Abstract
One of the major challenges in the field of regenerative medicine is how to optimize tissue regeneration in the body by therapeutically manipulating its natural ability to form scar at the time of injury or disease. It is often the balance between tissue regeneration, a process that is activated at the onset of disease, and scar formation, which develops as a result of the disease process that determines the ability of the tissue or organ to be functional. Using biomaterials as scaffolds often can provide a “bridge” for normal tissue edges to regenerate over small distances, usually up to 1 cm. Larger tissue defect gaps typically require both scaffolds and cells for normal tissue regeneration to occur without scar formation. Various strategies can help to modulate the scar response and can potentially enhance tissue regeneration. Understanding the mechanistic basis of such multivariate interactions as the scar microenvironment, the immune system, extracellular matrix, and inflammatory cytokines may enable the design of tissue engineering and wound healing strategies that directly modulate the healing response in a manner favorable to regeneration.
Introduction
A tissue’s natural response to injury or disease is associated with fibroblast deposition and scar formation. Scar tissue is often formed at the expense of normal tissue regeneration. The result of all significant injury and organ failure, regardless of the origin, is the presence of scar tissue. Often the balance between tissue regeneration, a process that is activated at the onset of injury or disease, and scar formation determines the ability of the tissue or organ to be functional. Using biomaterials as scaffolds often can provide a “bridge” for normal tissue edges to regenerate. Regenerative medicine uses various strategies for the management of disease or injury, including mechanisms that can enhance in situ tissue regeneration, the use of biomaterials, the use of cells for therapy, or the combination of biomaterials and cells for therapy or tissue engineering.
The immune system plays a key role during wound healing. It functions to sterilize the site of injury and remove dead or dying cells. Leukocytes also produce a host of inflammatory and anti-inflammatory cues that regulate the function of diverse cell types in vivo and can help or hinder wound healing, depending on the state of the local tissue microenvironment. In the context of regenerative medicine, the interaction of immune cells with both host and donor cells, the local matrix, and biomaterials must all be considered for successful tissue regeneration.
A critical prerequisite for successful tissue regeneration is appropriate extracellular matrix (ECM) remodeling. This process requires the tightly regulated synthesis and degradation of ECM components. Key players in this regulation are the matrix metalloproteinases (MMPs) and their endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). Their activity is considered to be the rate-limiting step in ECM degradation and preservation. As a function of their importance in the creation and maintenance of the ECM in both native and engineered tissues, recent attention has focused on the role of these proteins within the context of regenerating tissue.
Both surgical trauma and bacterial infections can lead to severe tissue injury that can be triggered by cell surface Toll-like receptor 4 (TLR4)-mediated receptor-ligand interactions. These polyvalent interactions occur between bacterially derived ligands as well as endogenous hyaluronan fragments. Hyaluronan is an inactive high molecular weight (≈2 million Da) nonsulfated glycosaminoglycan polymer made up of repeating units of (beta,1–4)-Dglucuronic acid-(beta,1–3)-N-acetyl-D-glucosamine. Hyaluronan fragments are essentially pieces of the full-length hyaluronan, and consist of hyaluronan oligomers of 12–16 disaccharides with molecular weights of ≈200,000 Da. TLR4-mediated interactions can lead to the release of life-threatening pro-inflammatory cytokines such as TNF (tumor necrosis factor)-α. As a result, this pathway is tightly regulated in all biological organisms. The checkpoints that initiate as well as arrest this tissue-damaging cascade are important, as the design of new molecules that could be used to manipulate this pathway is possible.
Regulatory roles of the immune response in wound healing and tissue regeneration, ECM remodeling, and the checkpoints that initiate as well as arrest tissue-damaging cascades are important interactions that require consideration when dealing with strategies that can help to modulate the scar response and can potentially enhance tissue regeneration in the field of regenerative medicine.
Principles of Wound Healing and Tissue Regeneration
The human body’s natural reaction to tissue injury is immediate activation of the wound healing cascade. The classic model of wound healing is divided into three sequential, yet overlapping, phases that occur in parallel with hemostasis: (1) inflammatory, (2) proliferative, and (3) remodeling.1,2 Upon injury, a set of complex biochemical events takes place in a closely orchestrated cascade to repair the damage.1 Within minutes post-injury, platelets or thrombocytes aggregate at the injury site to form a fibrin clot. This clot acts to control active bleeding through hemostasis.
In the inflammatory phase, bacteria and debris are phagocytized and removed, and factors are released that cause the migration and division of cells involved in the proliferative phase. The proliferative phase is characterized by angiogenesis (growth of new blood vessels from pre-existing vessels), collagen deposition, granulation tissue formation, cell growth, and wound contraction.3 In angiogenesis, new blood vessels are formed by vascular endothelial cells.4 In fibroplasia and granulation tissue formation, fibroblasts grow and form a new, provisional ECM by excreting collagen and fibronectin.3 Concurrently, cell growth occurs in which cells proliferate and “crawl” atop the wound bed, providing cover for the new tissue.5
In contraction, the wound is made smaller through myofibroblasts, which establish a grip on the wound edges and contract themselves using a mechanism similar to that in smooth muscle cells. In the maturation and remodeling phase, collagen is remodeled and realigned along tension lines, and apoptosis removes unnecessary cells.3
Although many parameters are responsible for scar formation, such as the wound healing cascade or increased tissue tension, the result in humans is often fibrous tissue deposition at the expense of tissue regeneration. A human’s initial response to injury favors isolation of the injury site from the outside environment and prevention of infection. The primary cause of death after injury or tissue disease in the early 1900s was infection. Antibiotics were not introduced for widespread clinical use until the 1940s. Humans have had over a six million year evolutionary history of depending primarily on their innate immune system to prevent or control infection at the time of injury or disease. Therefore, when taking into account the many parameters responsible for wound healing, it is evident that sealing our injuries from the outside environment with scar formation and the enhancement of our immune system to prevent infection occur somewhat at the expense of normal tissue regeneration.
Hemostasis and the wound repair process can often lead to scarless healing for small superficial wounds, such as shaving razor cuts. When the wounds are small and superficial, scars seldom form. For deeper wounds, scars are almost always present, even under conditions with a controlled environment, such as surgical scalpel incisions. The use of biomaterials and scaffold systems can enhance tissue regeneration and decrease scar formation. Nonetheless, it has been shown that the maximum distance for normal tissue regeneration to occur using biomaterial scaffolds alone is approximately 0.5 to 1 cm from any normal tissue edge (Figure 1).6 Larger tissue defect gaps require both scaffolds and tissue-appropriate cells for normal tissue regeneration to occur. This has been readily demonstrated with the engineering of several different tissue types using various scaffold systems created with both naturally derived and artificial biomaterials (Figure 2). In bladder tissue, naturally derived scaffolds created with decellularized bladder ECM alone can induce the regeneration of only limited amounts of normal tissue over short distances. However, if the defects are large, fibrosis and scar formation ensue.7,8
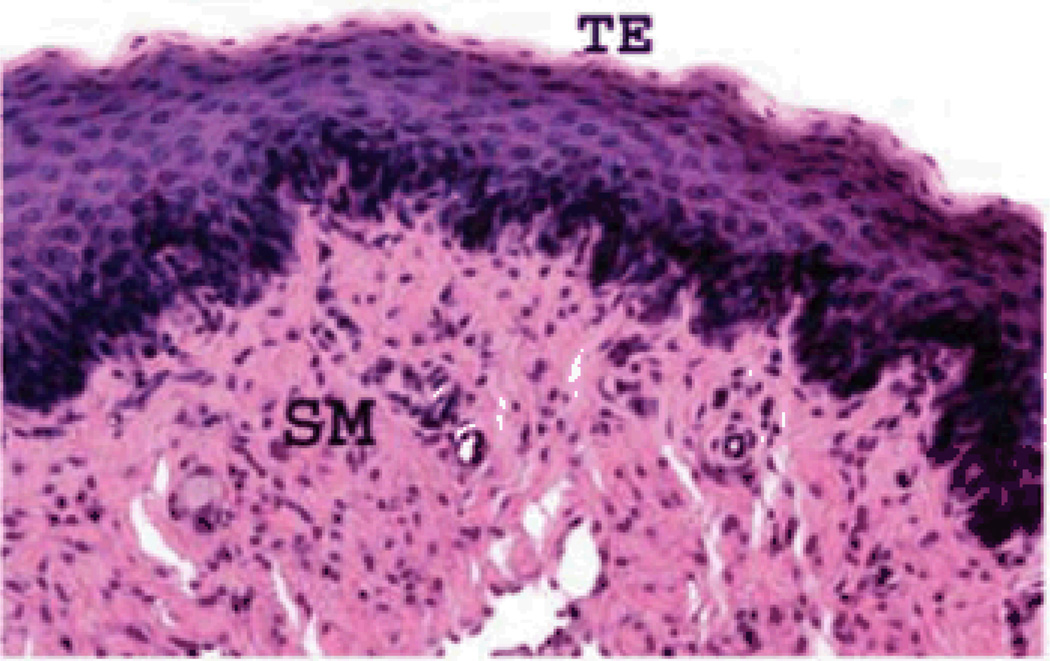
Figure 1.
Hematoxylin and eosin staining of 0.5 cm acellular urethral graft four weeks after implantation. A transitional epilthelial (TE) layer and a smooth muscle (SM) layer can be seen.
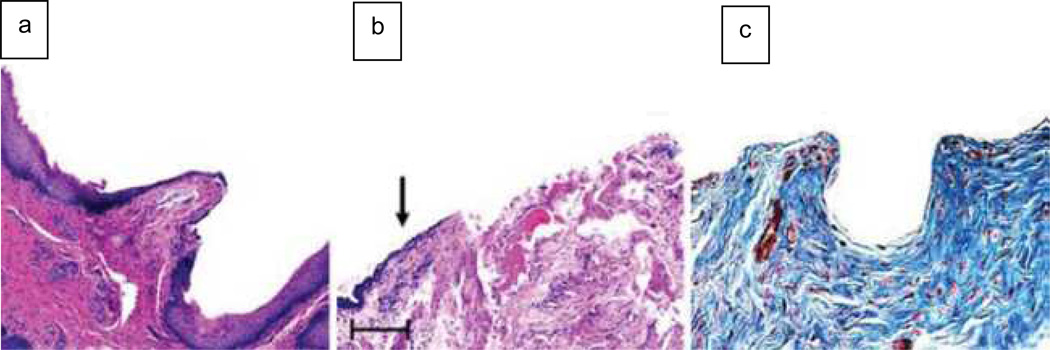
Figure 2.
Histology comparing short and long urethral grafts. (a) Hematoxylin and eosin staining of the anastomosis between normal urethral tissue (NU) and the acellular graft (GR) in the 0.5 cm defect, which indicates ingrowth of normal cell types. (b) Representative cross section of a longer graft. The arrow points to increased fibrosis and a friable epithelial surface, beginning approximately 0.5 cm from the tissue-to-graft anastomosis. (c) Masson’s trichrome staining of a longer graft confirms the increased collagen deposition that is characteristic of fibrosis.
In a similar manner, vaginal organs were engineered by using artificially created polyglycolic-acid matrices either alone or seeded with vaginal muscle and epithelial cells. The biomaterial scaffoldonly implants fibrosed and constricted over time. The cell-seeded matrices were able to regenerate the typical tri-layer vaginal tissue structure with adequate functional parameters long-term (Figure 3).9 Similar findings were present when engineering other tissues, such as blood vessels, heart valves, salivary glands, penile tissue, and nerves.10–14 These studies to date show that scars do not necessarily form for small or superficial wounds or injuries but may be present for deep or larger wounds, often over 3 mm in diameter. Scars for the larger wounds often can be minimized with the addition of biomaterials that can “bridge” the gap. Often, the biomaterials provide a readily available source of ECM that can act as the platform for native cells to “crawl” over the newly provided “wound bed.” However, as noted, there is a maximum distance for cells to regenerate over gaps greater than 1 cm, even if additional biomaterials or ECMs are provided. Fibroblasts tend to fill the larger gaps more rapidly than the native tissue cells, which are responsible for tissue regeneration and usually present at the edge of the injury. Therefore, for gaps 1 cm or larger, the addition of cells is needed for adequate regeneration to occur. Studies done to date suggest that having cells in place, in addition to biomaterials, leads to tissue formation and prevents the typical fibroblast deposition that is programmed to occur when injury is present. The wound healing response is essential for tissue regeneration, and providing the wound bed with additional matrix and cells enhances normal tissue formation.
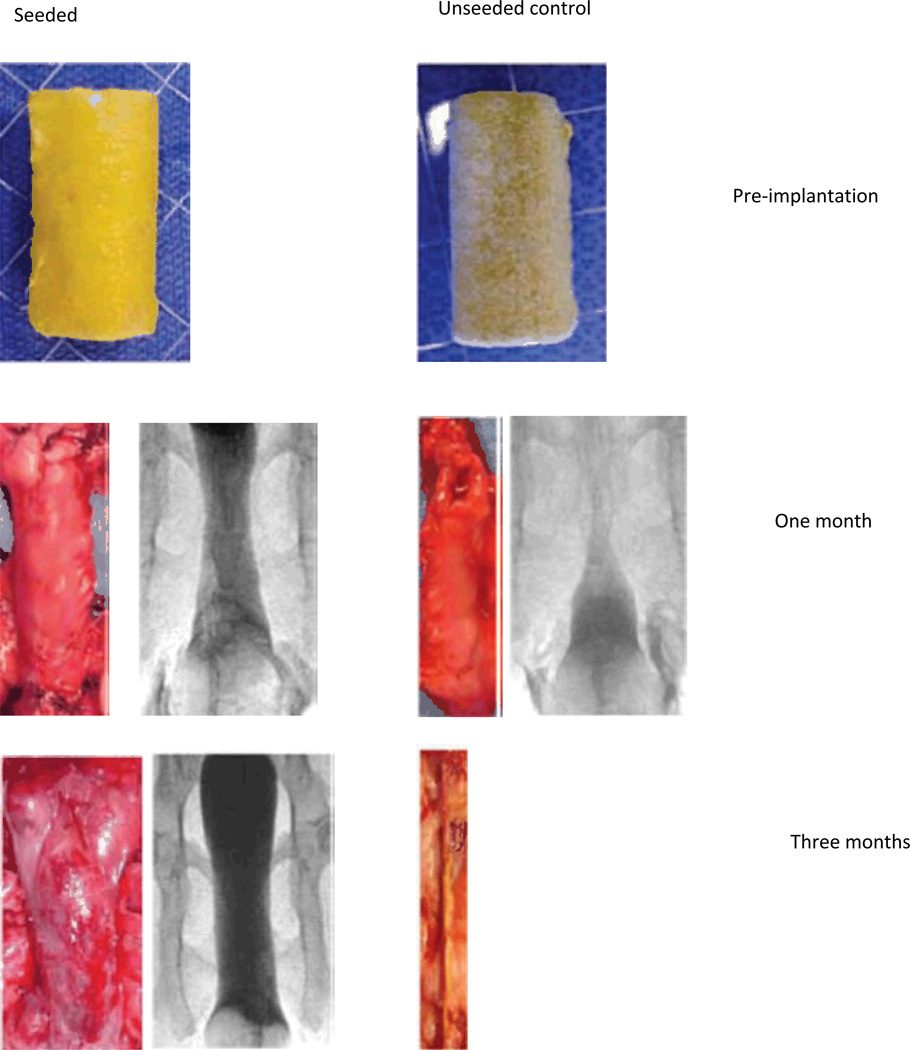
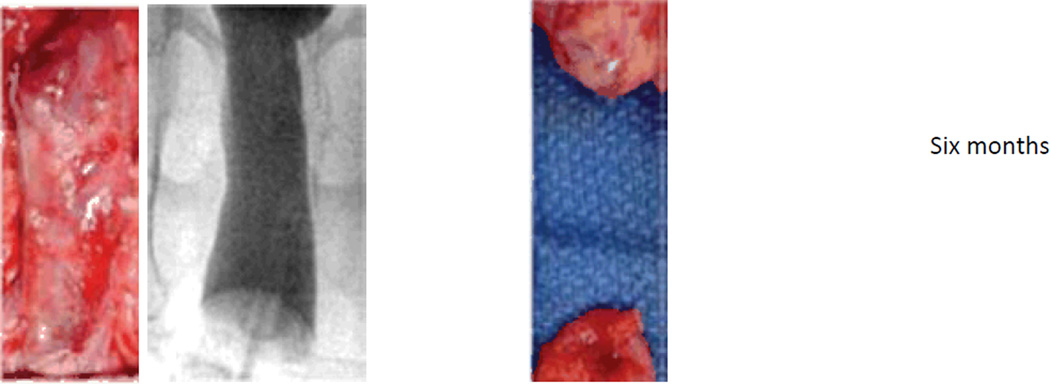
Figure 3.
Appearance of tissue-engineered vaginas at one, three, and six months after implantation in rabbits. The gross appearance of the unseeded and cell-seeded vaginal scaffolds after one week in culture is shown at the top of the figure. At each time point after implantation of these constructs, vaginography was performed, and then the construct was dissected out of the animal. A representative vaginogram and the gross appearance of the construct are shown for each time point. It can be seen through both vaginography and gross examination that the cell-seeded grafts resulted in patent tissue-engineered vaginas that are similar in structure to normal vaginal tissue. However, the unseeded grafts fibrosed and constricted with time, and tissue-engineered vaginas did not form, as evidenced by the absence of tissue in the photograph of the graft after six months in vivo.
In addition to the use of biomaterials and cells, there are many mechanisms that are able to optimize in situ regeneration. Understanding the scar microenvironment and being able to control the immune response, the matrix environment, and the inflammatory pathways may give us insight into the development of smarter biomaterials that can induce regeneration over larger distances and may create more efficient strategies for in situ tissue and organ regeneration after injury or disease.
Regulatory Roles of the Immune Response in Wound Healing and Tissue Regeneration Settings
The immune system plays a significant role in the natural process of wound healing, and thus immune cells and factors produced by these cells are also involved in the host response to tissue regeneration strategies. At least three distinct regulatory roles can be identified: (1) regulation of acute inflammation and induction of a foreign body response (if an implant is present), (2) regulation of angiogenesis and matrix remodeling, and (3) positive or negative feedback from interactions with host cells or transplanted donor cells.
Immune cells sterilize wounds and clear cellular debris at sites of tissue damage. Notably, immune cells have evolved to respond to not only conserved molecular signatures of pathogens (pathogen-associated molecular patterns) but also to signatures of tissue damage (danger-associated molecular patterns), such as extracellular ATP, heat shock proteins, and uric acid, which are present even at sterile sites of injury.15,16 Leukocytes are rapidly recruited to sites of tissue trauma and have an early interaction with scaffolds placed at a site to promote tissue regeneration. Biomaterials elicit a foreign body response that involves both immune cells and local tissue cells and leads to the generation of a fibrous capsule around biomaterial implants. Several recent excellent reviews have described our current understanding of the foreign body response in detail.17–19 Immediate release of chemoattractants, histamine, and inflammatory cytokines as blood clots in the wound site leads to neutrophil recruitment to the tissue (acute inflammation), followed by entry of monocytes and macrophages, which may fuse at the surface of implants over time, becoming foreign body giant cells that attempt to degrade/digest the biomaterial. Phagocyte recruitment can be supported by a positive feedback loop created as macrophages contacting implanted biomaterials produce additional chemoattractants for monocytes,20 which enter the site and differentiate into macrophages. Lymphocytes accumulate later in the response, and their presence defines the phase of chronic inflammation.17 The foreign body response is often viewed as a negative process that competes against therapeutic cells/scaffolds designed to drive tissue regeneration, but the growing appreciation for the immune system’s role in regulating repair processes—and its ability to either promote or inhibit key steps—makes this picture more complex. Unresolved chronic inflammation at a wound site favors scarring over tissue regeneration, and thus resolution of the early inflammatory response may be critical for successful regeneration strategies. Recently, an unappreciated role for adaptive immune cells in terminating inflammatory responses was discovered, revealing that CD4+ T cells actively shut down cytokine production by innate immune cells to terminate inflammatory responses.21 The identification of these and other negative regulatory pathways controlling inflammation22 could be important for promoting regeneration over scarring, and provides motivation for potentially engineering the recruitment of immune cells that might be beneficial for promoting tissue regeneration.
Macrophages and dendritic cells (DCs) are also sources of key cytokines and other factors regulating angiogenesis and ECM remodeling,23 and selective deletion of macrophages has been shown to impair collagen deposition, angiogenesis, and reepithelialization in mice.24 Interactions of macrophages with proteins adsorbed to biomaterial scaffolds (or bioactive molecules intentionally displayed from the surface of scaffolds) directly influence the production of matrix remodeling enzymes and angiogenic factors.20,25 Thus, attempts to regulate macrophage/dendritic cell function in tissue regeneration/wound healing therapies are of substantial interest, beyond mitigation of the foreign body response.
A final key regulatory role arises in the interaction between immune cells and donor cells that may be delivered with scaffolds in tissue regeneration or surrounding host cells. If donor cells are not autologous (derived from the same patient), recognition of the donor cells as foreign by host CD8+ T cells (and in some cases, CD4+T cells) can lead to direct killing of the transplanted cells or generation of strong local inflammation. Interestingly, studies have suggested that certain adult stem cell populations may have immunosuppressive properties capable of blocking allogeneic reactions, which could have important implications for regenerative medicine.26 In addition, a variety of cross-regulatory interactions are being revealed between immune cells/inflammatory mediators and parenchymal cells or progenitor cells. In bone fractures, an important role for inflammatory ctyokines and inflammatory factors has been identified in osteoblast/osteoclast (bone forming/remodeling cells, respectively) differentiation27 and bone healing.28,29 Activated macrophages secrete factors that favor regeneration of damaged optic nerves or and central nervous system neurons.30,31 In addition, inflammatory cytokines produced by innate immune cells promote neurogenesis from human mesenchymal stem cells.32 Strikingly, the liver, one of the few major organs capable of dramatic regeneration following damage in adult mammals, depends on two inflammatory cytokines, IL (interleukin)-6 and TNF-α, for regeneration.33 Complement, traditionally viewed as an inflammatory effector of innate immunity, has been implicated in guiding bone synthesis from fetal cartilage during development34 and limb regeneration in lower vertebrates35 and also plays a role in mammalian liver regeneration.36 Thus, immune cells and inflammatory factors may impact tissue regeneration therapies in many ways beyond their obvious roles in sterilization and tissue remodeling.
The ability of immune cells to influence the outcome in wound healing and to impact responses to tissue-engineering constructs has led to the concept that the immune system itself could be a target for therapeutic manipulation in wound healing and regenerative medicine settings.37–40 From this perspective, the same materials engineering and regenerative medicine concepts used to shape the microenvironment in support of parenchymal cells or vasculature could be employed to shape immune responses in the implant site. This could include the use of cytokine-releasing scaffolds,41 matrices with cell-releasable embedded factors,42,43 or co-delivery of drug-releasing microparticles/nanoparticles at the tissue site. However, applying such methods to manipulate the immune system is a relatively new concept where many potentially interesting avenues remain to be explored.
Controlling Immune Cell Recruitment to Tissue Sites
Because of the pro-regenerative and proangiogenic roles immune cells can play in wound healing, selective recruitment of targeted immune cell populations to tissue sites could provide a means to regulate regenerative processes. Immune cell trafficking in vivo is regulated by the expression of specific receptors for chemoattractants, proteins that stimulate directed cell migration when cells are exposed to concentration gradients of these factors.44,45 The chemoattractants that regulate leukocyte trafficking to tissues are well understood, making targeted attraction of specific cells to a tissue site possible. Biomaterials that release chemoattractants to create de novo chemoattractant gradients and elicit directed cell migration have been demonstrated in vitro and in vivo.46–50 Alternatively, selective exclusion of inflammatory cells from tissue sites, in some instances, also may be of interest in tissue regeneration to block chronic inflammatory processes and avoid unwanted inflammatory cell accumulation within/around tissue scaffolds. Interestingly, certain proteins can act as chemorepellents, driving immune cell migration away from tissue sites.51–53A more broad blockade of leukocyte (as well as other cells) entry into tissue sites from blood also might be achieved using molecules that block chemoattractant function, such as soluble tissue polysaccharide fragments or heparin, which bind to chemoattractants and block chemotaxis.54 Heparin analogues that lack anticoagulant activity but retain an ability to block the trafficking of cells through endothelial walls of blood vessels are of growing interest to regulate immune cell recruitment to inflammatory sites.55
Delivery of immune cell-guiding chemoattractant cues to a tissue site can be achieved by a number of strategies. Similar to the delivery of other therapeutic proteins, chemoattractants can be physically entrapped in solid polymer matrices,46 hydrogels,50 or biodegradable particles/scaffolds47,49 for diffusion/erosion-mediated release. Alternatively, strategies seeking to exploit the matrixbinding properties of chemokines can be employed; similar to cytokines such as basic fibroblast growth factor, many chemokines are basic, highly charged proteins that bind to glycosaminoglycans in the ECM.56,57 Thus, tissue scaffolds decorated with heparan sulfate proteoglycans or heparin can be loaded with chemoattractants by physisorption for slow release and gradient generation.48 Finally, vaccine studies have convincingly demonstrated the utility of plasmid DNA inoculation to locally transfect tissue cells to produce chemoattractants that draw immune cells to an injection site,58,59 and materials designed to promote plasmid delivery could likewise be tailored to this purpose in the setting of tissue regeneration.
Modulating the Function of Immune Cells at Tissue Regeneration Sites
In addition to regulating the recruitment of immune cells, tissue regenerative strategies could seek to modulate the function of immune cells present at the site. Treatment of implant sites with traditional anti-inflammatory drugs (or design of biomaterials that release these drugs in situ) has been attempted in an effort to minimize acute inflammation, but results of such strategies have been mixed, with some studies reporting beneficial effects and others suppression of tissue regeneration.39,60,61 This likely reflects the multiple positive and negative regulatory roles played by immune cells in the healing response discussed previously and the challenge that inflammatory cytokines often act on multiple cell types in vivo, sometimes in an opposing fashion. Notably, in any strategy aiming to modulate the function of immune cells at an implant site, careful attention must be paid to the purity of the materials employed. Trace contaminants such as bacterial products (especially endotoxin) are strong pro-inflammatory signals that directly stimulate innate immune cells and B cells, and these can be inadvertently introduced due to the use of impure starting materials from biologic sources or contamination during synthesis/handling. Also relevant is the fact that some materials have intrinsic immunostimulatory properties:18 Nanoparticles and microparticles are composed of many different materials ranging from poly(D,L-lactic acid-co-glycolic acid) (PLGA) to polystyrene to silica and have been recently shown to stimulate the inflammasome upon internalization by innate immune cells, leading to the production of inflammatory cytokines.62–64 Fragments of hyaluronan are signatures of tissue damage, and thus low molecular weight hyaluronan breakdown products from tissue matrices could also drive inflammatory cytokine production.65 Similarly, biomaterials based on natural polysaccharides, such as chitosan and alginate, may stimulate innate immune cells, though molecular weight, purity, and composition (ratio of guluronic versus mannuronic acid residues in alginate) effects play a significant role in whether these materials elicit inflammatory responses.66–69 Thus, a full understanding of the potential immunoregulatory triggers during synthesis, application, and eventual resorption of biomaterials in situ may be critical to successfully modulate immune responses without uncontrolled “background” inflammation.
As an alternative to general anti-inflammatory drug treatments, some studies have sought to tailor the composition of biomaterial scaffolds to directly alter the immune cell response to implanted scaffolds. One strategy has been to incorporate inflammation-blocking signals into the scaffold itself. For example, coupling of heparan sulfate or chondroitin sulfate glycosaminoglycans (GAGs) to cross-linked collagen tissue scaffolds reduced the foreign body response elicited in a rat model.70 While GAG incorporation could have many potential effects on the host response and the mechanisms underlying this result were not explored in this study, GAGs are known to bind the CD44 receptor of macrophages and provide signaling blocking foreign body giant cell formation.71 A similar reduction in the degree of fibrous tissue formation around PLGA scaffold implants was observed when acid-demineralized bone particles were embedded in scaffolds designed for bone tissue regeneration.72 These demineralized bone particles are known to contain a complex collection of proteins, including matrix collagen, bone morphogenetic protein, and other factors, and the precise signals from these particles involved in mitigating fibrous tissue formation in this instance remain unknown. In a more targeted strategy, van Putten et al. impregnated cross-linked collagen scaffolds with recombinant IL-10, one of the key cytokines involved in arresting inflammatory responses, which directly acts on macrophages, dendritic cells, and T cells.73 IL-10 release at the implant site did not block initial macrophage recruitment into the tissue site, but blunted collagenase activity and greatly reduced the formation of giant cells within the scaffolds.
As discussed previously, molecules traditionally viewed as pro-inflammatory also can promote tissue regeneration/healing in certain tissues. For example, biodegradable PLGA matrices releasing selective prostaglandin receptor agonists implanted in bone defects have been shown to promote bone healing in canine models while avoiding systemic side effects.28 To invoke the potential functions of complement in driving tissue regeneration in liver or bone regeneration, new materials being developed to control the activation of complement in situ may be of interest: For example, nanoparticles that carry functional groups capable of triggering complement activation have recently been shown to promote immune responses to vaccine antigens in immunization.74 Such materials might find new applications in tissue regeneration, particularly if coupled to the idea of co-functionalizing particles with matrix-binding peptides that anchor small particles in the ECM for prolonged periods.75 As illustrated by these examples, appropriate immunomodulatory strategies (either pro- or anti-inflammatory, depending on the situation) can have substantial benefits in tissue regeneration, and biomaterials can play an important role in maximizing the lifetime of key factors at the treatment site while limiting systemic exposure to potent signals.
Extracellular Matrix Remodeling
It is widely appreciated that tightly regulated ECM remodeling is a prerequisite for both successful wound healing and tissue regeneration. The most extensively studied mediators of this remodeling are the matrix metalloproteinase (MMP) family of enzymes, whose activity is the rate-limiting step in ECM remodeling. MMPs are a multigene family of zinc-dependent endopeptidases that share a relatively conserved structure. Their activity is regulated at both the transcriptional and the translational level and, once produced, predominantly by four endogenous inhibitors known as the tissue inhibitors of metalloproteases (TIMPs). Most MMPs are produced in a latent form and are activated extracellularly. The reader is referred to a number of comprehensive reviews of these important enzymes and their activities.76–81
As previously reviewed, the classic MMP domain structure includes A signal peptide domain, which guides the enzyme into the rough endoplasmic reticulum during synthesis.
A propeptide domain, which is responsible for maintaining the latent state of these enzymes until it is removed or disrupted.
The catalytic domain, which houses the highly conserved Zn2+ binding region and is responsible for the proteolytic activity.
The hemopexin domain, which determines the substrate specificity of MMPs.
A small hinge region, which enables the hemopexin domain to present substrate to the active core of the catalytic domain.
The subfamily of membrane-type MMPs (MT-MMPs) possesses an additional transmembrane domain, composed of a membrane-spanning segment and an intracellular domain.76
MMP activity is predominantly controlled by a group of structurally related, endogenous inhibitors known as TIMPs. TIMPs have been shown to specifically and reversibly inhibit the activity of MMPs. Four members of this family have been cloned and expressed to date: TIMP-1, -2, -3, and -4. These inhibitors can block the autocatalytic activation of latent MMPs as well as inhibit the proteolytic activity of activated MMPs due to their ability to bind both latent and active MMPs.82 Although originally distinguished from each other on the basis of their substrate specificity, which includes a wide variety of ECM components such as collagens, gelatins, elastin, and fibronectin, it is now known that these enzymes are capable of degrading other, non-ECM-related substrates as well,76 thereby increasing the complexity of their regulatory ability.
Although most extensively studied within the context of their regulation of angiogenesis, tumor growth, and metastasis, a growing literature has focused on MMPs and their roles in wound healing and tissue regeneration. It has long been appreciated that the successful and functional tissue remodeling that accompanies, and is required for, these latter two complex processes requires both degradation and synthesis of tissue components (i.e., both degenerative and regenerative processes).83–86 In the absence of tightly regulated ECM remodeling during these processes, significant physiological consequences result. For example, dysregulated ECM degradation in favor of excessive matrix formation or deposition can result in fibrotic disease,87,88 whereas excessive matrix degradation can result in chronic wounds that can be difficult or impossible to heal.89,90 Moreover, related processes necessary for normal wound healing and tissue regeneration, such as angiogenesis, are dependent upon the appropriate and controlled ECM remodeling mediated by these ECM-degrading MMPs76,91–93 and affected as well.
Successful tissue regeneration studies have clearly revealed the importance of appropriate ECM remodeling and angiogenesis in this complex process.92–94 For example, Alwayn and colleagues demonstrated that MMPs are required for successful post-hepatectomy liver regeneration in a murine model.93 Inhibition of MMP activity by a synthetic MMP inhibitor, marimastat, significantly inhibited regeneration in this system.93 Interestingly, the presence of urinary MMPs and their endogenous inhibitors, TIMPs, correlated with the progressive return of resected livers to their preoperative mass.93 These and other studies92,93,95,96 support a long-standing suggestion that the control of MMP activity (either positive or negative) in chronic wounds might represent a potential therapeutic strategy for this clinical problem.97–100
Tissue Damage Cascades
Both surgical trauma and bacterial infections can lead to severe tissue injury that can be triggered by cell surface TLR4-mediated receptor-ligand interactions. These polyvalent interactions between bacterially derived ligands as well as endogenous hyaluronan fragments can lead to the release of life-threatening proinflammatory cytokines such as TNF-α. As a result, this pathway is tightly regulated in all biological organisms. The checkpoints that initiate as well as arrest this tissue-damaging cascade are important, as the design of new molecules could be used to manipulate this pathway.
The Inflammatory Response Associated with Bacterial Infections
Fundamental to innate immunity are the pattern recognition receptors (TLRs) that recognize pathogen-associated molecular patterns. They allow the immune system to distinguish self-structures from pathogen-associated non-self molecules. They are the first line of host defense against invading pathogens.101 TLR4 on macrophages and dendritic cells is the key cell surface receptor. Antigen-mediated triggering leads to cytokine expression, dendritic cell maturation, and adaptive immune responses.
The outer membrane of all gram-negative bacteria, which cause infections in humans and have been extensively studied, is made up of a bilayer that consists of phospholipids on the inner leaflet, and the lipid anchor region of lipopolysaccharide (LPS) (i.e., lipid A) on the outer leaflet. Recognition of LPS, a potent pro-inflammatory stimulus and critical to discussion of these pathways, occurs as part of the TLR4-MD-2-CD14 receptor complex.102,103 In brief, the transport protein CD14 delivers LPS to MD-2, which has a hydrophobic pocket that is lined by a hydrophilic entrance. Lipid A binds to the hydrophilic entrance. Its lipid chains then enter MD-2’s hydrophobic pocket. The LPS-MD-2-TLR4 complex then undergoes a conformational change that enables TLR4 to dimerize. Intracellular signaling follows.103,104
Only a very short stimulation of TLR4 is required to lead to dendritic cell maturation and T cell stimulation. This contrasts with the prolonged and sustained stimulation of TLR4 that is required for the induction of pro-inflammatory cytokines such as TNF-α and IL-6. Distinct thresholds should exist within the TLR4-MD2-LPS complex (at cell surface level) for inducing the expression of CD markers of cellular differentiation compared to the release of cytokines.103 This unique nature of TLR4 compared to all other TLR receptors has only been recently recognized.105
The Inflammatory Response Associated with Surgery
The successful repair of injured tissues requires a coordinated host response to control the amount of structural damage. A major hallmark that alerts the host to tissue injury is the sudden increase in the turnover of hyaluronan in ECM.106 In its native form, hyaluronan exists as an inactive high molecular weight nonsulfated glycosaminoglycan polymer with a molecular weight of ≈2 million Da. It is made up of repeating disaccharide units of (beta,1–4)-Dglucuronic acid-(beta,1–3)-N-acetyl-D-glucosamine. At sites of acute inflammation and tissue injury, it is rapidly broken down by the local release of enzymes such as hyaluronidase, beta-glucuronidase, and hexosaminidase. Low molecular weight fragments are generated that consist of hyaluronan oligomers of 12–16 disaccharides, with a molecular weight of ≈200,000 Da. These fragments have all of the features of a pathogen-associated molecular pattern, and they mediate their biologically important effects on macrophages, dendritic cells, and endothelial cells.65,106 When compared to LPS, only high and localized concentrations of these hyaluronan fragments at sites of acute inflammation are capable of inducing pro-inflammatory chemokine and cytokine responses in dendritic cells. Resolution of the acute inflammatory response requires clearance of the hyaluronan fragments from their focal sites of accumulation by enzymatic degradation to hyaluronan disaccharides of ≈28,000 Da. These have no biological activity. In 2005, Jiang et al. showed that hyaluronan fragments trigger TLR-4 and TLR-2 and that signaling occurs via the MyD88/TIRAP pathway, which results in activation of the transcription factor NFκB and subsequent expression of pro-inflammatory genes.107 These fragments also can enhance T cell responses by activating and upregulating co-stimulatory molecules on immature dendritic cells. These observations have been confirmed in vitro and in vivo, as well as in mice and humans.108
In addition, it has recently been shown that high molecular weight hyaluronan (i.e., ≈2 million Da) has no effect on LPSmediated signaling via TLR4 and is an antagonist of TLR2. In contrast, low molecular weight hyaluronan (i.e., ≈200,000 Da) is an agonist of TLR4 and a partial agonist of TLR2. Taken together, these observations show that high molecular weight hyaluronan maintains homeostasis by downregulating pro-inflammatory responses, while the localized generation of low molecular weight hyaluronan in areas of tissue injury acts as an endogenous alert signal that triggers innate and acquired immune responses. It also means that the balance between high molecular weight hyaluronan and low molecular weight hyaluronan fragments critically controls the activation of the innate immune response in areas of tissue damage.109,110 These recent observations should not be confused with the previously well-established and essential role of CD44 in regulating the turnover of high molecular weight hyaluronan, because CD44 is not required for the expression of chemokines or cytokines by macrophages in vivo.111
Polyvalency
Bacterial infections and surgical tissue injury therefore trigger cell surface receptor-ligand interactions that are very specific in nature. They do not involve a single receptor-ligand interaction. Rather, these pro-inflammatory responses are mediated by polyvalent receptor-ligand interactions between bacterially derived ligands or hyaluronan and cell surface TLRs.106 The binding affinity of these ligands for their receptors increases exponentially as the number of receptor-ligand interactions increases.112 Also in this context, it is the cell surface–associated heparan sulfate proteoglycans that bind and concentrate the proteins, which play such a crucial role in cell-cell and cell-ECM interactions. These interactions between cell surface heparan sulfate proteoglycans, hyaluronan, and proteins ensure their central role in normal physiology and in the progression of many disease processes.113 Therefore, scientists are now adapting the concept of polyvalency to biomaterial design by creating materials that can interact with and modulate multiple tissue injury pathways at once.
Modulating Polyvalent Receptor-Ligand Interactions
As polyvalency requires multiple and cooperative receptor-ligand interactions, pharmacological intervention will require medicines based upon molecules that are also capable of multiple and co-operative interactions. This has already been achieved with protein-based medicines, which interact with multiple cell surface receptors with high affinity. For many years, the aim has been to achieve analogous co-operative interactions with synthetic macromolecules.
However, it has been found that in biological systems, the use of linear polymers has been less successful than anticipated. Attempts to use linear polymers have been impeded by (1) the structural heterogeneity of the macromolecules used; (2) an inability to control their molecular weight characteristics; and (3) the toxic side effects of activating complement and coagulation-triggered pathways.114
In the case of linear polymers that display saccharides, they have a tendency to self-associate and to form micelles because of the amphiphilic characteristics of many polymer-ligand combinations. In the case of polysaccharides, their structural heterogeneity and the complex nature of the chemistry involved in their preparation has impeded the scalable and reproducible synthesis of defined oligosaccharide-like molecules with the appropriate biological properties. In general, many synthetic steps are required, and the chemical intermediates and products made are very difficult to purify. These compounds are also very difficult to handle because they tend to be hygroscopic syrups, chemically labile, susceptible to rapid microbial degradation, and difficult to process into medicines. These fundamental problems have impaired the systematic study of saccharide-based structure-property correlations for rational drug design.
Dendrimers and Polyvalent Interactions
Dendrimers constitute an exciting component of the emerging nanotechnology revolution.115 They are an architectural class of hyperbranched synthetic nanomolecules that can be made by controlled sequential processes to give well-defined chemical structures. They are prepared from a starting core by a sequence of two reactions repeated sequentially to produce incremental “generations.” As dendrimers are hyperbranched, the ends of each branch define the molecular surface of the dendrimer. Notably, (1) their physicochemical properties are similar to those of conventional small molecule drugs; (2) they can be modified to exist as zwitterions at physiological pH; and (3) they have a considerable buffering capacity that makes them physicochemically “similar” to blood proteins (e.g.,albumin), and therefore biocompatible. However, unlike proteins, they (1) do not undergo proteolytic degradation in plasma; (2) are not immunogenic; (3) are not toxic after repeated intravenous administration; (4) can be optimized for their circulation time; and (5) show preferential accumulation in tissues containing inflammatory cells compared to healthy tissue at a ratio of 50:1.
Dendrimer Glucosamine Conjugates as TLR4 Antagonists
Until recently, the therapeutic potential of dendrimers was restricted to drug delivery and to their use as imaging agents.115,116 There is now an increasing recognition of the potential importance of making new and polyvalent dendrimerbased medicines for manipulating biologically well-defined cell surface–mediated immunoregulatory interactions between carbohydrates and proteins. Conventional small molecule drugs lack ligand-binding cooperativity (i.e., polyvalency) and therefore cannot enable such interactions at the cell surface.
Dendrimer glucosamine has been shown to inhibit hyaluronan—TLR4 cell surface–mediated pro-inflammatory cytokine production from human macrophages and dendritic cells while allowing the activation and maturation of dendritic cells.117 When monocytes and dendritic cells were cultured with dendrimer glucosamine for 30 minutes and then exposed to highly purified LPS for 21 hours, there was a significant reduction in the release of both chemokines (macrophage inflammatory proteins-1α and -1β, IL-8) and cytokines (TNF-α, IL-1β, and IL-6). When this experiment was repeated by incubating cells with LPS followed by the addition of dendrimer glucosamine after two or four hours, a significant inhibition of pro-inflammatory mediator release was still seen. Similar results were obtained with live bacteria. The antagonistic activity of dendrimer glucosamine was specific to TLR4-mediated pro-inflammatory responses.
Controlling Uncontrolled Immunological Trauma in Elective Surgery by Design
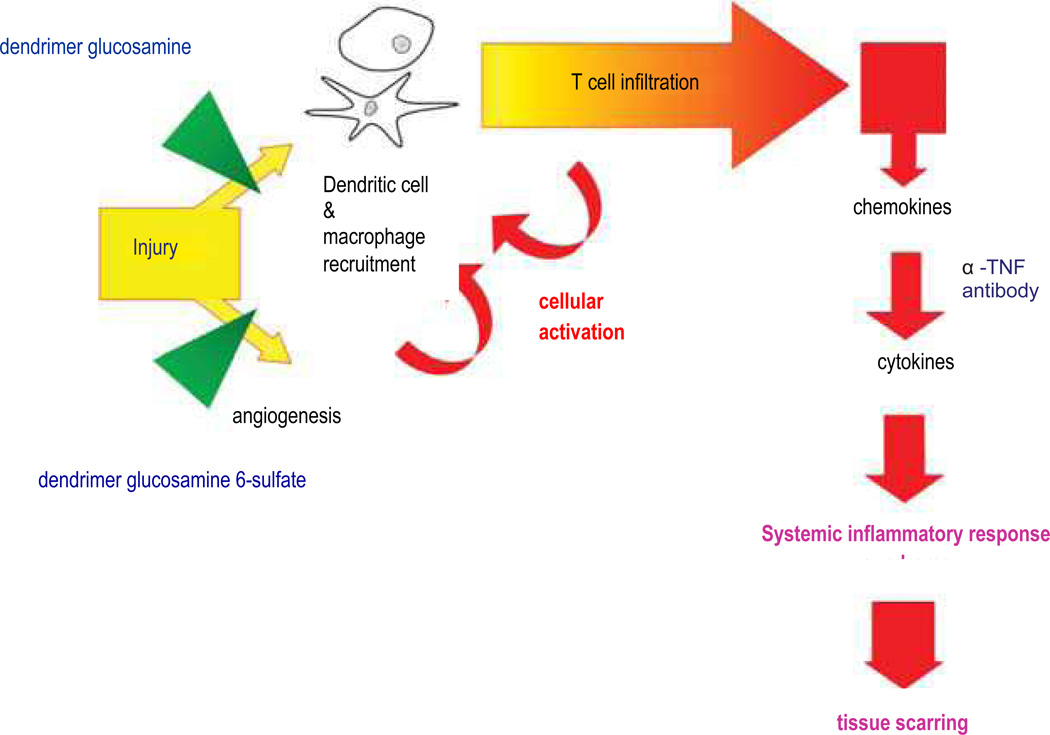
Elective surgery causes the release of tissue enzymes that degrade high molecular weight hyaluronan into low molecular weight hyaluronan. The small fragments trigger TLR4-mediated pro-inflammatory responses in a manner that is almost identical to bacterially derived LPS. An excessive pro-inflammatory cytokine release interferes with the normal phases of wound healing. The excessive angiogenesis that accompanies this host innate immune response increases pro-inflammatory monocyte recruitment to the wound site (Figure 4). Scarring is due to a persistent inflammatory response that promotes fibroblast proliferation. Shaunak postulated that early inhibition of an immuno-modulatory pathway and an anti-angiogenic pathway would enable physiological (rather than pathological) repair and regeneration of surgically induced injury without causing scar tissue formation (Figure 5).
Figure 4.
Cartoon of the pathogenic mechanisms responsible for scar tissue formation. The downstream sites of action of steroids and anti-TNF-α (tumor necrosis factor) antibodies are contrasted with the upstream sites of action of dendrimer glucosamine and dendrimer glucosamine 6-sulfate.
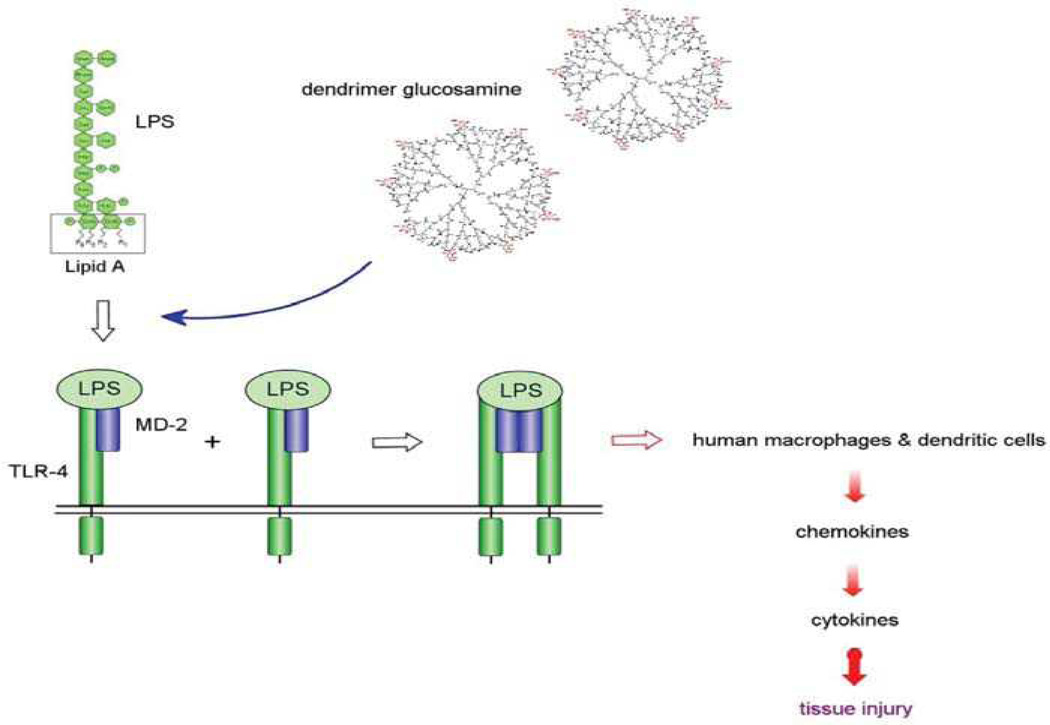
Figure 5.
Cartoon showing competition for cell surface Toll-like receptor 4 (TLR4) between the agonist (lipopolysaccharide [LPS])) and the antagonist (dendrimer glucosamine). MD-2 is a protein and R1, R2, R3, and R4 are acyl chains.
A rabbit model of glaucoma filtration surgery was chosen because the surgical intervention is precisely defined, and because surgical failure results from an excessive pro-inflammatory response combined with a neo-angiogenic response.
When used in combination, dendrimer glucosamine and dendrimer glucosamine 6-sulfate increased the success rate of glaucoma filtration surgery from 30% to 80% (P = 0.029; the P-value is the probability (0 ≤ P ≤ 1) that the observed results could have occurred by chance if the null hypothesis was true) in this clinically validated rabbit model. Therefore, this combination of dendrimer-based drugs safely and synergistically prevented scar tissue formation after surgery. Histological studies showed that the degree of tissue-based inflammatory cell infiltration and abnormal collagen formation was minimal.117 These studies with novel synthetic macromolecules provide clear-cut evidence that new chemical entities can be designed and synthesized that will enable the therapeutic manipulation of the early and critical stages of tissue repair and regeneration pathways.
Conclusion
Regulatory roles of the immune response, extracellular matrix remodeling processes, and the checkpoints that initiate as well as arrest tissue-damaging cascades are all important interactions that require consideration when dealing with strategies that can help to modulate the scar response and can potentially enhance tissue regeneration. Currently, novel biomaterials that can interact with aspects of all of these pathways are in development. In the future, in addition to serving as scaffolds to create the appropriate structure of a tissue, these new biomaterials will be able to inhibit scar formation and accelerate growth of new normal tissue in order to quickly regenerate organs in vivo. Advances in biomaterial design and fabrication are critical to the growth of the field of regenerative medicine.
Acknowledgments
D.J.I. is an investigator of the Howard Hughes Medical Institute. The authors wish to thank Dr. Jennifer Olson for editorial assistance with this manuscript.
References
- 1.Stadelmann WK, Digenis AG, Tobin GR. Am. J. Surg. 1998;176:26S. doi: 10.1016/s0002-9610(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 2.Quinn JV. Tissue Adhesives in Wound Care. Ontario: B.C. Decker, Hamilton; 1998. [Google Scholar]
- 3.Midwood KS, Williams LV, Schwarzbauer JE. Int. J. Biochem. Cell Biol. 2004;36:1031. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg HG, Longaker M. Scarless Wound Healing. New York: Marcel Dekker; 2000. [Google Scholar]
- 6.Dorin RP, Pohl HG, De Filippo RE, Yoo JJ, Atala A. World J. Urol. 2008;26:323. doi: 10.1007/s00345-008-0316-6. [DOI] [PubMed] [Google Scholar]
- 7.Yoo JJ, Meng J, Oberpenning F, Atala A. Urology. 1998;51:221. doi: 10.1016/s0090-4295(97)00644-4. [DOI] [PubMed] [Google Scholar]
- 8.Oberpenning F, Meng J, Yoo JJ, Atala A. Nat. Biotechnol. 1999;17:149. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo RE, Bishop CE, Filho LF, Yoo JJ, Atala A. Transplantation. 2008;86:208. doi: 10.1097/TP.0b013e31817f1686. [DOI] [PubMed] [Google Scholar]
- 10.Lee DJ, Steen J, Jordan JE, Kincaid EH, Kon ND, Atala A, Berry J, Yoo JJ. Tissue Eng. Part A. 2009;15:807. doi: 10.1089/ten.tea.2008.0250. [DOI] [PubMed] [Google Scholar]
- 11.Chen KL, Eberli D, Yoo JJ, Atala A. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3346. doi: 10.1073/pnas.0909367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joraku A, Sullivan CA, Yoo J, Atala A. Differentiation. 2007;75:318. doi: 10.1111/j.1432-0436.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 13.Stitzel J, Liu J, Lee SJ, Komura M, Berrya J, Sokerc S, Limc G, Dykec MV, Richard C, James JY. Biomater. 2006;27:1088. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 14.Kim BS, Yoo JJ, Atala A. J. Biomed. Mater. Res. Part A. 2004;68:201. doi: 10.1002/jbm.a.10045. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R. Nat. 2008;454:428. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi ME. J. Leukocyte Biol. 2007;81:1. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JM, Rodriguez A, Chang DT. Semin. Immunol. 2008;20:86. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babensee JE. Semin. Immunol. 2008;20:101. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Badylak SF, Gilbert TW. Semin. Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JA, Chang DT, Meyerson H, Colton E, Kwon K, Matsuda T, Anderson MM. J. Biomed. Mater. Res. Part A. 2007;83:585. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 21.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. Nat. 2009;460:269. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 22.Serhan CN, Savill J. Nat. Immunol. 2005;6:1191. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 23.Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Trends Immunol. 2007;28:385. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Mirza R, DiPietro LA, Koh TJ. Am. J. Pathol. 2009;175:2454. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung AS, Waldeck H, Schmidt DR, Kao WJ. J. Biomed. Mater. Res. Part A. 2009;91:742. doi: 10.1002/jbm.a.32259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batten P, Sarathchandra P, Antoniw JW, Tay SS, Lowdell MW, Taylor PM, Yacoub MH. Tissue Eng. 2006;12:2263. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 27.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Annu. Rev. Immunol. 2006;24:33. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 28.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, Owen TA, Li M, DaSilva-Jardine P, Zhou M, Dunn RL, Dumount F, Korsmeyer R, Krasney P, Brown TA, Plowchalk D, Vukicevic S, Thompson DD. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6736. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerstenfeld T-J, Cho LC, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. J. Bone Miner. Res. 2003;18:1584. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 30.Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Nat. Neurosci. 2006;9:843. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 31.Lu X, Richardson PM. J. Neurosci. 1991;11:972. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greco SJ, Rameshwar P. J. Immunol. 2007;179:3342. doi: 10.4049/jimmunol.179.5.3342. [DOI] [PubMed] [Google Scholar]
- 33.Michalopoulos GK, DeFrances MC. Science. 1997;276:60. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 34.Andrades JA, Nimni ME, Becerra J, Eisenstein R, Davis M, Sorgente N. Exp. Cell Res. 1996;227:208. doi: 10.1006/excr.1996.0269. [DOI] [PubMed] [Google Scholar]
- 35.Del Rio-Tsonis K, Tsonis PA, Zarkadis IK, Tsagas AG, Lambris JD. J. Immunol. 1998;161:6819. [PubMed] [Google Scholar]
- 36.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. J. Immunol. 2001;166:2479. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 37.Chan G, Mooney DJ. Trends Biotechnol. 2008;26:382. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Lumelsky NL. Tissue Eng. 2007;13:1393. doi: 10.1089/ten.2007.0100. [DOI] [PubMed] [Google Scholar]
- 39.Mountziaris PM, Mikos AG. Tissue Eng. Part B Rev. 2008 Apr;30 doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harty M, Neff AW, King MW, Mescher AL. Dev. Dyn. 2003;226:268. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- 41.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Nat. Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 42.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmökel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. FASEB J. 2003;17:2260. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 43.Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 44.Lauffenburger D, Farrell B, Tranquillo R, Kistler A, Zigmond S. J. Cell Sci. 1987;88(Pt 4):415. doi: 10.1242/jcs.88.4.415. [DOI] [PubMed] [Google Scholar]
- 45.Zigmond SH. J. Cell. Biol. 1977;75:606. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumamoto T, Huang EK, Paek HJ, Morita A, Matsue H, Valentini RF, Takashima A. Nat. Biotechnol. 2002;20:64. doi: 10.1038/nbt0102-64. [DOI] [PubMed] [Google Scholar]
- 47.Zhao X, Jain S, Benjamin Larman S, Gonzalez H, Irvine DJ. Biomater. 2005;26:5048. doi: 10.1016/j.biomaterials.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Stachowiak AN, Irvine DJ. J. Biomed. Mater. Res. Part A. 2008;85:815. doi: 10.1002/jbm.a.31661. [DOI] [PubMed] [Google Scholar]
- 49.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Nat. Mater. 2009;8:151. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh A, Suri S, Roy K. Biomater. 2009;30:5187. doi: 10.1016/j.biomaterials.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, Scadden DT. Nat. Med. 2000;6:543. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 52.Poznansky MC, Olszak IT, Evans RH, Wang Z, Foxall RB, Olson DP, Weibrecht K, Luster AD, Scadden DT. J. Clin. Invest. 2002;109:1101. doi: 10.1172/JCI13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tharp WG, Yadav R, Irimia D, Upadhyaya A, Samadani A, Hurtado S-Y, Liu O, Munisamy S, Brainard DM, Mahon MJ, Nourshargh S, van Oudenaarden A, Toner MG, Poznansky MC. J. Leukocyte Biol. 2006;79:539. doi: 10.1189/jlb.0905516. [DOI] [PubMed] [Google Scholar]
- 54.Christopherson KW, 2nd, Campbell JJ, Travers JB, Hromas RA. J. Pharmacol. Exp. Ther. 2002;302:290. doi: 10.1124/jpet.302.1.290. [DOI] [PubMed] [Google Scholar]
- 55.Perretti M, Page CP. Gut. 2000;47:14. doi: 10.1136/gut.47.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel DD, Koopmann W, Imai T, Whichard LP, Yoshie O, Krangel MS. Clin. Immunol. 2001;99:43. doi: 10.1006/clim.2000.4997. [DOI] [PubMed] [Google Scholar]
- 57.Proudfoot AE, Handel TM, Johnson Z, Lau EK, Wang PL, Clark-Lewis I, Borlat F, Wells TNC, Kosco-Vilbois MH. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1885. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barouch DH, McKay PF, Sumida SM, Santra S, Jackson SS, Gorgone DA, Lifton MA, Chakrabarti BK, Xu L, Nabel GJ, Letvin NL. J. Virol. 2003;77:8729. doi: 10.1128/JVI.77.16.8729-8735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song RJ, Leong KW. Mol. Ther. 2003;7:S257. [Google Scholar]
- 60.Haisch A, Wanjura F, Radke C, Leder-Jöhrens K, Gröger A, Endres M, Klaering S, Loch A, Sittinger M. Eur. Arch. Otorhinolaryngol. 2004;261:216. doi: 10.1007/s00405-003-0646-3. [DOI] [PubMed] [Google Scholar]
- 61.Ho ML, Chang JK, Wang GJ. Clin. Orthop. Relat. Res. 1995:270. [PubMed] [Google Scholar]
- 62.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Pétrilli V, Tschopp J, O’Neill LAJ, Lavelle EC. Proc. Natl. Acad. Sci. U.S.A. 2009;106:870. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Science. 2008;320:674. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Nat. Immunol. 2008;9:847. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. J. Exp. Med. 2002;195:99. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. J. Immunol. 2009;182:3573. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- 67.Yang D, Jones KS. J. Biomed. Mater. Res. Part A. 2009;90:411. doi: 10.1002/jbm.a.32096. [DOI] [PubMed] [Google Scholar]
- 68.Tam SK, Dusseault J, Polizu S, Menard M, Halle JP, Yahia L. Biomater. 2006;27:1296. doi: 10.1016/j.biomaterials.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 69.Klock G, Pfeffermann A, Ryser C, Grohn P, Kuttler B, Hahn JJ, Zimmermann U. Biomater. 1997;18:707. doi: 10.1016/s0142-9612(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 70.Pieper JS, Van Wachem PB, Van Luyn MJA, Brouwer LA, Hafmans T, Veerkamp JH, Van Kuppevelt TH. Biomater. 2000;21:1689. doi: 10.1016/s0142-9612(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 71.Sterling H, Saginario C, Vignery A. J. Cell Biol. 1998;143:837. doi: 10.1083/jcb.143.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon SJ, Kim SH, Ha HJ, Ko YK, So JW, Kim MS, Yang Y, Khang G, Rhee JM, Lee HB. Tissue Eng. Part A. 2008;14:539. doi: 10.1089/tea.2007.0129. [DOI] [PubMed] [Google Scholar]
- 73.van Putten SM, Wubben M, Hennink WE, van Luyn MJ, Harmsen MC. Biomater. 2009;30:730. doi: 10.1016/j.biomaterials.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 74.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Nat. Biotechnol. 2007;25:1159. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 75.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Nat. Mater. 2008;7:248. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 76.Roy R, Zhang B, Moses MA. Exp. Cell Res. 2006;312:608. doi: 10.1016/j.yexcr.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 77.Matrisian LM. Trends Genet. 1990;6:121. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 78.Nagase H, Woessner JF., Jr J. Biol. Chem. 1999;274:21491. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 79.Bode W. Biochem. Soc. Symp. 2003:1. doi: 10.1042/bss0700001. [DOI] [PubMed] [Google Scholar]
- 80.Visse R, Nagase H. Circ. Res. 2003;92:827. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 81.Woessner JF, Jr, Nagase H. Matrix Metalloproteinases and TIMPs. New York: Oxford University Press; 2000. [Google Scholar]
- 82.Bode W, Maskos K. Methods Mol. Biol. 2001;151:45. [PubMed] [Google Scholar]
- 83.Buckley-Sturrock A, Woodward SC, Senior RM, Griffin GL, Klagsbrun M, Davidson JM. J. Cell Physiol. 1989;138:70. doi: 10.1002/jcp.1041380111. [DOI] [PubMed] [Google Scholar]
- 84.Neely AN, Clendening CE, Gardner J, Greenhalgh DG, Warden GD. Wound Repair Regen. 1999;7:166. doi: 10.1046/j.1524-475x.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 85.Shapiro SD, Kobayashi DK, Welgus HG. J. Biol. Chem. 1992;267:13890. [PubMed] [Google Scholar]
- 86.Stock UA, Wiederschain D, Kilroy SM, Shum-Tim D, Khalil PN, Vacanti JP, Mayer JE, Moses MA. J. Cell Biochem. 2001;81:220. doi: 10.1002/1097-4644(20010501)81:2<220::aid-jcb1037>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 87.Karim MA, Ferguson AG, Wakim BT, Samarel AM. Am. J. Physiol. 1991;260:C316. doi: 10.1152/ajpcell.1991.260.2.C316. [DOI] [PubMed] [Google Scholar]
- 88.Peters CA, Freeman MR, Fernandez CA, Shepard J, Wiederschain DG, Moses MA. Am. J. Physiol. 1997;272:R1960. doi: 10.1152/ajpregu.1997.272.6.R1960. [DOI] [PubMed] [Google Scholar]
- 89.Wysocki AB, Staiano-Coico L, Grinnell F. J. Invest. Dermatol. 1993;101:64. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 90.Wysocki AB, Staiano-Coico L, Grinnell F. J. Cell Biol. 1993;115:137a. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 91.Moses MA, Harper J. Cancer: Cell Structures, Carcinogens and Tumor Pathogenesis, EXS. 2005;96:223. [Google Scholar]
- 92.Greene AK, Puder M, Roy R, Kilroy S, Louis G, Folkman J, Moses MA. Transplant. 2004;78:1139. doi: 10.1097/01.tp.0000137935.81103.a2. [DOI] [PubMed] [Google Scholar]
- 93.Alwayn IP, Verbesey JE, Kim S, Roy R, Arsenault DA, Greene AK, Novak K, Laforme A, Lee S, Moses MA, Puder M. J. Surg. Res. 2008;145:192. doi: 10.1016/j.jss.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bellayr I, Mu X, Li Y. Future Med. Chem. 2009;1:1095. doi: 10.4155/fmc.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moses MA, Marikovsky M, Harper JW, Vogt P, Eriksson E, Klagsbrun M, Langer R. J. Cell Biochem. 1996;60:379. doi: 10.1002/(SICI)1097-4644(19960301)60:3%3C379::AID-JCB9%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 96.Reiss MJ, Han YP, Garcia E, Goldberg M, Yu H, Garner WL. Surgery. 2010;147:295. doi: 10.1016/j.surg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grinnell F, Ho CH, Wysocki A. J. Invest. Dermatol. 1992;98:410. doi: 10.1111/1523-1747.ep12499839. [DOI] [PubMed] [Google Scholar]
- 98.Saarialho-Kere UK, Chang ES, Welgus HG, Parks WC. J. Clin. Invest. 1992;90:1952. doi: 10.1172/JCI116073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hennessey PJ, Black CT, Andrassy RJ. Arch. Surg. 1990;125:926. doi: 10.1001/archsurg.1990.01410190124021. [DOI] [PubMed] [Google Scholar]
- 100.Hasebe T, Harasawa S, Miwa T, Shibata T, Inayama S. Tokai J. Exp. Clin. Med. 1987;12:147. [PubMed] [Google Scholar]
- 101.Zuany-Amorim C, Hastewell J, Walker C. Nat. Rev. Drug Discov. 2002;1:797. doi: 10.1038/nrd914. [DOI] [PubMed] [Google Scholar]
- 102.Ohto U, Fukase K, Miyake K, Satow Y. Science. 2007;316:1632. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 103.Park BS, Song DH, Kim HM, Choi B-S, Lee H, Lee J-O. Nature. 2009;458:1191. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 104.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ. Cell. 2007;130:906. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 105.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. Nat. Immunol. 2008;9:361. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boros P, Bromberg JS. Am. J. Transplant. 2006;6:652. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 107.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA. Nat. Med. 2005;11:1173. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 108.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. J. Immunol. 2006;177:1272. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 109.Benhamron S, Nechushtan H, Verbovetski I, Krispin A, Abboud-Jarrous G, Zcharia E, Edovitsky E, Nahari E, Peretz T, Vlodavsky I, Mevorach D. J. Immunol. 2006;176:6417. doi: 10.4049/jimmunol.176.11.6417. [DOI] [PubMed] [Google Scholar]
- 110.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW. Am. J. Transplant. 2006;6:2622. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 111.Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A, Yamashita H, Heldin P. J. Biol. Chem. 2005;280:24195. doi: 10.1074/jbc.M411913200. [DOI] [PubMed] [Google Scholar]
- 112.Miller SI, Ernst RK, Bader MW. Nat. Rev. Microbiol. 2005;3:36. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 113.Parish CR. Nat. Rev. Immunol. 2006;6:633. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 114.Flo TH, Ryan L, Latz E, Takeuchi O, Monks BG, Lien E, Halaas O, Akira S. J. Biol. Chem. 2002;277:35489. doi: 10.1074/jbc.M201366200. [DOI] [PubMed] [Google Scholar]
- 115.Svenson S, Tomalia DA. Adv. Drug Deliv. Rev. 2005;57:2106. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 116.Dear JW, Hisataka K, Jo S-K, Holly MK, Hu X, Yuen PST, Brechbiel MW, Star RA. Kidney Int. 2005;67:2159. doi: 10.1111/j.1523-1755.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 117.Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E. Nat. Biotechnol. 2004;22:977. doi: 10.1038/nbt995. [DOI] [PubMed] [Google Scholar]