Abstract
OBJECTIVES
Our goal was to examine the association between parent-rated sleep problems during childhood and neuropsychological functioning during adolescence.
PARTICIPANTS AND METHODS
Longitudinal prospective data on an entire birth cohort from Dunedin, New Zealand, were obtained. One thousand thirty-seven children were enrolled in the study (52% male). Parents reported on sleep problems when the study members were 5, 7, and 9 years of age. Neuropsychological functioning was assessed by using 7 tests when the participants were 13 years of age.
RESULTS
After adjusting for gender and socioeconomic status, persistent sleep problems during childhood predicted scores on 2 neuropsychological tests: the copy score of the Rey-Osterrieth Complex Figure Test and 2 measures of performance on the Halstead Trail Making Test. These results were substantively replicated when sleep was assessed at the 5- and 9-year (but not 7-year) assessments separately.
CONCLUSIONS
Sleep problems during childhood may be associated with certain aspects of neuropsychological functioning during adolescence. This adds to the growing body of literature suggesting that childhood sleep problems may be a risk indicator of later difficulties.
Keywords: sleep, neuropsychological, longitudinal, prospective
Sleep and neuropsychological functioning are linked. In contrast to what is known about adults, less is known about these associations in children (for a review see ref 1). This is surprising given that childhood is a sensitive period in terms of development of sleep patterns and problems2 as well as cognitive development and brain maturation (for a review see ref 3). To add to the small body of literature addressing sleep and neuropsychological functioning in childhood, we focused on an inclusive measure of sleep problems during childhood, and examined links with neuropsychological functioning assessed during adolescence.
Previous research focusing on children has established links between sleep and neuropsychological functioning by (1) experimentally manipulating (eg, restricting or depriving) sleep and (2) comparing those with and without sleep problems. With regards to the first type of study, results have been mixed—although associations have been reported between sleep restriction and areas of neuropsychological functioning, including aspects of memory, verbal skills, and learning.4,5 The second type of study has focused almost exclusively on children with sleep-related breathing difficulties. Despite mixed results (for a review see ref 6), children experiencing these problems have been found to have a range of difficulties including problems with attention and executive functioning (eg, refs 7–10); language skills11–13; aspects of memory and learning13,14; and visual perception and construction.10,12 There is no doubt that sleep-related breathing difficulties provide an important clinical model for examining the effects of sleep disruption (see ref 15) and have been informative with regards to understanding associations with neuropsychological functioning. However, other, more common sleep problems such as difficulties initiating and maintaining sleep11 (see also ref 16) may also be linked to neuropsychological functioning (for a review see ref 17). Indeed, both poor and insufficient sleep as well as a composite of different sleep problems have been linked to poor academic performance.18–20 The frequency with which certain sleep difficulties occur (for the prevalence of different types of sleep problems in school children, see elsewhere refs 21 and 22) emphasizes the need to increase understanding of the links between sleep and neuropsychological functioning. An additional issue with regards to previous studies of sleep and neuropsychological functioning is that they have been almost exclusively cross-sectional—limiting understanding of longitudinal links. This is particularly salient as 1 study revealed that middle-school children who were performing poorly academically were more likely to retrospectively report snoring during early childhood as compared with those performing well.23 These results underscore the likely importance of prospective longitudinal studies for informing this area of research.
PARTICIPANTS AND METHODS
Participants
Participants were members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of the health and behavior of a complete birth cohort. The cohort of 1037 children (52% male) was constituted at 3 years of age when the investigators enrolled 91% of consecutive births from April 1, 1972, through March 31, 1973, in Dunedin, New Zealand. Cohort families were primarily white and represented the full range of socioeconomic status (SES) in the general population of New Zealand’s South Island. At each assessment age, participants (including emigrants living overseas) were brought back to the research unit for a full day of individual data collection. The study protocol was approved by the institutional review boards of the participating universities. After complete description of the study to the participants, written informed consent was obtained from parents up to age 15 and thereafter from the study members. Follow-ups were performed at 5, 7, 9, 11, 13, 15, 18, 21, 26, and, most recently, 32 years of age (n = 972 [96% of the living cohort members]). Here we focused on the 5-, 7-, 9-, and 13-year-old assessments as they provide the most thorough assessments of child sleep and neuropsychological functioning. Detailed information about sleep and neuropsychological functioning was not collected at the 11 year assessment.
Socioeconomic Status
SES of the study members’ families was measured on a 6-point scale that assessed parents’ self-reported occupational status. The scale allocated each occupation to 1 of 6 categories (6 = professional; 1 = unskilled laborer) on the basis of the educational levels and income associated with that occupation in data from the New Zealand census.
Sleep Problems
Parents reported on their children’s sleep problems at the 5-, 7-, and 9-year assessments. At both 5 and 7 years, 3 items explored sleep problems (Sleep problems last night? Typically has sleep problems? Does child have sleep problems?). At the 9-year-old assessment, 6 items addressed sleep problems (Sleep problems last night? Sleeping difficulties? Child has trouble falling asleep? Child awakens at night and can’t return to sleep? Child slept much more recently? Child wakens very early?). Although these items were originally coded on different scales, each item was recoded on a binary scale (0 = no sign of a problem; 1 = sign of a problem). A binary “persistent sleep problem” variable was developed from these items, with children scoring 1 if they showed signs of a sleep problem at the age 9 assessment and at 1 or more other times, and 0 otherwise. One hundred seventeen (12.4%) of the study members had a persistent sleep problem. This general measure of persistent sleep problems has been used previously (see ref 24) and was developed based on previous research highlighting the importance of the persistence of sleep problems in predicting later behavioral or emotional problems,25 and taking into account the principle of aggregation, which states that multiple measures more accurately assess a phenotype than does a single measure.26
To attempt internal replications of our results, we also examined sleep problems at ages 5, 7, and 9 years separately. At each of these ages, Study members were given a score of 1 if they had been reported to have a sleep problem, and a score of 0 if they had not. At age 5, 199 (19.7%) of the sample had a sleep problem; at age 7, 182 (19.2%) had a sleep problem; and at 9 years old, 244 (25.8%) had a sleep problem. The higher prevalence of problems at 9 as compared with 5 and 7 years may reflect the different methods used to assess sleep at the different ages.
As there is disagreement as to the best way to conceptualize sleep problems in children, we repeated analyses using alternative definitions of sleep problems (unreported). First, we redefined persistent sleep problems as those occurring at any 2 or more assessments and obtained substantively identical results to those obtained when using our alternative definition of persistent sleep problems. Second, because of the specific nature of the item “sleep problems last night” we remade all scales removing this item from each wave of data collection. Again, an extremely similar pattern of results emerged.
Neuropsychological Assessment
The Dunedin Study neuropsychological test battery was administered when the cohort was 13 years of age. The battery comprised the subtests of the revised version of the Wechsler Intelligence Scale for Children (WISC-R)27 and 7 additional neuropsychological tests. It constituted the then-current battery of the Neuropsychological Clinic at the University of California, Los Angeles Neuropsychiatric Institute. All of its tests are still in widespread use, and extensive literatures exist attesting to the reliability and validity of each test.28 Because adult norms for the 7 neuropsychological tests begin at 12 years old, and the cohort was 13 years old, adult versions of each test were used. Results from the battery have been reported frequently.29,30 The 7 tests are as follows:
WISC-R Mazes27: The WISC-R mazes involved presenting respondents with a series of increasingly difficult mazes, and requesting that they draw the way out. Here, we focused on a scaled score based on performance time and errors.
Wisconsin Card Sorting Test31,32: The Wisconsin Card Sorting Test required the respondent to sort a series of cards showing different numbers of colored shapes into bins on the basis of 1 of 3 possible principles: color, shape, or number. After 10 consecutive correct placements, the examiner covertly shifted the principle. Scores indicated the respondent’s ability to flexibly shift cognitive set, while inhibiting inappropriate perseverative responses.
Halstead Trail Making Test, Adult Worksheets A and B33
The Halstead Trail Making Test first required the respondent to connect a series of numbered dots on the A worksheet (1, 2, 3, …, an automatic processing task). On the B worksheet the respondent must connect dots by alternating between consecutive numbers and letters (1, A, 2, B, 3, C, …, an effortful processing task). Scores for the respondent’s increased time and errors from worksheet A to worksheet B represented the ability to inhibit overlearned behavior.
Rey-Osterrieth Complex Figure Test34,35
The Rey-Osterrieth Complex Figure Test commenced by the respondent being asked to copy a complex figure. After a 3-minute delay, the respondent drew the figure again from free recall. Scores for strategy assessed whether the respondent applied thoughtful planning to the task. Scores for copy accuracy assessed visuo-spatial processing. Scores for delayed recall assessed visual memory.
Rey Auditory Verbal Learning Test36
The Rey Auditory Verbal Learning Test involved the respondent hearing 5 learning-trial presentations of a list of 15 words, saying all the words they can remember after each trial. An interference trial of a different word list was presented, followed by a sixth recall trial of the original word list. Delayed recall for the original list was tested after a 15-minute delay, and recognition memory was tested by presenting the words embedded in a story.
Verbal Fluency Controlled Oral Word Association Test37
During the Verbal Fluency Controlled Oral Word Association Test, the respondents were asked to generate as many words as possible beginning with F in 1 minute. The task was repeated for letters A and S.
Grooved Pegboard Test38
The Grooved Pegboard test involved the respondent, as quickly as possible, picking up tiny metal pegs 1 by 1, and fitting each peg into a slot in a small board. The respondent worked first with the nondominant hand, and then with the dominant hand. Scores reflected lateral difference in fine-motor hand-eye coordination, and also speed of dexterity.
Data Preparation and Statistical Analyses
Only Dunedin Study members with data on both sleep problems and neuropsychological functioning could be included in these analyses. To examine specific cognitive functions within the context of broadly normal IQ, we excluded Dunedin Study members with IQ scores of >2 SD below the mean (n = 22). One participant who had suffered a severe head injury was also excluded. This resulted in a final data set of 720 individuals. Test scores were standardized before analyses. Regression analyses were used to predict different aspects of neuropsychological functioning from sleep problems adjusting for gender and family SES. Although numerous regressions were run we did not adjust for multiple testing as such adjustments are conservative and arguably inappropriate for research at the theory-generating (rather than the theory-testing) stage such as that reported here.
RESULTS
Persistent Sleep Problems in Childhood and Neuropsychological Functioning at 13 Years
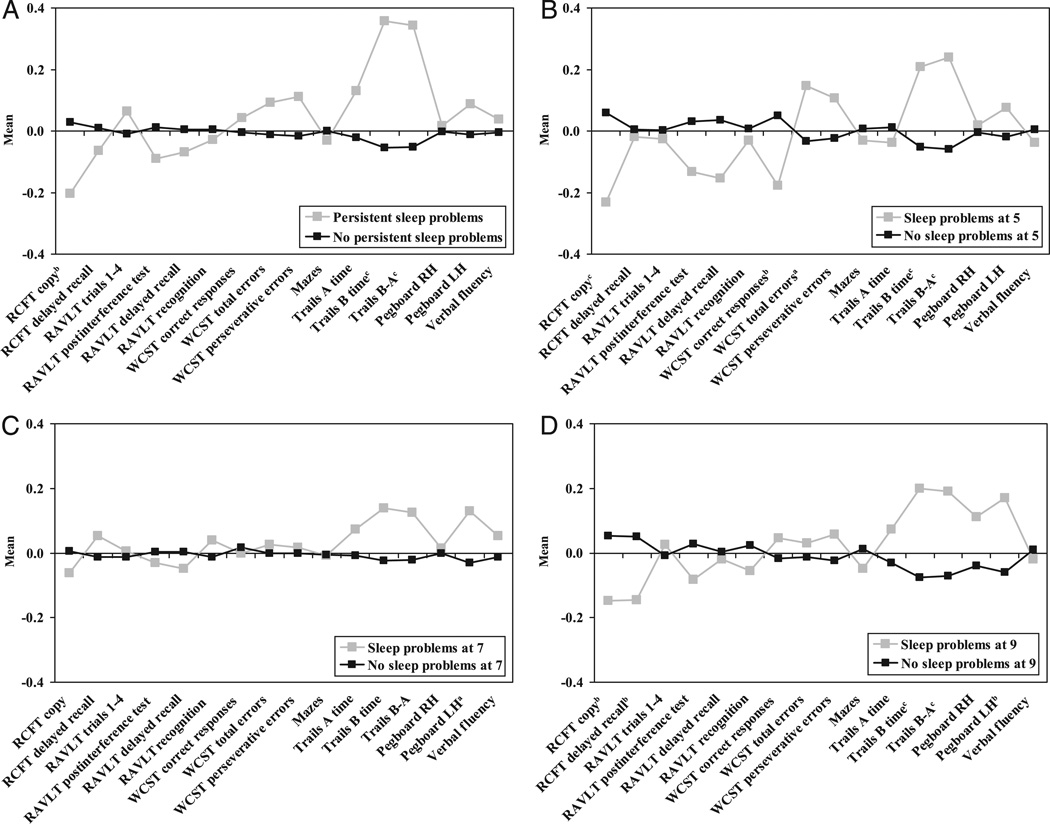
After controlling for gender and SES, persistent sleep problems at 9 years of age predicted significantly poorer scores on 3 measures of neuropsychological functioning: the Rey copy score (β = −.07 [95% confidence interval (CI): −.15 to .00], P = .047); the Trail Making test, part B score, time to completion (β = .14 [95% CI: .06 to .21], P < .001); and the Trail Making Test, part B score minus part A score (β = .13 [95% CI: .06 to .20], P = .001; see also Fig 1A).
FIGURE 1.
Sleep problems during childhood and neuropsychological functioning at 13 years. A, Persistent sleep problems at ages 5 to 9; B, sleep problems at age 5; C, sleep problems at age 7; D, sleep problems at age 9. a P < .10, b P < .05, and c P < .01 based on the statistical significance of sleep problems predicting neuropsychological functioning after controlling for gender and family SES.
Sleep Problems at 5, 7 and 9 and Neuropsychological Functioning at 13 Years
Sleep problems at separate ages of assessment were examined in association with later neuropsychological functioning. At 5 years of age, after controlling for gender and SES, sleep problems predicted poorer neuropsychological functioning indexed by: the Rey copy score (β = −.10 [95% CI: −.18 to −.03], P = .005); the number of correct responses on the Wisconsin Card Sorting Test (β = −.09 [95% CI: −.17 to −.02], P = .013); the Trail Making test, part B score, time to completion (β = .10 [95% CI: .02 to .17], P = .012); and the Trail Making Test, part B score minus part A score (β = .11 [95% CI: .04 to .18], P = .004; see Fig 1B). There was also a nonsignificant trend for sleep problems at 5 years of age to predict total error scores on the Wisconsin Card Sorting Test (β = .07 [95% CI: −.01–.14], P = .068). At the 7 year assessment, sleep problems did not significantly predict performance on any of the neuropsychological tasks (see Fig 1C), although there were trend differences in the same direction as at the other ages. After controlling for gender and SES, sleep problems at 9 years of age significantly predicted poorer neuropsychological functioning as indexed by: the Rey copy score (β = −.08 [95% CI: −.16 to −.01], P = .025); the Rey delayed recall score (β = −.08 [95% CI: −.16 to −.01], P = .023); the Trail Making test, part B score, time to completion (β = .12 [95% CI: .05–.19], P = .001); the Trail Making Test, part B score minus part A score (β = .11 [95% CI: .04 –.18], P = .003); and number of seconds to complete the Grooved Pegboard using the left hand (β = .09 [95% CI: .02–.17], P = .01, the effect persisted after exclusion of left-handers; Fig 1D). Of note, all of the significant differences between those with and without sleep difficulties were of small effect (β in the .1 range).
DISCUSSION
Summary
The results reported here are novel in demonstrating a prospective longitudinal association (albeit of small effect) between measures of sleep problems during childhood and neuropsychological functioning in adolescence. The results also emphasize the possibility that sleep difficulties are associated more strongly with certain aspects of neuropsychological functioning (eg, complex visual scanning and motor competence as measured by the Halstead Trail Making Test) than others (eg, verbal fluency).
Limitations
Two main limitations characterize this work. First, sleep problems were assessed using available parent-report items. Use of this measure was necessary as, with most other epidemiologic studies, the Dunedin study did not include a detailed measure of sleep problems during childhood. This limitation is noteworthy for numerous reasons. For example, results may differ as a function of rater21; and the inclusion of general items to measure sleep means that different sleep problems (eg, nightmares, sleep walking, insomnia) may have been reported. In addition, our crude (but necessary) method of classifying sleep problems as present versus absent will not have captured differences in the severity of difficulties being reported. In future studies, it would be beneficial to examine sleep more thoroughly (eg, using multiple raters and measures and assessing participants with difficulties in the full range as well as at the high extreme). Indeed, it would have been particularly informative to have included a measure of sleep disordered breathing, given the focus on this specific problem in association with neuropsychological functioning reported previously.6 A second limitation of this study is that we did not measure neuropsychological functioning in childhood or sleep problems in adolescence, meaning that hypotheses concerning the direction of effects between the links between sleep problems and neuropsychological functioning could not be tested.
Interpretation
Despite limitations, confidence in the results comes from noting strengths of this study, including the use of an entire birth cohort, the collection of sleep data at multiple time-points, and the thorough assessment of neuropsychological functioning. In particular, 3 points emerging from the results are worthy of discussion. First, an inclusive measure of sleep problems during childhood predicted later neuropsychological functioning. A possible explanation for the longitudinal associations reported here is that they simply reflect the known stability of sleep problems over time (eg, see ref 39) and the known concurrent associations between sleep difficulties and neuropsychological functioning.17 An alternative explanation is that sleep problems cause neuropsychological difficulties. This is consistent with a leading hypothesis for the link between sleep disordered breathing and neuropsychological functioning which suggests that sleep disruption could retard neural and skill development.6 Although previous studies have demonstrated that sleep problems are more likely to be found in children with severe intellectual difficulties as compared with others (eg, see ref 40), it is important to note that this study excluded study members with particularly low IQ, suggesting that links between sleep and neuropsychological functioning may hold within the context of normal functioning. The final explanation to be considered for the link between sleep and neuropsychological functioning is that certain factors which are not addressed here (eg, family disorganization, emotional difficulties) are linked to both sleep problems and later neuropsychological functioning and account for their association. Assessing both sleep and neuropsychological functioning at multiple time-points, as well as potentially co-occurring factors, would allow testing of hypotheses concerning the mechanisms by which sleep problems are associated with later neuropsychological functioning.
The second point of note concerns the assessment of sleep problems at multiple time-points. Sleep problems at 5, and also at 9 years of age predicted later neuropsychological functioning, increasing confidence in the results by providing an internal replication. What was more surprising is that the 7 year sleep data stand out for not being associated with any measure of later neuropsychological functioning. This finding could reflect the possibility that sleep problems are more or less significant at certain ages. For example, 7 years is the age at which many children transfer to new schools, and sleep problems in children of this age could therefore be associated with related changes (eg, changes in school start times). It is possible to speculate tentatively that such problems could be temporary and not associated with later difficulties. However, it is important to note that age differences were unexpected and therefore need to be replicated before they can be considered of importance.
Third, sleep problems seemed to be associated more strongly with certain aspects of neuropsychological functioning than with others. As demonstrated by a previous study focusing on sleep disordered breathing, there was an association between sleep difficulties and performance on the Rey Copy task.12 Sleep problems also seemed to be robustly associated with performance on the trail making test. This latter association seemed to be relatively specific in that sleep problems were associated with performance on trial B (an effortful processing task) as compared with trial A (an automatic processing task). This suggests that sleep problems may be related to the higher-level demands on working memory and mental flexibility imposed by trial B beyond the simpler demands on attention and vigilance imposed by trial A. This interpretation is consistent with suggestions by others that prefrontal cortical functioning is particularly sensitive to the effects of sleep deprivation (eg, see ref 41). In contrast to the associations reported, there did not seem to be much support for the associations between sleep problems and performance on other tasks such as the Rey auditory and mazes tasks. Some of the tasks included in the neuropsychological test battery are used to examine specific brain impairment, so given that we were not expecting specific brain damage in the Dunedin Study members, this pattern of results fits well with expectations.
Implications
The key findings reported here have implications for future research and clinical practice. With regards to future work, the results of this study concur with previous reports (eg, see ref 17) suggesting that when examining the links between sleep and neuropsychological functioning there may be scope to examine a wider range of sleep difficulties than just sleep disordered breathing (although of course it remains possible that the associations reported here could be driven by study members with sleep disordered breathing). With regards to clinical practice, this study adds to the previous body of literature suggesting that childhood sleep problems are not only associated with concurrent problems but with a range of difficulties later in life. Indeed, using data from the Dunedin Study we previously reported a link between the persistent measure of sleep reported here and anxiety disorders in adulthood.24 These findings lend support to the suggestion that sleep problems should be routinely assessed in children. Once identified, sleep problems should be addressed— as indicated by studies highlighting improved grades after treatment for obstructive sleep apnea syndrome23 and improved neurobehavioural functioning after sleep extension.42 Of note, it may be important to deal with sleep problems rapidly; 1 report presented results consistent with the idea that some of the effects of sleep problems on later neuropsychological functioning may be irreversible.23 Although the aforementioned report focused on sleep disordered breathing and proposed hypoxia as a potential cause of the irreversible neuropsychological impairment, other explanations remain possible. Increased understanding of and investment in childhood sleep problems seems to be an important way forward.
What’s Known on This Subject
There is a small body of literature to suggest that sleep and neuropsychological functioning are associated in childhood or adolescence. Most data informing this issue are cross-sectional, and there is a paucity of data from prospective longitudinal studies.
What This Study Adds
This study is, to our knowledge, the first to demonstrate that sleep problems assessed in childhood prospectively predict neuropsychological functioning during adolescence.
ACKNOWLEDGMENTS
The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research was supported by US National Institute of Mental Health grant MH 9414, MH 5070 and United Kingdom Medical Research Council grant G0100527. Dr Gregory was supported by a Leverhulme research fellowship. Drs Caspi and Moffitt are Royal Society Wolfson research merit award holders.
We thank the Dunedin Study members, unit research staff, Alan Taylor, HonaLee Harrington, and Phil Silva.
Abbreviations
- SES
socioeconomic status
- WISC-R
Wechsler Intelligence Scale for Children
- CI
confidence interval
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci. 2006;9(4):388–399. doi: 10.1111/j.1467-7687.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 2.Anders TF, Eiben LA. Pediatric sleep disorders: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(1):9–20. doi: 10.1097/00004583-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carskadon MA, Harvey K, Dement WC. Sleep loss in young adolescents. Sleep. 1981;4(3):299–312. doi: 10.1093/sleep/4.3.299. [DOI] [PubMed] [Google Scholar]
- 5.Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10–14. Sleep. 1998;21(8):861–868. [PubMed] [Google Scholar]
- 6.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115–1134. doi: 10.1093/sleep/29.9.1115. [DOI] [PubMed] [Google Scholar]
- 7.Beebe DW, Wells CT, Jeffries J, Chini B, Kalra M, Amin R. Neuropsychological effects of pediatric obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10(7):962–975. doi: 10.1017/s135561770410708x. [DOI] [PubMed] [Google Scholar]
- 8.Emancipator JL, Storfer-Isser A, Taylor HG, et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med. 2006;160(2):203–210. doi: 10.1001/archpedi.160.2.203. [DOI] [PubMed] [Google Scholar]
- 9.Lewin DS, Rosen RC, England SJ, Dahl RE. Preliminary evidence of behavioral and cognitive sequelae of obstructive sleep apnea in children. Sleep Med. 2002;3(1):5–13. doi: 10.1016/s1389-9457(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44–49. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Blunden S, Lushington K, Lorenzen B, Martin J, Kennedy D. Neuropsychological and psychosocial function in children with a history of snoring or behavioral sleep problems. J Pediatr. 2005;146(6):780–786. doi: 10.1016/j.jpeds.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Kurnatowski P, Putynski L, Lapienis M, Kowalska B. Neurocognitive abilities in children with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2006;70(3):419–424. doi: 10.1016/j.ijporl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes SK, Shimoda KC, Waid LR, et al. Neurocognitive deficits in morbidly obese children with obstructive sleep-apnea. J Pediatr. 1995;127(5):741–744. doi: 10.1016/s0022-3476(95)70164-8. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145(4):458–464. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Hill CM, Hogan AM, Karmiloff-Smith A. To sleep, perchance to enrich learning? Arch Dis Child. 2007;92:637–643. doi: 10.1136/adc.2006.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73(2):405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 17.Blunden SL, Beebe DW. The contribution of intermittent hypoxia, sleep debt and sleep disruption to daytime performance deficits in children: consideration of respiratory and nonrespiratory sleep disorders. Sleep Med Rev. 2006;10(2):109–118. doi: 10.1016/j.smrv.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Blunden SL, Chervin RD. Sleep problems are associated with poor outcomes in remedial teaching programmes: a preliminary study. J Paediatr Child Health. 2008;44(5):237–242. doi: 10.1111/j.1440-1754.2007.01237.x. [DOI] [PubMed] [Google Scholar]
- 19.Kahn A, Van de Merckt C, Rebuffat E, et al. Sleep problems in healthy preadolescents. Pediatrics. 1989;84(3):542–546. [PubMed] [Google Scholar]
- 20.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 21.Gregory AM, Rijsdijk FV, Eley TC. A twin-study of sleep difficulties in school-aged children. Child Dev. 2006;77(6):1668–1679. doi: 10.1111/j.1467-8624.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 22.Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. J Dev Behav Pediatr. 2000;21(1):27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Gozal D, Pope DW. Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107(6):1394–1399. doi: 10.1542/peds.107.6.1394. [DOI] [PubMed] [Google Scholar]
- 24.Gregory AM, Caspi A, Eley TC, Moffitt TE, O’Connor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- 25.Ford DE, Kamerow DB. Epidemiologic-study of sleep disturbances and psychiatric-disorders: an opportunity for prevention. JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 26.Rushton JP, Brainerd CJ, Pressley M. Behavioral development and construct validity: the principle of aggregation. Psychol Bull. 1983;94(1):18–38. [Google Scholar]
- 27.Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Revised. New York, NY: Psychological Corporation; 1974. Revised ed. [Google Scholar]
- 28.Lezak MD, Howieson DB, Loring DW, editors. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 29.Cannon M, Moffitt TE, Caspi A, Murray RM, Harrington H, Poulton R. Neuropsychological performance at the age of 13 years and adult schizophreniform disorder: prospective birth cohort study. B J Psychiatry. 2006;189(5):463–464. doi: 10.1192/bjp.bp.105.020552. [DOI] [PubMed] [Google Scholar]
- 30.Frost LA, Moffitt TE, McGee R. Neuropsychological correlates of psychopathology in an unselected cohort of young adolescents. J Abnorm Psychol. 1989;98(3):307–313. doi: 10.1037//0021-843x.98.3.307. [DOI] [PubMed] [Google Scholar]
- 31.Berg EA. A simple objective treatment for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 32.Heaton RK. Wisconsin Card Sorting Test (WCST) Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- 33.Reitan RM, Davison LA. Clinical Neuropsychology: Current Status and Applications. New York, NY: Winston/Wiley; 1974. [Google Scholar]
- 34.Osterrieth PA. The test of copying a complex figure [in French] Arch Psychol. 1944;30:206–356. [Google Scholar]
- 35.Waber DP, Holmes JM. Assessing children’s copy production of the Rey-Osterrieth complex figure. J Clin Exp Neuropsychol. 1985;7(3):264–280. doi: 10.1080/01688638508401259. [DOI] [PubMed] [Google Scholar]
- 36.Rey A. The Clinical Examination in Psychology [in French] Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- 37.Benton AL, Hamsher Kd. Multilingual Aphasia Examination. 3rd ed. Iowa City, Iowa: AJA Associates; 1989. [Google Scholar]
- 38.Bornstein RA. Consistency of intermanual discrepancies in normal and unilateral brain lesion patients. J Consult Clin Psychol. 1986;54(5):719–723. doi: 10.1037//0022-006x.54.5.719. [DOI] [PubMed] [Google Scholar]
- 39.Gregory AM, O’Connor TG. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry. 2002;41(8):964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Wiggs L, France K. Behavioural treatments for sleep problems in children and adolescents with physical illness, psychological problems or intellectual disabilities. Sleep Med Rev. 2000;4(3):299–314. doi: 10.1053/smrv.1999.0094. [DOI] [PubMed] [Google Scholar]
- 41.Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults: a model for healthy aging? Sleep. 2000;23(8):1067–1073. [PubMed] [Google Scholar]
- 42.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74(2):444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]