Abstract
Background
Chronic social instability during adolescence and early adulthood is known to produce a variety of long-lasting effects that may contribute to future psychiatric disorders. However, its potential to affect future generations has not been tested.
Methods
Female and male mice were exposed to chronic social stress involving social instability and disruption of social hierarchy from postnatal day 27 to 76. After treatment, a group of animals was used to evaluate long-term behavioral effects of the stress exposure, and other mice were used to generate F1, F2, and F3 offspring, to test for behavioral effects across generations.
Results
Chronic social instability during adolescence and early adulthood induces persistent behavioral alterations, including enhanced anxiety and social deficits that are transmitted predominantly to females across at least three generations. Both mothers and fathers can transmit all of these altered behaviors to their F1 offspring. However, only F1 fathers transmit all of them to their F2 and F3 daughters. In the F1 generation, enhanced anxiety and social deficits are associated with elevated serum corticosterone levels; however, in the F2 and F3 generations, they are not.
Conclusions
These findings support the idea that individual risk for psychiatric disorders that involve enhanced anxiety and/or social dysfunction may be dependent not only on the specific alleles of genes that are inherited from one’s parents and on one’s own experiences, but also on the experiences of one’s parents when they were young.
Keywords: Anxiety, germ-line, Rcan, social instability, social interactions, social stress, transgenerational
Chronic stress has been associated with the development of pathophysiologic conditions, adversely affecting normal neuronal, immune, cardiovascular, and metabolic functions (1–5). Included in these alterations are increased vulnerability to psychiatric diseases such as anxiety and depression (6,7).
A major source of chronic stress encountered by humans occurs during social interactions (8,9). As such, a variety of animal models have been developed to study the consequences and mechanisms underlying various forms of social stress. For example, a common model used to explore chronic hostility or aggression is social defeat stress, where animals are exposed repeatedly to larger and more aggressive individuals (9). To investigate the long-term effects of poor nurturing, paradigms including early maternal separation from offspring have commonly been used (10–12). Finally, to investigate how disruption of social networks affects behavior, models that generate social instability have been developed (7,13,14). These paradigms have revealed both common and distinct long-term consequences, suggesting they may be valuable tools to elucidate mechanisms that lead to behaviors found in numerous psychiatric disorders (15,16).
Here we used a chronic stress model of social instability to investigate both the long-term and possible transgenerational alterations on specific behaviors that are commonly found in affective disorders. The model is based on a complete disruption of social hierarchy achieved by randomly changing the cage composition of housed mice (7,17–19). Whereas social defeat stress involves a strong distinction between dominant and subordinate individuals, this model of social instability interferes with hierarchical separations (20) and may resemble what humans can experience in a continuously changing social environment (1,9).
We detected long-lasting behavioral alterations related to increased anxiety and defective social interactions that are transmitted predominantly to females across multiple generations. Interestingly, all of the phenotypes were transmitted through males, but only a subset through females. Moreover, we present evidence to suggest that at least some of these phenotypes may be due to deregulation of Rcan genes in the CA1 region of the hippocampus across generations.
Methods and Materials
Animals, Breeding, Corticosterone Measurement, RNA Analysis, Statistical Analysis
See Supplement 1.
Chronic Social Instability
The cage composition was changed twice per week for 7 weeks (starting at postnatal day 27), and each time, four mice from different cages were placed together in a clean cage. The rotation schedule was randomized (17). In contrast, control mice remained housed always with the same cage mates. At the end of the treatment, mice were separated and housed in pairs with cage mates from the last change. Two months after the stress treatment, behavioral tests were performed.
Behavioral Analysis
Mice from control and stressed groups and their offspring, were tested in a series of behavioral assays in the following order: elevated plus maze (EPM), open field test (OFT), and direct social interaction with a juvenile. These tests were done 3 days apart and were used to measure anxiety-related phenotypes. Mice were also used for the sociability and preference for social novelty tests, designed to measure social tendencies, and then for the forced swim test, designed to measure depression-like behaviors. To minimize litter effects, all behavioral measurements from animals belonging to the same litter, were averaged and considered as an individual value. At least two animals from the same litter were used for the behavioral tests. For details, see Supplement 1.
Results
To evaluate whether adverse effects of chronic social instability during adolescence and early adulthood can be transmitted across generations, female and male mice were submitted for 7 weeks to a previously described model of social instability (17), from Postnatal Day 27 to Day 76. The paradigm involves changing the mice group composition twice per week for 7 weeks. Two months later, some mice were used for assessment of behaviors known to result from social stress. Other stressed mice were used as breeding pairs to evaluate the transgenerational effects of this treatment.
Long-Term Effects of Chronic Social Instability During Adolescence and Early Adulthood
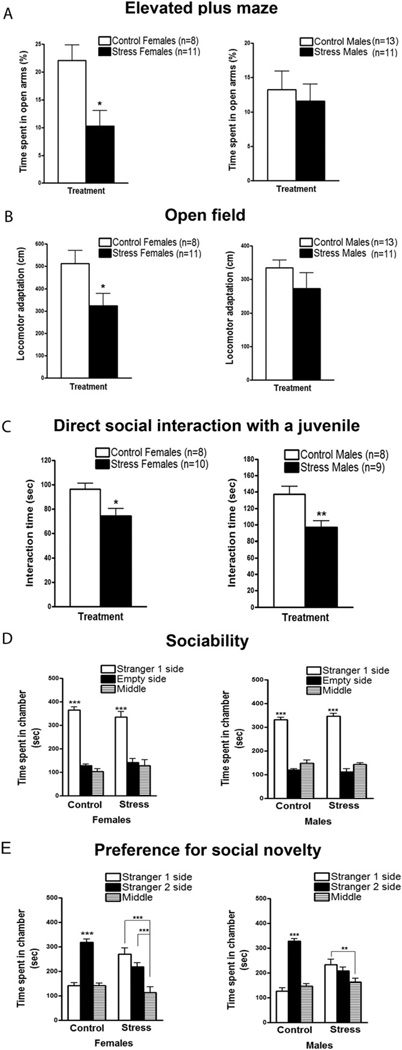
To investigate persistent anxiety-related behaviors caused by chronic social instability, stressed and control animals were tested 2 months after treatment in behavioral assays designed to measure anxiety, including the EPM and the OFT. In the EPM, stressed females spent significantly less time in the open arms of the maze than control females (Figure 1A), suggesting a higher than normal level of anxiety 2 months after social stress. In contrast, stressed males showed no significant differences in this test compared with their control counterparts.
Figure 1.
Long-term behavioral effects of chronic social instability exposure during adolescence and early adulthood. Two months after the end of the stress treatment, females and males were submitted to several behavioral assays for evaluation of anxiety-like phenotypes and social tendencies. (A) In the elevated plus maze, stressed females spent significantly less time in the open arms than control females [t(17) = 2.380, p < .05; left panel]. No differences in time spent in the open arms were found between stressed and control males [t(22) = .2790, p > .05; right panel]. (B) In the open field, stressed females showed a significant decrease in locomotor adaptation to a new environment in comparison with control females [t(17) = 2.201, p < .05; left panel]. Stressed males exhibited similar levels of locomotor adaptation in the open field as control males [t(22) = .6058, p > .05; right panel]. (C) In the direct social interaction test with a juvenile, both stressed females (left panel) and males (right panel) showed a significant reduction in the amount of time exploring and interacting with the unfamiliar juvenile in contrast to control females and males [t(16) = 2.795, p < .05; t(15) = 3.229, p < .01, respectively]. (D) In the sociability test, both stressed (n = 7) and control (n = 7) females (left panel) showed a significant preference [F(2,36) = 113.4, p < .001] for spending more time in the side containing the novel mouse (Stranger 1) in comparison with the empty side (p < .001) and the middle (p < .001). Similarly, both stressed (n = 7) and control (n = 7) males (right panel) showed a significant preference [F(2,36) = 263.7, p < .001] for spending more time in the side containing the novel mouse (Stranger 1) in comparison with the empty side (p < .001) and the middle (p < .001). (E) In the preference for social novelty test, control females (n = 7) exhibited a significant preference [F(2,36) = 32.16, p < .001] for spending more time in the side containing the Stranger 2 in comparison with Stranger 1’s side (p < .001) and the middle (p < .001; (left panel). However, stressed females (n = 7) displayed no preference for Stranger 2 compared with Stranger 1 (p > .05), and they spent significantly more time in the sides containing the Strangers 1 or 2 vs. the middle (p < .001). Similar to control females, control males (n = 7) also showed a significant preference [F(2,36) = 33.47, p < .001] for spending more time in the side containing the Stranger 2 compared with Stranger 1’s side (p < .001) and the middle (p < .001; (right panel). In contrast, stressed males (n = 7) did not show any preference between Strangers 1 and 2 (p > .05), and they spent significantly more time in the side containing the Stranger 1 versus the middle (p < .01). Error bars indicate SEM. *p < .05; **p < .01; ***p < .001.
Females also showed an enhanced anxiety-related phenotype in the OFT, in which locomotor adaptation to a new environment was examined (Figure 1B). Previously stressed females showed a significant decrease in locomotor adaptation in comparison to control females, an indication of high anxiety. Similar to the EPM, previously stressed males failed to exhibit a defect in locomotor adaptation in the open field.
Because an unstable social environment was used to induce chronic stress, social behaviors were also examined. First, a direct social interaction test was performed by quantifying time spent displaying affiliative behaviors with a same-sex juvenile. In this test, mice were placed individually in a new cage and left to habituate for 15 min. Then an unfamiliar same-sex juvenile was introduced into the same cage for 3 min. During that time, the extent of social interactions (e.g., sniffing, grooming, following the juvenile) was quantified. Both previously stressed females and males displayed a significant reduction compared with controls in the amount of time they spent interacting with the juvenile (Figure 1C), a sign of social anxiety or social withdrawal.
In a related assay, sociability was assessed by measuring time spent with a novel adult mouse in a three-chambered social box (21). In this case, the novel mouse located in one of the chambers was enclosed in a wire mesh container that prevented physical interaction, whereas an empty container was located in the opposite chamber. Strikingly, under these experimental conditions, control and previously stressed animals showed similar sociability by preferring to spend more time in the chamber containing the novel mouse over the chamber containing the empty cage (Figure 1D). The results in Figures 1C and D are consistent with the idea that chronic social instability produces long-lasting elevation of anxiety that can interfere with social interactions, particularly when physical contact with another animal is possible.
Interestingly, even when physical interactions are prevented in the three-chamber system described earlier, previously stressed mice did display a defect in a preference for social novelty test. In this case, mice are given a choice between the already explored novel mouse (Stranger 1) and a new unfamiliar enclosed mouse (Stranger 2). Whereas control mice spent significantly more time with the novel Stranger 2, stressed mice did not. Thus, previously stressed mice lacked preference for social novelty (Figure 1E).
Transmission of Enhanced Anxiety and Defective Social Interactions to Future Female Offspring
Offspring of stressed and control parents were raised in the absence of the chronic social instability paradigm and then submitted to the same behavioral tests as their parents at the age of 2 months.
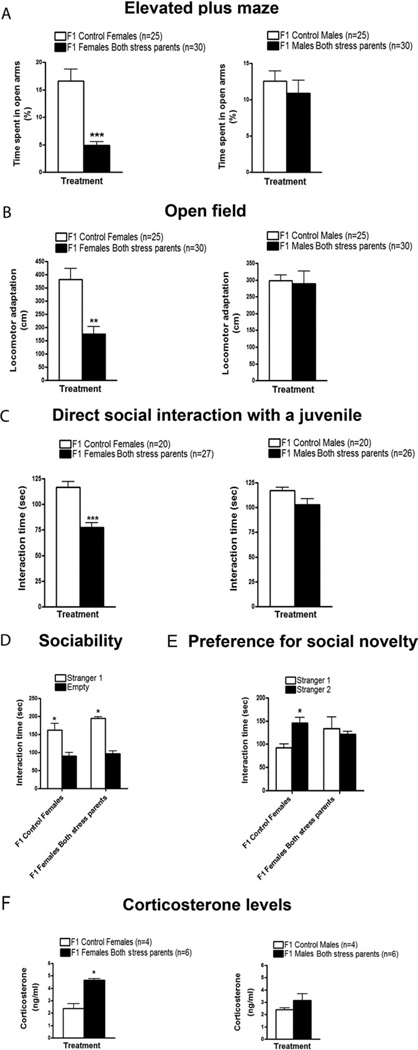
Similar to their stressed mothers, F1 females, but not males, spent significantly less time in the open arms of the EPM compared with the F1 females from control parents (Figure 2A). When tested on the OFT, F1 females, but not males, also behaved like their stressed mothers by displaying a significantly lower adaptation to the new environment compared with control mice (Figure 2B).
Figure 2.
Transmission of enhanced anxiety, defective social interactions, and elevated corticosterone levels to F1 female offspring. To investigate whether the phenotypes caused by direct exposure to chronic social instability during adolescence and early adulthood are transmitted to the F1 offspring, control and stressed females and males were mated with other control and stressed mice, respectively. Offspring were submitted to the same behavioral paradigms as the parents, at the age of 2 months. (A) In the elevated plus maze, F1 females from both stressed parents spent significantly less time in the open arms in comparison with F1 females from control mice [t(15) = 6.761, p < .0001; left panel]. F1 males from both stressed parents did not show significant differences in comparison with F1 control males in terms of time spent in the open arms [t(11) = .7265, p > .05; right panel]. (B) When tested in the open field, F1 females from both stressed parents displayed a significantly lower adaptation to the new environment in comparison with F1 control females [t(15) = 4.265, p < .01; left panel]. F1 males from both stressed parents showed similar levels of locomotor adaptation as F1 control males [t(11) = .1988, p > .05; right panel]. Number of litters used in A and B: 8–11 litters from both stressed females and males, and 5–6 litters from control females and males. (C) In the direct social interaction test with a juvenile, F1 females from both stressed parents spent significantly less time interacting with and exploring the juvenile in comparison with F1 control females [t(10) = 5.248, p < .0001; left panel]. F1 males from both stressed parents displayed similar interaction levels with the juvenile as F1 control males [t(9) = 1.385, p > .05; right panel]. Number of litters used in C: 7 litters from both stressed females and males, and 4–5 litters from control females and males. (D) In the sociability test, both F1 control females (n = 12) and F1 females from stressed parents (n = 10) spent significantly more time sniffing and interacting with the cage containing the Stranger 1 than with the empty cage [F(1,8) = 26.93, p < .05]. (E) In the preference for social novelty test, F1 control females (n = 12) spent significantly more time interacting with and sniffing the cage containing the Stranger 2 in comparison to the cage containing the Stranger 1 [F(1,8) = 5.743, p < .05]. In contrast, F1 females from both stressed parents (n = 10) did not show any difference in time spent interacting with both enclosed strangers (p > .05). Number of litters used in D and E: 3 litters from both stressed females and males, and 3–4 litters from control females and males. (F) Basal corticosterone levels in plasma were significantly increased in F1 females from both stressed parents in comparison to F1 control females [t(3) = 4.373, p < .05; (left panel).No differences in basal corticosterone levels were found between F1 males from both stressed parents and F1 control males [t(3) = 1.686, p > .05; (right panel). Number of litters used in F: 3 litters from both stressed females and males, and 2 litters from control females and males. Error bars indicate SEM. *p < .05; **p < .01; ***p < .001.
Next, social behaviors were assessed by exposing the animals to an unfamiliar juvenile in a direct social interaction test. Similar to their stressed parents, F1 females also spent significantly less time interacting with and exploring the juvenile compared to F1 control females (Figure 2C). Interestingly, although stressed male parents spent significantly less time interacting with the juvenile, their male offspring did not.
To assess social memory in F1 females, mice were reexposed to the same juvenile two days after the first encounter. In this case, both F1 control females and F1 females from stressed parents exhibited a significant decrease in social interaction compared with the first encounter, an indication of normal social memory (Figure S1A in Supplement 1). In addition, the interaction time displayed by F1 females from stressed parents during the second encounter remained significantly reduced in comparison to F1 control females.
Moreover, F1 females also behaved like their stressed parents in a sociability test by displaying normal interactions with a enclosed stranger mouse in comparison to an empty cage (Figure 2D), and in a test for preference for social novelty by displaying decreased interactions with an enclosed novel mouse when the choice was between Stranger 1 and a new unfamiliar enclosed mouse, or Stranger 2 (Figure 2E). No effects were observed for their male siblings (Figure S1B, C in Supplement 1).
Finally, elevated corticosterone levels were found in the female, but not male, offspring from stressed parents (Figure 2F). Because enhanced anxiety is frequently associated with depression, we tested F1 female and male offspring of stressed parents in a forced swim test. However, they did not respond differently than F1 control mice in this assay system (Figure S2 in Supplement 1).
Overall, these findings show that elevated anxiety and defective social behaviors acquired after exposure of mice to social instability during adolescence and early adulthood are inherited by their female offspring, even though the latter did not experience social instability stress.
Enhanced Anxiety and Defective Sociability Are Not Due to Altered Postnatal Behaviors of Parents
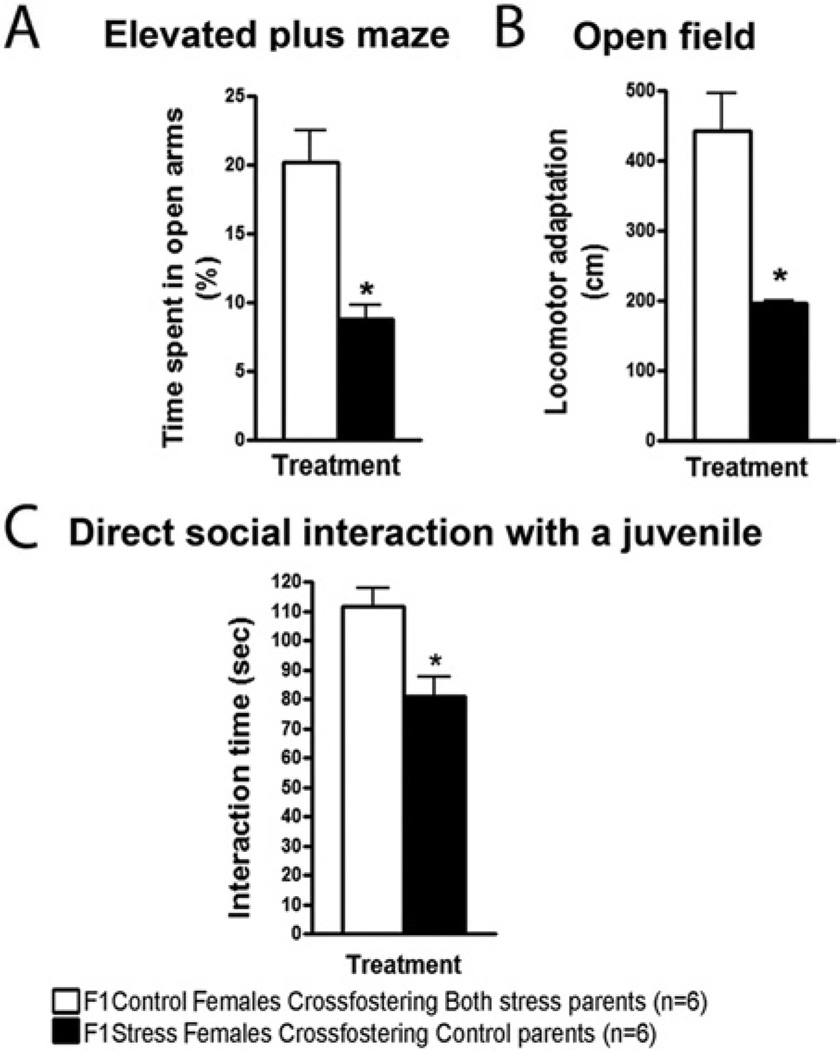
To test the possibility that changes in early postnatal nurturing were responsible for the transgenerational effects observed, cross-fostering experiments were performed. In particular, stressed parents were replaced at birth of their offspring with control foster parents, and the offspring were then raised by foster parents. When mice were 2 months old, they were tested in the EPM, the OFT, and the direct social interaction test with a juvenile. Figure 3A through 3C shows that females retained their anxiety phenotype even though they were raised by control parents. The converse experiment led to the same conclusion. When control female (or male; Figure S3 in Supplement 1) offspring were raised by stressed parents they performed normally on the previously cited tests. Thus, the transmission of these phenotypes from parents to the next generation was not caused by postnatal behavior alterations but rather occurred sometime before birth.
Figure 3.
F1 transgenerational effects of chronic social instability are not due to altered postnatal behaviors of stressed parents. Cross-fostering experiments were performed, and both stressed parents were replaced at birth of their offspring with control foster parents and vice versa. The female offspring of these crossings were tested when they were 2 months old. (A) In the elevated plus maze, F1 females born to stressed parents but raised by control parents (F1 stress females cross-fostering control parents) spent significantly less time in the open arms compared with F1 females born to control parents but raised by stressed parents [F1 control females cross-fostering both stress parents; t(2) = 4.674, p < .05]. (B) In the open field, F1 females born to stressed parents but raised by control parents displayed a significantly lower adaptation to the new environment in comparison with F1 females born to control parents but raised by stressed parents [t(2) = 5.319, p < .05]. (C) In the direct social interaction test with a juvenile, F1 females born to stressed parents but raised by control parents spent significantly less time interacting with and exploring the juvenile in comparison with F1 females born to control parents but raised by stressed parents [t(2) = 3.357, p < .05]. Number of litters used: 2 litters from both stressed females and males, and 2 litters from control females and males. Error bars indicate SEM. *p < .05.
F1 Female Offspring Inherit Altered Behaviors from Both Mothers and Fathers
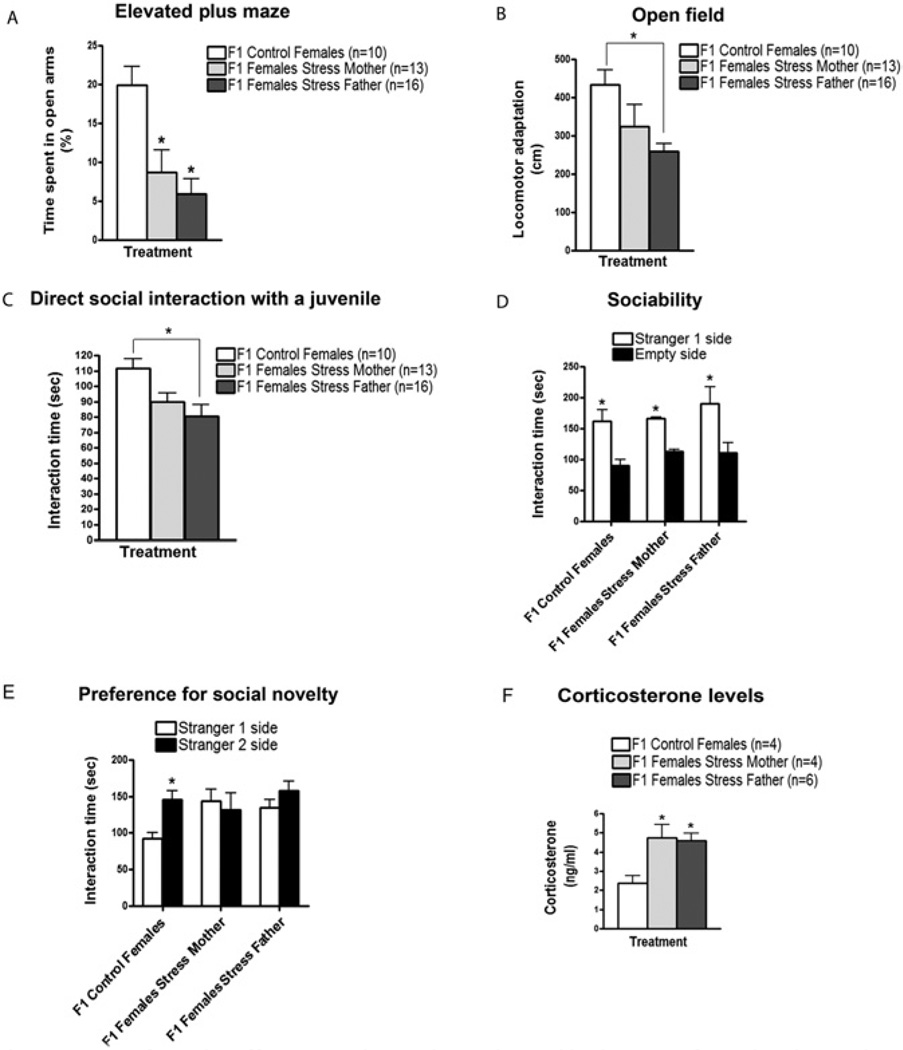
To discern whether these social instability-induced behaviors are transmitted to the next generation through the females and/or males, stressed females were mated with control males and vice versa. Female offspring were then tested in the behavioral assays described. In the EPM, female offspring of either stressed mothers or stressed fathers spent significantly less time in the open arms of the maze (Figure 4A). In the OFT, offspring of stressed fathers displayed a significant decrease in locomotor adaptation. However, the degree of inhibition was not as large as that found in offspring from both stressed mothers and fathers, suggesting a contribution from stressed mothers as well. In fact, offspring of stressed mothers did show a small decrease in locomotor adaptation; however, on its own, it was not statistically significant (Figure 4B).
Figure 4.
F1 female offspring inherit their altered behaviors from both mothers and fathers. Stressed females were mated with control males, and vice versa, and their female offspring were tested when they were 2 months old. (A) In the elevated plus maze, F1 females from single stressed mothers and F1 females from single stressed fathers spent significantly less time in the open arms compared with F1 females from control mice [F(2,10) = 5.743, p < .05]. (B) In the open field, F1 females from single stressed fathers displayed a significantly lower adaptation to the new environment in comparison with F1 control females [F(2,10) = 5.015, p < .05]. No significant difference was found in locomotor adaptation between F1 females from single stressed mothers and F1 control females (p > .05). (C) In the direct social interaction test with a juvenile, F1 females from single stressed fathers spent significantly less time interacting with and exploring the juvenile in comparison with F1 control females [F(2,10) = 5.085, p < .05]. Although no significant difference was detected in comparison to F1 control females (p > .05), F1 females from single stressed mothers displayed a tendency to have a reduced interaction time with the juvenile. Number of litters used in A, B, and C: 4 litters from stressed females and control males, 5 litters from stressed males and control females, and 4 litters from both control females and males. (D) In the sociability test, F1 control females (n = 8), F1 females from single stressed mothers (n = 7) and F1 females from single stressed fathers (n = 7) spent significantly more time sniffing and interacting with the cage containing Stranger 1 than with the empty cage [F(1,15) = 23.90, p < .05]. (E) In the preference for social novelty test, F1 control females (n = 8) spent significantly more time interacting with and sniffing the cage containing Stranger 2 compared with the cage containing Stranger 1 [F(1,15) = 29.98, p < .05]. In contrast, F1 females from single stressed mothers (n = 7) or fathers (n = 7) did not show any difference in time spent interacting with both enclosed strangers (p > .05). Number of litters used in D and E: 3 litters from stressed females and control males, 4 litters from stressed males and control females, and 4–5 litters from both control females and males. (F) Basal corticosterone levels in plasma were significantly increased in F1 females from single stressed mothers and single stressed fathers in comparison to F1 control females [F(2,8) = 6.505, p < .05]. Number of litters used in F: 3 litters from stressed females and control males, 3 litters from stressed males and control females, and 3 litters from both control females and males. Error bars indicate SEM. *p < .05.
Similar results were obtained in social behavior assays, in which F1 females from stressed fathers showed decreased interaction with a novel juvenile (Figure 4C). Again, F1 females from stressed mothers showed a small but not statistically significant decrease. In the preference for social novelty test, F1 females from either stressed fathers or mothers failed to prefer to interact with Stranger 2, as did offspring from both stressed mothers and fathers (Figure 4E). In addition, female offspring of either stressed mothers or stressed fathers had significantly higher levels of corticosterone, comparable to those found in F1 females from both stressed mothers and fathers (Figure 4F).
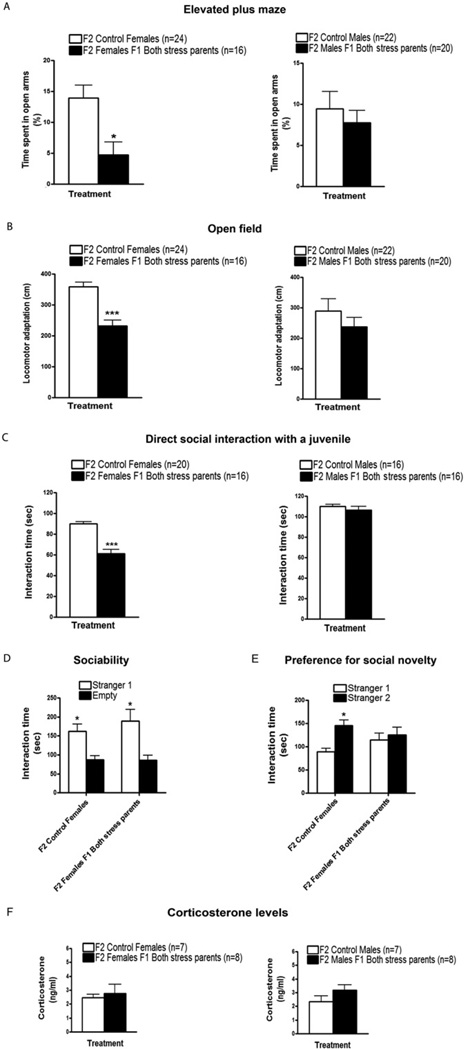
F2 Females Inherit Elevated Anxiety and Defective Social Interactions Predominantly from F1 Fathers, and Their Phenotype Is Independent of Elevated Corticosterone
F1 males and females from both stressed parents were mated with other F1 stressed or control animals. Their male and female offspring were then tested at 2 months of age in the behavioral assays described earlier. Like the F1 generation, female, but not male, F2 offspring from both F1 parents from stressed mice spent less time in the open arms of the EPM (Figure 5A). F2 females, but not F2 males, also displayed reduced locomotor adaptation in the OFT (Figure 5B). The same pattern was held for the direct social interaction test with a novel juvenile (Figure 5C). Finally, F2 female offspring also behaved normally in the sociability test (Figure 5D) but explored Stranger 2 significantly less than F2 female offspring from control mice in the preference for social novelty test (Figure 5E). Overall, F2 female, but not F2 males, faithfully inherited behavioral alterations found in their F1 mothers from stressed parents.
Figure 5.
Transmission of enhanced anxiety and defective social interactions to F2 female offspring. F1 females and males from both stressed parents were mated with others F1 mice from stressed parents and their offspring were tested when they were two months old. F2 offspring from control mice were also generated. (A) In the elevated plus maze, F2 females from both F1 mice from stressed parents spent significantly less time in the open arms in comparison with F2 females from control mice [t(12) = 2.839, p < .05; left panel]. F2 males from both F1 mice from stressed parents did not show significant differences in comparison with F2 control males in terms of time spent in the open arms [t(14) < .6636, p > .05; right panel]. (B) In the open field, F2 females from both F1 mice from stressed parents showed a significant reduction in locomotor adaptation in comparison with F2 control females [t(12) = 5.232, p < .001; left panel]. F2 males from both F1 mice from stressed parents showed similar levels of locomotor adaptation as F2 control males [t(14) = 1.053, p > .05; right panel]. Number of litters used in A and B: 5 litters from both F1 parents from stressed mice, and 9–11 litters from both F1 control mice. (C) In the direct social interaction test with a juvenile, F2 females from both F1 mice from stressed parents spent significantly less time interacting with and investigating the juvenile in contrast to F2 control females [t(10) = 7.353, p < .001; left panel]. F2 males from both F1 mice from stressed parents displayed similar interaction levels with the juvenile as F2 control males [t(6) = .8753, p > .05; right panel]. Number of litters used in C: 4–5 litters from both F1 parents from stressed mice, and 4–7 litters from both F1 control mice. (D) In the sociability test, F2 control females (n = 6) and F2 females from both F1 mice from stressed parents (n = 6) spent significantly more time sniffing and interacting with the cage containing Stranger 1 than with the empty cage [F(1,8) = 22.30, p < .05]. (E) In the preference for social novelty test, F2 control females (n = 6) spent significantly more time interacting with and sniffing the cage containing Stranger 2 compared with the cage containing Stranger 1 [F(1,8) = 7.084, p < .05]. In contrast, F2 females from both F1 mice from stressed parents (n = 6) did not show any difference in time spent interacting with both enclosed strangers (p > .05). Number of litters used in D and E: 3 litters from both F1 parents from stressed mice, and 3–4 litters from both F1 control mice. (F) Basal corticosterone levels in plasma were similar between F2 females from both F1 mice from stressed parents and F2 control females [t(11) = .3932, p > .05; left panel]. Likewise, no differences in basal corticosterone levels were found between F2 males from both F1 mice from stressed parents and F2 control males [t(11) = 1.464, p > .05; right panel]. Number of litters used in F: 5 litters from both F1 parents from stressed mice, and 8 litters from both F1 control mice. Error bars indicate SEM. *p < .05; ***p < .001.
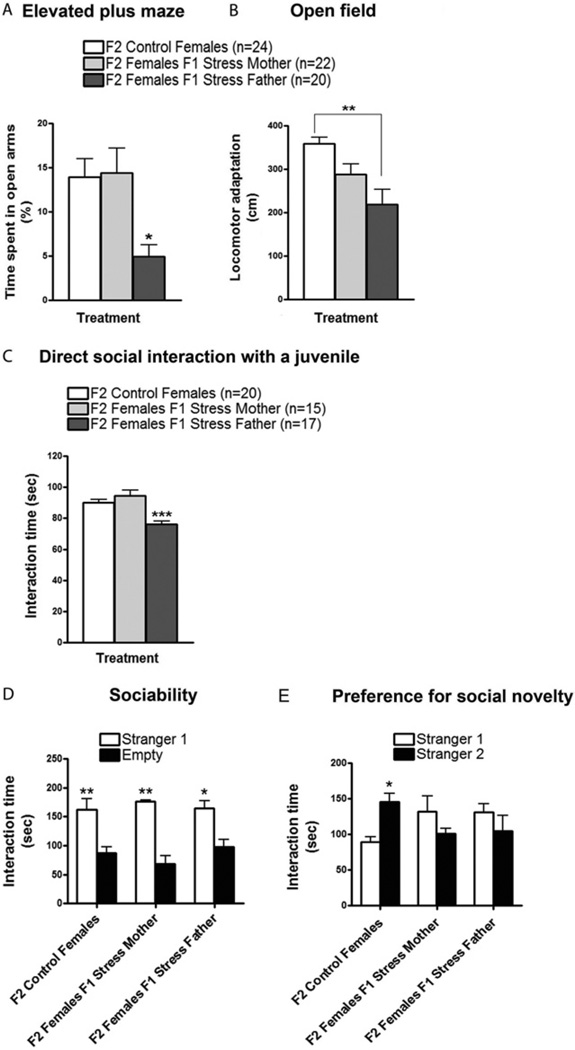
However, we found some striking differences in the transmission of the phenotypes from F1 to F2 versus transmission from F0 to F1 mice (see summary in Figure S5 in Supplement 1). First, unlike F1 offspring who could inherit elevated anxiety and defective behaviors in a direct social interaction test from either stressed mothers or fathers, F2 female mice displayed all these phenotypes only when they were the offspring of male descendants of stressed mice (Figure 6A–E). Remarkably, this occurs despite the fact that male F1 offspring of stressed mice displayed none of the altered behaviors they transmitted to their female offspring (Figure 5A–C). In contrast, F1 females were able to pass on defective preference for social novelty (Figure 6E) but not elevated anxiety (Figure 6A–C). The phenotype of these F2 females demonstrates that altered social behavior is not necessarily the consequence of elevated anxiety and thus could be transmitted independently across generations. In addition, unlike their F1 mothers, F2 female mice that inherited their altered behaviors from either their mother or their father did not display enhanced corticosterone levels (Figure 5F). Finally, F3 females, but not males, from the paternal lineage still displayed increased anxiety and abnormal social behaviors with no detectable increase in serum corticosterone (Figure S4 in Supplement 1).
Figure 6.
Transmission of enhanced anxiety and defective social interactions to F2 female offspring occurs predominantly through F1 fathers. F1 females from both stressed parents were mated with F1 control males and vice versa, and their female offspring were tested when they were 2 months old. (A) In the elevated plus maze, F2 females from single F1 fathers from stressed mice spent significantly less time in the open arms compared with F2 females from control mice and F2 females from single F1 mothers from stressed mice [F(2,21) = 4.935, p < .05]. (B) Intheopen field, F2 females from single F1 fathers from stressed mice showed a significant reduction in locomotor adaptation compared with F2 control females [F(2,21) = 7.428, p < .01] but not compared with F2 females from single F1 mothers from stressed mice. Number of litters used in A and B: 6 litters from F1 females from stressed mice and F1 control males, 6 litters from F1 males from stressed mice and F1 control females, and 10 litters from both F1 control females and males. (C) In the direct social interaction test with a juvenile, F2 females from single F1 fathers from stressed mice spent significantly less time interacting with and investigating the juvenile in contrast to F2 control females and F2 females from single F1 mothers from stressed mice [F(2,16) = 15.53, p < .001]. Number of litters used in C: 6 litters from F1 females from stressed mice and F1 control males, 6 litters from F1 males from stressed mice and F1 control females, and 5 litters from both F1 control females and males. (D) In the sociability test, F2 control females (n = 6), F2 females from single F1 mothers from stressed mice (n = 6), and F2 females from single F1 fathers from stressed mice (n = 6) spent significantly more time sniffing and interacting with the cage containing Stranger 1 than with the empty cage [F(1,11) = 40.80, p < .01, p < .05]. (E) In the preference for social novelty test, F2 control females (n = 6) spent significantly more time interacting with and sniffing the cage containing Stranger 2 compared with the cage containing Stranger 1 [F(1,11) = 6.836, p < .05]. In contrast, F2 females from single F1 mothers from stressed mice (n = 6) and F2 females from single F1 fathers from stressed mice (n = 6) did not show any difference in time spent interacting with both enclosed strangers (p > .05). Number of litters used in D and E: 3 litters from F1 females from stressed mice and F1 control males, 3 litters from F1 males from stressed mice and F1 control females, and 2–3 litters from both F1 control females and males. Error bars indicate SEM. *p < .05; **p < .01; ***p < .001.
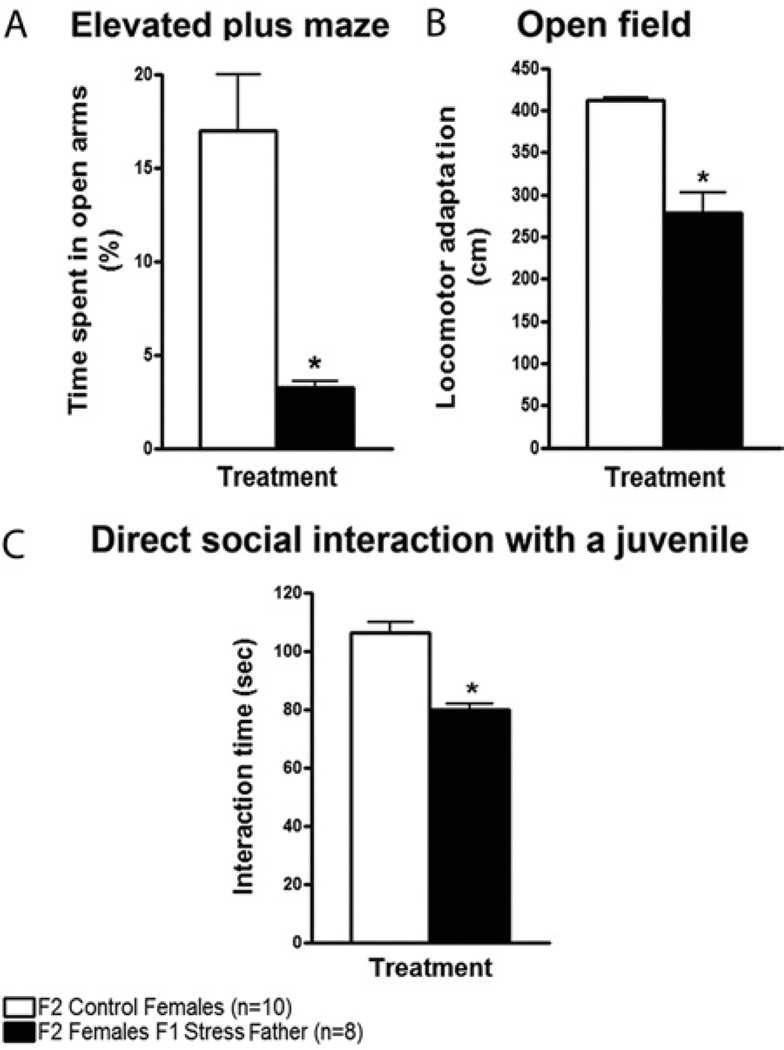
Although in these experiments male mice were present in the cages with their mating partner until pup weaning, the father’s presence was only needed during mating, because his removal after mating did not prevent offspring from displaying defective anxiety and social behaviors (Figure 7).
Figure 7.
F2 transgenerational effects of chronic social instability are not due to the presence of the F1 father during female pregnancy. F1 males (from stressed and control mice) were removed from the breeding cages after 2 weeks of mating, and F2 female offspring were tested as previously described. (A) In the elevated plus maze, F2 females from single F1 fathers from stressed mice spent significantly less time in the open arms compared with F2 control females [t(2) = 4.556, p < .05]. (B) In the open field, F2 females from single F1 fathers from stressed mice displayed a significantly lower adaptation to the new environment in comparison with F2 control females [t(2) = 3.865, p < .05]. (C) In the direct social interaction test with a juvenile, F2 females from single F1 fathers from stressed mice spent significantly less time interacting with and exploring the juvenile compared with F2 control females [t(2) = 6.420, p < .05]. Number of litters used: 2 litters from F1 fathers from stressed parents, and 2 litters from both F1 control mice. Error bars indicate SEM. *p < .05.
Overall, these results imply that maternal transmission of enhanced anxiety in females is associated with the induction of elevated corticosterone levels across one generation. These effects are likely mediated by changes in somatic, not germ, cells of the offspring, because transmission ends with the F1 generation. In contrast, maternal transmission of defective preference for social novelty carries to the F2 generation in the absence of elevated corticosterone. Moreover, paternal inheritance of elevated anxiety, defective social interactions, and preference for social novelty crosses at least 3 generations in the absence of elevated corticosterone levels, suggesting germ-line alterations.
Chronic Social Instability Enhances Rcan1 and Rcan2 Gene Expression in the CA1 of Females Across Generations
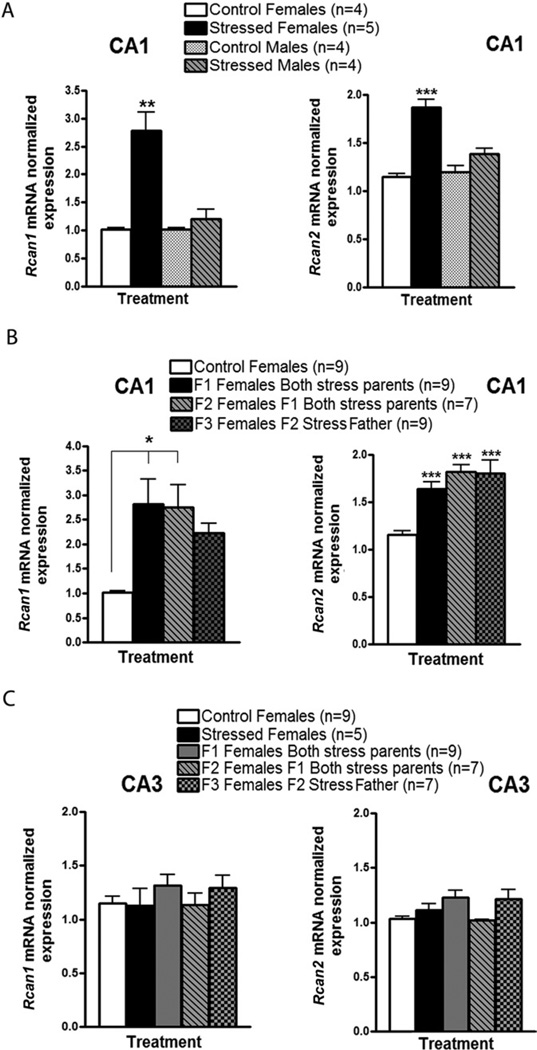
To begin to reveal how these behavioral alterations are induced across generations, we searched for genes whose expression changes in key regions of the brain across three generations, along with enhanced anxiety and defective social interactions. Thus, we first performed a microarray study to compare messenger (m)RNA from the CA1 region of the hippocampus of F1 female offspring from stressed and control parents. CA1 was chosen first because it is involved in deleterious effects of chronic stress (6,22). Two genes whose expression was found to rise in offspring of stressed mice were Rcan1 and Rcan2. Both encode inhibitors of calcineurin, a calcium activated phosphatase known to modulate synaptic plasticity (23–25). Quantitative real-time polymerase chain reaction showed that Rcan1 and Rcan2 mRNA levels were significantly upregulated (~2–3 fold) in the CA1 of stressed females, but not males (Figure 8A). Importantly, Rcan1 and Rcan2 levels remained elevated in the CA1 of F1, F2, and F3 females from stressed male predecessors compared to control female offspring (Figure 8B). Interestingly, their levels were not altered in the CA3 (Figure 8C). Overall, these results suggest that a stress-induced, transgenerational increase in the inhibition of calcineurin may contribute to the elevated anxiety and/or defective social interactions that are transmitted across generations.
Figure 8.
Chronic social instability-induced upregulation of Rcan1 and Rcan2 genes in the CA1 region of females across generations. Quantitative real-time polymerase chain reaction for Rcan1 and Rcan2 messenger (m)RNA detection was performed using CA1 and CA3 complementary DNA samples from socially stressed and control mice, as well as their female offspring (F1, F2, and F3). (A) Rcan1 (left panel) and Rcan2 (right panel) normalized mRNA levels in the CA1 of stressed females were significantly upregulated compared with control females, stressed males, and control males [for Rcan1 F(3,25) = 10.87, p < .01; for Rcan2 F(3,25) = 32.83, p < .001] 2 months after the end of the social stress treatment. No significant differences were detected in Rcan1 or Rcan2 mRNA levels between stressed and control males (p > .05). (B) Rcan1 (left panel) and Rcan2 (right panel) normalized mRNA levels in the CA1 of female offspring (F1, F2, and F3) from stressed parents were significantly increased compared with control female offspring [for Rcan1 F(3,33) = 3.205, p < .05; for Rcan2 F(3,33) = 11.19, p < .001]. Although Rcan1 mRNA levels in the CA1 of F3 females from the stressed male lineage were not statistically different from those in the control females, there is a clear tendency for Rcan1 upregulation in the former group. (C) Rcan1 (left panel) and Rcan2 (right panel) normalized mRNA levels in the CA3 of females directly exposed to social instability or in the female offspring from stressed parents were not significantly different from those of control females [for Rcan1 F(4,31) = .660, p > .05; for Rcan2 F(4,31) = 1.124, p > .05). The control groups depicted in panels B and C were composed of F1, F2, and F3 females from control mice. Number of litters used in B and C: 4–5 litters from both stressed females and males, 3 litters from F1 mice from both stressed parents, 3 litters from F2 mice from stressed fathers, and 2 litters from both control mice. Error bars indicate SEM. *p < .05; **p < .01; ***p < .001.
Discussion
Chronic social instability can cause long-term psychiatric alterations, particularly when experienced by youth (26,27). In fact, a mouse model of chronic social instability during adolescence and early adulthood produced long-term behavioral and endocrine alterations, including increased anxiety, deficits in locomotor adaptation, and elevated corticosterone levels (17–19,28). In addition to those previously reported phenotypes, we found that this stress treatment also induced long-term social defects including reduced physical interactions with a same-sex juvenile, implying social anxiety or social withdrawal, and loss of preference for social novelty. These defects were not due to the fact that stressed mice had defective neurologic reflexes in response to visual or auditory stimuli that are required for a normal social interaction, because previously stressed mice did interact normally with an enclosed novel mouse in a three-chambered compartment. Overall, these findings add to a growing body of work demonstrating that long-term changes in behaviors associated with a variety of psychiatric disorders such as enhanced anxiety and defective social interactions can be induced by stressful conditions during youth.
More dramatically, our results also demonstrate that these disorders can be transmitted across multiple generations. In the first generation, the transmission of enhanced anxiety and defective social interactions occurred through both maternal and paternal lines. However, although all of the alterations were transmitted through the paternal line across three generations, only the lack of preference for social novelty was transmitted through the second generation in the maternal line. In F1 through F3 generations, the abnormal behaviors were exhibited only by females (for a summary, see Figure S5 in Supplement 1).
Our detection of behavioral defects in the F1 offspring of female mice exposed to chronic social instability during adolescence and early adulthood was not particularly surprising. This is because we observed enhanced corticosterone levels in females for months after exposure to stress, time that includes pregnancy and nurturing of F1 pups after birth. A large body of evidence has shown that elevated stress signals during either pre- or postnatal periods lead to a variety of behavioral alterations throughout the life of offspring (29). In our case, the transmission of the stress response most likely occurred before birth because when we switched foster parents for stressed parents soon after birth, anxiety phenotypes in the offspring persisted.
Moreover, the behavioral defects associated with anxiety, including reduced time spent in the open arms of the EPM, decreased locomotor adaptation in the OFT, and decreased direct social interaction with a novel juvenile, were not transmitted from the F1 females to their F2 progeny consistent with changes in somatic but not germ cells. Presumably, epigenetic changes in the brains of F1 females occurred in utero in response to the stressed environment, which lead to the rise in corticosterone, enhanced anxiety, and defective social interactions observed in the F1 generation. Interestingly, one abnormality, loss of preference for social novelty, was passed to the F2 generation in the absence of enhanced corticosterone levels or anxiety, suggesting that gene changes normally regulated by stress hormones and involved in defective social interactions may be constitutively altered. This finding also indicates that the loss of preference for social novelty is not caused merely by elevated anxiety.
In contrast, our finding that all of these altered behaviors were also transmitted through stressed fathers was a surprise because although the stressed fathers displayed dysfunctional social behaviors, they did not display enhanced anxiety like their female counterparts did. Moreover, the F1 and F2 fathers transmitted all of these altered behaviors to their F2 and F3 female progeny, even though they themselves did not display any of the tested behavioral alterations. Importantly, the father’s F2 and F3 female offspring did not have elevated corticosterone levels that were associated with these behaviors in F1 females. These findings suggest that germ-line epigenetic changes had taken place in males that engrained the response to elevated corticosterone in their female offspring, even in the presence of normal levels of the hormone.
A previous study on the effects of a different form of stress, early postnatal disruption of mothering, demonstrated different transgenerational effects (30). In that case, transmission was also through the father, but unlike our studies, depressive-like phenotypes were observed. Analysis of sperm from these mice suggested that abundant changes in DNA methylation took place because alterations in the methylation status were detected in genes chosen merely by their potential to be involved in epigenetic regulation. However, whether these changes played a role in transmission of phenotypes across generations was not revealed. In addition, another study on the transgenerational effects of social defeat stress in adult mice found that some, but not all, of the inherited phenotypes were transmitted through sperm after in vitro fertilization (31). This finding raised the possibility that in addition to stressinduced alterations in sperm, behaviorally induced transmission of some phenotypes from the father to the mother may occur during the time of mating after exposure to social stress. Such a mechanism for our studies seems less likely because the males did not show significant defects in the behaviors we tested.
Another feature of our findings was that females were preferentially affected across three generations by exposure of adolescent and early adult mice to chronic social instability. In contrast, in a different study, where stress was induced in neonates by unpredictable separation of mothers who themselves were exposed to restraint stress, altered social behavior was detected preferentially in males (32). Also, in the study on the transgenerational effects of social defeat stress in adults, enhancement of anxiety and depression- related phenotypes was found mostly in male offspring (31). Sex differences in stress sensitivity, including preferential sensitivity of females to social instability (33), have been frequently found and are thought to contribute to sex-biases in certain neuropsychiatric disorders (34). In many cases, this occurs via differences in neuroendocrine responses (35). Although this might account for the specificity of F1 offspring of stressed parents in our study, as well as the results from a recently published social defeat study (31), it does not appear to account for the transmission to the F2 and F3 generations reported here, because these female mice displayed normal corticosterone levels even though they displayed elevated anxiety and dysfunctional social interactions.
We have initiated studies to reveal the mechanisms involved in the transgenerational effects of social instability during adolescence and early adulthood. One approach has been to search for genes whose expression is changed in key regions of the brain, along with behavior changes across generations. Interestingly, we found that the expression of both members of a family of calcineurin inhibitors, Rcan1 and Rcan2 (36) were elevated two- to threefold in the CA1 (but not CA3) region of the hippocampus of stressed females. Importantly, their expression was also elevated across three generations of behaviorally affected female offspring derived from stressed mice through the male lineage.
Rcan1 expression in the hippocampus has already been shown to be induced acutely by restraint stress, but this has been linked to neurodegeneration, not altered anxiety or social behaviors (37,38). Although Rcan2 has not been linked to stress previously, the fact that the expression of two inhibitors of calcineurin, a protein phosphatase regulator of synaptic plasticity, are elevated in the CA1 across generations by social instability suggests that a stress-induced, transgenerational increase in the inhibition of calcineurin in the CA1 may distort the way this region of the hippocampus influences the stress response and/or social behaviors across generations.
Overall, this study reveals that multiple adverse consequences of exposure of mice to chronic social instability during adolescence and early adulthood, including enhanced anxiety and dysfunctional social behavior, are transmitted to females across multiple generations. The types of defective behaviors observed here and their mode of transmission across generations differs substantially with those observed in other related studies that used different types of stress during different developmental periods. This implies that abundant mechanisms exist that lead to transgenerational consequences of the exposure to adverse environments. Therefore, this newly appreciated form of inheritance may play a significant role in the susceptibility of individuals to a variety of psychiatric disorders.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH; Grant Nos. RC1 AA019317 and 02R01MH083324 to LAF) and Tufts Center for Neuroscience Research (NIH; Grant No. P30 NS047243).
We would like to thank Dr. Terence Pang for detecting typographical errors in the pre-publication form of the manuscript that have been corrected.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
Editor’s Note: Minor editorial corrections and clarifications were incorporated into this final version of the article since the article-inpress version was made available online.
References
- 1.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 2.Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: Preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt MV, Czisch M, Sterlemann V, Reinel C, Samann P, Muller MB. Chronic social stress during adolescence in mice alters fat distribution in late life: Prevention by antidepressant treatment. Stress. 2009;12:89–94. doi: 10.1080/10253890802049343. [DOI] [PubMed] [Google Scholar]
- 4.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt MV, Sterlemann V, Muller MB. Chronic stress and individual vulnerability. Ann N Y Acad Sci. 2008;1148:174–183. doi: 10.1196/annals.1410.017. [DOI] [PubMed] [Google Scholar]
- 8.DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Tamashiro KL, Nguyen MM, Sakai RR. Social stress: From rodents to primates. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 11.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 12.Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: Role of neurotrophins from rodents to non-human primates. Neurosci Biobehav Rev. 2009;33:573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog CJ, Czéh B, Corbach S, Wuttke W, Schulte-Herbrüggen O, Hellweg R, et al. Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 14.McCormick CM. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiol Behav. 2010;99:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Sachser N, Hennessy MB, Kaiser S. Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci Biobehav Rev. 2011;35:1518–1533. doi: 10.1016/j.neubiorev.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–429. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Horm Behav. 2008;53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt MV, Scharf SH, Liebl C, Harbich D, Mayer B, Holsboer F, et al. A novel chronic social stress paradigm in female mice. Horm Behav. 2010;57:415–420. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 21.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 22.Joëls M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–31. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 23.Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- 24.Groth RD, Dunbar RL, Mermelstein PG. Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Commun. 2003;311:1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Mansuy IM. Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun. 2003;311:1195–1208. doi: 10.1016/j.bbrc.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 26.McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Morrissey MD, Mathews IZ, McCormick CM. Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol Learn Mem. 2011;95:46–56. doi: 10.1016/j.nlm.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt MV. Molecular mechanisms of early life stress—lessons from mouse models. Neurosci Biobehav Rev. 2010;34:845–852. doi: 10.1016/j.neubiorev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 30.Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 31.Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, et al. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin TB, Linder N, Russig H, Thony B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haller J, Fuchs E, Halász J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: Towards the development of a social stress model in female rats. Brain Res Bull. 1999;50:33–39. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 34.Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- 35.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in response to stress and expression of depressive-like behaviors in the rat. Curr Top Behav Neurosci. 2010;8:97–118. doi: 10.1007/7854_2010_94. [DOI] [PubMed] [Google Scholar]
- 36.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 37.Porta S, Martí E, de la Luna S, Arbonés ML. Differential expression of members of the RCAN family of calcineurin regulators suggests selective functions for these proteins in the brain. Eur J Neurosci. 2007;26:1213–1226. doi: 10.1111/j.1460-9568.2007.05749.x. [DOI] [PubMed] [Google Scholar]
- 38.Ermak G, Pritchard MA, Dronjak S, Niu B, Davies KJ. Do RCAN1 proteins link chronic stress with neurodegeneration? FASEB J. 2011;25:3306–3311. doi: 10.1096/fj.11-185728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.