., 2012). The viruses bud into the Golgi and then are released from the cell by exocytosis (Rusu et al., 2012). Bunyaviruses are relatively understudied, and less is known about their life cycle than the other arbovirus families.
Wild type RVFV is used in high containment making experiments difficult. Thus, many groups use the attenuated RVFV strain MP12 which can be handled under standard laboratory conditions (Caplen et al., 1985). This strain differs by 11 amino acids from the wild type strain ZH548, making it likely that cellular factors that impact MP12 replication are also needed for wild type strains (Vialat et al., 1997). As with other arboviruses, RVFV-infected mosquitoes present with little pathology when either fed or inoculated with virus (Bishop and Beaty, 1988). Adult fly inoculation also leads to productive replication with little mortality (Filone et al., 2010). RVFV can be observed in a wide variety of fly tissues including the fat body and visceral muscles of the gut (unpublished).
Additional bunyaviruses have been shown to infect and replicate in Drosophila. La Crosse virus, a member of the orthobunyavirus family, replicates with a low efficiency in cells and flies (Glaser and Meola, 2010). And Tahyna virus, another orthobunyavirus, also infects Drosophila cells poorly (Hannoun and Echalier, 1971). Because Tahyna virus and La Crosse are both strains of California encephalitis virus, it is possible that there is an underlying mechanistic explanation for this low level of replication.
West Nile virus and other Flaviviruses
Flaviviridae are a large family of enveloped non-segmented positive sense RNA viruses, most of which are arthropod-borne. West Nile Virus is another broadly distributed arbovirus that has caused numerous outbreaks, including many recently in the US ((CDC), 2012; Maxmen, 2012; Roehr, 2012). Kunjin virus is a less pathogenic strain of WNV found in Oceana (Griffin, 2007). Dengue virus is one the best-recognized human pathogens. More than a billion people are at risk and over 120 million people are infected annually (Ferreira, 2012; Griffin, 2007). Other Flaviviruses of clinical concern include Japanese encephalitis virus and yellow fever virus (Griffin, 2007; Weaver and Reisen, 2010).
Flavivirus virions are composed of a viral envelope (E) in a lipid bilayer surrounding a nucleocapsid, and encoding an ~11,000 nt genome with a 5’ cap but no poly(A) tail (Griffin, 2007). These viruses enter cells via receptor-mediated endocytosis dependent upon largely unknown cellular receptors. The low pH within the endosome induces fusion of the viral envelope with cellular membranes to allow uncoating. The viral genome serves as the mRNA for translation of the single ORF that is translated at the rough ER and processed into ~10 proteins. RNA replication occurs in cytoplasmic replication complexes that are associated with perinuclear membranes. Progeny virions assemble by budding into an intracellular membrane compartment, thought to be the endoplasmic reticulum, and are subsequently released by exocytosis.
WNV readily infects both Drosophila cells and adult flies and the infection is largely non-pathogenic (Chotkowski et al., 2008a; Glaser and Meola, 2010; Rose et al., 2011a). Furthermore, Dengue virus can infect Drosophila cells, and a Drosophila-adapted strain was used to perform a genome-wide RNAi screen that revealed many factors important for infection (Mukherjee and Hanley, 2010; Rancès et al., 2012; Sessions et al., 2009). Studies in adult flies have also been used to reveal important aspects of Dengue-insect interactions (Mukherjee and Hanley, 2010; Rancès et al., 2012).
6. Antiviral Innate Immune Mechanisms in Drosophila
Insects do not encode a classical acquired immune system and thus rely on innate defenses for antiviral immunity. At the molecular level, several viral recognition and innate antiviral signaling pathways have been identified in Drosophila. Many of these pathways have proven to be ancient and highly conserved, playing fundamental roles in the innate defenses of a broad range of insects as well as vertebrate hosts. To initiate an immune response, the first step is the recognition of pathogens via pattern recognition receptors (PRRs), which sense foreign entities as pathogen-associated molecular patterns (PAMPs). These receptors recognize conserved components of different pathogens, including viral nucleic acids and viral glycoproteins (Sabin et al., 2010; Takeuchi and Akira, 2010). Distinct classes of PRRs act as either membranebound sensors, such as the Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), or cytoplasmic sensors, such as the Retinoic acid-inducible gene (RIG)-I-like receptors (RLRs) and NOD-like receptors (NLRs) (Takeuchi and Akira, 2010). Drosophila encodes PRRs analogous to the TLRs, including transmembrane Toll and Toll-7 (Lemaitre et al., 1996; Nakamoto et al., 2012; Zambon et al., 2005). Recent evidence also suggests that Dcr-2 acts as a cytoplasmic sensor that detects viral dsRNA to trigger immune gene activation (Deddouche et al., 2008).
Once pathogen recognition occurs, the cell relays this information through host signaling programs to stimulate effector responses (Kawai and Akira, 2010; Takeuchi and Akira, 2010). It is clear that the sensing of PAMPs by PRRs induces the transcriptional activation of distinct subsets of genes (Medzhitov and Horng, 2009; Stetson and Medzhitov, 2006; Takeuchi and Akira, 2010). In mammals, these genes are largely interferon-dependent and encode proinflammatory cytokines, type I interferons (IFNs), chemokines, antimicrobial proteins, proteins involved in the modulation of PRR signaling, and many uncharacterized factors (Smale, 2010; Stetson and Medzhitov, 2006; Takeuchi and Akira, 2010). Insects do not appear to have an antiviral interferon system. Furthermore, while many PAMPs and PRRs that drive antibacterial and antifungal gene expression programs have been well-characterized in insects, recent studies are only beginning to reveal antiviral gene expression programs.
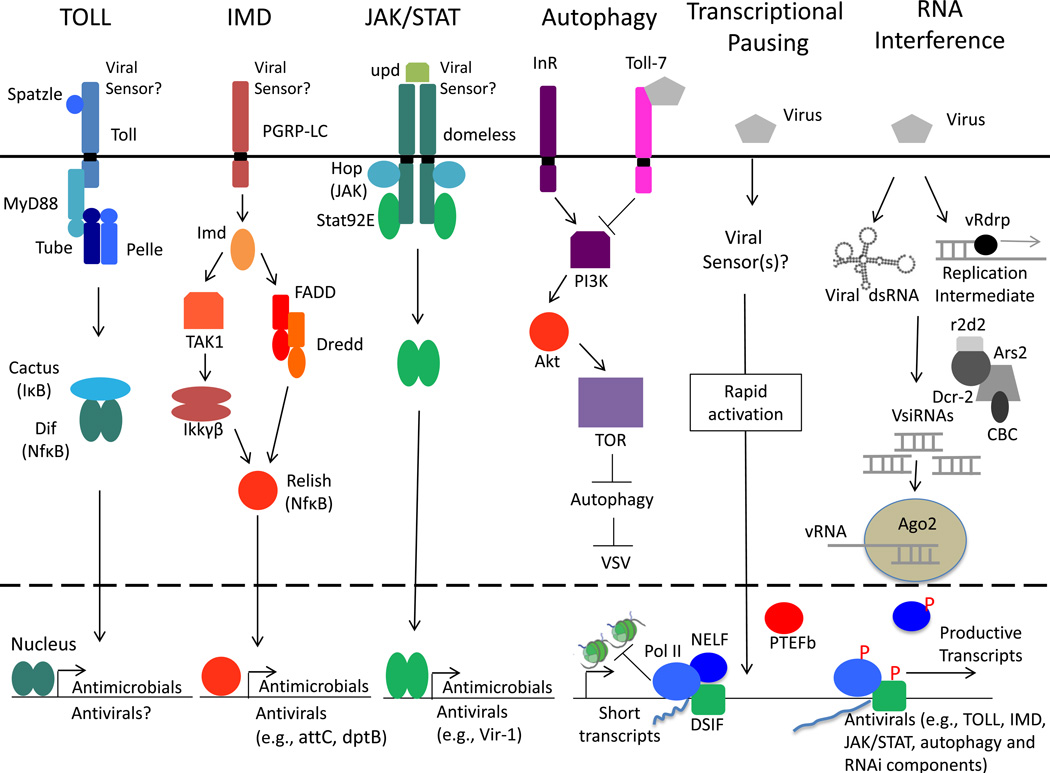
In the early 1990s, genes that encode antimicrobial peptides (AMPs) were cloned and promoter mapping experiments with the Cecropin A1 and Diptericin genes revealed the presence of DNA motifs required for immune induction (Kylsten et al., 1990; Samakovlis et al., 1990; Senger et al., 2004; Wicker et al., 1990) and prominent among these motifs are the κB response elements (Engström et al., 1993; Kadalayil et al., 1997). Genetic studies rapidly revealed that there is clear homology between mammals and insects; NFκB transcription factors are essential for immune defense across hosts (Ganesan et al., 2011; Hedengren et al., 1999; Hultmark, 1993; Ip et al., 1993). The Toll and IMD pathways are canonical and distinct NFκB-dependent pathways downstream of PRRs in Drosophila (Figure 1) (Riddell et al., 2009; Sabin et al., 2010). The Toll pathway shares similarities with the Toll-like receptor signaling pathway found in vertebrates, while the IMD pathway shares homology with the tumor necrosis factor pathway.
Figure 1. Antiviral Innate Immune Pathways in Drosophila.
The Toll pathway, IMD pathway, JAK/STAT pathway, Toll-7-autophagy pathway, transcriptional pausing pathway and the RNA silencing pathway are shown as they relate to antiviral immunity.
The Toll Pathway
The Toll receptor was initially discovered in Drosophila as required for development and subsequently shown to play an essential role in immune defense. This ultimately led to the discovery of mammalian Toll-like receptors (TLRs) (Fitzgerald and Chen, 2006; Lemaitre and Hoffmann, 2007; Lemaitre et al., 1996). In contrast to its mammalian counterparts, Drosophila Toll is not activated by direct interaction with microbial molecules, but through an endogenous ligand called the Nerve Growth Factor-related cytokine Spätzle (Spz) (Weber et al., 2003) (Figure 1). Following Spz–Toll interaction a receptor–adaptor complex including MyD88, which interacts with Toll through their respective Toll/Interleukin-1 receptor domains (Tauszig-Delamasure et al., 2002), recruits Tube via the death domain that in turn recruits the Drosophila IRAK homologue, Pelle (Towb et al., 2001) (Figure 1). This induces phosphorylation of the IκB homologue Cactus, which is subesquently targeted for degradation (Figure 1). Upon Cactus degradation, the NF-κB homologs Dorsal and Dif translocate to the nucleus and induce hundreds of target genes (Manfruelli et al., 1999; Rutschmann et al., 2000) (Figure 1). Hence, signaling pathways downstream of Toll and TLRs are conserved between insects and mammals and converge on the induction of NFκB-dependent genes (Figure 1).
Many studies have implicated the Toll pathway in antibacterial and antifungal defense (Ferrandon et al., 2007; Kemp and Imler, 2009). Pathogen recognition information is integrated through the activation of three recognition pathways via the Spätzle Processing Enzyme, SPE: one triggered by fungal or bacterial proteases that directly activate the host serine protease Persephone, which in this context acts as a sensor of virulence; another induced by recognition of fungal cell wall components; and a third activated by Lysine (Lys)-type bacterial peptidoglycan (El Chamy et al., 2008; Gottar et al., 2006; Ligoxygakis et al., 2002). In addition, the Toll pathway has also been found to protect against some viral infections in insects, although the mechanistic details are less well understood. Toll signaling is induced by and restricts DXV infection in Drosophila and Dengue infection in Aedes mosquitoes (Ramirez and Dimopoulos, 2010; Xi et al., 2008). Yet, the canonical Toll pathway NFκB-dependent genes are not induced by these viral infections. Therefore, it is likely that the Toll pathway confers antiviral activity through non-classical means, an area that remains to be elucidated. Consistent with this hypothesis, it was recently shown that another Toll receptor, Toll-7 in Drosophila, activates the antiviral cellular process of autophagy upon sensing VSV glycoproteins, which is independent of NFκB (Nakamoto et al., 2012; Shelly et al., 2009). Whether additional Toll receptors (Drosophila encodes nine) play a role in antiviral immunity awaits further study.
The IMD Pathway
The IMD pathway is the other canonical NFκB-dependent PRR pathway that has been well-characterized in insects, including Drosophila and mosquitoes. This pathway shares homology with the tumor necrosis factor pathway in mammals and converges on distinct NFκB transcription factors, inducing a largely distinct antimicrobial transcriptional program (Figure 1). IMD signaling is primarily activated by DAP-type bacterial peptidoglycan, which is present in the cell wall of Gram-negative bacteria as well as some Gram-positive Bacilli (Kaneko et al., 2004). Pathogen recognition is mediated by the PRRs PGRP-LC and PGRP-LE (Choe et al., 2005; Kaneko et al., 2006). Subsequent intracellular signaling is transduced by binding Imd, which in turn associates with Drosophila FADD (FAS-associated death-domain protein) homologue (Naitza et al., 2002) and recruits a DREDD, the homologue of mammalian caspase-8 (Leulier et al., 2000). DREDD cleaves Imd to unmask a domain of interaction with Drosophila Inhibitor of apoptosis-2 (IAP-2) (Paquette et al., 2010). IAP-2 ubiquitinates and stabilizes Imd, which then acts as a scaffold for the recruitment of downstream components. Once recruited, TAK1 triggers activation of the IκB-Kinase (IKK) complex, which in turn phosphorylates the NF-κB protein Relish (Ertürk-Hasdemir et al., 2009). DREDD likely cleaves Relish, allowing the N-terminal domain to translocate into the nucleus and induce gene expression (Ertürk-Hasdemir et al., 2009; Stöven et al., 2003).
Although IMD signaling has been extensively characterized in the context of bacterial infection, much less is understood about its potential involvement with viral infection. Recent studies suggest that the IMD pathway helps to control SINV and CrPV infection at the organismal level (Avadhanula et al., 2009b; Costa et al., 2009b). It is important to note that the mechanism by which IMD signaling is activated and how it restricts replication is still unclear (Avadhanula et al., 2009b; Costa et al., 2009b). While the antimicrobial peptides downstream of the pathway are only modestly induced during infection, a recent study suggests that antimicrobial peptides, such as DiptB, confers some resistance to SINV infection of flies (Huang et al., 2013).
JAK/STAT pathway
JAK/STAT signaling is a canonical mammalian antiviral signaling pathway and is an important component of the interferon response (Sen, 2001). The JAK/STAT pathway in flies was originally identified through its role in embryonic segmentation (Binari and Perrimon, 1994). The four main components of this pathway are the ligands, unpaired (Upd1–3), the receptor domeless (Dome), the kinase JAK (Hopscotch/Hop), and the transcription factor STAT (STAT92E/Marelle) (Figure 1). Upd proteins are transcriptionally induced and subsequently bind the receptor (Chen et al., 2002), which encodes a transmembrane protein that bears limited homology to the orthologous cytokine receptor, leukemia inhibitory factor receptor (Brown et al., 2001; Chen et al., 2002). Dome signals through Hop, which is a 120-kDa protein that is most similar to human JAK2, with 27% identity overall and higher levels of homology in the kinase and kinase-like domains (Binari and Perrimon, 1994). No other JAK proteins are present in the fly genome (Zeidler et al., 2000). Hop activation lead to phosphorylation of the only STAT protein encoded in the genome, STAT92E, an 83-kDa protein that is most similar to human STAT5, with 37% overall identity. STAT92E contains an SH2 domain, a DNA-binding domain, and the single C-terminal tyrosine residue found in all STAT-like genes and is phosphorylated (Hou et al., 1996; Yan et al., 1996). In addition to these four components, at least three types of proteins that attenuate JAK/STAT signal transduction in mammals have orthologs in flies, which include SOCS proteins (Socs16D, Socs36E, Socs44F), one PIAS ortholog (Su(var)2–10) and the phophatase PTP61F (Baeg et al., 2005; Souza-Neto et al., 2009; Waterhouse et al., 2007). Interestingly, it was recently shown that two different primary transcripts originate from the STAT92E locus and that there is alternative splicing in which some of the isoforms encode a truncated form of STAT92E that lack the N-terminal 133 amino acids and behaving as a dominant negative regulator of JAK/STAT signaling (Henriksen et al., 2002).
While interferon activity has not been demonstrated in insects, the JAK/STAT signaling pathway has been found to have antiviral activity in insects, including Drosophila and mosquitoes (Dostert et al., 2005; Paradkar et al., 2012; Souza-Neto et al., 2009). DCV and FHV in flies, and Dengue in Aedes, induce a transcriptional program that is in part dependent on JAK/STAT signaling (Avadhanula et al., 2009b). A subset of these genes has antiviral properties, and further studies will define the full spectrum of antiviral factors downstream of this pathway (Souza-Neto et al., 2009). In Drosophila, the JAK/STAT pathway is induced in bystander cells (Dostert et al., 2005), not the infected cells. How viruses are sensed to activate this pathway is unknown. Furthermore, the kinetics of gene induction by JAK/STAT signaling are slow (Dostert et al., 2005) perhaps suggesting a role in tolerance. While this pathway is broadly antiviral in mammals, recent studies found that while flies mutant for JAK are more susceptible to DCV and CrPV, they exhibit either no or a weak phenotype for other viruses including SINV, VSV, DXV and IIV-6 (Dostert et al., 2005; Kemp et al., 2013). This suggests that the JAK/STAT pathway-dependent inducible response is virus specific.
Transcriptional Pausing and Rapidly Activated Antiviral Responses
While the Toll, IMD, and JAK/STAT pathways clearly play a role in antiviral resistance, additional pathways also direct antiviral gene expression programs. Transcriptional profiling studies performed during infection with VSV, FHV, DXV, DCV, SINV, and Sigma viruses has begun to reveal the spectrum of host responses to viral infection (Castorena et al., 2007; Dostert et al., 2005; Mudiganti et al., 2010b; Roxström-Lindquist et al., 2004; Tsai et al., 2008a; Xu et al., 2012; Zambon et al., 2005) (Kemp et al., 2013). Studies of DCV, FHV, SINV, DXV, and Sigma virus-induced host responses were performed in adult flies (Dostert et al., 2005; Roxström-Lindquist et al., 2004; Tsai et al., 2008a; Zambon et al., 2005) (Kemp et al., 2013), while responses to VSV, FHV and SINV infections have been profiled in Drosophila cells (Castorena et al., 2007; Mudiganti et al., 2010b; Xu et al., 2012). This has uncovered large numbers of virus-induced genes that are largely non-overlapping with each other, or with previous profiling of bacterial and fungal infections (Tsai et al., 2008a). Furthermore, the majority of the genes induced by viral infection do not have kB or STAT binding sites (Carpenter et al., 2009a; Mudiganti et al., 2010b; Tsai et al., 2008a; Xu et al., 2012). Taken together, this suggests that there are additional pathways and PRRs, which sense viruses to direct specific transcriptional programs largely independent of NFκB and STAT.
Many of these transcriptional profiling studies were performed at relatively late time points (days post infection) compared to studies in mammalian systems that typically assess gene induction within a few hours. To determine whether rapidly-inducible host responses occur in insects, we recently performed expression profiling four hours post infection and observed robust gene induction by VSV in Drosophila cells (Xu et al., 2012). We found that this gene set included components of all known antiviral pathways (RNA silencing, autophagy, JAK/STAT, Toll, and IMD) and various Toll receptors (Xu et al., 2012). We found that many of these genes are induced by other viruses, including DCV and SINV, suggesting that this early response may be broadly antiviral, analogous to the mammalian interferon response. Mechanistically, we found that a large portion of this rapidly-inducible response is regulated by the transcriptional pausing pathway, including negative elongation factor (NELF) that pauses RNA polymerase II (Pol II) and positive elongation factor b (P-TEFb), which releases paused Pol II to produce full-length transcripts. Furthermore, these genes exhibit pausing-associated chromatin features (Xu et al., 2012). Importantly, transcriptional pausing is critical for antiviral immunity in insects, as NELF and P-TEFb are required to restrict viral replication in adult flies and vector mosquito cells (Figure 1) (Xu et al., 2012). Thus, transcriptional pausing primes virally induced genes to facilitate rapid gene induction and robust antiviral responses early during infection. Further studies are needed clarify the sensors involved and the spectrum of genes that are induced that have antiviral activity.
Autophagy
Autophagy is a highly conserved degradative mechanism by which cells break down cytoplasmic material through the lysosomal degradation pathway (Lum et al., 2005). Evolutionarily conserved in eukaryotic organisms ranging from yeast to flies to humans, autophagy is thought to have evolved as an adaptive response to cellular stress, such as nutrient deprivation, and it has rapidly emerged as a critical component of immunity and host defense (Levine et al., 2011). Studies have implicated autophagy in restricting the replication of diverse pathogens, and studies in Drosophila have revealed a role in immune defense against bacteria and viruses (Figure 1) (Nakamoto et al., 2012; Shelly et al., 2009; Yano et al., 2008). Upon infection of either cells or flies, we found that autophagy is activated via the attenuation of the PI3K/Akt pathway that normally controls autophagy in response to nutrient signaling to restrict infection (Shelly et al., 2009). The viral glycoprotein VSV G is the PAMP that binds to the PRR Toll-7 at the plasma membrane to induce the antiviral autophagy program (Shelly et al., 2009). Moreover, Toll-7 and autophagy genes restrict VSV replication in both cells and adult flies, and Toll-7 and autophagy mutant flies carry significantly increased viral replication and present with high mortality post-infection. Since Toll-7 is essential for inducing autophagy in response to VSV, this links virus recognition by a PRR to an antiviral autophagy program.
It remains unclear whether other viruses are restricted by this program. A role for autophagy against Sigma virus, a second rhabdovirus, is suggested by the polymorphism in ref(2)P, a well-known autophagy adapter in other systems (Contamine, 1981; Fleuriet, 1980, 1982; Nezis et al., 2008). However, direct evidence for autophagy as an antiviral pathway against Sigma virus is lacking. The inhibition of nutrient signaling by infection would not only impact autophagy but would also affect many other downstream pathways that may play roles in immunity. Attenuation of Akt signaling allows for the reallocation of resources from growth to immune defense against bacterial and fungal pathogens (DiAngelo et al., 2009; Dionne et al., 2006; Shelly et al., 2009). Activation of the Toll pathway via infection with these microbes led to the repression of Akt signaling and the inhibition of fat storage (DiAngelo et al., 2009). Whether or not the reallocation from nutrient storage to innate immune defense is also essential to combat viral infection has yet to be elucidated.
RNA recognition and degradation
In insects, RNA interference (RNAi) consists of three small RNA-dependent silencing pathways that regulate gene expression in a sequence-specific manner (Sabin et al., 2010). RNA silencing not only regulates endogenous gene expression, but also regulates exogenous sources of RNA, including viral RNAs (Figure 1) (Mueller et al., 2010). In Drosophila, virus infection leads to the generation of virus-derived siRNAs against large panel of viruses, including DNA viruses (Bronkhorst et al., 2012; Mueller et al., 2010; Sabin et al., 2013). Mutants in the core siRNA machinery display increased sensitivity to infection by all RNA viruses tested (DCV, CrPV, FHV, DXV, SINV, VSV,WNV, RVFV), except Nora virus (Chotkowski et al., 2008b; Mueller et al., 2010; Nayak et al., 2010; Sabin et al., 2009; van Mierlo et al., 2012; van Rij et al., 2006; Zambon et al., 2006) (unpublished results). Recent studies have shown that the DNA virus IIV-6 is both sensed by the system and restricted by RNA silencing (Bronkhorst et al., 2012; Kemp et al., 2013). Importantly, pathogenic RNA viruses of insects frequently encode suppressors of RNA silencing, further demonstrating that evasion of this response is essential to establish infection (Li et al., 2002; Nayak et al., 2010; Sabin et al., 2010; van Rij et al., 2006).
While the production of virus-derived siRNAs by Dcr-2 clearly plays an antiviral role through the RNA silencing pathway, recent evidence also suggests that Dcr-2 may also trigger a downstream antiviral signaling cascade upon binding and recognition of viral dsRNA (Deddouche et al., 2008). Dcr-2 belongs to the DExD/H-box helicase family and is the closest insect ortholog to the mammalian RIG-I-like receptors, which sense and respond to cytoplasmic viral RNA. Engagement of viral RNA by Dcr-2 not only leads to antiviral silencing, but also initiates a specific transcriptional response, including the induction of Vago, an antiviral effector both in Drosophila and mosquitoes (Deddouche et al., 2008; Paradkar et al., 2012). Although the full spectrum of genes induced by Dcr-2 is unknown, these data suggest that this insect cytoplasmic sensor can generate complex responses to viral replication in a manner perhaps more analogous to mammalian innate responses than previously imagined. Another potential sensor, the RNA methyltransferase Dnmt2, was recently shown to bind DCV RNA and control infection (Durdevic et al., 2013). This may be through the methylation of viral RNA. ADARs are another class of RNA editing enzymes that have been implicated in the hypermutation of viruses, and they have recently found to induce rare hypermutation of Sigma virus (Carpenter et al., 2009b).
Wolbachia
Many symbiotic bacteria confer fitness benefits to the organisms that they infect. Wolbachia are alpha-protobacterial symbionts that infect a wide variety of invertebrates, including more than 65% of arthropods (Hilgenboecker et al., 2008; Jeyaprakash and Hoy, 2000). These obligate intracellular bacteria are vertically transmitted (Glaser and Meola, 2010; Hedges et al., 2008). In insects, Wolbachia are mainly known for disrupting the reproductive biology of the host in order to increase its transmission through the female germline (Hertig and Wolbach, 1924; Werren et al., 2008). In Drosophila, although a strong and consistent effect of Wolbachia infection on cytoplasmic incompatibility has not been found, infection of Drosophila is widespread (Glaser and Meola, 2010). Recent data demonstrate that some Wolbachia species increase resistance of Drosophila to insect RNA virus infection as measured by increased survival, including DCV, FHV, CrPV and Nora virus (Teixeira et al., 2008). Furthermore, the arbovirus WNV was also highly restricted in Wolbachia-infected flies, while infection by La Crosse virus and Chikungunya virus were only modestly affected (Glaser and Meola, 2010). The strength of Wolbachia-induced suppression of viral replication varies widely and does not always correlate with increased survival. For instance, Wolbachia increases resistance of Drosophila to Nora virus and FHV, although the titers of Nora virus were only modestly affected and FHV were unaffected (Teixeira et al., 2008). Therefore, the mechanism(s) by which this bacterial infection protects flies from virus-induced mortality is largely unknown. While the density of bacteria correlates with protection, the Toll, IMD and RNA silencing pathways are dispensable for Wolbachia-mediated suppression of viral infection (Hedges et al., 2012). Nevertheless, the induced resistance to viral pathogens may explain the fitness advantage and prevalence of Wolbachia in natural populations. Studies have shown that Wolbachia induces resistance to viruses in other hosts, including vector mosquitoes, making this a promising new therapeutic approach to eradicate viruses in important vector insects (Hoffmann et al., 2011; Rasgon, 2011; Walker et al., 2011).
7. Summary Conclusions and Future Studies
All organisms, including insects, are confronted by viral pathogens. In order to successfully replicate, viruses encode a myriad of evasion strategies, many of which are beginning to be elucidated. Insects, including commercially important species, combat viral infections using a seemingly simple immune system. A clearer understanding of the factors and pathways involved in antiviral defense will not only aid in our understanding of fundamental aspects of immune defense in insects but may also expand our understanding of our own immunity, since many pathways are conserved. Furthermore, a wide variety of viral pathogens can be transmitted to humans and livestock via the bite of a blood-feeding insect. These arthropod-borne pathogens must successfully replicate in both vertebrate and invertebrate systems, evolving complex evasion strategies that are active in both hosts. Indeed, insects control these viruses effectively with little pathogenesis, while infection of many vertebrates presents with severe pathology. This is not only surprising, but also poorly understood. Since arboviruses are (re)emerging with few specific therapeutics available, identification of pathways that can help boost insect defenses may provide novel control strategies for vectors and agricultural pests. This is exemplified by the recent discovery that mosquitoes harboring Wolbachia are resistant to Dengue infection, and that Wolbachia can be introduced into wild mosquito populations with the goal of generating protective immunity to Dengue virus in the vector insect (Hoffmann et al., 2011; Walker et al., 2011). Future studies are will provide additional insights that can potentially be harnessed for the control of viral pathogens.
Highlights.
In vivo and in vitro tools to explore virus-host interactions in Drosophila are discussed.
The viral pathogens, including insect-restricted and vertebrate arboviruses, studied in Drosophila are described.
The major antiviral pathways are reviewed with an emphasis on non-RNAi-mediated responses
Acknowledgements
We would like to thank Leah Sabin and members of the Cherry lab for helpful discussions. We apologize to the many individuals who we were unable to cite. This work was supported by grants from the National Institutes of Health (R01AI074951, U54AI057168 and R01AI095500) to SC and a HHMI International Scholars award to J.X. S.C. is a recipient of the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (CDC), C.f.D.C.a.P. West nile virus disease and other arboviral diseases - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:510–514. [PubMed] [Google Scholar]
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Ahrens CH, Brunner E, Hafen E, Aebersold R, Basler K. A proteome catalog of Drosophila melanogaster: an essential resource for targeted quantitative proteomics. Fly (Austin) 2007;1:182–186. doi: 10.4161/fly.4532. [DOI] [PubMed] [Google Scholar]
- Ammar e-D, Tsai CW, Whitfield AE, Redinbaugh MG, Hogenhout SA. Cellular and molecular aspects of rhabdovirus interactions with insect and plant hosts. Annu Rev Entomol. 2009;54:447–468. doi: 10.1146/annurev.ento.54.110807.090454. [DOI] [PubMed] [Google Scholar]
- Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009a;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009b;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery OT, MacLeod CM, McCarty M 9.9624px, T.H.M.d.d.-c.-w.d.-f.-n.g.f.p.d.l.s.f.-s., serif,f.-f.,90.2534px, l., 366.475px,t., transform: scale(1.10723, 0px, t.-o., and ">. Biograph memoirs 1866–1945. Nat Acad Sci. 1959;33:283–325. [Google Scholar]
- Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 2005;19:1861–1870. doi: 10.1101/gad.1320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LA, White CN. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976;73:442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger MJ, Bruenn JA, Taylor DJ. Phylogeny, integration and expression of sigma virus-like genes in Drosophila. Mol Phylogenet Evol. 2012;65:251–258. doi: 10.1016/j.ympev.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Bangham J, Kim KW, Webster CL, Jiggins FM. Genetic variation affecting host-parasite interactions: different genes affect different aspects of sigma virus replication and transmission in Drosophila melanogaster. Genetics. 2008;178:2191–2199. doi: 10.1534/genetics.107.085449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B, Cherbas L. Drosophila cell lines as model systems and as an experimental tool. Methods Mol Biol. 2008;420:391–424. doi: 10.1007/978-1-59745-583-1_25. [DOI] [PubMed] [Google Scholar]
- Beaty BaMW. The Biology of Disease Vectors. 1996;Vol 1 (University Press of Colorado. P.O. Box 849 Niwot, Colorado 80544: University Press of Colorado.). [Google Scholar]
- Beckingham KM, Armstrong JD, Texada MJ, Munjaal R, Baker DA. Drosophila melanogaster--the model organism of choice for the complex biology of multi-cellular organisms. Gravit Space Biol Bull. 2005;18:17–29. [PubMed] [Google Scholar]
- Behura SK, Haugen M, Flannery E, Sarro J, Tessier CR, Severson DW, Duman-Scheel M. Comparative genomic analysis of Drosophila melanogaster and vector mosquito developmental genes. PLoS One. 2011;6:e21504. doi: 10.1371/journal.pone.0021504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstralh DT, St Johnston D. Epithelial cell polarity: what flies can teach us about cancer. Essays Biochem. 2012;53:129–140. doi: 10.1042/bse0530129. [DOI] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994;8:300–312. doi: 10.1101/gad.8.3.300. [DOI] [PubMed] [Google Scholar]
- Bishop DH, Beaty BJ. Molecular and biochemical studies of the evolution, infection and transmission of insect bunyaviruses. Philos Trans R Soc Lond B Biol Sci. 1988;321:463–483. doi: 10.1098/rstb.1988.0103. [DOI] [PubMed] [Google Scholar]
- Bishop DH, Gay ME, Matsuoko Y. Nonviral heterogeneous sequences are present at the 5' ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983;11:6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonami JR, Adams JR. Atlas of invertebrate viruses. Boca Raton: CRC Press; 1991. [Google Scholar]
- Bonami JR, Hasson KW, Mari J, Poulos BT, Lightner DV. Taura syndrome of marine penaeid shrimp: characterization of the viral agent. J Gen Virol. 1997;78(Pt 2):313–319. doi: 10.1099/0022-1317-78-2-313. [DOI] [PubMed] [Google Scholar]
- Bonning BC, Miller WA. Dicistroviruses. Annual Review of Entomology. 2010;55:129–150. doi: 10.1146/annurev-ento-112408-085457. [DOI] [PubMed] [Google Scholar]
- Boshra H, Lorenzo G, Busquets N, Brun A. Rift valley fever: recent insights into pathogenesis and prevention. J Virol. 2011;85:6098–6105. doi: 10.1128/JVI.02641-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M. Bunyaviridae: genome organization and replication strategies. Adv Virus Res. 1991;40:235–275. doi: 10.1016/s0065-3527(08)60281-x. [DOI] [PubMed] [Google Scholar]
- Bouloy M, Hannoun C. Studies on lumbo virus replication. I. RNA-dependent RNA polymerase associated with virions. Virology. 1976;69:258–264. doi: 10.1016/0042-6822(76)90212-9. [DOI] [PubMed] [Google Scholar]
- Bouloy M, Pardigon N, Vialat P, Gerbaud S, Girard M. Characterization of the 5' and 3' ends of viral messenger RNAs isolated from BHK21 cells infected with Germiston virus (Bunyavirus) Virology. 1990;175:50–58. doi: 10.1016/0042-6822(90)90185-t. [DOI] [PubMed] [Google Scholar]
- Bras-Herreng F. Changes in the properties of Sindbis virus during passage experiments in "Drosophila" (author's transl) Ann Microbiol (Paris) 1973;124A:507–533. [PubMed] [Google Scholar]
- Bras-Herreng F. Multiplication of sindbis virus in Drosophila cells cultivated in vitro (author's transl) Arch Virol. 1975;48:121–129. doi: 10.1007/BF01318145. [DOI] [PubMed] [Google Scholar]
- Bras-Herreng F. Adaptation of a Sindbis virus population to "Drosophila melanogaster" (author's transl) Ann Microbiol (Paris) 1976;127B:541–565. [PubMed] [Google Scholar]
- Bronkhorst AW, van Cleef KW, Vodovar N, Ince IA, Blanc H, Vlak JM, Saleh MC, van Rij RP. The DNA virus Invertebrate iridescent virus 6 is a target of the Drosophila RNAi machinery. Proc Natl Acad Sci U S A. 2012;109:E3604–E3613. doi: 10.1073/pnas.1207213109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes M. Fly the unsung hero of 20th century Science. Harper Collins Publishers; 2001. [Google Scholar]
- Brown S, Hu N, Hombrίa JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–1705. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- Brun G. A genetic study of the interaction of Piry virus with drosophila. Res Virol. 1991;142:313–331. doi: 10.1016/0923-2516(91)90018-x. [DOI] [PubMed] [Google Scholar]
- Brun G, Plus N. In The Genetics and Biology of Drosophila. New York: Academic Press; 1980. The viruses of Drosophila; pp. 625–702. [Google Scholar]
- Brunner E, Ahrens CH, Mohanty S, Baetschmann H, Loevenich S, Potthast F, Deutsch EW, Panse C, de Lichtenberg U, Rinner O, et al. A high-quality catalog of the Drosophila melanogaster proteome. Nat Biotechnol. 2007;25:576–583. doi: 10.1038/nbt1300. [DOI] [PubMed] [Google Scholar]
- Bushell M, Sarnow P. Hijacking the translation apparatus by RNA viruses. J Cell Biol. 2002;158:395–399. doi: 10.1083/jcb.200205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD, Malani N, Fernandes J, D'Orso I, Cagney G, Diamond TL, Zhou H, Hazuda DJ, Espeseth AS, König R, et al. Host cell factors in HIV replication: meta-analysis of genome-wide studies. PLoS Pathog. 2009;5:e1000437. doi: 10.1371/journal.ppat.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscereau F. The CO2 sensitivity induced by two rhabdoviruses, Piry and Chandipura, in Drosophila melanogaster. Ann Microbiol (Paris) 1975;126:389–403. [PubMed] [Google Scholar]
- Busserea F, Contamine D. Infectivity of two rhabdoviruses in Drosophila melanogaster: Rabies and isfahan viruses. Ann Virol. 1980;131 [Google Scholar]
- Bussereau F. Study of CO2 sensitivity symptom induced by sigma virus in Drosophila. I Influence of inoculation place on delay of symptom appearance. Ann Inst Pasteur (Paris) 1970a;118:367–385. [PubMed] [Google Scholar]
- Bussereau F. Study of the symptom of CO2 sensitivity induced by sigma virus in Drosophila II Comparative course of yield of nervous centers and of some organs after inoculation in the abdomen and thorax. Ann Inst Pasteur (Paris) 1970b;118:626–645. [PubMed] [Google Scholar]
- Campbell CL, Keene KM, Brackney DE, Olson KE, Blair CD, Wilusz J, Foy BD. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 2008;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplen H, Peters CJ, Bishop DH. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66(Pt 10):2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- Carmena A. Approaching Drosophila development through proteomic tools and databases: At the hub of the post-genomic era. Mech Dev. 2009;126:761–770. doi: 10.1016/j.mod.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Carpenter J, Hutter S, Baines JF, Roller J, Saminadin-Peter SS, Parsch J, Jiggins FM. The transcriptional response of Drosophila melanogaster to infection with the sigma virus (Rhabdoviridae) PLoS One. 2009a;4:e6838. doi: 10.1371/journal.pone.0006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JA, Keegan LP, Wilfert L, O'Connell MA, Jiggins FM. Evidence for ADAR-induced hypermutation of the Drosophila sigma virus (Rhabdoviridae) BMC Genet. 2009b;10:75. doi: 10.1186/1471-2156-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JA, Obbard DJ, Maside X, Jiggins FM. The recent spread of a vertically transmitted virus through populations of Drosophila melanogaster. Mol Ecol. 2007;16:3947–3954. doi: 10.1111/j.1365-294X.2007.03460.x. [DOI] [PubMed] [Google Scholar]
- Carré-Mlouka A, Gaumer S, Gay P, Petitjean AM, Coulondre C, Dru P, Bras F, Dezélée S, Contamine D. Control of sigma virus multiplication by the ref(2)P gene of Drosophila melanogaster: an in vivo study of the PB1 domain of Ref(2)P. Genetics. 2007;176:409–419. doi: 10.1534/genetics.106.063826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorena KM, Stapleford KA, Miller DJ. Complementary transcriptomic, lipidomic, and targeted functional genetic analyses in cultured Drosophila cells highlight the role of glycerophospholipid metabolism in Flock House virus RNA replication. BMC Genomics. 2010;11:183. doi: 10.1186/1471-2164-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorena KM, Weeks SA, Stapleford KA, Cadwallader AM, Miller DJ. A functional heat shock protein 90 chaperone is essential for efficient flock house virus RNA polymerase synthesis in Drosophila cells. J Virol. 2007;81:8412–8420. doi: 10.1128/JVI.00189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevallos RC, Sarnow P. Factor-independent assembly of elongation-competent ribosomes by an internal ribosome entry site located in an RNA virus that infects penaeid shrimp. J Virol. 2005;79:677–683. doi: 10.1128/JVI.79.2.677-683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HW, Chen X, Oh SW, Marinissen MJ, Gutkind JS, Hou SX. mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 2002;16:388–398. doi: 10.1101/gad.955202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S. Genomic RNAi screening in Drosophila S2 cells: what have we learned about host-pathogen interactions? Curr Opin Microbiol. 2008;11:262–270. doi: 10.1016/j.mib.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S, Doukas T, Armknecht S, Whelan S, Wang H, Sarnow P, Perrimon N. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 2005;19:445–452. doi: 10.1101/gad.1267905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S, Kunte A, Wang H, Coyne C, Rawson RB, Perrimon N. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog. 2006;2:e102. doi: 10.1371/journal.ppat.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S, Perrimon N. Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat Immunol. 2004;5:81–87. doi: 10.1038/ni1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S, Silverman N. Host-pathogen interactions in drosophila: new tricks from an old friend. Nat Immunol. 2006;7:911–917. doi: 10.1038/ni1388. [DOI] [PubMed] [Google Scholar]
- Chien S, Reiter LT, Bier E, Gribskov M. Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res. 2002;30:149–151. doi: 10.1093/nar/30.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Lee H, Anderson KV. Drosophila peptidoglycan recognition protein LC (PGRP-LC) acts as a signal-transducing innate immune receptor. Proc Natl Acad Sci U S A. 2005;102:1122–1126. doi: 10.1073/pnas.0404952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotkowski HL, Ciota AT, Jia Y, Puig-Basagoiti F, Kramer LD, Shi PY, Glaser RL. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology. 2008a;377:197–206. doi: 10.1016/j.virol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotkowski HL, Ciota AT, Jia Y, Puig-Basagoiti F, Kramer LD, Shi PY, Glaser RL. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology. 2008b;377:197–206. doi: 10.1016/j.virol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian PD, Scotti P. A suggested taxonomy and nomenclature for the cricket paralysis and Drosophila C virus complex. J Invertebr Pathol. 1994;63:157–162. doi: 10.1006/jipa.1994.1030. [DOI] [PubMed] [Google Scholar]
- Christian PDaS, P D. Dicistroviridae. London: Elsevier; 2008. Encyclopedia of Virology. [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, et al. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Collins PL, Fuller FJ, Marcus PI, Hightower LE, Ball LA. Synthesis and processing of Sindbis virus nonstructural proteins in vitro. Virology. 1982;118:363–379. doi: 10.1016/0042-6822(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Constantino M, Christian P, Marina CF, Williams T. A comparison of techniques for detecting Invertebrate iridescent virus 6. J Virol Methods. 2001;98:109–118. doi: 10.1016/s0166-0934(01)00356-1. [DOI] [PubMed] [Google Scholar]
- Contamine D. Role of the Drosophila genome in Sigma virus multiplication. I. Role of the ret(2)P gene; selection of host-adapted mutants at the nonpermissive allele Pp. Virology. 1981;114:474–488. doi: 10.1016/0042-6822(81)90227-0. [DOI] [PubMed] [Google Scholar]
- Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One. 2009a;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One. 2009b;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker B, Crozat K, Berger M, Xia Y, Sovath S, Schaffer L, Eleftherianos I, Imler JL, Beutler B. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat Genet. 2007;39:1453–1460. doi: 10.1038/ng.2007.25. [DOI] [PubMed] [Google Scholar]
- Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SPJ. Vesicular Stomatitis Virus Enters Cells through Vesicles Incompletely Coated with Clathrin That Depend upon Actin for Internalization. Plos Pathogens. 2009;5 doi: 10.1371/journal.ppat.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R, Free HM, Zietlow SL, Paskewitz SM, Aksoy S, Shi L, Fuchs J, Hu C, Christensen BM. Replication of flock house virus in three genera of medically important insects. J Med Entomol. 2007;44:102–110. doi: 10.1603/0022-2585(2007)44[102:rofhvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dasgupta R, Selling B, Rueckert R. Flock house virus: a simple model for studying persistent infection in cultured Drosophila cells. Arch Virol Suppl. 1994;9:121–132. doi: 10.1007/978-3-7091-9326-6_13. [DOI] [PubMed] [Google Scholar]
- Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis of Rift Valley Fever, an undescriptible virus disease of sheep, cattle and man from East Africa. Journal of Pathology and Bacteriology. 1931;34:543–579. [Google Scholar]
- Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, Imler JL. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- Delmas B, Mundt E, Vakharia VN, Wu JL. Birnaviridae Virus Taxonomy: Classification and Nomenclature of Viruses. In: King AMQ, Carstens EB, Lefkowitz EJ, editors. A.M.J. San Diego: Elsevier Academic Press; 2011. pp. 499–507. [Google Scholar]
- Denizot M, Neal JW, Gasque P. Encephalitis due to emerging viruses: CNS innate immunity and potential therapeutic targets. J Infect. 2012;65:1–16. doi: 10.1016/j.jinf.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Dezélée S, Blondel D, Wyers F, Petitjean AM. Vesicular stomatitis virus in Drosophila melanogaster cells: lack of leader RNA transport into the nuclei and frequent abortion of the replication step. J Virol. 1987;61:1391–1397. doi: 10.1128/jvi.61.5.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A. 2009;106:20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol. 2006;16:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Dobos P. The molecular biology of infectious pancreatic necrosis virus (IPNV) Annu Rev Fish Dis. 1995:25–54. [Google Scholar]
- Dobos P, Hill BJ, Hallett R, Kells DT, Becht H, Teninges D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979;32:593–605. doi: 10.1128/jvi.32.2.593-605.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nature Immunology. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Dru P, Bras F, Dezélée S, Gay P, Petitjean AM, Pierre-Deneubourg A, Teninges D, Contamine D. Unusual variability of the Drosophila melanogaster ref(2)P protein which controls the multiplication of sigma rhabdovirus. Genetics. 1993;133:943–954. doi: 10.1093/genetics/133.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdevic Z, Hanna K, Gold B, Pollex T, Cherry S, Lyko F, Schaefer M. Efficient RNA virus control in Drosophila requires the RNA methyltransferase Dnmt2. EMBO Rep. 2013;14:269–275. doi: 10.1038/embor.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton HE, Metcalf J, Penny E, Tcherepanov V, Upton C, Brunetti CR. Comparative genomic analysis of the family Iridoviridae: re-annotating and defining the core set of iridovirus genes. Virol J. 2007;4:11. doi: 10.1186/1743-422X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström JO, Habayeb MS, Srivastava V, Kieselbach T, Wingsle G, Hultmark D. Drosophila Nora virus capsid proteins differ from those of other picorna-like viruses. Virus Res. 2011;160:51–58. doi: 10.1016/j.virusres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- El Chamy L, Leclerc V, Caldelari I, Reichhart JM. Sensing of 'danger signals' and pathogen-associated molecular patterns defines binary signaling pathways 'upstream' of Toll. Nat Immunol. 2008;9:1165–1170. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherianos I, Schneider D. Drosophila immunity research on the move. Fly (Austin) 2011;5:247–254. doi: 10.4161/fly.5.3.17028. [DOI] [PubMed] [Google Scholar]
- Eleftherianos I, Won S, Chtarbanova S, Squiban B, Ocorr K, Bodmer R, Beutler B, Hoffmann JA, Imler JL. ATP-sensitive potassium channel (K(ATP))-dependent regulation of cardiotropic viral infections. Proc Natl Acad Sci U S A. 2011;108:12024–12029. doi: 10.1073/pnas.1108926108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y, Kadalayil L, Sun SC, Samakovlis C, Hultmark D, Faye I. kappa B-like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993;232:327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- Ertürk-Hasdemir D, Broemer M, Leulier F, Lane WS, Paquette N, Hwang D, Kim CH, Stöven S, Meier P, Silverman N. Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proc Natl Acad Sci U S A. 2009;106:9779–9784. doi: 10.1073/pnas.0812022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Spivak M. Socialized medicine: individual and communal disease barriers in honey bees. J Invertebr Pathol. 2010;103(Suppl 1):S62–S72. doi: 10.1016/j.jip.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nature Reviews Immunology. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Ferreira GL. Global dengue epidemiology trends. Rev Inst Med Trop Sao Paulo. 2012;54(Suppl 18):S5–S6. doi: 10.1590/s0036-46652012000700003. [DOI] [PubMed] [Google Scholar]
- Filone CM, Hanna SL, Caino MC, Bambina S, Doms RW, Cherry S. Rift valley fever virus infection of human cells and insect hosts is promoted by protein kinase C epsilon. PLoS One. 2010;5:e15483. doi: 10.1371/journal.pone.0015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine PE. Vectors and vertical transmission: an epidemiologic perspective. Ann N Y Acad Sci. 1975;266:173–194. doi: 10.1111/j.1749-6632.1975.tb35099.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Chen ZJ. Sorting out Toll signals. Cell. 2006;125:834–836. doi: 10.1016/j.cell.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Flegel TW. Hypothesis for heritable, anti-viral immunity in crustaceans and insects. Biol Direct. 2009;4:32. doi: 10.1186/1745-6150-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuriet A. Polymorphism of the Hereditary Sigma Virus in Natural Populations of DROSOPHILA MELANOGASTER. Genetics. 1980;95:459–465. doi: 10.1093/genetics/95.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuriet A. Comparison of various physiological traits in flies (Drosophila melanogaster) of wild origin, infected or uninfected by the hereditary Rhabdovirus sigma. Arch Virol. 1981;69:261–272. doi: 10.1007/BF01317341. [DOI] [PubMed] [Google Scholar]
- Fleuriet A. Factors affecting the frequency of infection by the sigma virus in experimental populations of Drosophila melanogaster. Arch Virol. 1982;73:121–133. doi: 10.1007/BF01314721. [DOI] [PubMed] [Google Scholar]
- Fleuriet A. Maintenance of a hereditary virus – the sigma virus in populations of its host, Drosophila melanogaster. Evol. Biol. 1988;23:1–30. [Google Scholar]
- Flick R, Bouloy M. Rift Valley fever virus. Curr Mol Med. 2005;5:827–834. doi: 10.2174/156652405774962263. [DOI] [PubMed] [Google Scholar]
- Friedman AA, Tucker G, Singh R, Yan D, Vinayagam A, Hu Y, Binari R, Hong P, Sun X, Porto M, et al. Proteomic and functional genomic landscape of receptor tyrosine kinase and ras to extracellular signal-regulated kinase signaling. Sci Signal. 2011;4:rs10. doi: 10.1126/scisignal.2002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZF. Genetic comparison of the rhabdoviruses from animals and plants. Curr Top Microbiol Immunol. 2005;292:1–24. doi: 10.1007/3-540-27485-5_1. [DOI] [PubMed] [Google Scholar]
- Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol. 2006a;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol. 2006b;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrey JL, Lee YY, Au HH, Bushell M, Jan E. Host and viral translational mechanisms during cricket paralysis virus infection. J Virol. 2010;84:1124–1138. doi: 10.1128/JVI.02006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P. [Drosophila genes which intervene in multiplication of sigma virus (author's transl)] Mol Gen Genet. 1978;159:269–283. doi: 10.1007/BF00268263. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go EP, Wikoff WR, Shen Z, O'Maille G, Morita H, Conrads TP, Nordstrom A, Trauger SA, Uritboonthai W, Lucas DA, et al. Mass spectrometry reveals specific and global molecular transformations during viral infection. J Proteome Res. 2006;5:2405–2416. doi: 10.1021/pr060215t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze S, Qeli E, Mosimann C, Staes A, Gerrits B, Roschitzki B, Mohanty S, Niederer EM, Laczko E, Timmerman E, et al. Identification and functional characterization of N-terminally acetylated proteins in Drosophila melanogaster. PLoS Biol. 2009;7:e1000236. doi: 10.1371/journal.pbio.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomariz-Zilber E, Poras M, Thomas-Orillard M. Drosophila C virus: experimental study of infectious yields and underlying pathology in Drosophila melanogaster laboratory populations. J Invertebr Pathol. 1995;65:243–247. doi: 10.1006/jipa.1995.1037. [DOI] [PubMed] [Google Scholar]
- Gordon KH, Waterhouse PM. Small RNA viruses of insects: expression in plants and RNA silencing. Adv Virus Res. 2006;68:459–502. doi: 10.1016/S0065-3527(06)68013-5. [DOI] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw JW, Krijgsveld J, Heck AJ. Quantitative proteomics by metabolic labeling of model organisms. Mol Cell Proteomics. 2010;9:11–24. doi: 10.1074/mcp.R900001-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Grumbling G, Strelets V. FlyBase: anatomical data, images and queries. Nucleic Acids Res. 2006;34:D484–D488. doi: 10.1093/nar/gkj068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habayeb MS, Ekengren SK, Hultmark D. Nora virus, a persistent virus in Drosophila, defines a new picorna-like virus family. J Gen Virol. 2006;87:3045–3051. doi: 10.1099/vir.0.81997-0. [DOI] [PubMed] [Google Scholar]
- Hannoun C, Echalier G. 3 Arbovirus multiplication in an established diploid cell line of Drosophila melanogaster. Curr Top Microbiol Immunol. 1971;55:227–230. doi: 10.1007/978-3-642-65224-0_37. [DOI] [PubMed] [Google Scholar]
- Hao L, Sakurai A, Watanabe T, Sorensen E, Nidom CA, Newton MA, Ahlquist P, Kawaoka Y. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–893. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DM, Rinderknecht JL, Nipp TL, Clarke NP, Snowder GD Fever, N.C.f.F.A.a.Z.D.D.A.G.o.R.V. Potential effects of Rift Valley fever in the United States. Emerg Infect Dis. 2011;17:e1. doi: 10.3201/eid1708.101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- Hedges LM, Yamada R, O'Neill SL, Johnson KN. The small interfering RNA pathway is not essential for Wolbachia-mediated antiviral protection in Drosophila melanogaster. Appl Environ Microbiol. 2012;78:6773–6776. doi: 10.1128/AEM.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti E, Bishop DH, Dubin DT, Stollar V. 5' nucleotide sequence of sindbis viral RNA. J Virol. 1975;17:149–159. doi: 10.1128/jvi.17.1.149-159.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen CU. IRES-induced conformational changes in the ribosome and the mechanism of translation initiation by internal ribosomal entry. Biochim Biophys Acta. 2009;1789:558–570. doi: 10.1016/j.bbagrm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen MA, Betz A, Fuccillo MV, Darnell JE. Negative regulation of STAT92E by an N-terminally truncated STAT protein derived from an alternative promoter site. Genes Dev. 2002;16:2379–2389. doi: 10.1101/gad.1020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertig M, Wolbach SB. Studies on Rickettsia-Like Micro-Organisms in Insects. J Med Res. 1924;44:329–374. 327. [PMC free article] [PubMed] [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?--A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hogenhout SA, Redinbaugh MG, Ammar e-D. Plant and animal rhabdovirus host range: a bug's view. Trends Microbiol. 2003;11:264–271. doi: 10.1016/s0966-842x(03)00120-3. [DOI] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996;84:411–419. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kingsolver MB, Avadhanula V, Hardy RW. An antiviral role for antimicrobial peptides during the arthropod response to Alphavirus replication. J Virol. 2013 doi: 10.1128/JVI.03360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993;9:178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Huszar T, Imler JL. Drosophila viruses and the study of antiviral host-defense. Adv Virus Res. 2008;72:227–265. doi: 10.1016/S0065-3527(08)00406-5. [DOI] [PubMed] [Google Scholar]
- Iconomidis J, L'Hertier P. [The relations of the sigma virus with its drosophilic host. Study of the spontaneous loss of the stabilized state] Ann Genet. 1961;2:53–58. [PubMed] [Google Scholar]
- Ip YT, Reach M, Engstrom Y, Kadalayil L, Cai H, González-Crespo S, Tatei K, Levine M. Dif a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Jakob NJ, Kleespies RG, Tidona CA, Müller K, Gelderblom HR, Darai G. Comparative analysis of the genome and host range characteristics of two insect iridoviruses: Chilo iridescent virus and a cricket iridovirus isolate. J Gen Virol. 2002;83:463–470. doi: 10.1099/0022-1317-83-2-463. [DOI] [PubMed] [Google Scholar]
- Jakob NJ, Müller K, Bahr U, Darai G. Analysis of the first complete DNA sequence of an invertebrate iridovirus: coding strategy of the genome of Chilo iridescent virus. Virology. 2001;286:182–196. doi: 10.1006/viro.2001.0963. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Ball LA. Replication of flock house virus RNAs from primary transcripts made in cells by RNA polymerase II. J Virol. 1997;71:3323–3327. doi: 10.1128/jvi.71.4.3323-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Ball LA. Induction and maintenance of autonomous flock house virus RNA1 replication. J Virol. 1999;73:7933–7942. doi: 10.1128/jvi.73.10.7933-7942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KN, Christian PD. The novel genome organization of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J Gen Virol. 1998;79(Pt 1):191–203. doi: 10.1099/0022-1317-79-1-191. [DOI] [PubMed] [Google Scholar]
- Johnson KN, van Hulten MC, Barnes AC. "Vaccination" of shrimp against viral pathogens: phenomenology and underlying mechanisms. Vaccine. 2008;26:4885–4892. doi: 10.1016/j.vaccine.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Jousset FX, Bergoin M, Revet B. Characterization of the Drosophila C virus. J Gen Virol. 1977;34:269–283. doi: 10.1099/0022-1317-34-2-269. [DOI] [PubMed] [Google Scholar]
- Jousset FX, Plus N. [Study of the vertical transmission and horizontal transmission of"Drosophila melanogaster" and"Drosophila immigrans" picornavirus (author's transl)] Ann Microbiol (Paris) 1975;126:231–249. [PubMed] [Google Scholar]
- Jousset FX, Plus N, Croizier G, Thomas M. [Existence in Drosophila of 2 groups of picornavirus with different biological and serological properties] C R Acad Sci Hebd Seances Acad Sci D. 1972;275:3043–3046. [PubMed] [Google Scholar]
- Kadalayil L, Petersen UM, Engström Y. Adjacent GATA and kappa B-like motifs regulate the expression of a Drosophila immune gene. Nucleic Acids Res. 1997;25:1233–1239. doi: 10.1093/nar/25.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampmueller KM, Miller DJ. The cellular chaperone heat shock protein 90 facilitates Flock House virus RNA replication in Drosophila cells. J Virol. 2005;79:6827–6837. doi: 10.1128/JVI.79.11.6827-6837.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/s1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- Kapun M, Nolte V, Flatt T, Schlötterer C. Host range and specificity of the Drosophila C virus. PLoS One. 2010;5:e12421. doi: 10.1371/journal.pone.0012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kemp C, Imler JL. Antiviral immunity in drosophila. Curr Opin Immunol. 2009;21:3–9. doi: 10.1016/j.coi.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C, Mueller S, Goto A, Barbier V, Paro S, Bonnay F, Dostert C, Troxler L, Hetru C, Meignin C, et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J Immunol. 2013;190:650–658. doi: 10.4049/jimmunol.1102486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A, Jan E. Modulation of stress granules and P bodies during dicistrovirus infection. J Virol. 2011;85:1439–1451. doi: 10.1128/JVI.02220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LAM, NF Evidence for the presence of a genome-linked protein in two insect picornaviruses, cricket paralysis and Drosophila C viruses. FEMS Microbiology Letters. 1988;50:41–44. [Google Scholar]
- Kingsolver MB, Hardy RW. Making connections in insect innate immunity. Proc Natl Acad Sci U S A. 2012;109:18639–18640. doi: 10.1073/pnas.1216736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kint G, Fierro C, Marchal K, Vanderleyden J, De Keersmaecker SC. Integration of 'omics' data: does it lead to new insights into host-microbe interactions? Future Microbiol. 2010;5:313–328. doi: 10.2217/fmb.10.1. [DOI] [PubMed] [Google Scholar]
- Kohler RE. Lords of the Fly: Drosophila genetics and the experimental life. Vol 1. London: The University of Chicago Press; 1994. [Google Scholar]
- Koles K, Lim JM, Aoki K, Porterfield M, Tiemeyer M, Wells L, Panin V. Identification of N-glycosylated proteins from the central nervous system of Drosophila melanogaster. Glycobiology. 2007;17:1388–1403. doi: 10.1093/glycob/cwm097. [DOI] [PubMed] [Google Scholar]
- König R, Chiang CY, Tu BP, Yan SF, DeJesus PD, Romero A, Bergauer T, Orth A, Krueger U, Zhou Y, Chanda SK. A probability-based approach for the analysis of large-scale RNAi screens. Nat Methods. 2007;4:847–849. doi: 10.1038/nmeth1089. [DOI] [PubMed] [Google Scholar]
- Konrad SK, Miller SN. A temperature-limited assessment of the risk of Rift Valley fever transmission and establishment in the continental United States of America. Geospat Health. 2012;6:161–170. doi: 10.4081/gh.2012.134. [DOI] [PubMed] [Google Scholar]
- Kopek BG, Perkins G, Miller DJ, Ellisman MH, Ahlquist P. Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle. PLoS Biol. 2007;5:e220. doi: 10.1371/journal.pbio.0050220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalev N, Pogany J, Nagy PD. A Co-Opted DEAD-Box RNA helicase enhances tombusvirus plus-strand synthesis. PLoS Pathog. 2012;8:e1002537. doi: 10.1371/journal.ppat.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela S, Manni T, Myllynen J, Vaheri A, Vapalahti O. Clinical and laboratory manifestations of Sindbis virus infection: prospective study, Finland 2002–2003. J Infect Dis. 2005;191:1820–1829. doi: 10.1086/430007. [DOI] [PubMed] [Google Scholar]
- Kylsten P, Samakovlis C, Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990;9:217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Heritier PH. Sensitivity to CO2 in Drosophila. Heredity. 1948;2:325–348. doi: 10.1038/hdy.1948.20. [DOI] [PubMed] [Google Scholar]
- L'Heritier PH, Teissier G. Une anomalie physiologique héréditaire chez la Drosophile. (Paris, CR Acad Sci.) 1937:192–194. [Google Scholar]
- Landry DM, Hertz MI, Thompson SR. RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev. 2009;23:2753–2764. doi: 10.1101/gad.1832209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanman J, Crum J, Deerinck TJ, Gaietta GM, Schneemann A, Sosinsky GE, Ellisman MH, Johnson JE. Visualizing flock house virus infection in Drosophila cells with correlated fluorescence and electron microscopy. J Struct Biol. 2008;161:439–446. doi: 10.1016/j.jsb.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent J, Lavay F. Oligosaccharide moieties of Drosophila-adapted vesicular stomatitis virus grown in different cells. Annates de Virologic. 1983;134E:301–308. [Google Scholar]
- Lautié-Harivel N, Thomas-Orillard M. Location of Drosophila C virus target organs in Drosophila host population by an immunofluorescence technique. Biol Cell. 1990;69:35–39. doi: 10.1016/0248-4900(90)90326-x. [DOI] [PubMed] [Google Scholar]
- Le Gall O, Christian P, Fauquet CM, King AM, Knowles NJ, Nakashima N, Stanway G, Gorbalenya AE. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T = 3 virion architecture. Arch Virol. 2008;153:715–727. doi: 10.1007/s00705-008-0041-x. [DOI] [PubMed] [Google Scholar]
- Lee T. New genetic tools for cell lineage analysis in Drosophila. Nat Methods. 2009;6:566–568. doi: 10.1038/nmeth0809-566. [DOI] [PubMed] [Google Scholar]
- Léger P, Lara E, Jagla B, Sismeiro O, Mansuroglu Z, Coppée JY, Bonnefoy E, Bouloy M. Dicer-2- and Piwi-mediated RNA interference in Rift Valley fever virus-infected mosquito cells. J Virol. 2013;87:1631–1648. doi: 10.1128/JVI.02795-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Lemm JA, Rice CM. Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J Virol. 1993;67:1916–1926. doi: 10.1128/jvi.67.4.1916-1926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemm JA, Rümenapf T, Strauss EG, Strauss JH, Rice CM. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 1994;13:2925–2934. doi: 10.1002/j.1460-2075.1994.tb06587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth GJ, Rodriguez LL, Del cbarrera J. Vesicular stomatitis. Vet J. 1999;157:239–260. doi: 10.1053/tvjl.1998.0303. [DOI] [PubMed] [Google Scholar]
- Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B. The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep. 2000;1:353–358. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- Liu B, Behura SK, Clem RJ, Schneemann A, Becnel J, Severson DW, Zhou L. P53-Mediated Rapid Induction of Apoptosis Conveys Resistance to Viral Infection in Drosophila melanogaster. PLoS Pathog. 2013;9:e1003137. doi: 10.1371/journal.ppat.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longdon B, Jiggins FM. Vertically transmitted viral endosymbionts of insects: do sigma viruses walk alone? Proc Biol Sci. 2012;279:3889–3898. doi: 10.1098/rspb.2012.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longdon B, Obbard DJ, Jiggins FM. Sigma viruses from three species of Drosophila form a major new clade in the rhabdovirus phylogeny. Proc Biol Sci. 2010;277:35–44. doi: 10.1098/rspb.2009.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longdon B, Wilfert L, Osei-Poku J, Cagney H, Obbard DJ, Jiggins FM. Host-switching by a vertically transmitted rhabdovirus in Drosophila. Biol Lett. 2011;7:747–750. doi: 10.1098/rsbl.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Maduro M, Li F, Li HW, Broitman-Maduro G, Li WX, Ding SW. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature. 2005;436:1040–1043. doi: 10.1038/nature03870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nature reviews. Molecular cell biology. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Maclachlan NJ. Bluetongue: history, global epidemiology, and pathogenesis. Prev Vet Med. 2011;102:107–111. doi: 10.1016/j.prevetmed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Magwire MM, Bayer F, Webster CL, Cao C, Jiggins FM. Successive increases in the resistance of Drosophila to viral infection through a transposon insertion followed by a Duplication. PLoS Genet. 2011;7:e1002337. doi: 10.1371/journal.pgen.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia LF, Soares MR, Valente AP, Almeida FC, Oliveira AC, Gomes AM, Freitas MS, Schneemann A, Johnson JE, Silva JL. Structure of a membrane-binding domain from a non-enveloped animal virus: insights into the mechanism of membrane permeability and cellular entry. J Biol Chem. 2006;281:29278–29286. doi: 10.1074/jbc.M604689200. [DOI] [PubMed] [Google Scholar]
- Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, Lemaitre B. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J. 1999;18:3380–3391. doi: 10.1093/emboj/18.12.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maori E, Lavi S, Mozes-Koch R, Gantman Y, Peretz Y, Edelbaum O, Tanne E, Sela I. Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honeybees in Israel: evidence for diversity due to intra- and inter-species recombination. J Gen Virol. 2007;88:3428–3438. doi: 10.1099/vir.0.83284-0. [DOI] [PubMed] [Google Scholar]
- Masoumi A, Hanzlik TN, Christian PD. Functionality of the 5'- and intergenic IRES elements of cricket paralysis virus in a range of insect cell lines, and its relationship with viral activities. Virus Res. 2003;94:113–120. doi: 10.1016/s0168-1702(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Maxmen A. The hidden threat of West Nile virus. Nature. 2012;489:349–350. doi: 10.1038/489349a. [DOI] [PubMed] [Google Scholar]
- Mead DG, Ramberg FB, Besselsen DG, Maré CJ. Transmission of vesicular stomatitis virus from infected to noninfected black flies co-feeding on nonviremic deer mice. Science. 2000;287:485–487. doi: 10.1126/science.287.5452.485. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, PrestonHurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Merkling SH, van Rij RP. Beyond RNAi: Antiviral defense strategies in Drosophila and mosquito. J Insect Physiol. 2012 doi: 10.1016/j.jinsphys.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Métras R, Collins LM, White RG, Alonso S, Chevalier V, Thuranira-McKeever C, Pfeiffer DU. Rift Valley fever epidemiology, surveillance, and control: what have models contributed? Vector Borne Zoonotic Dis. 2011;11:761–771. doi: 10.1089/vbz.2010.0200. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Ahlquist P. Flock house virus RNA polymerase is a transmembrane protein with amino-terminal sequences sufficient for mitochondrial localization and membrane insertion. J Virol. 2002;76:9856–9867. doi: 10.1128/JVI.76.19.9856-9867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Schwartz MD, Ahlquist P. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells. J Virol. 2001;75:11664–11676. doi: 10.1128/JVI.75.23.11664-11676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Schwartz MD, Dye BT, Ahlquist P. Engineered retargeting of viral RNA replication complexes to an alternative intracellular membrane. J Virol. 2003;77:12193–12202. doi: 10.1128/JVI.77.22.12193-12202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S, Bakal C, Perrimon N. Genomic screening with RNAi: results and challenges. Annu Rev Biochem. 2010;79:37–64. doi: 10.1146/annurev-biochem-060408-092949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr SE, Perrimon N. RNAi screening: new approaches, understandings, and organisms. Wiley Interdiscip Rev RNA. 2012;3:145–158. doi: 10.1002/wrna.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N KJ. Viruses of Invertebrates. New York: CRC Press; 1991. Identification, Pathology, Structure, and Replication of Insect Picornaviruses; pp. 287–300. [Google Scholar]
- Moore NF, Tinsley TW. The small rna-viruses of insects. Arch Virol. 1982;72:229–245. doi: 10.1007/BF01315220. [DOI] [PubMed] [Google Scholar]
- Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010;6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudiganti U, Hernandez R, Brown DT. Insect response to alphavirus infection-- establishment of alphavirus persistence in insect cells involves inhibition of viral polyprotein cleavage. Virus Res. 2010a;150:73–84. doi: 10.1016/j.virusres.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Mudiganti U, Hernandez R, Brown DT. Insect response to alphavirus infection-- establishment of alphavirus persistence in insect cells involves inhibition of viral polyprotein cleavage. Virus Res. 2010b;150:73–84. doi: 10.1016/j.virusres.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Mueller S, Gausson V, Vodovar N, Deddouche S, Troxler L, Perot J, Pfeffer S, Hoffmann JA, Saleh MC, Imler JL. RNAi-mediated immunity provides strong protection against the negative-strand RNA vesicular stomatitis virus in Drosophila. Proc Natl Acad Sci U S A. 2010;107:19390–19395. doi: 10.1073/pnas.1014378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Hanley KA. RNA interference modulates replication of dengue virus in Drosophila melanogaster cells. BMC Microbiol. 2010;10:127. doi: 10.1186/1471-2180-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali T, Pacifico S, Yu J, Guest S, Roberts GG, Finley RL. DroID 2011: a comprehensive, integrated resource for protein, transcription factor, RNA and gene interactions for Drosophila. Nucleic Acids Res. 2011;39:D736–D743. doi: 10.1093/nar/gkq1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MA, Summers MD, editors. Virus Taxonomy: Sixth Report of the International Committee on Taxonomy of Viruses. New York & Vienna: Spirnger-Verlag; 1995. [Google Scholar]
- Myers EW, Sutton GG, Delcher AL, Dew IM, Fasulo DP, Flanigan MJ, Kravitz SA, Mobarry CM, Reinert KH, Remington KA, et al. A whole-genome assembly of Drosophila. Science. 2000;287:2196–2204. doi: 10.1126/science.287.5461.2196. [DOI] [PubMed] [Google Scholar]
- Nagy E, Dobos P. Synthesis of Drosophila X virus proteins in cultured Drosophila cells. Virology. 1984;134:358–367. doi: 10.1016/0042-6822(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Naitza S, Rossé C, Kappler C, Georgel P, Belvin M, Gubb D, Camonis J, Hoffmann JA, Reichhart JM. The Drosophila immune defense against gram-negative infection requires the death protein dFADD. Immunity. 2002;17:575–581. doi: 10.1016/s1074-7613(02)00454-5. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Moy RH, Xu J, Bambina S, Yasunaga A, Shelly SS, Gold B, Cherry S. Virus Recognition by Toll-7 Activates Antiviral Autophagy in Drosophila. Immunity. 2012 doi: 10.1016/j.immuni.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A, Berry B, Tassetto M, Kunitomi M, Acevedo A, Deng C, Krutchinsky A, Gross J, Antoniewski C, Andino R. Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat Struct Mol Biol. 2010;17:547–554. doi: 10.1038/nsmb.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, Rusten TE, Stenmark H, Brech A. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol. 2008;180:1065–1071. doi: 10.1083/jcb.200711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu T, Gaff HD, Papelis YE, Hartley DM. An epidemiological model of rift valley fever with spatial dynamics. Comput Math Methods Med. 2012;2012:138757. doi: 10.1155/2012/138757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Odegard A, Banerjee M, Johnson JE. Flock house virus: a model system for understanding non-enveloped virus entry and membrane penetration. Curr Top Microbiol Immunol. 2010;343:1–22. doi: 10.1007/82_2010_35. [DOI] [PubMed] [Google Scholar]
- Odegard AL, Kwan MH, Walukiewicz HE, Banerjee M, Schneemann A, Johnson JE. Low endocytic pH and capsid protein autocleavage are critical components of Flock House virus cell entry. J Virol. 2009;83:8628–8637. doi: 10.1128/JVI.00873-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanessian A. Vertical transmission of the Piry rhabdovirus by sigma virus-infected Drosophila melanogaster females. J Gen Virol . 1989;70(Pt 1):209–212. doi: 10.1099/0022-1317-70-1-209. [DOI] [PubMed] [Google Scholar]
- Oliveira DC, Hunter WB, Ng J, Desjardins CA, Dang PM, Werren JH. Data mining cDNAs reveals three new single stranded RNA viruses in Nasonia (Hymenoptera: Pteromalidae) Insect Mol Biol. 2010;19(Suppl 1):99–107. doi: 10.1111/j.1365-2583.2009.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KE, Myles KM, Seabaugh RC, Higgs S, Carlson JO, Beaty BJ. Development of a Sindbis virus expression system that efficiently expresses green fluorescent protein in midguts of Aedes aegypti following per os infection. Insect Mol Biol. 2000;9:57–65. doi: 10.1046/j.1365-2583.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- Oura C. Bluetongue: new insights and lessons learnt. Vet Rec. 2011;168:375–376. doi: 10.1136/vr.d2192. [DOI] [PubMed] [Google Scholar]
- Panda D, Cherry S. Cell-based genomic screening: elucidating virus-host interactions. Curr Opin Virol. 2012 doi: 10.1016/j.coviro.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, Ertürk-Hasdemir D, Reichhart JM, Meier P, Silverman N. Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol Cell. 2010;37:172–182. doi: 10.1016/j.molcel.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar PN, Trinidad L, Voysey R, Duchemin JB, Walker PJ. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc Natl Acad Sci U S A. 2012;109:18915–18920. doi: 10.1073/pnas.1205231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RK, Hardy RW. Role for the phosphatidylinositol 3-kinase-Akt-TOR pathway during sindbis virus replication in arthropods. J Virol. 2012;86:3595–3604. doi: 10.1128/JVI.06625-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JT. Segmented Double-Stranded RNA Viruses: Structure and Molecular Biology. Caister Academic Press; 2008. [Google Scholar]
- Perrimon N, Ni JQ, Perkins L. In vivo RNAi: today and tomorrow. Cold Spring Harb Perspect Biol. 2010;2:a003640. doi: 10.1101/cshperspect.a003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A, Mossel E, Sanchez-Vargas I, Foy B, Olson K. Alphavirus transducing system: tools for visualizing infection in mosquito vectors. J Vis Exp. 2010 doi: 10.3791/2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plus N, Croizier G, Reinganum C, Scott PD. Cricket paralysis virus and drosophila C virus: serological analysis and comparison of capsid polypeptides and host range. J Invertebr Pathol. 1978;31:296–302. doi: 10.1016/0022-2011(78)90219-7. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Price BD, Rueckert RR, Ahlquist P. Complete replication of an animal virus and maintenance of expression vectors derived from it in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1996;93:9465–9470. doi: 10.1073/pnas.93.18.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344:9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ramadan N, Flockhart I, Booker M, Perrimon N, Mathey-Prevot B. Design and implementation of high-throughput RNAi screens in cultured Drosophila cells. Nature Protocols. 2007;2:2245–2264. doi: 10.1038/nprot.2007.250. [DOI] [PubMed] [Google Scholar]
- Ramirez JL, Dimopoulos G. The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev Comp Immunol. 2010;34:625–629. doi: 10.1016/j.dci.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancés E, Ye YH, Woolfit M, McGraw EA, O'Neill SL. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon JL. Dengue fever: Mosquitoes attacked from within. Nature. 2011;476:407–408. doi: 10.1038/476407a. [DOI] [PubMed] [Google Scholar]
- Reinganum C. The isolation of cricket paralysis virus from the emperor gum moth, Antheraea eucalypti Scott, and its infectivity towards a range of insect species. Intervirology. 1975;5:97–102. doi: 10.1159/000149886. [DOI] [PubMed] [Google Scholar]
- Reinganum C, O'Loughlin GT, Hogan TW. A nonoccluded virus of theoccations of dina oceanicus and T commodus (Orthoptera: Gryllidae) Journal of Invertebrate Pathology. 1970;16:214–220. [Google Scholar]
- Reiter LT, Bier E. Using Drosophila melanogaster to uncover human disease gene function and potential drug target proteins. Expert Opin Ther Targets. 2002;6:387–399. doi: 10.1517/14728222.6.3.387. [DOI] [PubMed] [Google Scholar]
- Reiter LT, Potocki L, Chien S, Gribskov M, Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Petit V, Affolter M. Signaling systems, guided cell migration, and organogenesis: insights from genetic studies in Drosophila. Dev Biol. 2003;260:1–8. doi: 10.1016/s0012-1606(03)00211-2. [DOI] [PubMed] [Google Scholar]
- Riddell C, Adams S, Schmid-Hempel P, Mallon EB. Differential expression of immune defences is associated with specific host-parasite interactions in insects. PLoS One. 2009;4:e7621. doi: 10.1371/journal.pone.0007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DBe. Drosophila: a practical approach. Oxford: Oxford University Press; 1998. [Google Scholar]
- Roehr B. Texas records worst outbreak of West Nile virus on record. BMJ. 2012;345:e6019. doi: 10.1136/bmj.e6019. [DOI] [PubMed] [Google Scholar]
- Rose JK, Schubert M. The rhabdoviruses. New York, NY: Plenum Press; 1987. Rhabdovirus genomes and their products; pp. 129–166. [Google Scholar]
- Rose PP, Hanna SL, Spiridigliozzi A, Wannissorn N, Beiting DP, Ross SR, Hardy RW, Bambina SA, Heise MT, Cherry S. Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and mammalian hosts. Cell Host Microbe. 2011a;10:97–104. doi: 10.1016/j.chom.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PP, Hanna SL, Spiridigliozzi A, Wannissorn N, Beiting DP, Ross SR, Hardy RW, Bambina SA, Heise MT, Cherry S. Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and Mammalian hosts. Cell Host Microbe. 2011b;10:97–104. doi: 10.1016/j.chom.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L. Carbon dioxide sensitivity in mosquitoes infected with sigma, vesicular stomatitis, and other rhabdoviruses. Science. 1980;207:989–991. doi: 10.1126/science.6101512. [DOI] [PubMed] [Google Scholar]
- Roxström-Lindquist K, Terenius O, Faye I. Parasite-specific immune response in adult Drosophila melanogaster: a genomic study. EMBO Rep. 2004;5:207–212. doi: 10.1038/sj.embor.7400073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P. Bluetongue virus genetics and genome structure. Virus Res. 1989;13:179–206. doi: 10.1016/0168-1702(89)90015-4. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Lewis EB. A brief history of Drosophila's contributions to genome research. Science. 2000;287:2216–2218. doi: 10.1126/science.287.5461.2216. [DOI] [PubMed] [Google Scholar]
- Rusu M, Bonneau R, Holbrook MR, Watowich SJ, Birmanns S, Wriggers W, Freiberg AN. An assembly model of rift valley Fever virus. Front Microbiol. 2012;3:254. doi: 10.3389/fmicb.2012.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Hetru C, Reichhart JM, Hoffmann JA, Ferrandon D. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity. 2000;12:569–580. doi: 10.1016/s1074-7613(00)80208-3. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Jouanguy E, Dostert C, Zachary D, Dimarcq JL, Bulet P, Imler JL. Pherokine-2 and-3. Eur J Biochem. 2003;270:3398–3407. doi: 10.1046/j.1432-1033.2003.03725.x. [DOI] [PubMed] [Google Scholar]
- Sabin LR, Cherry S. Small creatures use small RNAs to direct antiviral defenses. Eur J Immunol. 2013;43:27–33. doi: 10.1002/eji.201243201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin LR, Hanna SL, Cherry S. Innate antiviral immunity in Drosophila. Current Opinion in Immunology. 2010;22:4–9. doi: 10.1016/j.coi.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin LR, Zheng Q, Thekkat P, Yang J, Hannon GJ, Gregory BD, Tudor M, Cherry S. Dicer-2 processes diverse viral RNA species. PLoS One. 2013;8:e55458. doi: 10.1371/journal.pone.0055458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin LR, Zhou R, Gruber JJ, Lukinova N, Bambina S, Berman A, Lau CK, Thompson CB, Cherry S. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell. 2009;138:340–351. doi: 10.1016/j.cell.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samakovlis C, Kimbrell DA, Kylsten P, Engström A, Hultmark D. The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J. 1990;9:2969–2976. doi: 10.1002/j.1460-2075.1990.tb07489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-San Martin C, Liu CY, Kielian M. Dealing with low pH: entry and exit of alphaviruses and flaviviruses. Trends Microbiol. 2009;17:514–521. doi: 10.1016/j.tim.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane J, Guedes S, Kurkela S, Lyytikäinen O, Vapalahti O. Epidemiological analysis of mosquito-borne Pogosta disease in Finland 2009. Euro Surveill. 2010;15 doi: 10.2807/ese.15.02.19462-en. [DOI] [PubMed] [Google Scholar]
- Sasaki J, Nakashima N. Methionine-independent initiation of translation in the capsid protein of an insect RNA virus. Proc Natl Acad Sci U S A. 2000;97:1512–1515. doi: 10.1073/pnas.010426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann A, Reddy V, Johnson JE. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv Virus Res. 1998;50:381–446. doi: 10.1016/s0065-3527(08)60812-x. [DOI] [PubMed] [Google Scholar]
- Schneider D. Using Drosophila as a model insect. Nat Rev Genet. 2000;1:218–226. doi: 10.1038/35042080. [DOI] [PubMed] [Google Scholar]
- Schüler M, Connell SR, Lescoute A, Giesebrecht J, Dabrowski M, Schroeer B, Mielke T, Penczek PA, Westhof E, Spahn CM. Structure of the ribosome-bound cricket paralysis virus IRES RNA. Nat Struct Mol Biol. 2006;13:1092–1096. doi: 10.1038/nsmb1177. [DOI] [PubMed] [Google Scholar]
- Schwientek T, Mandel U, Roth U, Müller S, Hanisch FG. A serial lectin approach to the mucin-type O-glycoproteome of Drosophila melanogaster S2 cells. Proteomics. 2007;7:3264–3277. doi: 10.1002/pmic.200600793. [DOI] [PubMed] [Google Scholar]
- Scotti PD, Dearing S, Mossop DW. Flock House virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae) Arch Virol. 1983;75:181–189. doi: 10.1007/BF01315272. [DOI] [PubMed] [Google Scholar]
- Scotti PD, Hoefakker P, Dearing S. The production of cricket paralysis virus in suspension cultures of insect cell lines. J Invertebr Pathol. 1996;68:109–112. doi: 10.1006/jipa.1996.0067. [DOI] [PubMed] [Google Scholar]
- Scotti PD, Longworth JF, Plus N, Croizier G, Reinganum C. The biology and ecology of strains of an insect small RNA virus complex. Adv Virus Res. 1981;26:117–143. doi: 10.1016/s0065-3527(08)60422-4. [DOI] [PubMed] [Google Scholar]
- Seecof RL. Deleterious effects on Drosophila development associated with sigma virus infection. Virology. 1964;22:142–148. [Google Scholar]
- Selling BH, Allison RF, Kaesberg P. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proc Natl Acad Sci U S A. 1990;87:434–438. doi: 10.1073/pnas.87.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GC. Viruses and interferons. Annual Review of Microbiology. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- Senger K, Armstrong GW, Rowell WJ, Kwan JM, Markstein M, Levine M. Immunity regulatory DNAs share common organizational features in Drosophila. Mol Cell. 2004;13:19–32. doi: 10.1016/s1097-2765(03)00500-8. [DOI] [PubMed] [Google Scholar]
- Seo JK, Kwon SJ, Rao AL. A physical interaction between viral replicase and capsid protein is required for genome-packaging specificity in an RNA virus. J Virol. 2012;86:6210–6221. doi: 10.1128/JVI.07184-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, Garcia-Blanco MA. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles EW, Friesen PD. Flock house virus induces apoptosis by depletion of Drosophila inhibitor-of-apoptosis protein DIAP1. J Virol. 2008;82:1378–1388. doi: 10.1128/JVI.01941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Sasvari Z, Nagy PD. Inhibition of phospholipid biosynthesis decreases the activity of the tombusvirus replicase and alters the subcellular localization of replication proteins. Virology. 2011;415:141–152. doi: 10.1016/j.virol.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AE, Veronesi E, Maurin G, Ftaich N, Guiguen F, Rixon F, Ratinier M, Mertens P, Carpenter S, Palmarini M, et al. Drosophila melanogaster as a model organism for bluetongue virus replication and tropism. J Virol. 2012;86:9015–9024. doi: 10.1128/JVI.00131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer DA, Rosen L. Extrachromosomal inheritance of carbon dioxide sensitivity in the mosquito Culex quinquefasciatus. Genetics. 1983;104:649–659. doi: 10.1093/genetics/104.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MA. Signal transduction during the development of the Drosophila R7 photoreceptor. Dev Biol. 1994;166:431–442. doi: 10.1006/dbio.1994.1327. [DOI] [PubMed] [Google Scholar]
- Siomi H, Siomi MC. On the road to reading the RNA-interference code. Nature. 2009;457:396–404. doi: 10.1038/nature07754. [DOI] [PubMed] [Google Scholar]
- Smale ST. Selective Transcription in Response to an Inflammatory Stimulus. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAKSTAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn CM, Jan E, Mulder A, Grassucci RA, Sarnow P, Frank J. Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor. Cell. 2004;118:465–475. doi: 10.1016/j.cell.2004.08.001. [DOI] [PubMed] [Google Scholar]
- St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet. 2002;3:176–188. doi: 10.1038/nrg751. [DOI] [PubMed] [Google Scholar]
- Stapleford KA, Rapaport D, Miller DJ. Mitochondrion-enriched anionic phospholipids facilitate flock house virus RNA polymerase membrane association. J Virol. 2009;83:4498–4507. doi: 10.1128/JVI.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Antiviral defense: interferons and beyond. J Exp Med. 2006;203:1837–1841. doi: 10.1084/jem.20061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, Maniatis T, Hultmark D. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci U S A. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. THE ALPHAVIRUSES - GENE-EXPRESSION, REPLICATION, AND EVOLUTION. Microbiological Reviews. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JH, Wang KS, Schmaljohn AL, Kuhn RJ, Strauss EG. Host-cell receptors for Sindbis virus. Arch Virol Suppl. 1994;9:473–484. doi: 10.1007/978-3-7091-9326-6_46. [DOI] [PubMed] [Google Scholar]
- Sylvester ESaR, J . Aphid-borne rhabdoviruses- relationship with their aphid vectors. Springer-Verlag: Advances in Disease Vector Research; 1992. pp. 313–341. [Google Scholar]
- Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tate J, Liljas L, Scotti P, Christian P, Lin T, Johnson JE. The crystal structure of cricket paralysis virus: the first view of a new virus family. Nat Struct Biol. 1999;6:765–774. doi: 10.1038/11543. [DOI] [PubMed] [Google Scholar]
- Tauszig-Delamasure S, Bilak H, Capovilla M, Hoffmann JA, Imler JL. Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nat Immunol. 2002;3:91–97. doi: 10.1038/ni747. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Hurlbut HS, Work TH, Kingston JR, Frothingham TE. Sindbis virus: a newly recognized arthropod transmitted virus. Am. J Trop. Med. Hyg. 1955;4:844–862. doi: 10.4269/ajtmh.1955.4.844. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hagen KG, Zhang L, Tian E, Zhang Y. Glycobiology on the fly: developmental and mechanistic insights from Drosophila. Glycobiology. 2009;19:102–111. doi: 10.1093/glycob/cwn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teninges D, Ohanessian A, Richard-Molard C, Contamine D. Isolation and biological properties of Drosophila X virus. J Gen Virol. 1979;42:241–254. [Google Scholar]
- Thomas-Orillard M, Jeune B, Cusset G. Drosophila-host genetic control of susceptibility to Drosophila C virus. Genetics. 1995;140:1289–1295. doi: 10.1093/genetics/140.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson TC, Schneemann A, Johnson J. Oocyte destruction is activated during viral infection. Genesis. 2012;50:453–465. doi: 10.1002/dvg.22004. [DOI] [PubMed] [Google Scholar]
- Towb P, Bergmann A, Wasserman SA. The protein kinase Pelle mediates feedback regulation in the Drosophila Toll signaling pathway. Development. 2001;128:4729–4736. doi: 10.1242/dev.128.23.4729. [DOI] [PubMed] [Google Scholar]
- Tsai CW, McGraw EA, Ammar ED, Dietzgen RG, Hogenhout SA. Drosophila melanogaster mounts a unique immune response to the Rhabdovirus sigma virus. Appl Environ Microbiol. 2008a;74:3251–3256. doi: 10.1128/AEM.02248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CW, McGraw EA, Ammar ED, Dietzgen RG, Hogenhout SA. Drosophila melanogaster mounts a unique immune response to the Rhabdovirus sigma virus. Appl Environ Microbiol. 2008b;74:3251–3256. doi: 10.1128/AEM.02248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvila J, Vanha-Aho LM, Rämet M. Drosophila phagocytosis - still many unknowns under the surface. APMIS. 2011;119:651–662. doi: 10.1111/j.1600-0463.2011.02792.x. [DOI] [PubMed] [Google Scholar]
- van Mierlo JT, Bronkhorst AW, Overheul GJ, Sadanandan SA, Ekström JO, Heestermans M, Hultmark D, Antoniewski C, van Rij RP. Convergent evolution of argonaute-2 slicer antagonism in two distinct insect RNA viruses. PLoS Pathog. 2012;8:e1002872. doi: 10.1371/journal.ppat.1002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJ, Bellen HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet. 2005;6:167–178. doi: 10.1038/nrg1553. [DOI] [PubMed] [Google Scholar]
- Venter PA, Schneemann A. Recent insights into the biology and biomedical applications of Flock House virus. Cell Mol Life Sci. 2008;65:2675–2687. doi: 10.1007/s00018-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraksa A. When peptides fly: advances in Drosophila proteomics. J Proteomics. 2010;73:2158–2170. doi: 10.1016/j.jprot.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Vialat P, Muller R, Vu TH, Prehaud C, Bouloy M. Mapping of the mutations present in the genome of the Rift Valley fever virus attenuated MP12 strain and their putative role in attenuation. Virus Res. 1997;52:43–50. doi: 10.1016/s0168-1702(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Walker PJ, BA, Calisher CH, Dietzgen R, Fang RX, Jackson AO, Kurath G, Leong JC, Nadin-Davies S, Tesh RB, Tordo N. Family Rhabdoviridae. Berlin, Heidelberg, New York: Springer; 2000. [Google Scholar]
- Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- Walter CT, Barr JN. Recent advances in the molecular and cellular biology of bunyaviruses. J Gen Virol. 2011;92:2467–2484. doi: 10.1099/vir.0.035105-0. [DOI] [PubMed] [Google Scholar]
- Walukiewicz HE, Johnson JE, Schneemann A. Morphological changes in the T=3 capsid of Flock House virus during cell entry. J Virol. 2006;80:615–622. doi: 10.1128/JVI.80.2.615-622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne ML, Blohm GM, Brooks ME, Regan KL, Brown BY, Barfield M, Holt RD, Bolker BM. The prevalence and persistence of sigma virus, a biparentally transmitted parasite of Drosophila melanogaster. (Evol Ecol Res) 2011:323–345. [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral research. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AN, Tauszig-Delamasure S, Hoffmann JA, Lelièvre E, Gascan H, Ray KP, Morse MA, Imler JL, Gay NJ. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- Weeks SA, Miller DJ. The heat shock protein 70 cochaperone YDJ1 is required for efficient membrane-specific flock house virus RNA replication complex assembly and function in Saccharomyces cerevisiae. J Virol. 2008;82:2004–2012. doi: 10.1128/JVI.02017-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Wicker C, Reichhart JM, Hoffmann D, Hultmark D, Samakovlis C, Hoffmann JA. Insect immunity Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem. 1990;265:22493–22498. [PubMed] [Google Scholar]
- Wilfert L, Jiggins FM. Disease association mapping in Drosophila can be replicated in the wild. Biol Lett. 2010;6:666–668. doi: 10.1098/rsbl.2010.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE, Pestova TV, Hellen CU, Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000a;102:511–520. doi: 10.1016/s0092-8674(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Powell MJ, Hoover SE, Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol Cell Biol. 2000b;20:4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers F, Richard-Molard C, Blondel D, Dezelee S. Vesicular stomatitis virus growth in Drosophila melanogaster cells: G protein deficiency. J Virol. 1980;33:411–422. doi: 10.1128/jvi.33.1.411-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Grant G, Sabin LR, Gordesky-Gold B, Yasunaga A, Tudor M, Cherry S. Transcriptional pausing controls a rapid antiviral innate immune response in Drosophila. Cell Host Microbe. 2012;12:531–543. doi: 10.1016/j.chom.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yampolsky LY, Webb CT, Shabalina SA, S KA. Rapid accumulation of a vertically transmitted parasite triggered by relaxation of natural selection among hosts. (Evol. Ecol. Res.) 1999:581–589. [Google Scholar]
- Yan R, Small S, Desplan C, Dearolf CR, Darnell JE. Identification of a Stat gene that functions in Drosophila development. Cell. 1996;84:421–430. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]
- Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, Takada H, Goldman WE, Fukase K, Silverman N, et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol. 2008;9:908–916. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci U S A. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon RA, Vakharia VN, Wu LP. RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell Microbiol. 2006;8:880–889. doi: 10.1111/j.1462-5822.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- Zeidler MP, Bach EA, Perrimon N. The roles of the Drosophila JAK/STAT pathway. Oncogene. 2000;19:2598–2606. doi: 10.1038/sj.onc.1203482. [DOI] [PubMed] [Google Scholar]
- Zeller H, Bouloy M. Infections by viruses of the families Bunyaviridae and Filoviridae. Rev Sci Tech. 2000;19:79–91. doi: 10.20506/rst.19.1.1208. [DOI] [PubMed] [Google Scholar]