ABSTRACT
Non-mydriatic ocular fundus photography is a promising alternative to direct ophthalmoscopy, particularly when combined with telemedicine. This review discusses these technologies from a longitudinal perspective: past, present, and future. The focus is directed to the role that non-mydriatic fundus photography and telemedicine have played in medical research and patient care, with emphasis on the major advances to date. Also discussed are the challenges to their widespread application and their substantial promise for revitalizing the importance of the ocular fundus examination in patient care, providing improved access to ophthalmic consultative services, and facilitating clinical and epidemiologic research.
Keywords: Fundus photography, neurology, ophthalmology, review, telemedicine
INTRODUCTION
Examination of the ocular fundus is a fundamental component of the general physical examination and critical to the diagnosis of life- and sight-threatening medical conditions among patients with certain presenting complaints, such as headache.1,2 Yet, the examination of the ocular fundus is infrequently and inadequately performed in most non-ophthalmic settings.3–7 Non-mydriatic ocular fundus photography offers a promising alternative to the most commonly used general examination method, direct ophthalmoscopy, by removing most of the technical barriers to adequate examination of the ocular fundus. In addition, the digital nature of the photographs obtained allows them to be easily stored and transmitted to another location anywhere in the world for consultation. The combination of non-mydriatic fundus photography with telemedicine has substantial promise for revitalizing the importance of the ocular fundus examination in patient care, providing access to ophthalmic consultative services in underserved areas, and facilitating clinical and epidemiologic research.
THE PAST
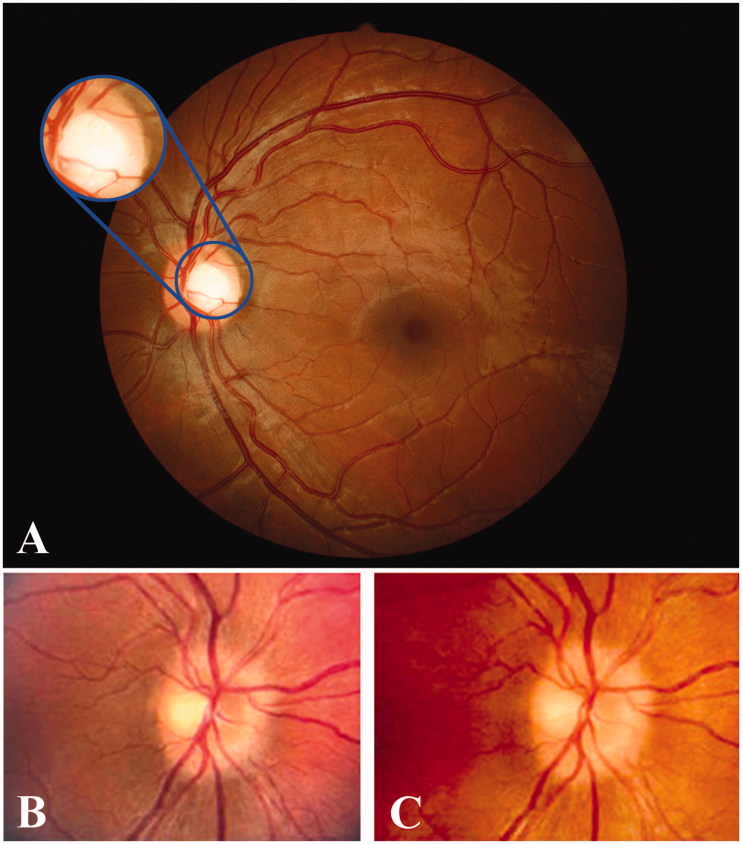
Imaging has been essential to ophthalmology since Jackman and Webster first published a human fundus photograph in 1886.8,9 Over the next 50 years, techniques improved very gradually until a new era dawned with the development of electronic flashtubes and the convenient 35 mm film format.8 Another revolution occurred when digital photography became commercially available in the early 1990s, providing remarkable advantages over film: immediate display of the photographs taken (allowing real-time adjustments to composition and quality), essentially no incremental cost to obtain additional images or reprints, and the ability to easily archive and transmit the photographs to remote locations.8,9 Advances in optics over a similar time frame increased the field of view obtained by ocular fundus cameras to about 45 degrees, even without pupillary dilation (i.e., non-mydriatic) (Figure 1A).
FIGURE 1.
Top panel: (A) Typical view of posterior pole using non-mydriatic fundus photography showing the optic nerve, the macula, and major retinal vessels. The insert depicts the typical view during direct ophthalmoscopy. Lower panel: Fundus photograph of an optic disc with pseudo-oedema taken by a professional ocular photographer with a mydriatic camera (B), and a picture of the same optic disc taken by a nurse practitioner with a non-mydriatic fundus camera (C). Note: Figure 1 of this article is available in colour online at www.informahealthcare.com/oph.
The capabilities, indeed advantages, of non-mydriatic digital retinal imaging compared with funduscopic examination have been documented by several studies. For example, in diabetic retinopathy screening (the most extensively studied area in retinal imaging), studies have found that non-mydriatic fundus photography has higher sensitivity, specificity, and inter-examination agreement than ophthalmoscopy, even among ophthalmologists.10,11
In contrast to ophthalmoscopy, non-medical personnel can assist by obtaining high-quality images for later review, even after only limited training. One study comparing images obtained by a trained ophthalmic photographer (with 20 years of experience) and two non-professional photographers (one with 2 days and the other with 1 hour of training) found no difference in the image quality based on the ratings of two retina specialists.12 These findings concur with the more recent Fundus photography versus Ophthalmoscopy Trial Outcomes in the Emergency Department (FOTO-ED) study, in which nurse practitioners obtained photographs of diagnostic value in 97% of patients with a non-mydriatic digital fundus camera after only 15–30 minutes of formal training (Figure 1B and C).6,13
Non-mydriatic fundus photography is routinely used to screen for treatable, sight-threatening eye diseases within at-risk populations, such as diabetic retinopathy.14 Indeed, there is level I evidence that single-field fundus photography can identify patients with diabetic retinopathy who require referral for ophthalmic evaluation and management.15 The capabilities of ocular fundus photography have also been shown both in the diagnosis of referral-warranted retinopathy of prematurity and in the telemedical diagnosis of cytomegalovirus retinitis in human immunodeficiency virus (HIV)-positive patients in underserved countries, although assessment of both of these conditions requires pupillary dilation.16,17
In the past, the use of retinal photography other than in ophthalmology services has largely been confined to large-scale, population-based epidemiologic studies that have demonstrated associations among retinal microvascular abnormalities and various cardiovascular and neurological diseases. In cardiology, elevated blood pressure and various retinal changes have been consistently and strongly associated.18–20 The retinal changes generally fall into two categories: either chronic arteriolar changes or retinopathic changes.21 The chronic arteriolar changes, characterized by generalized arteriolar narrowing and arteriovenous (AV) nicking, are markers of long-term, cumulative damage from hypertension based on their association with blood pressure measured 5–8 years before retinal photography.19 Conversely, the retinopathic changes of focal arteriolar narrowing, retinal haemorrhages, microaneurysms, and cotton-wool spots are markers of acute hypertension based on their association only with concurrently obtained blood pressure measurements.19,21 As would be expected, retinal changes are also associated with increased risks of left ventricular hypertrophy,21 ischaemic heart disease,21 congestive heart failure,22 renal dysfunction,23 and cardiovascular mortality.23,24
In neurology, chronic arteriolar versus retinopathic changes also have different implications. In the case of cerebrovascular cognitive impairment, which represents 20% of all dementia, chronic retinal arteriolar changes correlate with cerebral white-matter lesions on magnetic resonance imaging (MRI).25 The Atherosclerosis Risk in Communities (ARIC) study investigated the relationship between retinal microvascular abnormalities and cognitive impairment in a large, stroke-free, middle-aged, population-based cohort.26 The ARIC study found that retinopathic abnormalities were independently associated with lower cognitive function, particularly microaneurysms and retinal haemorrhages.26 The ARIC investigators also found that retinopathy and arteriovenous nicking on baseline photography were independently associated with 10-year cerebral ventricular enlargement, but not 10-year sulcal widening, suggesting a microvascular aetiology for subcortical, but not cortical, cerebral atrophy.27
It is unsurprising that stroke and retinal microvascular changes are associated, since they share the common risk factors of hypertension and diabetes. However, even after controlling for the confounding effects of hypertension and diabetes, the ARIC study and other large cohort studies found that retinal microvascular anomalies, particularly microaneurysms and soft exudates, predict both incident and subclinical strokes.21,28–30
THE PRESENT
Recently, two notable transitions have occurred in the use of non-mydriatic fundus photography. First, it has been applied to areas where direct ophthalmoscopy is particularly challenging: children and patients in the emergency department. Second, non-ophthalmologists are increasingly the primary reader of photographs.
It is challenging to perform ophthalmoscopy in children, especially without pupillary dilation. In fact, ophthalmologists sometimes have difficulty visualizing the fundi of young, uncooperative patients despite pupillary dilation. In a recent study of 212 children (median age, 6 years; range, 1–18 years),31 non-mydriatic photographs of at least one eye were obtained in 190 children (89.6%) and in both eyes in 181 (85.3%). The study found that it was feasible to obtain non-mydriatic fundus photographs of adequate quality in children >3 years of age and in children as young as 22 months. Since the American Academy of Pediatrics states that direct ophthalmoscopy “may be possible to perform in very cooperative 3 to 4 year olds,”32 the study authors suggested that non-mydriatic fundus photography may expand the age range of children who can have an evaluation of the ocular fundus areas generally visualized by direct ophthalmoscopy to almost all 3-year-olds and some cooperative 2-year-olds.31
In the emergency department (ED), the stakes of missing key information obtainable only from the ocular fundus are higher in terms of both patient outcomes and physician medicolegal liability, yet funduscopic examination is rarely performed in this setting.7 Indeed, among the 350 patients from a university emergency department enrolled in the first phase of the FOTO-ED study with a presentation warranting ocular fundus examination (chief complaint of headache, acute focal neurological deficit, or visual change; or a diastolic blood pressure 120 mm Hg or greater), only 14% underwent direct ophthalmoscopy by the emergency physician. Disturbingly, none of the 44 patients (13%; 95% confidence interval [CI]: 9–17%) with findings that would have potentially altered the course of the patient’s emergency department course were detected by the emergency physicians’ direct ophthalmoscopy.6,7 Routine ED evaluations (e.g., including consultations) missed 80% of patients with abnormal ocular fundus findings (optic disc oedema, optic atrophy, retinal vascular occlusions, and grade III/IV hypertensive retinopathy) that were unknown upon presentation to the ED; these findings were all identified by neuro-ophthalmologist review of the fundus photographs.6,7 In several cases, the discovery of these findings resulted in patients being recalled to the emergency department and admitted to the hospital.7
In the second phase of the FOTO-ED study,33 an additional 354 patients were enrolled with the same presentations as the first phase, but during the second phase photographs were made available to the emergency department physicians during routine clinical care. There was a similar frequency of relevant findings in the second phase (35 findings, 10% of enrolled patients), but the emergency physicians viewed the ocular fundus on photographs significantly more often (239 patients, 68% of enrolled) and detected significantly more of the relevant abnormalities (16 of 35, 46%) than they had by direct ophthalmoscopy in the first phase. The increased frequency of both viewing the fundus and diagnosing abnormalities was particularly remarkable given that the emergency physicians had not received any additional training. The emergency physicians also reported that the photographs were helpful in over a third of cases, including when the photographs were normal (e.g., the absence of papilloedema in a patient with potential shunt malfunction).
Non-ophthalmic physicians have also read non-mydriatic photographs for diabetic retinopathy screening in two large-scale projects.34,35 The first study reported the characteristics of 742 patients referred for ophthalmic care by 24 trained general practitioners who reviewed the photographs for evidence of diabetic retinopathy within a nationwide screening program in Singapore, but the article did not discuss false negatives (missed diagnoses).34 In the other study,35 four trained general practitioners in Spain deemed the photographs of 2036 of 2750 patients (74%) normal and sought ophthalmologic consultation for the remainder. Among those sent for review, 392 (55%) did not have diabetic retinopathy, suggesting that the general practitioners had a low threshold for referral to avoid false negatives. Ophthalmologists also reviewed a sample of 240 of the patients that the general practitioners had read as normal and found that 16 of these (7%) had diabetic retinopathy, but that only two patients (1%) had treatable diabetic retinopathy. The authors concluded that the general practitioners had acceptable sensitivity (particularly relevant for a screening technique) based on the British Diabetic Association’s guidelines, which required at least 80% sensitivity. However, the authors were concerned about specificity in their study (recommended to be 95% by the British Diabetic Association) and recommended additional training to avoid inappropriate referrals.35
THE FUTURE
The demonstration that non-ophthalmic physicians are capable of reviewing photographs for key conditions in various settings, combined with the technical advantages of non-mydriatic fundus photography over direct ophthalmoscopy, suggests that non-mydriatic photography may be an acceptable (and in some cases a better) alternative to direct ophthalmoscopy. Moreover, it seems likely that educational efforts at the allied health, medical school, and post-graduate levels may be best directed at teaching students and clinicians how to read photographs rather than how to perform the technical skills of direct ophthalmoscopy. Two longitudinal studies by Lippa et al. of a sustained, multi-year ophthalmology curriculum emphasise the difficulties of teaching direct ophthalmoscopy in a way that has lasting impact.36,37 For example, while there was initially a 46% documentation rate of ophthalmoscopy in one of the medical students’ third year rotations, there were no documented funduscopic examinations during their fourth year internal medicine clerkship. In addition, only 23% of the students had purchased an ophthalmoscope by completion of medical school.37 Of further concern, 13% to 16% of students stated that a direct ophthalmoscope was not important for clinical duties, and 5% to 6% stated that there was a “dearth of opportunities” for its use in clinical encounters.37
One can ask the controversial question of whether the general physician of the future will even need to be capable of basic fundus interpretation skills. Even now, the push toward non-ophthalmic readers of clinically obtained non-mydriatic fundus photography has moved beyond physicians. Indeed, Bhargava et al.37a recently reported on 367 diabetic patients assessed by both non-physician graders and family physicians compared with a reference standard of a retinal specialist. They found that the non-physician graders with 1 year of rigorous training followed by yearly auditing had better agreement with the retinal specialists (κ = 0.66) than the family physicians who had 2 hours of training followed by re-education every 2 years (κ = 0.40). The non-physician graders also had better sensitivity (70%) than the family physicians.
While it is promising that the majority of diabetic retinopathy screening could be offloaded from retinal specialists and general practitioners to non-ophthalmic readers, developments in automated, computerized reading go even further, by potentially taking the task of reading photographs out of the hands of human reviewers. Although work has been on-going in automatic methods to identify features of diabetic retinopathy for over 20 years,38 only recently have they begun to achieve levels of diagnostic capability comparable to ophthalmologists.39 For example, two groups have recently reported sensitivity of at least 90% with 100% specificity using their comprehensive assessment algorithms.40,41 As noted above, the primary goal of screening programs is high sensitivity in order to avoid missed cases. However, automated systems can have a dramatic practical impact even if only high specificity is maintained under real-world conditions because such a system would dramatically reduce the number of normal images that a human would need to review. Beyond diabetic retinopathy, a recent study of a technique for automated detection and severity assessment of optic disc oedema on photographs has shown promising results that can bring the fruits of decades of research in diabetic retinopathy to neuro-ophthalmology and emergency medicine.42
Nevertheless, substantial challenges remain for the widespread clinical use of non-mydriatic photography regardless of how well it performs with or without automatic screening algorithms. The issues are particularly notable in the acute care arena: (1) how to adequately photograph patients who are too ill or too young to sit at a tabletop camera and (2) how to review photographs in a timely fashion.
The availability of several new, portable devices for retinal photography, in some cases non-mydriatic, such as EyeQuick (Eye Quick, El Paso, TX, USA; http://www.iexam.com), iExaminer (Intuitive Medical Technologies, Shreveport, LA, USA; http://www.eyequick.com), and Pictor (Volk Optical, Mentor, OH, USA; http://www.volk.com/catalog/index.php?cPath=53) are part of the solution to the first major barrier. However, these devices are substantially more difficult to use than a tabletop camera and can only obtain photographs of lower quality. Further scientific and technological progress in imaging science and engineering will be required to produce the ideal “digital ophthalmoscope.”
Advancements in telemedicine, particularly via nearly ubiquitous mobile devices (e.g., smartphones and tablets), likely hold part of the solution to the second challenge, that of reviewing emergent images in a timely fashion. For example, in a subanalysis of the FOTO-ED study, the 5-point overall quality rating assigned by two reviewers to the same 100 photographs on a desktop computer and the iPhone 3G was compared. A very high intra- and inter-rater agreement on the iPhone (kappa 0.96) and high agreement of the same reviewer between the two devices (0.82–0.91) was found. Notably, both reviewers on average rated the same image as higher quality on the iPhone compared with the desktop computer (χ2 > 36, p < 0.001).43 Likewise, Kumar et al. found that the ophthalmologists who reviewed images of patients for the telemedical diagnosis of diabetic retinopathy had very high agreement (κ = 0.9) and gave high scores to the image quality on the iPhone 4.44 On the neurology side, an agreement study of a bedside reviewer’s National Institutes of Health Stroke Scale (NIHSS) versus that of a reviewer remotely directing and observing the examination with an iPhone 4 demonstrated excellent agreement for 10 items (level of consciousness, month and age, visual fields, right motor arm, left motor arm, right motor leg, left motor leg, sensation, language, neglect), moderate agreement for 3 items (gaze, facial palsy, dysarthria), and poor agreement for only 1 item (ataxia). The overall NIHSS scores obtained at bedside and remotely showed excellent agreement (intraclass correlation coefficient, 0.98).45
Finally, advancements in non-mydriatic fundus photography and telemedicine not only have the potential to improve patient care, but also may facilitate clinical research in areas that are currently intractable. For example, the advantages of telemedicine have already been demonstrated in the process of administering emergent therapies in acute stroke.46 The expansion and validation of non-mydriatic fundus photography and its interpretation by non-ophthalmic reviewers and by telemedicine will offer the early diagnosis required for analogous therapeutic studies in neuro-ophthalmology, such as novel therapies for central retinal artery occlusion, anterior ischaemic neuropathy, and traumatic optic neuropathy. In addition, these techniques hold promise for risk stratification and predictive health in both acute and chronic diseases, but their role remains to be fully elucidated.
CONCLUSION
Major advances have occurred in both non-mydriatic fundus photography and telemedicine, but to reach their full potential, a broad and sustained multidisciplinary approach to research and implementation will be required. If that full potential is reached, these technologies will impact all areas of vision health and research with transformative ripple effects throughout the rest of medicine.
Declaration of interest: This study was supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology). Dr. Bruce receives research support from the NIH/NEI (K23-EY019341). Dr. Newman is a recipient of the Research to Prevent Blindness Lew R. Wasserman Merit Award. The authors have no relevant financial disclosures. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Sobri M, Lamont AC, Alias NA, Win MN. Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol 2003;76:532–535 [DOI] [PubMed] [Google Scholar]
- 2.Thulasi P, Fraser CL, Biousse V, Wright DW, Newman NJ, Bruce BB. Non-mydriatic ocular fundus photography among headache patients in an emergency department. Neurology 2013;80:432–437. [DOI] [PMC free article] [PubMed]
- 3.Stern GA. Teaching ophthalmology to primary care physicians. The Association of University Professors of Ophthalmology Education Committee. Arch Ophthalmol 1995;113:722–724 [DOI] [PubMed] [Google Scholar]
- 4.Roberts E, Morgan R, King D, Clerkin L. Funduscopy: a forgotten art? Postgrad Med J 1999;75:282–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mottow-Lippa L. Ophthalmology in the medical school curriculum: reestablishing our value and effecting change. Ophthalmology 2009;116:1235–1236 [DOI] [PubMed] [Google Scholar]
- 6.Bruce BB, Lamirel C, Biousse V, Ward A, Heilpern KL, Newman NJ, Wright DW. Feasibility of nonmydriatic ocular fundus photography in the emergency department: phase I of the FOTO-ED study. Acad Emerg Med 2011;18:928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce BB, Lamirel C, Wright DW, Ward A, Heilpern KL, Biousse V, Newman NJ. Nonmydriatic ocular fundus photography in the emergency department. N Engl J Med 2011;364:387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett TJ, Barry CJ. Ophthalmic imaging today: an ophthalmic photographer's viewpoint—a review. Clin Exp Ophthalmol 2009;37:2–13 [DOI] [PubMed] [Google Scholar]
- 9.Bernardes R, Serranho P, Lobo C. Digital ocular fundus imaging: a review. Ophthalmologica 2011;226:161–181 [DOI] [PubMed] [Google Scholar]
- 10.Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol 2002;134:204–213 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed J, Ward TP, Bursell SE, Aiello LM, Cavallerano JD, Vigersky RA. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care 2006;29:2205–2209 [DOI] [PubMed] [Google Scholar]
- 12.Maberley D, Morris A, Hay D, Chang A, Hall L, Mandava N. A comparison of digital retinal image quality among photographers with different levels of training using a non-mydriatic fundus camera. Ophthalmic Epidemiol 2004;11:191–197 [DOI] [PubMed] [Google Scholar]
- 13.Lamirel C, Bruce BB, Wright DW, Delaney KP, Newman NJ, Biousse V. Quality of nonmydriatic digital fundus photography obtained by nurse practitioners in the emergency department: the FOTO-ED study. Ophthalmology 2012;119:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulze-Dobold C, Erginay A, Robert N, Chabouis A, Massin P. Ophdiat((R)): five-year experience of a telemedical screening programme for diabetic retinopathy in Paris and the surrounding area. Diabetes Metab 2012;38:450–457 [DOI] [PubMed] [Google Scholar]
- 15.Williams GA, Scott IU, Haller JA, Maguire AM, Marcus D, McDonald HR. Single-field fundus photography for diabetic retinopathy screening: a report by the American Academy of Ophthalmology. Ophthalmology 2004;111:1055–1062 [DOI] [PubMed] [Google Scholar]
- 16.Salcone EM, Johnston S, VanderVeen D. Review of the use of digital imaging in retinopathy of prematurity screening. Semin Ophthalmol 2010;25:214–217 [DOI] [PubMed] [Google Scholar]
- 17.Ausayakhun S, Skalet AH, Jirawison C, Ausayakhun S, Keenan JD, Khouri C, Nguyen K, Kalyani PS, Heiden D, Holland GN, Margolis TP. Accuracy and reliability of telemedicine for diagnosis of cytomegalovirus retinitis. Am J Ophthalmol 2011;152:1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, de Jong PT. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004;45:2129–2134 [DOI] [PubMed] [Google Scholar]
- 19.Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, Pinsky JL, Klein R. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 1999;150:263–270 [DOI] [PubMed] [Google Scholar]
- 20.Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull 2005;73–74:57–70 [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, McIntosh R. Systemic associations of retinal microvascular signs: a review of recent population-based studies. Ophthalmic Physiol Opt 2005;25:195–204 [DOI] [PubMed] [Google Scholar]
- 22.Wong TY, Rosamond W, Chang PP, Couper DJ, Sharrett AR, Hubbard LD, Folsom AR, Klein R. Retinopathy and risk of congestive heart failure. JAMA 2005;293:63–69 [DOI] [PubMed] [Google Scholar]
- 23.Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, McNamara M, Thom SA, Klein R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension 2006;47:975–981 [DOI] [PubMed] [Google Scholar]
- 24.Mimoun L, Massin P, Steg G. Retinal microvascularisation abnormalities and cardiovascular risk. Arch Cardiovasc Dis 2009;102:449–456 [DOI] [PubMed] [Google Scholar]
- 25.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat 2005;206:319–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong TY, Klein R, Sharrett AR, Nieto FJ, Boland LL, Couper DJ, Mosley TH, Klein BE, Hubbard LD, Szklo M. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke 2002;33:1487–1492 [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki R, Cheung N, Mosley T, Islam AF, Sharrett AR, Klein R, Coker LH, Knopman DS, Shibata DK, Catellier D, Wong TY. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:1826–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, Couper DJ, Heiss G, Sorlie PD. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke 2006;37:82–86 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology 2005;65:1005–1009 [DOI] [PubMed] [Google Scholar]
- 30.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology 2003;110:933–940 [DOI] [PubMed] [Google Scholar]
- 31.Toffoli D, Bruce BB, Lamirel C, Henderson AD, Newman NJ, Biousse V. Feasibility and quality of nonmydriatic fundus photography in children. J AAPOS 2011;15:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Committee on Practice and Ambulatory Medicine Section on Ophthalmology; American Association of Certified Orthopedists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Eye examination in infants, children, and young adults by pediatricians: organizational principles to guide and define the child health care system and/or improve the health of all children. Ophthalmology 2003;110:860–865 [DOI] [PubMed]
- 33.Bruce BB, Thulasi P, Fraser CL, Keadey MT, Ward A, Heilpern KL, Wright DW, Newman NJ, Biousse V. Diagnostic accuracy and use of non-mydriatic ocular fundus photography by emergency department physicians: phase II of the FOTO-ED study. Ann Emerg Med in press [DOI] [PMC free article] [PubMed]
- 34.Lim MC, Lee SY, Cheng BC, Wong DW, Ong SG, Ang CL, Yeo IY. Diabetic retinopathy in diabetics referred to a tertiary centre from a nationwide screening programme. Ann Acad Med Singapore 2008;37:753–759 [PubMed] [Google Scholar]
- 35.Andonegui J, Zurutuza A, de Arcelus MP, Serrano L, Eguzkiza A, Auzmendi M, Gaminde I, Aliseda D. Diabetic retinopathy screening with non-mydriatic retinography by general practitioners: 2-year results. Primary Care Diabetes 2012;6:201–205 [DOI] [PubMed] [Google Scholar]
- 36.Lippa LM, Boker J, Duke A, Amin A. A novel 3-year longitudinal pilot study of medical students' acquisition and retention of screening eye examination skills. Ophthalmology 2006;113:133–139 [DOI] [PubMed] [Google Scholar]
- 37.Mottow-Lippa L, Boker JR, Stephens F. A prospective study of the longitudinal effects of an embedded specialty curriculum on physical examination skills using an ophthalmology model. Acad Med 2009;84:1622–1630 [DOI] [PubMed] [Google Scholar]
- 37a.Bhargava M, Cheung CY, Sabanayagam C, Kawasaki R, Harper CA, Lamoureux EL, Chow WL, Ee A, Hamzah H, Ho M, Wong W, Wong TY. Accuracy of diabetic retinopathy screening by trained non-physician graders using non-mydriatic fundus camera. Singapore Med J 2012;53:715–9 [PubMed] [Google Scholar]
- 38.Ward NP, Tomlinson S, Taylor CJ. Image analysis of fundus photographs. The detection and measurement of exudates associated with diabetic retinopathy. Ophthalmology 1989;96:80–86 [PubMed] [Google Scholar]
- 39.Faust O, Acharya UR, Ng EY, Ng KH, Suri JS. Algorithms for the automated detection of diabetic retinopathy using digital fundus images: a review. J Med Syst 2012;36:145–157 [DOI] [PubMed] [Google Scholar]
- 40.Nayak J, Bhat PS, Acharya R, Lim CM, Kagathi M. Automated identification of diabetic retinopathy stages using digital fundus images. J Med Syst 2008;32:107–115 [DOI] [PubMed] [Google Scholar]
- 41.Yun WL, Acharya UR, Venkatesh YV, Chee C, Min LC, Ng EYK. Identification of different stages of diabetic retinopathy using retinal optical images. Inform Sci (Ny) 2008;178:106–121 [Google Scholar]
- 42.Echegaray S, Zamora G, Yu H, Luo W, Soliz P, Kardon R. Automated analysis of optic nerve images for detection and staging of papilledema. Invest Ophthalmol Vis Sci 2011;52:7470–7478 [DOI] [PubMed] [Google Scholar]
- 43.Lamirel C, Bruce BB, Wright DW, Newman NJ, Biousse V. Nonmydriatic digital ocular fundus photography on the iPhone 3G: the FOTO-ED study. Arch Ophthalmol 2012;130:939–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Wang EH, Pokabla MJ, Noecker RJ. Teleophthalmology assessment of diabetic retinopathy fundus images: smartphone versus standard office computer workstation. Telemedicine J E-Health 2012;18:158–162 [DOI] [PubMed] [Google Scholar]
- 45.Anderson ER, Smith B, Ido M, Frankel M. Remote assessment of stroke using the iPhone 4. J Stroke Cerebrovasc Dis 2011. [in press] [DOI] [PubMed] [Google Scholar]
- 46.Wu O, Langhorne P. The challenge of acute-stroke management: does telemedicine offer a solution? Int J Stroke 2006;1:201–207 [DOI] [PubMed] [Google Scholar]