Abstract
Globoid cell leukodystrophy (GLD) is a neurological disease caused by deficiency of the lysosomal enzyme galactosylceramidase (GALC). In the absence of GALC, the cytotoxic glycosphingolipid, psychosine (psy), accumulates in the nervous system. Psychosine accumulation preferentially affects oligodendrocytes, leading to progressive demyelination and infiltration of activated monocytes/macrophages into the CNS. GLD is characterized by motor defects, cognitive deficits, seizures, and death by 2–5 years of age. It has been hypothesized that psychosine accumulation, primarily within lipid rafts, results in the pathogenic cascade in GLD. However, the mechanism of psychosine toxicity has yet to be elucidated. Therefore, we synthesized the enantiomer of psychosine (ent-psy) to use as a probe to distinguish between protein-psy (stereo-specific enantioselective) or membrane-psy (stereo-insensitive nonenantioselective) interactions. The enantiomer of psychosine has equal or greater toxicity compared with psy, suggesting that psy exerts its toxicity through a nonenantioselective mechanism. Finally, in this study we demonstrate that psy and ent-psy localize to lipid rafts, perturb natural and artificial membrane integrity, and inhibit protein Kinase C translocation to the plasma membrane. Although other mechanisms may play a role in disease, these data strongly suggest that psy exerts its effects primarily through membrane perturbation rather than through specific protein-psy interactions.
Keywords: membrane alteration, enantiomer, Krabbe disease
Globoid cell leukodystrophy (GLD) is a rapidly progressing pediatric neurodegenerative disease caused by missing or dysfunctional lysosomal enzyme galactosylceramidase (GALC). Hallmarks of thi s disease include macrophage (globoid cell) infiltration into the brain parenchyma, loss and dysfunction of myelin and oligodendrocytes, and axonal damage (1, 2). The GALC enzyme is responsible for cleaving the glycosydic linkage of galactosylceramide and galatosylsphingosine (psychosine or psy) (3). Although galactosylceramide can be degraded by other enzymes, psy cannot and subsequently accumulates to high levels in the brains of GALC-deficient individuals (4).
Psychosine is a highly cytotoxic lipid, capable of inducing cell death in a wide variety of cell types including, most relevantly to GLD, oligodendrocytes (5). Psychosine accumulation was postulated to be the pathogenic mechanism leading to GLD nearly 40 years ago (4, 6, 7). However, the mechanism of psychosine toxicity has remained elusive (8). Psychosine induces pleiotropic effects, including dysfunctions in several cellular pathways and compartments with no clear mechanistic connection between them (9–13). No unified cause for these dysfunctions has been proposed. Given the wide-ranging nature of psy's effects, it seems unlikely that psy toxicity is mediated through a single protein-binding partner, such as a receptor. It is similarly unlikely that psy specifically binds many proteins independently.
As an amphipathic molecule, psy would be expected to partition largely into cellular membranes. Indeed, White et al. (14) demonstrated that psy preferentially partitions into detergent-resistant membrane microdomains rich in cholesterol. Cholesterol has a chemical affinity for sphingolipids such as psy (15); therefore, this association is not surprising. It is possible that psy disrupts the biophysical properties of membranes it inserts into, thereby causing widespread downstream effects. Membrane perturbations have been implicated in other diseases, including Alzheimer's (16), cancer (17), and diabetes (18), providing evidence that this mechanism is possible and perhaps even common.
Enantiomers are powerful tools with which to investigate biological processes (19). Enantioselectivity has recently been used to distinguish between the protein-mediated and membrane-mediated effects of cholesterol and other steroids (20–22). Similarly, enantioselectivity can be used to study psy modes of action. Enantiomers (psy and ent-psy) have identical physicochemical properties but are mirror images of each other. Typically, only one enantiomer of lipids, proteins, sugars, nucleic acids, and cofactors are found within cells. Proteins are chiral molecules with a defined three-dimensional structure and generally only recognize one enantiomer of their binding partners (psy or ent-psy) (19). Accordingly, psy interactions with proteins are expected to be largely enantioselecitve. Conversely, the lipid membrane, even though it is composed of chiral molecules, is fluid and does not maintain well-defined “binding sites” for other lipids or sterols. Thus, lipid-lipid and lipid-sterol membrane interactions of psy are nonenantioselective. Additionally, any membrane property altered by psy (i.e., polarity, fluidity, and packing capacity) will be altered equivalently by ent-psy because of its identical chemical and physical properties. This point is important because if psy and ent-psy had different physicochemical properties then they might alter membrane properties differently even if their membrane interactions were not enantioselective. Because of this fundamental difference between membranes and proteins, protein-mediated actions are usually enantiosensitive, whereas membrane-mediated actions are not. Therefore, enantiomers of natural molecules can be used as probes to distinguish between enantio-specific (protein-mediated) and nonenantio-specific (lipid membrane-based) functions (19). We previously synthesized the unnatural enantiomer of psy (ent-psy) (23). In this study, the underlying causes of psy toxicity are investigated using ent-psy as a probe to distinguish between protein- and membrane-based mechanisms.
Materials and Methods
Chemicals and reagents ent
Psychosine was synthesized as previously described (23) Psychosine was obtained from Matreya (Pleasant Gap, PA). Both lipids were dissolved in DMSO to 20 mM before use unless otherwise noted.
Cell viability measurements
Cells from the human glial (oligodendrocytic) cell line MO3.13 (Cedarlane Labratories, Burlington, NC) were maintained in DMEM with 10% FBS. Before lipid treatment, cells were plated to a density of 2 × 104 cells/cm2 on uncoated plastic dishes. After 24 h, the media was replaced with serum-free media. After an additional 24 h, media containing lipids or vehicle was added. Cells were exposed to the lipids for 24 h before measuring cell survival. Cell viability was measured using the commercially available MTT assay (ATCC, Manassas, VA). This procedure is based on the cleavage of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to a blue product by mitochondrial dehydrogenase in viable cells. Absorbance was measured at 570 nm and compared with the reference wavelength of 650 nm to determine cell viability. Cell survival after lipid treatment was normalized to the vehicle (DMSO) control.
Psy- and ent-psy-mediated apoptosis
The proportion of cells undergoing apoptosis mediated by exposure to psy or its ent-psy was measured using Annexin V according to the manufacturer's instructions. Briefly, oligodendrocyte cells, M03.13, were plated at ∼80% confluence on day 1. The cells were serum-starved for 24 h, and then the appropriate concentration (10, 20, and 40 μM) of psychosine or the enantiomer was added for an additional 24 h in the absence of fetal calf serum. The cells were harvested, washed once in PBS, and resuspended at ∼1 × 106 cells/ml in Annexin V binding buffer (10 mM, HEPES [7.4], 140 mM NaCl, 2.5 mM CaCl2). Allophycocyanin-conjugated Annexin V (cat# A35110; Life Technologies, Eugene, OR) and 7AAD (viability) were added to the cell suspensions and incubated 15 min at room temperature. The Annexin V-positive cells were detected by fluorescence activated cell sorting using a Beckman Coulter Gallios instrument (Brea, CA) and analyzed using FloJo software (Ashland, OR).
Psy and ent-Psy degradation
MO3.13 cells were pulsed with 10 μM psy, ent-psy or an equivalent volume of vehicle for 30 min, after which they were washed and returned to normal media at 37°C. At 0, 15, 30, and 60 min postpulse, cells were washed, harvested, spun down, and flash frozen. Once thawed, cells were quickly homogenized in 0.04 M citric acid by passing five times through a 25 gauge needle . Fifty microliters of each sample was added to 20 μl of N,N-dimethylpsychosine (250 ng/ml) internal standard and 200 μl MeOH. Samples were vortexed and centrifuged, and the supernatant was collected. This extraction was repeated on the remaining pellet and the supernatants pooled. Psy or ent-psy concentrations were obtained using a column-switching LC-MS/MS method. Detection was achieved using an AB SCIEX 4000QTRAP tandem mass spectrometer (Applied Biosystems/MDS Sciex Inc., Ontario, Canada) using ESI in the positive ion mode along with multiple reaction monitoring. Analyst software (version 1.5.1; Applied Biosystems/MDS Sciex Inc.) was used for the data analysis. The calibration curves (analyte peak area/internal standard peak area for y-axis and analyte concentration for x-axis) of psychosine were obtained using the least square linear regression fit (y = ax + b) and a weighting factor of 1/x2. The coefficient of determination (r2) was set as >0.98 for acceptance criteria of calibration curves.
Immunocytochemistry
HeLa cells were plated at a density of 2 × 104 cells/cm2 on poly-L-lysine (Sigma, St. Louis MO) coated glass coverslips in serum-free standard media (DMEM-F12). After 24 h of growth, the cells were exposed to lipids or vehicle for an additional 24 h. After this time, stimulated cells were treated with 20 ng/ml platelet-derived growth factor for 15 min at 37°C. After stimulation, the cells were fixed with 4% paraformaldehyde. Plasma membranes were stained with fluorescently labeled cholera toxin B (CTXB, Sigma) before incubation with anti-phosphoPKC primary antibody (Cell Signaling, Danvers, MA). After incubation with secondary antibodies (Invitrogen, Grand Island, NY), the coverslips were mounted and imaged with a Zeiss confocal microscope in the Alafi Neuroimaging Core (Washington University in St. Louis, Hope Center). Images from random fields were acquired. Membrane translocation was quantified by an independent observer blinded to treatment who scored each cell as “membrane associated” or “cytoplasmic.” More than 30 cells per treatment were scored in this manner.
Liposome preparation
Carboxyfluorescein (CF)-loaded DOPC (Sigma) liposomes were generated essentially as previously described (24). Dried DOPC was dissolved in 0.5 ml ethyl ether, and an equal volume of 20 mM CF (Molecular Probes, Eugene, OR) in elution buffer (100 mM KCl, 10 mM HEPES [pH 7.0], 1 mM EDTA) was added. This mixture was sonicated three times for 20 s each to generate a thick emulsion before evaporation of the ether under reduced pressure. The evaporated solution was passed five times through a 22 guage needle and a mini-extruder (Avanti, Alabaster, AL) containing a 200 nM membrane to limit liposome size (Nuclepore, Pleasanton, CA). All CF not contained within a liposome was removed by passing the solution over a Sephadex G-25-80 column. Liposome concentration was normalized against the maximum fluorescence achieved after the vesicles were lysed with 20% Triton-X-100.
Liposome swelling
Liposomes were diluted in elution buffer to a concentration appropriate for the assay (generally 1:500 to 1:2,000) to a final volume of 1 ml. Baseline fluorescence was measured for 10 min. Lipids were then added, and the change in fluorescence was measured for an additional 60 min. At this time, 10 μl of 20% Triton-X-100 was added to lyse all liposomes so that the maximal fluorescence could be obtained. Data are presented as the percent maximal fluorescence after the baseline is subtracted.
Detergent-resistant membrane composition
Detergent-resistant membranes (DRMs) were isolated and analyzed essentially as previously reported (14). Briefly, HeLa cells were treated with lipid at a concentration of 10 μM for 6 h. The cells were scraped from the plates, pelleted, then resuspended in 2 ml cold lysis buffer (25 mM Tris-HCL [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.5% Lubrol, protease inhibitor cocktail, 1 mM PMSF, 1 mM okadaic acid, and 2 mM sodium orthovanadate). This suspension was passed through a 25 gauge needle five times to lyse the cells. An equal volume of 90% sucrose was added to the cellular lysate to give a final concentration of 45% sucrose. Four milliliters of 35% sucrose then 5% sucrose was loaded on top of the homogenates using an auto densi-flow density gradient fractionator (Labonco, Kansas City, MO). Tubes were ultracentrifuged using a SW-41 rotor (Beckman-Coulter, Brea, CA) at 39,000 rpm overnight at 4°C. Fractions were collected in 1ml volumes from the top of the fractions down, with raft markers found in fractions 4 and 5. Psy or ent-psy was measured using LC-MS. To prepare the samples, each fraction was extracted with chloroform/methanol/water then analyzed as described (25). Positive ion electrospray precursor ion scanning was performed using a triple quadrapole mass spectrometer (API 4000, Applied Biosystems) equipped with a Shimadzu HPLC system and Leap autosampler (26). Ceramide was quantified in these samples using an AB SCIEX 4000QTRAP tandem mass spectrometer (Applied Biosystems/MDS Sciex Inc.).
Western blot of membrane fractions
Western blots of membrane fractions were carried out essentially as described previously (14). Pooled aliquots from DRMs (fractions 4 and 5) were solubilized in 0.25% SDS. Volume of sample loaded was normalized, and samples were resolved in a precast 7.5% polyacrylamide gel (Biorad, Hercules, CA) using the MiniProtean electrophoresis system (Biorad). Proteins were then transferred to a PVDF membrane (Biorad), blocked with 5% nonfat milk, and probed with primary antibodies for phospho-PKC (Cell Signaling) or total PKC (Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with peroxidase-labeled secondary antibodies, the blots were visualized with chemiluminescent ECL substrate (Pierce, Rockford, IL). All treatment groups (DMSO, psy, and ent-psy) were exposed to the same film for the same amount of time. Signal was quantified using ImageJ software.
Cholesterol extractability with cyclodextrin
CHO cells were grown on uncoated plastic dishes under standard conditions in DMEM-F12 media containing 5% FBS. Twenty-four hours before testing, cells were loaded with 1 μCi/ml 3H-Cholesterol (PerkinElmer, Waltham, MA). At this time, cells were also treated with 20 μM lipids or vehicle control. After 24 h, the media was washed off, and cells were trypsinized and pelleted. The cell pellet was washed three times with HEPES/DMEM (50 mM HEPES in DMEM) to remove any remaining 3H-cholesterol and resuspended in HEPES/DMEM at 4 × 106 cells/ml. Six hundred microliters of this cell suspension was added to 3 ml acceptor medium (50 ml 2-hydroxypropyl-β-cyclodextrin in HEPES/DMEM 50% cholesterol saturated) and incubated at 37°C. Aliquots (200 μl) were removed after 0, 0.5, 1, 2, 4, 6, 10, and 20 min. These aliquots were filtered to remove the cells, and the flow-through containing CD-extracted 3H-cholesterol was collected. The amount of 3H-cholesterol in the samples was measured by scintillation counting and normalized to the time zero measurement. Control samples were treated with lyso-phosphatidylserine (25 μM; decreases cholesterol extractability), 25-hydroxycholesterol (1 μM; increases cholesterol extractability), or 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (1 μM; has no effect on cholesterol extractability).
Statistical analysis
Graphpad Prism software (GraphPad Software, Inc., La Jolla, CA) was used to generate all graphs and perform all statistical analyses. All data are the average of at least three experiments and are presented as the mean ± SEM unless otherwise noted. Student's t-tests, one-way ANOVA, or repeated measures ANOVA were used where appropriate. P values <0.05 were considered significant.
Results
Ent-psy-protein interactions
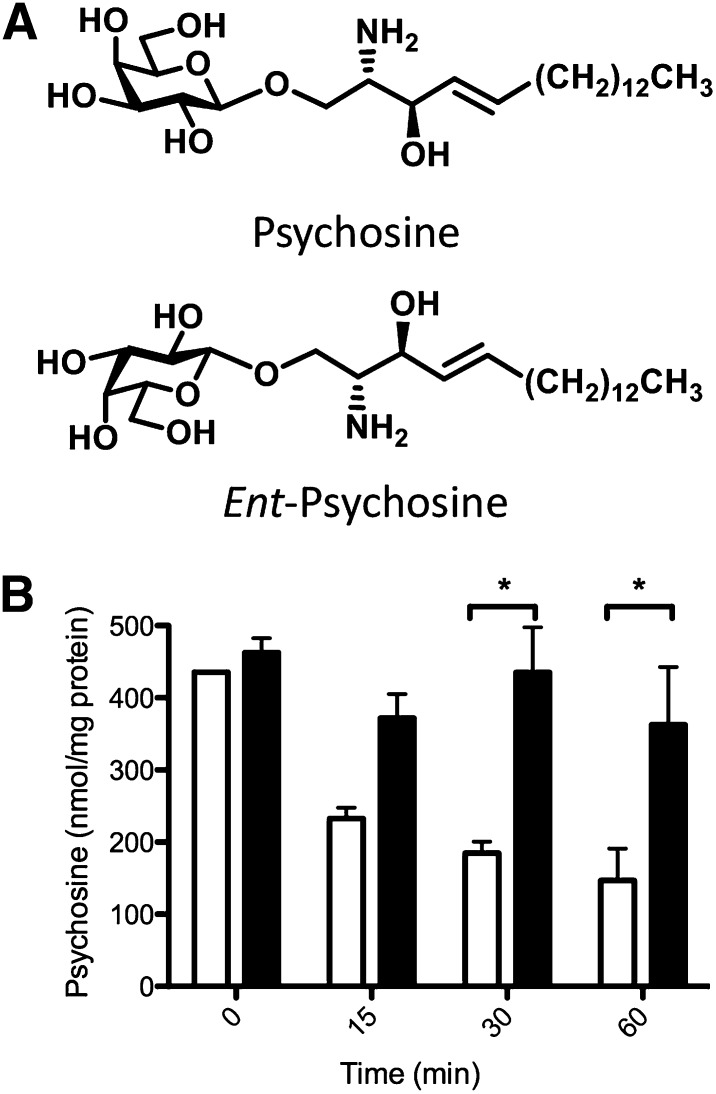
We first confirmed that ent-psy (Fig. 1A) could not interact with the only known psy-binding protein, GALC, as predicted. We measured the degradation of psy and ent-psy over time in MO3.13 cells (which have functional GALC). Whereas psy was rapidly degraded to a steady-state level after 1 h, levels of ent-psy remained high over this time course (Fig. 1B). Therefore, GALC does not recognize and degrade ent-psy. These data also clearly demonstrate that exogenously added lipid does enter cells and lysosomes because it could not otherwise be available to the lysosomal enzyme GALC for degradation.
Fig. 1.
A: Psychosine and its enantiomer. B: Psy (open bars) is degraded by GALC over time in normal MO3.13 cells, but ent-psy (filled bars) is not. By 30 min after a lipid pulse, the amount of ent-psy remaining in cells is significantly more than the amount of undigested psy. As expected, GALC is incapable of degrading the enantiomer of its normal substrate. *P < 0.05.
Cell viability
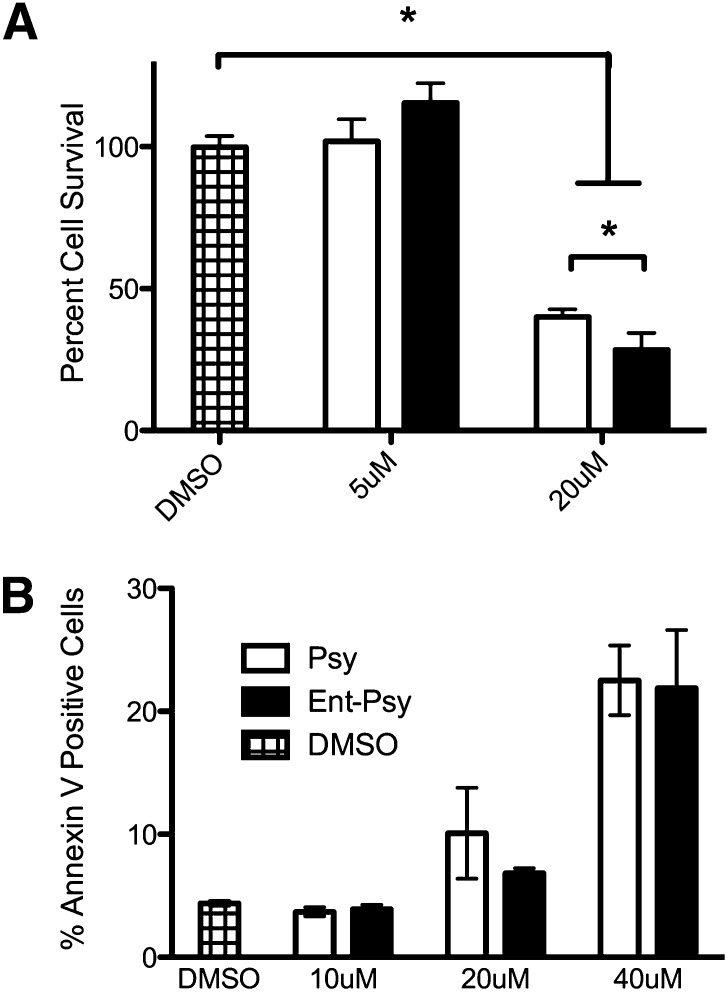
The oligodendrocytic cell line MO3.13 has been used frequently for psy-toxicity studies. After a 24-hr serum-starvation, these cells were treated with psy, ent-psy, or vehicle (DMSO) at a lethal (20 μM) or sublethal (5 μM) dose. These concentrations were empirically determined. Psy at 20 μM has been shown to be toxic, and a dose response curve revealed that 5 μM is not. After 24 h, cell viability was assessed (Fig. 2A). As expected, neither psy nor ent-psy demonstrated toxicity at 5 μM. Both lipids were highly cytotoxic at 20 μM, showing only 20–30% cell viability after 24 h. At concentrations higher than 20 μM, 0% cell viability was observed. A small but significant increase in toxicity of ent-psy relative to psy was observed.
Fig. 2.
A: Both psy and ent-psy cause significant cell death in an oliogodendrocytic cell line, MO3.13, at 20 μM. Neither lipid induces cell death at 5 μM. ent-Psy causes a small but significant increase in cell death compared with psy at 20 μM. B: Both psy and ent-psy induce apoptosis in a dose-dependent manner in MO3.13 cells. There is no statistical difference in the percentage of Annexin-V-positive cells after treatment with psy or ent-psy at any concentration tested.
Psy- and Ent-Psy-mediated apoptosis
Psychosine causes cell death at least in part via apoptosis (10, 27, 28). It was theoretically possible that psy and ent-psy could cause cell death through different mechanisms. To investigate this, we treated cells with psy or ent-psy and measured the percentage of cells staining positively for the apoptosis marker Annexin V. Treatment with either lipid resulted in a dose-dependent increase in Annexin V-positive cells, and both lipids induced apoptosis equivalently (Fig. 2B). Therefore, not only is psy toxicity not enantioselective, but both enantiomers likely induce death through the same mechanism(s).
Translocation of PKC
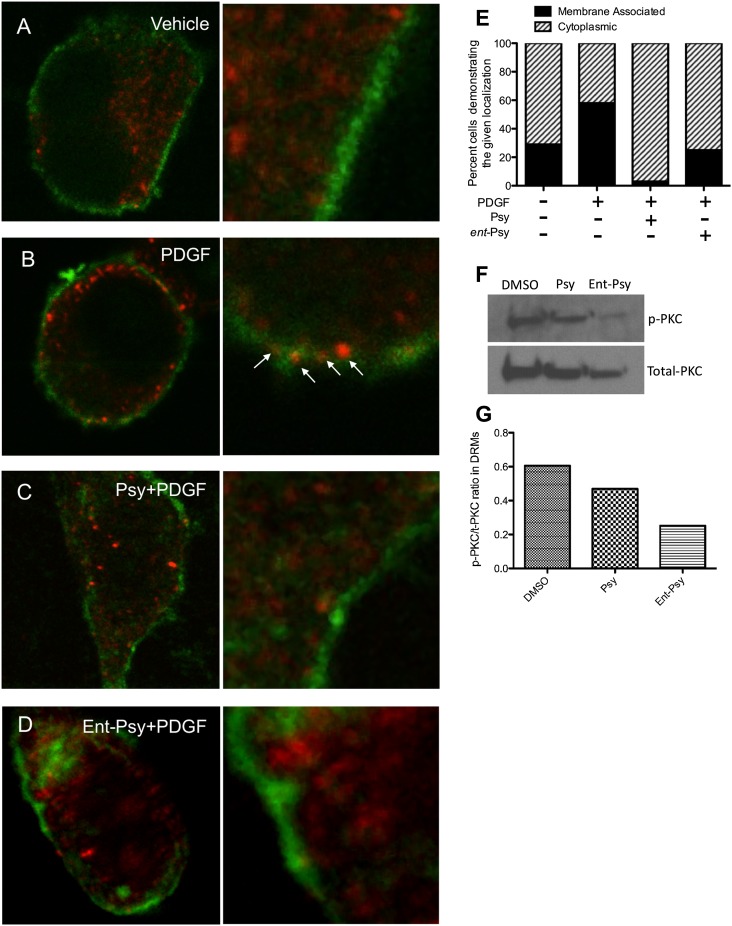
Protein kinase C is an important signal transduction protein involved in regulating numerous cellular functions. Psychosine is a known inhibitor of PKC. White et al. (14) demonstrated that phosphorylated PKC (p-PKC, active) does not translocate to the plasma membrane of cells treated with psy under PKC stimulating conditions. However, it was unclear whether this is due to direct binding of psy to either of PKC's lipid-binding domains. Phospholipid binding is required for PKC translocation to the membrane and function. Although it is unlikely that psy would bind in a pocket optimized for phospholipids, it is possible that psy may be able to aberrantly enter the lipid-binding site of PKC. It is equally possible that psy may act by altering the plasma membrane in such a way as to exclude PKC association without binding to PKC. To test this, we treated cells with psy or ent-psy to determine whether psy bound to PKC directly because ent-psy should not be capable of such an interaction. The lipid-binding domain of PKC is stereospecific and would be predicted not to recognize the enantiomer of a lipid binding partner (29). We observed that psy and ent-psy inhibit the translocation of PKC to the membrane surface under stimulating conditions (Fig. 3). Previously, activated PKC (p-PKC) has been shown to be diminished at the DRMs in the brains of Twi mice compared with normal animals (14). Significantly less p-PKC is found associated with the DRM fraction of psy or ent-psy treated cells, so this inhibition of association, even in an unstimulated cell, is nonenantioselective. These data suggest that psy inhibition of PKC is most likely due to an alteration of the lipid environment rather than a direct binding of psy to PKC itself.
Fig. 3.
Phospho-PKC translocation in response to platelet-derived growth factor (PDGF) after lipid exposure. Phospho-PKC (red) is normally intracellular (A). When cells are stimulated with PDGF, PKC translocates to the cell surface, where it is functionally active (B). Treatment with 10 μM of psy (C) or ent-psy (D) abolished the translocation of PKC to the cell surface. Plasma membrane is labeled with fluorescently conjugated cholera toxin b (green). This translocation was quantified by a blinded observer scoring randomly selected cells as having membrane-associated or cytoplasmic p-PKC distribution (E). The percentage of cells with membrane associated PKC was increased after PDGF compared with unstimulated cells (∼60% vs. ∼25%). Treatment with psy or ent-psy before PDGF stimulation reduced the percentage of cells with translocated PKC to approximately that of unstimulated cells. The amount of p-PKC (active) relative to total PKC associated with the DRMs of lipid-treated cells was assessed. Treatment with psy or ent-psy inhibits the association of p-PKC with the DRMs (F, G).
Liposome swelling
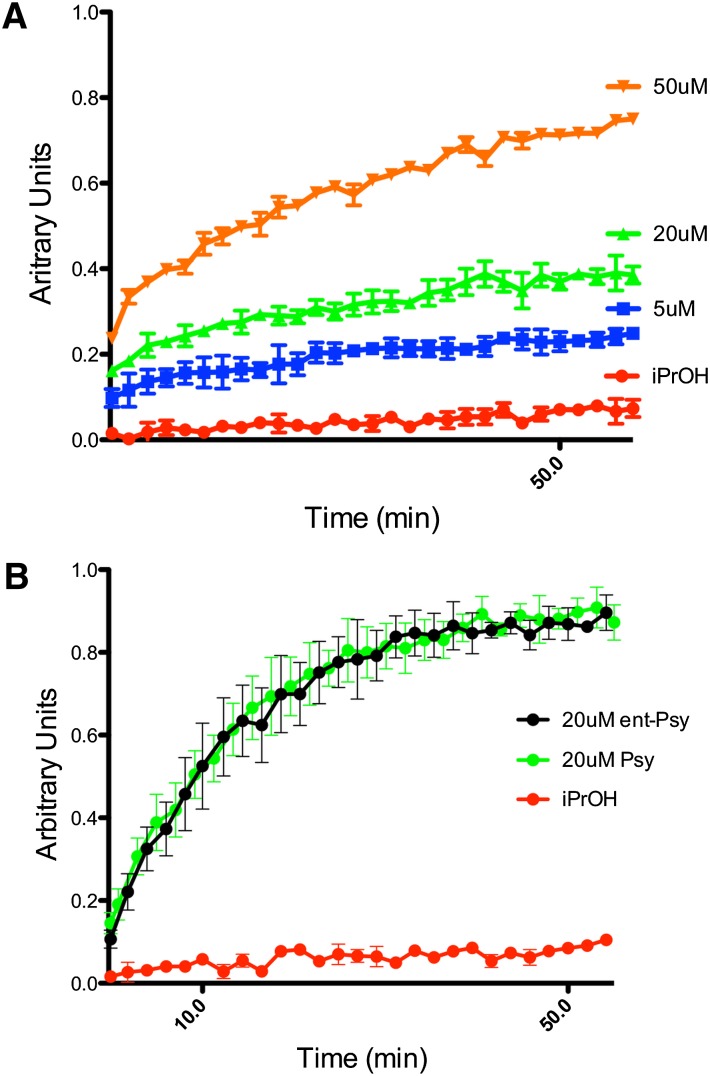
To further investigate what effects psy may have on membranes, we used a liposome-based system. Disruptive lipids cause the liposomes to swell, whereas more inert lipids have no effect (30). Lipid vesicles composed of DOPC and filled with CF at a self-quenching concentration were exposed to psy at concentrations of 5, 20, and 50 μM. These concentrations of lipid are physiologically relevant because they represent nontoxic, moderately toxic, and extremely toxic levels of psy in this study, respectively. A dose-dependent increase in fluorescence indicating liposome swelling was observed at all psy concentrations studied (Fig. 4A). This was repeated with ent-psy, and, as expected, the same result was observed (Fig. 4B).
Fig. 4.
A: Psy induces significant swelling in CF-loaded DOPC liposomes in a dose-dependent manner. The addition of psy at 5 μM (blue), 20 μM (green), and 50 μM (orange) caused disruption of the liposomal membrane sufficient to allow water influx, which resulted in CF dequenching. B: Psy (green) and ent-psy (black) have an indistinguishable effect on liposomes at 20 μM. Both lipids caused significant swelling and CF dequenching.
Distribution of Psy and ent-Psy
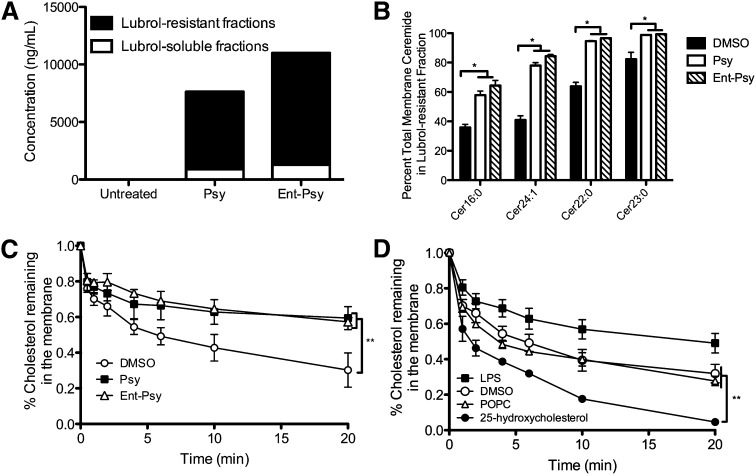
Psychosine accumulates in cholesterol-rich microdomains and may have some effect on the protein composition of these domains (14). We determined that ent-psy, like psy, localizes to DRM microdomains in treated cells (Fig. 5A). This was expected given the identical physical properties of these two molecules.
Fig. 5.
A: ent-Psy partitions into DRMS (black), rather than detergent-soluble fractions (white), in the same manner as psy. A slight increase in ent-psy concentration is observed, although the same quantity of lipid was added to each cohort of cells. This is likely due to the lack of ent-psy degradation over the course of the experiment (Figure 2). B: A significant increase in the percentage of total cellular ceramides is found in the DRMs of psy and ent-psy treated cells relative to vehicle controls, indicating an alteration of DRM composition after lipid exposure. Other classic DRM lipids (sphingomyelin) were unaffected, suggesting that this is a specific phenomenon. C: Psy (closed squares) and ent-psy (open triangles) inhibit the extraction of cholesterol by CD from membranes of CHO cells. Significantly more 3H-cholesterol remains in the membranes of psy or ent-psy treated cells after 4 min of exposure to CD-containing media. **P < 0.01. D: Control lipids LPC, 25-hydroxycholesterol, and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) decrease, increase, and do not affect (respectively) cholesterol extraction from the membrane, as expected.
DRM microdomain composition
Because psy is known to accumulate in Lubrol-resistant membrane microdomains and to increase the percentage of cellular cholesterol there, we investigated whether other lipids typically associated with these domains were similarly altered. We found that several ceramide species were significantly enriched in the detergent-resistant fractions after psy or ent-psy exposure relative to vehicle-treated cells (Fig. 5B). To account for any changes in absolute lipid concentration, the percentage of total cellular ceramides found in the lubrol-resistant fractions is reported. The fraction of total cellular sphingomyelins found in the DRMs was unchanged after psy or ent-psy exposure (data not shown).
Cholesterol extractability
To investigate the effect of psy on the condition of the plasma membrane, we used a cholesterol extractability assay. The heptameric sugar molecule 2-hydroxyproyl-β-cyclodextrin (CD) is a large cyclic molecule capable of removing cholesterol from membranes (31). The extent to and speed with which cholesterol is removed by CD can be used to assess the environment of the membrane (31). Cholesterol was significantly less extractable when cells were treated with psy or ent-psy relative to the vehicle control (Fig. 5C). Several lipids with known effects on cholesterol extractability were tested as controls. As expected, lysophosphatidylserine decreased extractability, 25-hydroxycholesterol increased extractability, and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine had no effect on cholesterol extractability (Fig. 5D). The total cholesterol of the cells was unchanged across all treated groups (data not shown).
DISCUSSION
Much effort has been expended searching for the cellular pathway(s) or receptor(s) responsible for psy toxicity. One possibility that has been largely unrecognized, however, is that psy may have a global and physicochemical effect on the membrane environment and little or no direct binding to specific proteins. Indeed, several lines of evidence would seem to suggest a membrane-based toxicity mechanism. Psy causes hemolysis (32), causes the inhibition of cytochrome c oxidase irrespective of the protein's orientation (33, 34), and has never been shown to bind directly to any receptor or signaling protein despite significant investigation. Furthermore, a large number of proteins and cellular systems are affected by psy, making the likelihood that psy directly interacts with every one of them unlikely. The enantiomer of psy, which was recently synthesized (23), is a powerful probe to test this possibility (19).
We first sought to confirm that ent-psy is not recognized as a binding partner by the only known psy-binding protein, GALC. As expected, ent-psy is not recognized by GALC and therefore is not degraded (Fig. 1B). This simple observation validates the use of ent-psy as a probe to investigate the mechanism(s) of psy toxicity.
We observed that ent-psy has an equal or greater toxicity profile to the natural lipid. This finding strongly supports a nonenantio-specific mechanism for the majority of psy toxicity because ent-psy is incapable of any enantio-specific interactions with proteins that psy may have. Because it was possible that the two lipids were causing cell death through different pathways, we confirmed that both enantiomers are initiating cell death at least in part through apoptosis, as was previously reported for psy (10, 27, 28). In addition, the failure of GALC to degrade ent-psy causes treated cells to be exposed to a higher concentration of that lipid relative to psy. These data suggest a membrane perturbation-based mode of action for psy.
This is further supported by the finding that PKC, a known psy-sensitive protein capable of directly binding lipids (normally DAG and phospholipid such as phosphatidylserine), is equally inhibited by either enantiomer of psy. Of all the proteins previously implicated in psy toxicity, PKC was perhaps the most likely psy-binding candidate given its two lipid binding domains. If psy bound to PKC directly, one would expect that ent-psy should not interfere with normal p-PKC localization and translocation because that interaction should be stereo-specific and therefore unavailable to ent-psy. Therefore, the observation that ent-psy still inhibits translocation argues that the defect preventing translocation lies in the membrane, not within a PKC-lipid complex. This is not a stimulation-dependent phenomenon because both psy and ent-psy reduce the amount of p-PKC (active PKC) associated with the DRM even in a resting cell, consistent with previous reports (14). We believe that this is further evidence supporting a perturbation in membrane integrity after psy exposure. Disruption of membrane microdomains by other means have been shown to alter PKC activity, further supporting this interpretation (35). Although all known psy-sensitive proteins could not be tested, given the fact that psy toxicity is nonenantio-selective, it is anticipated that many, if not most, psy-sensitive proteins are affected in a similarly indirect manner.
These data do not exclude possible nonspecific lipid-protein associations. Therefore, it was vital to directly investigate the effect of psy on membranes. Through in vitro experiments we observed that psy is indeed capable of affecting model membrane systems devoid of protein components, such as liposomes. Any effects observed in such a system can only be due to the biophysical properties of the lipid. Psy and its enantiomer altered bilayers of liposomes, making them more water permeable and allowing the vesicles to expand their volume as the added sphingolipid partitioned into the membrane (21, 36). It is clear that psy and ent-psy alter the integrity of model membrane systems. Given its enantio-insensitive toxicity, psy is likely deleterious to more complex cell membranes as well.
Even in the complex systems of whole cells, psy alters the plasma membrane. psy reduces the extractability of cholesterol from the membrane, demonstrating a clear alteration of normal membrane properties. Ent-psy affected this measurement in an identical direction and magnitude when compared with psy. This is consistent with previous reports showing no change in total cholesterol in GLD brains or psy-treated cells but an increased proportion of total cholesterol in the raft fractions compared with nonraft fractions (14). Because these DRMs are more condensed, the cholesterol is likely protected from CD extraction in these structures. Increased DRM cholesterol could be a result of favorable psy-cholesterol interactions (15), wherein psy has affinity for the already cholesterol-rich DRM and in turn stabilizes additional cholesterol within that domain. This is consistent with previous reports that a greater percentage of total cellular cholesterol is located within the detergent-resistant membrane domains from psy-treated cells and the brains of GALC-deficient animals (14).
Because cholesterol localization had previously been reported to be altered after psy treatment, we investigated whether other classic DRM lipids were similarly altered. We found that several ceramide species were enriched in the DRM fractions. We also observed that there were no changes in sphingomyelin distribution between the DRMs and the detergent soluble membranes. This demonstrates that the changes in membrane composition after psy treatment are specific and not simply an artifact of lipid addition to these cells. Although the in vivo relevance of DRMs is under investigation, these data, combined with those previously generated, strongly suggest that psy exposure results in alterations in membrane distribution of certain lipids. Although we cannot, at this point, determine precisely how these alterations initiate or contribute to the toxicity of psy, it is very likely that these events are involved.
These findings open up a wide range of future studies to determine the precise biophysical alterations imposed upon the membrane by psy. These data do not exclude the possibility of nonspecific psy-protein binding, which would be available to psy as well as ent-psy. These types of interactions are less common than enantio-specific lipid-protein binding but are certainly possible (19). However, given the observations that toxicity is nonenantioselective and that psy disrupts in vivo and in vitro membranes in a number of ways, the preponderance of evidence suggests a membrane-based toxicity mechanism. It is also possible that specific protein-mediated effects coexist with membrane alterations in mediating psy toxicity, and this possibility should be further investigated. However, the nonenantioselective component of toxicity is sufficient to cause cell death, which supports the assertion that psy toxicity is mediated largely, if not entirely, through effects on the lipid bilayer.
These studies have also not investigated how the effects of psy and ent-psy may differ in individual cellular compartments. For example, what are the effects of these lipids on mitochondrial membranes or on lysosomal membranes? This would be a very interesting avenue of future investigation because these studies have investigated primarily psy's effects on plasma membranes and whole cells. Finally, all of these studies have been performed by exogenously adding psy or ent-psy to cells or liposomes. Psy added this way enters the cell because it is degraded in the lysosome by GALC (Fig. 2A). However, it is possible that lipid added in this manner may have somewhat different effects from endogenously produced psy. Previous work has indicated that the exogenously added psy produces similar, if not identical, effects to those seen in GALC-deficient mice (14). Future investigations into the state of membranes in GALC-deficient animals should be undertaken.
Given the vast number of cellular pathways originating at or including cell membranes, it is perhaps not surprising that membrane disruption by psy would be so widely toxic. It is possible that drugs or other therapies could be designed to counteract these effects and could be a promising avenue for GLD treatment. In theory, molecules similar to cyclodextrin could be used to extract psy from membranes. This approach has shown great promise in treating Neimann-Pick type C, a cholesterol storage disorder, where cyclodextrin has been successfully used to treat disease (37, 38). An approach of this type, perhaps coupled with therapies targeted at the underlying genetic defect, may hold great potential for treating GLD. These data also indicate that any GLD therapies directed toward downstream effects of psy toxicity, such as PKC activation, are likely to be only modestly efficacious at best. Because membrane disruption is such a wide-reaching mechanism, multiple cascades capable of inducing cell death are likely initiated. Targeting only one would likely be of limited benefit.
The lipid phase is increasingly appreciated as an active player in cellular events originating at the membrane. Changes in membrane properties can affect receptor functioning, protein-membrane associations, protein-protein associations, as well as ion and small molecule gradients. Perturbation of all of these cellular functions has been observed in cells exposed to psy or in GLD tissues. Membrane composition and function is altered in a number of common diseases, including Alzheimer's (16), cancer (17), and diabetes (18). As our understanding of membrane organization and functionality improves, it is likely more diseases may be added to this list.
Acknowledgments
The authors thank Doug Covey, Ph.D., for critical reading of the manuscript and Stephanie Schneider for technical assistance with the FACS analysis. Mass spectrometry analyses were performed in Washington University Metabolomics Facility and supported by P60 DK020579.
Footnotes
Abbreviations:
- CF
- carboxyfluorescein
- DRM
- detergent-resistant membrane
- ent-psy
- enantiomer of psychosine
- GALC
- galactosylceramidase
- GLD
- globoid cell leukodystrophy
- psy
- psychosine
This study was supported by NIH grants R01 HD055461 (M.S.S.) and by the Alafi Neuroimaging Laboratory, the Hope Center for Neurological Disorders, and NIH Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University.
REFERENCES
- 1.Wenger D., Suzuki K., Suzuki Y. 2001. Galactosylceramide Lipidosis: Globoid Cell Leukodystrophy (Krabbe Disease). In The Metabolic & Molecular Basis of Disease. C. Scriver, A. Beaudet, W. Sly, D. Valle, B. Childs, K. Kinzler, and B. Vogelstein, editors. McGraw-Hill Medical Publishing Division, New York. 2669–3694. [Google Scholar]
- 2.Castelvetri L. C., Givogri M. I., Zhu H., Smith B., Lopez-Rosas A., Qiu X., van Breemen R., Bongarzone E. R. 2011. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 122: 35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenger D. A., Sattler M., Hiatt W. 1974. Globoid cell leukodystrophy: deficiency of lactosyl ceramide beta-galactosidase. Proc. Natl. Acad. Sci. USA. 71: 854–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svennerholm L., Vanier M. T., Mansson J. E. 1980. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J. Lipid Res. 21: 53–64 [PubMed] [Google Scholar]
- 5.Nagara H., Ogawa H., Sato Y., Kobayashi T., Suzuki K. 1986. The twitcher mouse: degeneration of oligodendrocytes in vitro. Brain Res. 391: 79–84 [DOI] [PubMed] [Google Scholar]
- 6.Miyatake T., Suzuki K. 1973. Additional deficiency of psychosine galactosidase in globoid cell leukodystrophy: an implication to enzyme replacement therapy. Birth Defects Orig. Artic. Ser. 9: 136–140 [PubMed] [Google Scholar]
- 7.Vanier M., Svennerholm L. 1976. Chemical pathology of Krabbe disease: the occurrence of psychosine and other neutral sphingoglycolipids. Adv. Exp. Med. Biol. 68: 115–126 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K. 1998. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem. Res. 23: 251–259 [DOI] [PubMed] [Google Scholar]
- 9.Yamada H., Suzuki K. 1999. Responses to cyclic AMP is impaired in the twitcher Schwann cells in vitro. Brain Res. 816: 390–395 [DOI] [PubMed] [Google Scholar]
- 10.Haq E., Giri S., Singh I., Singh A. K. 2003. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J. Neurochem. 86: 1428–1440 [DOI] [PubMed] [Google Scholar]
- 11.Khan M., Haq E., Giri S., Singh I., Singh A. K. 2005. Peroxisomal participation in psychosine-mediated toxicity: implications for Krabbe's disease. J. Neurosci. Res. 80: 845–854 [DOI] [PubMed] [Google Scholar]
- 12.Contreras M. A., Haq E., Uto T., Singh I., Singh A. K. 2008. Psychosine-induced alterations in peroxisomes of twitcher mouse liver. Arch. Biochem. Biophys. 477: 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri S., Khan M., Nath N., Singh I., Singh A. K. 2008. The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: implication for Krabbe disease. J. Neurochem. 105: 1820–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White A. B., Givogri M. I., Lopez-Rosas A., Cao H., van Breemen R., Thinakaran G., Bongarzone E. R. 2009. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J. Neurosci. 29: 6068–6077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leventis R., Silvius J. R. 2001. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys. J. 81: 2257–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Askarova S., Lee J. C. 2010. Membrane biophysics and mechanics in Alzheimer's disease. Mol. Neurobiol. 41: 138–148 [DOI] [PubMed] [Google Scholar]
- 17.Hendrich A. B., Michalak K. 2003. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr. Drug Targets. 4: 23–30 [DOI] [PubMed] [Google Scholar]
- 18.Waczuliková I., Cagalinec M., Ulicna O., Slezak P., Ziegelhoffer A. 2010. Biophysical investigation on left ventricular myocytes in rats with experimentally induced diabetes. Physiol. Res. 59(Suppl 1): S9–S17 [DOI] [PubMed] [Google Scholar]
- 19.Covey D. F. 2009. ent-Steroids: novel tools for studies of signaling pathways. Steroids. 74: 577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langmade S. J., Gale S. E., Frolov A., Mohri I., Suzuki K., Mellon S. H., Walkley S. U., Covey D. F., Schaffer J. E., Ory D. S. 2006. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA. 103: 13807–13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale S. E., Westover E. J., Dudley N., Krishnan K., Merlin S., Scherrer D. E., Han X., Zhai X., Brockman H. L., Brown R. E., et al. 2009. Side chain oxygenated cholesterol regulates cellular cholesterol homeostasis through direct sterol-membrane interactions. J. Biol. Chem. 284: 1755–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielska A. A., Schlesinger P., Covey D. F., Ory D. S. 2012. Oxysterols as non-genomic regulators of cholesterol homeostasis. Trends Endocrinol. Metab. 23: 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parameswar A. R., Hawkins J. A., Mydock L. K., Sands M. S., Demchenko A. V. 2010. Consise synthesis of the unnatural sphingosine and psychosine enantiomer. Eur. J. Org. Chem. 3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szoka F., Jr, Papahadjopoulos D. 1978. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA. 75: 4194–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galbiati F., Basso V., Cantuti L., Givogri M. I., Lopez-Rosas A., Perez N., Vasu C., Cao H., van Breemen R., Mondino A., et al. 2007. Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. J. Neurosci. 27: 13730–13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitfield P. D., Sharp P. C., Taylor R., Meikle P. 2001. Quantification of galactosylsphingosine in the twitcher mouse using electrospray ionization-tandem mass spectrometry. J. Lipid Res. 42: 2092–2095 [PubMed] [Google Scholar]
- 27.Jatana M., Giri S., Singh A. K. 2002. Apoptotic positive cells in Krabbe brain and induction of apoptosis in rat C6 glial cells by psychosine. Neurosci. Lett. 330: 183–187 [DOI] [PubMed] [Google Scholar]
- 28.Zaka M., Wenger D. A. 2004. Psychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activation. Neurosci. Lett. 358: 205–209 [DOI] [PubMed] [Google Scholar]
- 29.Dekker L. V. 2004. Protein Kinase C. 2nd ed. Landes Bioscience/Eurekah.com United States, Georgetown, TX. [Google Scholar]
- 30.Pajewski R., Djedovic N., Harder E., Ferdani R., Schlesinger P. H., Gokel G. W. 2005. Pore formation in and enlargement of phospholipid liposomes by synthetic models of ceramides and sphingomyelin. Bioorg. Med. Chem. 13: 29–37 [DOI] [PubMed] [Google Scholar]
- 31.Lange Y., Ye J., Duban M. E., Steck T. L. 2009. Activation of membrane cholesterol by 63 amphipaths. Biochemistry. 48: 8505–8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igisu H., Hamasaki N., Ito A., Ou W. 1988. Inhibition of cytochrome c oxidase and hemolysis caused by lysosphingolipids. Lipids. 23: 345–348 [DOI] [PubMed] [Google Scholar]
- 33.Igisu H., Nakamura M. 1986. Inhibition of cytochrome c oxidase by psychosine (galactosylsphingosine). Biochem. Biophys. Res. Commun. 137: 323–327 [DOI] [PubMed] [Google Scholar]
- 34.Cooper C. E., Markus M., Seetulsingh S. P., Wrigglesworth J. M. 1993. Kinetics of inhibition of purified and mitochondrial cytochrome c oxidase by psychosine (beta-galactosylsphingosine). Biochem. J. 290: 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X. Q., Yan Q., Sun P., Liu J. W., Go L., McDaniel S. M., Paller A. S. 2007. Suppression of epidermal growth factor receptor signaling by protein kinase C-alpha activation requires CD82, caveolin-1, and ganglioside. Cancer Res. 67: 9986–9995 [DOI] [PubMed] [Google Scholar]
- 36.Nachtergaele S., Mydock L. K., Krishnan K., Rammohan J., Schlesinger P. H., Covey D. F., Rohatgi R. 2012. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat. Chem. Biol. 8: 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B., Ramirez C. M., Miller A. M., Repa J. J., Turley S. D., Dietschy J. M. 2010. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 51: 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez C. M., Liu B., Taylor A. M., Repa J. J., Burns D. K., Weinberg A. G., Turley S. D., Dietschy J. M. 2010. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 68: 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]