Abstract
Numerous antiretroviral therapy (ART) regimens are recommended for first-line and subsequent HIV care, but regimen selection for clinical use may not represent the full range of options. We hypothesized that despite an increase in available antiretrovirals, clinical trial data on regimen efficacy and fixed-dose combination options have lead to uniformity in initial ART. We evaluated regimen selection for ART-naïve patients at the University of Alabama at Birmingham (UAB) 1917 Clinic between January 2000 and December 2007. The annual number of unique initial regimens was quantified. Initial regimen variability was expressed as regimens per 100 patients. Subsequent ART regimens were characterized for complexity via regimen sequence trees detailing the first three generations of regimens for patients starting the two most common initial combinations. Four hundred eighty-two ART-naïve patients were treated with 39 unique initial regimens (8.0 regimens per 100 patients). Variability in initial regimen selection was highest in the first 6 years (14.9–24.4 regimens per 100 patients). A sharp decline was observed in 2006 (16.1 regimens per 100 patients) and 2007 (6.5 regimens per 100 patients). The most dramatic shift in drug selection involved an increase in emtricitabine plus tenofovir plus efavirenz, from 0% in 2003 to 85% in 2007. During the study period, 205 of 482 (43%) patients required a change in initial therapy. Of these, 156 of 205 (76%) had a unique sequence of regimens. A shift toward homogeneity of initial ART was observed (85% of patients received the same first-line regimen in 2007). In contrast, regimen sequencing beyond the first regimen remained complex. These shifts in ART prescribing patterns may have implications for collaborative HIV care.
Introduction
Selection of antiretroviral therapy for HIV-infected patients has traditionally been a complicated process, punctuated by rapidly evolving guidelines and a continuous stream of new therapeutic options and drug combinations. Early in the antiretroviral therapy (ART) era providers made treatment decisions between multiple novel antiretroviral agents with only limited understanding of long-term side effect profiles and few clinical trials that compared individual agents. The first few years of the ART era saw treatment strategies such as “mega-HAART,” triple nucleoside reverse transcriptase inhibitor (NRTI) regimens, and structured treatment interruptions fall from favor in a short period of time.1–4 With the rapid publication of landmark trials, the early ART era can be characterized as a period of progressive evolution in our understanding of optimal combination therapy for HIV. More recently, the advent of fixed-dose combinations of more tolerable agents, comparative trials of initial regimens, and the clear recognition of uninterrupted therapy as the preferred treatment strategy, have greatly advanced our understanding of ART and impacted regimen selection in treatment-naïve patients.5–8
We hypothesized that these recent findings have lead to greater standardization in the selection of initial ART regimens despite a growing number of antiretroviral options. Consistency and homogenization in regimen selection should clarify the perceived complexity of ART and could indicate an opportunity for primary care providers to play a larger, more direct role in the care of HIV infected patients in the United States. In order to characterize the evolution of regimen selection in the context of these considerable changes and test our hypothesis, we performed a retrospective review of the first and subsequent ART regimens prescribed in treatment-naïve patients starting initial therapy at the University of Alabama at Birmingham (UAB) 1917 HIV/AIDS Clinic from January 2000 to December 2007.
Methods
The UAB 1917 Clinic Cohort (www.uab1917cliniccohort.org) database contains detailed sociodemographic, psychosocial, and clinical information from approximately 7000 clinic patients dating back to 1988.9 The clinic currently serves a population of 1211 patients who are 49.4% white and 76.8% male. The payer distribution for the clinic is 25% from Ryan White, 40% from private insurance, and the remainder are covered by Medicare, Medicaid, or charity care. The cohort data are 100% quality assured, whereby all provider notes are reviewed within 72 hours of entry into the clinic electronic medical record to ensure appropriate data capture regarding diagnoses and medications. Using this database, we identified antiretroviral-naïve patients initiating therapy during the study period of January 1, 2000 to December 31, 2007. Two teams of independent medical record abstractors performed chart review to confirm antiretroviral (ARV)-naïve status for all patients. Discrepancies between teams (<2%) were arbitrated by a third team (J.H.W and M.J.M.) who made the final determination on ARV exposure status.
Patients enrolled in research studies evaluating ARV agents were excluded, as provider preference was not the sole determinant for regimen selection. All ARV medications prescribed to patients treated through routine clinical care were electronically extracted from the cohort database. Additional targeted chart review was undertaken for all ARV combinations that did not conform to established HIV therapeutic guidelines. A regimen was defined as any combination of medications active against the HIV virus that was used continuously for 14 days or more. Subsequent ART was defined as the addition or subtraction of 1 or more antiretroviral drug to an existing ARV combination for any reason. The transition of individual drugs to fixed-dose combinations was not considered a regimen change (e.g., a change from zidovudine and lamivudine to Combivir®, GlaxoSmithKline, Research Triangle Park, NC).
Statistical analysis
Initial regimen variability
The crude number of unique regimens prescribed in each calendar year was quantified (Count Model). Initial regimen variability for each year of the study was expressed as the number of regimens prescribed per hundred patients (unique regimens/patients starting ARVs × 100) to allow for the comparison of regimen variability over time (Normalized Model). Because we are studying the count of unique ARV regimens, Poisson regression models were fit. The count models were fit without an offset. The normalized models were fit with an offset which reflects the differing number of patients each year. To test for a trend over time, year was treated as a continuous measure.
Initial regimen treatment share
The percentage of patients starting a unique regimen each year was defined as the regimens' “treatment share,” which was plotted over time. We conducted a Cochrane-Armitage test of trend using year as the ordinal variable and the two-level variable of tenofovir plus emtricitabine plus efavirenz versus other therapy.
Regimen sequence analysis—the regimen tree
Data on the two initial regimens with the largest overall treatment shares—zidovudine plus lamivudine plus efavirenz and emtricitabine plus tenofovir plus efavirenz—are presented as “regimen sequence trees.” This was used to assess the variability in subsequent regimen sequences among patients with a common initial regimen. The figures start with the initial regimen and branch out to display all unique second- and third-line regimens utilized in the subsequent therapy of these patients.
Results
Initial regimen selection
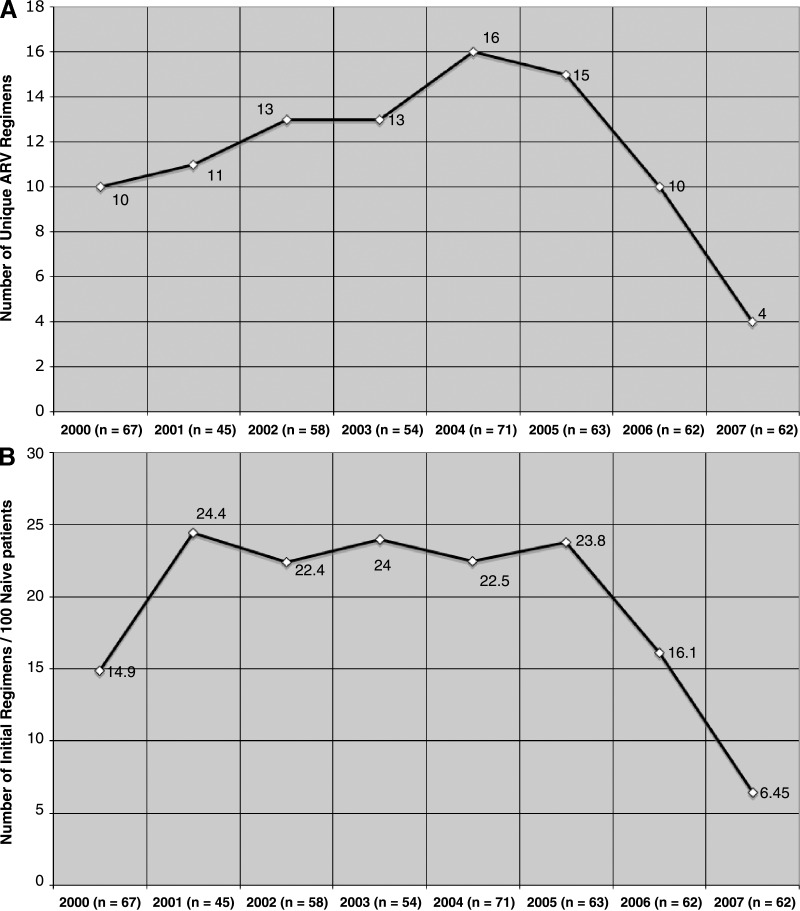
A total of 482 antiretroviral naïve patients initiated ART as part of routine care at the 1917 Clinic between January 2000 and December 2007. The number of patients starting therapy annually remained relatively stable whereas the total number of unique ART regimens prescribed per calendar year declined dramatically during the latter part of the study period (Fig. 1). Between 2000 and 2006, the number of unique ART regimens used was high (10–16 ART regimens; 14.9–24.4 regimens per 100 patients), indicating considerable variability in initial regimen choice during this time period. Subsequently, the annual variability in initial regimen selection abruptly declined, from 16.1 regimens per 100 patients in 2006 (10 ARV regimens) to 6.5 regimens per 100 patients in 2007 (4 ART regimens, Fig. 1). Poisson regression analysis showed no evidence of a linear relationship between year and number of unique ARV regimens. There was a statistically significant quadratic relationship in both the count (p < 0.01) and the normalized (p < 0.01) models.
FIG. 1.
Crude number of initial regimens (A) and initial regimen variability (number of initial regimens per 100 patients) (B) of 482 antiretroviral-naïve patients at the University of Alabama 1917 HIV Clinic starting therapy January 1, 2000 to December 31, 2007. A decline in the number of unique regimens utilized over time is observed.
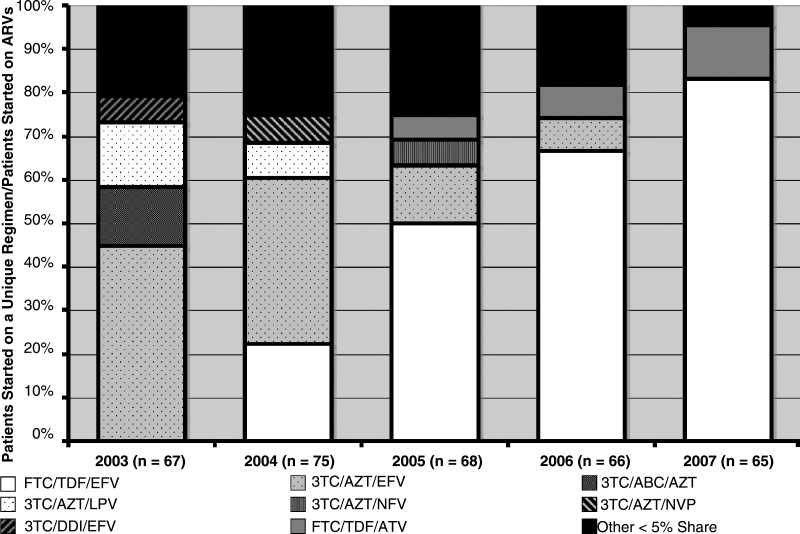
A descriptive analysis of treatment share also demonstrated notable changes in initial regimen selection (Fig. 2). The initial regimen of tenofovir plusemtricitabine plus efavirenz experienced the greatest growth, from 0% in 2003 to 85% of initial ARV combinations in 2007. This shift coincided with a marked decline in zidovudine plus lamivudine plus efavirenz use, which dropped from 46% in 2003, to 13% in 2005, and then to 0% in 2006 and 2007. The Cochrane-Armitage test of trend indicated that the increase in tenofovir plus emtricitabine plus efavirenz was highly significant (p < 0.001). In 2000 and 2001, seven unique regimens accounted for 95% of the total initial ARV treatment share. By 2007, only two unique regimens were used in the treatment of over 95% of patients starting ARV combinations, reflecting a trend toward homogenization of initial treatment selection.
FIG. 2.
Annual treatment share for unique antiretroviral regimens prescribed as initial therapy at the University of Alabama 1917 HIV Clinic from January 1, 2003 to December 31, 2007. Several trends including the cessation of initial triple NRTI (3TC/ABC/AZT) use (2003–2004), the decreasing utilization of 3TC/AZT/EFV (2003–2006), and the rise of FTC/TDF/EFV as the preferred option at the end of 2007 can be observed.
Regimen sequence
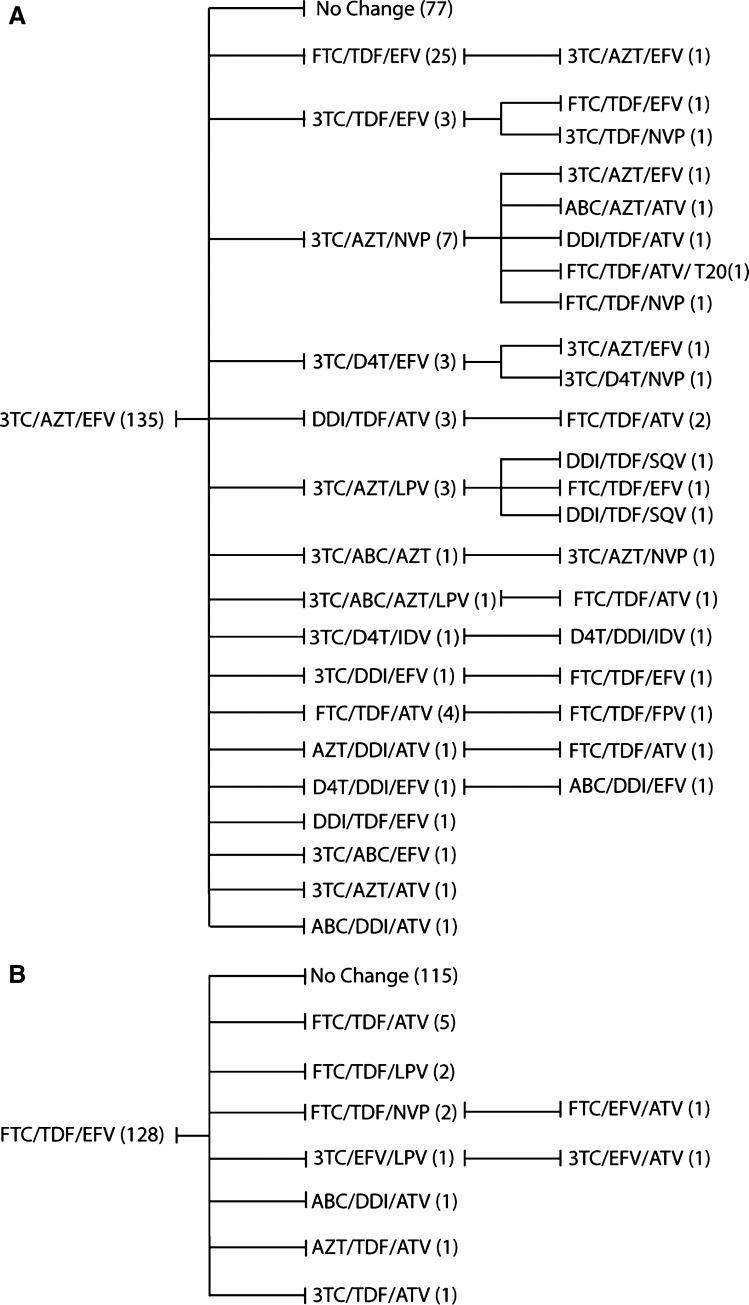
Among the 482 patients, 205 (43%) required second-line therapy during the study period. Overall, 156 of 205 patients (76%) had a unique sequence of consecutive regimens. To capture the individual variability of treatment history, we utilized regimen sequence trees to detail the first three consecutive ARV combinations for the two most commonly prescribed initial regimens (zidovudine plus lamivudine plus efavirenz [n = 135]; tenofovir plus emtricitabine plus efavirenz [n = 128]; Fig. 3). Among patients started on zidovudine plus lamivudine plus efavirenz, 58 individuals who required second-line ART were switched to 17 distinct regimens. Clinicians subsequently used 21 unique regimens for 22 individuals who required a third ARV combination from this common starting point (Fig. 3A). Similar heterogeneity was observed among patients changing from an initial regimen of emtricitabine plus tenofovir plus efavirenz, although fewer of these patients required changes, in part due to the relatively recent emergence of this combination. Among the 13 patients who changed from this initial ART regimen, 7 unique second-line regimens were prescribed (Fig. 3B). These findings point to the persistence of interindividual heterogeneity in subsequent ARV regimen choice despite the trend toward the homogenization of initial ARV therapy.
FIG. 3.
Regimen sequence analysis for the two most common initial ART regimens; lamivudine, zidovudine, with efavirenz (A) and emtricitabine, tenofovir, with efavirenz (B). This figure outlines the unique treatment course followed by the majority of patients who require a regimen change, despite a common initial regimen.
Discussion
This study describes the dynamic evolution of ART for treatment-naïve HIV-infected patients in clinical practice since 2000. Despite an expanding number of treatment options created by the approval of over 25 antiretroviral drugs, the number and variability of regimens prescribed as initial regimens decreased markedly between 2000 and 2007. Notably, a single regimen accounted for 85% of all initial therapies started in 2007. In contrast, subsequent ART among patients changing regimens remained highly individualized and heterogeneous (Fig. 3).
The observed homogenization of initial therapy appears to be due to both the confluence of clinical trial outcomes and the greater convenience afforded by once-daily, fixed-dose combinations of commonly used ARV agents.3,5–7,10 A strong shift away from the NRTI backbone of zidovudine plus lamivudine toward emtricitabine plus tenofovir began in 2004. This change was coincident with reports from a randomized trial showing improved outcomes with emtricitabine-tenofovir versus zidovudine-lamivudine regimen backbones.6,11 Similarly, the sharp reduction in zidovudine plus lamivudine plus abacavir followed the publication of an AIDS Clinical Trial Group (ACTG) study showing that this triple NRTI regimen was inferior to an efavirenz-based strategy (ACTG 5095).7 The simultaneous emergence of clinical trial data and more convenient therapeutic options has led to a dramatically more streamlined approach to initial therapy.
In contrast to the increasing uniformity of initial therapy, patients who required a subsequent regimen painted a more complex picture (Fig. 3). The regimen sequence trees for the two most common initial ARV regimens (ZDV/3TC/EFV and TDF/FTC/EFV) highlight the divergence of individual treatment histories over time. This heterogeneity of subsequent regimens is influenced by several factors, including: available drugs; temporal popularity of each ARV regimen (provider and patient preference); and the reason for changing ARV therapy (side effects, resistance, attempts to improve dosing schedule, or reduce pill burden). In addition, the lack of consensus regarding the sequencing of optimal salvage regimens plays an important role, contributing to the high degree of variability among individual patient experiences.12
At a time when HIV-infected patients are increasingly being cared for by HIV specialists due to the perceived complexity of ART, our findings suggest that initial HIV therapy is becoming more uniform. Should one or two regimens remain the most popular and effective choices for initial therapy, expanding the role of primary care providers in this patient population could be considered. Successfully treated patients are now expected to live for decades but are known to suffer from complications of ARV therapy and other chronic conditions including accelerated cardiovascular disease, hyperlipidemia, hypertension, and glucose intolerance/diabetes.10,13 A system designed to share responsibility for patients, whereby experienced HIV providers are responsible for ARV selection and primary care practitioners provide internal medicine expertise and treatment follow-up as part of a collaborative multidisciplinary team, would create a coordinated, comprehensive model of care for HIV infected individuals.14–16 In the context of expanded HIV testing and anticipated increases in the number of newly diagnosed patients requiring treatment, a system of bidirectional collaborative care would also improve capacity of HIV specialty clinics to meet the expanding needs of people living with HIV/AIDS.17–21
The results of this study should be interpreted within the context of its limitations. As a single, academic HIV-treatment center, in the southeastern United States, results may not be generalizable to other populations. In addition, most treatment decisions were made before resistance testing of naïve patients became standard of care. It remains to be seen how this testing will impact initial regimen selection. Furthermore, due to the small number of patients changing therapy in recent years, quantification of regimen variability for subsequent ART was limited among patients initially treated with TDF/FTC/EFV. However, evaluation of patients treated with the most popular first-line regimen in the early 2000s (ZDV/3TC/EFV) displayed considerable heterogeneity of subsequent ART regimen selection.
In conclusion, initial ART regimen selection has become increasingly homogenized in recent years, while subsequent treatment remains divergent, heterogeneous and highly individualized. These findings have implications for the future provision of HIV care. An expanded role for primary care providers in the management of HIV-infected patients would allow patients to benefit from the internal medicine expertise of these providers and help meet the expanding care needs for people living with HIV/AIDS. The inevitable need for subsequent regimen changes and the persistent complexity of subsequent therapy mandate effective comanagement with experienced HIV providers. While there may be a number of approaches to incorporate more providers in routine care of this disease, any model for HIV care requires careful consideration of the psychosocial and unique case management needs of newly diagnosed HIV patients.
Acknowledgments
We thank the University of Alabama at Birmingham 1917 Clinic HIV/AIDS Clinic Cohort management team for their assistance with this project and Dustin Rhinehart for his assistance with the creation of the figures for the regimen sequence trees.
1917 Clinic Cohort Team Steering Committee
Michael S. Saag, Michael J. Mugavero, James H. Willig, James L. Raper, Paul Goepfert, Jeroan J. Allison, Mirjam-Colette Kempf, Joseph E. Schumacher, Inmaculada B. Aban.
Faculty Investigators
Hui-Yi Lin, Maria Pisu, Linda Moneyham, David Vance, Susan L. Davies, Eta Berner, Edward Acosta, Jennifer King, Richard A. Kaslow, Eric Chamot, Andrew O. Westfall.
Research Support Team
Karen Savage, Christa Nevin, Frances B. Walton, Malcolm L. Marler, Sarah Lawrence, Barbara Files-Kennedy, D. Scott Batey.
Informatics Team
Manoj A. Patil, Ujavala Patil, Mohit Varshney, Eugene Gibson, Suneetha Thogaripally, Alfredo Guzman, Dustin Rinehart, Ridha T. Bagana.
Current Trainees
James McKinnell, Paula Seal, Jessica Pullins, David Jackson, Rebecca Wylie, Cynthia Baffi, Noah Godwin.
Dr. James McKinnell had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Presented in part at the 48th annual ICAAC/IDSA 46th annual meeting in Washington D.C., October 2008.
The UAB 1917 Clinic Cohort is supported by the UAB CFAR (P30-AI27767), CNICS (1R24-AI067039-1), and the Mary Fisher CARE Fund.
This study was approved by the University of Alabama at Birmingham Institutional Review Board.
Dr. James McKinnell, M.D. contributed to this work by assisting with conception and design, acquisition of data, analysis of data, and drafting of the manuscript.
Dr. James Willig, M.D. contributed to this work by assisting with conception and design, acquisition of data, analysis of data, and drafting of the manuscript.
Andrew Westfall, M.S. contributed to this work by assisting with conception and design, analysis of data, and revision of the manuscript.
Christa Nevin, M.S.P.H contributed to this work by assisting with conception and design, acquisition of data, and revision of the manuscript.
The remaining authors provided substantial contributions to the design, data acquisition or interpretation of data and critically revised the intellectual content of the manuscript. All authors approved the final version of the manuscript.
Author Disclosure Statement
Dr. McKinnell has received research funding from the Bristol-Myers Squibb Virology Fellows Research Program for the 2008-2009 Academic Years.
Dr. Saag has received research support from and has served as a consultant to Achillion Pharmaceuticals, Avexa, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Monogram Biosciences, Panacos, Pfizer, Progenics, Roche, Serono, Tanox, Tibotec, Trimeris, and Vertex.
Dr. Willig has received research funding from the Bristol-Myers Squibb Virology Fellows Research Program for the 2006–2008 Academic Years and has consulted for Bristol-Myers Squibb and Gilead Sciences.
Dr. Mugavero has received recent research funding from Tibotec Therapeutics and has consulted for Bristol-Myers Squibb and Gilead Sciences.
References
- 1.Montaner JS. Reiss P. Cooper D, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: The INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 2.Carr A. Samaras K. Chisholm DJ. Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 3.Shafer RW. Smeaton LM. Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2304–2315. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz L. Martinez-Picado J. Romeu J, et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS. 2000;14:397–403. doi: 10.1097/00002030-200003100-00013. [DOI] [PubMed] [Google Scholar]
- 5.Walmsley S. Bernstein B. King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 6.Gallant JE. DeJesus E. Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 7.Gulick RM. Ribaudo HJ. Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren JD. Babiker A. El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: Role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 9.Chen RY. Accortt NA. Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 10.Willig JH. Abroms S. Westfall AO, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22:1951–1960. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazzard B. DeJesus E. Campo R, et al. Interscience Conference of Antimicrobial Agents and Chemotherapy. Washington, D.C.: 2004. The combination of tenofovir (TDF), emtricitabine (FTC) and efavirenz (EFV) has significantly greater responses vs fixed dose zidovudine/lamivudine (CBV) and EFV in antiretroviral naive patients: A 24 week preliminary analysis [Abstract H1137c] [Google Scholar]
- 12.Hammer SM. Eron JJ., Jr. Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society—USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 13.The Antiretroviral Therapy Cohort Committee. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine (U.S.) Primary Care: America's Health in a New Era. Washington, D.C.: National Academy Press; 1996. Division of Health Care Services. Committee on the Future of Primary Care., Donaldson MS. [Google Scholar]
- 15.Barr M. Ginsburg J. The advanced medical home: A patient-centered, physician-guided model of health care. American College of Physicians: Current Policy Papers. 2006.
- 16.Redesigning the practice model for general internal medicine. A proposal for coordinated care: A policy monograph of the Society of General Internal Medicine. J Gen Intern Med. 2007;22:400–409. doi: 10.1007/s11606-006-0082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall HI. Song R. Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branson BM. Handsfield HH. Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11–14. [PubMed] [Google Scholar]
- 19.Reddy S. Sabatino S. Coordination of Care: Missed Opportunity? Synthesis of the Literature on Care Coordination. ABIM Foundation Summer Forum. Aug, 2007.
- 20.Saag MS. Opt-out testing: who can afford to take care of patients with newly diagnosed HIV infection? Clin Infect Dis. 2007;45(Suppl 4):S261–S265. doi: 10.1086/522548. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. HIV prevalence, unrecognized infection, and HIV testing among men who have sex with men—Five U.S. cities, June 2004–April 2005. MMWR Morb Mortal Wkly Rep. 2005;54:597–601. [PubMed] [Google Scholar]