Abstract
Prostate cancer (PC) is both an age and androgen-dependent disease. Paradoxically, systemic levels of androgens decline with age as the risk of PC rises. While there is no correlation between systemic androgen levels and the risk of PC, systemic androgen levels do not reflect the levels of androgen in prostate tissue. In metastatic PC, changes in the androgen biosynthesis pathway during hormone therapy cause increased levels of androgens in cancer tissue and contribute to continued androgen receptor (AR) signaling. It is possible that similar changes occur in normal prostate tissue as androgens decline with age and that this contributes to tumorigenesis. We sought to determine if the rat prostate is able to maintain functional levels of androgens despite low serum testosterone (T). Rats were castrated and implanted with capsules to achieve castrate, normal, sub- and supra-physiological levels of T. After six weeks of treatment, LC-MS/MS was used to quantify the levels of T and dihydrotestosterone (DHT) in serum and prostate tissue. QRT-PCR was used to quantify expression of genes involved in the androgen/AR signaling axis. Despite having significantly different levels of T and DHT in the serum, T and DHT concentrations in prostate tissue from different T treatment groups were very similar. Furthermore, the expression of androgen-regulated genes in the prostate was similar among all T treatment groups, demonstrating that the rat prostate can maintain a functional level of androgens despite low serum T levels. Low T treatment resulted in significant alterations in the expression of androgen biosynthesis genes, which may be related to maintaining functional androgen levels.
Keywords: androgen, prostate, prostate cancer, androgen receptor
Introduction
Androgens and the androgen receptor (AR) signaling pathway are intimately associated with prostate carcinogenesis and androgen deprivation therapy remains the most effective way to prevent the growth of metastatic prostate cancer (Miyamoto, et al. 2004). However, the relationship between androgens and the initiation of prostate cancer is still unclear. The risk of prostate cancer, an androgen-dependent disease, is highly associated with age, but serum levels of androgens are known to decline with age (Feldman, et al. 2002). Many groups have studied the relationship between serum androgen levels and prostate cancer risk, and the consensus is that elevated systemic androgen levels do not correlate with prostate cancer incidence (Morgentaler 2009; Roddam, et al. 2008). However, several cross-sectional studies have found that a low testosterone (T) level at the time of prostate cancer diagnosis is correlated with more aggressive disease (Schatzl, et al. 2001). Additionally, in one of the largest and most thorough analyses to date, low serum dihydrotestosterone (DHT) levels were associated with an increased risk of prostate cancer (Goldenberg, et al. 2011). Furthermore, a study in a genetically engineered mouse model of human cancer (Nkx 3.1; Pten mutant mouse) showed that prolonged exposure to low levels of T accelerated tumor progression and led to more aggressive disease than exposure to normal or castrate levels of T (Banach-Petrosky, et al. 2007). While these studies are far from conclusive, it appears that low systemic androgen levels may be associated with an increased risk of prostate cancer incidence and aggressiveness.
A major shortcoming of all of these studies is that they use the serum androgen levels as a surrogate for prostatic androgen levels. It has been clearly demonstrated that prostate and prostate cancer tissues are only modestly influenced by serum levels of androgens. Studies on the molecular impact of testosterone replacement therapy (TRT) as well as of male hormonal contraception (MHC) on intraprostatic androgen concentration and androgen action indicate that intraprostatic level of androgen is not affected by testosterone supplementation(Goldenberg et al. 2011; Marks, et al. 2006; Morgentaler and Traish 2009; Mostaghel, et al. 2012; Page, et al. 2006). A small study in medically castrated healthy men further reveals that despite a 94% decrease in serum T with medical castration, intraprostatic T and DHT levels remained 20-30% of control values, and prostate cell proliferation, apoptosis and androgen regulated protein expression were not affected(Page et al. 2006). These results suggest that the prostate may behave like a buffer which can maintain the intraprostatic level of androgen despite a wide range of serum levels. However, how the prostate buffers androgen concentrations in the milieu of a wide range of systemic androgens is still unclear.
A similar phenomenon occurs in metastatic prostate cancer tissue where, despite castrate levels of androgens in the blood, T and DHT levels in the cancer tissue are sufficient to activate AR signaling and drive cancer growth(Nadiminty and Gao 2012). Several mechanisms have been proposed to explain the continued AR signaling in castration-resistant prostate cancer (CRPC), chief among them changes that allow for the increased uptake of adrenal androgens(Bosland 2000; Chen, et al. 2004; Labrie, et al. 2005; Nadiminty and Gao 2012; Stanbrough, et al. 2006), de novo intracellular androgen synthesis(Cheng, et al. 2010; Locke, et al. 2008; Montgomery, et al. 2008; Nadiminty and Gao 2012), and down-regulation of steroid metabolism(Soronen, et al. 2004), as well as amplification and over-expression of the AR and its splice variants(Chen et al. 2004; Guo, et al. 2009; Hornberg, et al. 2011; Shiota, et al. 2011; Waltering, et al. 2009). We reasoned that many of these same mechanisms may be responsible for the ability of the normal prostate to maintain relatively constant levels of T and DHT despite a wide range of these androgens in the blood, especially in the setting of low T that is often observed in older men.
To test our hypothesis, we first sought to determine if the rat prostate could be used to model intraprostatic androgen maintenance. We also comprehensively examined the expression of genes in the androgen/AR signaling pathway to determine if changes in these genes correlated with the ability of the rat prostate to maintain androgens. We found that the normal rat prostate, much like CRPC tissue, has altered expression of genes in the androgen/AR signaling axis in response to low serum T levels which results in the ability of the prostate to sustain functional intraprostatic androgens levels.
Materials and Methods
Animal protocol
These studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the City of Hope IACUC (11036). A total of 28 male Sprague Dawley rats (8 weeks old, postpubertal) were purchased from Harlan labs and were fed water and rat chow ad libitum. A cohort of rats (n=4) was kept intact as control for the experiments. The remaining rats were randomly divided into 4 groups (6 rats/group) and castrated to eliminate endogenous androgens. The manipulation of testosterone (T) was achieved using silastic capsules, which were implanted subcutaneously via a small skin incision at the same time as castration. Testosterone was purchased from Steraloids (Newport, RI). According to published results, the T capsules were designed to have a release rate of 11.5μg/d for T 0.5cm group, 46μg/d for T 2cm group, or 110 μg/d for T 5cm group, which allowed us to administer sub, normal or supra-physiological levels of T (Moger 1976; Vanderschueren, et al. 2000). Six weeks later, the rats were euthanized, serum was collected by cardiac puncture and T and DHT levels were measured using HPLC/MS/MS. Prostates were also harvested, weighed, and prepared for gene expression analysis or immediately frozen for measurement of T and DHT concentrations.
Androgen concentration measurements
T and DHT levels were determined by mass spectrometry (MS) using methods adapted from those that have been described previously (Montgomery et al. 2008). In brief, frozen tissue samples were individually thawed, weighed, and homogenized in H2O at a concentration of 100mg/mL. 100μL of homogenates or serum were spiked with internal standard Td3 (3-deuteride-T) and DHT d3, diluted with 400μL water and extracted with 5mL hexane. The organic phase was transferred and evaporated to dryness. Each individual concentrated extract was dissolved in 0.1M hydroxylamine hydrochloride in 50% methanol/water and incubated for 1 hour. Standards for DHT and T were prepared in parallel. The resulting oximes were analyzed by HPLC-MS-MS using an Agilent Technologies LC 1100 series system (Palo Alto, CA, USA) interfaced with a Micromass Quattro Ultima Triple Quadrupole Mass Spectrometer (Micromass, Inc., Milford, MA, USA). MassLynx version 4.1 software was used to acquire and analyze data. Separation of analytes was achieved on a Kinetex 2.6 μm 100 × 2.1 mm C18 column (Phenomenex, Torrance, CA, USA). Ions monitored were m/z 304.5>124.25 and 307.5>112.25 for T and T-d3 respectively, and m/z 347.55>306.45 and 350.55>309.45 for DHT and DHT-d3 respectively. Limits of detection (defined as a S/N ≥ 5) for T and DHT were 0.02 and 0.01ng/ml respectively.
Estradiol And dhea Elisa
Dehydroepiandrosterone (DHEA) and estradiol (E2) concentrations were determined by ELISA (DHEA: APLCO, Salem, NH; E2: Diagnositc Automation, INC., Calabasas, CA). The sensitivities of the DHEA and estradiol ELISA assays were 00.108ng/mL and 10pg/mL, respectively. Cross-reactivity of DHEA assay with other steroid is less than <0.1%, and for estradiol, cross-reactivity with other steroids is less than 2%. The measurable ranges for DHEA and E2 are 0-30ng/mL and 0-1000pg/mL, respectively.
RNA Isolation and Quantitative RT-PCR
Total RNA was isolated using a PARIS kit (Ambion), followed by treatment with DNAse using the Qiagen RNase-Free DNase Set (Qiagen Inc, Valencia, CA). cDNA was generated from each sample using 250ng of total RNA in an oligo dT-primed reverse transcription reaction. Quantitative PCR (qRT-PCR) reactions were performed in triplicate using a StepOne Real Time PCR system (Applied Biosystems) with approximately 2.5ng cDNA, 1μM of each primer pair, SYBR Green (Invitrogen) as the detecting dye, Rox (Invitrogen) as the reference dye and Taq PCR kit (Qiagen). Primers specific for genes of interest were designed using the Web-based primer design service PrimerQuest provided by the Integrated DNA technologies. To analyze Ar variant mRNA expression, we adapted the primers previously reported specific to AR splice variants in human (Dehm, et al. 2008; Guo et al. 2009; Hu, et al. 2009b; Sun, et al. 2010). Sequences are provided in Supplementary Table 1.
Cloning and sequencing of Ar variant transcripts
2% cDNA product from 250ng input total RNA was used for each sample. PCR products derived from the primer pairs that span the region of exon 1 to exon 3 and exon 1 to exon 8 of Ar gene (Supplementary Table 1) were cloned into TopoTA vector (invitrogen) and subjected to sequencing analysis by DNA sequencing core facility (City of Hope).
Statistical Analysis
The serum and tissue androgen concentrations were expressed as the mean ± SD. The significance of differences was determined using ANOVA followed by a Tukey correction. P values < 0.05 were considered significant. For analysis of the qRT-PCR data, the mean cycle threshold (Ct) obtained for each gene was normalized to the expression of the housekeeping gene RPL19 in the same sample (the delta Ct). The specificity of amplification in each reaction was assessed based on the melting point of the dissociation curve. The fold change was calculated by taking the inverse log of the difference in mean delta Ct's between the treatment groups and the intact group. One-way ANOVA followed by a Tukey correction was used for planned comparisons between the treatment groups that were defined by linear contrast statements. A p-value < 0.05 was considered significant.
Results
Functional levels of androgens are maintained in the prostate despite low serum testosterone
To determine the relationship between serum and prostate tissue androgen levels in the rat, rats were castrated to remove the endogenous source of testicular androgens and implanted with silastic capsules for delivery of zero, low, normal, or high levels of testosterone. We maintained a group of rats that had not been castrated or otherwise manipulated, which provided a control for endogenous levels of T and DHT (the intact group). After six weeks, the serum and intraprostatic concentrations of T and DHT were assessed by LC-MS/MS analysis.
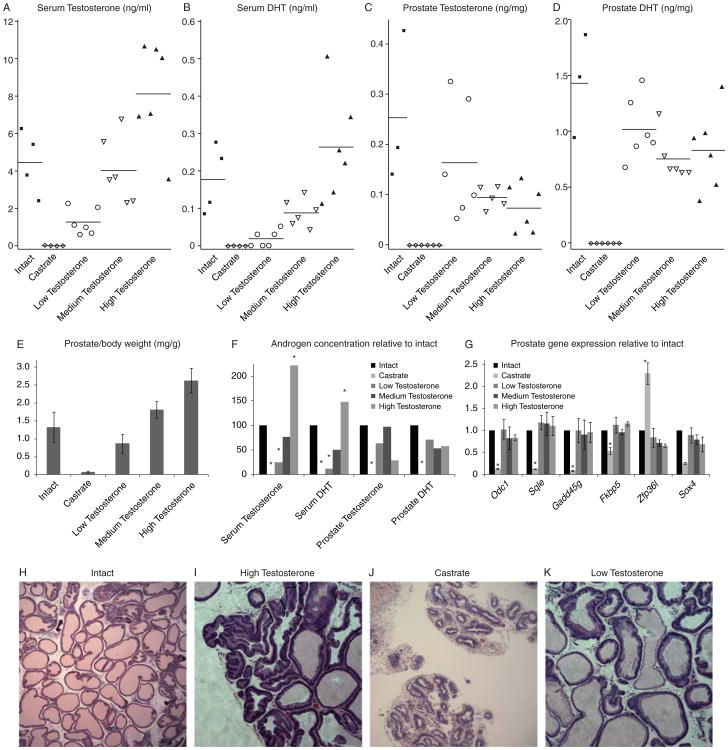
The serum and prostate levels of T and DHT in each rat are shown in Fig 1. Intact rat serum T levels were 4.48±1.72ng/mL and DHT levels were 0.18±0.09ng/mL. Serum DHT was below the level of detection (0.03 ng/mL) in all castrate rats and serum T was below the level of detection in 2/6 rats, with the remainder averaging only 0.04 ng/mL. T values were corroborated by ELISA (data not shown). We observed a linear correlation between size of T implant and serum T and DHT levels, as expected. While the normal T-treated group had similar serum T and DHT levels compared to the intact group (3.98±1.63ng/mL for T and 0.09±0.03ng/mL for DHT), serum levels of T (1.30±0.25ng/mL, p=0.0305) and DHT (0.02±0.02ng/mL, p=0.0171) were significantly lower in the low T-treated group compared to the intact group. The group receiving high T implants had serum T (9.95±2.77ng/mL, p<0.0001) and DHT (0.26±0.14ng/mL, p<0.0001) concentrations significantly higher than those in the intact group or in rats implanted with either low or normal doses of T. The differences in serum androgens were reflected in the wet weight of the prostates, although these differences were not statistically significant from intact animals (Figure 1E). High T treatment resulted in widespread hypertrophy as indicated by increased glandular folding and hyperchromatic nuclei (Figure 1I, compare to intact in 1H), while castration resulted in severely atrophied and compact glands devoid of secretory cells (Figure 1J). The low T treated rat prostates had multiple atrophic foci, as evidenced by glands with flattened epithelial layers (Figure 1K), but there were no other major abnormalities in low-T treated rat prostates.
Figure 1. Serum and prostate levels of androgens.
The levels of serum T (A), serum DHT (B), prostate T (C), and prostate DHT (D) are shown for each rat. (E) Average wet prostate weight from each treatment group. (F) The average concentration of T and DHT in each treatment group compared to intact rats. (G) Relative gene expression for a panel of androgen-responsive genes in the prostates of each treatment group compared to intact rats. Though serum androgen levels are vastly different, prostate androgen levels are very similar in all T-treatment groups, leading to equivalent androgen-dependent gene expression. (H-K) Representative H&E (20× magnification) staining from intact (H), high T (I), castrate (J) and low T (K) rat prostates. Statistically significant differences from intact group (ANOVA, p < 0.05) are indicated by *.
While serum T and serum DHT levels were correlated (r=0.87), neither prostate T nor prostate DHT levels correlated with serum T levels (r=0.06, r=-0.02, respectively). Despite varied serum androgen levels, intraprostatic concentrations of T and DHT were not significantly different among treatment groups (Figure 1 F). Although the serum levels of T and DHT in the low T group were only 29% and 12% of the intact group, the levels of T and DHT in the prostates of the low T group were 64% and 71% as much as the intact group. The converse phenomenon was observed in the high T group. Although serum levels were markedly higher, tissue levels of T and DHT were only 29% and 58% of the intact group – lower than in the low T group. Although these comparisons were made on a per mg of tissue basis, adjusting for prostate size to calculate the total androgenic content of the prostate in each animal lead to the same conclusions. Our results are consistent with other studies that have shown that the relationship between serum and prostate levels of androgens is not linear. Furthermore, the levels of androgens in the prostate of all T-treatment groups appear to be functional as there is no significant difference in the expression of androgen-regulated genes among the groups while the expression of these genes was significantly different in the castrated group (Figure 1G). Our results suggest the rat prostate can preserve a functional level of T and DHT despite low serum levels and is therefore an adequate model for studying the mechanism of intraprostatic androgen maintenance.
Altered expression of AR and genes mediating androgen uptake in the low T environment
To investigate the mechanisms responsible for maintenance of androgens in the prostate in the setting of low serum T, we used qPCR to quantify the expression of genes known to be involved in the androgen/AR signaling axis (Table 1 and supplementary table 1). We first examined the expression of AR and known steroid-transporter genes including the Slco gene family (Slco2b1, Slco1b3, Slco1a5,and Slco2a1), androgen binding protein (Abp), which is the homolog of human steroid hormone binding globulin (Shbg) and low density lipoprotein-related protein 2 (Lrp2, or megalin). Notably, in the Low-T group, AR expression was increased (p=.0099), while the expression of AR in other groups was comparable to the intact group. This suggests that there is pressure to increase AR expression in normal prostate in response to low serum androgen levels much like in metastatic prostate cancer tissue. We also attempted to detect the expression of AR splice variants documented in human CRPC, including AR-V1, AR-V7, AR3-5, and AR567es (Dehm et al. 2008; Guo et al. 2009; Hu et al. 2009b; Sun et al. 2010); however, we were not able to detect the expression of any of these variants, which may be because rats do not have equivalent AR variants as humans. To further identify novel AR splice variants in rats, we amplified the regions spanning from exon 1 to 3 and exon 1 to 8 of Ar gene, but we did not detect any transcripts of unexpected lengths, which suggests there may be no detectable AR splice variants in our rat prostate samples. The only steroid-transporter gene to show any significant differences among treatment groups was Slco2b1, which was significantly increased in the castrate group compared to the intact group (p<0.0001). This suggests that in the rat prostate, a prime response to castration is specific up-regulation of Slco2b1. In humans, the protein this gene encodes regulates the uptake of sulfated steroids (Smith, et al. 2005), though what molecules it normally transports in rats is unknown. One of its major substrates in humans, DHEA-S, is only produced at very low levels in rats (Cutler, et al. 1978). Furthermore, we were not able to detect any DHEA in rat prostate tissues by ELISA. However, it appears that the increased expression of Slco2b1 is not important for the ability of the rat prostate to maintain androgen levels in our low T treated group as its expression in these rats is very similar to that of intact animals. Although we found slight increases in the levels of Lrp2 as well as Abp in response to low serum T levels, the changes were not significant (Lrp2: p=0.4272, Abp: p=0.1218).
Table 1.
Relative expression of genes in different T treatment groups (fold change) versus the intact group (p values in the low T-treated group are indicated; statistically significant differences (ANOVA, p < 0.05) from other groups are indicated by *). Additional genes examined without significant difference from intact group: Cyp11a1, Cyp17a1, Hsd3b2, Akr1c1, Akr1c2, Akr1c3, Srd5a1, Srd5a2, Slco1b3, Slco1a5, Slco2a1
| Gene | Low T (p value) | Med T | High T | Castrate |
|---|---|---|---|---|
| Ar | 1.7(0.009) | 0.93 | 1.15 | 0.96 |
| Slco2b1 | 1.1(0.998) | 0.82 | 0.69 | 4.48* |
| Abp | 1.87 (0.121) | 1.00 | 1.72 | 0.59 |
| Lrp2 | 1.8 (0.427) | 1.02 | 0.98 | 1.50 |
| Hsd3b1 | 2.03(0.042) | 1.36 | 1.16 | 0.40 |
| Hsd17b1 | 2.74(0.008) | 1.91 | 2.06 | 1.22 |
| Hsd17b3 | 3.67(0.029) | 2.98 | 2.76 | 3.21 |
| Hsd17b6 | 2.92(0.005) | 1.19 | 0.52 | 0.16 |
| Hsd17b9 | 2.25(0.041) | 2.04 | 1.60 | 1.37 |
| Srd5a3 | 1.68(0.083) | 1.46 | 1.59 | 0.80 |
| Hsd17b7 | 1.06(0.998) | 0.98 | 1.23 | 0.33* |
| Hsd17b10 | 1.56(0.015) | 0.98 | 1.27 | 0.97 |
| Cyp19a1 | 1.60(0.037) | 1.20 | 1.37 | 0.83 |
| Sult1e1 | 3.32(0.112) | 1.85 | 1.21 | 0.69 |
| Hsd17b2 | 3.07(0.140) | 2.14 | 2.13 | 1.98 |
| Gusb | 4.06(0.635) | 3.50 | 1.98 | 2.72 |
| Arsc1 | 1.72 (0.081) | 1.30 | 1.21 | 1.20 |
Alternations in Genes Encoding Steroidogenic Enzymes in low-T treated Group
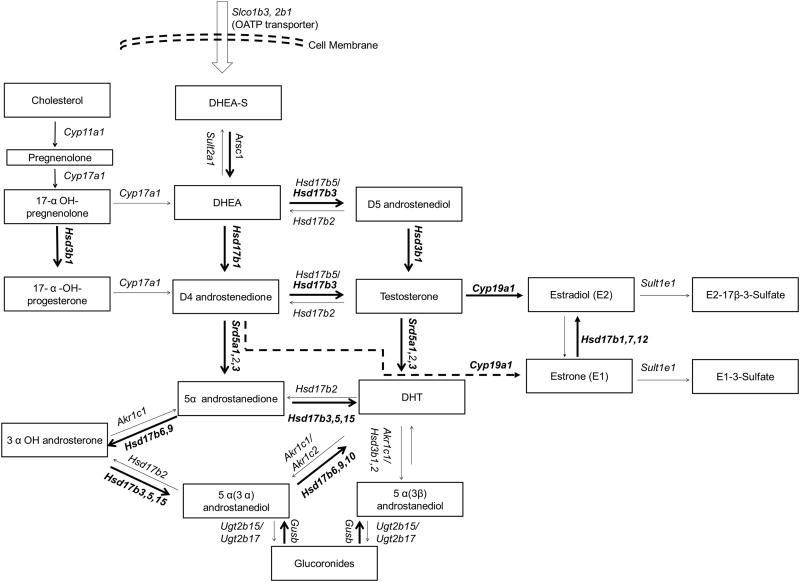
To investigate the role of steroid synthesis enzymes in intraprostatic androgen maintenance, we quantified transcripts encoding many enzymes involved in this process. Compared to the intact group, the low-T treated group demonstrated significant increases in the expression of Hsd3b1, Hsd17b3, Hsd17b6, Hsd17b9, Srd5a3, Hsd17b10, key enzymes required for metabolism of cholesterol to T and DHT (Table 1). The expression of Cyp11a1, Hsd17b2, Gusb, and Arsc1 increase in the low T group relative to the intact group as well, but the changes did not reach statistical significance. Nearly all of these changes were more pronounced in the low T group compared to the normal and high T groups, suggesting a stronger selection for up-regulation of these genes in the low T environment. The changes in expression of these genes would be predicted to lead to increased synthesis of T and/or DHT (Figure 2). Interestingly, Cyp19a1, the gene encoding the aromatase enzyme that converts T to estradiol (E2), Hsd17b1, encoding the enzyme involved in the conversion of estrone (E1) to E2, and Sult1e1, the gene encoding the enzyme that metabolizes E2 to E2-sulfate, were also up-regulated in the low T treated group. However, E2 was below the limit of detection by ELISA (5pg/mg) in all our rat prostate samples, so it is unclear how the changes in the estrogen pathway affect tissue hormone levels. Taken together, our results suggest that low serum levels of androgens may lead to changes in the steroidogenic pathway in the prostate that allow it to synthesize T and DHT de-novo or convert unidentified precursor androgens to maintain functional levels of androgens.
Figure 2. Changes in steroid biosynthesis pathway lead to T and DHT accumulation.
Genes in which statistically significant changes occurred in Low T-treated rats vs. intact rats are in bold. These changes would be predicted to lead to increased levels of T and DHT.
Discussion
The complex relationship between serum and prostate androgen levels and the risk of prostate cancer is not well understood. Here we confirm the results of others that show that the rat can provide a useful model for understanding the relationship between serum and prostate androgens (Kyprianou and Isaacs 1987a). We found that despite a wide range of serum T levels, the levels of androgen in the prostate were fairly constant and not related to serum T levels, which has been shown previously by Kyprianou and lsaacs (Kyprianou and Isaacs 1987b). Kyprianou and lsaacs treated castrate rats with various sizes of silastic T capsules to demonstrate that “androgen-induced increase in prostatic cell number occurs as a quantal process which can only begin when the concentration of prostatic DHT is above a critical threshold value (ie, 0.4 ng/10(8) cells for the rat ventral prostate).” We anticipate that a similar threshold exists for maintaining androgen-dependent signaling in the prostate and furthermore, that a threshold exists for the concentration of blood androgens necessary to maintain tissue androgens. We also suspect that this threshold impacts tumorigenesis because in the Nkx3.1/pten knockout model, castration reduces prostate cancer incidence but low T increases incidence (Abate-Shen, et al. 2003).
Our findings in the rat also parallel previous findings in humans (Goldenberg et al. 2011; Morgentaler 2009; Morgentaler and Traish 2009; Page et al. 2006). Page and colleagues found that either medical castration or androgen supplementation in healthy men resulted in substantial changes in the serum T level while intraprostatic T or DHT concentrations were relatively unaffected (Page, et al. 2011). Likewise, in a study of 44 men, Marks et al demonstrate that six months of TRT normalizes serum androgen levels but does not affect prostate tissue androgen levels and cellular functions (Marks et al. 2006). In the setting of prostate cancer, several studies have found that intraprostatic androgen levels are preserved despite castrate serum T and DHT levels following androgen deprivation therapy (Mohler, et al. 2004; Montgomery et al. 2008). Moreover, these levels of intraprostatic androgens are sufficient to maintain important biological processes within the gland as evidenced by the continued expression of androgen-regulated genes. Likewise, we found that androgen levels in the prostates of low T-treated rats were sufficient to maintain the expression of androgen-regulated genes. Together, these data support the hypothesis that in humans and rats, serum androgen levels are not equivalent to prostate androgen levels and that the prostate is able to adapt to variable androgen environments to maintain a functional level of androgen.
In humans, several mechanisms have been shown to contribute to androgen maintenance in the setting of CRPC, many of which may be relevant in intraprostatic androgen maintenance in healthy human and rat prostate as well. Chen and colleagues have shown that increased AR expression is instrumental in the progression from androgen-dependent to castration-resistant growth, with the degree of AR up-regulation observed sufficient to allow tumor cell proliferation in 80% lower androgen concentrations (Chen et al. 2004). We also observed a slight but significant increase in the expression of AR when the prostate was exposed to low systemic T. While this certainly contributes to maintaining AR-dependent signaling, it could also contribute to the increased prostatic androgen levels by simply increasing the amount of androgen bound and retained in the prostate. However, the slight increase in AR expression, the estimated femtomolar concentration of AR, and the presence of a plethora of other androgen binding proteins in the prostate suggests that increased androgen retention is not the major mechanism of androgen maintenance in normal rat prostate tissue.
Increased uptake of androgens from the serum is another potential contributor to intraprostatic androgen maintenance. We assessed several genes reported to mediate androgen delivery and uptake in humans, but found none to be significantly altered in the setting of low T. We did however observe slight increases in the expression of Abp and megalin. It is possible that with larger cohorts of animals, these values would have reached statistical significance. Abp, the SHBG homolog, is a well-characterized plasma protein that regulates the level of bioavailable androgens in the blood and has also been shown to be produced by prostate cells and to facilitate androgen entry into cells via interaction with endocytotic receptors such as megalin (Hammes, et al. 2005), signaling receptors which remain to be identified (Heinlein and Chang 2002; Kahn, et al. 2008), or proteins in the extra-cellular matrix, such as fibulin (Ng, et al. 2006). Thus, increased expression of Abp/SHBG and its receptors such as megalin in prostate tissue may contribute to the cellular uptake of steroid hormones and intraprostatic androgen maintenance.
In addition to the uptake of circulating T and DHT, the conversion of circulating adrenal androgen precursors, primarily DHEA-S, has also been reported as a potential source of prostate androgens in CRPC tissue (Bartsch, et al. 1990; Labrie, et al. 2001; Stanbrough et al. 2006). However, in agreement of previous studies that indicate that rodent species only have very low levels of circulating DHEA-S due to a lack of adrenal CYP17 expression (Ando, et al. 1988; Cutler et al. 1978; Hu, et al. 2009a), we could not detect any DHEA in serum or in the prostate by ELISA. While an unknown adrenal androgen could contribute to the androgenic content of the rat prostate, the fact that T and DHT were not detected in prostates from castrate rats argues against an adrenal androgen source, since the adrenal gland was intact in these animals. Thus, at least in rats, conversion of adrenal androgens does not appear to contribute to intraprostatic androgen maintenance.
We did observe many significant changes in the expression of enzymes involved in the biosynthesis and metabolism of androgens in response to low serum T. Many of these changes were very similar to those that have been reported in prostate cancer cells as they transitionto castration resistance. We observed an up-regulation of Hsd3b1, Hsd17b1, Hsd17b3, Hsd17b6, Hsd17b9, Hsd17b10, and Srd5a3, all of which would be expected to lead to an increase of T and DHT (Figure 2). In one of the most comprehensive studies to date, Montgomery and colleagues saw a similar up-regulation in the expression of several of these enzymes in castration-resistant versus androgen-sensitive prostate cancer tissue (Montgomery et al. 2008). They also observed a decrease in SRD5A2 expression and an increase in SRD5A1 expression. While we did not see significant changes in these genes, we did see a significant increase in the expression of Srd5a3, an enzyme that can mediate the same conversion of androgens to their 5-alpha metabolites (Uemura, et al. 2008). This alternative enzyme usage might be specific to rats vs. humans or to the process of intraprostatic androgen maintenance versus the process of castration resistance. Of note, we did not observe the expression of any unusual AR transcripts or AR splice variants, as has been reported in CRPC tissue (Guo et al. 2009; Hornberg et al. 2011; Hu et al. 2009b). This suggests that the expression of AR splice variants may be unique to humans and/or the process of castration resistance. The altered expression of genes encoding enzymes in the androgen biosynthetic pathway suggests that increased synthesis of androgens in the prostate may be major factor in androgen maintenance in the rat prostate in a low systemic T environment, much as it is in the setting of CRPC. Whether the normal rat prostate can synthesis T and DHT de novo from cholesterol remains to be determined.
The Montgomery study also noted significant changes in the metabolic enzymes responsible for the conjugation of glucuronide to androgens, UGT2B15 and UGT2B17. These proteins do not have direct homologs in rats, but we did observe changes in the expression of two genes involved in the sulfate and glucuronide metabolic pathways: Gusb, which encodes the β-glucuronidase enzyme responsible for the hydrolysis of steroid glucuronides, and Arsc1, which encodes the enzyme responsible for the hydrolysis 17β-estradiol-3-sulfate as well as DHEA-sulfate. Of note, β-glucuronidase has been reported to be increased in prostatic carcinoma compared to in prostatic hyperplasia (Pearson, et al. 1989). Thus, it is possible that these metabolic enzymes participate in the maintenance of intraprostatic androgens.
The Montgomery study also noted a strong increase in the expression of CYP19A1, the gene encoding aromatase. We also observed a significant increase in the expression of Cyp19a1, as well as increased expression of Sult1e1 and Hsd17b1, enzymes involved in estrogen synthesis pathway (Falany, et al. 1995; Ghosh, et al. 2009; Labrie, et al. 1997). These changes should lead to increased concentrations of estrogens in the prostate; however, levels of estradiol were below the level of detection by ELISA in rat prostates in our study. Tissue estrogen levels have not been comprehensively examined in the setting of CRPC, but several studies have shown that estrone and estradiol are not the major estrogens in prostate and prostate cancer tissue (Krieg, et al. 1993; Shibata, et al. 2000). As aromatase expression and estrogens have been implicated in the development of prostate cancer (Ellem and Risbridger 2010; Kozak, et al. 1982; Krieg et al. 1993; Thompson, et al. 2002), there is need for further exploration of estrogens in the response to low systemic T and in prostate tumorigenesis as well as the development of CRPC.
While the rat model of intraprostatic androgen maintenance has important limitations including differences between the rodent and human endocrine systems and prostate structure (Cutler et al. 1978; Sharma and Schreiber-Agus 1999), and the use of continuous T administration which does not reflect the normal diurnal variation or the gradual decline of systemic androgens with age, our data demonstrate that the healthy rat prostate is able to maintain intraprostatic androgens in the presence of low serum androgens through mechanisms similar to those that occur in human CRPC.
Although the development of prostate cancer is androgen-dependent, the incidence of prostate cancer actually increases as systemic androgen levels decline with age. Thus, the relationship between prostate cancer and aging suggests that it may be a decrease rather than an elevation of systemic androgen levels that facilitates prostate oncogenesis. However, there is no direct mechanistic evidence supporting this hypothesis. In light of our current findings and the findings of others, one interpretation is that low serum androgen levels may put a selective pressure on the prostate and confer a growth advantage upon cells that maintain androgen levels and active AR signaling. Selection for cell survival in the setting of declining systemic T has been previously proposed (Prehn 1999), but here we suggest that instead of changes that lead to the selection of androgen-independent cells, low systemic T selects for cells with enhanced sensitivity to androgens and greater dependence on AR-signaling. Changes in AR signaling might result in a switch from regulation of cell differentiation genes to regulation of growth-stimulatory genes, as occurs in CRPC (Hoimes and Kelly 2010). If such changes occur in prostate stem cells, it could lead to the expansion of proliferation-enabled, AR-dependent cells and lead to distinct cancer foci. While we have demonstrated that changes in the androgen/AR-signaling axis likely contribute to intraprostatic androgen maintenance, further studies are required to determine if and how the mechanisms involved in maintaining intraprostatic androgen levels are responsible for prostate tumorigenesis.
Supplementary Material
Acknowledgments
Funding: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Footnotes
Declaration of interest: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP, Borowsky AD, Cardiff RD, Shen MM. Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 2003;63:3886–3890. [PubMed] [Google Scholar]
- Ando S, Canonaco M, Beraldi E, Valenti A, Maggiolini M, Piro A, Tavolaro R, Dessi Fulgheri F. The evaluation of androgen circulating levels following castration in adult male rats. Exp Clin Endocrinol. 1988;91:311–318. doi: 10.1055/s-0029-1210763. [DOI] [PubMed] [Google Scholar]
- Banach-Petrosky W, Jessen WJ, Ouyang X, Gao H, Rao J, Quinn J, Aronow BJ, Abate-Shen C. Prolonged exposure to reduced levels of androgen accelerates prostate cancer progression in Nkx3.1; Pten mutant mice. Cancer Res. 2007;67:9089–9096. doi: 10.1158/0008-5472.CAN-07-2887. [DOI] [PubMed] [Google Scholar]
- Bartsch W, Klein H, Schiemann U, Bauer HW, Voigt KD. Enzymes of androgen formation and degradation in the human prostate. Ann N Y Acad Sci. 1990;595:53–66. doi: 10.1111/j.1749-6632.1990.tb34282.x. [DOI] [PubMed] [Google Scholar]
- Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wu Y, Mohler JL, Ip C. The transcriptomics of de novo androgen biosynthesis in prostate cancer cells following androgen reduction. Cancer Biol Ther. 2010;9:1033–1042. doi: 10.4161/cbt.9.12.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler GB, Jr, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978;103:2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J Steroid Biochem Mol Biol. 2010;118:246–251. doi: 10.1016/j.jsbmb.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Falany CN, Krasnykh V, Falany JL. Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol. 1995;52:529–539. doi: 10.1016/0960-0760(95)00015-r. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457:219–223. doi: 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg SL, Koupparis A, Robinson ME. Differing levels of testosterone and the prostate: a physiological interplay. Nat Rev Urol. 2011;8:365–377. doi: 10.1038/nrurol.2011.79. [DOI] [PubMed] [Google Scholar]
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Hoimes CJ, Kelly WK. Redefining hormone resistance in prostate cancer. Ther Adv Med Oncol. 2010;2:107–123. doi: 10.1177/1758834009356433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, Bergh A, Wikstrom P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Xin D, Chen J, Sun G, Wang Y, Na Y. Changes in the androgen levels in the ventral prostate of spontaneously hypertensive rats after castration. BJU Int. 2009a;104:406–411. doi: 10.1111/j.1464-410X.2009.08442.x. [DOI] [PubMed] [Google Scholar]
- Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009b;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SM, Li YH, Hryb DJ, Nakhla AM, Romas NA, Cheong J, Rosner W. Sex hormone-binding globulin influences gene expression of LNCaP and MCF-7 cells in response to androgen and estrogen treatment. Adv Exp Med Biol. 2008;617:557–564. doi: 10.1007/978-0-387-69080-3_57. [DOI] [PubMed] [Google Scholar]
- Kozak I, Bartsch W, Krieg M, Voigt KD. Nuclei of stroma: site of highest estrogen concentration in human benign prostatic hyperplasia. Prostate. 1982;3:433–438. doi: 10.1002/pros.2990030503. [DOI] [PubMed] [Google Scholar]
- Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1993;77:375–381. doi: 10.1210/jcem.77.2.7688377. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT. Biological significance of measurable androgen levels in the rat ventral prostate following castration. Prostate. 1987a;10:313–324. doi: 10.1002/pros.2990100405. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT. Quantal relationship between prostatic dihydrotestosterone and prostatic cell content: critical threshold concept. Prostate. 1987b;11:41–50. doi: 10.1002/pros.2990110106. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Belanger A. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, Veltri RW, Makarov DV, Partin AW, Bostwick DG, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351–2361. doi: 10.1001/jama.296.19.2351. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61:332–353. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- Moger WH. Effect of testosterone implants on serum gonadotropin concentrations in the male rat. Biol Reprod. 1976;14:665–669. doi: 10.1095/biolreprod14.5.665. [DOI] [PubMed] [Google Scholar]
- Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2009;181:972–979. doi: 10.1016/j.juro.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–320. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Mostaghel EA, Lin DW, Amory JK, Wright JL, Marck BT, Nelson PS, Matsumoto AM, Bremner WJ, Page ST. Impact of male hormonal contraception on prostate androgens and androgen action in healthy men: a randomized, controlled trial. J Clin Endocrinol Metab. 2012;97:2809–2817. doi: 10.1210/jc.2012-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadiminty N, Gao AC. Mechanisms of persistent activation of the androgen receptor in CRPC: recent advances and future perspectives. World J Urol. 2012;30:287–295. doi: 10.1007/s00345-011-0771-3. [DOI] [PubMed] [Google Scholar]
- Ng KM, Catalano MG, Pinos T, Selva DM, Avvakumov GV, Munell F, Hammond GL. Evidence that fibulin family members contribute to the steroid-dependent extravascular sequestration of sex hormone-binding globulin. J Biol Chem. 2006;281:15853–15861. doi: 10.1074/jbc.M512370200. [DOI] [PubMed] [Google Scholar]
- Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, Nelson PS, Matsumoto AM, Bremner WJ. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- Page ST, Lin DW, Mostaghel EA, Marck BT, Wright JL, Wu J, Amory JK, Nelson PS, Matsumoto AM. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab. 2011;96:430–437. doi: 10.1210/jc.2010-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JP, Pretlow TP, Bradley EL, Jr, McGinnis MC, Pretlow TG. Beta-glucuronidase activity in prostatic carcinoma and benign prostatic hyperplasia. Cancer. 1989;64:911–915. doi: 10.1002/1097-0142(19890815)64:4<911::aid-cncr2820640425>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Prehn RT. On the prevention and therapy of prostate cancer by androgen administration. Cancer Res. 1999;59:4161–4164. [PubMed] [Google Scholar]
- Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzl G, Madersbacher S, Thurridl T, Waldmuller J, Kramer G, Haitel A, Marberger M. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47:52–58. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- Sharma P, Schreiber-Agus N. Mouse models of prostate cancer. Oncogene. 1999;18:5349–5355. doi: 10.1038/sj.onc.1203037. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Ito K, Suzuki K, Nakano K, Fukabori Y, Suzuki R, Kawabe Y, Honma S, Yamanaka H. Changes in the endocrine environment of the human prostate transition zone with aging: simultaneous quantitative analysis of prostatic sex steroids and comparison with human prostatic histological composition. Prostate. 2000;42:45–55. doi: 10.1002/(sici)1097-0045(20000101)42:1<45::aid-pros6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Shiota M, Yokomizo A, Naito S. Increased androgen receptor transcription: a cause of castration-resistant prostate cancer and a possible therapeutic target. J Mol Endocrinol. 2011;47:R25–41. doi: 10.1530/JME-11-0018. [DOI] [PubMed] [Google Scholar]
- Smith NF, Figg WD, Sparreboom A. Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination. Expert Opin Drug Metab Toxicol. 2005;1:429–445. doi: 10.1517/17425255.1.3.429. [DOI] [PubMed] [Google Scholar]
- Soronen P, Laiti M, Torn S, Harkonen P, Patrikainen L, Li Y, Pulkka A, Kurkela R, Herrala A, Kaija H, et al. Sex steroid hormone metabolism and prostate cancer. J Steroid Biochem Mol Biol. 2004;92:281–286. doi: 10.1016/j.jsbmb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CJ, Tam NN, Joyce JM, Leav I, Ho SM. Gene expression profiling of testosterone and estradiol-17 beta-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology. 2002;143:2093–2105. doi: 10.1210/endo.143.6.8846. [DOI] [PubMed] [Google Scholar]
- Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, Nakagawa H. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99:81–86. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschueren D, Vandenput L, Boonen S, Van Herck E, Swinnen JV, Bouillon R. An aged rat model of partial androgen deficiency: prevention of both loss of bone and lean body mass by low-dose androgen replacement. Endocrinology. 2000;141:1642–1647. doi: 10.1210/endo.141.5.7472. [DOI] [PubMed] [Google Scholar]
- Waltering KK, Helenius MA, Sahu B, Manni V, Linja MJ, Janne OA, Visakorpi T. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 2009;69:8141–8149. doi: 10.1158/0008-5472.CAN-09-0919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.