Abstract
The purpose of this study was to test the effects of intense tone exposure on the spontaneous activity of multiunit clusters in the mammalian dorsal cochlear nucleus (DCN). Adult hamsters (60-101 days of age) were exposed to a 10 kHz tone at levels between 125 and 130 dB SPL for a period of 4 hours. The effects of tone exposure were studied following a recovery period of 30-58 days and were quantified by measuring the spontaneous rates, response thresholds and frequency tuning properties of neural clusters at the surface of the DCN. Measures were performed at each of 10-15 sites along the tonotopic axis of the DCN. The effects of the tone exposure were examined by comparison with identical measures obtained from normal unexposed animals. Results indicate that tone exposure induced major chronic increases in the spontaneous activity of the DCN. Such increases were broadly distributed across the tonotopic range of the DCN and were generally found in tonotopic map areas characterized by tone-induced elevations of neural thresholds. Mean spontaneous rate reached its maximum value at or close to the tonotopic locus which normally represents the frequency of the exposure tone. The increased activity induced by tone exposure resembled the heightened activity in normal animals during presentation of a moderate level continuous tone. These changes in spontaneous activity indicate that central auditory neurons are in a state of elevated activity for extended periods following intense sound exposure and suggest that the affected neurons may signal the presence of acoustic stimulation even though such stimulation is not present. Possible mechanisms of these changes and their relation to the clinical problem of tinnitus are discussed.
Keywords: Tinnitus, noise-induced tinnitus, noise-induced hearing loss, cochlear nucleus, spontaneous activity, tinnitus mechanisms
INTRODUCTION
Exposure to intense sound causes a number of well-known anatomical alterations in the cochlea such as damage and/or loss of hair cells, reductions of cochlear vessel diameter and damage to the cochlear lateral wall (see reviews of Saunders et al., 1985; Saunders et al., 1991). Such alterations induce changes in the physiology of auditory nerve fibers, the most notable being the loss of acoustic sensitivity and changes in frequency tuning properties. These peripheral disturbances can, in turn, have important higher order effects at central levels of the auditory pathway. Some of these effects have been described previously and include transneuronal degeneration of second-order neurons (Morest and Bohne, 1983), reductions in cell size and number (Hall, 1974, 1976; Sowinski, 1975; Tarmas, 1974), enhancements of excitatory responses (Salvi et at., 1990; Willott and Lu, 1982) and reorganizations of tonotopic maps (Harrison et at., 1991; Robertson and Irvine, 1989; Rajan et al., 1993).
Most physiological studies concerned with the central effects of acoustic trauma have concentrated on changes in acoustic response properties of auditory neurons. In contrast, very little is known about the effects of acoustic trauma on the baseline properties, or spontaneous activity, of central auditory neurons. These latter effects may be clinically important since intense sound exposure often results in tinnitus, a phantom auditory percept which occurs in the absence of acoustic stimulation. Several investigators have suggested that tinnitus may reflect changes in the level of spontaneous activity across a subset of neurons in the auditory system (see review by Jastreboff, 1990). This hypothesis is supported by several findings. For example, salicylate, which is a potent inducer of acute tinnitus, also induces acute increases in spontaneous activity in auditory structures including the auditory nerve (Evans et al., 1981: Evans and Borerwe, 1982; Katahara et al., 1995: Martin et al., 1993; Schreiner and Snyder, 1987; Schreiner et al.. 1990) and inferior colliculus (Jastreboff and Sasaki, 1986). In addition, animals showing salicylate-induced increases in spontaneous activity in the inferior colliculus also appear to experience phantom auditory percepts akin to human tinnitus (Jastreboff and Sasaki, 1986; Jastreboff et al., 1988, 1989). Finally, the increase in spontaneous rate of auditory nerve fibers induced by salicylate treatment is transiently reduced by lidocaine (Schreiner et al., 1990; Lenarz and Schreiner, 1990), a substance known to reduce the severity of tinnitus (Gejrot, 1963; Melding et al., 1978; Martin and Coleman, 1980).
The effects of intense sound, another inducer of tinnitus, have been well-studied at the auditory nerve level. However, most of these studies indicate that intense sound exposure causes a decrease in spontaneous activity, the decrease invariably being found among fibers showing elevated response thresholds (Liberman and Kiang, 1978; Liberman and Dodd, 1984). These peripheral changes cannot easily account for the spontaneous percept of sound that underlies tinnitus. Indeed, many instances have been found in which tinnitus persists or is worsened after auditory nerve transection (Dandy, 1941; House and Brackman, 1981). Other studies indicate that monaural tinnitus often can be masked by contralateral sound at levels below the threshold of interaural cross-talk (Feldman, 1971; Tyler and Conrad-Arms, 1980). These findings suggest that tinnitus involves important mechanisms at central levels of the auditory pathway.
Thus far, only a few studies have considered the effects of intense sound on spontaneous activity in the central auditory system. Salvi (1976) found acute increases in spontaneous activity of neurons in the inferior colliculus following intense noise exposure. Decreases in spontaneous activity were found in the ventral cochlear nucleus following longer term recovery from noise exposure, probably reflecting the decreases seen peripherally (Salvi et al., 1978). Gerken et al., 1984) studied the short- and long-term effects of intense tone exposure on background EEG-like potentials recorded from several brainstem auditory nuclei. These exposures were found to induce decreases in background activity in the ventral and posteroventral cochlear nucleus but increases in activity of the pericentral nucleus of the inferior colliculus. As yet, there is little information concerning the effects of intense sound on the spontaneous activity of neurons in the dorsal cochlear nucleus (DCN). A previous study by Koerber et al. (1966) indicates that DCN neurons retain their spontaneous activity following cochlear removal. A view of their data suggests that partial cochlear removal may produce changes in the interspike intervals of spontaneous activity in the DCN (Koerber et al., 1966, Fig. 2a-b). These findings suggest that acoustic injury might cause changes in the spontaneous discharge rates of DCN neurons.
In the present study we measured the long term effects of intense tone exposure on the spontaneous activity of the DCN. The level of the exposure was adjusted to induce hair cell damage to the basal half of the cochlear partition. Measures of spontaneous activity were combined with studies of neural tuning properties using a method employed previously to map the tonotopic organization of the DCN in normal and tone-exposed animals (Kaltenbach and Lazor, 1991; Kaltenbach et al., 1992a). This approach allowed us to pinpoint tone-induced changes in spontaneous activity relative to well-defined tonotopic coordinates. The results indicate that tone exposure consistently produced major changes in the spontaneous activity of the DCN.
METHODS
Treatment Groups
Animal subjects were Syrian Golden hamsters (LVG strain) obtained from Charles River Laboratory. The chronic effects of sound exposure were assessed by comparing recordings from 2 animal groups: the first group consisted of unexposed normal animals; the second group consisted of animals studied 30-58 days after exposure to an intense tone.
Tone Exposure
Conditions for intense sound exposure were similar to those described previously (Kaltenbach et al., 1992b). Tone exposures were carried out in animals anesthetized by intramuscular injection of ketamine (26mg) and xylazine (4mg). Each anesthetized animal was mounted in a head brace and exposed to an intense 10 kHz tone. Tone exposure was conducted in a double walled IAC booth. Body temperature was maintained thermostatically at 37°C. The tone was delivered through a Beyer DT-48 transducer coupled to the left ear by a plastic speculum. A probe tube microphone (Etymotic ER-7C), placed at the entrance to the ear canal, was used to measure the tone level throughout the exposure period. The tone was presented at a level of 125-130 dB SPL for a period of 4 hours, conditions which have been shown to cause well-defined hair cell lesions in the basal half of the hamster cochlea (Kaltenbach et al., 1992b). The animals were exposed at 60-101 days of age. Exposed animals were returned to the animal holding facility for a recovery periods of 30-58 days, after which electrophysiological recordings were carried out in the DCN.
Recording System
Following the postexposure recovery period, animals were prepared surgically for electrophysiological recordings. Again, the animals were anesthetized by intramuscular injection of ketamine (26mg) and xylazine (4mg). A tracheotomy and parieto-occipital craniotomy were performed, and a portion of the cerebellum was aspirated to expose the left DCN. Micropipette electrodes were pulled on a Brown-Flaming puller and filled with 0.3 M KCl. Tip impedances measured between 0.1 and 0.3 megohms. The electrode was mounted on an hydraulic microdrive, then manually positioned just above the DCN. Further movement of the electrode was controlled remotely using a Narashige XYZ micromanipulator. A video camera, mounted on the microscope, provided output to a video screen which displayed the electrode over the DCN surface. The output from the electrode was passed through a preamplifier (1000X, filter bandwidth = 300 - 10,000 Hz) then channeled outside the acoustic booth to an oscilloscope, audio monitor and level discriminator. The output of the level discriminator consisted of negative voltage pulses which were fed to a universal counter and an 8 bit input port which served as an interface to a Compaq 386/125 computer. The universal counter was used for counting spontaneous neural voltage events while the computer was used to control a program customized for tonotopic mapping. Counts were read directly off a digital readout on the face of the universal counter. Acoustic feedback to the electrode from the stimulus system was eliminated by shielding the transducer with aluminum foil and connecting the foil to ground. Neurophonic responses were not seen in the frequency range over which neural responses to acoustic stimuli were tested.
Tonotopic Mapping
Tonotopic mapping was carried out using the same video display system employed in previous studies (Kaltenbach and Lazor, 1991; Kaltenbach et al., 1992b). The camera mounted onto the overhead microscope provided a means for viewing both the DCN and the recording electrode during each experiment. Recordings were obtained at each of 10-15 sites along the tonotopic axis of the DCN. These sites were labeled on a transparency which included a drawing of principal DCN boundaries for each animal. In most animals, the tonotopic axis was found to be parallel to the medial-lateral axis of the DCN (Kaltenbach and Lazor, 1991). The electrode was lowered until contact was made with the DCN surface. This contact was evident visually on the TV monitor displaying the DCN and in the sudden appearance of multiunit activity on the oscilloscope. The level discriminator was then adjusted to trigger on spontaneous neural voltage events that exceeded −100 millivolts. Examples of output pulses from the level discriminator and their relation to the neural voltage waveform are shown in Figure 1. The onset of each pulse was triggered each time a neural voltage event exceeded −100 mV while the pulse offset occurred when the neural voltage fell back below −100 mV. Once adjusted, the trigger level was maintained throughout the recording session, and was chosen in order to capture only the tallest voltage events in the field of activity in normal animals and to avoid artifactually high counts in exposed animals due to the higher fluctuations in electrical baseline. At each recording site, a spectral response plot was obtained. The method for generating the spectral response plot has been described previously (Kaltenbach and Saunders, 1987). Briefly, the method tested the response of a neuron or neuron cluster to each of 800 different frequency/intensity combinations. For each combination, the response was recorded by computer and plotted as a vertical bar whose height was proportioned to the number of events collected during the steady state interval of stimulation. Responses to all 800 stimuli were displayed in real time on a graph that spanned a frequency range of 3-32 kHz and an intensity range of 6-96 dB SPL. The response area was apparent in such graphs as the frequency and intensity range over which bar heights were consistently elevated above the general background. The spectral response plot was used to measure response thresholds as defined by the low intensity boundary of the response area. Generally, the lower edge of the response area was characterized by bar heights which were judged visually to be at least 100% greater than those in the background. Threshold measures were usually obtained at the characteristic frequency (CF), defined as the frequency of lowest response threshold. In some cases wherein spontaneous activity was very high or the response area was very distorted due to the tone exposure, CFs were indeterminate, and the lowest response threshold was measured irrespective of whether a sharply defined tip was apparent. Response areas were collected from each of the 10-15 recording sites, and CFs mapped on a drawing of the DCN surface. Maps in normal animals provided a frame of reference for localization of recordings of spontaneous activity as described in the Results section.
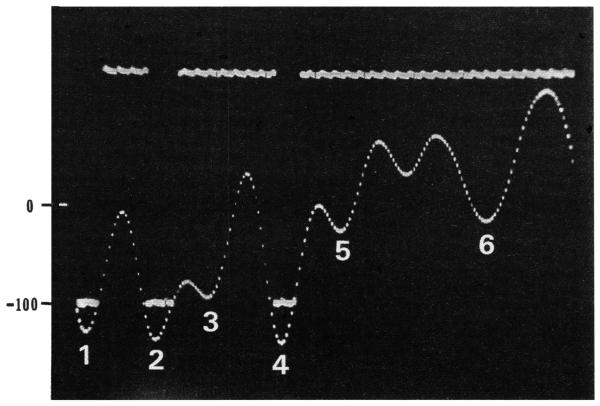
FIGURE 1.
Oscillographic trace showing an amplified neural waveform characterized by 3 successive voltage events that exceeded −100 mV. The corresponding level discriminator output pulses are show by horizontal bars at the −100 mV criterion level. Note that the trigger of the level discriminator was adjusted until its output pulse width exactly matched the width of the neural waveform at −100 mV (see peaks 1, 2 and 4). Neural voltage events under −100 mV (e.g., peaks 3 and 5-6) did not produce output pulses. The 0 and −100 mV levels are shown by numbers on the left. The full width of the trace spans 2 msec.
Recordings of Spontaneous Activity
At each recording site, measures of neural tuning were followed within a few minutes by measures of spontaneous activity without moving the electrode. Trains of neural events were converted to pulse trains which were then relayed to the universal counter. The number of pulse events was counted over a period of 90 seconds and converted to a spontaneous rate in counts/second. This procedure was repeated until spontaneous rates were measured at each of the 10-15 recording sites. In some animals, photographs were taken of representative traces showing the appearance of multiunit activity at the 10 kHz locus of the DCN.
Cochlear Histology
Following recordings, each animal, while still under anesthesia, was decapitated. The temporal bone was removed and the cochlea was perfused through the round and oval windows with 3% glutaraldehyde. The tissue was postfixed in OsO4 for 30 minutes. The cochlea was dissected into apical and basal segments in distilled water. Postfixation in OsO4 was followed by two alternate exchanges between thiocarbohydrazide and OsO4 followed by rinsing in distilled water. Cochleas were dehydrated in acetone and critical-point dried. Cochleograms were generated employing a system in which each hair cell was examined with the SEM and ranked according to the percent survival of its tallest row of stereocilia (Kaltenbach et al., 1992b). Photomicrographs, taken along the entire length of the cochlea at a magnification of 100X, were assembled into a montage. The image of the organ of Corti in this montage was then divided at the level of the spiral limbus with tic marks into numbered bins 1 cm in width. This montage served as a guide to locate the boundaries of specific bin numbers when the organ of Corti was studied at 2000X magnification. At this magnification, each hair cell was ranked on a scale of 0-4 according to the following stereocilia/hair cell survival criteria: grade 4 was given to hair cells with a normal or near-normal tuft of stereocilia (90-100% stereocilia survival), and grade 1 was given to cells with either complete or nearly complete loss or fusion of their tallest stereocilia (0-9% stereocilia survival). A grade 0 was assigned to bins in which hair cells were missing and their positions taken over by neighboring Dieter’s cells, Hensen’s cells or Claudius’ cells. The average grade for all hair cells in a given bin were computed and plotted as a bar whose height was proportioned to the hair cell grade designated on the vertical scale. Plots of bar height for all bins showing the condition of hair cells along the entire length of the cochlea were presented as cochleograms for each of the four rows of hair cells.
Data Analysis and Evaluation
Spontaneous rates, expressed in counts/second, were plotted as a function of distance relative to the 5 kHz isofrequency contour line on the surface of the DCN for each animal. This contour typically lay within 25% of the medial distance from the lateral margin of the DCN surface as viewed dorsally. The topographic width of the DCN spanned by the full range of recording sites from all animals was then divided into 13 bins, each representing 0.01 mm of tissue. All spontaneous rates falling within a given bin were pooled both within and across animals and used to calculate an average rate expressed in counts per second. Comparisons between the two groups were made by plotting the mean rate of spontaneous activity ± 1 S.E.M. The effects of tone exposure were evaluated by computing the differences between mean spontaneous rates and CF thresholds in tone-exposed and normal animal groups for each of the 13 bins.
RESULTS
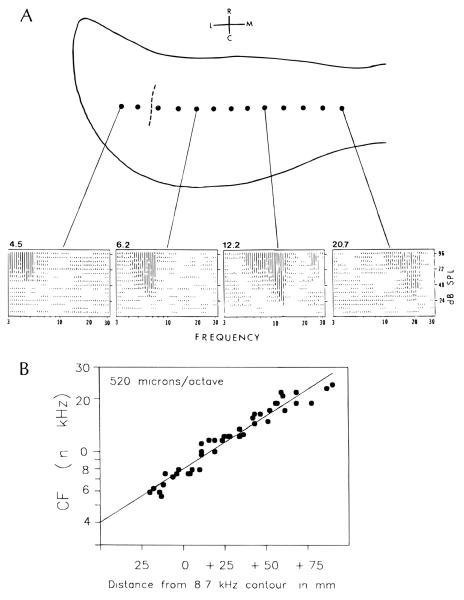
The results presented here are based on recordings from 13 animals, 6 normal and 7 tone-exposed. In all these animals, spontaneous activity and responses to tones were recorded directly at the surface of the DCN. The locations of recording sites relative to the principal DCN boundaries, along with representative response areas from selected sites in a normal animal, are shown in Figure 2. The response areas show the systematic increase in CFs as the electrode was moved toward the medial direction (numbers above graphs in Figure 2A). A plot of CF versus distance showing the topographic representation of frequency based on data pooled from 3 normal animals is shown in Figure 2B. In tone-exposed animals, the original CFs above 7 kHz could not be easily discerned because response thresholds were severely elevated and were commonly accompanied by major alterations of frequency tuning properties. For this reason, the locations of all recording sites, both in normal and tone-exposed animals, were mapped topographically rather than tonotopically. This mapping was achieved by measuring distance to each site from the 5 kHz isofrequency contour line (see dashed line in top panel of Figure 2A). The CFs normally represented at each topographic position in exposed animals were inferred from the regression line for normal animals in Figure 2B which sloped at a rate of 0.52 mm of tissue per octave.
FIGURE 2.
A. Spectral responses obtained from selected locations along a single medial-lateral row of 13 recording sites on the surface of the hamster DCN. Note the increase in CFs (represented by the numbers at the top left of each graph) toward the medial direction. The dashed line represents the 5 kHz isofrequency contour which served as a reference for topographic mapping. B. The tonotopic gradient along the medial-lateral axis of the DCN. The data were pooled from 3 consecutively recorded animals. The regression line represents the best fit to the data points shown, and provides a basis for inferring tonotopic coordinates in tone-exposed animals at locations where CFs were otherwise indeterminate. The number in the upper left inside the graph denotes the slope of the regression line expressed in microns/octave of tissue. Abbreviations in A: M, medial; L, lateral; R, rostral; and C, caudal
Multiunit Events in Normal and Tone-Exposed Animals
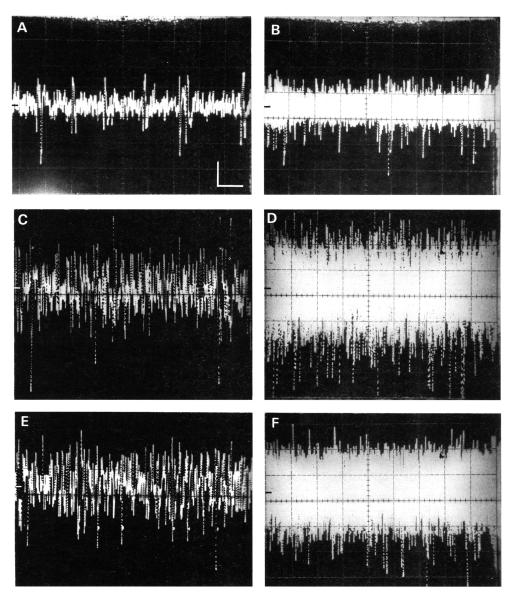
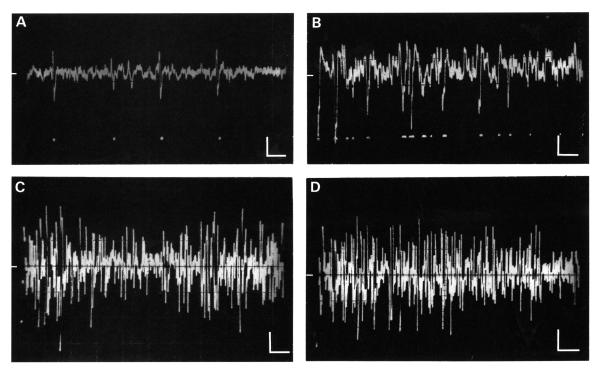
A comparison is presented in Figure 3 between trains of spontaneous events in a normal. unexposed animal (panels A and B and those obtained from a corresponding location in a tone exposed animal (panels C and D). Representative single traces are shown on the left side of the figure while the envelopes of multiple overlapping sweeps are shown on the right. This figure illustrates two features which were typical of spontaneous events in exposed animals. First, the amplitudes of those events were generally larger than those recorded in normal animals. The amplitudes of most voltage events in the normal animal (panel B) were under 150 mV peak-to-peak, with only an occasional event reaching 200 mV. In contrast, the amplitudes of most events in the tone-exposed animal (panel D) were over 150 mV peak-to-peak, and a sizeable fraction of events exceeded 200 mV. Second, tone-exposed animals generally showed higher spontaneous rates than did normal animals. This increase in rate was evident in the relatively denser field of spontaneous events in traces recorded from exposed animals (compare Figures 3A and 4A with Figures 3C and 4B, respectively). In addition, there was a tendency for neuron clusters in exposed animals to generate more frequent burst-like barrages of events. The edges of these barrages were captured in some oscillographic traces (Fig. 4), and appeared as spindles of high amplitude activity which were either separated (panel C) or followed by (panel D) intervals of lower amplitude activity.
FIGURE 3.
Oscillographic traces showing multiunit spontaneous activity in representative normal (A-B) and tone-exposed (C-D) animals. E-F show activity from the same animal represented in A and B but during the presentation of a continuous 10 kHz tone that induced a vigorous response. All recordings were obtained from the portion of the DCN which normally represents 10 kHz. Traces on the left show single sweeps while traces on the right show multiple sweeps (B and D: 20 sweeps; F: 10 sweeps). In each frame, the time axis was 10 ms/div and the amplitude axis was 50 mV/div, as indicated by the calibration bars in A. The 0 voltage line is shown by the bar along the middle of the left margin of each panel.
FIGURE 4.
A-B. Spontaneous activity characterized by voltage events from only a few different neurons. A. A normal animal; B. A tone-exposed animal. The higher incidence of voltage events shown in B was typical of records from tone-exposed animals. C-D. Spontaneous multiunit activity in a tone-exposed animal. In C, the voltage events occurred in spindle-like bursts; in D the burst of activity was followed by an interval of low amplitude activity. Recordings in A and B were obtained with a higher impedance electrode than those in C and D. In each frame the time axis was 10 ms/div and the amplitude axis 50 ms/div, as indicated by the calibration bars. The 0 voltage line is shown by the bar along the middle of the left margin of each panel.
Comparison of Spontaneous Activity in Tone-Exposed Animals with Tone-Evoked Activity In Normal Animals
One might initially interpret the higher voltage amplitudes of events in tone-exposed animals as suggesting that the exposure caused a direct increase in the magnitude of spikes from a given set of neurons. Alternatively, high amplitude voltage events might emerge as a result of a tone-induced recruitment of neurons. The latter possibility predicts that a similar emergence of higher amplitude voltage events should occur in normal animals when neurons are induced to fire in response to sound. For this reason we compared trains of spontaneous events from tone-exposed animals with trains obtained from normal animals before and during presentation of a continuous CF tone sufficient in level to evoke a vigorous response. The results of this test are shown in Figure 3E-F. Again, spontaneous activity from the normal, unexposed animal before stimulation (Figs. 3A and 3B) was characterized by voltage events whose amplitudes generally fell below 100 mV peak-to-peak. In contrast, when a continuous CF tone was presented, the trains of events from the same neuron cluster showed amplitudes which reached between 200 and 300 mV (Fig. 3E-F). The latter traces resembled those showing spontaneous activity in tone-exposed animals (Fig. 3C-D). Curiously, presentation of a high level tone to the exposed animal in Figure 3 did not evoke further increases in voltage amplitude than was characteristic of spontaneous activity (data not shown). These comparisons demonstrate that the emergence of high amplitude voltage events qualitatively similar to those seen in tone-exposed animals, can also be evoked in normal animals by recruitment of activity using a continuous tone. They further suggest that the amplitude of spontaneous activity in some exposed animals may already be near their maximal achievable levels.
Quantitative Analysis of Spontaneous Activity in Normal and Exposed Animals
Although spontaneous activity in tone-exposed animals appeared to have higher amplitudes as well as higher rates, it was apparent that the increased rate was dominant over higher amplitude. Not only did changes in rate appear more dramatic, but they varied more with position. In contrast, voltage event amplitudes were similar across most of the tonotopic region characterized by elevated activity, despite the fact that rates of event occurrence were quite variable. For these reasons, we chose to use measures of multiunit event rate rather than amplitude to map the effects of tone exposure on spontaneous activity. Simple spike-like events as well as multiple events composing more complex waveforms that may have resulted from temporal overlap between successive spike events were counted if they exceeded −100 mV (see Fig. 1). The results of these counts were expressed as event rates and are presented in Figures 5 and 6. Raw data plots for normal, unexposed animals are presented in Figure 5A, while their average rates are plotted in Figure 5B.
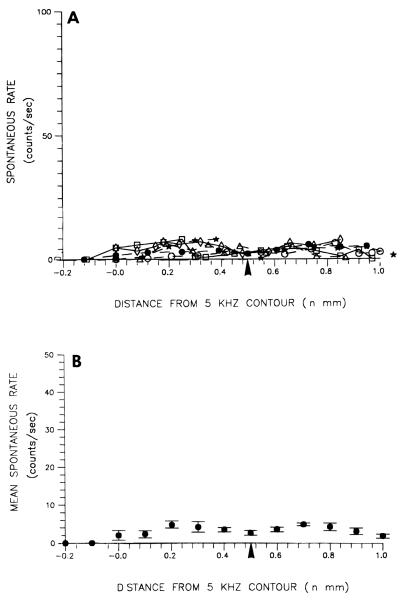
FIGURE 5.
Topographic distribution on the DCN surface of multiunit spontaneous rates in control animals. A. Raw spontaneous rates for all 6 animals. B. Spontaneous rates averaged across all 6 control animals. Each point in (B) represents the mean ± S.E.M. of the 6 animals represented in A. The 5 kHz contour marks the location along the tonotopic axis of the DCN where neurons had characteristic frequencies of 5 kHz. The arrowhead under the abscissa in each graph marks the location of neurons tuned to 10 kHz.
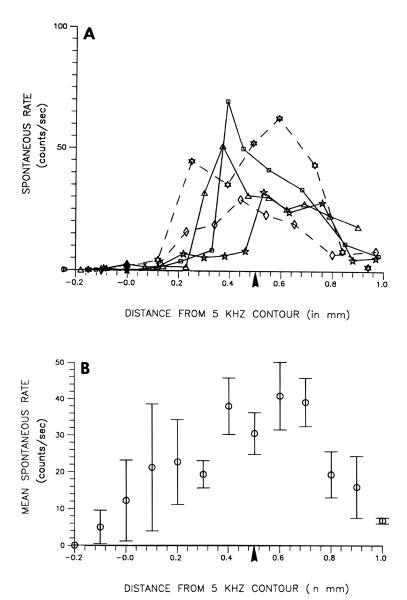
FIGURE 6.
Topographic distributions on the DCN surface of multiunit spontaneous rates in tone-exposed animals. A) Raw spontaneous rates for 5 representative animals. B) Spontaneous rates averaged across all animals. Each point in (B) represents the mean ± S.E.M. of 7 animals. The 5 kHz contour marks the location along the tonotopic axis of the DCN where neurons had characteristic frequencies of 5 kHz. The arrowhead under the abscissa in each graph corresponds to the relative locations of neurons tuned to 10 kHz in normal animals (see panel B in Fig. 2).
At this trigger level, spontaneous rates did not exceed 10 counts per second in normal animals, and in the majority of recordings, seldom exceeded 5 counts per second. Average rates in normal animals varied between 4 and 5 counts per second except near the medial and lateral extremities of the DCN where rates lower than 2 counts per second were recorded.
The raw data plots for 5 representative tone-exposed animals and average event rates for all 7 tone-exposed animals are presented in Figure 6A and B, respectively. In general, the data proved to be much more variable in this group than in normal animals. Nevertheless, the raw data (Fig. 6A) revealed that tone exposure induced major increases in spontaneous rates in most cases. Of 7 tone-exposed animals examined, 5 showed rates which were at least 10-fold higher than normal; two others showed rates that were more than 7 times higher than normal. In the 5 examples shown, the maximal rates were found at or near the middle of the tonotopic range representing frequencies within a quarter octave of the exposure tone.
The average spontaneous rates for all 7 tone-exposed animals (Fig. 6B) showed a systematic increase toward the middle of the DCN. The peak spontaneous rate in this region averaged 42 counts per second at a location of 0.6 mm medial to the 5 kHz isofrequency contour line (Fig. 6B). This value contrasted with only 4.2 counts per second at the corresponding location in normal animals (Fig. 5B). The location of maximal rate in exposed animals corresponded tonotopically to the 12 kHz isofrequency contour line in normal animals (derived from regression line in Figure 2B). Thus, the maximal average spontaneous rate was a full order of magnitude higher than that seen at the corresponding position in control animals, and occurred in a portion of the tonotopic map close to the frequency (10 kHz) of the exposure tone.
Relation between Changes In Spontaneous Rate and Threshold
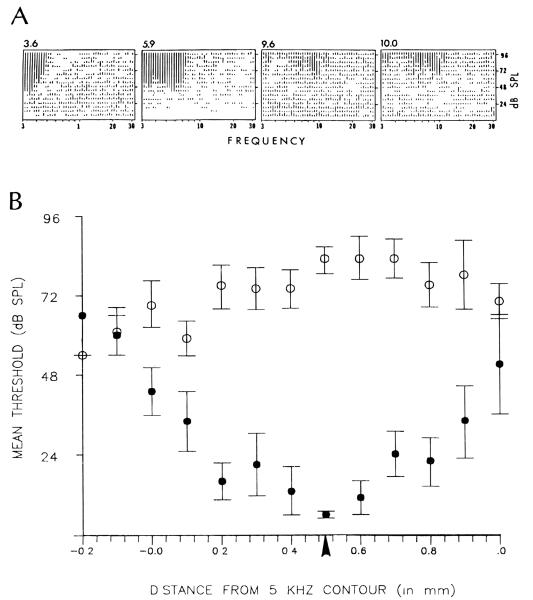
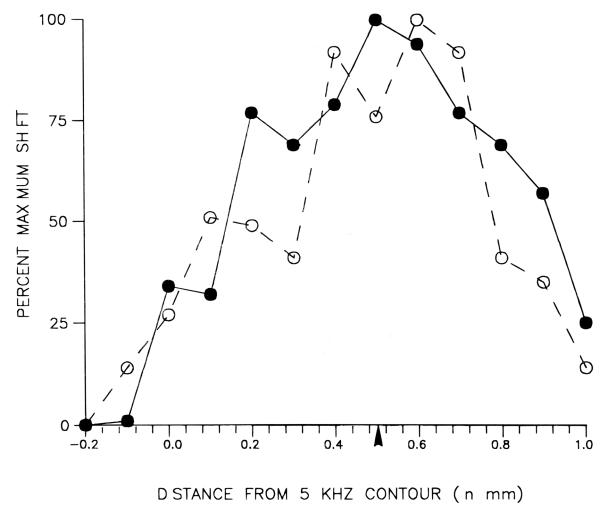
Since intense tone-exposure also is known to cause an increase in response threshold, we wished to determine whether the increase in spontaneous rate was correlated with the degree of threshold shift. Indeed, threshold shifts were a consistent finding in our sample of tone-exposed animals. Examples of response areas collected from a single tone-exposed animal are presented in Figure 7A. These examples were selected to show the characteristic elevation of response thresholds following exposure; although the spontaneous activity in these examples was elevated above the mean normal levels, the responses to sound were sufficiently strong to stand out well above this elevated background activity. Figure 7B compares the average threshold curve for normal animals with that of tone-exposed animals. The normal curve (filled circles) is a broad U-shaped function of distance with the lowest thresholds found in the mid-frequency region of the DCN. The thresholds for tone-exposed animals showed dramatic increases above normal values, especially in the mid-frequency region of the DCN near the 10kHz isofrequency band (see arrow below abscissa). In the extreme low and high frequency regions, thresholds in tone-exposed animals were closer to those obtained from normal animals. Both the total average shift in spontaneous activity and the average shift in threshold are plotted versus tonotopic position in Figure 8. The two curves were normalized by computing the shifts as a percent of the maximal shift for each parameter. The data show that the mean increase in spontaneous rate approximately paralleled the mean threshold shift, although it should be noted that in individual animals the relationship between spontaneous rates and response thresholds was not always predictable.
FIGURE 7.
Representative spectral response areas collected along the medio-lalteral axis of the DCN of a single tone-exposed animal. Graphs are arranged from the lateral to the medial direction of the DCN toward the right. Response area tip thresholds became severely elevated as the electrode was moved toward the medial direction. Numbers above the graphs represent the characteristic frequencies. B. Comparison of the topographic distributions of mean neural thresholds in control (filled circles) and tone-exposed (open circles) animals. Each point represents the mean ± S.E.M. from the same group of 6 normal or 7 tone-exposed animals that were analyzed in Figures 5 and 6. Thresholds were measured at the characteristic frequency or, in cases wherein well-defined response area peaks were absent due to tone-exposure, at the lowermost boundary of the response area. The arrowhead under the abscissa corresponds to the location along the tonotopic axis of the DCN where neurons were tuned to 10 kHz in normal animals (see panel B in Fig. 2). Vertical scale for bar heights are not equal for all 4 graphs in panel A.
FIGURE 8.
Comparison of average shift of spontaneous activity (open circles) in tone-exposed animals with average shift of neural threshold (filled circles), in the same animals, as a function of distance along the tonotopic axis. The comparison is normalized by representing shifts as a percent of maximal values. Each point represents the difference between the mean values from 7 tone-exposed animals and 6 control animals. Thresholds were measured at the characteristic frequency or, in cases wherein well-defined response area peaks were absent due to tone exposure, at the lowermost boundary of the response area. The arrowhead under the abscissa corresponds to the location of neurons tuned to 10 kHz in control animals.
Cochlear Pathology of Tone-Exposed Animals
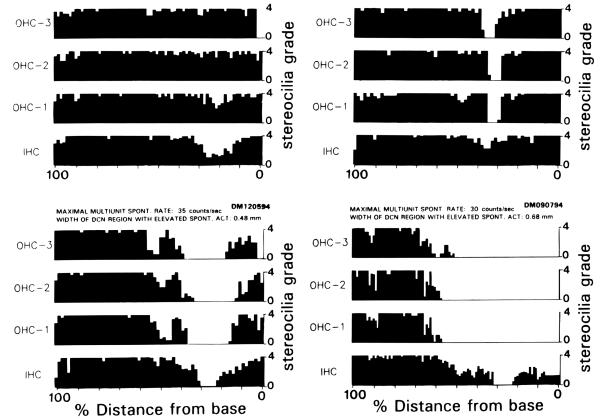
Six of the animals showing tone-induced increases in spontaneous activity were analyzed histologically to evaluate the type and extent of cochlear damage. The results of this analysis are presented in Figure 9 for 4 representative animals. Each cochleogram plots the condition of hair cells as a function of % distance from the base to the apex of the cochlear spiral. The 4 examples in the figure were selected to show the range of lesion patterns observed in our sample of tone-exposed animals. They are arranged in order of severity of outer hair cell damage. Although each of these examples was characterized by damage to both inner (IHCs) and outer hair cells (OHCs) in the basal half of the cochlea, one of these animals (DM121694) showed a punctate lesion characterized by complete loss of OHCs over a narrowly defined portion of the cochlea while IHCs remained intact, albeit damaged; some of the charactenstics of this lesion can be appreciated more fully by inspection of the photomicrographs in Figure 10. Two other animals (DM120594 and DM090794) showed more severe lesions characterized by a patch of completely missing IHCs and OHCs skirted by broader areas of damaged but intact hair cells. On both of these animals, damage to OHCs was more severe and/or more broadly distributed than damage to IHCs. The fourth animal (DM091994) showed major damage only to IHCs and 1st row of OHCs while the second and third row OHCs were only marginally damaged.
FIGURE 9.
Cochleograms plotting the condition of hair cells as a function of distance from the cochlear base (right side of graph). Hair cell condition was based on the grade of the stereocilia bundles as described in Methods. A grade of 4 represents a full tuft of stereocilia while a grade of 0 represents complete or nearly complete loss of the stereocilia bundle and/or hair cell. Spontaneous activity measures are indicated above each set of 4 histograms. The numbers above each histogram represent the maximal counts/second and width of DCN within which spontaneous rates were above the control average (i.e., > 10 counts/sec).
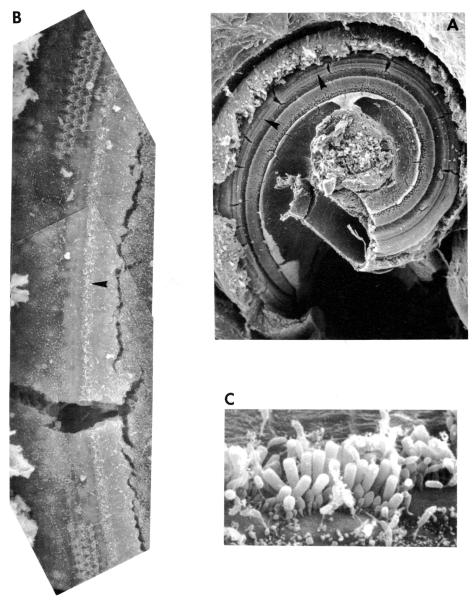
FIGURE 10.
A representative example of the type of injury sustained by the cochlea (DM121694) following tone exposure. Injury was characterized by a region in the basal turn, bracketed by arrows in A, shown at higher magnification in B, over which outer hair cells were either partially or completely missing but inner hair cells remained intact. B. Micrograph in C illustrates condition of intact inner hair cells at locations shown by arrow in B.
In attempting to assess the relationship between the pattern of cochlear pathology and change in spontaneous activity in exposed animals, attention should be directed to the numbers above the cochleograms in Figure 9 which show the maximal spontaneous rates and the spatial width of the DCN region characterized by above-average spontaneous rates (ie., >10 counts/second) recorded from each corresponding animal. In viewing the differing cochlear widths of the lesions for these 4 animals, it is apparent that these variations were not associated with equivalent variations in the widths of DCN areas with elevated spontaneous activity. There was also no proportional relationship between the magnitude of the hair cell lesion and the maximal spontaneous rates. For example, the animal which had the least amount of OHC loss (DM091994) showed the highest maximal spontaneous rate (68 counts/second), whereas the animal which had the most amount of OHC loss DM090794) had the lowest maximal rates of spontaneous activity (30 counts/second) among our tone-exposed animals. Similarly, there was no proportional relationship between the magnitude of spontaneous rates and the amount of IHC loss. The animals having the most severe and least severe IHC lesions (DM090794 and DM121694. respectively) had similar maximal rates (30 counts/second and 33 counts/second) and spatial spreads (0.68 mm and 0.48 mm) of elevated spontaneous activity. These results demonstrate that, while elevations of spontaneous activity may be associated with stereocilia damage, neither the degree of stereocilia damage nor the total amount of hair cell loss correlated directly with the magnitude of changes in spontaneous activity in the DCN. However, this finding should be interpreted with caution since our sample was very small.
DISCUSSION
The results presented in the preceding section demonstrate that high intensity tone-exposure produces major chronic increases in multiunit spontaneous activity in the hamster DCN. The degree to which mean spontaneous rates were increased was correlated with the mean shift in response threshold, the highest mean rates occurring in the portion of the DCN having the highest threshold shift and approximating the tonotopic map areas which normally had CFs near the frequency of the exposure tone. These increases in spontaneous activity were consistently associated with cochlear pathologies, although there was no simple correlation between the level of spontaneous activity or threshold shift and the magnitude of the cochlear lesion. Interestingly, dramatic increases in spontaneous activity occurred in some animals showing only mild injury, involving damage to the stereocilia bundles, but the hair cells remained otherwise intact. Thus, it is apparent that the increase in spontaneous activity at the DCN level can be triggered by very subtle changes in the condition of the cochlear transducer without necessarily involving degeneration of hair cells or auditory nerve fibers
Origins of Changes in Spontaneous Activity
These results differ substantially from previously described effects of noise on the spontaneous activity of auditory nerve fibers. Whereas the DCN showed chronic increases in spontaneous activity following tone exposure, comparable studies at the auditory nerve level have shown that intense sound exposure causes a chronic decrease in mean spontaneous rates (Liberman and Kiang, 1978; Liberman and Dodds, 1984). In one of these investigations, the amount by which average spontaneous rates were reduced in the auditory nerve was found to be correlated with the amount of damage to the IHC stereocilia tufts (Liberman and Dodds, 1984). A similar study by Salvi and Ahroon (1983) also examined the long term effects of noise exposure on spontaneous unit activity of the auditory nerve. However, their study showed no significant change across the population of units sampled. The 10-fold increase in spontaneous activity of DCN neurons following long term recovery from intense sound exposure thus has no known counterpart at the peripheral level and thus probably does not represent a simple relay of changes expressed in individual auditory nerve fibers.
The tone-induced changes we have observed in the DCN may explain a discrepancy that has been identified in previous studies of brainstem auditory nuclei following intense sound exposure. Intense sound exposure has been found to cause a chronic decrease in background EEG-like activity at the level of the ventral cochlear nucleus (VCN), but a chronic increase in this activity at the level of the inferior colliculus (IC) (Gerken eral., 1984). Since the IC receives direct input from the DCN, we suggest that the increase in background activity in the IC may reflect changes originating in the DCN. In contrast, the induced decrease in background activity seen in the VCN probably reflects changes originating in the auditory nerve. The fact that the DCN does not reflect these peripheral effects is consistent with results of a previous study showing that, unlike the VCN, the spontaneous activity of the DCN is not dependent on input from the auditory nerve (Koerber et al., 1966).
Neural Substrates underlying Increases in Multiunit Activity
What changes do the increases in spontaneous multiunit activity following tone exposure represent at the neuronal level? Although we cannot yet give a definitive answer to this question, a clue concerning what changes might be expected at the single unit level may be gained by considering the data in Figure 3. A similarity was found between the observed increases in spontaneous activity induced by intense sound (panels C and D) and the increase in activity observed in normal animals during presentation of a continuous acoustic stimulus (panels E and F). It as well known that acoustic stimulation causes increases both in the number of active neurons and in the rates of discharge of individual neurons. If these changes account for the increased multiunit activity seen during stimulation in normal animals, then it is reasonable that the increased spontaneous activity induced by tone exposure might likewise reflect increases in neuronal discharge rates and/or numbers of active neurons. Increases in the number of voltage events generated by neurons would also increase the opportunities for temporal overlaps and summation of spike events from different neurons; this summation could produce an enhancement of voltage amplitudes at the recording site, such as those demonstrated in Figure 3C-F. Since trains of multiunit events in tone-exposed animals showed increases in both rate and amplitude, it is also possible that at least part of the increase in event rate might have been an artifact of a change in spike amplitudes of DCN neurons rather than an increase in neuronal discharge rate, per se. Such increases might be expected if the initial lesion results indirectly in a change in membrane conductance or in the electrical insulation of central target neurons. Both types of change have been observed in somatosensory nerves following peripheral injury, and has been linked to degeneration of the myelin sheath (see review of Devor, 1994; Devor et al., 1993). Although degeneration has been reported previously in the DCN following noise exposure (Morest and Bohne, 1983), such changes were usually found only after profound damage to the cochlea involving loss of hair cells and degeneration of auditory nerve fibers. In contrast, loss of hair cells was not a consistent finding in the tone-exposed animals with increases in spontaneous activity in the present study. Thus, although the possibility of increases in single neuron spike amplitude cannot be ruled out entirely, our data do not provide any clue as to how such increases could occur. Systematic studies are now in progress to test the amplitudes and rates of single unit discharges directly.
Other factors which could contribute to changes in spike amplitudes include neurophonic potentials and voltages that are presynaptic to the recorded neurons. Phase-locked neural activity can give rise to a neurophonic response when low-frequency stimuli are presented at high sound levels, and this might be suggested as a possible basis of the higher amplitude potentials that were recorded in normal animals during stimulation (Fig. 3E-F). However, this explanation is dubious since phase-locked responses are generally restricted to frequencies below 5 kHz. In contrast, the increased activity typified by the example in Figure 3E-F was observed in the presence of a 10 kHz tone, well outside the range in which neurophonic responses would be expected. Presynaptic potentials have been reported as a component of extracellular recordings. For example, extracellular recordings from the VCN may include prepotentials which are thought to represent the presynaptic input from the auditory nerve (Bourke, 1976). However, these prepotentials are generally very low in amplitude and are thought to reflect the unusually large size of the auditory nerve axon terminals (i.e., the end bulbs of Held). It is dubious that prepotentials are a significant component of our multiunit signals since the DCN does not possess large synaptic inputs analogous to the end bulbs of Held, and to our knowledge, no evidence has been reported previously that presynaptic potentials can be recorded in the DCN using the methods applied in the present study
Possible Mechanisms Underlying Increased Spontaneous Activity
There are several mechanisms which could mediate an increase in spontaneous activity following tone exposure. One possibility is that the observed increases might result from a reorganization or expansion in the functional connections of the DCN. The potential for reorganization following peripheral injury has been demonstrated in several subcortical sensory pathways and may involve a number of different alterations, including expansions in the size of topographic map areas representing a given sensory input, increases in the number of synapses, changes in the shapes of receptive fields, and modulations in the number and/or sensitivity of neurotransmitter receptors (see review of Snow and Wilson, 1991). The DCN may share the potential for one or more of these modes of reorganization. However, if reorganization occurs following tone-exposure, it is not expressed as a plasticity of the tonotopic map of the DCN. In none of the animals examined in this study was there any indication that particular regions of the tonotopic map characterized by normal thresholds were expanded. We have previously presented evidence that tonotopic maps of the DCN of some tone-exposed animals show expanded map areas, but those areas are always characterized by distorted frequency tuning curves with elevated thresholds, changes which are normally ascribed to alterations at the peripheral level (see Kaltenbach et al., 1992a; Kaltenbach et al., 1996). A second possibility is that increased spontaneous activity in the DCN following tone exposure results from shifts in the balance of excitation and inhibition in the DCN due to loss of normal input. A similar explanation has been cited as a possible basis for noise-induced hyper-responsiveness to sound in the inferior colliculus (Gerken et al., 1984; Salvi et al., 1990; Willott and Lu, 1982). Cochlear injury might bring about the expected reduction of spontaneous activity in the auditory nerve as demonstrated previously (Liberman and Dodds, 1984), but this reduction might diminish tonic drive to inhibitory interneurons resulting in a disinhibition of their target cells. Such a mechanism could be triggered by reduced spontaneous activity in either the type I auditory nerve fibers originating from IHCs or type II auditory nerve fibers originating from OHCs. However, if such a mechanism is involved, it probably is reflected at a more subtle level than we were able to judge by looking only at the presence or absence of stereocilia. Previous studies have, in fact, shown that there does not appear to be a tight link between the degree of hair cell damage and the degree of physiological change, at least at the eighth nerve level, unless the means of observation is sensitive enough to detect noise-related disarray of stereocilia (Liberman and Dodds. 1984). In general, we were not able to distinguish the amount of stereocilia disarray induced by sound exposure from that resulting artifactually from critical point drying. And indeed, in the present study, no correlation was found between the rate of spontaneous activity and the amount of damage to either hair cell population. For example, the animal with the highest rate of spontaneous events showed the least amount of OHC damage; conversely, the animal with the most amount of OHC damage showed the lowest spontaneous rates among our tone-exposed animals (Figure 9). Thus, although a relationship may still exist between the amount and type of hair cell injured by sound and the changes seen in the DCN, further analysis using the approach of Liberman and Dodds (1984) will be required to test this possibility more directly.
A third mechanism which could underlie tone induced increases in spontaneous activity in the DCN is a change in the level of activity of descending pathways projecting from higher brainstem nuclei to the DCN. There are a number of nuclei that project to the DCN or to the DCN-associated granule cell domain including the superior olivary complex (Brown et al., 1988; Godfrey et al., 1983, 1987; Shore et al., 1991), the nuclei of the lateral lemniscus and inferior colliculus (Kane and Finn, 1977; Kane and Conlee, 1979; Shore et al., 1991) and dorsal column nuclei (Itoh et at., 1987; Weinberg and Rustioni, 1987). Intense sound exposure might cause a chronic change in the activity of one or more of these efferent pathways, and such changes might feed back to the DCN to cause an increase in the activity levels of certain populations of its contained neurons. Although the effects of intense sound exposure on these pathways are not known, evidence has been presented previously that heightened activity of efferents from the superior olivary complex does affect the spontaneous activity of neurons in the cochlear nucleus with some showing an enhancement and others showing a decrement in their spontaneous discharge rates (Comis and Whitfield, 1968; Starr and Wernick, 1968). If efferent fiber projections contribute to the increase in spontaneous activity of the DCN following intense tone exposure, it is possible that they do so either directly through feedback activation or indirectly through disinhibition.
Clinical Relevance
The increases in neural activity observed in the DCN may be an important contributing factor underlying some forms of tinnitus. Intense noise exposure is the single most frequently cited cause of chronic tinnitus (Axelsson and Barrenas, 1995; Coles, 1995; MeikIe and Taylor-Walsh, 1984), and evidence has been cited that tinnitus, in many cases, has an important central component. For example, House and Brackman (1981) found that 55% of patients with chronic tinnitus continued to experience their tinnitus after surgical excision of the auditory nerve. A further indication of a central component of tinnitus is the demonstration that monaural tinnitus can, in many instances, be masked by a contralateral sound (Tyler and Conrad-Arms, 1980). More recently, studies in the inferior colliculus of rats suggest that increases in spontaneous activity may be an important component of acute tinnitus. For example, when rats were given intravascular injections of the tinnitus-inducing agent, sodium salicylate, those rats showed acute increases in spontaneous activity of single units in the IC. Results of behavioral tests suggest that the same animals also experienced tinnitus-like percepts (Jastreboff and Sasaki, 1986). In the present study, increased spontaneous activity of the DCN was consistently observed following intense tone exposure, and this hyperactivity resembled the heightened neural activity evoked in a normal, unexposed animal during presentation of a continuous CF tone (Fig. 3). If stimulus-evoked increases in activity signal the presence of sound in a normal animal, then elevated levels of spontaneous activity in the same neural population resulting from intense tone exposure might also be interpreted by the brain as representing a sound, even though an acoustic stimulus is not present. The percept resulting from this activity would presumably correspond to the tonotopic locus in the DCN where activity is maximal as predicted by the ‘place principle’ of auditory frequency analysis. In the present study, maximal activity occurred in the 8-12 kHz region of the DCN. A recent study by Meikle (1995) found that the majority of tinnitus patients matched the pitch of their tinnitus to high frequencies, with 20% matching to frequencies above 8 kHz and half matching to frequencies between 3500 and 8500 Hz. The pitch of tinnitus was found, in general, to be either at the edge of the hearing loss or close to the frequency of maximum hearing loss, which was generally lower than 8 kHz. Thus, the fact that most patients match their tinnitus to frequencies lower than the CF range in which spontaneous activity was found to be maximal in the present study may reflect differences in the topographic locus of the hearing loss rather than differences in underlying mechanisms.
A key question is whether animals which show tone-induced increases in spontaneous activity also experience phantom auditory percepts. If a link could be demonstrated following intense sound exposure, similar to that demonstrated in rats following salicylate treatment (Jastreboff and Sasaki, 1986), the DCN would provide an excellent locus for investigating tinnitus-producing mechanisms and for developing and testing potential therapeutic drugs. Further research on the DCN, combined with behavioral tests would help shed light on these issues.
ACKNOWLEDGMENTS
The authors thank Dr. Donald A. Godfrey for his critical suggestions concerning the manuscript and Dr. Michael Church and Pamela Falzarano for their technical assistance. Support for this work was provided by The American Tinnitus Association and The National Organization for Hearing Research.
REFERENCES
- Axelsson A, Barrenas M-L. Tinnitus In Noise-induced hearing loss. In: Dancer AL, Henderson D, Salvi RJ, Hamernik RP, editors. Noise-induced Hearing Loss. Mosby Year Book; Boston: 1992. pp. 269–276. [Google Scholar]
- Brown MC, Liberman MC, Benson TE, Ryugo DK. Brainstem branches from olivocochlear axons in cats and rodents. J. Comp. Neurol. 1988;278:591–603. doi: 10.1002/cne.902780410. [DOI] [PubMed] [Google Scholar]
- Bourke TR. Unpublished doctoral dissertation. MIT; Cambridge, Massachusetts: 1976. Electrical responses of neural units in the anteroventral cochlear nucleus of the cat. [Google Scholar]
- Coles RA. Classification of causes, mechanisms of patients disturbance and associated counseling. In: Vernon JA, Moller AR, editors. Mechanisms of Tinnitus. Allyn and Bacon; Boston: 1995. pp. 11–20. [Google Scholar]
- Comis SD, Whitfield IC. Influence of centrifugal pathways on unit activity in the cochlear nucleus. J. Neurophysiol. 1968;31:62–68. doi: 10.1152/jn.1968.31.1.62. [DOI] [PubMed] [Google Scholar]
- Dandy WE. Surgical treatments of Meniere’s disease. Surg. Gyn. and Obstet. 1941;72:421–425. [Google Scholar]
- Devor M. The pathophysiology of damaged peripheral nerves. In: Wall PD, Melzack R, editors. Mechanisms of Pain. Plenum Press; New York: 1994. pp. 79–100. [Google Scholar]
- Devor M, Keller CH, Deerinck TJ, Levinsonm SR, Ellisman MH. Sodium channel accumulation on axolemma of afferent endings of nerve end neuromas in Apteronotus. Neurosci. Letters. 1993;102:149–154. doi: 10.1016/0304-3940(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Evans EF, Borerwe TA. Ototoxic effects of salicylates on the responses of single cochlear fibers and on cochlear potentials. Brit. J. of Audiol. 1982;16:101–108. doi: 10.3109/03005368209081454. [DOI] [PubMed] [Google Scholar]
- Evans EF, Wilson JP, Borerwe TA. Animal models of tinnitus. In: Evered D, Lawrenson G, editors. CIBA Foundation Symposium 85: Tinnitus; London. Pitman; 1981. pp. 108–129. [DOI] [PubMed] [Google Scholar]
- Feldman H. Homolateral and contralateral masking of tinnutus by noise bands and pure tones. Audiology. 1971;10:138–144. doi: 10.3109/00206097109072551. [DOI] [PubMed] [Google Scholar]
- Gejrot T. Intravenous xylocaine in the treatment of attacks of Meniere’s disease. Acta Otolaryngol. 1963;(Suppl. 188):190–195. [PubMed] [Google Scholar]
- Gerken GM, Saunders SS, Paul RE. Hypersensitivity to electrical stimulations of auditory nuclei follows hearing loss in cats. Hear. Res. 1984;13:249–259. doi: 10.1016/0378-5955(84)90078-9. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Park JL, Rabe JR, Ross CD. Effects of large brainstem lesions on the cholinergic system in rat cochlear nucleus. Hear. Res. 1983;11:133–156. doi: 10.1016/0378-5955(83)90076-x. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Park-Hellendall JL, Dunn JP, Ross CD. Effects of trapezoid body and superior olive lesions on choline acetyltransferase activity in the rat cochlear nucleus. Hear. Res. 1987;28:253–270. doi: 10.1016/0378-5955(87)90053-0. [DOI] [PubMed] [Google Scholar]
- Hall JG. Pathological changes in second order auditory neurons after noise exposure and peripheral denervation. Scand. Audiol. 1974;4:31–38. [Google Scholar]
- Hall JG. The cochlea, nuclei in monkeys after dehydrostreptomycin or noise exposure. Acta Oto-laryngol. 1976;81:344–352. doi: 10.3109/00016487609119972. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Nagasawa A, Smith DV, Stanton S, Mount RJ. Reorganization of auditory cortex after neonatal high frequency hearing loss. Hear. Res. 1991;54:11–19. doi: 10.1016/0378-5955(91)90131-r. [DOI] [PubMed] [Google Scholar]
- House JW, Brackman DE. Tinnitus Surgical treatment. In: Evered D, Lawrenson G, editors. CIBA Foundation Symposium 85: Tinnitus; London. Pitman; 1981. pp. 204–212. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kamiya H, Mitani A, Yasui Y, Takada M, Mizuno N. Direct projections from the dorsal column nuclei and the spinal trigeminal nucleus to the cochlear nuclei in the cat. Brain Res. 1987;400:145–150. doi: 10.1016/0006-8993(87)90662-7. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennen JF, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behavioral Neurosci. 1988;102:811–822. doi: 10.1037//0735-7044.102.6.811. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Sasaki CT. Salicylate induced changes in spontaneous activity of single units in the inferior colliculus. J. Acoust. Soc. Am. 1986;80:1384–1391. doi: 10.1121/1.394391. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Czaja JM, Kaplan CR. Changes in the tonotopic map of the dorsal cochlear nucleus following induction of cochlear lesions by exposure to intense sound. Hear. Res. 1992;59:149–160. doi: 10.1016/0378-5955(92)90118-7. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Lazor J. Tonotopic maps obtained from the surface of the dorsal cochlear nucleus of the hamster and rat. Hear. Res. 1991;59:149–160. doi: 10.1016/0378-5955(91)90013-y. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Meleca RJ, Falzarano PR, Salvi RJ, Henderson D, et al. Auditory System Plasticity and Regeneration. Theime Medical Publishers; New York: 1996. Alterations in the tonotopic map of the cochlear nucleus following cochlear damage; pp. 317–332. [Google Scholar]
- Kaltenbach JA, Saunders JC. Spectral and temporal response patterns of single units in the chinchilla dorsal cochlear nucleus. Exp. Neurol. 1987;96:406–419. doi: 10.1016/0014-4886(87)90058-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Schmidt RN, Kaplan CR. Tone-induced stereocilia lesions as a function of exposure level and duration in the hamster cochlea. Hear. Res. 1992b;60:205–215. doi: 10.1016/0378-5955(92)90022-f. [DOI] [PubMed] [Google Scholar]
- Kane ES, Conlee JW. Descending inputs to the caudal cochlear nucleus of the cat: degeneration and auto radiographic studies. J. Comp. Neurol. 1979;187:739–784. doi: 10.1002/cne.901870408. [DOI] [PubMed] [Google Scholar]
- Kane ES, Finn RC. Descending and intrinsic inputs to dorsal cochlear nucleus of cats; a horseradish peroxidase study. Neurosci. 1977;2:897–912. [Google Scholar]
- Kitahara M, Kitano H, Suzuki M, Kitajima K. Tinnitus and spontaneous activity. In: Vernon JA, Moller AR, editors. Mechanisms of Tinnitus. Allyn and Bacon; Boston: 1995. pp. 95–99. [Google Scholar]
- Koerber KC, Pfieffer WB, Kiang NYS. Spontaneous spike discharges from single units in the cochlear nucleus after destruction of the cochlea. Exp. Neurol. 1966;1:119–130. doi: 10.1016/0014-4886(66)90091-4. [DOI] [PubMed] [Google Scholar]
- Lenarz TH, Schreiner CE. Pharmacological modifications of abnormal ensemble spontaneous activity of cat auditory nerve. Abs. Assoc. Res. Otolaryngol. 1990;13:198. [Google Scholar]
- Liberman MC. Response properties of cochlea efferent neurons: monaural vs. binaural stimulation studies. J. Neurophysiol. 1988;60:1779–1790. doi: 10.1152/jn.1988.60.5.1779. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rate. Hear. Res. 1984;16:43–45. doi: 10.1016/0378-5955(84)90024-8. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NYS. Acoustic trauma in cats: Cochlear pathology and auditory nerve activity. Acta Otolaryngol. Supp. 1978;358:5–63. [PubMed] [Google Scholar]
- Martin FW, Coleman BH. Tinnitus: a double blind crossover controlled trial to evaluate the use of lodocaine. Clin. Otolaryngol. 1980;5:3–11. doi: 10.1111/j.1365-2273.1980.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Martin WH, Schwegler JV, Scheibethoffer J, M. L. Salicylate-induced changes in cat auditory nerve activity. Laryngoscope. 1993;103:600–604. doi: 10.1288/00005537-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Meikel M. The interaction of central and peripheral mechanisms in tinnitus. In: Vernon JA, Moller AR, editors. Mechanisms of Tinnitus. Allyn and Bacon; Boston: 1995. pp. 181–206. [Google Scholar]
- Meikle M, Taylor-Walsh E. Characteristics of tinnitus and related observations in over 1800 tinnitus patients. J. Laryngol. and Otol. Suppl. 1984;9:1–21. doi: 10.1017/s1755146300090053. [DOI] [PubMed] [Google Scholar]
- Melding PS, Goodey RJ, Thorne PR. The use of intravenous lignocaine in the diagnosis and treatment of tinnitus. J. of Laryngol. and Otol. 1978;92:115–121. doi: 10.1017/s002221510008511x. [DOI] [PubMed] [Google Scholar]
- Morest DK, Bohne BA. Noise-induced degeneration in the brain and representation of inner and outer hair cells. Hear. Res. 1983;9:145–151. doi: 10.1016/0378-5955(83)90024-2. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvin DRF, Wise LV, Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J. Comp. Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- Robertson D, Irvine DRF. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J. Comp. Neurol. 1989;338:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Salvi RJ. Central components of the temporary threshold shift. In: Henderson D, Hamernik RP, Dosanjh DS, Mills JH, editors. Effects of Noise on Hearing. Raven; New York: 1976. pp. 247–260. [Google Scholar]
- Salvi RJ, Ahroon WA. Tinnitus and neural activity. J. of Speech and Hear. Res. 1983;26:629–632. doi: 10.1044/jshr.2604.629. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Hamernik RP, Henderson D. Discharge patterns in the cochlea nucleus of the chinchilla following noise-induced asymptotic threshold shift. Exp. Brain Res. 1978;32:301–302. doi: 10.1007/BF00238704. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculous of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–258. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Cohen YE, Szymko YM. The structural and functional consequences of acoustic injury in the cochlea and peripheral auditory system: a five year update. J. Acoust. Soc. Am. 1991;90:136–146. doi: 10.1121/1.401307. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: a review and tutorial. J. Accoust. Soc. Am. 1985;78:833–860. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. The effects of noise on the physiology of hearing: a review and tutorial. J. Acoust. Soc. Am. 1984;76:1293–1317. doi: 10.1121/1.391446. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Snyder RL. A physiological animal model of tinnitus. Abs. Assoc. Res. Otolaryngol. 1987;10:195. [Google Scholar]
- Schreiner CE, Snyder RL, Lenarz TH. Spectral and temporal characteristics of abnormal ensemble spontaneous activity of cat auditory nerve. Abs. Assoc. Res. Otolaryngol. 1990;13:197. [Google Scholar]
- Shore SE, Helfert RH, Bledsoe SC, Altschuler RA. Descending projections to the dorsal and ventral divisions of the cochlear nucleus in guinea pig. Hear. Res. 1991;52:255–268. doi: 10.1016/0378-5955(91)90205-n. [DOI] [PubMed] [Google Scholar]
- Snow PJ, Wilson P. Plasticity in the somatosensory system of developing and mature mammals - The effects of injury to the central peripheral nervous system. In: Autrum H, Ottoson D, Prel ER, Schmidt RF, Shimazu H, Willis WD, editors. Progress in Sensory Physiol. Vol. 11. Springer-Vertag; New York: 1991. pp. 1–482. [Google Scholar]
- Sowinski H. Histochemical and Morphological changes in the Organ of Corti and pathways of hearing induced by experimental acoustic trauma. Neuroipathol. Pol. 1975;13:367–373. [PubMed] [Google Scholar]
- Starr A, Wernick JS. Olivocochlear bundle stimulation: effects on spontaneous and tone-evoked activities of single units in the cat cochlear nucleus. J. Neurophysiol. 1968;31:5–12. doi: 10.1152/jn.1968.31.4.549. [DOI] [PubMed] [Google Scholar]
- Tarmas J. Morphological changes in nerve cells of the rabbit brain caused by industrial noise. Folia. Morphol. 1974;33:5–12. [PubMed] [Google Scholar]
- Tyler RS, Conrad-Arms D. Procedural variables and interpretations of tinnitus loudness estimates. J. Am. Speech and Hear. Assoc. 1980;22:759. [Google Scholar]
- Weinberg RJ, Rustioni A. A cuneocerebellar pathway in the rat. Neurosci. 1987;20:209–219. doi: 10.1016/0306-4522(87)90013-3. [DOI] [PubMed] [Google Scholar]
- Willott JF, Lu SM. Noise induced hearing loss can alter neural coding and increase excitability in the central nervous system. Science. 1982;216:1331–1332. doi: 10.1126/science.7079767. [DOI] [PubMed] [Google Scholar]