Abstract
BACKGROUND
The HPTN 046 trial evaluated the efficacy of extended infant nevirapine (NVP) administration for prevention of HIV transmission through breastfeeding. Infants received daily NVP to 6 weeks of age. HIV-uninfected infants (the intent-to-treat group) received daily NVP or placebo up to 6 months of age. We analyzed emergence of NVP resistance in infants who acquired HIV-infection despite prophylaxis.
METHODS
HIV genotyping was performed using the ViroSeq HIV Genotyping System. Medians and proportions were used to summarize data. Two-sided Fisher’s exact tests were used to evaluate associations between categorical variables.
RESULTS
NVP resistance was detected in 12 (92.3%) of 13 infants who were HIV-infected by 6 weeks and in seven (28%) of 25 infants who were HIV-uninfected at 6 weeks and HIV-infected at 6 months of age (6/8=75% in the NVP arm, 1/17=5.9% in the placebo arm, P=0.001). Among those 25 infants, 4 had mothers who initiated an antiretroviral (ARV) treatment regimen by 6 months postpartum. In all 4 cases, the treatment regimen included a non-nucleoside reverse transcriptase inhibitor (NVP or efavirenz). NVP resistance was detected in all four of those infants by 6 months of age (4/4=100%). In contrast, only three (14.2%) of the remaining 21 HIV-infected infants whose mothers did not initiate ARV treatment developed NVP resistance (P=0.003).
CONCLUSIONS
Extended NVP prophylaxis significantly increased the risk of NVP resistance in infants who acquired HIV infection after 6 weeks of age. Treatment of maternal HIV infection was also associated with emergence of NVP resistance in HIV-infected, breastfed infants.
Keywords: Nevirapine resistance, prevention of mother-to-child transmission, extended nevirapine, HIV
The risk of mother-to-child transmission (MTCT) of HIV through breastfeeding can be reduced by providing infants with daily infant nevirapine (NVP) prophylaxis [1–3] or by providing the mother with a triple-drug regimen during the postpartum period, either for prevention of MTCT of HIV (PMTCT), or as treatment for her own HIV infection [4–8].
Unfortunately, infants who are HIV-infected despite NVP prophylaxis often acquire NVP resistance that may compromise their ability to respond to NVP-containing treatment regimens [9–14]. NVP resistance often emerges in HIV-infected infants after exposure to single dose NVP (sdNVP) or other short course regimens for PMTCT [15, 16]. Provision of extended daily NVP to infants for PMTCT further increases the risk of NVP resistance in the subset of infants who are HIV-infected despite prophylaxis. In the Six Week Extended-Dose NVP (SWEN) trial [1], 21 (84.0%) of 25 HIV-infected Ugandan infants [10] and 11 (91.7%) of 12 HIV-infected Indian infants [11] who received up to 6 weeks of daily NVP had NVP resistance at 6 weeks of age [1]. In the Post-Exposure Prophylaxis in Infants trial in Malawi (PEPI-Malawi) [2], 44 (83.0%) of 53 HIV-infected infants who received up to 14 weeks of daily NVP had NVP resistance at 14 weeks of age [12]. In both the SWEN and PEPI-Malawi trials, NVP resistance mutations were still detectable in most infants at 6 months of age [10–12, 17].
As ARV drugs become more widely available in resource-constrained settings for PMTCT and for maternal and infant HIV treatment, analysis of ARV resistance in perinatal HIV prevention trials becomes more complex. HIV-infected infants may acquire resistance as a result of exposure to study drugs, but may also acquire resistance as a result of exposure to ARV drugs administered to the mother or infant outside of the study for PMTCT or treatment. Our previous studies demonstrated that breastfeeding infants who were HIV-infected despite receiving ARV prophylaxis can acquire resistance to ARV drugs in their mother’s treatment regimen. When women initiated highly active ARV treatment (HAART) in the first year after delivery, six (85.7%) of seven HIV-infected infants in the SWEN trial [18] and 11 (29.7%) of 37 HIV-infected infants in the PEPI-Malawi trial [13] acquired multi-class ARV resistance (resistance to NVP, a non-nucleoside reverse transcriptase inhibitor [NNRTI] plus resistance to nucleoside or nucleotide reverse transcriptase inhibitors [NRTIs]). In those studies, it was not possible to determine whether the infant acquired NNRTI resistance from the PMTCT regimen or from exposure to an NNRTI in the mother’s treatment regimen. Therefore, it is important to consider all sources of ARV exposure when evaluating drug resistance in infants who received an ARV regimen for PMTCT.
HPTN 046 was a randomized, placebo-controlled clinical trial performed in South Africa, Tanzania, Uganda, and Zimbabwe [3] that assessed the safety and efficacy of extending daily infant NVP from 6 weeks to 6 months for prevention of postnatal HIV transmission. In HPTN 046, most women received sdNVP at the time of delivery and most infants received sdNVP with a short course of zidovudine (ZDV) shortly after birth, according to local treatment guidelines at each site [3]. Some women also received ZDV and/or lamivudine (3TC) for PMTCT. Between birth and one week of age, all infants initiated a 6-week course of daily, open-label NVP. At 6 weeks of age, HIV-uninfected infants (intent-to-treat group) were randomized to either receive daily NVP or placebo through six months of age or until the cessation of breastfeeding, whichever was first. Study drug was stopped in infants with confirmed HIV infection and those infants were referred for ARV treatment. Some women in HPTN 046 initiated HAART outside of the study for their own health [3]. In this report, we analyzed factors associated with emergence of ARV resistance in infants in the HPTN 046 trial who were HIV-infected despite extended daily NVP prophylaxis and were also exposed to maternal ARV drugs for PMTCT or treatment.
MATERIALS AND METHODS
Samples used for analysis
Samples were obtained from HPTN 046 (NCT 00074412) version 3.0 [3]. Infants were followed to 18 months of age and plasma was stored within 7 days of birth, at 2, 5, 6, and 8 weeks, and 3, 6, 9, 12 and 18 months. Infants were tested for HIV infection with an HIV-1 DNA PCR test up to and including 12 months of age. At 18 months, infants were tested for HIV infection using an HIV enzyme immunoassay or HIV rapid test. This report includes analysis of NVP resistance at 6 months for HIV-infected infants in the intent-to-treat group (those who were HIV-uninfected at 6 weeks of age). NVP resistance was also analyzed at 6 weeks for infants who were HIV-infected by 6 weeks of age; this included infants who were randomized in the trial at 6 weeks of age and subsequently diagnosed with HIV infection that seemingly occurred between 6 weeks and 8 weeks, as well as infants who were not randomized because they were diagnosed with HIV-infection by 6 weeks of age. Longitudinal plasma and breast milk samples from women who started HAART by 6 months postpartum were also analyzed for ARV resistance.
HIV Genotyping
Infant plasma, maternal plasma, and breast milk samples were analyzed using the ViroSeq HIV-1 Genotyping System v2.8 (Celera, Alameda, CA). Breast milk samples with HIV viral loads >400 copies/ml were also analyzed. HIV genotyping was performed according to the manufacturer’s instructions, with the following exceptions: infant samples were tested using 100 µl of plasma, and a nested PCR procedure [19] was used to amplify plasma and breast milk samples that failed PCR amplification with standard test procedures. HIV sequences were analyzed for the presence of mutations associated with resistance to NNRTIs, NRTIs, and protease inhibitors (PIs). HIV subtypes were determined by phylogenetic analysis as previously described [20] using PHYLIP v3.66.
Statistical Analysis
Medians and proportions were used to summarize the data. Two-sided Fisher’s exact tests were used to test hypotheses about associations between categorical variables.
Ethical considerations
The study was approved by ethics review committees at each study site, and institutional review boards of partner institutions in the United States, if applicable. Written informed consent for participation in the HPTN 046 trial was obtained from the infants’ parents or legal guardians.
GenBank Accession Numbers
Sequences analyzed in this report were submitted to GenBank (accession numbers: JQ730752-JQ730813).
RESULTS
Overview of the study cohort
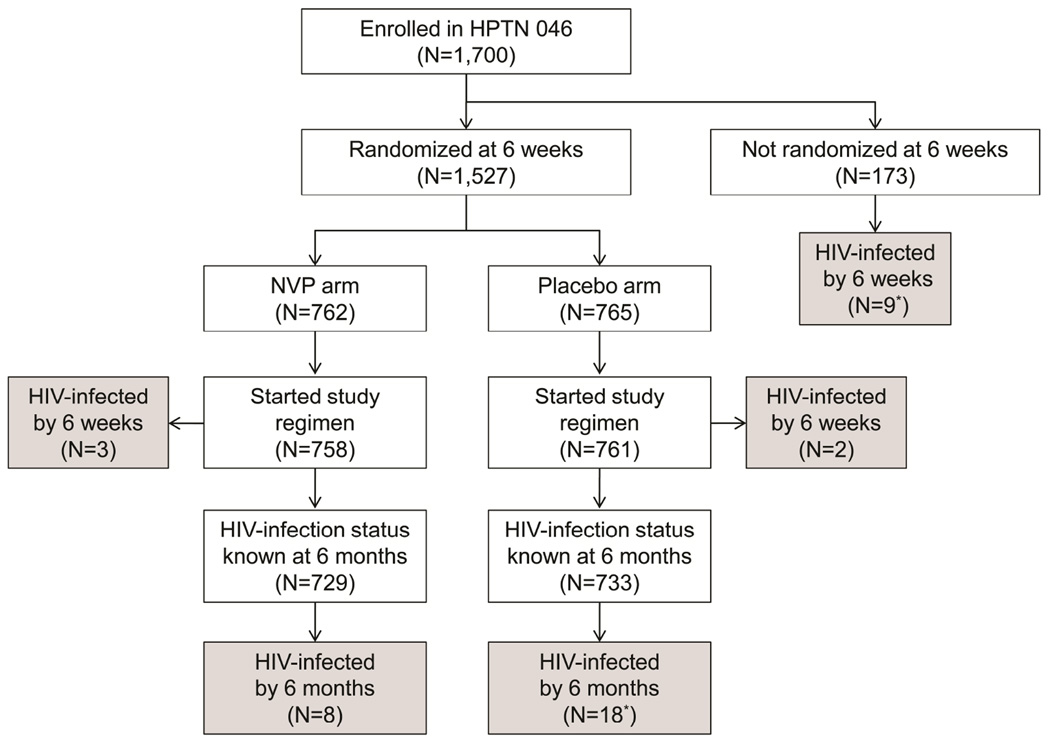
One-thousand seven-hundred infants were enrolled in HPTN 046 and initiated a course of daily open-label NVP (Figure 1). At six weeks of age, 1,527 breastfeeding infants were randomized to receive either daily NVP prophylaxis (NVP arm) or placebo (placebo arm) for up to six months of age or until the cessation of breastfeeding if stopped earlier than six months. This included 1,522 infants who were HIV-uninfected at 6 weeks of age (intent-to treat group, Figure 1) and five infants who were randomized before HIV infection was diagnosed. The study regimen (NVP or placebo) was initiated in 1,519 infants, and HIV infection status was known for 1,462 (96.2%) of those infants at 6 months of age (729 in the NVP arm, 733 in the placebo arm). Twenty-six (1.8%) of the 1,462 infants who were randomized acquired HIV infection by 6 months of age (8 in the NVP arm, 18 in the placebo arm, Figure 1). In addition, nine (5.2%) of the 173 infants who were not randomized were HIV-infected by 6 weeks of age (Figure 1).
Figure 1.
HIV genotyping results obtained for maternal plasma, breast milk, and infant plasma samples from eight mother-infant pairs where the mother started highly active antiretroviral therapy (HAART) by 6 months postpartum; all available samples were tested. Samples were collected at 6 weeks, and at 3 and 6 months postpartum. Shaded boxes indicate the study visits the mother was on HAART. HIV genotyping was not performed for maternal samples that had <400 copies/ml (c/ml) HIV RNA (VL<400c/ml). (a) A nested polymerase chain reaction (PCR) procedure was required for amplification. (b) HIV genotyping results for this infant did not include amino acids 8 to 32 in HIV reverse transcriptase. No major non-nucleoside reverse transcriptase inhibitor (NNRTI) or nucleoside / nucleotide reverse transcriptase inhibitor (NRTI) resistance mutations occur in that region of the reverse transcriptase protein.
ARV drug resistance was assessed in 38 infants. This included 25 of the 26 infants who were HIV-uninfected at 6 weeks of age (Table 1) and 13 of the 14 infants who were HIV-infected at 6 weeks of age (Supplemental Digital Content). The majority of women and their infants studied received non-study ARV drugs for PMTCT according to the standard of care in their country. Thirty-six (94.7%) of the 38 women received sdNVP in labor, and 26 (68.4%) of the 38 women received additional ARV prophylaxis; 17 (44.7%) received zidovudine (ZDV, starting 1–137 days prior to delivery and ending near the time of delivery) and nine (23.7%) received a combination of ZDV and 3TC (starting 0–66 days prior to delivery and extending up to 11 days postpartum). All but one of the infants received sdNVP, and 26 (68.4%) of the infants received a 1-week or 4-week regimen of ZDV (Tables 1 and 3). In addition, eight (21.1%) of the women and seven (18.4%) of the infants initiated HAART within the first 6 months after delivery (Tables 1 and 3). The women and infants in this study, who started HAART by 6 months after birth initiated an NNRTI-containing HAART regimen and a co-formulated lopinavir/ritonavir (LPV-r)-containing HAART regimen, respectively.
Table 1.
Antiretroviral drug resistance in infants who were HIV-uninfected at 6 weeks of age and HIV-infected by 6 months of age.*
| # | Regimens for PMTCTa | Maternal HAARTa |
Infant HAARTa |
Age at HIV dx (days) |
BF at 6 months |
Resistance at 6 monthsb |
ST | ||
|---|---|---|---|---|---|---|---|---|---|
| Mat/Inf sdNVP |
Maternal regimen | Infant regimen | |||||||
| 1 | Yes/Yes | ZDV (−54 to 0) | NVP (6 to 36) + NVP (43 to 105) | 91 | No | WT | C | ||
| 2 | Yes/Yes | ZDV/3TC (−66 to 11) | ZDV (1 to 6) NVP (4 to 104) | 3TC/ZDV/NVP (157) | 96 | Yes | A98G K103N Y181C | A | |
| 3 | Yes/Yes | ZDV/3TC (−37 to 7) | ZDV (0 to 7) NVP (4 to 98) | 3TC/d4T/LPV-r (168) | 139 | No | WT | A | |
| 4 | Yes/Yes | NVP (7 to 182) | 3TC/d4T/NVP (159) | 182 | Yes | K103N V106M Y181C | C | ||
| 5 | Yes/Yes | ZDV (−18 to 0) | ZDV (3 to 10) NVP (7 to 183) | 3TC/d4T/NVP (18) | 183 | Yes | K103N Y181C | C | |
| 6 | Yes/Yes | ZDV (1 to 28) NVP (5 to 183) | 184 | Yes | K103N | C | |||
| 7 | Yes/Yes | ZDV (1 to 28) NVP (5 to 188) | 190 | Yes | K103N Y181C | C | |||
| 8 | Yes/Yes | ZDV (−84 to 0) | ZDV (1 to 7) NVP (7 to 177) | 177 | Yes | K103N Y181C | C | ||
| 9 | Yes/Yes | ZDV (−137 to 0) | ZDV (1 to7) NVP (5 to 43) | 3TC/d4T/EFV (154) | 87 | Yes | V106M | C | |
| 10 | Yes/Yes | ZDV/3TC (−50 to 7) | ZDV (0 to 7) NVP (5 to 43) | 3TC/ZDV/LPV-r (111 to 407) | 91 | Yes | WT | A | |
| 11 | Yes/Yes | ZDV/3TC (−14 to 6) | NVP (5 to 43) | 91 | Yes | WT | A | ||
| 12 | Yes/Yes | NVP (7 to 43) | 91 | Yes | WT | C | |||
| 13 | No/Yes | ZDV (−41 to −1) | ZDV (2 to 8) NVP (6 to 43) | 91 | Yes | WT | C | ||
| 14 | Yes/Yes | ZDV (−75 to 0) | NVP (16 to 42) | 92 | No | WT | C | ||
| 15 | Yes/Yes | NVP (6 to 44) | 177 | Yes | WT | C | |||
| 16 | Yes/Yes | NVP (7 to 43) | 182 | Yes | WT | C | |||
| 17 | Yes/Yes | ZDV (−70 to 8) 3TC (−1 to 8) | ZDV (0 to 5) NVP (5 to 42) | 182 | Yes | WT | A | ||
| 18 | Yes/Yes | ZDV (−36 to 0) | ZDV (1 to7) NVP (6 to 42) | 182 | Yes | WT | C | ||
| 19 | Yes/Yes | ZDV (−22 to 0) | ZDV (1 to 7) NVP (6 to 43) | 182 | Yes | WT | C | ||
| 20 | Yes/Yes | ZDV (−28 to 7) 3TC (0–7) | ZDV (1 to 7) NVP (7 to 43) | 183 | Yes | WT | C | ||
| 21 | No/Yes | ZDV (1 to 28) NVP (5 to 43) | 183 | Yes | WT | C | |||
| 22 | Yes/Yes | ZDV (−65 to 5) | ZDV (1 to 3) NVP (5 to 43) | 183 | Yes | WT | C | ||
| 23 | Yes/Yes | ZDV/3TC (−29 to 7) | ZDV (1 to 7) NVP (5 to 43) | 184 | Yes | WT | A | ||
| 24 | Yes/Yes | NVP (7 to 43) | 185 | Yes | WT | C | |||
| 25 | Yes/Yes | ZDV (−35 to 0) | ZDV (2 to 8) NVP (6 to 43) | 238 | No | WT | C | ||
Characteristics and HIV genotyping results from infants who were randomized to the nevirapine arm (infants #1–8) and the placebo arm (infants #9–25) of HPTN 046 who were HIV infected between 6 weeks and 6 months of age. All infants received open-label nevirapine from around birth to 6 weeks of age. Infants received study drug (nevirapine or placebo) daily from 6 weeks of age up to 6 months of age. Nevirapine was stopped in infants who were diagnosed with HIV infection.
Abbreviations: PMTCT: prevention of mother-to-child transmission of HIV; HAART: highly active antiretroviral therapy; dx: age at diagnosis (days); BF: breastfeeding; ST: subtype; Mat/Inf: maternal/infant; sdNVP: single dose nevirapine; ZDV: zidovudine; NVP: nevirapine; WT: wild type (no mutations detected); 3TC: lamivudine; d4T: stavudine; LPV-r: co-formulated lopinavir/ritonavir; EFV: efavirenz.
Antiretroviral regimens administered to the mother or infant as prophylaxis (for prevention of mother-to-child transmission of HIV) or for treatment of HIV infection (highly active antiretroviral treatment) are shown; the numbers in parentheses indicate the days each regimen was administered, where negative numbers indicate days prior to delivery, 0 indicates the day of delivery, and positive numbers indicate days after delivery.
Resistance mutations detected in infant plasma HIV at 6 months of age are shown (Resistance at 6 months).
Table 3.
Factors associated with detection of NVP resistance in infants HIV-infected between 6 weeks and 6 months of age.*
| Factor | N (%) | Relative Risk (95 %CI [IQR]) |
P-value | |
|---|---|---|---|---|
| ARV exposure from study | NVP arm | 6/8 (75.0 %) | 12.8 (1.8 – 89.0) | 0.001 |
| regimen | Placebo arm | 1/17 (5.9 %) | ||
| ARV exposure for PMTCT | Maternal ZDV prophylaxis: | 0.5 (0.2 – 1.8) | 0.355 | |
| Yes | 4/18 (22.2 %) | |||
| No | 3/7 (42.9 %) | |||
| Maternal 3TC prophylaxis: | 0.4 (0.1 – 2.9) | 0.626 | ||
| Yes | 1/7 (5.9 %) | |||
| No | 6/18 (75.0 %) | |||
| Infant ZDV prophylaxis: | 2.3 (0.3 – 16.1) | 0.626 | ||
| Yes | 6/18 (33.3 %) | |||
| No | 1/7 (14.3 %) | |||
| ARV exposure for maternal or infant treatment | Maternal HAART by 6 months postpartum | 7.0 (2.5 – 20.0) | 0.003 | |
| Yes | 4/4 (100.0 %) | |||
| No | 3/21 (14.2 %) | |||
| Infant HAART by 6 months of age | -- | 1.000 | ||
| Yes | 0/2 (0.0 %) | |||
| No | 7/23 (30.4 %) | |||
| Other factors | HIV-infected by 3 months | 0.4 (0.1 – 3.0) | 0.626 | |
| Yes | 1/7 (14.3 %) | |||
| No | 6/18 (75.0 %) | |||
| HIV subtype | 1.9 (0.3 – 12.8) | 0.637 | ||
| C | 6/19 (31.6 %) | |||
| A | 1/6 (16.7 %) | |||
Analysis of NVP resistance in infants who were HIV-infected between 6 weeks and 6 months of age
Eight infants in the NVP arm and 18 infants in the placebo arm who were HIV-uninfected at 6 weeks acquired HIV infection by 6 months of age (Figure 1); 25 (96.2%) of those infants had a 6-month sample available for analysis (Table 1). The infants were diagnosed with HIV infection at a median of 182 days of age (range 87–238 days). Infants in the NVP arm received a median of 173 days (range 92–182 days) of NVP (open-label NVP plus NVP as study drug). Infants in the placebo arm received a median of 37 days (range 26–38 days) of open-label NVP. Six (75.0%) of the eight HIV-infected infants in the NVP arm had NVP resistance, compared to only one (5.9%) of the 17 HIV-infected infants in the placebo arm (P=0.001, Table 3).
There was no association between emergence of NVP resistance in infants at 6 months of age and any of the following factors: off-study NRTI exposure for PMTCT (maternal ZDV prophylaxis, maternal 3TC prophylaxis, or infant ZDV prophylaxis) or initiation of infant HAART by 6 months of age, timing of infant HIV infection (infection by 3 months of age), or HIV subtype (C vs. other subtypes, Table 3). In contrast, emergence of NVP resistance in infants at 6 months of age was associated with initiation of maternal HAART by 6 months postpartum (P=0.003, Table 3). All infants whose mothers initiated an NNRTI-containing regimen by 6 months postpartum had NVP resistance; three of the infants were in the NVP arm (infants 2, 4, and 5) and one infant was in the placebo arm (infant 9). In the placebo arm, the only infant who had NVP resistance had a mother who was receiving NNRTI-based HAART. In the NVP arm, three (50%) of the six infants who had NVP resistance had a mother who was receiving NNRTI-based HAART.
Analysis of NVP resistance in infants who were HIV-infected by 6 weeks of age
Fourteen of the infants studied were HIV-infected by 6 weeks of age; five were diagnosed with HIV infection after randomization (3 in the NVP arm, 2 in the placebo arm) and nine were not randomized to study drug (Figure 1). Thirteen (92.9%) of those infants had a 6-week plasma sample available for analysis (Supplemental Digital Content). The median age at HIV diagnosis was 36 days (range 14–85 days). Infants who were randomized to NVP or placebo at 6 weeks of age received a median of 93 days (range: 37–97 days) of NVP (open-label NVP with or without NVP as study drug). Infants who were not randomized received a median of 35 days (range: 12–39 days) of daily NVP prophylaxis, begun at an average of 6 days after birth. NVP resistance was detected in 12 (92.3%) of the 13 infants who were HIV-infected by 6 weeks of age (Supplemental Digital Content).
Analysis of resistance to other antiretroviral drugs
Many infants were exposed to ARV drugs other than NVP, including NRTI’s administered to mothers and/or infants for PMTCT, and HAART administered to mothers and/or infants for treatment of HIV infection. In four cases where resistance testing was performed at 6 months (infants 2, 4, 5 and 9), the mother initiated HAART before the 6-month visit while she was still breastfeeding (Table 1). None of the 25 infants in the intent-to-treat group had resistance to drugs other than NVP at 6 months of age (Table 1). Two infants in the intent-to-treat group (infants 3 and 10) initiated HAART by 6 months; none of those infants had ARV resistance detected at 6 months.
The mothers of two of the 13 infants who had resistance assessed at 6 weeks initiated HAART before the 6-week visit while their infants were still breastfeeding (infants 26 and 27). Neither of those infants (and none of the other 11 infants infected by 6 weeks of age) had resistance to drugs other than NVP at 6 weeks. Five of the 13 infants who were HIV-infected by 6 weeks were not diagnosed by the 6-week visit and were randomized in the HPTN 046 trial. All five of those infants initiated HAART by 6 months. Four of those five infants had a sample collected at the 6-month visit. Three of the infants had ARV resistance detected at 6 months of age; two infants had NVP resistance detected and one infant had 3TC resistance (M184V mutation) detected at 6 months of age (infant 30, Supplemental Digital Content). That infant did not receive 3TC prophylaxis and the mother did not receive 3TC as prophylaxis or as part of HAART. However, the infant initiated a 3TC-containing HAART regimen before the 6-month visit (at 156 days of age).
More detailed analysis was performed for the eight infants whose mothers started HAART by 6 months postpartum (Table 2). All eight infants were exclusively breastfeeding when their mothers started HAART. For these eight mother-infant pairs, HIV genotyping was performed for all available maternal plasma, breast milk, and infant plasma samples collected after infant HIV infection up to 6 months postpartum (Table 2). Six of the eight women had a genotyping result for at least one plasma sample; four of those women had NVP resistance detected. Five of the eight women had a genotyping result for at least one breast milk sample; three of those women had NVP resistance detected. Seven (88%) of the eight infants had NVP resistance detected. In addition to NVP resistance, one of the eight infants (infant 34) had the K65R mutation, associated with resistance to NRTIs, including 3TC and stavudine (d4T), detected at 3 months of age [21]. In this mother-infant pair, neither mother nor infant received 3TC for PMTCT, and the infant did not receive HAART. However, the mother started a regimen of 3TC, d4T, and NVP at 63 days postpartum, after the infant was diagnosed with HIV infection while she was still breastfeeding. In this case, exposure to 3TC and/or d4T in the mother’s HAART regimen was the likely explanation for emergence of multi-class (NNRTI plus NRTI) resistance in the infant.
Table 2.
| # | Sample type | Age at HIV diagnosis |
6 weeks | 3 months | 6 months |
|---|---|---|---|---|---|
| 2 | Maternal plasma | A98G, V108V/I | |||
| Breast milk | A98Ga | ||||
| Infant plasma | 3 mo | A98G, K103N, Y181C | |||
| 4 | Maternal plasma | VL < 400c/ml | |||
| Breast milk | VL < 400c/ml | ||||
| Infant plasma | 6 mo | K103N, V106M,Y181C | |||
| 5 | Maternal plasma | K103N | |||
| Breast milk | failed PCR | ||||
| Infant plasma | 6 mo | K103N, Y181Ca | |||
| 9 | Maternal plasma | WT | |||
| Breast milk | WT | ||||
| Infant plasma | 3 mo | V106M | |||
| 26 | Maternal plasma | WT | VL < 400 c/ml | WT | |
| Breast milk | WTa | WT | WT | ||
| Infant plasma | 6 wk | WT | WT | WT | |
| 27 | Maternal plasma | K103N | K103N | ||
| Breast milk | K103Na | K103N | |||
| Infant plasma | 6 wk | Y188C | K103N, V106M, Y188C | ||
| 33 | Maternal plasma | K103N | |||
| Breast milk | K103Na | ||||
| Infant plasma | 6 wk | K103N | K103N | ||
| 34 | Maternal plasma | VL< 400 c/ml | |||
| Breast milk | VL< 400 c/ml | ||||
| Infant plasma | 6 wk | K103N | K103N, Y181C, K65R | ||
Fisher’s exact test was used to assess the association of various factors with emergence of nevirapine resistance in HIV-infected infants. Relative risk for infant exposure to highly active antiretroviral therapy (HAART) was undefined, because none of the two infants exposed to HAART had resistance. Note that none of the infants received a non-nucleoside reverse transcriptase inhibitor (NNRTI)-containing HAART regimen.
Abbreviations: CI: confidence intervals; ARV: antiretroviral; NVP: nevirapine; PMTCT: prevention of mother-to-child transmission; ZDV: zidovudine; 3TC: lamivudine; HAART: highly active ARV treatment.
HIV genotyping results obtained for maternal plasma, breast milk and infant plasma samples from 8 mother–infant pairs where the mother started HAART by 6 months postpartum; all available samples were tested. Samples were collected at 6 weeks, and at 3 and 6 months postpartum. Mothers started HAART at 6 weeks (infants 5, 26, 27), 3 months (infants 33, 34), and 6 months (infants 2, 4, 9). HIV genotyping was not performed for maternal samples that had <400 copies/mL (c/mL) HIV RNA (VL < 400 c/mL).
A nested polymerase chain reaction (PCR) procedure was required for amplification.
HIV genotyping results for this infant did not include amino acids 8–32 in HIV reverse transcriptase. No major NNRTI or nucleoside/NRTI resistance mutations occur in that region of the reverse transcriptase protein.
DISCUSSION
We analyzed ARV resistance in HIV-infected infants in the HPTN 046 trial. Among HIV-infected infants who were HIV-uninfected at 6 weeks, the frequency of NVP resistance was significantly higher in the NVP arm than in the placebo arm at 6 months (75% of the infants had resistance in the NVP arm, compared to only 5.9% in the placebo arm). Notably, almost all (92%) of the infants who were HIV-infected by 6 weeks and received extended daily NVP prophylaxis had NVP resistance at 6 weeks of age. This is consistent with the high frequency of NVP resistance observed at 6 and 14 weeks of age among infants in the SWEN and PEPI-Malawi trials [10–12, 22].
In HPTN 046, many women and infants received ARV drugs for PMTCT or HIV treatment (maternal or infant HAART) in addition to the study regimen. Emergence of NVP resistance in infants at 6 months was not associated with exposure to ZDV or 3TC administered to the mother or infant near the time of birth for PMTCT, to initiation of infant HAART, timing of infant HIV infection (detected by vs. after 3 months), or HIV subtype (C vs. non-C). A failure to see an association of NVP resistance with these factors may have been due to the relatively small number of infants analyzed, particularly in some subgroups. In contrast, emergence of NVP resistance at 6 months in the intent-to-treat group was associated with initiation of maternal HAART including NVP or EFV before 6 months postpartum. In the placebo arm, the only infant who had NVP resistance had a mother who was receiving NNRTI-containing HAART. In the NVP arm three (50%) of six infants with NVP resistance had mothers who had initiated NNRTI-containing HAART.
The increased risk of NVP resistance in infants whose mothers initiate NNRTI-containing HAART postpartum may be due to NVP exposure through breast milk and/or transmission of NVP-resistant HIV strains from the mother to the infant. When nursing mothers receive NVP, NVP concentrations in breast milk are around 70% of those in maternal plasma, and the infants receive a sufficient NVP dose via breast milk to achieve infant plasma NVP concentrations of around 1000 ng/mL, well above the IC50 of 10 ng/mL. [23, 24]. Transmission of NVP-resistant HIV from a woman to her infant has been documented [10, 25]. In this study, NVP-resistant HIV was detected in breast milk in three of the five women who had HIV genotyping results obtained for breast milk samples. In one case, it is possible that a NVP-resistant strain was transmitted from the mother to her infant.
In this cohort, emergence of resistance to drugs other than NVP was uncommon. One infant acquired the M184V mutation, most likely as a result of exposure to the infant’s own HAART regimen. In addition, one of eight breastfeeding infants who were exposed to maternal HAART acquired multi-class resistance: NVP resistance and the K65R mutation, which is associated with resistance to NRTIs such as 3TC, d4T. In this case, acquisition of the K65R mutation was likely to have resulted from exposure to 3TC and/or d4T in the mother’s HAART regimen. 3TC is concentrated in breast milk, with average concentrations about 2.5 fold higher than in maternal plasma, and 3TC concentrations in the plasma of breastfeeding infants average 20 ng/mL, just above the upper limit of the range of the 3TC IC50 for wild-type HIV (0.6 to 21 ng/mL) [23, 25]. The K103N mutation was selected in the infant prior to maternal HAART exposure. Other NVP resistance mutations were detected in samples collected at later study visits; these NVP resistance mutations may have been selected in the infant either from the infant’s NVP prophylaxis or from NVP exposure in the mother’s HAART regimen. Acquisition of multi-class resistance in breastfeeding infants exposed to maternal HAART was less frequent in this study (1/8 infants, 12.5%) than in other studies [13, 14, 18]. This may reflect the relatively short period of time between maternal HAART initiation and collection of the infant samples used for testing (maximum 3 months). In previous studies, infants were tested for resistance 6 months or more after maternal HAART initiation [13, 18]. Another factor that may have contributed to the relatively low frequency of multi-class resistance in infants in this study is that three (38%) of the eight women studied initiated HAART more than 3 months after delivery. In the PEPI-Malawi trial, emergence of multi-class resistance was more frequent in infants whose mothers initiated HAART closer to the time of delivery [13].
This study extends findings from the SWEN trial [10, 11, 17] and the PEPI-Malawi trial [12, 22] by examining NVP resistance in infants who received up to 6 months of extended daily NVP. This study also identifies an additional risk factor for acquisition of NVP resistance in breastfeeding infants: initiation of maternal HAART by 6 months postpartum. The World Health Organization (WHO) currently recommends more wide-spread use of HAART in pregnant and breastfeeding women who meet the criteria for treatment [26]. The WHO also recommends that women who start HAART within 12 months of sdNVP exposure start a non-NNRTI-containing HAART regimen [26]. However, NVP is commonly included in first-line regimens used to treat HIV-infected mothers and infants in these settings, despite previous sdNVP exposure. HIV-infected infants who were exposed to NVP prophylaxis prior to HIV diagnosis are not likely to respond to a NVP-containing treatment regimen [27]. Resistance testing is not readily available in most resource-constrained settings. Therefore, it is often not possible to screen infants for NVP resistance before starting an ARV treatment regimen. Findings from this study support the current guidelines of the WHO that HIV-infected infants should be treated with a regimen that does not include an NNRTI if they were exposed to sdNVP, other NVP-containing regimens used for PMTCT, or a maternal HAART regimen that included NVP or another NNRTI [28]. This study also provides further evidence that initiation of maternal HAART can induce multi-class resistance in breastfeeding infants who become HIV-infected. Infants who develop NRTI resistance, in addition to NVP resistance, may not respond to any of the treatment regimens that are available in resource-limited settings. Further studies are needed to evaluate factors associated with emergence of multi-class resistance in HIV-infected infants.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the women and infants in the HPTN 046 for their participation in the trial, and thank the laboratory staff at the study sites and at the HPTN Network Laboratory for their assistance with sample and data management.
Sources of Funding
This work was funded by: (1) the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group (U01AI068632), which receives support from the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), of the US National Institutes of Health (NIH), and (2) 1R01AI087139 funded by NIAID, NIH. The HIV Prevention Trials Network (HPTN) 046 was funded by the NIH, initially through the HIV Prevention Trials Network (HPTN) and later through the IMPAACT group. The HPTN (U01AI46749) has been funded by the NIAID, NICHD, NIMH, and the National Institute of Drug Abuse (NIDA). The study products used in the HPTN 046 trial were provided free of charge by Boehringer Ingelheim Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None of the authors has a conflict of interest.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health. Use of trade names is for identification purposes only and does not constitute endorsement by the National Institutes of Health or the Department of Health and Human Services.
REFERENCES
- 1.Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 2.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 3.Coovadia HM, Brown ER, Fowler MG, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:221–228. doi: 10.1016/S0140-6736(11)61653-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 6.Marazzi MC, Nielsen-Saines K, Buonomo E, et al. Increased infant human immunodeficiency virus-type one free survival at one year of age in sub-saharan Africa with maternal use of highly active antiretroviral therapy during breast-feeding. Pediatr Infect Dis J. 2009;28:483–487. doi: 10.1097/INF.0b013e3181950c56. [DOI] [PubMed] [Google Scholar]
- 7.Taha TE, Kumwenda J, Cole SR, et al. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J Infect Dis. 2009;200:1490–1497. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 8.Thomas TK, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding--the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorthy A, Kuhn L, Coovadia A, et al. Induction therapy with protease-inhibitors modifies the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children. Clin Infect Dis. 2011;52:514–521. doi: 10.1093/cid/ciq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church JD, Omer SB, Guay LA, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198:1075–1082. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moorthy A, Gupta A, Bhosale R, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS ONE. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogel J, Hoover DR, Sun J, et al. Analysis of nevirapine resistance in HIV-infected infants who received extended nevirapine or nevirapine/zidovudine prophylaxis. AIDS. 2011;25:911–917. doi: 10.1097/QAD.0b013e328344fedc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel J, Li Q, Taha TE, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin Infect Dis. 2011;52:1069–1076. doi: 10.1093/cid/cir008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011;8:e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 16.Eshleman SH, Jackson JB. Nevirapine resistance after single dose prophylaxis. AIDS Rev. 2002;4:59–63. [PubMed] [Google Scholar]
- 17.Persaud D, Bedri A, Ziemniak C, et al. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother-to-child HIV transmission. AIDS Res Hum Retroviruses. 2011;27:823–829. doi: 10.1089/aid.2010.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lidstrom J, Guay LA, Musoke P, et al. Multi-class drug resistance arises frequently in HIV-infected breastfeeding infants whose mothers initiate highly active antiretroviral therapy (HAART) postpartum. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Abstract T-124]. [Google Scholar]
- 19.Mackie N, Dustan S, McClure MO, Weber JN, Clarke JR. Detection of HIV-1 antiretroviral resistance from patients with persistently low but detectable viraemia. J Virol Methods. 2004;119:73–78. doi: 10.1016/j.jviromet.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Eshleman SH, Guay LA, Mwatha A, et al. Characterization of nevirapine resistance mutations in women with subtype A vs. D HIV-1 6–8 weeks after single-dose nevirapine (HIVNET 012) J Acquir Immune Defic Syndr. 2004;35:126–130. doi: 10.1097/00126334-200402010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Stanford University. HIV drug resistance database. [Accessed: January 23, 2012];2012 Available at: http://hivdb.stanford.edu/. [Google Scholar]
- 22.Lidstrom J, Li Q, Hoover DR, et al. Addition of extended zidovudine to extended nevirapine prophylaxis reduces nevirapine resistance in infants who were HIV-infected in utero. AIDS. 2010;24:381–386. doi: 10.1097/QAD.0b013e3283352ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53:1170–1176. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunz A, Frank M, Mugenyi K, et al. Persistence of nevirapine in breast milk and plasma of mothers and their children after single-dose administration. J Antimicrob Chemother. 2009;63:170–177. doi: 10.1093/jac/dkn441. [DOI] [PubMed] [Google Scholar]
- 25.Coates JA, Cammack N, Jenkinson HJ, et al. (−)-2'-deoxy-3'-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO) Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. [Accessed: March 12, 2012];2010 Available at: http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [PubMed]
- 27.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO) Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach. [Accessed December 22, 2011];2010 Available at: http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.