Abstract
Background
Sudden cardiac death (SCD), the cause of 250,000-450,000 deaths per year, is a major public health problem. The majority of those affected do not have a prior cardiovascular diagnosis. Elevated B-type natriuretic peptide levels have been associated with the risk of heart failure and mortality, as well as sudden death in women.
Objective
To examine the relationship between N-terminal pro-B-type natriuretic peptide (NT-proBNP) and SCD in the Cardiovascular Health Study population.
Methods
The risk of SCD associated with baseline NT-proBNP was examined in 5447 participants. Covariate-adjusted Cox model regressions were used to estimate the hazard ratios of developing SCD as a function of baseline NT-proBNP
Results
Over a median follow-up of 12.5 years (maximum of 16), there were 289 cases of SCD. Higher NT-proBNP levels were strongly associated with SCD, with an unadjusted hazard ratio of 4.2 (95% CI: 2.9, 6.1, p<0.001) in the highest quintile compared to the lowest. NT-proBNP remained associated with SCD even after adjustment for numerous clinical characteristics and risk-factors (age, sex, race, and other associated conditions), with an adjusted hazard ratio for the 5th versus the 1st quintile of 2.5 (95% CI: 1.6, 3.8, p<0.001).
Conclusion
NT-proBNP provides information regarding the risk of sudden cardiac death in a community based population of older adults, beyond other traditional risk factors. This biomarker may ultimately prove useful in targeting the population at risk with aggressive medical management of comorbid conditions.
Keywords: Sudden cardiac death, B-type natriuretic peptide, BNP, NT-proBNP
Introduction
Sudden cardiac death (SCD) is a major cause of mortality in the United States, affecting approximately 250,000-450,000 people a year (1-2). The majority of SCD events are attributable to coronary heart disease, and over 50% of cardiac disease deaths are sudden(2). Known risk factors for SCD therefore overlap with those for coronary artery disease, and include smoking, obesity, hypertension, diabetes, and hypercholesterolemia(3-4). Unfortunately, the majority of people who die suddenly do not have prior cardiovascular diagnoses, or indications of high risk(2). Identification of markers that predate the occurrence of SCD may permit early treatment of those at risk and facilitate the development of therapeutic strategies aimed at preventing these tragic events.
The neurohormone B-type natriuretic peptide is a regulator of cardiovascular function(5). B-type natriuretic peptide is produced by the ventricular myocardium, with additional production in the atrial myocardium and the brain. Although most widely used as a marker of clinical heart failure, elevated B-type natriuretic peptide levels are also associated with prognosis after acute coronary syndrome and in heart failure and chronic coronary artery disease patients(6-7), and was recently shown to predict risk of SCD in white women(8).
The Cardiovascular Health Study (CHS), a longitudinal study of older men and women, is an ideal population in which to assess the association between levels of NT-proBNP and SCD in a large, biracial cohort.
Methods
Study Population
The design and objectives of CHS have been described(9). The CHS is a longitudinal study of 5,888 men and women aged 65 and older, randomly selected from four communities in the United States and enrolled during two time periods. The ‘original’ cohort was enrolled from 1989-1990 (N=5201) and the ‘minority’ cohort during 1992-1993 (N=687, African-Americans). Each participant gave informed consent, and each center underwent institutional review board approval. The baseline examination included a standardized questionnaire assessing a variety of behavioral and health risk factors, including smoking, alcohol intake, history of diabetes, stroke, coronary heart disease, heart failure, self-reported health status, medication use, and history of prior cardiovascular disease The physical examination included measurements of height, weight, and seated blood pressure10.
Evaluation included a resting 12-lead electrocardiogram (ECG), which was repeated annually through the ninth year of follow-up. In a subset of the population (n= 3412), an echocardiogram which assessed left ventricular dimensions, ventricular septal thickness, posterior wall thickness, aortic root dimension, left atrial dimension, percent fractional shortening, left ventricular mass, and end systolic stress was performed at enrollment(11). The echocardiogram was not performed until year 2 in the minority cohort. Laboratory examinations included measurement of total cholesterol, high density lipoprotein, cholesterol, fasting glucose, C-reactive protein, and serum creatinine(12). Participants were contacted every 6 months for follow-up, alternating between a telephone interview and a clinic visit for the first 10 years, and by telephone interview only thereafter. Discharge diagnoses for all hospitalizations were collected. Adjudication of cardiovascular events was performed by a centralized events committee(10). The maximum follow-up was 16 years (median 12.5 years).
Determination of Incident Cardiovascular Events and Sudden Cardiac Death
New cardiovascular events were reported during semiannual contacts with participants, family members, or other designated informants by telephone or at a clinic visit. Medical records were obtained to confirm diagnoses, and events were adjudicated by committee. Details of cardiovascular event or death ascertainment and adjudication has previously been published (10). Death certificates, inpatient records, nursing home or hospice records, physician questionnaires, interviews with next-of-kin, and autopsy reports where available, were reviewed, to determine the cause of death. Sudden cardiac death was defined as a sudden pulseless condition, presumed due to a cardiac arrhythmia, in a previously stable individual, occurring out of the hospital or in the emergency room. For unwitnessed deaths, the participant must have been seen within 24 hours of the arrest in a stable condition and without evidence of a non-cardiac cause of cardiac arrest. SCD cases could not be under hospice or nursing home care, or have a life-threatening non-cardiac comorbidity.
Natriuretic hormone assays
NT-proBNP was measured in serum collected at baseline in the original CHS trial cohort (1989 to 1990) and the minority cohort (1992 to 1993). A second measure of NT-proBNP was performed on sera collected 3 years later for the original cohort (1992 to 1993), and 2 years later for the minority cohort (1994 to 1995). Measurement of NT-proBNP was performed using an FDA approved commercially available immuno-assay (Roche Diagnostics Elescys® proBNP Assay, Indianapolis, Indiana) on the Elescys 2010 instrument. Serum was frozen and maintained at -70 C until testing. All measurements were performed in a CLIA certified laboratory accredited by the College of American Pathologists. The core laboratory was blinded as to patient outcome and reported certified data to the central data repository.
NT-proBNP levels were determined in 5,447 participants. From the original cohort of 5,201 subjects, 3,979 participants had NT-proBNP measured at baseline, and an additional 832 had only a NT-proBNP measurement 3 years later, for a total of 4,811. From the 687 participants in the minority cohort, 545 had NT-proBNP measured at baseline and an additional 91 had a measurement 2 years later, for a total of 636. Follow-up NT-proBNP measures were available in 2770 participants of the original cohort from the exam 3 years later and in 545 participants of the minority cohort from the exam 2 years later.
Statistical analysis
NT-proBNP levels were analyzed both as a continuous variable, where the natural log of NT-proBNP was used, and as categorized into quintiles. In initial analyses we examined the risk of SCD associated with baseline NT-proBNP among the 5,447 subjects. We present unadjusted Kaplan-Meier survival curves comparing cumulative risk of SCD across quintiles of baseline NT-proBNP.
We used covariate-adjusted Cox model regressions to estimate the hazard ratios of developing SCD by quintile of baseline NT-proBNP and using the natural log of NT-proBNP as a continuous predictor. Failure time for the Cox models for those who developed SCD was the date of death. For those who did not have SCD, failure time was the date of last known follow-up or the date of death.
Cox models were adjusted for the statistically significant predictors selected by backward elimination from the variables shown in Table 1. Interactions were tested for all covariates that were found to be statistically significant using a p-value of 0.01 for significance. This analysis was repeated separately for those participants with and without evidence of cardiovascular disease at baseline (CVD, defined as having no history of myocardial infarction, heart failure, or stroke at baseline),. We also carried out time dependent Cox models additionally adjusting for incident myocardial infarction, heart failure and stroke in those free of CVD at baseline. Finally, Cox model analyses were conducted of the change in NT-proBNP after adjustment for baseline covariates and the average of the two NT-proBNP at the two time points.
Table I.
Selected baseline characteristics of 5447 CHS participants according to Sudden Cardiac Death status (n and % for categorical variables and mean and standard deviation for continuous variables).
| No Sudden Cardiac Death (n=5158) |
Sudden Cardiac Death (n=289) |
p-value No vs. ECG |
|
|---|---|---|---|
| Age (years) | 72.7 ± 5.6 | 73.4 ± 5.6 | < 0.05 |
| Male | 2090 (40.5) | 165 (57.1) | < 0.001 |
| African-American | 793 (15.4) | 56 (19.4) | n.s. |
| Body mass index (kg/m2) | 26.7 ± 4.7 | 27 ± 4.4 | n.s. |
| Smoking | <0.05 | ||
| Current | 565 (11) | 38 (13) | |
| Former | 2150 (41.7) | 136 (47.1) | |
| Never | 2438 (47.3) | 115 (39.8) | |
| Number of pack years | 17.2 (26) | 23.5 (32.7) | < 0.001 |
| Diabetes Mellitus | 412 (8) | 40 (13.8) | < 0.001 |
| Myocardial Infarction | 456 (8.8) | 64 (22) | < 0.001 |
| Heart failure | 230 (4.5) | 30 (10.4) | < 0.001 |
| Stroke | 208 (4) | 27 (9.3) | < 0.001 |
| Hypertension | 2225 (43.7) | 148 (51.2) | <0.05 |
| Systolic blood pressure (mm Hg) | 136 ± 21.6 | 140 ± 23.4 | < 0.005 |
| Diastolic blood pressure (mm Hg) | 70.9 ± 11.2 | 72 ± 11.7 | n.s. |
| Total cholesterol (mg/dl) | 211 ± 38.7 | 207 ± 48.1 | n.s. |
| HDL cholesterol (mg/dl) | 54.3 ± 15.6 | 50.2 ± 14.7 | < 0.001 |
| Glucose (mg/dl) | 111 (37.2) | 117 (41.9) | < 0.005 |
| Creatinine (mg/dl) | 1.05 ± 0.41 | 1.12 ± 0.32 | < 0.005 |
| C-reactive protein (mg/l) | 3.55 ± 6.06 | 4.28 ± 6.3 | < 0.05 |
| ACE-inhibitor user | 406 (7.9) | 33 (11.4) | < 0.05 |
| Beta Blocker user | 655 (12.7) | 49 (17) | <0.05 |
| Digoxin user | 434 (8.4) | 38 (13.1) | < 0.005 |
| Diuretic user | 559 (10.8) | 39 (13.5) | n.s. |
| K+ sparing diuretic user | 441 (8.5) | 18 (6.2) | n.s. |
| Potassium level (mEq/L) | 4.17 (0.4) | 4.15 (0.4) | n.s. |
| Heart rate (beats per minute) | 65.5 (12.1) | 65.8 (12.3) | n.s. |
| ECG atrial fibrillation | 137 (2.7) | 11 (3.8) | n.s. |
| Abnormal Q waves | 270 (5.2) | 34 (11.8) | <0.001 |
| Prolonged QT interval | 722 (14) | 66 (22.8) | < 0.001 |
| Ventricular conduction delay | 495 (5.6) | 56 (19.4) | < 0.001 |
| ln NT-proBNP | 4.81 (1.2) | 5.27 (1.4) | < 0.001 |
In supplementary analyses, the echocardiographic variables left ventricular ejection fraction ((LVEF) coded as normal (≥ 55%), borderline (45-54%), or abnormal (<45%)), left atrial size, left ventricular dimension, ventricular septal thickness, posterior wall thickness, aortic root dimension, percent fractional shortening, left ventricular mass, and end systolic stress were examined. The echocardiographic data was obtained from an M-mode echocardiogram.
For NT-proBNP measures at exams other than the baseline exam, the covariate values were taken from the same exam if the variable was measured at that exam or from the nearest exam in time prior to the NT-proBNP determination if not available at that exam. We report hazard ratios from the Cox models, along with 95% confidence intervals (CI) and p-values. All analyses were conducted with STATA 10.0.
Results
The demographic and medical characteristics of the CHS participants according to SCD status are compared in Table 1. Over the course of 16 years of follow-up (median 12.5 years), 289 subjects experienced SCD. Affected subjects were more likely to be older, male, and have conventional cardiovascular risk factors (smoking history, diabetes, dyslipidemia), coronary heart disease, or heart failure. There was a trend toward a higher risk of SCD in African-Americans compared with the Caucasians. Although only a small percentage of subjects were treated with cardiovascular medications at baseline, beta-blockers, digoxin, and angiotensin converting enzyme inhibitors were prescribed significantly more often in the group that experienced SCD. Subjects who suffered SCD were also more likely to have abnormalities on baseline electrocardiogram, including abnormal Q waves, a prolonged QT interval, and any ventricular conduction defect (Table 1).
The baseline NT-proBNP was significantly higher in subjects who suffered from SCD: the mean ln NT-proBNP was 5.3 (standard deviation 1.4) vs. 4.8 (standard deviation 1.2), in the unaffected subjects (median NTproBNP of 198 ng/dl with interquartile range 79-472 ng/dl in SCD group vs. 117 ng/dl with interquartile range of 60-236 ng/dl). The relevant conditions associated with SCD (age, gender, race, comorbid medical conditions, and medications) were also associated with quintile of NT-proBNP, with an increasing hazard ratio with increasing NT-proBNP (Table 2). In the unadjusted Cox model, the hazard ratio for a standard deviation increment in ln NT-proBNP was 1.9 (95% CI: 1.7, 2.2) (table 3). The association of NT-proBNP with SCD remained strong after adjustment for significant covariates by backwards elimination: HR 1.5 per standard deviation of ln NT-proBNP (95% CI 1.3, 1.7, p< 0.001) (Table 3). There were no significant interactions of any of the selected covariates with ln NT-proBNP.
Table 2.
Association of selected baseline characteristics with quintile of NT-proBNP (n and % for categorical variables and mean and standard deviation for continuous variables).
| Quintile of NT-proBNP (range ng/dl) | 1 (5.00-50.81) | 2 (50.82-91.78) | 3 (91.79-156.09) | 4 (156.1-298.3) | 5 (>290.3) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 70.5 (4.1) | 71.2 (4.6) | 72.3 (4.9) | 73.7 (5.7) | 75.9(6.3) | <0.001 |
| Male | 516 (47.4) | 431 (39.5) | 418 (38.4) | 378 (34.6) | 512 (47) | <0.001 |
| African-American | 239 (22) | 179 (16.4) | 146 (13.4) | 146 (13.4) | 139 (12.8) | <0.001 |
| Body mass index (kg/m2) | 27.6 (4.6) | 27 (4.7) | 26.8 (4.8) | 26.3 (4.7) | 25.8 (4.6) | <0.001 |
| Smoking | <0.05 | |||||
| Current | 130 (12) | 122 (11.2) | 113 (10.4) | 111 (10.2) | 127 (11.7) | |
| Former | 497 (45.7) | 452 (41.5) | 473 (43.5) | 430 (39.5) | 434 (39.9) | |
| Number of pack years | 18.4 (25.7) | 17.2 (26.7) | 17.2 (25.4) | 15.5 (25.4) | 19.3 (28.9) | <0.05 |
| Diabetes Mellitus | 100 (9.2) | 73 (6.7) | 94 (8.6) | 77 (7.1) | 108 (9.9) | <0.05 |
| Myocardial Infarction | 43 (4) | 51 (4.7) | 82 (7.5) | 95 (8.7) | 249 (22.8) | <0.001 |
| Heart failure | 19 (1.8) | 18 (1.7) | 25 (2.3) | 30 (2.8) | 168 (15.4) | <0.001 |
| Stroke | 24 (2.2) | 27 (2) | 39 (3.6) | 51 (4.7) | 94 (8.8) | <0.001 |
| Hypertension | 391 (36) | 441 (40.5) | 438 (40.2) | 519 (47.6) | 614 (56.3) | <0.001 |
| Systolic blood pressure (mm Hg) | 131 (18) | 133.4 (19.6) | 135.4 (20.4) | 139.2 (21.8) | 144.3 (25.5) | <0.001 |
| Diastolic blood pressure (mm Hg) | 71.5 (10.5) | 71 (10.4) | 70.4 (11) | 70.5 (11.5) | 71.4 (12.7) | n.s. |
| Total cholesterol (mg/dl) | 214.9 (38.1) | 216 (37.3) | 210.9 (38.4) | 209.4 (38.7) | 202.4 (40.5) | <0.001 |
| HDL cholesterol (mg/dl) | 53.1 (15) | 54.2 (15.2) | 54.9 (16) | 55.4 (15.7) | 52.8 (15.7) | <0.001 |
| Glucose (mg/dl) | 116.1 (44.7) | 111.4 (37.4) | 110.2 (33.6) | 106.8 (32.9) | 110.6 (37.1) | <0.001 |
| Creatinine (ng/dl) | 1 (0.25) | 0.99 (0.24) | 1.01 (0.31) | 1.04 (0.31) | 1.24 (0.68) | <0.001 |
| C-reactive protein (mg/l) | 3.04 (3.92) | 3.29 (5.83) | 3.05 (4.46) | 3.67 (5.59) | 4.9 (9.04) | <0.001 |
| ACE-inhibitor user | 76 (7) | 76 (7) | 70 (6.4) | 75 (6.9) | 142 (13) | <0.001 |
| Beta Blocker user | 69 (6.4) | 98 (9) | 124 (11.4) | 174 (16) | 239 (22) | <0.001 |
| Digoxin user | 37 (3.4) | 45 (4.1) | 68 (6.3) | 89 (8.2) | 233 (21.4) | <0.001 |
| Diuretic user | 108 (9.9) | 132 (12.1) | 112(10.3) | 132 (12.1) | 114 (10.5) | n.s. |
| K+ sparing diuretic user | 76 (7) | 76 (7) | 101 (9.3) | 101 (9.3) | 105 (9.6) | <0.05 |
| Potassium level (mEq/L) | 4.16 (0.39) | 4.15 (0.35) | 4.17 (0.37 | 4.14 (0.38) | 4.22 (0.41) | < 0.001 |
| Heart rate (beats per minute) | 92 (8.6) | 106 (9.8) | 141 (13) | 172 (15.9) | 277 (26.7) | <0.001 |
| ECG atrial fibrillation | 1 (0.1) | 3 (0.3) | 7 (0.6) | 9 (0.8) | 128 (11.8) | < 0.001 |
| Abnormal Q waves | 30 (2.8) | 37 (3.4) | 47 (4.4) | 64(5.9) | 126 (12.2) | < 0.001 |
| Prolonged QT interval | 92 (8.6) | 106 (9.8) | 141 (13) | 172 (15.9) | 277 (26.7) | < 0.001 |
| Ventricular conduction delay | 65 (6) | 72 (6.7) | 91 (8.4) | 104 (9.6) | 219 (21.2) | <0.001 |
Table 3.
Cox proportional hazards regression results for NT-proBNP as a predictor of sudden death
| Quintile of NT- proBNP | Unadjusted | Adjusted* | Adjusted† | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Hazard Ratio | 95% Confidence Interval | p-value | Hazard Ratio | 95% Confidence Interval | p-value | Hazard Ratio | 95% Confidence Interval | p-value | |
| Entire Sample | |||||||||
| 1 | 1.0 | 1.0 | 1.0 | ||||||
| 2 | 0.9 | (0.6,1.5) | 0.93 | 1.0 | (0.6,1.6) | 0.98 | 1.0 | (0.6,1.5) | 0.85 |
| 3 | 1.4 | (0.9,2.0) | 0.16 | 1.3 | (0.8,1.9) | 0.30 | 1.1 | (0.7,1.8) | 0.59 |
| 4 | 1.9 | (1.3,2.8) | 0.001 | 1.8 | (1.2,2.7) | 0.006 | 1.6 | (1.0,2.5) | 0.04 |
| 5 | 4.2 | (2.9,6.1) | <0.001 | 2.5 | (1.6,3.8) | <0.001 | 1.7 | (1.0,2.6) | 0.04 |
| ln NTproBNP ‡ | 1.9 | (1.7,2.1) | <0.001 | 1.5 | (1.3,1.7) | <0.001 | 1.3 | (1.1,1.5) | 0.002 |
| No CVD | |||||||||
| 1 | 1.0 | 1.0 | 1.0 | ||||||
| 2 | 1.1 | (0.7, 1.7) | 0.83 | 1.0 | (0.6, 1.7) | 0.86 | 0.9 | (0.6, 1.6) | 0.81 |
| 3 | 1.6 | (1.0, 2.5) | 0.05 | 1.5 | (1.0, 2.4) | 0.07 | 1.3 | (0.8, 2.2) | 0.25 |
| 4 | 1.5 | (0.9, 2.3) | 0.12 | 1.3 | (0.8, 2.2) | 0.26 | 1.1 | (0.7, 1.9) | 0.67 |
| 5 | 3.3 | (2.1, 5.2) | < 0.001 | 2.2 | (1.4, 3.8) | 0.001 | 1.7 | (1.0, 3.0) | 0.05 |
| ln NTproBNP‡ | 1.6 | (1.3, 1.9) | < 0.001 | 1.4 | (1.1, 1.6) | 0.001 | 1.2 | (1.0, 1.5) | 0.05 |
| CVD | |||||||||
| 1 | 1.0 | 1.0 | |||||||
| 2 | 0.6 | (0.2,1.9) | 0.40 | 0.7 | (0.2,2.2) | 0.55 | 0.9 | (0.3,3.2) | 0.86 |
| 3 | 0.4 | (0.1,1.3) | 0.13 | 0.4 | (0.1,1.4) | 0.17 | 0.4 | (0.1,1.7) | 0.24 |
| 4 | 2.4 | (1.0,5.5) | 0.04 | 2.7 | (1.1,6.3) | 0.02 | 3.1 | (1.1,8.5) | 0.03 |
| 5 | 2.3 | (1.0,5.1) | 0.04 | 2.4 | (1.0,5.5) | 0.04 | 1.7 | (0.6,4.6) | 0.32 |
| ln NTproBNP‡ | 1.6 | (1.3,1.9) | <0.001 | 1.6 | (1.3,1.9) | <0.001 | 1.4 | (1.1,1.8) | 0.02 |
Adjusted for the significant predictors by backwards elimination from among the variables listed in table 1 (age, gender, race, diabetes, smoking, systolic blood pressure, serum potassium level, ECG conduction delay and CVD for entire sample regressions)
Adjusted for left ventricular ejection fraction (categorized as normal ≥ 55%, borderline 45-54%, and abnormal <45%).
Standard deviation units
The hazard ratio for SCD increased in a dose dependent manner with each quintile of baseline NT-proBNP (Table 3). The hazard ratio for the highest quintile (unadjusted) was 4.2 (95% CI: 2.9, 6.1, p <0.001) compared to the lowest quintile. This association remained after adjustment for an extensive number of covariates; the hazard ratio in the fully adjusted Cox model for the highest quintile was 2.5 (95% CI: 1.6, 3.8, p< 0.001) compared to the lowest. After 15 years of follow-up, the cumulative incidence of SCD in the highest quintile was approximately 14%, compared with 4% for those with NT-proBNP in the lowest quintile; follow-up shown in the figure was restricted to 15 years because of the small number of subjects followed beyond that point (Figure 1).
Figure 1.
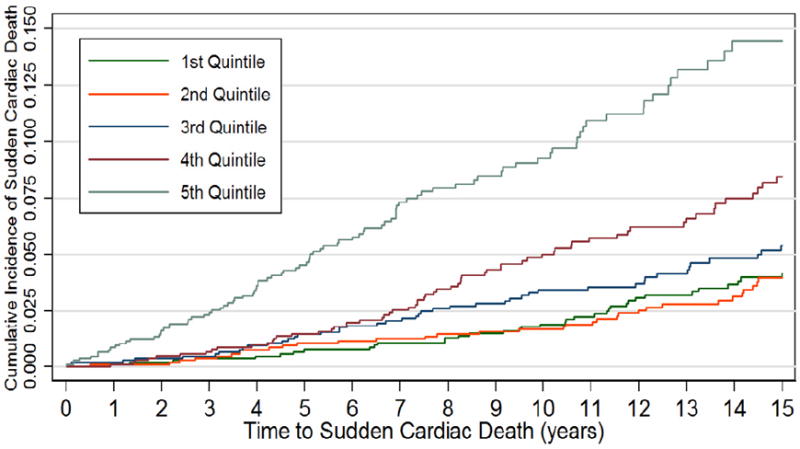
Cumulative Sudden Cardiac Death Rate by quintile of NT-proBNP
Since only the main cohort had echocardiograms performed at enrollment, coincident with NT-proBNP, the sample size for analysis was reduced to 3412 with 143 SCD events. LVEF was the only echocardiographic variable that was selected in the regression. Including LVEF in the Cox model reduced the coefficient for NT-proBNP, yet it remained statistically significant (Table 3).
In the subset of subjects without a prior history of myocardial infarction, heart failure, or stroke, 195 SCD occurred in 4606 participants. The unadjusted HR for ln NT-proBNP was 1.6 per standard deviation increase (95% CI 1.3, 1.9, p< 0.001). Although the hazard ratios were less in this group, in all quintiles compared with the entire cohort, higher NT-proBNP remained associated with an increased risk of SCD even after adjustment for clinical and echocardiographic variables (Table 3). In the cohort of subjects with known CVD (n=841, 94 SCD), the HR for the ln NT-proBNP was also statistically significant in the unadjusted and adjusted models. The hazard ratios for ln NT-proBNP for those with and without CVD were not statistically significantly different (test for interaction).
In time dependent Cox models in participants free of CVD at baseline adjusting individually for incident myocardial infarction, heart failure or stroke, both myocardial infarction and heart failure were strongly associated with SCD while stroke was non-significant. However, only the addition of heart failure to the model had any effect on the magnitude of the association of NT-proBNP and SCD, reducing the coefficient for ln NT-proBNP from 1.4 to 1.2 per standard deviation. Nonetheless, the coefficient remained statistically significant (p<0.025).
In Cox models including both the average of the ln NT-proBNP (standard deviation units) at baseline and follow-up and the change in ln NT-proBNP between the two time points, only the average of the values was significantly associated with SCD after adjustment for covariates (average ln NT-proBNP HR 1.52 (CI 1.3, 1.8, P<0.001), change in NT-proBNP HR 1.13 (CI 0.95, 1.34, p=0.18).
Discussion
In this large, population based study of older adults, higher levels of baseline NT-proBNP were positively associated with an increased risk of SCD. Subjects in the highest quintile of NT-proBNP had a 2.5 fold increased risk of SCD after adjustment for an extensive array of comorbid conditions. The association between NT-proBNP and SCD was modestly attenuated by adjustment for other risk factors, signifying that NT-proBNP may perform as a marker for these other conditions. However, this association persisted even after these adjustments, suggesting that either NT-proBNP itself influences SCD risk, or NT-proBNP serves as a marker of physiologic risk that is more strongly related to the underlying cause of SCD than this set of covariates. This hypothesis was reinforced by our finding that higher NT-proBNP levels remained associated in the large cohort of elder participants without underlying severe cardiovascular disease. Elevated NTproBNP levels should trigger suspicion regarding the threat of SCD in subjects who would otherwise be considered low-risk.
Of the more than 250,000 Americans that suffer a cardiac arrest each year, less than 10% will survive(13), and over half of the survivors will experience anoxic brain injury with variable sequellae(14). The significance of the morbidity and costs of SCD from a public health standpoint are widely understood; efforts to reduce the burden of SCD rely on improved primary prevention strategies, which must include identification of subpopulations at risk. Although conventional cardiac risk factors are also strong predictors of increased risk for SCD, the majority of those affected do not have a prior diagnosis of cardiovascular disease or risk equivalents(1). In our population of older adults, a single baseline measure of NT-proBNP provided important information regarding sudden death risk in addition to comorbid medical conditions, and prior to the development of incident medical conditions.
B-type natriuretic peptide is a well described biomarker predictive of cardiovascular outcomes(16-18). In 3346 asymptomatic subjects followed in the Framingham Study, an increase in B-type natriuretic peptide levels was associated with an increased risk of overall mortality, a first cardiovascular event, heart failure, atrial fibrillation, and stroke, independent of traditional risk factors(17). NT-proBNP was shown to be predictive of mortality, coronary events, heart failure, and stroke in a population based study in Copenhagen of 658 older adults, also independent of conventional risk factors(18).
In addition to overall mortality and cardiovascular events, B-type natriuretic peptide has been associated with SCD. In 452 ambulatory subjects with an ejection fraction of <35%, and pre-existing heart failure, B-type natriuretic level was the only significant predictor of sudden death after controlling for 16 neurohormonal, clinical, and hemodynamic variables(19). The Nurses’ Health Study recently reported a 1.49 relative risk increase for SCD for each 1-standard deviation increment in NT-proBNP in a nested, case control analysis(8). The relationship between NT-proBNP and SCD was strengthened when the model was adjusted for coronary heart disease risk factors, and when the analysis was limited to definite arrhythmic sudden death(8). However, the generalizability of these results has been unclear, since prior studies have revealed sex-specific differences in etiology of and genetic predisposition to cardiac arrest (20-21).
The limitations of this study include restriction to a population aged ≥65 years, with attendant comorbid conditions. Since the causes of SCD are likely to differ significantly when compared with a much younger population, extrapolation of these results may not be possible. It is important to note that in any observational study, causality is impossible to determine. While we found that incident heart failure is a confounder of the relationship of NT-proBNP to SCD, this doesn’t necessarily prove that heart failure alone explains the relationship. In addition, as in all studies regarding SCD, the phenotype itself is difficult to ascertain, and may be particularly so in the extremely aged. In a prior study in the CHS population, NTpro-BNP levels in the highest quintile were associated with a three-fold greater risk of incident heart failure and cardiovascular mortality 22, and the specificity of NT-proBNP elevations for SCD is therefore uncertain. However, many conditions associated with both SCD and cardiovascular mortality (i.e., diabetes and abnormal systolic ejection fraction) have shown themselves to be clinical useful, and have suggested future avenues for mechanistic study. The import of finding an association of NT-proBNP with SCD in the absence of known prior myocardial infarction, heart failure, or stroke may be in the potential etiologic clues it provides. For example, this finding in the ambulatory elderly population of the CHS may reflect an underlying substrate, such as cardiac fibrosis and not necessarily increases in intracardiac pressure or stretch23.
Our findings of an increased risk of SCD associated with higher NT-proBNP levels broaden our understanding of a useful biomarker. Although we cannot rule out modest interactions, baseline NT-proBNP levels had similar associations with SCD in both men and women, and in African-Americans as well as white Americans (both interactions were non-significant); emphasizing its potential global utility in a large population of older adults. In addition, there was no evidence of an interaction between cardiovascular disease status and the association with higher levels of NT-proBNP. That higher baseline NT-proBNP levels were associated with SCD over a very long follow-up period, in the absence of association with echocardiographic abnormalities or the clinical diagnoses of heart failure or coronary disease, suggests that peptide level elevations are a subclinical marker of underlying myocardial dysfunction, or may signal a predisposition towards arrhythmogenesis. Further investigation regarding the electrophysiologic effects of B-type natriuretic peptide and the consequences of genetic variations of NT-proBNP is of interest.
Conclusion
Few prior studies have focused specifically on NT-proBNP and the risk of SCD. Higher baseline levels of NT-proBNP in a large population based study of older adults were associated with an increased risk of SCD over a follow-up period of 16 years, independent of other comorbid clinical conditions. Further research is needed to determine whether the use of this biomarker to identify older adults at higher risk and target interventions for comorbidities reduces the risk of sudden cardiac arrest among older adults in the community.
Acknowledgments
A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Funding Support: The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Roche Diagnostics provided funding and laboratory reagents for the NT pro-BNP assay.
Abbreviations
- NT-proBNP
Amino terminal pro B-type natriuretic peptide
- SCD
sudden cardiac death
- CHS
Cardiovascular Health Study
- N
number
- ECG
electrocardiogram
- CVD
cardiovascular disease
- LVEF
left ventricular ejection fraction
- CI
confidence interval
- ln
natural log
- ng
nanogram
- dl
deciliter
- HR
hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–97. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Z-J, Croft JB, Giles WH, Mensah GA. Sudden Cardiac Death in the United States, 1989 to 1998. Circ. 2001;104:2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, McGee DL. Epidemiology of sudden death: insights from the Framingham Study. Cardiovascular Clinics. 1985;15:93–105. [PubMed] [Google Scholar]
- 4.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. European Heart J. 2005;26:2142–7. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 5.Levin ER, Gardner DG, Samson WK. Natriuretic Peptides. New Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 6.Omland T, Sabatine MS, Jablonski KA, et al. Prognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Col Cardi. 2007;50:205–14. doi: 10.1016/j.jacc.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. New Engl J Med. 2005;352:666–75. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 8.Korngold EC, Januzzi JL, Jr, Gantzer ML, Moorthy MV, Cook NR, Albert CM. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein as predictors of sudden cardiac death among women. Circ. 2009;119:2868–76. doi: 10.1161/CIRCULATIONAHA.108.832576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 10.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 11.Gardin J, Wong N, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 12.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–70. [PubMed] [Google Scholar]
- 13.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. New Engl J Med. 2009;361:605–11. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 15.Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–12. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. New Engl J Med. 2006;355:2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. New Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 18.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–16. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 19.Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circ. 2002;105:2392–7. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 20.Albert CM, Nam EG, Rimm EB, et al. Cardiac sodium channel gene variants and sudden cardiac death in women. Circ. 2008;117:16–23. doi: 10.1161/CIRCULATIONAHA.107.736330. [DOI] [PubMed] [Google Scholar]
- 21.Chugh SS, Chung K, Zheng ZJ, John B, Titus JL. Cardiac pathologic findings reveal a high rate of sudden cardiac death of undetermined etiology in younger women. Am Heart J. 2003;146:635–9. doi: 10.1016/S0002-8703(03)00323-5. [DOI] [PubMed] [Google Scholar]
- 22.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Col Cardi. 2010;55:441–50. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstam MA. Natriuretic peptides and cardiovascular events: more than a stretch. JAMA. 2007;297:212–4. doi: 10.1001/jama.297.2.212. [DOI] [PubMed] [Google Scholar]


