Abstract
This review examines the growing literature on the role of peroxisome proliferator-activated receptors (PPARs) in addiction. There are two subtypes of PPAR receptors that have been studied in addiction: PPAR-α and PPAR-γ. The role of each PPAR subtype in common models of addictive behavior, mainly pre-clinical models, is summarized. In particular, studies are reviewed that investigated the effects of PPAR-α agonists on relapse, sensitization, conditioned place preference, withdrawal and drug intake, and effects of PPAR-γ agonists on relapse, withdrawal and drug intake. Finally, studies that investigated the effects of PPAR agonists on neural pathways of addiction are reviewed. Taken together this preclinical data indicates that PPAR agonists are promising new medications for drug addiction treatment.
Keywords: Self-administration, Abuse, Dependence, Stress, Nuclear Receptors, Opioids, Nicotine, Alcohol, Psychostimulants, PPAR
Drug dependence is a chronic, relapsing disorder in which compulsive drug-seeking and drug-taking behavior persists despite serious negative consequences (1). Addictive substances, such as opioids, ethanol, psychostimulants, alcohol and nicotine, induce pleasant states or relieve distress, effects that contribute to their recreational use. Drug use can be elicited by the positive motivational effects of drugs or their ability to relieve physical withdrawal or the negative affect associated with drug abstinence in dependent individuals (2). Those aspects are termed positive and negative reinforcement, respectively. Animal models for the study of addiction have been developed to investigate the rewarding and reinforcing effects of drugs of abuse through, for example, operant drug self-administration and reinstatement studies, while withdrawal studies investigate the somatic signs of withdrawal that occur after abrupt cessation of drug use following dependence induction or the negative affect (i.e., anxiety- or depressive-like behaviors) that may occur during protracted abstinence.
Although addictive drugs produce their effects through actions at various receptors in the brain, it is thought that their common effects on activity of dopaminergic brain reward pathways is primarily responsible for their addictive properties (3–6). Notably, the mesocorticolimbic system, which projects from the ventral tegmental area to the nucleus accumbens, cortical areas and the amygdala, is implicated in the rewarding/reinforcing effects of psychostimulants and other drugs of abuse, as well as the effects of non-drug natural rewards such as food (7) (see (6) for a recent review). The involvement of dopamine in the rewarding effects of drugs of abuse is suggested by findings that most drugs abused by humans increase extracellular levels of dopamine in the nucleus accumbens (NAcc) (8, 9) and that blockade of dopamine transmission reduces the rewarding effects of psychostimulants (10). In particular, all addictive drugs are thought to activate the shell subregion of the NAcc (11, 12).
The peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins which serve primarily to regulate gene expression through their role as ligand-activated transcription factors (13). Originally discovered as orphan nuclear receptors in the early 1990s, PPARs were found to be targets of a group of compounds known as peroxisome proliferators (14) due to their ability to induce a proliferation of cellular organelle peroxisomes in rodents (15). Though these receptors were eventually found to not be involved in the induction of peroxisome proliferation in humans, the name has been maintained. Three PPAR isoforms have been identified (alpha, delta, and gamma), with each being transcribed from different genes (16). The primary function of PPAR-α is as a fatty acid sensor that regulates lipid and lipoprotein metabolism and energy homeostasis through the activation of several target genes, it is a major regulator of lipid and lipoprotein metabolism and energy homeostasis (17).
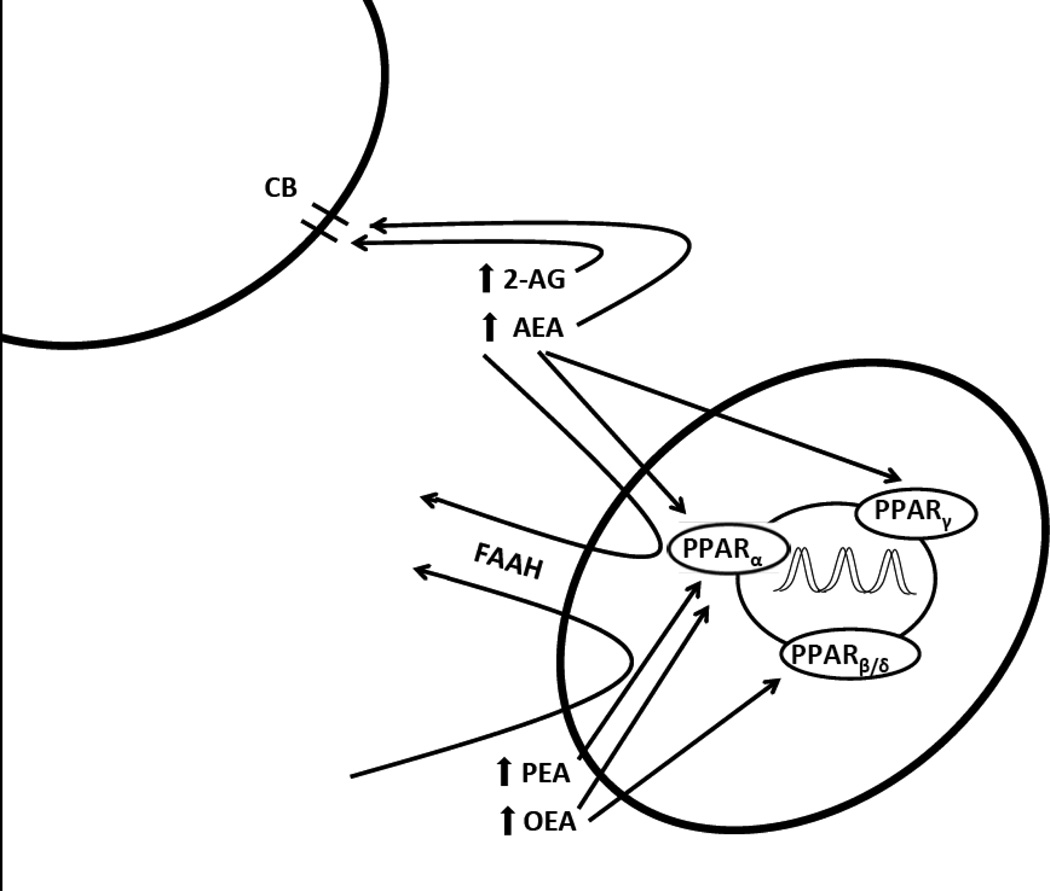
Interest in PPARs as a target for treating drug addiction arose from the study of the related cannabinoid system (see Figure 1), which is believed to be involved in the addictive properties of drugs, including cocaine and nicotine (18–23). There are two main endogenous ligands for cannabinoid receptors, anandamide (AEA) and 2-arachindonoylglycerol (2-AG), which are broken down by fatty acid amide hydrolase (FAAH) and by monoacyglycerol lipase (MAGL), respectively. FAAH also breaks down the endogenous ligands for PPAR receptors, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). Thus FAAH inhibiting drugs increase endogenous levels of both endocannabinoids and PPAR agonists. Studies with the FAAH inhibitor URB597 have identified FAAH inhibition as a promising target for the treatment of addiction (24–26), and cannbinoid receptors and PPAR may both play a role in these effects.
Figure 1.
Biological effects of PPARs. CB: cannabinoid receptor; 2-AG: 2-arachindonoylglycerol; AEA: anandamide; FAAH: fatty acid amide hydrolase; OEA: oleoylethanolamide; PEA: palmitoylethanolamide; PPAR: peroxisome proliferator activated receptor
PPARs are distributed in many areas throughout the brain (27–29). One study found their expression highest in the cerebral cortices (29), while another found that PPARα mRNA expression was also high in the dentate gyrus of the hippocampus and the olfactory tubercle. Another study (27) provided a detailed description of the localization of PPARs in the brain, finding PPARα in most areas except the hypothalamus, rhombencephalon and spinal cord, and finding PPARγ in most areas except the olfactory bulb, and some parts of the olfactory cortex and thalamus. Interestingly for addiction, PPARγ was also found in the ventral tegmental area, where it colocalizes with tyrosine hydroxylase positive signals, which suggests it is expressed in dopaminergic neurons (30).
Recently, the role of PPARs in addiction has received increasing attention (31, 32). This review focuses on the role of PPAR agonists on preclinical models of addictive behaviors; the findings are summarized in Table 1, and will be reviewed here. Table 2 provides a list of agonists and antagonists for PPAR receptors that are referred to in this review. We will first summarize the main animal models used to assess subjective and rewarding/reinforcing effects of drugs of abuse (23) and then summarize the preclinical and clinical findings related to PPAR agonism and the subjective and rewarding/reinforcing effects of different drugs of abuse in these models. The results obtained will be presented by animal model. The putative neurobiological mechanisms underlying these effects will also be discussed.
Table 1.
Summary of findings of pre-clinical studies reviewed.
| receptor subtype |
antagonist/agonist | effect | behaviour | reference |
|---|---|---|---|---|
| α | agonist | ↓ | acquisition of nicotine self-administration | 67 |
| α | agonist | ↓ | maintenance of nicotine self-administration FR schedule | 67, 68 |
| α | agonist | - | responding for food FR schedule | 25, 67, 68 |
| α | agonist | - | drug discrimination | 68 |
| α | agonist | ↓ | ethanol intake | 69 |
| α | agonist | ↑ | ethanol intake | 70 |
| α | agonist | ↓ | nicotine-induced reinstatement | 67, 68 |
| α | agonist | ↓ | cue-induced reinstatement of nicotine-seeking | 67 |
| α | agonist | - | sensitization to cocaine | 71 |
| α | agonist | ↑ | sensitization to morphine | 71 |
| γ | agonist | ↓ | intake of alcohol | 73 |
| γ | agonist | ↑ | food intake | 73 |
| γ | agonist | - | water intake | 73 |
| γ | agonist | ↓ | self-administration of ethanol | 73 |
| γ | agonist | ↓ | self-administration of heroin FR schedule | 75 |
| γ | agonist | ↓ | self-administration of heroin PR schedule | 75 |
| γ | agonist | - | cue-induced reinstatement of alcohol-seeking | 73 |
| γ | agonist | ↓ | yohimbine-induced reinstatement of alcohol-seeking | 73 |
| γ | agonist | ↓ | Methamphetamine sensitization | 78 |
| γ | antagonist | ↑ | Methamphetamine sensitization | 78 |
Table 2.
Agonists and antagonists at PPARα and PPARγ receptors reviewed.
| PPARα | agonists | endogenous | oleoylethanolamide (OEA) palmitoylethanolamide (PEA) |
| exogenous | clofibrate gemfibrozil WY14643 |
||
| antagonists | exogenous | MK886 | |
| PPARγ | agonists | exogenous | pioglitazone rosiglitazone ciglitazone |
Animal models for studying effects of drugs of abuse
A variety of animal models are available to study the cardinal features of drug dependence (23, 33–45). The effects of PPAR agonists have been evaluated using animals models for the subjective effects of drugs (drug discrimination), their rewarding/reinforcing effects (intravenous drug self-administration), the influence of environmental factors on drug-seeking behavior (reinstatement of extinguished drug-seeking behavior and other relapse models, sensitization) and the withdrawal states associated with abrupt termination of drug action. We will review the results obtained so far with these various procedures, focusing on operant drug self-administration models, the preeminent animal model of drug abuse.
Drug discrimination
Humans abusing psychoactive drugs report characteristic subjective effects, and drug discrimination procedures in rats and monkeys are extensively used to model these effects. The organism’s ability to perceive and identify the characteristic interoceptive effects of drugs is thought to play a role in drug-seeking, encouraging the development of this behavior and directing it towards one substance rather than another, on the basis of relative potencies and effects (46). To assess the discriminative effects of drugs, animals are trained with response-contingent food-pellet delivery or stimulus-shock termination to respond on one lever after an injection of a training dose of a drug and on the other lever after an injection of vehicle. Once animals learn to reliably make this discrimination, the subjective effects of different drugs can be compared and the modulation of subjective effects of drugs of abuse by various pharmacological ligands can be studied.
Operant drug self-administration
Natural rewards, such as water, food, and drugs of abuse may serve as positive reinforcers, increasing the frequency of the response that produces them. To study intravenous drug self-administration, a permanent catheter is implanted in a vein to allow the animal to self-inject a small amount of drug by pressing a lever. The administration of drug constitutes the event that positively reinforces the lever-pressing behavior. Various schedules of reinforcement of drug self-administration behavior have been developed, defined by the response requirements for obtaining each injection.
Fixed-ratio and progressive-ratio schedules
Under a fixed-ratio schedule, a fixed number of lever-presses are necessary to obtain each injection (e.g., 1 lever press for a fixed-ratio 1, i.e. FR1, schedule). In contrast, under a progressive-ratio schedule, the number of lever-press responses required to obtain a drug injection increases after each injection (47) until the subject fails to emit the required number of responses. The highest ratio that is completed is termed the “breaking point”, and higher breaking points are considered to be an indicator of higher reinforcing effectiveness of the drug.
Reinstatement
The main animal models of relapse are reinstatement paradigms that model the ability of re-exposure to environmental stimuli (48) or drugs (49–51) to induce drug-seeking after a period of abstinence. Relapse is of clear importance to the study of addiction, and the reinstatement model has been studied in rats (53, 54) and humans (55) and found to have high predictive validity (52). In this model, the animal first learns to self-administer a drug, and then the response is extinguished by discontinuing availability of the drug. Following extinction of the drug-seeking behavior, the behavior is reinstated by exposing the subject to environmental cues that have been associated with drugs (48, 53, 54, 56–58), stress (59–61) and by acute priming injection of drugs (49, 53, 54, 62).
Sensitization
With repeated exposure to drugs of abuse, animals can show either a heightened or decreased response to a dose of drug. Termed sensitization and tolerance, respectively, the occurrence of one or the other can be dependent on the manner in which the drug of abuse is given. That is, repeated high doses with little time intervening between administrations can lead to tolerance, whereas intermittent exposure can lead to sensitization (63, 64). Sensitization —which in animals is usually studied as increased locomotor activity when the drug is given— has been demonstrated for all drugs of abuse, and the incentive sensitization theory posits that sensitization functions to transition drug ‘liking’ to drug ‘wanting’ (65). Specifically it is the incentive motivational properties of the drug that are sensitized (66) and this is thought to lead to approach to drugs of abuse.
PPAR-α agonists and addiction
The following review of the literature investigating effects of PPAR-α agonists on animal models of drug addiction are presented by behavior.
Drug intake
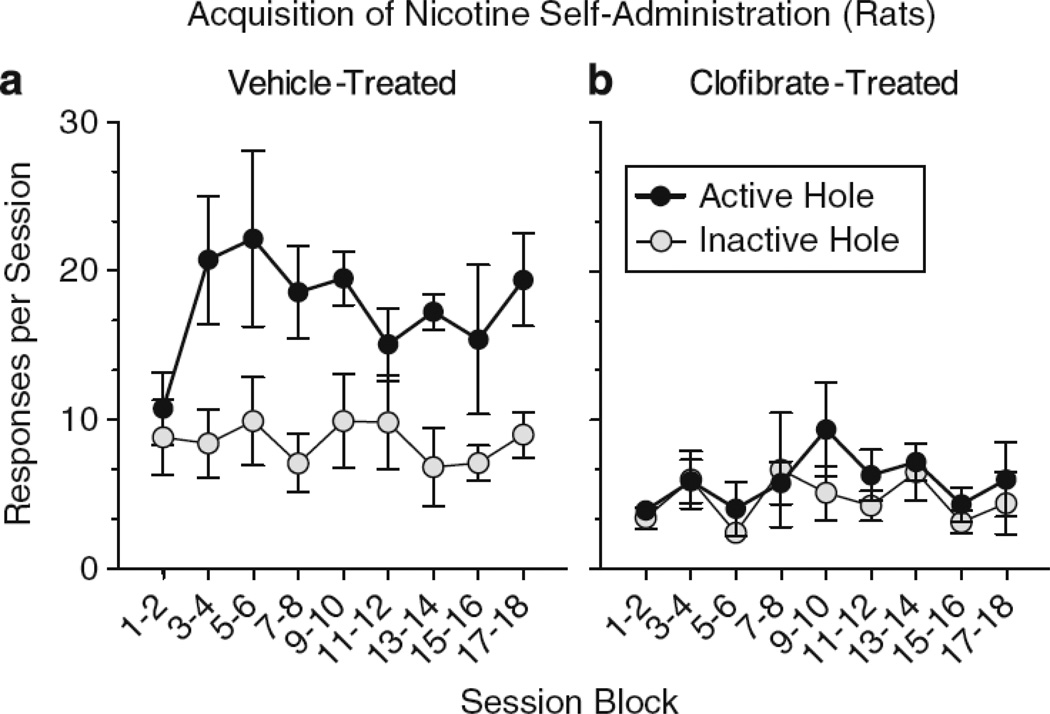
When the PPAR-α agonist clofibrate was administered prior to daily sessions when rats were first allowed to self-administer nicotine, rats did not did not develop nicotine self-administration behavior (67). When nicotine self-administration was established in rats or monkeys before they were ever treated with the PPAR-α agonists WY1463 or clofibrate, these PPAR-α treatments decreased nicotine intake (67, 68); these effects were reversed by the PPAR-α antagonist MK886 (67, 68), which had no effect on nicotine self-administration on its own. As clearly indicated in Figure 2, the number of active lever presses by rats for nicotine was lower following pretreatment with clofibrate than following pretreatment with vehicle. Clofibrate did not affect pressing of an inactive lever that was used to detect possible non-specific changes in activity levels during acquisition of nicotine self-administration. Although clofibrate (67) and WY1463 (68) blocked the rewarding effects of nicotine, they did not block all of the interoceptive effects of nicotine (some of which may actually be aversive) in the nicotine drug-discrimination test. In sum, these studies suggest that PPAR-α agonists specifically reduce nicotine intake by interfering directly with nicotine’s rewarding/reinforcing pharmacological effects.
Figure 2.
Clofibrate prevented the acquisition of nicotine self-administration in nicotine-naïve rats. Left panel: Vehicle-treated control rats acquired nicotine self-administration and responses in the active nose-poke hole were greater than in the inactive nose-poke hole that had no consequences. Right panel: Clofibrate pre-treatment decreased the acquisition of nicotine self-administration; responses in the inactive and active nose-poke holes were the same. Taken from Panlilio et al. (2012).
Drug intake has also been studied with a two bottle choice paradigm in which animals are given alcohol solution in one bottle and water in another, and measuring the amount of each that is consumed. In one study (69), it was found that the PPAR-α agonist gemfibrozil decreased ethanol intake for a 7% solution, with no effect on the daily intake of rat chow, suggesting that PPAR-α agonists may be effective in decreasing alcohol drinking. However, it should be noted that another study (70) found the opposite effect when the concentration of alcohol was gradually increased to 30%; after clofibrate administration, rats increased their intake by 127% as compared to controls that increased their intake by 27%. The latter effect was accompanied by an increase in the amount of calories consumed from alcohol and a concomitant decrease in the caloric intake from chow. The reasons for the discrepancy in the literature are unclear, but it may be related to differences in concentration of alcohol, with 7% alcohol used in the former study and 30% alcohol used in the latter. Also, dietary factors may have played a role at the higher alcohol concentration, leading to increased intake.
Reinstatement
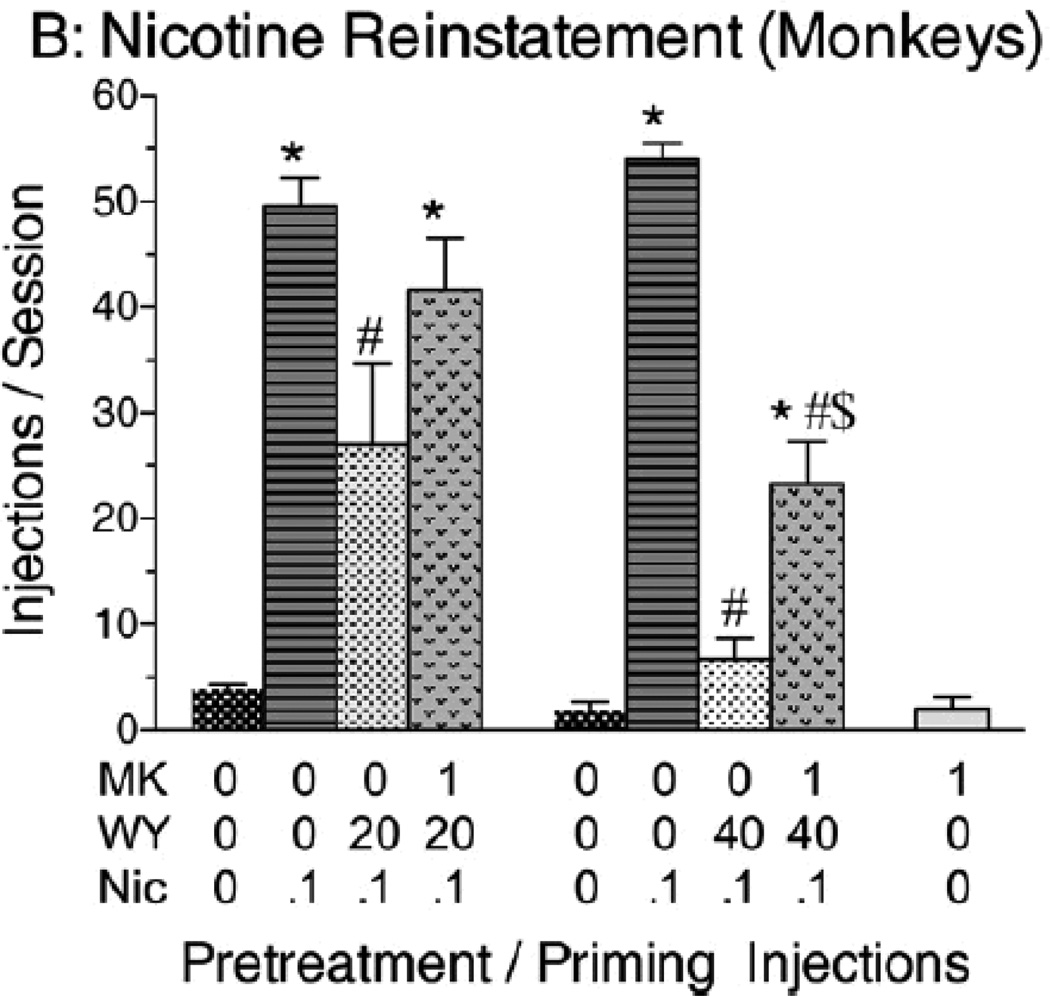
The effects of PPAR-α agonists have been tested in both the cue-induced reinstatement and drug-induced reinstatement paradigms. That is, after acquisition of self-administration responding for nicotine under either a FR-1 or FR-5 schedule of reinforcement followed by extinction of the responding, responding was reinstated by priming injections of nicotine (25). In our studies (67, 68), pretreatment with the PPAR-α agonists WY14643 and clofibrate prior to nicotine-induced reinstatement attenuated reinstatement in rats and monkeys, as illustrated in monkeys in Figure 3; administration of the PPAR-α antagonist MK886 reversed the effects of WY14643 on nicotine-induced reinstatement (67). MK886 also reversed the effects of clofibrate on nicotine-induced reinstatement (67). However, unlike drug intake, where the effects of PPAR-α agonists seemed to be selective for nicotine, and perhaps alcohol, both nicotine-induced and cue-induced reinstatement to nicotine-seeking behavior in monkeys were blocked by clofibrate, and both of these effects were reversed by MK886 (67).
Figure 3.
Reinstatement of nicotine-seeking by a priming injection of nicotine after extinction in monkeys. The PPAR-α agonist WY14643 (20 or 40 mg/kg i.p.) dose-dependently reduced the reinstatement of extinguished nicotine-seeking responses. This effect of WY14643 was prevented by pretreatment with the PPAR-α antagonist MK886 (1 mg/kg i.m.). Data are presented as mean ± SEM. *Significant difference from vehicle pretreatment during a saline prime session. #Significant difference from vehicle pretreatment during a nicotine prime session. Taken from Masica et al. (2011).
Sensitization
Two studies have looked at the role of PPAR-α receptors in sensitization. In one study, mutant mice lacking the PPAR-α gene were tested for sensitization to injections of morphine and cocaine. It was found that, in these mutant mice, sensitization to cocaine was unaffected (71). However, in mutant mice lacking the PPAR-α gene, sensitization to morphine was potentiated (71). Increased sensitization to morphine was observed at a dose of 5 mg/kg but, at a higher dose of 10 mg/kg, sensitization in the mutant was not different from that in the wild-type. Therefore, deletion of PPAR-α gene may enhance sensitization. Further, these effects may be evident only after the acquisition of sensitization, because when the PPAR-α agonist WY14643 was administered prior to either induction or expression of sensitization, it blocked the expression but not the acquisition of sensitization to morphine.
PPAR-γ and addiction
In a relatively recent study looking at the expression of PPAR-γ protein in circulating monocytes and macrophages from healthy smokers and non-smokers, it was found that PPAR-γ protein expression is increased in smokers as compared to non-smokers (72). To confirm these findings, cells from healthy non-smokers were treated in vitro with nicotine and PPAR-γ protein quantified; a dose-dependent increase in expression was found after nicotine treatment. These effects were reversed by bungarotoxin. Although indicative of a link between nicotine effects and PPAR-γ protein, clear evidence for a role of this receptor in addiction came from studies on alcohol carried out in our laboratory (73).
Intake
In one series of experiments, the effects of PPAR-γ agonists on multiple measures of alcohol drinking were examined. The PPAR-γ agonists pioglitazone and rosiglitazone decreased voluntary consumption of a 10% alcohol solution in rats genetically selected for high alcohol consumption when these rats were given a choice between the alcohol solution and water (73). This effect lasted the duration of the 7 day treatment phase and drinking returned to normal after the treatments were abated. Water consumption was unchanged while food intake was increased by pioglitazone but not rosiglitazone; this effect decreased over time. These results suggest that changes in alcohol intake were specific and not due to any general inhibition of feeding behavior. Similarly, when rats had to perform an operant task to receive alcohol, pioglitazone significantly reduced alcohol self-administration while lever pressing for saccharin was not modified. These results not only suggest a selective effect of PPAR-γ agonists on intake of alcohol, as opposed to natural reinforcers, they also suggest that decreases in alcohol self-administration were not due to a non-specific inhibition of behavior or a decrease in the ability to perform a response. Importantly, in this study, it was also demonstrated that PPAR-γ agonists, while reducing alcohol drinking, did not modify blood glucose levels nor did they affect alcohol metabolism, ruling out the possibility that metabolic effects might have contributed to drug effects. Rather, PPAR-γ agonists appear to have affected the motivation to take alcohol. In a subsequent study it was also shown that combining pioglitazone with naltrexone, a drug currently used for alcohol addiction treatment in humans, leads to a more pronounced inhibition of drinking compared to the two drugs given alone (74). More recently, experiments were conducted to evaluate the effect of PPARγ agonists on opiate intake. Results revealed that treatment with pioglitazone significantly reduced intravenous self-administration of heroin under both fixed-ratio and progressive-ratio schedules of reinforcement. This effect was maintained over repeated days of treatment (75).
Reinstatement and withdrawal
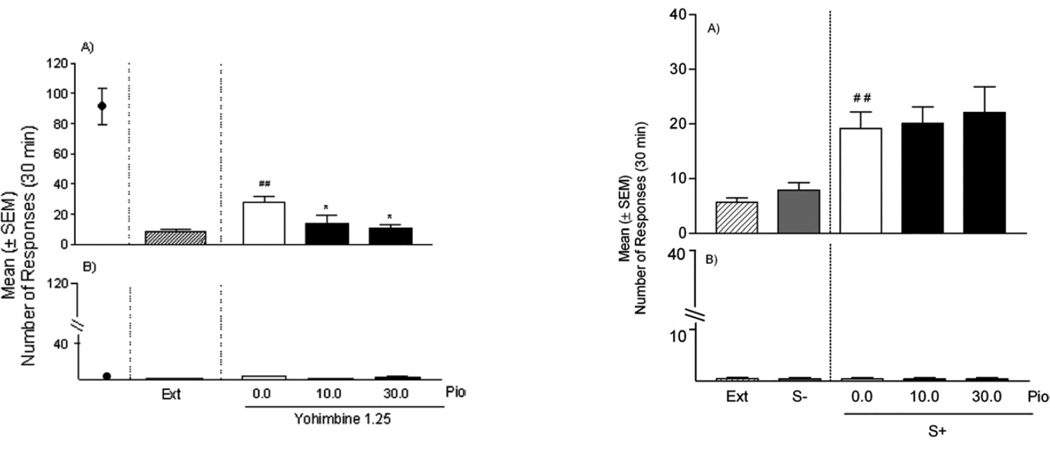
By contrast to the effects on intake, the PPAR-γ agonist pioglitazone had no effect on cue-induced reinstatement of alcohol-seeking behavior (73), suggesting that PPAR-γ agonists may function to abate primary responses for drugs and not those conditioned to the environment. As can be seen in Figure 4, reexposure to environmental stimuli induced increases in alcohol-seeking and these increases were similar following administration of pioglitazone. By comparison, as can also be seen in Figure 4, pioglitazone decreased reinstatement induced by yohimbine stress. Yohimbine is an α2 adrenoceptor antagonist that acts as a pharmacologic stressor in animals and in humans. In animals, it potently reinstates alcohol–seeking behavior (76), while in abstinent alcoholics it elicits intense craving that correlates with alcoholism severity (77). Most notably, contrary to pioglitazone, naltrexone reduced reinstatement of drug-seeking triggered by cues but not by yohimbine stress (74). However if the two drugs were combined at relatively low doses, they were able to prevent both forms of relapse (74). This provides further evidence for the potential of this drug combination in the treatment of alcohol addiction. In additional experiments with pioglitazone, activation of PPARγ markedly reduced the expression of somatic withdrawal signs in rats made dependent on alcohol following chronic intragastric alcohol administration (73).
Figure 4.
The effect of pioglitazone on yohimbine-induced reinstatement (left panel) and cue-induced reinstatement (right panel). During training, rats consumed alcohol prior to extinction (Ext) of this response. Compared with extinction, both yohimbine (left panel) and cues predictive of alcohol (S+; right panel) induced reinstatement of alcohol-seeking. Responding for the alcohol-predictive cues (S+) was also higher than responding for a stimulus predictive of water availability (S−). Yohimbine-induced reinstatement was reduced following treatment with pioglitazone (Pio), while cue-induced reinstatement was not affected. *Significant difference from vehicle (p<0.05 for yohimbine-induced reinstatement and p<0.01 for the cue-induced reinstatement data). Data are presented as mean ± S.E.M. Taken from Stopponi et al. (2011).
Sensitization
Repeated daily administration of methamphetamine led to development of locomotor sensitization associated with an increased level of PPARγ protein in the nuclear fraction from whole brain tissue, suggesting an increased translocation of the receptor in the nucleus (78). Most notably, repeated intracerebroventricular administration of two distinct PPARγ agonists, pioglitazone and ciglitazone, prevented the expression of methamphetamine sensitization. This protective effect of pioglitazone was synergistically facilitated by concomitant administration of 9-cis-retinoic acid, an agonist for the retinoid X receptor which is another nuclear receptor that forms heterodimers with PPARγ. On the other hand, treatment with the PPARγ antagonist GW9662 during the withdrawal period prior to methamphetamine challenge increased the expression of behavioral sensitization (78).
In light of the ability of PPARγ to reduce glia-mediated inflammatory response in the brain and to protect from NMDA mediated excitotoxic damage (79, 80), it was hypothesized that the effect of pioglitazone on methamphetamine sensitization was the result of its neuroprotective effects (78). However, as discussed in the following paragraph, an alternative possibility is that pioglitazone blocked the locomotor sensitizing effects of methamphetamine by reducing the ability of this drug to activate corticomesolimbic dopamine (DA).
PPARs and dopamine neurons
The endogenous PPAR-α agonists, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) can block nicotine’s ability to stimulate mesolimbic dopamine neurons, thereby blocking nicotine’s rewarding and addictive properties (81). These effects of OEA and PEA are inhibited by the PPAR-α antagonist MK886, demonstrating specific PPAR-α involvement. Further, incubation of brain slices with the tyrosine hydroxylase inhibitor, genistein, also inhibited the effects of OEA on nicotine induced changes in firing rate of dopamine neurons, suggesting that tyrosine kinases contribute to the effects of PPARs on nicotine-induced changes in firing rate; phosphorylation of the nicotine receptor appears to mediate the PPAR-α inhibition of nicotine’s effects. The rapid onset of OEA/PEA effects suggested a nongenomic mechanism of PPAR-α stimulation of tyrosine kinases. Further work showed that PPAR-α agonists caused negative modulation of dopamine neurons, as WY14643, a PPAR-α agonist, reduced the number of spontaneously active dopamine neurons, while MK886, a PPAR-α antagonist, increased spontaneous activity of VTA dopamine neurons, an effect that was blocked by WY14643 and by nicotinic antagonists (82). The addictive potential of nicotine is believed to be mediated by dopamine neurons (6, 83), and thus these effects are key to its addictive potential.
Indeed, PPAR-α agonists were also effective at the terminal regions in the nucleus accumbens, an area believed to be critical to abuse potential (84), especially the shell subregion (11, 12). That is, PPAR-α agonists blocked nicotine’s ability to induce excitation of dopamine neurons in the VTA, at doses that did not alter spontaneous firing rates; this effect was reversed by the PPAR-α antagonist MK886 (67, 68). PPAR-α agonists also attenuated nicotine-induced increases in dopamine levels in the nucleus accumbens shell at doses that had no effect on DA on their own; these effects were also reversed by MY886 at doses that were ineffective on its own (67, 68). Further, MK886, a PPAR-α antagonist, blocked URB597’s effects on nicotine-induced inhibition of medium spiny neuron activation induced by stimulation of the baso-lateral amygdala, an effect that was also found with cocaine- induced inhibition of medium spiny neuron activation induced by stimulation of the baso-lateral amygdala.
In relation to PPARγ it has been recently demonstrated that this receptor is present in the VTA, where it co-localizes with tyrosine hydroxylase (TH), suggesting its expression in dopaminergic cells (30). The VTA dopamine system has a well established role in several aspects of drug dependence including sensitization, drug intake and relapse, which may explain the findings with alcohol, methamphetamine and heroin reviewed above. In this respect, recent data from our laboratory demonstrated that activation of PPARγ by pioglitazone reduced morphine-induced increases in the firing rate of VTA dopamine cells and dopamine release in the shell portion of the nucleus accumbens.
Summary
Despite the fact that PPAR research in the context of addiction is relatively new and unexplored, there is already robust evidence supporting an important role of this system in drug abuse (31, 32). Current data point to the possibility that agonism at these receptors may offer a potentially valuable pharmacotherapeutic approach to drug addiction.
Acknowledgments
Part of the description of addictive models has been reproduced with permission from (23). Preparation of this review supported in part by the Intramural Research Program, of the National Institute on Drug Abuse, NIH, DHHS.
References
- 1.American Psychiatric Association. revised version: American Psychiatric Association. Ed. 4. Washington CD: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF. Dopamine, addiction and reward. Seminars in the Neurosciences. 1992;4:139–148. [Google Scholar]
- 4.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. Epub 2004/05/21. [DOI] [PubMed] [Google Scholar]
- 5.Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS medicine. 2006;3(11):e437. doi: 10.1371/journal.pmed.0030437. Epub 2006/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20(1):1–17. doi: 10.1097/FBP.0b013e3283242f05. Epub 2009/01/31. [DOI] [PubMed] [Google Scholar]
- 7.Wise RA. Neuroleptics and operant behavior: The anhedonia hypothesis. Behavioral and Brain Sciences. 1982;5:39–87. [Google Scholar]
- 8.Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132(2–3):337–338. doi: 10.1016/0014-2999(86)90629-1. Epub 1986/12/16. [DOI] [PubMed] [Google Scholar]
- 9.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390(6658):401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 10.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends in Pharmacological Sciences. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 11.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92(26):12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382(6588):255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 13.Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58(4):726–741. doi: 10.1124/pr.58.4.5. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 14.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. Epub 1990/10/18. [DOI] [PubMed] [Google Scholar]
- 15.Lalwani ND, Reddy MK, Qureshi SA, Sirtori CR, Abiko Y, Reddy JK. Evaluation of selected hypolipidemic agents for the induction of peroxisomal enzymes and peroxisome proliferation in the rat liver. Human toxicology. 1983;2(1):27–48. doi: 10.1177/096032718300200103. Epub 1983/01/01. [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. Epub 2002/01/31. [DOI] [PubMed] [Google Scholar]
- 17.van Raalte DH, Li M, Pritchard PH, Wasan KM. Peroxisome proliferator-activated receptor (PPAR)-alpha: a pharmacological target with a promising future. Pharmaceutical research. 2004;21(9):1531–1538. doi: 10.1023/b:pham.0000041444.06122.8d. Epub 2004/10/23. [DOI] [PubMed] [Google Scholar]
- 18.Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav. 2005;81(2):396–406. doi: 10.1016/j.pbb.2005.02.015. Epub 2005/06/01. [DOI] [PubMed] [Google Scholar]
- 19.Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326(2):483–492. doi: 10.1124/jpet.108.138321. Epub 2008/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, et al. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol. 2012;17(1):47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- 21.Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One. 2012;7(1):e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration--comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205(4):613–624. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- 23.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312(3):875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 24.Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, et al. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6(11):e28142. doi: 10.1371/journal.pone.0028142. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, et al. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3’-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008;327(2):482–490. doi: 10.1124/jpet.108.142224. Epub 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2009;60(3):119–125. Epub 2009/10/15. [PubMed] [Google Scholar]
- 27.Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. doi: 10.1016/j.neuroscience.2003.08.064. Epub 2003/12/12. [DOI] [PubMed] [Google Scholar]
- 28.Kainu T, Wikstrom AC, Gustafsson JA, Pelto-Huikko M. Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport. 1994;5(18):2481–2485. doi: 10.1097/00001756-199412000-00019. Epub 1994/12/20. [DOI] [PubMed] [Google Scholar]
- 29.Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131(2):405–418. doi: 10.1016/j.cell.2007.09.012. Epub 2007/10/25. [DOI] [PubMed] [Google Scholar]
- 30.Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, et al. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150(2):707–712. doi: 10.1210/en.2008-0899. Epub 2008/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panlilio LV, Justinova Z, Goldberg SR. Fatty acid amide hydrolase inhibition and activation of peroxisome proliferator-activated receptors: New and complementary approaches to the treatment of cognitive dysfunction and drug dependence. Pharmacology and Therapeutics. 2012 in press. [Google Scholar]
- 32.Mascia P, Tanda G, Yasar S, Heishman SJ, Goldberg SR. Peroxisome proliferator-activated nuclear receptors (PPAR) and drug addiction. In: RG Sorenson, ME Abood, N Stella., editors. endoCANNABINOIDS: Actions at Non-CB1/CB2 Cannabinoid Receptors. New York: Springer; 2012. [Google Scholar]
- 33.Schuster CR, Woods JH. The conditioned reinforcing effects of stimuli associated with morphine reinforcement. International Journal of Addictions. 1968;3:223–230. [Google Scholar]
- 34.Goldberg SR, Kelleher RT, Morse WH. Second-order schedules of drug injection. Fed Proc. 1975;34(9):1771–1776. [PubMed] [Google Scholar]
- 35.Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacological Review. 1976;27:325–340. [PubMed] [Google Scholar]
- 36.Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu Rev Pharmacol Toxicol. 1978;18:313–339. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg SR, Spealman RD, Kelleher RT. Enhancement of drug-seeking behavior by environmental stimuli associated with cocaine or morphine injections. Neuropharmacology. 1979;18(12):1015–1017. doi: 10.1016/0028-3908(79)90169-2. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214(4520):573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- 39.Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112(2–3):163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 40.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153(1):17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 41.Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol. 2005;526(1–3):186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 2002;163(3–4):327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- 43.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 44.Katz JL, Goldberg SR. Preclinical assessment of abuse liability of drugs. Agents and actions. 1988;23(1–2):18–26. doi: 10.1007/BF01967174. Epub 1988/02/01. [DOI] [PubMed] [Google Scholar]
- 45.Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology (Berl) 2006;184(3–4):367–381. doi: 10.1007/s00213-005-0155-8. Epub 2005/10/06. [DOI] [PubMed] [Google Scholar]
- 46.Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12(12):467–473. doi: 10.1016/0165-6147(91)90638-9. Epub 1991/12/01. [DOI] [PubMed] [Google Scholar]
- 47.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134(3483):943–944. doi: 10.1126/science.134.3483.943. Epub 1961/09/29. [DOI] [PubMed] [Google Scholar]
- 48.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 49.Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3(6):1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 50.de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 1983;79(1):29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- 51.Stretch R, Gerber GJ. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can J Psychol. 1973;27(2):168–177. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- 52.Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 2003;168(1–2):31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology. 1996;127:102–107. doi: 10.1007/BF02805981. [DOI] [PubMed] [Google Scholar]
- 54.Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 1997;130(4):396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- 55.McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14(1):99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68(3):265–271. doi: 10.1016/j.biopsych.2010.01.029. Epub 2010/03/20. [DOI] [PubMed] [Google Scholar]
- 57.Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, et al. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2010;13(2):181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- 58.Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol. 2011:1–10. doi: 10.1017/S1461145711001398. Epub 2011/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132(3):289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- 60.Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144(2):183–188. doi: 10.1007/s002130050992. Epub 1999/07/08. [DOI] [PubMed] [Google Scholar]
- 61.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Annals of the New York Academy of Science. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 62.Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, et al. Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology. 2012;37(3):685–696. doi: 10.1038/npp.2011.245. Epub 2011/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 64.Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23(3):135–191. Epub 1971/09/01. [PubMed] [Google Scholar]
- 65.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 66.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–269. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 67.Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology. 2012;37(8):1838–1847. doi: 10.1038/npp.2012.31. Epub 2012/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69(7):633–641. doi: 10.1016/j.biopsych.2010.07.009. Epub 2010/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, et al. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol. 2009;43(6):433–441. doi: 10.1016/j.alcohol.2009.07.003. Epub 2009/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlicht I. Enhancement of voluntary alcohol consumption in rats by clofibrate feeding. Alcohol. 1987;4(3):199–206. doi: 10.1016/0741-8329(87)90043-7. Epub 1987/05/01. [DOI] [PubMed] [Google Scholar]
- 71.Fernandez-Espejo E, Ramiro-Fuentes S, Rodriguez de Fonseca F. The absence of a functional peroxisome proliferator-activated receptor-alpha gene in mice enhances motor sensitizing effects of morphine, but not cocaine. NeuroScience. 2009;164(2):667–675. doi: 10.1016/j.neuroscience.2009.08.023. Epub 2009/08/25. [DOI] [PubMed] [Google Scholar]
- 72.Amoruso A, Bardelli C, Gunella G, Fresu LG, Ferrero V, Brunelleschi S. Quantification of PPAR-gamma protein in monocyte/macrophages from healthy smokers and non-smokers: a possible direct effect of nicotine. Life Sci. 2007;81(11):906–915. doi: 10.1016/j.lfs.2007.07.017. Epub 2007/09/04. [DOI] [PubMed] [Google Scholar]
- 73.Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 2011;69(7):642–649. doi: 10.1016/j.biopsych.2010.12.010. Epub 2011/02/01. [DOI] [PubMed] [Google Scholar]
- 74.Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, et al. Activation of PPAR gamma by the anti-diabetic agent pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in the rat. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12091. in progress. [DOI] [PubMed] [Google Scholar]
- 75.Ciccocioppo R, De Guglielmo G, Melis M, De Luca MA, Li A, Cippitelli A, et al. Activation of PPAR gamma by the anti-diabetic agent pioglitazone reduces opioid reinforcement and opioid-induced actiavtion of the mesolimbic dopamine system. Soc Neurosci Abst. 2012;668:16. [Google Scholar]
- 76.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179(2):366–373. doi: 10.1007/s00213-004-2036-y. Epub 2004/11/20. [DOI] [PubMed] [Google Scholar]
- 77.Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, et al. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36(6):1178–1186. doi: 10.1038/npp.2010.253. Epub 2011/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S. Peroxisome proliferator-activated receptor gamma activation relieves expression of behavioral sensitization to methamphetamine in mice. Neuropsychopharmacology. 2007;32(5):1133–1140. doi: 10.1038/sj.npp.1301213. Epub 2006/10/05. [DOI] [PubMed] [Google Scholar]
- 79.Landreth GE, Heneka MT. Anti-inflammatory actions of peroxisome proliferator-activated receptor gamma agonists in Alzheimer’s disease. Neurobiol Aging. 2001;22(6):937–944. doi: 10.1016/s0197-4580(01)00296-2. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 80.Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Frontiers in bioscience : a journal and virtual library. 2008;13:1813–1826. doi: 10.2741/2802. Epub 2007/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, et al. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 2008;28(51):13985–13994. doi: 10.1523/JNEUROSCI.3221-08.2008. Epub 2008/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, et al. Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol Psychiatry. 2010;68(3):256–264. doi: 10.1016/j.biopsych.2010.04.016. Epub 2010/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107(2–3):285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 84.Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12(5):781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]