Abstract
Background
Bioelectrical impedance analysis (BIA) is a simple tool to assess total body water (TBW), from which body composition can be estimated using statistical equations. However, standard BIA equations have not been sufficiently validated during pregnancy, in HIV infection, or in sub-Saharan Africa. We therefore compared TBW estimates from multifrequency BIA with those from the reference method deuterium isotope dilution (Deut) in a cohort of 30 HIV-uninfected and 30 HIV-infected pregnant women from Tanzania.
Methods
We enrolled pregnant women presenting for routine antenatal care and collected data on pregnancy outcomes. At each trimester of gestation and once at 10-wk post-partum, we measured maternal anthropometry, TBWBIA, and TBWDeut.
Results
TBWBIA was highly correlated at each time point with TBWDeut among HIV-infected (all P ≤0.001) and HIV-uninfected women (all P <0.0001). During pregnancy, mean TBWBIA progressively underestimated TBWDeut in the overall cohort; trimester-specific differences (mean ±SD) were −1.02 ±2.36 kg, −1.47 ±2.43 kg, and −2.42 ±2.63 kg, respectively. The difference at 10-wk postpartum was small (−0.24 ±2.07 kg). In Bland-Altman and regression models, TBWBIA was subject to a systematic predictive bias at each antenatal and postnatal time point (all P ≤0.038). Among HIV-positive women, TBWDeut measured during the first (P =0.02) and second trimester (P =0.03) was positively related to birthweight.
Conclusions
The validity of current BIA equations to assess TBW during pregnancy and in the postpartum period among women from sub-Saharan Africa remains uncertain. Deuterium dilution may assess aspects of maternal body composition relevant for pregnancy outcomes among HIV-infected women.
Keywords: Bioelectrical impedance analysis, deuterium dilution, pregnancy, Tanzania, HIV
Introduction
Multifrequency bioelectrical impedance analysis (BIA) is a safe and noninvasive method to estimate body composition. It measures the impedance (opposition) to the flow of an electrical current through the body and uses empirical linear regression models to predict total body water (TBW) and fat-free mass [1]. Such models are obtained from populations in whom a reference method was employed. BIA has been successfully used to estimate fat-free mass among non-pregnant and HIV-uninfected Caucasian populations [1-3]. However, the validity of current BIA equations is uncertain among different ethnicities, especially African populations [3-5] as well as during pregnancy and HIV-infection, which are conditions of altered body composition and water distribution [2].
The use of the deuterium dilution method is a reference method to assess the validity of BIA. The dose of deuterium given (as deuterated water) and its subsequent enrichment in urine can be used to calculate TBW and subsequently fat-free mass. The difference between the body weight and the fat-free mass yields the fat mass. The method is safe and has been validated widely among different populations including pregnant women [6], but it requires timed sample collection and is technically demanding [7].
Maternal body composition during pregnancy may be related to pregnancy outcomes. Lean body mass and total body water during pregnancy were related to intrauterine growth in several studies [8, 9], and short maternal stature and low pre-pregnancy weight to increased risk of low birth weight, intra-uterine growth retardation [10], and preterm delivery [11]. The ability to accurately determine body composition with simple methods such as BIA among pregnant women could help identify women at high risk of adverse pregnancy outcomes, including low birth weight.
Body composition changes profoundly during the course of HIV infection, as evidenced by progressive losses of lean or fat mass [12]. In sub-Saharan Africa, the prevalence of wasting is high among HIV-infected persons [13] and this change in body composition has become one of the most common AIDS-defining conditions [14, 15]. Among HIV-infected women, low body mass index before the third trimester of gestation appears to be associated with increased risk of mother-to-child transmission of HIV [16].
Based on the importance of body composition during pregnancy and HIV-infection, as well as the current research gaps, we compared the performance of multifrequency BIA against deuterium dilution in a cohort composed of HIV-infected and HIV-uninfected pregnant Tanzanian women. In addition, we examined the relationship between maternal body composition during gestation and infant birth weight in both groups of women.
Subjects and methods
Study design, study population, and setting
Pregnant women seeking antenatal care in Dar es Salaam, Tanzania were offered HIV-1 testing as part of the existing standard of care tests. Women screened in their first trimester (≤13 wk) were invited to visit a study clinic at the Muhimbili National Hospital. Women received post-test counseling and were invited to give informed consent for the current study. Those infected with HIV were also offered to participate in a multivitamin trial on child health. According to that trial protocol, their children were randomized at 6 wk of age to receive daily supplements containing vitamins B, C, and E or placebo. The study protocol was approved by the Institutional Review Boards at Muhimbili University Health and Allied Sciences and the Harvard School of Public Health.
Women were enrolled into this study during the first trimester of pregnancy (≤14 wk gestation) and were asked to return to the clinic for study visits during the second (20-30 wk gestation) and third trimester (30 wk gestation until delivery). A postpartum visit was scheduled 10 wk after delivery. Isotope dilution and BIA methods were employed at each study visit alongside the determination of anthropometry.
Data collection
At baseline, trained research nurses collected data on client health, socioeconomic status, and obstetric history. Study physicians performed clinical examinations.
Anthropometric measurements
Trained staff measured height, weight, mid-upper arm circumference (MUAC), and triceps skinfold thickness (TSF) in a standardized manner using calibrated instruments [17]. Height and weight were measured to the nearest 0.1 cm and 100 g, respectively. MUAC was obtained at the midpoint between the olecranon and the acromion process with a non-stretchable tape, to the nearest 0.1 cm. TSF measurements were performed in triplicates according to a standardized protocol and the median of the three measurements was chosen for analysis. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Isotope dilution method
Women were asked to report to the study clinic in the morning after an overnight fast. In case a woman had eaten in the last 4 h or had experienced diarrhea, vomiting, or fever in the last 24 h, she was not eligible to undergo body composition measurements and was asked to return to the clinic within 3 days.
Women received a dose of deuterated water of 0.2 g/kg body weight. The dose was measured to the nearest 0.01 g using a digital scale (Explorer Pro Precision Balance, Ohaus Corporation, Pine Brook, NJ, USA). A 2-3 ml aliquot of the dose was placed into a 4 ml centrifuge tube for later analysis. Immediately before the deuterium dose, and 3 h and 4 after receiving the dose, women were asked to provide urine samples into separate 120 ml urine collection bottles. Four ml aliquots of urine were stored in 5 ml Nunc collection tubes with a sealed screw-top. Tubes were stored at −20° C until they were packed on dry ice and shipped to Bangalore, India, for analyses.
BIA
Multifrequency bioelectrical impedance analyses were performed with the Quadscan 4000 (Bodystat Inc, Isle of Man, British Isles, United Kingdom). Women were asked to remove all metal objects and to lie on an examination table with arms and legs spread out. Two distal current-introducing electrodes were placed on the dorsal surfaces of the right hand and foot proximal to the metacarpal phalangeal and metatarsal phalangeal joints, respectively. Two voltage-sensing electrodes were placed at the pisiform prominence of the wrist and between the medial and lateral malleoli of the ankle. Impedance was measured with a multi-frequency bioelectrical impedance analyzer using 5, 50, 100, and 200 kHz at oscillating current. An undisclosed proprietary equation developed by the manufacturer calculated TBW using the impedance at 5 kHz and 200 kHz, body weight, height, age and gender (information provided by manufacturer). After the measurement, research staff recorded the calculated TBW and the measured impedance values, and study participants received a small standard meal with 250 ml of water or tea.
Estimates of TBWBIA (l) were converted to kg by using a conversion factor equivalent to the density of water at 36°C (0.9937 g/cm3). The ratio of 200 kHz to 5 kHz impedance was calculated as an indicator of illness and cell membrane impairments [18].
Pregnancy outcomes
Immediately after delivery, research midwives measured the weight of neonates to the nearest 50 g using a standard mechanical scale. Gestational age was based on the date of the last menstrual period obtained at enrollment.
Fetal deaths before 28 wk of gestation were classified as miscarriages and those thereafter as stillbirths. Women were discharged from the study in the event of a fetal death.
Standard of care
Study participants received standard pre- and postnatal care, including daily doses of ferrous sulfate (200 mg, equivalent to 60 mg ferrous Fe) and folic acid (0.25 mg) and malaria prophylaxis in the form of sulfadoxine-pyrimethamine tablets (Fansidar, Roche) at 20 and 30 wk of gestation. Based on earlier findings [19], HIV-infected participants received 20 mg of vitamin B1, 20 mg of vitamin B2, 25 mg of vitamin B6, 100 mg of niacin, 50 μg of vitamin B12, 500 mg of vitamin C, 30 mg of vitamin E, and 0.8 mg of folic acid from enrollment until delivery.
HIV-infected women were offered antiretroviral therapy (ART) in line with national treatment guidelines [20]. To prevent transmission of HIV from mother to child, a 200 mg single dose of nevirapine was given orally to all HIV-infected women during labor who were not receiving ART. A dose of 2 mg nevirapine/kg syrup was given to all children within 72 h of delivery who were born to HIV-infected mothers [21].
Laboratory analyses
HIV status was determined by using HIV enzyme-linked immunosorbent assay testing algorithm as evaluated previously [22]. Among HIV-infected women, T-cell subset counts (CD4, CD8 and CD3) were enumerated at enrollment with the use of the FACSCount system (Becton-Dickinson, San Jose, CA). Urine samples were analyzed for deuterium enrichment by the zinc reduction technique [23], using a dual inlet isotope ratio mass spectrometer (Europa Scientific Ltd, Crewe, UK). The coefficient of variation of repeated measurements of deuterium enrichment was < 0.02%. The measurement of TBW (in kg) as the ratio of the dose of deuterated water administered and the enrichment of deuterium in the urine was corrected by 4% overestimation because of deuterium-hydrogen exchange. The highest enrichment measured in either the 3 h or 4 h sample was used for the calculation of TBW. The difference in TBW (in kg) between the two samples was 8.4% ± 10.6%.
Statistical analyses
The Wilcoxon rank-sum test was used to compare baseline characteristics by HIV status. For each study visit, Pearson correlation coefficients were constructed to evaluate the correlation between TBW determined by BIA (TBWBIA) and by deuterium dilution (TBWDeut); paired t-tests were used to evaluate whether the mean values of TBWBIA and TBWDeut differed significantly.
In linear regression models with TBWDeut as the dependent variable, we examined whether the coefficient for TBWBIA differed by HIV status during pregnancy and at the postpartum visit. For this, we created an interaction term between TBWBIA and HIV status and used the Wald test to evaluate statistical significance.
The Bland-Altman model was used to evaluate agreement between TBWBIA and TBWDeut [24, 25]. In this model, the difference between the two methods was plotted against the mean of the methods. To examine whether there was a trend in the bias, mixed regression models with an empirical variance estimator were used.
To examine the proportion of variability of TBWDeut that is explained by other predictors, linear regression models were used to calculate the coefficient of determination R2. Models were fit separately for the impedance quotient (Ht2/R50) only, for anthropometric variables only, both Ht2/R50 and anthropometric variables, and a full model with Ht2/R50, anthropometric variables, and other predictors. Models were fit separately for trimesters 1, 2, and 3 and for the 10 week postpartum visit.
The relation of TBWDeut, MUAC, and TSF measured during pregnancy with birthweight was examined in each trimester using mixed regression models with an empirical variance estimator.
All P values reported are two-sided; statistical significance was defined as P < 0.05. Statistical analyses were carried out using the SAS system version 9.1 (SAS Institute, Carey, NC). Unless otherwise noted, values are means ± SD or percentages.
Results
Participants were enrolled between April 2006 and February 2007. Of the 60 women enrolled, two were not at ≤14 wk gestation and their baseline measurements for deuterium dilution, bioelectrical impedance, and anthropometry were therefore set to missing. Of the eight fetal losses, seven occurred after the first trimester measurement and one after the second trimester measurement. On average, women had 3.0 (range 1-4) TBWDeut samples available.
Women entered the study at a mean gestational age of 12.7 ± 2.0 wk. At baseline, women weighed on average 58.2 ± 11.6 kg, had a mean BMI of 23.8 ± 4.5, and a mean MUAC of 26.9 ± 3.9 cm. Mean TBWDeut and TBWBIA were 30.0 ± 4.3 kg and 28.8 ± 3.0 kg, respectively. HIV-infected women had a mean CD4 cell count of 454 ± 226 cells/μl. Baseline characteristics between HIV-uninfected and HIV-infected women did not differ (Table 1). There was no ART use at any of the study visits.
Table 1.
Background characteristics at the first trimester baseline visit.
| Characteristic1 | HIV-negative | HIV-positive | P-value2 |
|---|---|---|---|
| Background information | |||
| Gestational Age (wk) | 12.3 ± 2.0 | 13.1 ± 2.0 | 0.14 |
| Age (y) | 25.0 ± 5.3 | 27.3 ± 5.3 | 0.08 |
| Formal education (y) | 7.2 ± 3.7 | 7.5 ± 3.0 | 0.98 |
| Money spent on food/day (TSh)3 | 849 ± 250 | 1023 ± 443 | 0.13 |
| Immunology 4 | |||
| CD4 count (/μl) | – | 454 ± 226 | |
| Anthropometry | |||
| Weight (kg) | 57.8 ± 12.0 | 58.7 ± 11.3 | 0.63 |
| Height (cm) | 155.4 ± 7.1 | 158.0 ± 6.3 | 0.10 |
| Body mass index | 23.9 ± 4.5 | 23.7 ± 4.5 | 0.82 |
| Triceps skinfold thickness (mm) | 19.3 ± 7.3 | 19.0 ± 6.2 | 0.97 |
| Mid-upper arm circumference (cm) | 26.9 ± 3.9 | 26.8 ± 4.0 | 0.83 |
| Body composition | |||
| Total body water (kg)Deuterium | 29.4 ± 4.8 | 30.6 ± 3.7 | 0.20 |
| Total body water (kg)Bioelectrical Impedance Analysis | 28.5 ± 3.3 | 29.2 ± 2.8 | 0.26 |
| Ratio of 200 kHz to 5 kHz Impedance | 0.81 ± 0.02 | 0.81 ± 0.02 | 0.40 |
n=49-60;
From Wilcoxon rank-sum test;
Per household member;
n=18 (measured among HIV-infected women only).
TSh = Tanzanian Shilling. At the time of the study, 1 U.S.$ ≈ 1250 Tanzanian Shillings.
TBWDeut and TBWBIA were highly correlated in HIV-uninfected (r ≥ 0.74) and HIV-infected (r ≥ 0.80) women at all four time points (Table 2). From baseline to the third trimester, mean TBWDeut increased by 4.5 kg and 4.4 kg among HIV-uninfected and HIV-infected women, respectively (Table 3). TBWBIA was consistently lower than TBWDeut in the overall cohort and among subgroups defined by HIV status. This difference was statistically significant in the overall cohort and among HIV-infected women at each visit during pregnancy but not at the postpartum visit; among HIV-uninfected women, the difference was only statistically significant at the 3rd trimester visit.
Table 2.
Correlation between total body water values obtained by using deuterium dilution and bioelectrical impedance analysis.
| Time point | HIV-negative | HIV-positive | ||||
|---|---|---|---|---|---|---|
| n | r 1 | P-value1 | n | r | P-value | |
| 1st Trimester | 30 | 0.85 | <0.0001 | 28 | 0.85 | <0.0001 |
| 2nd Trimester | 22 | 0.74 | <0.0001 | 19 | 0.90 | <0.0001 |
| 3rd Trimester | 20 | 0.77 | <0.0001 | 17 | 0.80 | P = 0.001 |
| 10 wks postpartum | 21 | 0.87 | <0.0001 | 20 | 0.92 | <0.0001 |
Based on Pearson correlation analyses.
Table 3.
Comparison in total body water values obtained by using deuterium dilution (TBWDeut) and bioelectrical impedance analysis (TBWBIA).
| Time point | Mean ± SD1 |
HIV-negative Difference ± SD |
P 2 | Mean ± SD |
HIV-positive Difference ± SD |
P | Mean ± SD |
Combined Difference ± SD |
P 2 |
|---|---|---|---|---|---|---|---|---|---|
| 1st Trimester | |||||||||
| TBWDeut | 29.4 ± 4.8 | 30.6 ± 3.7 | 30.0 ± 4.3 | ||||||
| TBWBIA | 28.5 ± 3.3 | −0.95 ± 2.64 | 0.06 | 29.2 ± 2.8 | −1.47 ± 2.04 | 0.0007 | 28.8 ± 3.0 | −1.20 ± 2.36 | 0.0003 |
| 2nd Trimester | |||||||||
| TBWDeut | 31.0 ± 3.4 | 33.9 ± 4.9 | 32.4 ± 4.4 | ||||||
| TBWBIA | 30.2 ± 2.8 | −0.78 ± 2.29 | 0.13 | 31.2 ± 4.0 | −2.69 ± 2.23 | <0.0001 | 30.7 ± 3.4 | −1.66 ± 2.43 | <0.0001 |
| 3rd Trimester | |||||||||
| TBWDeut | 33.9 ± 4.1 | 35.0 ± 4.5 | 34.4 ± 4.2 | ||||||
| TBWBIA | 31.3 ± 3.2 | −2.54 ± 2.61 | 0.0003 | 32.3 ± 4.1 | −2.71 ± 2.72 | 0.0008 | 31.8 ± 3.6 | −2.62 ± 2.62 | <0.0001 |
| 10 wks postpartum | |||||||||
| TBWDeut | 29.9 ± 3.1 | 29.1 ± 5.1 | 29.5 ± 4.2 | ||||||
| TBWBIA | 29.3 ± 2.4 | −0.64 ± 1.59 | 0.08 | 28.9 ± 3.2 | −0.20 ± 2.51 | 0.72 | 29.1 ± 2.8 | −0.43 ± 2.07 | 0.19 |
All values shown in kg;
Based on paired t-test.
In regression analyses between TBWDeut and TBWBIA, the coefficient for TBWBIA did not differ by HIV status during (P-value for interaction = 0.72) or after pregnancy (P-value for interaction = 0.11) (data not shown).
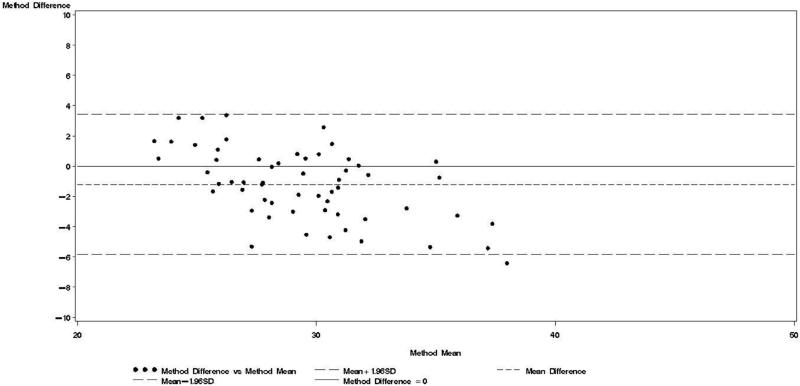
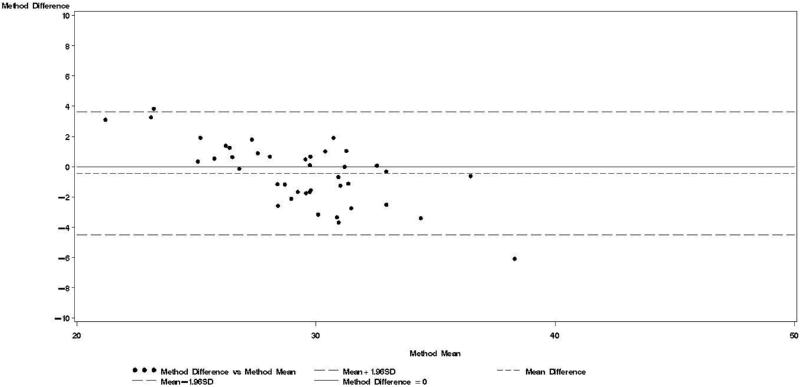
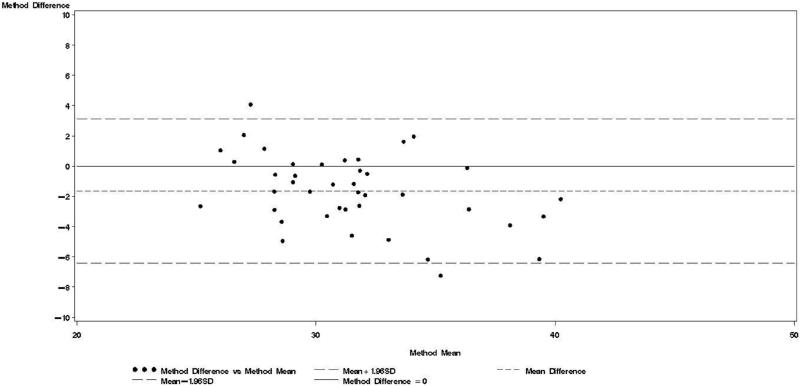
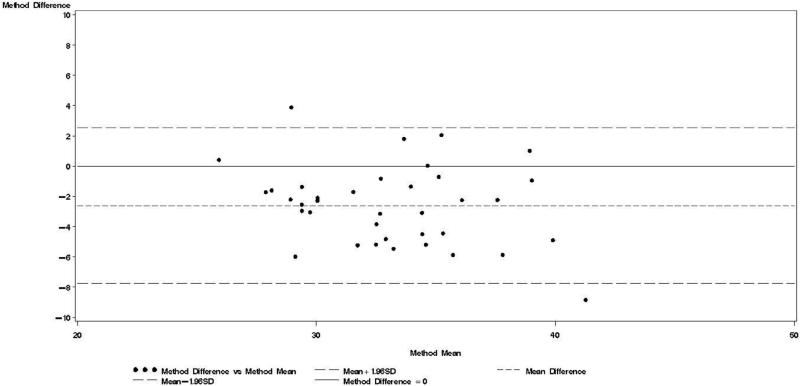
Bland-Altman models indicated that during each trimester and during the postpartum visit, the mean bias in TBWBIA increased with increasing mean TBW (Figs 1-4). This observation was validated in linear regression models, which indicated statistically significant negative slopes at trimesters 1, 2, 3, and the postpartum visit (data not shown).
Figure 1.
Bland & Altman plot for TBWDeut and TBWBIA – 1st trimester.
Figure 4.
Bland & Altman plot for TBWDeut and TBWBIA – 10 wk postpartum.
The impedance quotient Ht2/R50 explained about 2/3 of variability in TBWDeut at the first and second trimester and the postpartum visits (Table 4), and 49% in the third trimester. In the first and second trimester, Ht2/R50 explained about the same variability as all anthropometric variables combined. In the third trimester, anthropometry explained considerably more variability than Ht2/R50 (86% vs. 49%, respectively), whereas the difference was less pronounced at the 10-week postpartum visit (74% vs. 67%, respectively). Addition of week of gestation, age, and gestational age did not explain more variability than a model containing anthropometry and Ht2/R50.
Table 4.
Prediction equations for total body water values obtained by using deuterium dilution during trimesters 1, 2, and 3 and 10-weeks postpartum1.
| Model type | Time point | R2 |
|---|---|---|
| 1st Trimester | ||
| Ht2/R50 only | TBWDeut = 5.31 + 0.65 Ht2/R50 | 0.653 |
| Anthropometry only | TBWDeut = −7.72 + 0.25 Wt + 0.15 Ht + 0.34 BMI − 0.27 MUAC – 0.09 TSF | 0.634 |
| Anthropometry + Ht2/R50 | TBWDeut = −41.41 − 0.28 Wt + 0.32 Ht + 1.36 BMI − 0.50 MUAC – 0.06 TSF + 0.46 Ht2/R50 | 0.750 |
| Full model | TBWDeut = −35.34 + 0.45 Ht2/R50 + 0.31 Ht – 0.44 MUAC −0.11 | |
| Age – 0.20 gestation + 0.61 HIVpos + 1.22 BMI + 0.06 TSF −0.24 Wt | 0.769 | |
| 2nd Trimester | ||
| Ht2/R50 only | TBWDeut = 9.67 + 0.54 Ht2/R50 | 0.637 |
| Anthropometry only | TBWDeut = 11.86 + 0.44 Wt + 0.03 Ht − 0.32 BMI − 0.05 MUAC – 0.10 TSF | 0.672 |
| Anthropometry + Ht2/R50 | TBWDeut = 3.72 + 0.25 Wt + 0.06 Ht − 0.16 BMI − 0.12 MUAC – 0.04 TSF + 0.30 Ht2/R50 | 0.748 |
| Full model | TBWDeut = −48.58 + 0.34 Ht2/R50 + 0.42 Ht – 0.40 MUAC − 0.03 | |
| Age – 0.13 gestation + 1.13 HIVpos + 1.19 BMI + 0.05 TSF − 0.28 Wt | 0.771 | |
| 3rd Trimester | ||
| Ht2/R50 only | TBWDeut = 15.77 + 0.42 Ht2/R50 | 0.489 |
| Anthropometry only | TBWDeut = 43.82 + 0.73 Wt − 0.18 Ht − 0.99 BMI − 0.04 MUAC – 0.11 TSF | 0.862 |
| Anthropometry + Ht2/R50 | TBWDeut = 42.49 + 0.70 Wt − 0.17 Ht − 0.97 BMI − 0.05 MUAC – 0.09 TSF + 0.04 Ht2/R50 | 0.864 |
| Full model | TBWDeut = 31.05 + 0.58 Wt − 0.76 BMI – 0.09 TSF – 0.08 Ht + 0.02 MUAC – 0.06 | |
| Age + 0.06 Ht2/R50 −0.04 gestation – 0.16 HIVpos | 0.851 | |
| 10-weeks postpartum | ||
| Ht2/R50 only | TBWDeut = 2.00 + 0.70 Ht2/R50 | 0.665 |
| Anthropometry only | TBWDeut = 2.42 + 0.30 Wt − 0.06 Ht + 0.26 BMI − 0.15 MUAC – 0.11 TSF | 0.741 |
| Anthropometry + Ht2/R50 | TBWDeut = −15.20 + 0.004 Wt − 0.12 Ht + 0.63 BMI − 0.18 MUAC – 0.01 TSF + 0.42 Ht2/R50 | 0.850 |
| Full model | TBWDeut = −9.42 + 0.07 Wt + 0.40 Ht2/R50 −0.06 | |
| Age + 0.09 Ht + 0.47 BMI – 0.14 MUAC – 0.04 TSF −0.04 HIVpos | 0.855 |
Based on linear regression models
The mean birth weight among HIV-uninfected women (3224 ± 436 g) was higher than the birth weight among HIV-infected women (2887 ± 594 g) (P = 0.04). TBWDeut measured during the first (P = 0.02) and second (P = 0.03) trimester was positively related to birth weight among HIV-infected but not among HIV-uninfected women (Table 5), despite similar increments in TBW observed during pregnancy. TBWDeut, TSF, or MUAC were not related to birth weight among HIV-infected or HIV-uninfected women.
Table 5.
Relation of pregnancy total body water and anthropometry with birth weight among HIV-negative and HIV-positive women1.
| Time point | TBWDeut(kg)2 | HIV-negative MUAC (cm)3 |
TSF (mm)4 | TBWDeut(kg) | HIV-positive MUAC (cm) |
TSF (mm) |
|---|---|---|---|---|---|---|
| 1st Trimester | 12 (−42,66) | 24 (−41,90) | 24 (−13,60) | 80 (12,147)5 | 53 (−33,139) | 29 (−29,87) |
| 2nd Trimester | 11 (−56,77) | 55 (−28,138) | 34 (−12,79) | 81 (9,153)6 | 57 (−114,227) | 2 (−86,89) |
| 3rd Trimester | 13 (−39,66) | 47 (−12,107) | 38 (10, 66) | 64 (−23,152) | 58 (−48,163) | 14 (−47,74) |
Based on linear regression models. β (95% CI) indicates the mean increase in birth weight in g related to a one unit increase in the variable of interest. All models adjusted for age and gestational age at baseline;
Total body water determined by deuterium dilution;
Mid-upper arm circumference;
Triceps skinfold thickness;
P= 0.02;
P= 0.03.
Discussion
In this cohort of 30 HIV-infected and 30 HIV-uninfected women who were enrolled in the first trimester of pregnancy and followed until 10 wk postpartum, we compared the performance of BIA against the reference method deuterium dilution for determining TBW.
There was a strong correlation between TBWBIA and TBWDeut during gestation and in the postpartum period. Among lactating women from South Africa, correlations between TBW obtained by bioimpedance spectroscopy (BIS) and TBWDeut were slightly higher among the HIV-uninfected than among the HIV-infected subgroup [26]. The authors hypothesized that the HIV-infected women in the study had fat free mass of different quality and thus altered body water compartments compared to HIV-uninfected women [27]. In contrast to the results from the South African study, correlations were slightly higher among HIV-infected than among HIV-uninfected in the current study. This may be due to the slightly larger range of values among the HIV-infected group, or the fact that HIV-infected women were largely asymptomatic and had therefore not yet experienced any changes in body water compartments. In a study among ten healthy women from the US, the correlation between TBWBIA and TBWDeut in the first, second, and third trimesters of pregnancy, as well as in the postpartum period (4-6 wk) obtained were remarkably similar to the coefficients observed in our study [28]. Among healthy women from the US, different BIA prediction equations yielded good correlations between TBWBIA and TBWDeut [29]. Our findings and these previous findings indicate that high correlations can be obtained between TBWBIA and TBWDeut among pregnant women and during asymptomatic HIV infection. However, high correlations do not prove that TBWBIA is an unbiased estimator of TBWDeut.
Bland-Altman analyses are better suited than correlation coefficients to determine agreement between a new and a reference method [24]. Our findings indicate TBWBIA during pregnancy consistently underestimated TBWDeut, especially in the third trimester of pregnancy among HIV-uninfected women and in the second and third trimester among HIV-infected women. On the other hand, differences during the postpartum period were small and statistically non-significant. There was evidence of a predictive bias, which led to greater underestimations of TBW with increasing TBW both during and after pregnancy. Among healthy women in the US, there was good agreement between TBWBIA and TBWDeut during all three trimesters of pregnancy and during the postpartum period [29]. In the study from South Africa among lactating women, TBWBIA underestimated TBWDeut by 1.8 kg among HIV-infected and by 1.5 kg among HIV-uninfected women [26]. Even though these differences were statistically significant, the authors concluded that BIS provides an estimate of body composition comparable to that obtained by deuterium dilution. This conclusion is in line with the non-significant respective −0.64 kg and −0.20 kg mean biases among HIV-uninfected and HIV-infected women during the postpartum visit in our study. Nevertheless, the presence of the systematic predictive bias raises concerns about the validity of BIA among pregnant women from sub-Saharan Africa.
The impedance quotient (Ht2/R50) was not better than simple anthropometric measurements at explaining variability in TBWDeut. Combination of both sets of parameters led to modest increases in variability explained. Among pregnant women from the US, the impedance quotient explained 90% of variability in TBWDeut and addition of anthropometric variables explained additional variability [29]. It therefore appears that BIA performed better in the US study population than in our cohort.
First and second trimester TBWDeut was related to birth weight among HIV-infected women. Because TBW reflects fat-free mass, this finding suggests benefits of greater fat free mass for birth weight among HIV-infected women, who are at a higher risk for low birth weight than HIV-uninfected women [30]. It thus appears that TBWDeut better captures physiological states and processes relevant for birth weight among HIV-infected women than MUAC, a combined measure of muscle and subcutaneous fat, or TSF, a measure of subcutaneous fat. In later gestation, TBW reflects increases in amount of fat-free mass, including expansions of plasma, fetal and placental tissue, and amniotic fluid [31-33]. Among healthy women, the plasma volume increases on average 1250 mL during pregnancy [31], of which approximately 90% is water. The plasma expansion thus accounted for about ¼ of the increase in TBW observed in this study. Plasma expansion is a known maternal hemodynamic adaptation during pregnancy, which is a known determinant of fetal growth [33]. It is unclear why TBWDeut measured during the third trimester or changes in TBWDeut were not significantly related to birth weight. Low statistical power, which is the main limitation of this study, may be one potential explanation. In a study among 200 women from the US with mixed ethnicities and a study among 224 Chilean women, TBWDeut measured near term was related to birth weight [8, 9].
In summary, TBWBIA correlated well with TBWDeut during and after pregnancy. TBWBIA yielded an increasingly biased mean estimate of TBWDeut as pregnancy proceeded, and was also subject to a systematic prediction bias. Multivariate regression analysis identified BIA and standard anthropometric data as significant predictors of TBW, and, among HIV-infected women, TBW was a significant predictor of infant birth weight. Future studies should examine which components of TBW expansion might be related to higher birth weight among HIV-exposed infants, and body composition analysis holds promise as an important outcome in nutritional intervention trials during pregnancy.
Figure 2.
Bland & Altman plot for TBWDeut and TBWBIA – 2nd trimester.
Figure 3.
Bland & Altman plot for TBWDeut and TBWBIA – 3rd trimester.
Acknowledgements
The authors thank Anna Acosta and Rehema Mtonga for supervising the implementation of the study, Eduardo Villamor for comments during the design and analysis stages, and Sakki Meeuwsen for technical assistance on bioelectrical impedance data.
RK, CD, KM, and WWF designed research; RK, CD, KM, EW, and SA conducted research; AVK and SA performed laboratory analyses; RK, CD, RB, and AVK analyzed data; RK wrote paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by the International Atomic Energy Agency Coordinated Research Program, Research Contract 12778 RO; and the Eunice Kennedy Shriver National Institute of Child Health & Human Development, Grants R01HD043688 and 1K24HD058795. Bodystat Ltd, Isle of Man, UK donated the Quadscan 4000 bioelectrical impedance machine.
Footnotes
Conflict of interest – The authors declare no conflict of interest.
A modified version of this manuscript was submitted for presentation at the 2009 International Congress of Nutrition and published in abstract form in Ann Nutr Metab 55 (suppl 1) Abstract P 177-20, p.617.
The opinions and statements in this article are those of the authors, and may not reflect official UNICEF policies.
References
- 1.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis – part I: review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis – part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–53. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Dioum A, Gartner A, Cisse AS, et al. Validity of impedance-based equations for the prediction of total body water as measured by deuterium dilution in African women. Am J Clin Nutr. 2005;81:597–604. doi: 10.1093/ajcn/81.3.597. [DOI] [PubMed] [Google Scholar]
- 4.Deurenberg P, Wolde-Gebriel Z, Schouten FJ. Validity of predicted total body water and extracellular water using multifrequency bioelectrical impedance in an Ethiopian population. Ann Nutr Metab. 1995;39:234–41. doi: 10.1159/000177868. [DOI] [PubMed] [Google Scholar]
- 5.Deurenberg P, Deurenberg-Yap M. Validity of body composition methods across ethnic population groups. Forum Nutr. 2003;56:299–301. [PubMed] [Google Scholar]
- 6.Lof M, Forsum E. Hydration of fat-free mass in healthy women with special reference to the effect of pregnancy. Am J Clin Nutr. 2004;80:960–5. doi: 10.1093/ajcn/80.4.960. [DOI] [PubMed] [Google Scholar]
- 7.Hopkinson JM, Butte NF, Ellis KJ, Wong WW, Puyau MR, Smith EO. Body fat estimation in late pregnancy and early postpartum: comparison of two-, three-, and four-component models. Am J Clin Nutr. 1997;65:432–8. doi: 10.1093/ajcn/65.2.432. [DOI] [PubMed] [Google Scholar]
- 8.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Maternal body fat and water during pregnancy: do they raise infant birth weight? Am J Obstet Gynecol. 1999;180:235–40. doi: 10.1016/s0002-9378(99)70181-x. [DOI] [PubMed] [Google Scholar]
- 9.Mardones-Santander F, Salazar G, Rosso P, Villarroel L. Maternal body composition near term and birth weight. Obstet Gynecol. 1998;91:873–7. doi: 10.1016/s0029-7844(98)00109-4. [DOI] [PubMed] [Google Scholar]
- 10.A WHO Collaborative Study Maternal anthropometry and pregnancy outcomes. Bull World Health Organ. 1995;73(Suppl):1–98. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraz EM, Gray RH, Cunha TM. Determinants of preterm delivery and intrauterine growth retardation in north-east Brazil. Int J Epidemiol. 1990;19:101–8. doi: 10.1093/ije/19.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Macallan DC. Wasting in HIV infection and AIDS. J Nutr. 1999;129:238S–242S. doi: 10.1093/jn/129.1.238S. [DOI] [PubMed] [Google Scholar]
- 13.Serwadda D, Mugerwa RD, Sewankambo NK, et al. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet. 1985;2:849–52. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 14.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindan CP, Allen S, Serufilira A, et al. Predictors of mortality among HIV-infected women in Kigali, Rwanda. Ann Intern Med. 1992;116:320–8. doi: 10.7326/0003-4819-116-4-320. [DOI] [PubMed] [Google Scholar]
- 16.Burns DN, FitzGerald G, Semba R, et al. Women and Infants Trans-mission Study Group Vitamin A deficiency and other nutritional indices during pregnancy in human immunodeficiency virus infection: prevalence, clinical correlates, and outcome. Clin Infect Dis. 1999;29:328–34. doi: 10.1086/520210. [DOI] [PubMed] [Google Scholar]
- 17.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics; Chicago, IL: 1988. [Google Scholar]
- 18.Gonzalez MC, Maslonek J, Uliano GL, Munareto MM, Teixeira LO. Illness marker as a prognostic tool in intensive care unit: A prospective study. J Parenter Enteral Nutr. 2009;33:219. abstr. [Google Scholar]
- 19.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–82. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 20.National AIDS Control Programme . National Guidelines for the Clinical Management of HIV and AIDS. 2nd ed 2005. [Google Scholar]
- 21.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 22.Aboud S, Urassa W, Lyamuya E, Mhalu F, Biberfeld G. Evaluation of HIV antibody and antigen/antibody combination ELISAs for use in an alternative confirmatory HIV testing strategy in Dar es Salaam, Tanzania. J Virol Methods. 2006;135:192–6. doi: 10.1016/j.jviromet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Wong W, Schoeller D. Mass spectrometric analysis. In: Prentice AM, editor. The Doubly-labelled Water Method for Measuring Energy Expenditure: A consensus Report by the IDECG working group. International Atomic Energy Agency; Vienna: 1990. pp. 20–47. [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 25.Cheng NF, Gansky SA. [Accessed 09/12/2009];A SAS macro to produce a Bland-Altman reliability plot. UCSF CAN-DO website. http://dentistry.ucsf.edu/cando/resources/software/BlandAltmanPlot.sas.txt.
- 26.Papathakis PC, Rollins NC, Brown KH, Bennish ML, Van Loan MD. Comparison of isotope dilution with bioimpedance spectroscopy and anthropometry for assessment of body composition in asymptomatic HIV-infected and HIV-uninfected breastfeeding mothers. Am J Clin Nutr. 2005;82:538–46. doi: 10.1093/ajcn.82.3.538. [DOI] [PubMed] [Google Scholar]
- 27.Paton NI, Macallan DC, Jebb SA, et al. Longitudinal changes in body composition measured with a variety of methods in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:119–27. doi: 10.1097/00042560-199702010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Van Loan MD, Kopp LE, King JC, Wong WW, Mayclin PL. Fluid changes during pregnancy: use of bioimpedance spectroscopy. J Appl Physiol. 1995;78:1037–42. doi: 10.1152/jappl.1995.78.3.1037. [DOI] [PubMed] [Google Scholar]
- 29.Lukaski HC, Siders WA, Nielsen EJ, Hall CB. Total body water in pregnancy: assessment by using bioelectrical impedance. Am J Clin Nutr. 1994;59:578–85. doi: 10.1093/ajcn/59.3.578. [DOI] [PubMed] [Google Scholar]
- 30.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105:836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 31.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–12. [PubMed] [Google Scholar]
- 32.Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw. 1973;80:884–7. doi: 10.1111/j.1471-0528.1973.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 33.Ghezzi F, Franchi M, Balestreri D, et al. Bioelectrical impedance analysis during pregnancy and neonatal birth weight. Eur J Obstet Gynecol Reprod Biol. 2001;98:171–6. doi: 10.1016/s0301-2115(01)00330-x. [DOI] [PubMed] [Google Scholar]