Abstract
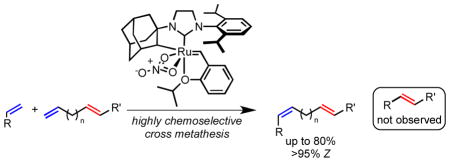
Chelated ruthenium catalysts have achieved highly chemoselective olefin metathesis reactions. Terminal and internal Z olefins were selectively reacted in the presence of internal E olefins. Products were produced in good yield and high stereoselectivity for formation of a new Z olefin. No products of metathesis with the internal E olefin were observed. Chemoselectivity for terminal olefins was also observed over both sterically hindered and electronically deactivated alkenes.
Keywords: metathesis, chemoselectivity, ruthenium, unconjugated dienes, macrocycle
Olefin metathesis catalyzed by transition metal carbene complexes has developed as an important technique in the manipulation of carbon-carbon double bonds. [1] The development and subsequent commercial availability of highly active catalyst complexes has allowed olefin metathesis to have an important synthetic impact on fields ranging from materials science,[2] to biochemistry,[3] to natural products synthesis. [4]
Chemoselectivity, in general, is a current and outstanding problem in organic synthesis.[5] The synthetic utility of olefin metathesis is underscored by the orthogonal reactivity of carbon-carbon double bonds to many organic functional groups. However, in contrast to ring-closing metathesis, cross metathesis has received relatively fewer synthetic applications because of the low stereo- and chemoselectivity observed when previous generations of metathesis catalysts such as 1 and 2 are used.[6] Selective olefin metathesis methods would be useful for the construction of new carbon-carbon double bonds; however, chemoselective cross metathesis reactions of dienes are rare. [7,8] Until recently, metathesis of dienes where one olefin was inert to reaction was limited to olefins differentiated by either electronic or steric properties,[9] with some limited examples in macrocyclic ring-closing metathesis.[10]
Recent advances in catalyst development have produced new metathesis catalysts that are highly Z-selective (3–6, Figure 1). The Schrock and Hoveyda groups initially reported molybdenum and tungsten monoaryloxide pyrrolide (MAP) catalysts (e.g. 3 and 4) that have now been shown to conduct Z-selective dimerization and cross metathesis of terminal olefins[11], ring-opening metathesis polymerization,[12] as well as macrocyclic ring-closing metathesis[13] with high levels of Z-selectivity. Our group then reported chelated catalysts 5 and 6 as the most effective ruthenium catalysts for Z-selective olefin metathesis of terminal and internal Z-olefins, with turnover numbers as high as 7400 and Z-selectivity of >95% in many cases. [14] The synthetic promise of these ruthenium complexes has been presented in the synthesis of macrocyclic musk[15] and insect pheromone natural products.[16]
Figure 1.
Prominent Olefin Metathesis Catalysts (Ar = 2,6-(2,4,6-iPr3C6H2)2C6H3; SIMes = 1,3-Bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene)
The Z-selectivity exhibited by all known Z-selective catalysts is believed to be because of steric crowding around the transition metal center. Large ligands held over a single face of the metal center force the intermediate metallacyclobutane to be formed exclusively in an all-cis orientation (8), in turn producing olefin products highly enriched in the Z isomer (7, Scheme 1).[13c] In this way, catalysts 3–6 produce olefin products with kinetic selectivity for the higher-energy cis olefins. In the case of ruthenium catalysts 5 and 6, this is accomplished by chelation of the NHC ligand, which positions the N-aryl group over the metallacyclobutane.[17]
Scheme 1.
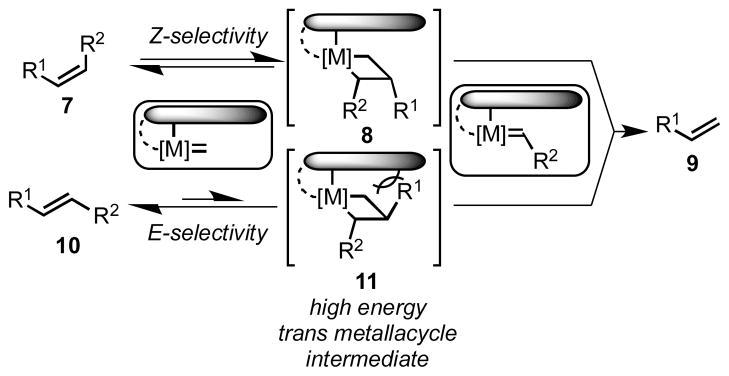
Destabilized trans metallacyclobutane 11 disfavors metathesis of E-olefins
The kinetic selectivity inherent in Z-selective olefin metathesis catalysts provides a platform for enhanced olefin metathesis chemoselectivity. The preference for the formation of Z olefins indicates that the reverse reaction should also be highly preferred for Z olefins. [11c,15,18] As a result, otherwise unbiased E olefins (10) should be significantly less reactive to metathesis as the intermediate metallacycle 11 is destabilized (Scheme 1). In 2012, the Schrock and Hoveyda groups made use of this principle with MAP catalysts of molybdenum (3) and tungsten (4) that selectively dimerized terminal E-1,3-dienes to E, Z, E-trienes with high Z-selectivity and good chemoselectivity for terminal olefins over internal E olefins.[19] Herein, we report the general use of chelated ruthenium catalysts (5 and 6) to perform chemoselective cross metathesis.[16,20] Catalyst 6 was discovered to provide products of cross metathesis with essentially complete chemo- and stereoselectivity with a wide substrate scope.
Initial catalyst investigations were conducted with 8-nonenol (9a) and trans-1,4-hexadiene (Table 1). Reactions were conducted in open vessels to facilitate efficient ethylene removal.[21] Ruthenium complex 5 provided cross product 12a in good yield and Z-selectivity (entry 1). Catalyst 6, bearing the bulkier 2,6-diisopropylphenyl group, has recently been shown to exhibit superior reactivity and Z-selectivity.[14d] Indeed, complex 6 provided diene 12a in similar yields with essentially complete stereoselectivity (entry 2). Production of diene 12a was observed with catalyst loadings as low as 0.1 mol %, though the conversion was diminished (entries 3 and 4). Unchelated dichloride catalyst 1 provided no observable amount of the desired cross product (entry 5), producing primarily oligomeric products of unselective cross metathesis of 1,4-hexadiene. Less reactive catalyst 2, provided some 12a, however the yield and stereoselectivity were low (entry 6). For future investigations, we chose 1 mol % of catalyst 6 in a 0.5 M solution of substrate in 1:1 THF:hexadiene as optimal conditions to achieve sufficient reaction rate with most substrates.
Table 1.
Catalyst investigation for chemoselective olefin metathesis.[a]

| ||||
|---|---|---|---|---|
| entry | catalyst | mol % | yield (%) | Z (%)[b] |
| 1 | 5 | 1 | 87 | 89 |
| 2 | 6 | 1 | 80 (70)[c] | >95 |
| 3 | 6 | 0.5 | 77 | >95 |
| 4 | 6 | 0.1 | 50 | >95 |
| 5 | 1 | 1 | <5[d] | – |
| 6[e] | 2 | 1 | 28[d][f] | 29 |
Reaction conditions: 9a (0.2 mmol), 0.2 mL trans-1,4-hexadiene, 0.2 mL THF.
Determined by 1H NMR analysis.
Reaction conditions: 9a (1 mmol), 1 mL trans-1,4-hexadiene, 1 mL THF.
Major products were oligomeric olefin material.
Allyl methyl carbonate was used instead of 9a.
Determined by 1H NMR analysis with 1,3,5-trimethoxybenzene as an external standard.
The scope was further investigated with trans-1,4-hexadiene as the metathesis partner (Table 2). A number of functionalized terminal olefins were reacted in modest to good yields. Carbonyl functionality such as aldehydes (entry 2), ketones (entry 3), and esters (entry 4) were well tolerated. Allylic amine functionality did not significantly compromise the conversion or overall yields (entries 5–6). Synthetically useful Z-allylic carbonates (12h) and boronic esters (12i) could also be produced in modest yields (entries 7–8). In all cases no products derived from cross metathesis of the internal E-olefin were observed, providing complete chemoselectivity for the cross metathesis of terminal olefins in the presence of unbiased E-olefins. Comparable yields were also obtained on reactions conducted on 1 mmol scale, indicating the robustness of this methodology (Table 1, entry 2 and Table 2, entries 2 and 6).
Table 2.
Scope of Cross Metathesis with trans-1,4-Hexadiene[a]
| ||||
|---|---|---|---|---|
| entry | R | 12 | yield[b] (%) | Z (%)[c] |
| 1 | CH2Ph | 12b | 63[d] | >95 |
| 2 | (CH2)8CHO | 12c | 70 (65)[e] | >95 |
| 3 | (CH2)2COCH3 | 12d | 49 | >95 |
| 4 | (CH2)7CO2Me | 12e | 82 | >95 |
| 5 | CH2NHPh | 12f | 68 | >95 |
| 6 | CH2NHBoc | 12g | 54 (64)[e] | >95 |
| 7 | CH2OCO2Me | 12h | 79 | >95 |
| 8 | CH2BPin | 12i | 65 | >95 |
Reaction conditions: 9 (0.2 mmol), 0.2 mL trans-1,4-hexadiene, 0.2 mL THF, 0.002 mmol 6.
Isolated yield.
Determined by 1H NMR analysis.
Yield determined by 1H NMR analysis with 1,3,5-trimethoxybenzene as an external standard.
Reaction conditions: 9 (1 mmol), 1 mL trans-1,4-hexadiene, 1 mL THF, 0.01 mmol 6.
The diene partner could also be used as the limiting reagent (Table 3). The terminal olefin of diene 13a reacted preferentially over the E-α,β-unsaturated ester in good yield (entry 1). Cross metathesis of diene 13b with 1-decene occurred in similarly good yield and Z-selectivity (entry 1). The proximity of a functionalized trans olefin effects the efficiency of the reaction. Diene 13c required higher catalyst loading to provide a modest yield of E, Z-diene 12l (entry 3). Interestingly, trans-2,5-hexadiene-1-ol (13d) was unreactive towards cross metathesis (entries 4). This lack of reactivity is due to the oxygen functionality rather than protic substrates, as related benzoate ester 13e was also unreactive (entry 5). Furthermore, 1,3-dienes, used as the limiting reagent or in excess, were similarly unreactive to metathesis (e.g. 13f, entry 6).
Table 3.
Scope of Cross Metathesis with 1-Decene[a]
| |||||
|---|---|---|---|---|---|
| entry | R (13) | n | 12 | yield[b] (%) | Z(%)[c] |
| 1 | CO2Me(13a) | 8 | 12j | 80 | >95 |
| 2 | CH2OH (13b) | 8 | 12k | 60 | >95 |
| 3[d] | CH2OH (13c) | 2 | 12l | 51 | >95 |
| 4 | CH2OH(13d) | 1 | 12m | <5 | – |
| 5 | CH2OBz(13e) | 1 | 12n | <5 | – |
| 6 | n-C10H21(13f) | 0 | 12o | <5 | – |
Reaction conditions: 13 (0.2 mmol), 0.2 mL 1-decene, 0.2 mL THF, 0.002 mmol 6.
Isolated yield.
Determined by 1H NMR analysis.
2 mol% 6.
Chelated ruthenium catalyst 6 is sensitive to the steric environment of the reacting olefin. As a result, monosubstituted terminal olefins are also preferentially reacted over disubstituted alkenes (eq 1). When diene 14 was reacted with 8-nonenol (9a), as either the limiting reagent or in excess, metathesis only occured at the less substituted olefin to produce diene 15 as the major product.[22]
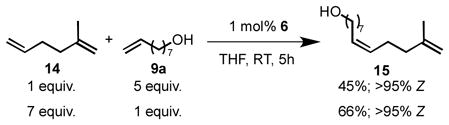 |
(1) |
Chemoselectivity can also be driven by electronic factors. α,β-Unsaturated ester 13g was preferentially metathesized at the terminal olefin to provide Z, Z-diene 12p in good yield and selectivity (eq 2). The electron-withdrawing nature of the methyl ester was sufficient to deactivate the internal Z olefin towards metathesis with catalyst 6. As a result, both E- and Z-α,β-unsaturated esters are unreactive to metathesis with catalyst 2.
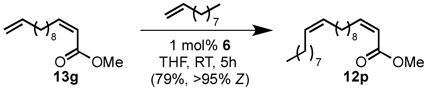 |
(2) |
Chemoselective cross metathesis can also be performed from internal Z-olefins. Cis-6-nonen-1-ol (16) underwent cross metathesis with trans-1,4-hexadiene to provide diene 12q as the major cross metathesis product (eq 3). Cross metathesis in this case likely occurred by initial ethenolysis of 16 to 6-hepten-1-ol by a ruthenium methylidene generated by dimerization of trans-1,4-hexadiene.[16] The terminal alkene was then able to undergo further metathesis to afford E, Z-diene 12q.
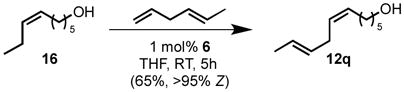 |
(3) |
Chemoselective macrocyclic ring-closing metathesis was also possible with catalyst 5 (eq 4). Utilizing conditions previously optimized for Z-selective macrocyclic ring-closing metathesis of dienes (7.5 mol % catalyst loading under static vacuum of 100 mTorr),[15] the terminal alkenes of triene 17 were selectively reacted to provide 17-membered lactone 18, which contains both internal E and Z double bonds. For this transformation, less hindered catalyst 5 was necessary for sufficient conversion.[23] High Z selectivity was observed for the newly formed olefin, and no products of metathesis of the E olefin were observed. It is important to note that this ring-closing metathesis would be extremely challenging using previous generations of metathesis catalysts (e.g. 1 or 2). Furthermore, the mass balance of this transformation was primarily recovered triene 17, with small amounts of oligomeric material.
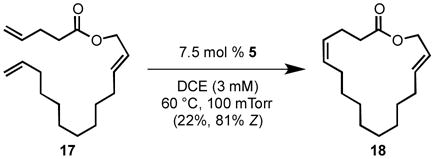 |
(4) |
In summary, chemoselective cross metathesis has been demonstrated with Z-selective ruthenium metathesis catalysts. Catalyst 2 preferentially reacts with terminal and internal Z-olefins to prepare E, Z-dienes in good yields with complete stereoselectivity. Additionally, this preference was extended to sterically and electronically deactivated olefins. Chemoselective macrocyclic ring-closing metathesis was also achieved. Ruthenium complex 6 exhibits broad substrate compatibility and scope. While there are some limitations in compatible dienes, as new catalysts are discovered, the utility and scope of these chemoselective transformations will improve. The chemoselectivity of ruthenium catalysts 5 and 6 provide new avenues for the synthesis of polyenes.
Supplementary Material
Acknowledgments
This work was financially supported by the NIH (R01-GM031332 and N.R.S.A. to J.S.C.: GM103002), and the NSF (CHE-1212767). NMR spectra were obtained by instruments supported by the NIH (RR027690). Materia Inc. is acknowledged for the generous donation of metathesis catalysts 1, 2, 5, and 6.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author. dihydroimidazol-2-ylidene)
References
- 1.a) Trnka TM, Grubbs RH. Acc Chem Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]; b) Fürstner A. Angew Chem. 2000;112:3140–3172. [Google Scholar]; Angew Chem, Int Ed. 2000;39:3012–3043. [PubMed] [Google Scholar]; c) Schrock RR. Chem Rev. 2002;102:145–180. doi: 10.1021/cr0103726. [DOI] [PubMed] [Google Scholar]; d) Vougioukalakis GC, Grubbs RH. Chem Rev. 2010;110:1746–1787. doi: 10.1021/cr9002424. [DOI] [PubMed] [Google Scholar]; e) Samojowicz C, Bieniek M, Grela K. Chem Rev. 2009;109:3708–3742. doi: 10.1021/cr800524f. [DOI] [PubMed] [Google Scholar]
- 2.a) Leitgeb A, Wappel J, Slugovc C. Polymer. 2010;51:2927–2946. [Google Scholar]; b) Sutthasupa S, Shiotsuki M, Sanda F. Polym J. 2010;42:905–915. [Google Scholar]; c) Liu X, Basu A. J Organomet Chem. 2006;691:5148–5154. [Google Scholar]
- 3.Binder JB, Raines RT. Curr Opin Chem Biol. 2008;12:767–773. doi: 10.1016/j.cbpa.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Fürstner A. Chem Commun (Camb) 2011;47:6505–6511. doi: 10.1039/c1cc10464k. [DOI] [PubMed] [Google Scholar]; b) Cossy J, Arseniyadis S, Meyer C. Metathesis in Natural Product Synthesis: Strategies, Substrates, and Catalysts. Wiley–VCH; Weinheim, Germany: 2010. [Google Scholar]
- 5.Shenvi RA, O’Malley DP, Baran PS. Acc Chem Res. 2009;42:530–541. doi: 10.1021/ar800182r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grubbs RH. Handbook of Metathesis. Wiley–VCH; Weinheim, Germany: 2003. [Google Scholar]
- 7.a) Nolan SP, Clavier H. Chem Soc Rev. 2010;39:3305–3316. doi: 10.1039/b912410c. [DOI] [PubMed] [Google Scholar]; b) Zhang Y, Arpin CC, Cullen AJ, Mitton-Fry MJ, Sammakia T. J Org Chem. 2011;76:7641–7653. doi: 10.1021/jo2012658. [DOI] [PubMed] [Google Scholar]; c) Carter KP, Moser DJ, Storvick JM, O’Neil GW. Tetrahedron Lett. 2011;52:4494–4496. [Google Scholar]
- 8.Diver ST, Giessert AJ. Chem Rev. 2004;104:1317–1382. doi: 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]
- 9.a) Funk TW, Efskind J, Grubbs RH. Org Lett. 2005;7:187–190. doi: 10.1021/ol047929z. [DOI] [PubMed] [Google Scholar]; b) Ferrié L, Amans D, Reymond S, Bellosta V, Capdevielle P, Cossy J. J Organomet Chem. 2006;691:5456–5465. [Google Scholar]; c) Moura-Letts G, Curran DP. Org Lett. 2007;9:5–8. doi: 10.1021/ol062017d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Garbaccio RM, Stachel SJ, Baeschlin DK, Danishefsky SJ. J Am Chem Soc. 2001;123:10903–10908. doi: 10.1021/ja011364+. [DOI] [PubMed] [Google Scholar]; b) Dvorak CA, Schmitz WD, Poon DJ, Pryde DC, Lawson JP, Amos RA, Meyers AI. Angew Chem. 2000;112:1730–1732. doi: 10.1002/(sici)1521-3773(20000502)39:9<1664::aid-anie1664>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2000;39:1664–1666. doi: 10.1002/(sici)1521-3773(20000502)39:9<1664::aid-anie1664>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; c) Wagner J, Martin Cabrejas LM, Grossmith CE, Papageorgiou C, Senia F, Wagner D, France J, Nolan SP. J Org Chem. 2000;65:9255–9260. doi: 10.1021/jo001296u. [DOI] [PubMed] [Google Scholar]
- 11.a) Meek SJ, O’Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Flook MM, Jiang AJ, Schrock RR, Müller P, Hoveyda AH. J Am Chem Soc. 2009;131:7962–7963. doi: 10.1021/ja902738u. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Marinescu SC, Levine DS, Zhao Y, Schrock RR, Hoveyda AH. J Am Chem Soc. 2011;133:11512–11514. doi: 10.1021/ja205002v. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Jiang AJ, Zhao Y, Schrock RR, Hoveyda AH. J Am Chem Soc. 2009;131:16630–16631. doi: 10.1021/ja908098t. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Yu M, Ibrahem I, Hasegawa M, Schrock RR, Hoveyda AH. J Am Chem Soc. 2012;134:2788–2799. doi: 10.1021/ja210946z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flook MM, Ng VWL, Schrock RR. J Am Chem Soc. 2011;133:1784–1786. doi: 10.1021/ja110949f. [DOI] [PubMed] [Google Scholar]
- 13.a) Wang C, Yu M, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Chem—Eur J. 2013;19:2726–2740. doi: 10.1002/chem.201204045. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yu M, Wang C, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Nature. 2011;479:88–93. doi: 10.1038/nature10563. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang C, Haeffner F, Schrock RR, Hoveyda AH. Angew Chem. 2013;125:1993–1997. doi: 10.1002/anie.201209180. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2013;52:1939–1943. doi: 10.1002/anie.201209180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Endo K, Grubbs RH. J Am Chem Soc. 2011;133:8525–8527. doi: 10.1021/ja202818v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Keitz BK, Endo K, Patel PR, Herbert MB, Grubbs RH. J Am Chem Soc. 2012;134:693–699. doi: 10.1021/ja210225e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Keitz BK, Endo K, Herbert MB, Grubbs RH. J Am Chem Soc. 2011;133:9686–9688. doi: 10.1021/ja203488e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Rosebrugh LE, Herbert MB, Marx VM, Keitz BK, Grubbs RH. J Am Chem Soc. 2013;135:1276–1279. doi: 10.1021/ja311916m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx VM, Herbert MB, Keitz BK, Grubbs RH. J Am Chem Soc. 2013;135:94–97. doi: 10.1021/ja311241q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert MB, Marx VM, Pederson RL, Grubbs RH. Angew Chem. 2013;125:328–332. doi: 10.1002/anie.201206079. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2013;52:310–314. [Google Scholar]
- 17.Liu P, Xu X, Dong X, Keitz BK, Herbert MB, Grubbs RH, Houk KN. J Am Chem Soc. 2012;134:1464–1467. doi: 10.1021/ja2108728. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki H, Herbert MB, Liu P, Dong X, Xu X, Keitz BK, Ung T, Mkrtumyan G, Houk KN, Grubbs RH. J Am Chem Soc. 2013;135 doi: 10.1021/ja4010267. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend EM, Schrock RR, Hoveyda AH. J Am Chem Soc. 2012;134:11334–11337. doi: 10.1021/ja303220j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Our group has reported a single example of the chemoselective cross metathesis of trans-1,4-hexadiene with oleyl alcohol using catalyst 5. See ref. 16.
- 21.Reactions conducted in closed vessels would not reach complete conversion due to metathesis with ethylene generated by dimerization of the terminal alkene used in excess.
- 22.The low yield obtained when diene 14 was used as the limiting reagent was likely associated with the evaporation of the relatively volatile 14 when run in an open vessel.
- 23.It has been shown that catalyst 6 generally provides lower conversions for macrocyclic ring-closing metathesis than catalyst 5. See ref. 14d for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



