Abstract
Recent reports of retinal stem cells being present in several locations of the adult eye have sparked great hopes that they may be used to treat the millions of people worldwide who suffer from blindness as a result of retinal disease or injury. A population of proliferative cells derived from the ciliary body epithelium (CE) has been considered one of the prime stem cell candidates, and as such they have received much attention in recent years. However, the true nature of these cells in the adult human eye has still not been fully elucidated, and the stem cell claim has become increasingly controversial in light of new and conflicting reports. In this paper, we will try to answer the question of whether the available evidence is strong enough for the research community to conclude that the adult human CE indeed harbors stem cells.
1. Introduction
In the retina, light is translated into electrical impulses that are processed and transmitted further into the brain through a complex neuronal chain. This sensory pathway is damaged in common eye diseases such as retinal degenerative diseases, diabetic retinopathy, arterial occlusions, traumas, and glaucoma. Stem cell-based therapies still hold great promise to treat several neurodegenerative diseases and/or injuries, and the retina may be an ideal candidate for regenerative medicine due to its relatively small size and immunity, as well as recent discoveries in retinal microsurgery and visualization [1]. There are three main categories of human stem cells which are currently being investigated for retinal regenerative therapy: embryonic stem cells (ESCs) [2], induced pluripotent stem cells (iPS cells) [3], and somatic or adult neural stem cells (NSCs) [1, 4]. One of the putative advantages of adult NSCs is the possibility for autologous transplantation without reprogramming, whereby NSCs may be harvested from adult patients, expanded or modified in vitro, and re-transplanted into the original patient [5]. However, most studies regarding isolation and characterization of NSCs in the adult eye have until recently been performed in lower vertebrates and rodents [4]. In this review, we will focus on the adult human eye.
The neuroretina—like the rest of the central nervous system (CNS)—is considered to have limited regenerative potential in adult humans, and severe injuries can lead to permanent damage for which currently there are no definitive curative treatment options. Until the 1990s, it was a central dogma of neuroscience that no new neurons could be formed in the adult human CNS. This doctrine was best formulated in the words of the histologist Ramon y Cajal: “Once the development was ended, the fountains of growth and regeneration of the axons and dendrites dried up irrevocably. In the adult centers, the nerve paths are something fixed, ended, and immutable. Everything may die, nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree.” [6]. However, despite this dogma, researchers have continuously tried to identify NSCs in humans that are both able to self-renew and differentiate into functional retinal cell types to treat patients with retinal disorders, and one of the key scientific questions has been whether such NSCs exist in the patient's own eye. In this search for retinal stem cells (RSCs), the ciliary body epithelium (CE) has been considered as one of the prime niches. There is also evidence that both Müller glia [7–10] and retinal pigment epithelial (RPE) cells [11] can have properties of NSCs in the adult human eye, but these important topics will not be addressed in the current review.
2. The RSC Hypothesis
The development of the retina forms the theoretical background for the RSC hypothesis. During embryogenesis, the optic cup forms as a double-layered extension of the forebrain, with which it is continuous [12]. The inner layer of the optic cup differentiates into the neural retina centrally and into the nonpigmented layer of the CE, and iris peripherally, while the outer layer gives rise to three types of pigmented epithelial cells: RPE, pigmented CE and iris pigmented epithelium (IPE). Thus, all of these tissues—although diverse—share a common origin from multipotent NSCs and form a structural and developmental continuum with the brain.
The second element that lead to the RSC hypothesis is the observation that in many lower vertebrates new retinal cells are constantly produced throughout life from multipotent cells residing in the ciliary marginal zone (CMZ) [13]. In mammals, this zone anatomically corresponds to the area around the peripheral margin of the retina and contains NSCs during retinogenesis. However, after birth, this stem cell population appears to have been depleted or remains in a quiescent state in vivo [8, 12]. Based on the above considerations, it was postulated that it is possible to isolate and propagate a rare/small population of quiescent RSCs from the adult mammalian CE. The first evidence to support this claim was put forward by Tropepe et al. and Ahmad et al. in 2000 [14, 15], and a number of studies on the proliferative and differentiation potential of these cells have since been performed to date [4, 8].
3. The Problem of Identifying a Stem Cell
If a subpopulation of CE cells is to be labeled as stem cells, they must fulfill certain criteria. A stem cell is commonly defined as a cell that has the ability to (a) self-renew, (b) proliferate to form progenitor cells with a higher degree of lineage commitment, and (c) ultimately give rise to all the terminally differentiated and functional cells of the tissue from which it is derived [16]. In contrast to stem cells, progenitor cells could have a more restricted lineage potential. When trying to judge whether a population of cells meet these criteria, we face several problems. There are few, if any, genetic markers or morphological characteristics that precisely identify a stem cell as such. Thus, one can only conclude that stem cells are present in a tissue sample retrospectively, based on the functional criteria of proliferation, self-renewal, and production of differentiated cells [17].
When studying NSCs in vitro, it is common to use the neurosphere assay, first described by Reynolds and Weiss [18], where the tissue is prepared to form a single-cell suspension and cultured in a defined medium containing mitogens. After a few days, free-floating clusters of cells with a characteristicALLY rounded appearance, known as neurospheres, are formed (Figure 1(a)). These are thought to represent stem cells and their progeny [18]. Through repeated passaging, where the spheres are dissociated and replated as single cells, the stem/progenitor cell population may be expanded. This assay has also become an important method to strengthen the presence of a stem/progenitor cell population in a tissue. The continued formation of neurospheres over the course of many passages is interpreted as an expression of the stem cell's capacity for self-renewal and the expression of mature neural and glial markers as the stem cell's multipotency [17, 19].
Figure 1.
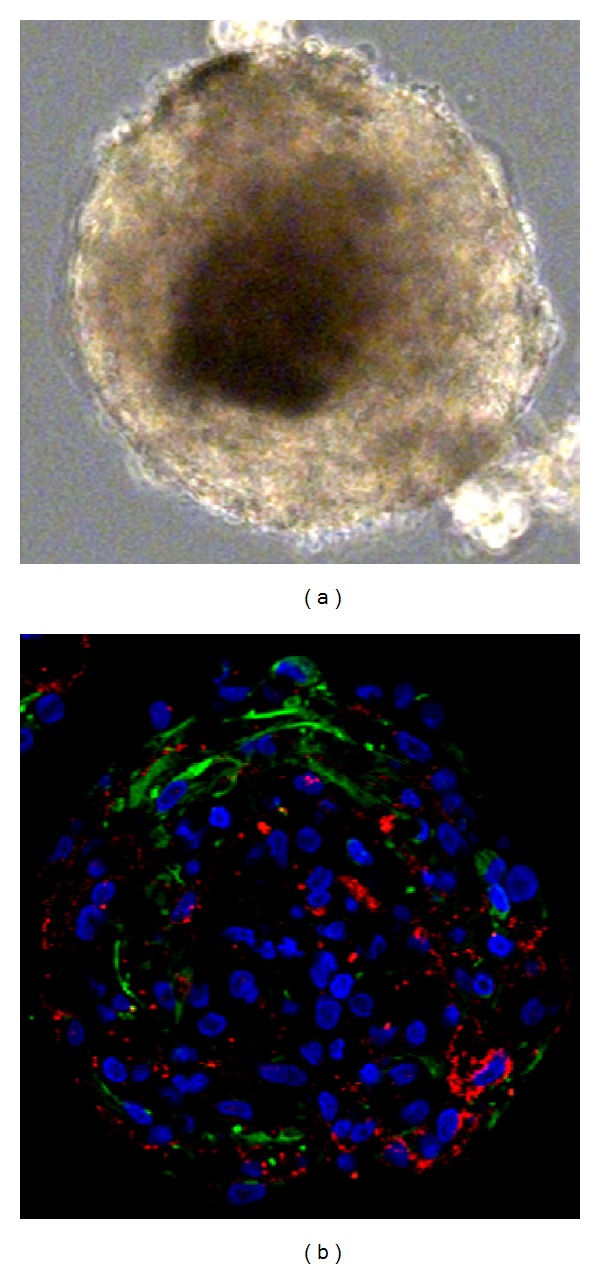
Light microscopy of secondary sphere derived from postmortem CE (a). Immunocytochemical analysis of secondary spheres derived from postmortem CE (b), costaining of Nestin (green) and Claudin-1 (red). Nuclear staining with Hoechst (blue).
However, the neurosphere assay has limitations. The population of cells within a sphere is heterogenous, consisting of cells at many different stages of differentiation and committed to different lineages [20–22]. Also, the neurosphere culturing method is sensitive to variations in factors such as cell density, concentrations of added mitogens, and number of passages. This can make it difficult to compare results between research groups and may account for the great variation in published results [16, 19]. In vivo, stem cells are thought to reside in a so-called stem cell niche, where their properties are carefully regulated by the structural and functional conditions of the extracellular matrix, cell-cell interactions, and complex signaling cascades [23]. The sphere may be viewed as an in vitro niche which provides different stimuli and cues to the cells therein. Thus, depending on the inherent plasticity of the cells, they may display different potency in vitro than they would be capable of in vivo [19]. It has recently been shown that sphere formation in culture, and CE spheres in particular, may grow nonclonally by incorporating other spheres and adherent cells. [24, 25]. Therefore, we can strictly only use sphere formation and repeated passaging as a test of the cells' ability to survive and proliferate in culture for extended periods of time, and not as a test of “stemcellness.” Lastly, evidence has also been presented that nonstem cells may be capable of forming clonogenic spheres in culture [26]. Since most of the evidence for the existence of RSCs in the adult ciliary body is based on the neurosphere assay, it is important to have a clear understanding of the benefits and limitations of this culture method.
4. Evidence Favoring the Presence of RSCs in the Adult Human CE
Coles et al. attempted to culture cells isolated from the neural retina, pars plana and pars plicata of the ciliary body, RPE, and iris using the neurosphere assay and found that spheres were formed only from the ciliary body and iris. Of these, only spheres from the ciliary body could be passaged to form secondary spheres, indicating that only cells from this location exhibited the capacity for self-renewal. Multipotency was inferred by the immunohistochemical detection of markers for mature retinal cells of all lineages. Finally, cells were transplanted into developing mouse retinas, where a number of them showed signs of migration and integration into the host retina, as well as expression of mature retinal markers [27]. Mayer et al. found sphere-forming cells in both the pars plana and the neural retina itself (in contrast to the study cited above). These spheres consisted of cells expressing immature neuronal and glial markers. When exposed to differentiation conditions, a subset of cells expressing rhodopsin—a photoreceptor marker—was identified [28]. The same group later performed a study showing that adult human retina consistently gave rise to spheres in culture irrespective of age, sex, or postmortem time [29]. Xu et al. characterized spheres derived from the ciliary body, confirming earlier findings that they consist of proliferating cells that express certain immature neuronal and glial markers, while mature retinal markers could not be identified. Differentiation was not attempted [30].
Whilst the results of these studies partly support the adult RSC hypothesis, they have obvious weaknesses. The capability of sphere-forming CE cells for proliferation and self-renewal is well documented, but their multipotency is less so. To date, it has only been shown that these cells express certain mature retinal markers in culture. In order to conclude that functional retinal neurons have been formed, it would be necessary to demonstrate that they are postmitotic, have the correct morphology, and are capable of firing action potentials and releasing neurotransmitters [31]. Also, it is important to remember that these putative stem cells are derived from a nonneural tissue (but with neuroepithelial origin)—the CE. None of these papers investigated whether the CE-derived spheres contained a pure population of neural and glial cells—like neurospheres from the brain—or if they retained part of the epithelial phenotype of the tissue from which they were derived. This would have an important impact on their status as RSCs, as well on their potential use in cell-based therapy.
5. RSC or CE Cells?
Recently, several studies have questioned the existence of NSCs in the CE of the adult human eye [7, 9, 26, 34, 35]. Initially, we examined how the morphological characteristics and gene expression profiles of sphere-forming cells of the CE compared to those of brain-derived neural stem cells and found that CE spheres contained a population of proliferative epithelial-like cells with decreased expression of neural stem cell markers compared to CNS neurospheres [34] (Table 1). These results are partially in agreement with a recent study by Cicero et al., showing that although cells of the CE are able to clonally proliferate to form spheres and express certain markers of retinal stem/progenitor cells in culture, each cell still contained pigment and displayed membrane interdigitations and epithelial junctions, characteristic of differentiated ciliary epithelial phenotype [26]. Another recent study demonstrated that although CE cells in culture expressed significant levels of pluripotent and retinal progenitor markers, they consistently failed to differentiate into photoreceptors [35]. A study that separated the pigmented and nonpigmented CE found that only the nonpigmented CE proliferated to form spheres in culture, expressing high levels of epithelial markers, very limited numbers and levels of neural progenitor markers, and could not be induced to show signs of proper neural differentiation [7].
Table 1.
Comparison of gene expression profile of four key neural stem cell (NSC) markers.
| Gene | WT CE | CE spheres | PVR spheres | SVZ spheres |
|---|---|---|---|---|
| GFAP | − | −/+ | ++ | +++ |
| Sox-2 | − | + | + | ++ |
| Nestin | + | + | + | ++ |
| Nanog | + | + | + | + |
Semiquantitative comparison of RT-PCR expression in adult human ciliary body epithelium (CE) whole tissue samples (WT CE) [32] and cultivated spheres from the adult human CE [32], proliferative vitreoretinopathy (PVR) samples [33], and subventricular zone (SVZ) biopsies [34]. No detectable expression: (−), very low: (−/+), low: (+), middle: (++), and high: (+++) expression.
In light of this recent evidence, it is necessary to re-evaluate the RSC hypothesis regarding the adult human CE. The three stem cell functions described earlier: self-renewal, ability to form progenitor cells, and functional terminal differentiation are characteristically triggered by tissue injury. The self-renewal and proliferative capacity of CE cells is well documented [15, 27, 30, 34]. There is also little doubt that CE spheres contain a population of cells that display certain characteristics of neuroepithelial progenitors. Several studies have shown that CE spheres do express a range of immature neural and retinal markers [15, 27, 30, 34]. Importantly, we found that the spheres contain two distinct populations of cells: one Nestin+ and one Claudin-1+, while no double positive cells were detected (Figure 1(b)) [32]. This suggests that, in contrary to the conclusion drawn by Cicero et al., CE-derived spheres consist of a homogenous population of ciliary epithelial cells, they contain both epithelial cells and cells with a more neural progenitor-like phenotype.
However, expression of certain progenitor markers in vitro is not sufficient evidence of the presence of true stem cells. Kohno et al. showed that CE-derived spheres initially consist of Nestin− epithelial-like cells that begin to express Nestin during cultivation. These spheres had the ability to grow nonproliferatively by incorporating adherent Nestin− cells, which then became Nestin+ [24]. It was later shown that CE cells rapidly upregulate this protein during the first 24 hrs in culture, before they have time to clonally proliferate [26]. Thus, it is possible that the cell population in CE spheres with neuroepithelial properties is not derived from true NSCs residing within the CE but rather from a trans-/de-differentiation process where CE cells respond to stem cell culture conditions by shifting their gene expression profile to an immature direction. In order to shed further light on this, we performed RT-PCR [32] (Table 1) and immunostaining [33] on wild-type adult human CE tissue and compared the expression of neural and epithelial genes to that of CE spheres. Immunostaining showed that most Nestin+ cells were found around peripheral cysts of the retina (Figure 2). While these cells also stained for the glial marker GFAP in the peripheral retina, they were GFAP− in the adjacent proximal pars plana region [33]. Interestingly, we found no major clusters of Nestin+ cells or other putative NSC markers in the peripheral pars plana or pars plicata regions of the adult human CE (Figure 2).
Figure 2.
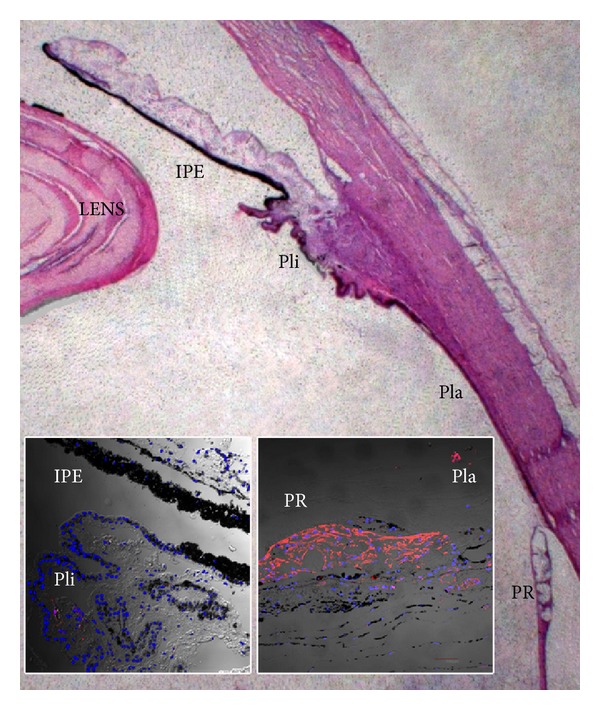
Light microscopic overview for illustrational purposes of the ciliary body epithelium (CE) consisting of pars plicata (Pli) and pars plana (Pla), as well as surrounding tissue including the lens and the iris pigmented epithelium (IPE) of the adult human eye. Almost complete absence of immunohistochemical Nestin-staining of IPE and Pli of the normal adult human eye (left inset), while there is intense Nestin-staining in the nonlaminated far peripheral retina (PR) and around peripheral cysts extending into the most posterior pars plana of the CE (right inset).
The final test of a stem cell is in its capacity for producing differentiated cells. Some research groups have shown that CE cells can be induced to express markers of mature retinal neurons [14, 15, 27, 36–38], although only one of these studies was performed on human tissue. Moreover, others have shown recently that CE cells exposed to differentiation conditions tend to revert to a differentiated state of CE cells and not retinal cells [7, 26, 35]. This lack of consistence in results could be caused by differences in the culture protocols but could also be due to the fact that only the latter studies have looked for morphological and genetic characteristics of epithelial cells, while the earlier ones exclusively focused on neural and retinal markers. Ballios et al. recently demonstrated that a subpopulation of cells derived from the CE and sorted out on the basis of size and pigmentation criteria could be induced to express high levels of immature and mature photoreceptor markers in a sequential manner when cultured under specific differentiation conditions for extended periods of time (up to 40 days). These cells then no longer displayed a CE morphology, as judged by their lack of pigmentation and ciliation, and were indistinguishable from photoreceptor cultures in vitro [36]. However, photoreceptors do not display their characteristic outer segment morphology in vitro, leaving it unclear whether these cells possess the ability to adopt the correct structure in vivo. In order to reach a final conclusion on this topic, it would be necessary to perform functional studies to show that CE cells not only are capable of upregulating certain mature retinal markers in vitro but also possess the intracellular structures necessary to mature functionally. There are several criteria that are necessary to determine whether a (stem) cell has generated a functional neuron such as a photoreceptor [31, 39]; the cell should be (1) postmitotic, (2) polarized with developed cellular processes, (3) capable of proper electrophysiological activity, and (4) able to communicate with other neurons through synapses. Inoue et al. have shown that adult human CE cells may develop some functional properties of photoreceptor cells; however, their approach required transduction of several key regulator genes of photoreceptor formation [40]. In addition, Jasty et al. recently showed development of functional ionotropic glutamate receptors upon differentiation of adult human CE spheres [41]. There is also evidence that many key properties of retinal cell polarization and function are environment dependent. For instance, in the primate retina, progressive degeneration of the outer segments is observed during the first two weeks after a retinal detachment, and production of outer segments is only initiated after repositioning of the sensory retina in contact with the RPE. Thus, lack of fully functional differentiation of retinal stem/progenitor cells in vitro does not predict how these cells may differentiate in a proper in vivo environment.
6. Stem Cells Should Be Able to Respond to Injury
One final way of assessing the stem cell-potential of CE cells is to examine their response to retinal injury. We hypothesized that if RSCs indeed reside within the CE, they would be activated in eyes suffering from proliferative vitreoretinopathy (PVR) and respond by migrating towards the damaged areas (Table 1) [33]. Retinal injury did induce cell proliferation in the CE, but NSC-markers such as Sox2, Pax6, and Nestin could not be detected in the major parts of the CE, both in injured and uninjured eyes. The only part of the adult human CE that showed some upregulation of NSC markers upon PVR was the most posterior part close to the retinal edge surrounding peripheral cysts. In contrast, we found cellular hyperplasia and Nestin upregulation in the CE of mouse eyes with PVR, suggesting that there may be important species differences in the neural potential of the CE. This is especially of interest since most of the studies supporting the RSC hypothesis in the adult CE have been performed on rodents. Our results partially concur with a recent in situ report of 3 human eyes with PVR. In this study, hyperplasia of the CE, forming “neurosphere-like” structures was found. There were no GFAP+ cells, but unlike our study, a few rhodopsin+ cells were found in the vicinity of the CE [42]. However, finding rhodopsin+ cells in the adult CE does not prove that these cells are in fact photoreceptors, as expression of markers usually found in retinal cell types can also be induced in other cells [43]. Future analysis of the adult human CE in patients with retinal damage, including studies using an endoscopic technique during vitreoretinal surgery, would give important new information regarding this controversy [44].
7. Future Directions
In order to reach a final ruling on this topic, more knowledge is needed. Results vary greatly between research groups, which may be partly due to the lack of a standardized method for isolation and culturing of CE cells. Variations in culture supplements, protocols for passaging and differentiating cells, and time points at which cells are studied may all affect the cells' phenotype in vitro. However, this highlights the problem of solely relying on morphologic and genetic markers for identifying the presence of stem cells. It is possible that cells upregulate certain genes in response to the culture conditions and that this may be misinterpreted as presence of true stem/progenitor cells. Functional studies are thus needed in order to validate the RSC hypothesis. In light of the available evidence, it seems most likely that the adult human CE does not contain bona fide NSCs but rather consists of a population of epithelial cells which display a remarkable plasticity in vitro reflecting their neuroepithelial developmental origin. Perhaps our current sum of knowledge thus indicates a shift in focus away from studies of the adult human CE for cell-based therapy to restore vision, as stated by Cicero et al. already in 2009 [26].
Authors' Contribution
Goran Petrovski and Morten C. Moe are shared last co-authors.
Acknowledgments
This work has been supported by the Research Council of Norway, the Norwegian Association of the Blind and Partially Sighted, Blindemissionen IL, Faculty of Medicine, University of Oslo, and Oslo University Hospital. Furthermore, support was provided by a grant from the TÁMOP-4.2.2A-11/1/KONV-2012-0023 project implemented through the New Hungary Development Plan, co-financed by the European Social Fund. The authors would like to thank Kristiane Haug, Eli Gulliksen, and Geir Quale (Center for Eye Research, OUS) for their technical assistance and support. All tissue harvesting used for figure illustrations were approved by the Local Committees for Medical Research Ethics.
References
- 1.Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140:2576–2585. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skottman H, Narkilahti S, Hovatta O. Challenges and approaches to the culture of pluripotent human embryonic stem cells. Regenerative Medicine. 2007;2(3):265–273. doi: 10.2217/17460751.2.3.265. [DOI] [PubMed] [Google Scholar]
- 3.Osakada F, Hirami Y, Takahashi M. Stem cell biology and cell transplantation therapy in the retina. Biotechnology and Genetic Engineering Reviews. 2009;26:297–334. doi: 10.5661/bger-26-297. [DOI] [PubMed] [Google Scholar]
- 4.Wohl SG, Schmeer CW, Isenmann S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Progress in Retinal and Eye Research. 2012;31(3):213–242. doi: 10.1016/j.preteyeres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Liu CY, Westerlund U, Svensson M, et al. Artificial niches for human adult neural stem cells: possibility for autologous transplantation therapy. Journal of Hematotherapy and Stem Cell Research. 2003;12(6):689–699. doi: 10.1089/15258160360732713. [DOI] [PubMed] [Google Scholar]
- 6.Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. 1913. (translated by R. M. Day from the 1913 Spanish edition). [Google Scholar]
- 7.Bhatia B, Jayaram H, Singhal S, Jones MF, Limb GA. Differences between the neurogenic and proliferative abilities of Müller glia with stem cell characteristics and the ciliary epithelium from the adult human eye. Experimental Eye Research. 2011;93(6):852–861. doi: 10.1016/j.exer.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia B, Singhal S, Jayaram H, Khaw PT, Limb GA. Adult retinal stem cells revisited. Open Journal of Ophthalmology. 2010;4:30–38. doi: 10.2174/1874364101004010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia B, Singhal S, Lawrence JM, Khaw PT, Limb GA. Distribution of Müller stem cells within the neural retina: evidence for the existence of a ciliary margin-like zone in the adult human eye. Experimental Eye Research. 2009;89(3):373–382. doi: 10.1016/j.exer.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25(8):2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 11.Salero E, Blenkinsop TA, Corneo B, et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012;10(1):88–95. doi: 10.1016/j.stem.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2(6):538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perron M, Harris WA. Retinal stem cells in vertebrates. Bioessays. 2000;22(8):685–688. doi: 10.1002/1521-1878(200008)22:8<685::AID-BIES1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochemical and Biophysical Research Communications. 2000;270(2):517–521. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 15.Tropepe V, Coles BLK, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 16.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 17.Deleyrolle LP, Reynolds BA. Isolation, expansion, and differentiation of adult mammalian neural stem and progenitor cells using the neurosphere assay. Methods in Molecular Biology. 2009;549:91–101. doi: 10.1007/978-1-60327-931-4_7. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 19.Jensen JB, Parmar M. Strengths and limitations of the neurosphere culture system. Molecular Neurobiology. 2006;34(3):153–161. doi: 10.1385/MN:34:3:153. [DOI] [PubMed] [Google Scholar]
- 20.Arsenijevic Y, Villemure J, Brunet J, et al. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Experimental Neurology. 2001;170(1):48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- 21.Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from neurogenic regions of adult human brain. Experimental Neurology. 1999;156(2):333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 22.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 24.Kohno R, Ikeda Y, Yonemitsu Y, et al. Sphere formation of ocular epithelial cells in the ciliary body is a reprogramming system for neural differentiation. Brain Research. 2006;1093(1):54–70. doi: 10.1016/j.brainres.2006.03.093. [DOI] [PubMed] [Google Scholar]
- 25.Singec I, Knoth R, Meyer RP, et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nature Methods. 2006;3(10):801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- 26.Cicero SA, Johnson D, Reyntjens S, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6685–6690. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coles BLK, Angénieux B, Inoue T, et al. Facile isolation and the characterization of human retinal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15772–15777. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer EJ, Carter DA, Ren Y, et al. Neural progenitor cells from postmortem adult human retina. The British Journal of Ophthalmology. 2005;89(1):102–106. doi: 10.1136/bjo.2004.057687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter DA, Mayer EJ, Dick AD. The effect of postmortem time, donor age and sex on the generation of neurospheres from adult human retina. The British Journal of Ophthalmology. 2007;91(9):1216–1218. doi: 10.1136/bjo.2007.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Sta Iglesia DD, Kielczewski JL, et al. Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Investigative Ophthalmology and Visual Science. 2007;48(4):1674–1682. doi: 10.1167/iovs.06-1034. [DOI] [PubMed] [Google Scholar]
- 31.Reh TA. Neural stem cells: form and function. Nature Neuroscience. 2002;5(5):392–394. doi: 10.1038/nn0502-392. [DOI] [PubMed] [Google Scholar]
- 32.Frøen RC, Johnsen EO, Petrovski G, et al. Pigment epithelial cells isolated from human peripheral iridectomies have limited properties of retinal stem cells. Acta Ophthalmologica. 2011;89(8):e635–e644. doi: 10.1111/j.1755-3768.2011.02198.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnsen EO, Frøen RC, Albert R, et al. Activation of neural progenitor cells in human eyes with proliferative vitreoretinopathy. Experimental Eye Research. 2012;98(1):28–36. doi: 10.1016/j.exer.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Moe MC, Kolberg RS, Sandberg C, et al. A comparison of epithelial and neural properties in progenitor cells derived from the adult human ciliary body and brain. Experimental Eye Research. 2009;88(1):30–38. doi: 10.1016/j.exer.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Gualdoni S, Baron M, Lakowski J, et al. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. Stem Cells. 2010;28(6):1048–1059. doi: 10.1002/stem.423. [DOI] [PubMed] [Google Scholar]
- 36.Ballios BG, Clarke L, Coles BL, Shoichet MS, van der Kooy D. The adult retinal stem cell is a rare cell in the ciliary epithelium whose progeny can differentiate into photoreceptors. Biology Open. 2012;1:237–246. doi: 10.1242/bio.2012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das AV, James J, Rahnenführer J, et al. Retinal properties and potential of the adult mammalian ciliary epithelium stem cells. Vision Research. 2005;45(13):1653–1666. doi: 10.1016/j.visres.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 38.MacNeil A, Pearson RA, MacLaren RE, Smith AJ, Sowden JC, Ali RR. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells. 2007;25(10):2430–2438. doi: 10.1634/stemcells.2007-0035. [DOI] [PubMed] [Google Scholar]
- 39.Moe MC, Varghese M, Danilov AI, et al. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain. 2005;128(9):2189–2199. doi: 10.1093/brain/awh574. [DOI] [PubMed] [Google Scholar]
- 40.Inoue T, Coles BLK, Dorval KIM, et al. Maximizing functional photoreceptor differentiation from adult human retinal stem cells. Stem Cells. 2010;28(3):489–500. doi: 10.1002/stem.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jasty S, Srinivasan P, Pasricha G, Chatterjee N, Subramanian K. Gene expression profiles and retinal potential of stem/progenitor cells derived from human iris and ciliary pigment epithelium. Stem Cell Reviews. 2012;8(4):1163–1177. doi: 10.1007/s12015-012-9394-3. [DOI] [PubMed] [Google Scholar]
- 42.Ducournau Y, Boscher C, Adelman RA, et al. Proliferation of the ciliary epithelium with retinal neuronal and photoreceptor cell differentiation in human eyes with retinal detachment and proliferative vitreoretinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology. 2012;250(3):409–423. doi: 10.1007/s00417-011-1797-3. [DOI] [PubMed] [Google Scholar]
- 43.Tomita M, Adachi Y, Yamada H, et al. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002;20(4):279–283. doi: 10.1634/stemcells.20-4-279. [DOI] [PubMed] [Google Scholar]
- 44.Boscher C, Ducournau Y, Adelman RA, Guillaubey C, Ducournau D. Retinal progenitor cells contingents in the adult human eye with retinal injury: a specific differentiation potential according to the localization? Experimental Eye Research. 2012;105:79–80. doi: 10.1016/j.exer.2012.09.013. [DOI] [PubMed] [Google Scholar]


