Abstract
Systemic sclerosis (SSc) is an autoimmune inflammatory disease with unknown etiology characterized by microvascular injury and fibrosis of the skin and internal organs. A growing body of evidence suggests that deficiency of the transcription factor Fli1 (Friend leukemia integration-1) has a pivotal role in the pathogenesis of SSc. Fli1 is expressed in fibroblasts, endothelial cells, and immune cells, and has important roles in the activation, differentiation, development, and survival of these cells. Previous studies demonstrated that Fli1 is downregulated in SSc fibroblasts by an epigenetic mechanism and a series of experiments with Fli1-deficient animal models revealed that Fli1 deficiency in fibroblasts and endothelial cells reproduces the histopathologic features of fibrosis and vasculopathy in SSc, respectively. In this article, we review the impact of Fli1 deficiency on the pathogenesis of SSc and discuss a new therapeutic strategy for SSc by targeting the transcription factor Fli1.
Keywords: Systemic sclerosis, Fli1, Fibroblasts, Endothelial cells, Immune cells, Imatinib
1. Introduction
Systemic sclerosis (SSc) or scleroderma is an autoimmune inflammatory disease characterized by fibrosis of the skin and internal organs as well as microvessel injury. Although the pathogenesis of SSc is not yet fully elucidated, recent studies suggest that the transcription factor Fli1 (Friend leukemia integration-1) has a pivotal role in the development of skin fibrosis and vasculopathy in SSc [1–8]. In this article, we review the role of Fli1 in the pathogenesis of SSc and discuss a new therapeutic strategy for SSc by targeting this transcription factor.
2. The characteristics of transcription factor Fli1
Fli1 is a member of the Ets (E26 transformation-specific) transcription factor family characterized by a winged helix-turn-helix DNA binding domain that is responsible for nuclear targeting and specific binding to a DNA element containing a purine-rich GGAA/T core sequence [9,10]. Fli1 was initially identified as a proto-oncogene in Friend virus-induced erythroleukemia in mice [11]. In humans, the Fli1 gene is rearranged in 90% of Ewing sarcomas, a pediatric tumor with neuroectodermal origin [12,13]. Thus, dysregulation of the Fli1 gene is involved in the progression of at least two distinct tumors. A comparative gene expression profile analysis of various human cell lines revealed that Fli1 is expressed at high levels in both endothelial and hematopoietic cells under physiologic conditions [9]. Fli1 is also expressed in dermal fibroblasts, although the levels are relatively low compared with those in endothelial cells and hematopoietic cells [5,14]. Thus, Fli1 is expressed both in endothelial cells and fibroblasts, two cells types that have important roles in the pathogenesis of SSc. Importantly, a growing body of evidence from our laboratory and others links the aberrant expression of Fli1 to the pathogenesis of skin fibrosis and microvessel injury in SSc [1–5,8,14].
3. The role of Fli1 in fibrosis
3.1. Fli1 regulates the expression of the type I collagen gene in dermal fibroblasts
Type I collagen is the most abundant component of extracellular matrix (ECM) proteins in the dermis, and SSc dermal fibrosis is characterized by excessive deposition of fibrillar collagen type I [5,15–32]. Type I collagen comprises two pro-α1(I) chains that are produced from the COL1A1 gene and one pro-α2(I) encoded by the α2(I) collagen (COL1A2) gene. The role of Fli1 in transcriptional regulation of the human COL1A2 gene in human dermal fibroblasts is well-studied. Fli1 binds to the GGAT motif at the −285 to −282 bp region of the COL1A2 promoter where it interacts with Sp1, suppressing the transcriptional activity of the COL1A2 gene [14]. Stable transfection of Fli1 into dermal fibroblasts markedly decreases the production of type I collagen protein [14], while gene silencing of Fli1 using small interference RNA (siRNA) results in a robust increase of type I collagen protein [33]. Furthermore, the expression levels of type I collagen protein are inversely correlated with those of Fli1 protein in mouse embryonic fibroblasts derived from wild-type mice, Fli1 heterozygous mice, and Fli1 null mice [5]. Together, these results indicate that Fli1 is a potent suppressor of the type I collagen gene.
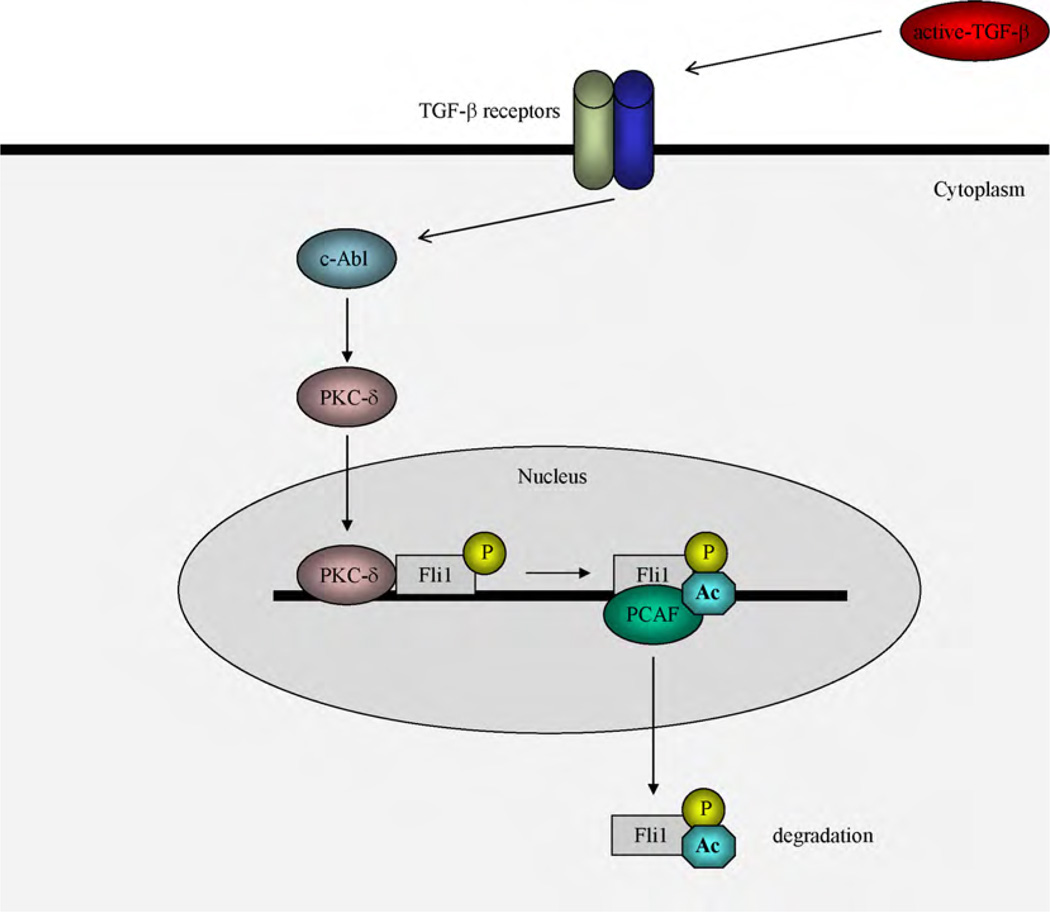
The transcriptional activity of Fli1 is tightly regulated by post-translational modification, such as phosphorylation and acetylation [1,4]. This signaling cascade is well studied in human dermal fibroblasts stimulated with transforming growth factor (TGF)-β (Fig. 1). Upon TGF-β stimulation, protein kinase C (PKC)-δ is activated and subsequently translocates into the nucleus. Nuclear PKC-δ directly interacts with Fli1 at the COL1A2 promoter, phosphorylating Fli1 at the threonine 312 residue, which is located in the consensus phosphorylation motif of PKC (KXT motif). Phosphorylation at threonine 312 of Fli1 increases its affinity for p300/CREB-binding protein-associated factor (PCAF), which has histone acetyltransferase activity, leading to acetylation of Fli1 at lysine 380 [4]. Upon acetylation, Fli1 is released from the COL1A2 promoter, probably due to a conformational change [2]. Once dissociated from the COL1A2 promoter, Fli1 is rapidly degraded through a proteasomal pathway (Asano et al. unpublished data). As a result, Fli1 no longer inhibits the promoter activity of the COL1A2 gene. Thus, TGF-β regulates the transcriptional activity of Fli1 through a “phosphorylation-acetylation cascade”.
Fig. 1.
Schematic model of the TGF-β-induced post-translational modifications of Fli1. In quiescent fibroblasts, collagen gene expression is repressed by Fli1 occupancy of the collagen promoter. In response to TGF-β stimulation, PKC-δ is translocated into the nucleus and recruited to the COL1A2 promoter leading to phosphorylation of Fli1 at threonine 312. Phosphorylation of Fli1 is required for the subsequent PCAF-mediated acetylation of Fli1 at lysine 380. Acetylated Fli1 dissociates from the collagen promoter and is rapidly degraded through the proteasomal pathway, thereby enhancing collagen gene transcription.
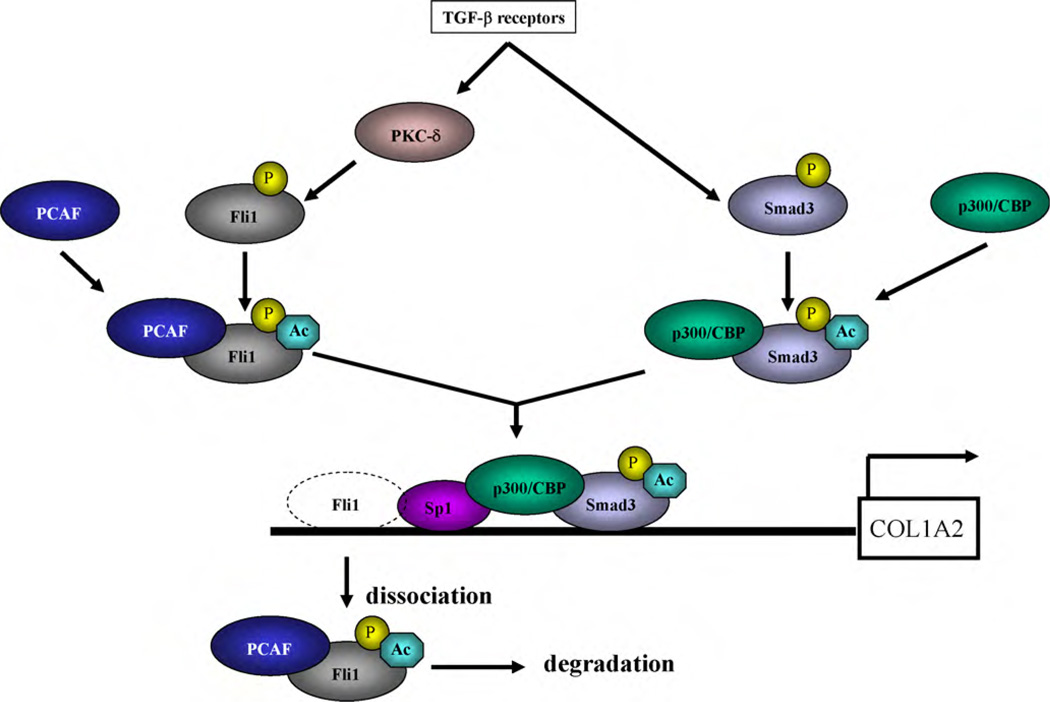
A similar phosphorylation–acetylation cascade regulates the transcriptional activity of Smads, the principal intracellular second messengers in the TGF-β signaling pathway. Upon TGF-β stimulation, Smad2/3 are phosphorylated by a serine/threonine kinase of the TGF-β type I receptor and then translocate into the nucleus, where p300/CREB-binding protein and/or PCAF acetylate Smad2/3 and increase their DNA binding affinity [34–36]. Thus, the phosphorylation–acetylation cascade is the critical post-translational modification whereby TGF-β coordinately regulates the DNA binding activity of transcriptional activators such as Smad3 and repressors such as Fli1, resulting in increased extracellular matrix production (Fig. 2).
Fig. 2.
TGF-β coordinately regulates DNA binding activity of the transcriptional activator “Smad3” and the repressor “Fli1”. Upon TGF-β stimulation, Fli1 loses the transcriptional inhibitory effect on the COL1A2 promoter through a phosphorylation-acetylation cascade, as described in Fig. 1. In contrast, in response to TGF-β stimulation, Smad3 is phosphorylated by the TGF-β type I receptor and translocated into the nucleus, where p300/CREB-binding protein acetylates Smad3. Acetylation of Smad3 increases its DNA binding ability for the COL1A2 promoter. Thus, TGF-β coordinately regulates DNA binding activity of transcriptional activator “Smad3” and the repressor “Fli1”, leading to increased extracellular matrix production.
In addition to regulating COL1A2 gene expression, Fli1 also functions as a transcriptional repressor of the COL1A1 gene. Rottlerin, a specific PKC-δ inhibitor, strongly suppresses COL1A1 gene expression at the transcriptional level and the corresponding responsive element is located at the 129-bp segment, encompassing nucleotides −675 to −804 of the COL1A1 gene promoter [37]. This region contains a putative Ets transcription factor binding site at nucleotides −709 to −712 and Fli1 occupies this site in quiescent dermal fibroblasts [4]. Furthermore, inhibition of endogenous PKCδ enhances Fli1 binding to the COL1A1 promoter [4]. These data indicate that PKC-δ-dependent phosphorylation of Fli1 coordinately regulates COL1A1 and COL1A2 expression in dermal fibroblasts.
3.2. Impact of Fli1 on the expression of genes related to ECM remodeling
Connective tissue growth factor (CTGF)/CCN2 is a member of the CCN family of the multifunctional matricellular factors, which may operate in tandem with or downstream of the TGF-β or endothelin-1 fibrotic pathways [38,39]. Whereas TGF-β or CCN2 alone produce only a transient fibrotic response in animal models, these cytokines act together to promote sustained fibrosis [40]. CCN2 is expressed at low levels in dermal fibroblasts under physiologic conditions [39], but it is constitutively overexpressed in SSc fibroblasts [41,42]. This may be at least in part due to the autocrine activation of TGF-β signaling in SSc fibroblasts, as TGF-β is the most potent inducer of CCN2 identified so far. Given that CCN2 promotes ECM deposition and fibroblast adhesion and proliferation, its overexpression in fibrotic lesions is likely to contribute to the development of the SSc phenotype. Interestingly, apart from being a negative regulator of type I collagen gene expression, Fli1 is also involved in the transcriptional regulation of the CCN2 gene. A series of reporter analyses with Fli1 siRNA or forced expression of Fli1 demonstrated that Fli1 is a potent suppressor of CCN2 gene [33]. Therefore, Fli1 downregulation may contribute to the mechanism responsible for the TGF-β-dependent upregulation of CCN2. Fli1 might affect fibrosis by regulating the expression of a key enzyme responsible for degradation of type I collagen protein, matrix metalloproteinase-1 (MMP-1). Thus, treatment with Fli1 siRNA markedly decreases mRNA and protein levels of MMP-1 in normal dermal fibroblasts [33]. Interestingly, however, ectopic expression of Fli1 also decreases MMP-1 gene expression at the transcriptional level [29]. The detailed mechanism responsible for the Fli1-dependent regulation of MMP-1 gene expression requires further investigation. Further supporting its role as an antifibrotic molecule, depletion of Fli1 results in the upregulation of α-smooth muscle actin (α-SMA) mRNA, to a greater extent than the levels obtained after exogenous TGF-β stimulation [33]. Therefore, TGF-β induced downregulation of Fli1 may, at least in part, be responsible for the myofibroblastic differentiation of dermal fibroblasts during wound healing and in certain pathologic conditions. Collectively, these in vitro findings indicate that Fli1 coordinately regulates the fibrotic process through the upregulation of type I collagen, CCN2, and α-SMA, and potentially the downregulation of MMP-1.
3.3. Fli1 deficiency in SSc fibroblasts
The SSc fibroblast phenotype shares many similarities with TGF-β stimulated fibroblasts, and because Fli1 downregulation also mimics TGF-β-induced profibrotic gene program, it is likely that Fli1 deficiency contributes to the activation of SSc fibroblasts. In support of this hypothesis, Fli1 protein levels are markedly decreased in lesional SSc fibroblasts compared with dermal fibroblasts of healthy control skin in vivo [5]. Furthermore, in the lesional skin of SSc patients, Fli1 protein expression levels are inversely correlated with the mRNA levels of the COL1A2 gene [5]. Moreover, cultured SSc fibroblasts derived from lesional skin produce excessive amounts of type I collagen when compared to normal fibroblasts derived from healthy control skin, while Fli1 protein levels are consistently decreased [5,29]. Together, these observations support the notion that Fli1 deficiency contributes to the constitutive activation of SSc fibroblasts.
3.4. The mechanisms of Fli1 downregulation in SSc fibroblasts
Although it is well established that decreased levels of Fli1 contribute to the SSc phenotype, the mechanisms of Fli1 downregulation are not entirely clear. Our recent findings that Fli1 is phosphorylated and targeted for degradation as a result of activation of TGF-β signaling raise the possibility that Fli1 is downregulated in SSc fibroblasts by post-translational mechanisms [1,4]. On the other hand, Wang et al. [8] reported that Fli1 mRNA levels are decreased in cultured SSc fibroblasts and further verified that the acetylation levels of histone H3 and H4 are decreased in the promoter region of the Fli1 gene in SSc fibroblasts, while the methylation levels of CpG islands in the promoter region of the Fli1 gene are markedly increased in SSc fibroblasts. These results suggest that Fli1 gene expression is suppressed at the transcriptional level by an epigenetic mechanism in SSc fibroblasts. Epigenetic regulation of Fli1 gene expression can explain why SSc fibroblasts maintain their profibrogenic phenotype in an in vitro culture system, where they are totally free from inflammation, autoimmune attack, and vascular damage. Given that elevated levels of TGF-β could only be detected in the early stages of SSc, Fli1 may be downregulated at both the transcriptional and post-translational levels in the early stage, but at only the transcriptional level at later stages.
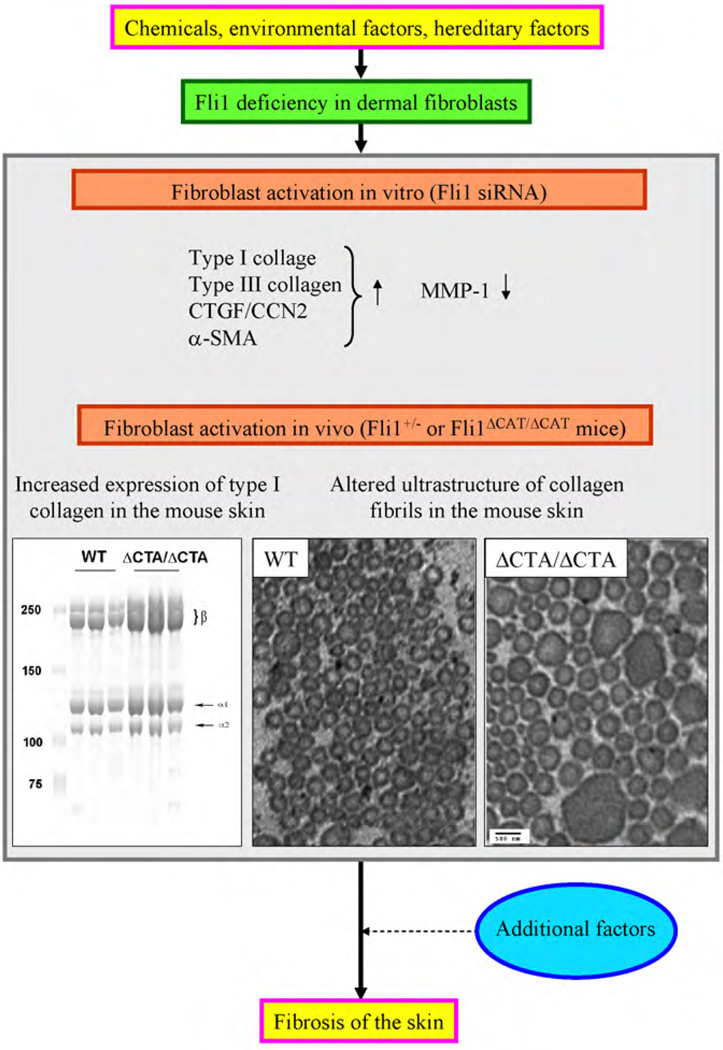
3.5. Fibroblast activation in Fli1+/− mice and Fli1ΔCTA/ΔCTA mice
The early lethality of Fli1 null mice limits functional studies in vivo, but heterozygous Fli1+/− mice develop normally and do not show obvious phenotypic alterations [43,44]. To investigate whether an Fli1 deficiency contributes to the fibroblast activation in vivo, mice with targeted deletion of the Fli1 carboxy terminal activation (CTA) domain (Fli1ΔCTA) were generated and used to further study the role of Fli1 in maintaining skin homeostasis [2]. Skin analyses of the Fli1ΔCTA/ΔCTA mice by quantitative real time-PCR, acetic acid extraction, and hydroxyproline assays revealed a significant upregulation of fibrillar collagen genes at the mRNA levels, as well as an increased collagen content measured by acetic acid extraction and hydroproline assays. In addition, collagen fibrils contained ultrastructural abnormalities including immature thin fibrils and very thick irregularly-shaped fibrils, which are quite similar to the ultrastructural features of collagen fibers in the skin of SSc patients [45–48]. Clinically and histopathologically, however, Fli1ΔCTA/ΔCTA mice do not develop skin fibrosis. These results suggest that although Fli1 has an important role in fibroblast activation, additional factors are required for the development of skin fibrosis in this animal model and in human disease (Fig. 3). This notion is consistent with the conventional view of the pathogenesis of SSc that environmental and genetic factors interact in the development of SSc. Fli1 deficiency may represent one of the genetic alterations in SSc, which can be caused by a polymorphism in the Fli1 regulatory pathway or induced postnatally by the exposure to chemicals or other environmental factors [8].
Fig. 3.
The impact of Fli1 deficiency on dermal fibrosis in SSc. Fli1 deficiency due to an epigenetic mechanism in dermal fibroblasts results in the upregulation of type I collagen, CTGF/CCN2, and α-SMA, and the downregulation of MMP-1. Fli1 deficiency activates dermal fibroblasts in vivo in two distinct animal models, where the collagen fibers have an altered ultrastructure similar to that in SSc. To develop dermal fibrosis characteristic of SSc, some additional factors may be required.
4. Vasculopathy
4.1. The impact of Fli1 on endothelial integrity
The first reports describing the impact of Fli1 on endothelial integrity were published by Hart et al. [43] and Spyropoulos et al. [44] in 2000. These authors reported that Fli1 null mice die at E11.5 during embryogenesis due to cranial and spinal hemorrhage. Embryonic day (E)11.5 is the time point when development of the vasculature shifts from “vasculogenesis”, a de novo synthesis of the vascular network from hemangioblasts, to “angiogenesis”, the progression of new vessels from pre-existing vessels, thus implicating Fli1 in angiogenesis. Consistently, recent studies of zebrafish and Xenopus embryos demonstrated that Fli1 functions as a master regulator of the transcriptional network driving blood and endothelial cell lineages [49]. In humans, Fli1 is expressed in healthy skin microvasculature [9], but its presence is greatly reduced in endothelial and peri-endothelial cells in SSc skin [5]. Together, these observations suggest that vasculopathy in SSc is caused by aberrant angiogenesis due to Fli1 deficiency.
4.2. Vascular phenotype of endothelial cell-specific Fli1 knockout mice
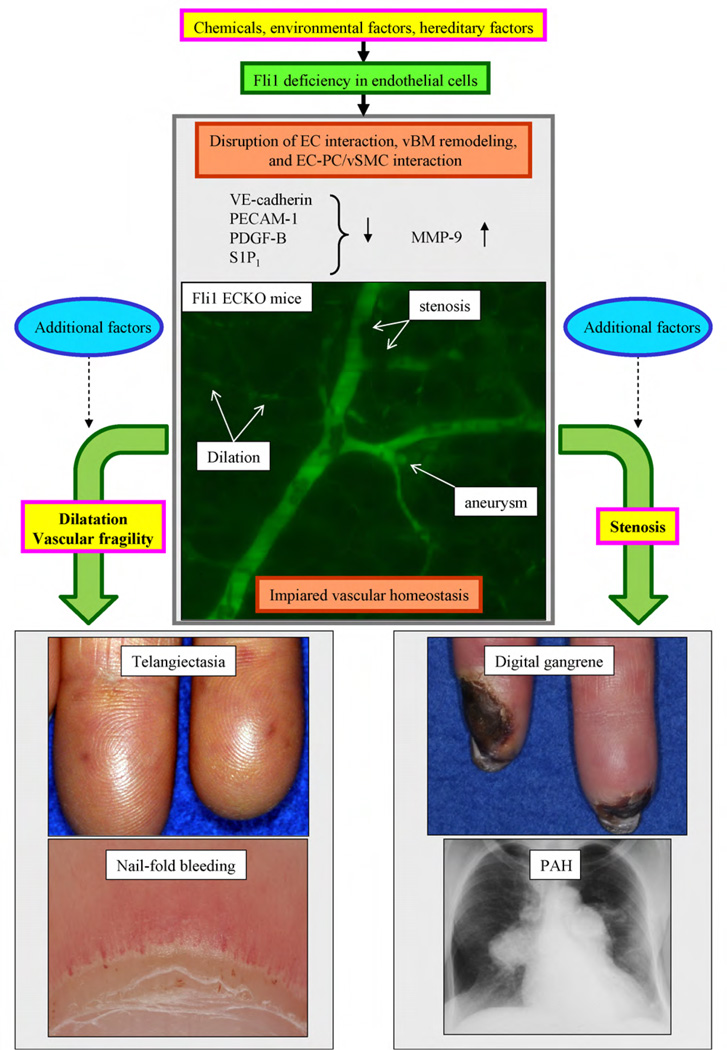
The role of Fli1 in endothelial cells was evaluated in endothelial cell-specific conditional Fli1 knockout mice (Fli1 ECKO mice) generated by crossing Fli1flox/flox mice with Tie2-Cre transgenic mice [3]. The vascular network structure in the skin is ill-organized in Fli1 ECKO mice. Importantly, arteriole stenosis, micro-aneurysm formation, and capillary dilation are prominent features in Fli1 ECKO mice (Fig. 4), whereas these features are absent in wild-type mice. In addition, vascular permeability is dramatically increased and vessels are quite fragile in Fli1 ECKO mice. The altered vascular phenotype in Fli1 ECKO mice is likely due to the decreased expression of vascular endothelial (VE)-cadherin, platelet/endothelial cell adhesion molecule (PECAM)-1, platelet derived growth factor (PDGF)-B, and sphingosin 1 phosphate (S1P)1 receptors, and the increased expression of MMP-9 in endothelial cells. The absence of VE-cadherin and PECAM-1 may result in a weak endothelial cell-cell interaction, leading to capillary dilation, vascular fragility, and increased vascular permeability. Increased MMP-9 expression may lead to alterations in vascular basement remodeling, such as the loss of type IV collagen and a compensatory increase of proteoglycans. The altered phenotype of pericytes characterized by decreased α-SMA expression in Fli1 ECKO mice might in part be caused by the alterations of the endothelial cells, including the downregulation of VE-cadherin, PDGF-B, and S1P1 receptors (Fig. 4). Previous in vivo and in vitro studies demonstrated that pericyte loss induces the dilation of small vessels, micro-aneurysm formation, and apoptosis or proliferation of endothelial cells [50]. As these vascular features are observed in Fli1 ECKO mice, pericyte loss might be the main determinant of the vascular phenotype in Fli1 ECKO mice.
Fig. 4.
The impact of Fli1 deficiency on SSc vasculopathy. Fli1 deficiency due to an epigenetic mechanism in endothelial cells results in the downregulation of VE-cadherin, PECAM-1, PDGF-B, and S1P1, and the upregulation of MMP-9. Endothelial cell-specific Fli1 knockout mice develop capillary dilation, vascular fragility, and arteriole stenosis, which are the histopathologic features of SSc vasculopathy. To develop the clinical symptoms of SSc vasculopathy, some additional factors may be required.
4.3. Vascular phenotype of Fli1 ECKO mice recapitulates that of SSc patients
The vascular phenotype of SSc patients is quite similar to that of Fli1 ECKO mice [3]. Cell surface molecules regulating endothelial cell interaction, such as VE-cadherin and PECAM-1, are markedly decreased in endothelial cells of the small dermal vessels of SSc patients compared with those of closely matched healthy controls. The type IV collagen levels are also decreased in the vascular basement membranes of the small dermal vessels of SSc patients, which might be due to the increased production of MMP-9 by SSc endothelial cells because Fli1 does not influence the type IV collagen mRNA levels in cultured human and murine dermal microvascular endothelial cells. Consistent with this notion, serum MMP-9, but not MMP-2, levels are increased in SSc patients compared with healthy controls [51]. The pericyte coverage of small dermal vessels, as evaluated by immunostaining for α-SMA, is uniformly decreased in SSc skin, especially the capillaries, compared with healthy control skins. Thus, Fli1 ECKO mice recapitulate the vascular phenotype of SSc patients, supporting the involvement of endothelial Fli1 deficiency in the pathogenesis of SSc vasculopathy.
4.4. The impact of Fli1 deficiency on the development of clinical symptoms of SSc vasculopathy
As summarized above, an endothelial Fli1 deficiency reproduces the histopathologic features of SSc vasculopathy [3]. Thus, some of the clinical symptoms in SSc patients might be explained by an endothelial deficiency of Fli1. For example, the absence of Fli1 might increase vascular permeability leading to the swelling of fingers and extremities in the early stage of SSc. Other consequences of reduced levels of Fli1, such as capillary dilation, may be manifested by the abnormality of nailfold capillaries and telangiectasias, whereas blood vessel fragility may be related to nailfold bleeding. Stenosis of the arterioles due to endothelial proliferation may lead to the development of digital gangrene and pulmonary arterial hypertension (PAH) in SSc. Importantly, imaging studies, such as magnetic resonance angiography, of hands of patients with SSc often reveal stenosis of the arterioles and a lack of blood flow at the distal areas of the fingers, suggesting proliferation and apoptosis of endothelial cells due to pericyte loss [52,53]. It is important to note, however, that Fli1 ECKO mice do not display clinical symptoms of SSc vasculopathy, suggesting the contribution of additional factors in the development of SSc vasculopathy (Fig. 4). Further studies are required to test this hypothesis in SSc.
5. Autoimmunity
5.1. The role of Fli1 in hematopoietic cells
Fli1 has a central role in megakaryocytic differentiation. Studies by Hart et al. [43] and Spyropoulos et al. [44] revealed that targeted disruption of Fli1 results in severe dysmegakaryopoiesis and embryonic lethality due to impaired vascular integrity. Importantly, Hart et al. [43] reported that dysmegakaryopoiesis in Fli1 null embryos resembles that of patients with Jacobsen or Paris-Trousseau Syndrome [54–56], which is a rare congenital disorder that often includes growth and mental retardation, cardiac defects, dysmorphogenesis of the digits and face, pancytopenia, and thrombocytopenia. The authors mapped the megakaryocytic defects in 14 Jacobsen patients to a minimal region on 11q that includes the Fli1 gene [56]. This elegant study suggests that dysmegakaryopoiesis in patients with Jacobsen syndrome is caused by a hemizygous loss of Fli1.Masuya et al. [57] further demonstrated that Fli1 has important roles in granulocytic, erythroid, and natural killer cell proliferation and differentiation. Based on these findings, Fli1 is an important regulator of hematopoietic cells as well as endothelial cells, consistent with the observation that Fli1 is expressed at relatively high levels in those cells [9].
5.2. The role of Fli1 in autoimmune diseases
The impact of transcription factor Fli1 on the pathogenesis of autoimmune diseases was initially demonstrated by Zhang et al. [58] in H-2Kk-Fli1 transgenic mice in which Fli1 is overexpressed in various tissues, especially the thymus and spleen. Fli1 transgenic mice develop a progressive immunologic renal disease similar to systemic lupus erythematosus and ultimately die of renal failure due to tubulointerstitial nephritis and immune-complex glomerulonephritis. Importantly, Fli1 protein expression levels in the lymphoid tissues of these mice positively correlate with the prevalence of renal involvement. A series of elegant studies by the authors revealed hypergammaglobulinemia, splenomegaly, B cell hyperplasia, accumulation of abnormal CD3 + B220 + T lymphoid cells, and CD5 + B220 + B cells in peripheral lymphoid tissues, and various autoantibodies in Fli1 transgenic mice, suggesting the involvement of an immune dysfunction in the pathogenesis of the renal disease in these mice. Furthermore, splenic B cells from Fli1 transgenic mice exhibit increased proliferation and prolonged survival in vitro in response to mitogens. Together, these observations suggest that Fli1 regulates normal lymphoid cell function and cell survival, and its dysregulation might lead to the development of autoimmune diseases, such as systemic lupus erythematosus.
5.3. The impact of Fli1 deficiency on T cells
The impact of Fli1 deficiency on T cells has not yet been well studied. In 1994, Mao et al. [59] elucidated the potential role of Fli1 deficiency in T cells by demonstrating that resting mature T cells in G0 phase express high levels of Fli1 mRNA, while stimulation with anti-CD3 antibody, which promotes quiescent cells to enter the cell cycle, dramatically decreases Fli1 mRNA levels within 2–4 h. Zhang and Watson [60] also reported that the levels of newly synthesized Fli1 proteins are decreased after stimulating T cells with phorbol myristate acetate and ionomycin. These previous observations suggest that the downregulation of Fli1 is involved in the mechanism responsible for T cell activation. Further investigation is required to clarify the role of Fli1 in the activation of certain T cell subsets, such as CD4+ and CD8+ T cells.
5.4. The impact of Fli1 deficiency on B cells
A series of studies using Fli1+/−MRL/lpr, Fli1+/−C57BL/6, and Fli-1ΔCTA/ΔCTA C57BL/6 mice demonstrated that Fli1 deficiency affects B cell proliferative responses to various mitogens, including phorbol myristate acetate and ionomycin, and this abnormality is independent of B cell receptor and Toll-like receptor expression [58,61–63]. Another study demonstrated that Fli1 has anti-apoptotic activity in lymphoid cells and that apoptosis of murine preB leukemic cells is accompanied by the specific cleavage of Fli1 by caspase-like activity [64]. The potential role of Fli1 in cells of immune origin in SSc should be investigated, but, to the best of our knowledge, such studies have not been yet performed.
6. A new therapeutic strategy for SSc by targeting Fli1
6.1. Post-translational modification of Fli1 in dermal fibroblasts
As described in the first part of this article, Fli1 has a critical role in the repression of type I collagen genes and inhibition of the TGFβ-induced profibrotic gene program [1,2,4,14,33,42,65]. TGF-β abrogates the function of this repressor through post-translational modifications that involve PKC-δ-dependent phosphorylation and subsequent PCAF-dependent acetylation of Fli1 [1,4]. As a result, Fli1 dissociates from the promoter of the type I collagen gene and is targeted for degradation through a proteasomal pathway, resulting in a decreased steady-state level of Fli1 protein and a derepression of collagen gene transcription. PKC-δ has a close association with c-Abl tyrosine kinase in collagen synthesis of stellate cells [66], apoptosis induction in Hep3B cells [67], type I interferon signaling in chronic myelocytic leukemia cells [68], the oxidative stress response in osteoblasts [69], and tumor growth in a fibroblast carcinogenesis model using Rat1 cells [70]; therefore, we focused on the relationship between PKC-δ and c-Abl in dermal fibroblasts and our preliminary results indicated that TGF-β-dependent activation of PKC-δ is mediated by c-Abl (Bujor et al. unpublished data). This finding is consistent with the profibrotic function of c-Abl in the non-canonical TGF-β signaling pathway [71–73]. A novel non-canonical pathway of TGF-β signaling “c-Abl – PKC-δ – Fli1” would be an attractive therapeutic target in SSc.
6.2. Imatinib mesylate modulates the transcriptional activity of Fli1 by affecting the phosphorylation-acetylation cascade
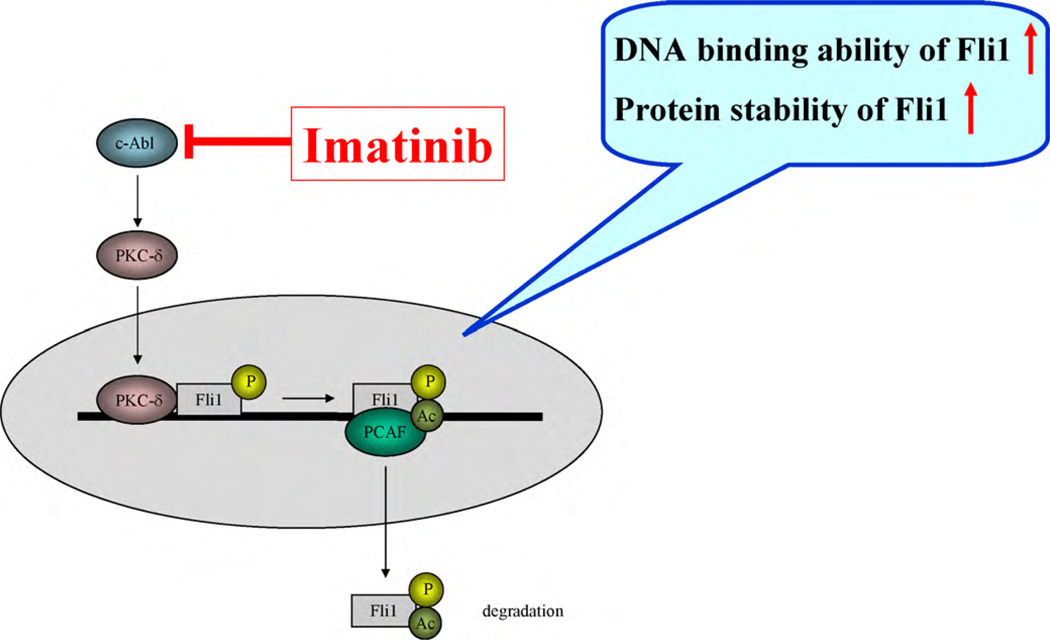
Imatinib mesylate, a selective protein tyrosine kinase inhibitor against c-Abl, as well as the PDGF receptor and c-kit, is now widely used to treat various diseases, including chronic myeloid leukemia, gastrointestinal stromal tumors, acute lymphoblastic leukemia with Philadelphia chromosome, myelodysplastic syndrome/mye-loproliferative disease, aggressive systemic mastcytosis, hypereosinophilic syndrome/chronic eosinophilic leukemia, and dermatofibrosarcoma protuberans [74–79]. Imatinib blocks the induction of c-Abl tyrosine kinase activity and fibrotic gene responses elicited by TGF-β, and normalizes collagen overproduction in cultured SSc fibroblasts [42]. Until recently, the anti-TGF-β effects of imatinib were thought to be associated with blockade of the activation of Smad1 and early growth response protein 1 [42,80]. Our latest study, however, identified a novel mechanism by which imatinib blocks the TGF-β-dependent upregulation of type I collagen genes. As discussed above, upon TGF-β stimulation, c-Abl and PKC-δ are sequentially activated and Fli1 consequently dissociates from the promoters of type I collagen genes. As imatinib inhibits tyrosine kinase activity of c-Abl and subsequently the serine/threonine kinase activity of PKC-δ, imatinib increases the binding activity of Fli1 to type I collagen promoters and increases the protein stability of Fli1 through dephosphorylation and deacetylation of Fli1, resulting in the suppression of type I collagen gene expression (Fig. 5).
Fig. 5.
Imatinib increases the DNA binding ability and protein stability of Fli1 in the context of dermal fibrobasts. Upon TGF-β stimulation, c-Abl and PKC-d are sequentially activated and Fli1 loses its inhibitory effect on the COL1A2 promoter through the phosphorylation-acetylation cascade. Imatinib inhibits c-Abl tyrosine kinase activity and subsequently increases the DNA binding and protein stability of Fli1 by blocking the phosphorylation-acetylation cascade.
6.3. Fli1 as a new therapeutic target for SSc
Numerous reports suggest that imatinib is effective for ameliorating skin fibrosis in some patients with SSc and in fibrosing conditions, such as chronic graft-versus-host disease, nephrogenic systemic fibrosis, and localized scleroderma [81–86]. The most remarkable case was reported by Sfikakis et al. [85] in 2008. A patient with SSc who was resistant to the conventional immunosuppressive therapies was highly responsive to imatinib (400 mg/day). The skin score of the patient improved from 44 to 28 and histopathologic examination revealed a decrease in type I collagen deposition and restored aberrant type III collagen deposition, indicating that imatinib reversed fibrosis. In addition, imatinib ameliorates PAH (a frequent complication of limited cutaneous SSc) in animal models, in individual case reports, and in a small phase II clinical trial [87–90]. The first clinical case revealing the efficacy of imatinib for SSc-related PAH was reported by ten Freyhaus et al. [91] in 2009. The patient was resistant to the conventional treatments for PAH, including bosentan and sildenafil, but was successfully treated with imatinib (400 mg/day). Because imatinib blocks PDGF receptor tyrosine kinase as well as c-Abl tyrosine kinase, the reversal of Fli1 gene expression is likely to be one of the mechanisms by which imatinib improves the clinical symptoms of SSc. Thus, imatinib is a promising candidate for the treatment of SSc and many clinical trials are currently under way [92] (Fig. 6).
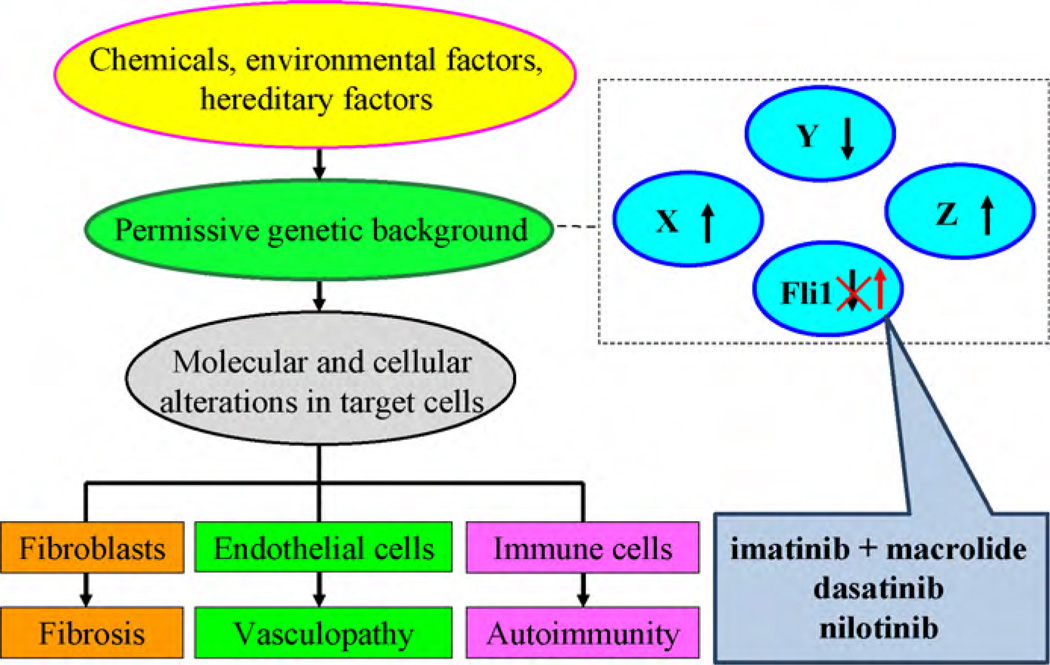
Fig. 6.
A new therapeutic strategy for SSc by targeting the transcription factor Fli1. A certain set of genetic factors, including Fli1 deficiency, may be required for the development of SSc. The reversal of Fli1 expression by tyrosine kinase inhibitors may be a promising therapeutic strategy. Combination treatment of imatinib with macrolides, which block the inhibitory effect of α1-acid glycoprotein for imatinib, would be another therapeutic option for SSc.
6.4. The potential of new derivatives of imatinib and the combination of imatinib with macrolide antibiotics for the treatment of SSc
New derivatives of imatinib, such as dasatinib and nilotinib, are active against multiple members of the Src family of kinases as well as c-Abl and the PDGF receptor [93–95], and are potential drugs for the treatment of fibrosis in SSc. Akhmetshina et al. [96] demonstrated that dasatinib or nilotinib inhibit the TGF-β-induced expression of type I collagen and fibronectin in normal and SSc fibroblasts. Furthermore, they showed that bleomycin-induced skin fibrosis in mice is improved by injecting dasatinib or nilotinib simultaneously with bleomycin in a dose-dependent manner. Therefore, new derivatives of imatinib are promising candidates for the treatment of SSc, and dasatanib is now in early-stage clinical trials for SSc [92].
There is an ongoing clinical trial of imatinib for idiopathic pulmonary fibrosis [92], but preliminary results do not show a treatment advantage [97]. This observation is quite contrary to the experimental data of the efficacy of tyrosine kinase inhibitors in animal models of fibrosis [71–73]. A study by Azuma et al. [98] provided an important clue to the possible causes of this discrepancy. Bleomycin-induced pulmonary fibrosis was inhibited by imatinib when the treatment was started on Day 1. In contrast, imatinib did not affect bleomycin-induced pulmonary fibrosis when the treatment was started at Day 15. Importantly, the levels of α1-acid glycoprotein, one of the acute phase proteins, which is a potent inhibitor of imatinib, were rapidly upregulated by inflammation and were increased in serum and lung homogenates in bleomycin-treated mice. Combination treatment of imatinib with erythromycin or clarithromycin, which interact with α1-acid glycoprotein and block its inhibitory effect on imatinib, reversed the antifibrotic effect of imatinib even when the treatment was started at Day 15. A study by Kucharz et al. [99] reported increased levels of α1-acid glycoprotein in the serum and broncho-alveolar lavage fluid of patients with SSc. Thus, the different efficacy of imatinib treatment in individual SSc cases might, at least in part, be due to variations in the levels of α1-acid glycoprotein. Combined administration of imatinib with erythromycin or clarithromycin might be beneficial for SSc patients (Fig. 6).
7. Conclusion
In this review article, we focused on the role of Fli1 in the pathogenesis of SSc. Over the past 3 years, a number of candidate genes that increase the susceptibility for the development of SSc, such as CTGF, BANK1, C8 or f13-BLK, IL-23R, IRF5, STAT4, TBX21, and TNFSF4, have been identified by large case-control studies, although some of them are still controversial [100]. Further studies of the possible crosstalk between Fli1 and other susceptibility genes could help to clarify the pathogenesis of SSc and lead to successful therapies.
Biography

Yoshihide Asano graduated from The University of Tokyo and received his MD in 1998. He received his PhD in 2004 at Graduate School of Medicine and Faculty of Medicine, The University of Tokyo. He trained as a Postdoctoral Fellow under Prof. Maria Trojanowska in the Department of Rheumatology and Immunology at Medical University of South Carolina from 2006 to 2008. Since 2009, he has been an assistant professor in the Department of Dermatology at Graduate School of Medicine and Faculty of Medicine, The University of Tokyo. He continues to conduct research in the area of collagen disease, especially systemic sclerosis, with a focus on the mechanisms of pathological fibrosis and vasculopathy.
References
- 1.Asano Y, Czuwara J, Trojanowska M. Transforming growth factor-beta regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J Biol Chem. 2007;282:34672–34683. doi: 10.1074/jbc.M703907200. [DOI] [PubMed] [Google Scholar]
- 2.Asano Y, Markiewicz M, Kubo M, Szalai G, Watson DK, Trojanowska M. Transcription factor Fli1 regulates collagen fibrillogenesis in mouse skin. Mol Cell Biol. 2009;29:425–434. doi: 10.1128/MCB.01278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano Y, Stawski L, Hant F, Highland K, Silver R, Szalai G, et al. Endothelial Fli1 deficiency impairs vascular homeostasis. A role in scleroderma vasculopathy. Am J Pathol. 2010;176:1983–1998. doi: 10.2353/ajpath.2010.090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asano Y, Trojanowska M. Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta. Mol Cell Biol. 2009;29:1882–1894. doi: 10.1128/MCB.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubo M, Czuwara-Ladykowska J, Moussa O, Markiewicz M, Smith E, Silver RM, et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayes MD, Trojanowska M. Genetic factors in systemic sclerosis. Arthritis Res Ther. 2007;9:S5. doi: 10.1186/ar2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga JA, Trojanowska M. Fibrosis in systemic sclerosis. Rheum Dis Clin North Am. 2008;34:115–143. doi: 10.1016/j.rdc.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Fan PS, Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–2279. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]
- 9.Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–5702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91:896–903. doi: 10.1002/jcb.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-David Y, Giddens EB, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5:908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- 12.Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 13.Ida K, Kobayashi S, Taki T, Hanada R, Bessho F, Yamamori S, et al. EWS-FLI-1 and EWS-ERG chimeric mRNAs in Ewing’s sarcoma and primitive neuroectodermal tumor. Int J Cancer. 1995;63:500–504. doi: 10.1002/ijc.2910630407. [DOI] [PubMed] [Google Scholar]
- 14.Czuwara-Ladykowska J, Shirasaki F, Jackers P, Watson DK, Trojanowska M. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J Biol Chem. 2001;276:20839–20848. doi: 10.1074/jbc.M010133200. [DOI] [PubMed] [Google Scholar]
- 15.Asano Y, Ihn H, Asashima N, Yazawa N, Mimura Y, Jinnin M, et al. A case of diffuse scleroderma successfully treated with high-dose intravenous immune globulin infusion. Rheumatology (Oxford) 2005;44:824–826. doi: 10.1093/rheumatology/keh600. [DOI] [PubMed] [Google Scholar]
- 16.Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126:1761–1769. doi: 10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- 17.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Differential effects of the immunosuppressant FK-506 on human alpha2(I) collagen gene expression and transforming growth factor beta signaling in normal and scleroderma fibroblasts. Arthritis Rheum. 2005;52:1237–1247. doi: 10.1002/art.20934. [DOI] [PubMed] [Google Scholar]
- 18.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 19.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005;52:2897–2905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- 20.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Phosphatidylinositol 3-kinase is involved in alpha2(I) collagen gene expression in normal and scleroderma fibroblasts. J Immunol. 2004;172:7123–7135. doi: 10.4049/jimmunol.172.11.7123. [DOI] [PubMed] [Google Scholar]
- 21.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499–510. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Impaired Smad7-Smurf-mediated negative regulation of TGF-beta signaling in scleroderma fibroblasts. J Clin Invest. 2004;113:253–264. doi: 10.1172/JCI16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Increased expression levels of integrin alphavbeta5 on scleroderma fibroblasts. Am J Pathol. 2004;164:1275–1292. doi: 10.1016/s0002-9440(10)63215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihn H, Yamane K, Asano Y, Jinnin M, Tamaki K. Constitutively phosphorylated Smad3 interacts with Sp1 and p300 in scleroderma fibroblasts. Rheumatology (Oxford) 2006;45:157–165. doi: 10.1093/rheumatology/kei124. [DOI] [PubMed] [Google Scholar]
- 25.Ihn H, Yamane K, Kubo M, Tamaki K. Blockade of endogenous transforming growth factor beta signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor beta receptors. Arthritis Rheum. 2001;44:474–480. doi: 10.1002/1529-0131(200102)44:2<474::AID-ANR67>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Jinnin M, Ihn H, Mimura Y, Asano Y, Tamaki K. Involvement of the constitutive complex formation of c-Ski/SnoN with Smads in the impaired negative feedback regulation of transforming growth factor beta signaling in scleroderma fibroblasts. Arthritis Rheum. 2007;56:1694–1705. doi: 10.1002/art.22588. [DOI] [PubMed] [Google Scholar]
- 27.Jinnin M, Ihn H, Mimura Y, Asano Y, Tamaki K. Potential regulatory elements of the constitutive up-regulated alpha2(I) collagen gene in scleroderma dermal fibroblasts. Biochem Biophys Res Commun. 2006;343:904–909. doi: 10.1016/j.bbrc.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 28.Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. Effects of hepatocyte growth factor on the expression of type I collagen and matrix metalloproteinase-1 in normal and scleroderma dermal fibroblasts. J Invest Dermatol. 2005;124:324–330. doi: 10.1111/j.0022-202X.2004.23601.x. [DOI] [PubMed] [Google Scholar]
- 29.Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. Matrix metalloproteinase-1 up-regulation by hepatocyte growth factor in human dermal fibroblasts via ERK signaling pathway involves Ets1 and Fli1. Nucleic Acids Res. 2005;33:3540–3549. doi: 10.1093/nar/gki648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeRoy EC. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974;54:880–889. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Constitutive phosphorylation of focal adhesion kinase is involved in the myofibroblast differentiation of scleroderma fibroblasts. J Invest Dermatol. 2005;124:886–892. doi: 10.1111/j.0022-202X.2005.23701.x. [DOI] [PubMed] [Google Scholar]
- 32.Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Constitutive thrombospondin-1 overexpression contributes to autocrine transforming growth factor-beta signaling in cultured scleroderma fibroblasts. Am J Pathol. 2005;166:1451–1463. doi: 10.1016/s0002-9440(10)62362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakerakanti SS, Kapanadze B, Yamasaki M, Markiewicz M, Trojanowska M. Fli1 and Ets1 have distinct roles in connective tissue growth factor/CCN2 gene regulation and induction of the profibrotic gene program. J Biol Chem. 2006;281:25259–25256. doi: 10.1074/jbc.M600466200. [DOI] [PubMed] [Google Scholar]
- 34.Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku H, Fukamizu A, et al. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26:500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- 35.Itoh S, Ericsson J, Nishikawa J, Heldin CH, ten Dijke P. The transcriptional co-activator P/CAF potentiates TGF-beta/Smad signaling. Nucleic Acids Res. 2000;28:4291–4298. doi: 10.1093/nar/28.21.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonsson M, Kanduri M, Gronroos E, Heldin CH, Ericsson J. The DNA binding activities of Smad2 and Smad3 are regulated by coactivator-mediated acetylation. J Biol Chem. 2006;281:39870–39878. doi: 10.1074/jbc.M607868200. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez SA, Gaidarova S, Saitta B, Sandorfi N, Herrich DJ, Rosenbloom JC, et al. Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J Clin Invest. 2001;108:1395–1403. doi: 10.1172/JCI12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagares D, Garcia-Fernandez RA, Jimenez CL, Magan-Marchal N, Busnadiego O, Lamas S, et al. Endothelin 1 contributes to the effect of transforming growth factor beta1 on wound repair and skin fibrosis. Arthritis Rheum. 2010;62:878–889. doi: 10.1002/art.27307. [DOI] [PubMed] [Google Scholar]
- 39.Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- 40.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Grotendorst GR, et al. Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J Invest Dermatol. 1995;105:280–284. doi: 10.1111/1523-1747.ep12318465. [DOI] [PubMed] [Google Scholar]
- 42.Pannu J, Asano Y, Nakerakanti S, Smith E, Jablonska S, Blaszczyk M, et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58:2528–2537. doi: 10.1002/art.23698. [DOI] [PubMed] [Google Scholar]
- 43.Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, et al. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–177. doi: 10.1016/s1074-7613(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 44.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, et al. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun-Falco O, Rupec M. Collagen fibrils of the scleroderma in ultra-thin skin sections. Nature. 1964;202:708–709. doi: 10.1038/202708a0. [DOI] [PubMed] [Google Scholar]
- 46.Fleischmajer R, Damiano V, Nedwich A. Alteration of subcutaneous tissue in systemic scleroderma. Arch Dermatol. 1972;105:59–66. [PubMed] [Google Scholar]
- 47.Fleischmajer R, Gay S, Meigel WN, Perlish JS. Collagen in the cellular and fibrotic stages of scleroderma. Arthritis Rheum. 1978;21:418–428. doi: 10.1002/art.1780210404. [DOI] [PubMed] [Google Scholar]
- 48.Sakakibara N, Sugano S, Morita A. Ultrastructural changes induced in cutaneous collagen by ultraviolet-A1 and psoralen plus ultraviolet A therapy in systemic sclerosis. J Dermatol. 2008;35:63–69. doi: 10.1111/j.1346-8138.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18:1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 50.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 51.Montagnana M, Volpe A, Lippi G, Caramaschi P, Salvagno GL, Biasi D, et al. Relationship between matrix metalloproteinases/tissue inhibitors of matrix metalloproteinases systems and autoantibody patterns in systemic sclerosis. Clin Biochem. 2007;40:837–842. doi: 10.1016/j.clinbiochem.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 52.Allanore Y, Seror R, Chevrot A, Kahan A, Drape JL. Hand vascular involvement assessed by magnetic resonance angiography in systemic sclerosis. Arthritis Rheum. 2007;56:2747–2754. doi: 10.1002/art.22734. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Yarnykh VL, Molitor JA, Nash RA, Chu B, Wilson GJ, et al. Micro magnetic resonance angiography of the finger in systemic sclerosis. Rheumatology (Oxford) 2008;47:1239–1243. doi: 10.1093/rheumatology/ken215. [DOI] [PubMed] [Google Scholar]
- 54.Breton-Gorius J, Favier R, Guichard J, Cherif D, Berger R, Debili N, et al. A new congenital dysmegakaryopoietic thrombocytopenia (Paris-Trousseau) associated with giant platelet alpha-granules and chromosome 11 deletion at 11q23. Blood. 1995;85:1805–1814. [PubMed] [Google Scholar]
- 55.Penny LA, Dell’Aquila M, Jones MC, Bergoffen J, Cunniff C, Fryns JP, et al. Clinical and molecular characterization of patients with distal 11q deletions. Am J Hum Genet. 1995;56:676–683. [PMC free article] [PubMed] [Google Scholar]
- 56.Tunnacliffe A, Jones C, Le Paslier D, Todd R, Cherif D, Birdsall M, et al. Localization of Jacobsen syndrome breakpoints on a 40-Mb physical map of distal chromosome 11q. Genome Res. 1999;9:44–52. [PMC free article] [PubMed] [Google Scholar]
- 57.Masuya M, Moussa O, Abe T, Deguchi T, Higuchi T, Ebihara Y, et al. Dysregulation of granulocyte, erythrocyte, and NK cell lineages in Fli-1 gene-targeted mice. Blood. 2005;105:95–102. doi: 10.1182/blood-2003-12-4345. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Eddy A, Teng YT, Fritzler M, Kluppel M, Melet F, et al. An immunological renal disease in transgenic mice that overexpress Fli-1, a member of the ets family of transcription factor genes. Mol Cell Biol. 1995;15:6961–6970. doi: 10.1128/mcb.15.12.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao X, Miesfeldt S, Yang H, Leiden JM, Thompson CB. The FLI-1 and chimeric EWS-FLI-1 oncoproteins display similar DNA binding specificities. J Biol Chem. 1994;269:18216–18222. [PubMed] [Google Scholar]
- 60.Zhang XK, Watson DK. The FLI-1 transcription factor is a short-lived phosphoprotein in T cells. J Biochem. 2005;137:297–302. doi: 10.1093/jb/mvi032. [DOI] [PubMed] [Google Scholar]
- 61.Bradshaw S, Zheng WJ, Tsoi LC, Gilkeson G, Zhang XK. A role for Fli-1 in B cell proliferation: implications for SLE pathogenesis. Clin Immunol. 2008;129:19–30. doi: 10.1016/j.clim.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang XK, Gallant S, Molano I, Moussa OM, Ruiz P, Spyropoulos DD, et al. Decreased expression of the Ets family transcription factor Fli-1 markedly prolongs survival and significantly reduces renal disease in MRL/lpr mice. J Immunol. 2004;173:6481–6489. doi: 10.4049/jimmunol.173.10.6481. [DOI] [PubMed] [Google Scholar]
- 63.Zhang XK, Moussa O, LaRue A, Bradshaw S, Molano I, Spyropoulos DD, et al. The transcription factor Fli-1 modulates marginal zone and follicular B cell development in mice. J Immunol. 2008;181:1644–1654. doi: 10.4049/jimmunol.181.3.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarrazin S, Bonod-Bidaud C, Remy P, Mehlen P, Morle F. Caspase cleavage of the transcription factor FLI-1 during preB leukemic cell death. Biochim Biophys Acta. 2002;1592:123–127. doi: 10.1016/s0167-4889(02)00290-2. [DOI] [PubMed] [Google Scholar]
- 65.Markiewicz M, Asano Y, Znoyko S, Gong Y, Watson DK, Trojanowska M. Distinct effects of gonadectomy in male and female mice on collagen fibrillogenesis in the skin. J Dermatol Sci. 2007;47:217–226. doi: 10.1016/j.jdermsci.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceni E, Crabb DW, Foschi M, Mello T, Tarocchi M, Patussi V, et al. Acetaldehyde inhibits PPARgamma via H2O2-mediated c-Abl activation in human hepatic stellate cells. Gastroenterology. 2006;131:1235–1252. doi: 10.1053/j.gastro.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Lasfer M, Davenne L, Vadrot N, Alexia C, Sadji-Ouatas Z, Bringuier AF, et al. Protein kinase PKC delta and c-Abl are required for mitochondrial apoptosis induction by genotoxic stress in the absence of p53, p73 and Fas receptor. FEBS Lett. 2006;580:2547–2552. doi: 10.1016/j.febslet.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 68.Kaur S, Parmar S, Smith J, Katsoulidis E, Li Y, Sassano A, et al. Role of protein kinase C-delta (PKC-delta) in the generation of the effects of IFN-alpha in chronic myelogenous leukemia cells. Exp Hematol. 2005;33:550–1557. doi: 10.1016/j.exphem.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Li B, Wang X, Rasheed N, Hu Y, Boast S, Ishii T, et al. Distinct roles of c-Abl and Atm in oxidative stress response are mediated by protein kinase C delta. Genes Dev. 2004;18:1824–1837. doi: 10.1101/gad.1223504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kin Y, Shibuya M, Maru Y. Inhibition of protein kinase C delta has negative effect on anchorage-independent growth of BCR-ABL-transformed Rat1 cells. Leuk Res. 2001;25:821–825. doi: 10.1016/s0145-2126(01)00031-5. [DOI] [PubMed] [Google Scholar]
- 71.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Distler JH, Jungel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56:311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 73.Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. Faseb J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 74.Cortes J, Kantarjian H. Beyond chronic myelogenous leukemia: potential role for imatinib in Philadelphia-negative myeloproliferative disorders. Cancer. 2004;100:2064–2078. doi: 10.1002/cncr.20211. [DOI] [PubMed] [Google Scholar]
- 75.Handolias D, McArthur GA. Imatinib as effective therapy for dermatofibrosarcoma protuberans: proof of concept of the autocrine hypothesis for cancer. Future Oncol. 2008;4:211–217. doi: 10.2217/14796694.4.2.211. [DOI] [PubMed] [Google Scholar]
- 76.Orfao A, Garcia-Montero AC, Sanchez L, Escribano L. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12–30. doi: 10.1111/j.1365-2141.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 77.Ravandi F, Kebriaei P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:1043–1063. doi: 10.1016/j.hoc.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85:158–164. doi: 10.4065/mcp.2009.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2010;184:3–20. doi: 10.1007/978-3-642-01222-8_1. [DOI] [PubMed] [Google Scholar]
- 80.Bhattacharyya S, Ishida W, Wu M, Wilkes M, Mori Y, Hinchcliff M, et al. A non-Smad mechanism of fibroblast activation by transforming growth factor-beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene. 2009;28:1285–1297. doi: 10.1038/onc.2008.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kay J, High WA. Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum. 2008;58:2543–2548. doi: 10.1002/art.23696. [DOI] [PubMed] [Google Scholar]
- 82.Magro L, Catteau B, Coiteux V, Bruno B, Jouet JP, Yakoub-Agha I. Efficacy of imatinib mesylate in the treatment of refractory sclerodermatous chronic GVHD. Bone Marrow Transplant. 2008;42:757–760. doi: 10.1038/bmt.2008.252. [DOI] [PubMed] [Google Scholar]
- 83.Moreno-Romero JA, Fernandez-Aviles F, Carreras E, Rovira M, Martinez C, Mascaro JM., Jr Imatinib as a potential treatment for sclerodermatous chronic graft-vs-host disease. Arch Dermatol. 2008;144:1106–1109. doi: 10.1001/archderm.144.9.1106. [DOI] [PubMed] [Google Scholar]
- 84.Sabnani I, Zucker MJ, Rosenstein ED, Baran DA, Arroyo LH, Tsang P, et al. A novel therapeutic approach to the treatment of scleroderma-associated pulmonary complications: safety and efficacy of combination therapy with imatinib and cyclophosphamide. Rheumatology (Oxford) 2009;48:49–52. doi: 10.1093/rheumatology/ken369. [DOI] [PubMed] [Google Scholar]
- 85.Sfikakis PP, Gorgoulis VG, Katsiari CG, Evangelou K, Kostopoulos C, Black CM. Imatinib for the treatment of refractory, diffuse systemic sclerosis. Rheumatology (Oxford) 2008;47:735–737. doi: 10.1093/rheumatology/ken104. [DOI] [PubMed] [Google Scholar]
- 86.van Daele PL, Dik WA, Thio HB, van Hal PT, van Laar JA, Hooijkaas H, et al. Is imatinib mesylate a promising drug in systemic sclerosis? Arthritis Rheum. 2008;58:2549–2552. doi: 10.1002/art.23648. [DOI] [PubMed] [Google Scholar]
- 87.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 88.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med. 2006;145:152–153. doi: 10.7326/0003-4819-145-2-200607180-00020. [DOI] [PubMed] [Google Scholar]
- 89.Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:81–88. doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 90.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ten Freyhaus H, Dumitrescu D, Bovenschulte H, Erdmann E, Rosenkranz S. Significant improvement of right ventricular function by imatinib mesylate in scleroderma-associated pulmonary arterial hypertension. Clin Res Cardiol. 2009;98:265–267. doi: 10.1007/s00392-009-0752-3. [DOI] [PubMed] [Google Scholar]
- 92.ClinicalTrials.gov. [accessed 27 January 2009]; [ http://clinicaltrials.gov/ct2/show/NCT00764309]
- 93.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 94.Kantarjian H, Jabbour E, Grimley J, Kirkpatrick P, Dasatinib Nat Rev Drug Discov. 2006;5:717–718. doi: 10.1038/nrd2135. [DOI] [PubMed] [Google Scholar]
- 95.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 96.Akhmetshina A, Dees C, Pileckyte M, Maurer B, Axmann R, Jungel A, et al. Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. Faseb J. 2008;22:2214–2222. doi: 10.1096/fj.07-105627. [DOI] [PubMed] [Google Scholar]
- 97.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Azuma M, Nishioka Y, Aono Y, Inayama M, Makino H, Kishi J, et al. Role of alpha1-acid glycoprotein in therapeutic antifibrotic effects of imatinib with macrolides in mice. Am J Respir Crit Care Med. 2007;176:1243–1250. doi: 10.1164/rccm.200702-178OC. [DOI] [PubMed] [Google Scholar]
- 99.Kucharz EJ, Grucka-Mamczar E, Mamczar A, Brzezinska-Wcislo L. Acute-phase proteins in patients with systemic sclerosis. Clin Rheumatol. 2000;19:165–166. doi: 10.1007/s100670050039. [DOI] [PubMed] [Google Scholar]
- 100.Agarwal SK, Reveille JD. The genetics of scleroderma (systemic sclerosis) Curr Opin Rheumatol. 2010;22:133–138. doi: 10.1097/BOR.0b013e3283367c17. [DOI] [PubMed] [Google Scholar]