Abstract
Dietary intake of long-chain fatty acids (LCFAs) plays a causative role in insulin resistance and risk of diabetes. Whereas LCFAs promote lipid accumulation and insulin resistance, diets rich in medium-chain fatty acids (MCFAs) have been associated with increased oxidative metabolism and reduced adiposity, with few deleterious effects on insulin action. The molecular mechanisms underlying these differences between dietary fat subtypes are poorly understood. To investigate this further, we treated C2C12 myotubes with various LCFAs (16:0, 18:1n9, and 18:2n6) and MCFAs (10:0 and 12:0), as well as fed mice diets rich in LCFAs or MCFAs, and investigated fatty acid-induced changes in mitochondrial metabolism and oxidative stress. MCFA-treated cells displayed less lipid accumulation, increased mitochondrial oxidative capacity, and less oxidative stress than LCFA-treated cells. These changes were associated with improved insulin action in MCFA-treated myotubes. MCFA-fed mice exhibited increased energy expenditure, reduced adiposity, and better glucose tolerance compared with LCFA-fed mice. Dietary MCFAs increased respiration in isolated mitochondria, with a simultaneous reduction in reactive oxygen species generation, and subsequently low oxidative damage. Collectively our findings indicate that in contrast to LCFAs, MCFAs increase the intrinsic respiratory capacity of mitochondria without increasing oxidative stress. These effects potentially contribute to the beneficial metabolic actions of dietary MCFAs.
Keywords: metabolic disease, mitochondrial metabolism, oxidative stress, insulin signalling
The most common metabolic disorders in our Western society (including obesity and insulin resistance) have arisen to a great extent from excess nutrient intake, especially in the form of fat. Intake of diets rich in long-chain fatty acids (LCFAs) (>C16), the most common fatty acid (FA) type in Western diets, is associated with disturbances in glucose homeostasis and insulin action (1, 2). Many mechanisms have been proposed to underpin the deleterious effects of LCFAs on metabolic health, including increased inflammation, overactivation of stress-related pathways, and inappropriate lipid accumulation in nonadipose tissues (1, 3).
Interestingly, not all dietary fats induce the same degree of metabolic dysfunction. Our group and others have shown that intake of equal-caloric diets rich in medium-chain fatty acids (MCFAs) (C8–C12) decreases adiposity (4–6), increases energy expenditure (7, 8), and avoids many of the detrimental effects associated with LCFA intake. The increased energy expenditure suggests that FAs are funneled into oxidative versus storage pathways, and this has previously been suggested to be due to enhanced cellular uptake and entry of MCFAs into mitochondria (especially in the liver) for oxidation. In a recent study we showed that high-fat diets enriched with MCFAs caused a marked induction in mitochondrial oxidative capacity in muscle, over and above that induced by a LCFA high-fat diet, suggesting that enhanced myocellular oxidation of MCFAs might also be a key pathway for oxidative disposal of this class of FAs (8). In conjunction with the increase in markers of mitochondrial metabolism, MCFAs also prevented lipid accumulation and insulin resistance in muscle, with similar glucose uptake and muscle triglyceride levels in MCFA-fed animals compared with lean animals fed a low-fat diet (8).
In this study, we have used in vitro and in vivo systems to further characterize the disparate metabolic effects of MCFAs and LCFAs in muscle. First, we have investigated whether in addition to affecting mitochondrial content in muscle, MCFAs and LCFAs have differential effects on the intrinsic bioenergetic capacity of mitochondria. Additionally, as mitochondria are a major site for reactive oxygen species (ROS) production and LCFAs have been linked with oxidative stress (9, 10), we have determined the effect of MCFAs and LCFAs on ROS production and markers of oxidative damage.
METHODS
Cell culture
C2C12 myoblasts were grown in 1:1 Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 nutrient mix (Life Technologies, Mulgrave, VIC, Australia) containing 10% HyClone bovine calf serum (Thermo Fisher Scientific, Scoresby, VIC, Australia) and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). Differentiation was induced by serum-starvation using 2% horse serum (Life Technologies) once 90–100% confluence was reached. Cells were differentiated for 5 days, and differentiated myotubes were incubated for 18 h with individual FAs. For the lipid treatments, 200 μM FAs in ethanol were conjugated to 1% FA-free BSA at 55°C for 1.5 h in differentiation medium; the medium was filter-sterilized and applied to the cells. Control cells were incubated with an equal concentration of BSA and ethanol. For insulin stimulations, myotubes were serum-starved for 4 h (in the presence of the respective FA), stimulated with 10 nM insulin for 30 min, and lysed immediately in RIPA buffer for immunoblotting (see below).
Succinate dehydrogenase activity
Cell lysates were used to measure succinate dehydrogenase (SDH) activity. Lysate (10 μl) was mixed with 250 μl of reaction cocktail containing 50 mM KH2PO4, 20 mM succinate, 2.5 μM antimycin A, 2.5 mM sodium cyanide, and 0.45 mM phenazine methosulfate in a 96-well plate. Dichloroindophenol (50 μl, 0.72 mM) was added to the wells, and change in absorbance measured at 600 nm and 30°C. Protein concentration was measured using the Bradford method.
FA uptake
For the determination of FA uptake in cells, C2C12 myotubes were grown in 12-well plates and treated for 18 h with 200 μM FAs as described above. On the day of the assay, 200 μM lauric acid-conjugated medium containing 1 μCi/ml [1-14C]lauric acid (MP Biomedicals, Singapore) was applied to cells pretreated with MCFAs and 200 μM palmitic acid-conjugated medium containing 1 μCi/ml [1-14C]palmitic acid (Perkin Elmer, Melbourne, VIC, Australia) was applied to cells pretreated with LCFAs for exactly 2 h. Control cells received both treatments. After the 2 h incubation period, the medium was acidified with 1 M perchloric acid, and evolved 14CO2 was captured in 1 M NaOH. Acid-soluble metabolites (ASMs) in the medium labeled with 14C were also determined. Cells were scraped into 200 μl 1× PBS + 0.05% SDS, sonicated, a subsample (20 μl) taken for protein determination, and the remainder used for lipid extraction. Lipids were extracted using 2:1 chloroform:methanol following phase separation with 0.6% NaCl, 14C in the lipid phase and aqueous phase (representing cell ASMs) were determined. Total FA uptake was calculated as the sum of FAs that were funneled into oxidation (CO2 + ASMs in medium + ASM cell counts) and the lipid fraction. Protein content was determined using the BCA assay (Pierce, Thermo Fisher Scientific).
Superoxide production in cells
For the determination of mitochondrial as well as cytosolic superoxide production, C2C12 myoblasts were grown in black (clear-bottom) 96-well plates, differentiated, and treated with MCFAs and LCFAs for 18 h as described above. On the day of the experiment, FA-containing differentiation medium was replaced with HBSS buffer (Life Technologies) also containing FAs (conjugated to BSA) and either the mitochondrial fluorophor MitoSOX Red (5 μM, in nitrogen-bubbled DMSO), the cytosolic superoxide marker dihydroethidium (DHE) (20 μM, in nitrogen-bubbled DMSO), or DMSO itself. MitoSOX Red and DHE were added from stock solutions in DMSO with the final concentration of DMSO being less than 0.2% (v/v). Cytosolic superoxide generation with DHE was measured in the absence or presence of the NAD(P)H oxidase inhibitor VAS2870 (10 μM). Time-resolved fluorescence was determined for 1 h using a BMG Labtech FLUOstar OPTIMA plate reader and 485/590 nm excitation/emission filters. Changes in fluorescence in the MitoSOX Red- or DHE-treated cells were corrected for slope observed in the DMSO-treated cells (for each individual FA treatment). Results are expressed as change in fluorescence/min/mg protein. For the determination of protein content, medium was removed from the wells, 10 μl 1 M KOH added to lyse the cells, topped up with 190 μl of ddH2O, and protein concentration measured using the Bradford method (Bio-Rad Laboratories, Regents Park, NSW, Australia).
Glycogen synthesis
C2C12 myotubes were grown in 12-well plates and treated with 200 μM FAs as described above. On the day of the assay, cells were serum-starved for 4 h in the presence of the respective FA, followed by a 1 h incubation in serum-free medium containing FAs and 4 μCi/ml 14C-glucose (Perkin Elmer), and in the absence (basal state) or presence (insulin-stimulated state) of 100 nM insulin. Cells were washed three times in PBS, scraped into 100 μl 1M KOH, and heated for 15 min to 75°C. A subsample (10 μl) was taken for protein determination. To the remainder, 30 μl 25 mg/ml glycogen, 20 μl saturated Na2SO4, and 450 μl ice-cold ethanol was added, the mix vortexed and frozen for 30 min at −80°C, followed by a centrifugation step (10 min, 13,000 rpm, 4°C). Pellets were washed by resuspension in 50 μl ddH2O, followed by addition of 1 ml ice-cold ethanol and recentrifugation. The final pellet was resuspended in 50 μl ddH2O and counted for radioactivity.
Animal maintenance
Eight-week-old male C57BL/6J mice were purchased from the Australian Resource Centre (Perth, Australia). Mice were maintained in a temperature-controlled room (22 ± 1°C) with a 12 h light/dark cycle and ad libitum access to food and water. After 1 week on a standard low-fat chow diet (CHOW; 8% of calories from fat, 21% of calories from protein, 71% of calories from carbohydrate; Gordon's Specialty Stock Feeds, Yanderra, NSW, Australia), mice were randomly allocated to remain on the CHOW diet or to receive a diet enriched in either MCFAs or LCFAs ad libitum for 8 weeks. The high-fat diet [45% of calories from fat (from hydrogenated coconut oil for the MCFA diet and from lard for the LCFA diet), 20% of calories from protein, 35% of calories from carbohydrate] was made in-house and was based on rodent diet number D12451 (Research Diets, New Brunswick, NJ). The dietary FA composition was described previously (8). Tissue samples were collected from mice at 0900–1000 h without any prior fasting period. All experiments were approved by the Garvan Institute/St. Vincent's Hospital Animal Experimentation Ethics Committee, and followed guidelines issued by the National Health and Medical Research Council of Australia.
Determination of body composition and energy expenditure
Lean and fat mass were measured in mice using dual-energy X-ray absorptiometry (Lunar PIXImus2 densitometer; GE Healthcare, Little Chalfont, UK). The rate of oxygen consumption (VO2) and respiratory exchange ratio (RER) of individual mice were measured using an Oxymax indirect calorimeter over 24 h following an overnight acclimatization period in the Oxymax cages and a 2 h settling period (Columbus Instruments, Columbus, OH) as previously described (11).
Glucose tolerance and insulin levels
Mice were fasted overnight and injected ip with glucose (2 g/kg), and blood glucose levels were monitored using an Accucheck II glucometer (Roche Diagnostics, Castle Hill, NSW, Australia) for 90 min following glucose injection. Plasma insulin levels after overnight fast were determined by radioimmunoassay (Linco Research, St. Charles, MO).
Isolation of mitochondria
Muscle mitochondria (from mixed hindlimb muscle) were isolated by differential centrifugation as described previously (12). Briefly, muscle was diced in CP-1 medium [100 mM KCl, 50 mM Tris/HCl (pH 7.4), and 2 mM EGTA], digested on ice for 3 min in CP-2 medium [CP-1, to which was added 0.5% (w/v) BSA, 5 mM MgCl2, 1 mM ATP, and 2.45 units/ml−1 protease type VIII (Sigma P 5380)] and homogenized using an Ultra Turrax homogenizer. The homogenate was spun for 5 min at 500 g and 4°C. The resulting supernatant was subjected to a high-speed spin (10,600 g, 10 min, 4°C) and the pellet was resuspended in CP-1. The high-speed spin cycle was repeated twice. Protein content was measured using the Bradford method.
Respiration in cells and isolated mitochondria
For the determination of respiration in C2C12 myotubes, cells were incubated with FAs for 18 h as described above, trypsinized into 1 ml FA-containing media, and oxygen consumption was measured at 37°C in a Clark-type oxygen electrode (Strathkelvin Instruments, Motherwell, Scotland). In isolated mitochondria, oxygen consumption was measured in air-saturated respiration buffer [120 mM KCl, 5 mM K2HPO4, 3 mM HEPES, 1 mM EGTA, and 0.3% (w/v) defatted BSA (pH 7.2)], which was calculated to contain 406 nmol oxygen/ml (13), and to which was added either succinate (5 mM) and rotenone (2 μM) or palmitoyl carnitine (40 μM) + 2 mM malate as substrates. Oxygen consumption was measured in state 2 (in the presence of substrate), state 3 (after addition of 200 μM ADP), and in state 4 (after addition of 2.5 μg/ml oligomycin). The respiratory control ratios (state 3/state 4 respiration) were above 3.5 for succinate and above 5.0 for palmitoyl carnitine as substrates, indicating well-coupled mitochondria.
Mitochondrial H2O2 production
Hydrogen peroxide production in isolated mitochondria was determined by monitoring the oxidation of Amplex Ultra Red (Invitrogen, Mount Waverley, VIC, Australia) using a FLUOstar OPTIMA fluorescence plate reader maintained at 37°C. Mitochondria were incubated at 0.25 mg/ml in assay medium [120 mM KCl, 3 mM HEPES, 1 mM EGTA, and 0.3% BSA (pH 7.2) at 37°C] containing 6 u/ml horseradish peroxidase, 30 u/ml superoxide dismutase, and 0.1 mM Amplex Ultra Red, as described previously (14). H2O2 production was determined under three conditions: in the presence of succinate (5 mM), in the presence of succinate and rotenone (2 μM, to inhibit electron backflow toward complex I), and in the presence of palmitoyl carnitine (40 μM). In separate wells containing assay medium, H2O2 was injected (using onboard injectors) at concentrations between 0 and 500 nM and a standard curve calculated.
H2O2 production and state 2 oxygen consumption were used to calculate the percentage of electrons which leak out of sequence producing superoxide and subsequently hydrogen peroxide (15). Whereas two electrons are needed for the reduction of 1 mol of O2 to H2O2, four electrons are transferred in the reduction of 1 mol of O2 to water. Therefore, the percent free radical leak (FRL) was calculated as the rate of H2O2 production divided by twice the rate of oxygen consumption, and the result was multiplied by 100 (16).
Lipid accumulation
Triacylglycerol (TAG) content was determined using a colorimetric assay kit (Triglycerides GPO-PAP; Roche Diagnostics, Indianapolis, IN) as previously described (17). For diacylglycerol (DAG) and ceramide measurements, lipids were extracted from muscle in solvents containing 2 nmol ceramide (17:0) and 10 nmol DAG (17:0/17:0) (18). DAG and ceramide levels were measured using a hybrid linear ion trap-triple quadrupole mass spectrometer (QTRAP 5500; AB Sciex, Foster City, CA). Ceramide molecular lipids were analyzed by precursor-ion scanning for protonated dehydrated sphingosine at m/z 264.3. DAG molecular lipids were analyzed by multiple neutral-loss scanning for ammoniated FAs. Data were analyzed and quantified with LipidView (AB Sciex) version 1.1 after isotope correction (19).
Immunoblotting
Lysates were prepared from FA-treated C2C12 myotubes and mouse muscle mitochondria. Cells were collected in RIPA buffer (20), sonicated for 5 s, rotated at 4°C for 2 h and centrifuged for 10 min at 16,000 g. Mitochondria were isolated as described above, mixed 1:1 in RIPA buffer, and sonicated for 5 s. For both cells and mitochondria, 20 μg of protein was denatured in Laemmli buffer at 65°C for 15 min, resolved by SDS-PAGE, electrotransferred, and immunoblotted, as described previously (21). Immunolabeled bands were quantified using ImageJ 1.44p software.
Measurement of antioxidant protection and oxidative damage
Glutathione peroxidase (GPx) activity was measured as the decrease in NADPH absorption at 340 nm and 30°C. A reaction cocktail was prepared by mixing azide buffer (9.2 ml) [50 mM NaH2PO4, 0.4 mM EDTA (pH 7.0 at 30°C), addition of 1 mM Na-azide], glutathione reductase (100 μl, 100 u/ml), and GSH (50 μl, 200 mM) into a 1 mg β-NADPH vial (Sigma N0411). Sample and reaction cocktail were mixed and H2O2 [5 μl, 0.042% (v/v)] was added to start the reaction.
Lipid hydroperoxides (LOOHs) were measured according to the method of Bou et al. (22). Thiobarbituric acid reactive substances (TBARS) and protein carbonyls were measured in homogenates as described previously (14). Homogenate protein content was measured using the Bradford method.
Statistical analysis
All results are presented as mean ± SEM. Results were compared using a one-way ANOVA with P < 0.05 considered significant, followed by a Fisher's least significant differences test. For both the cell culture and animal experiments, the mean of each treatment group was compared with the mean of every other treatment group.
RESULTS
Effect of FA subtypes on oxidative metabolism and lipid accumulation in C2C12 myotubes
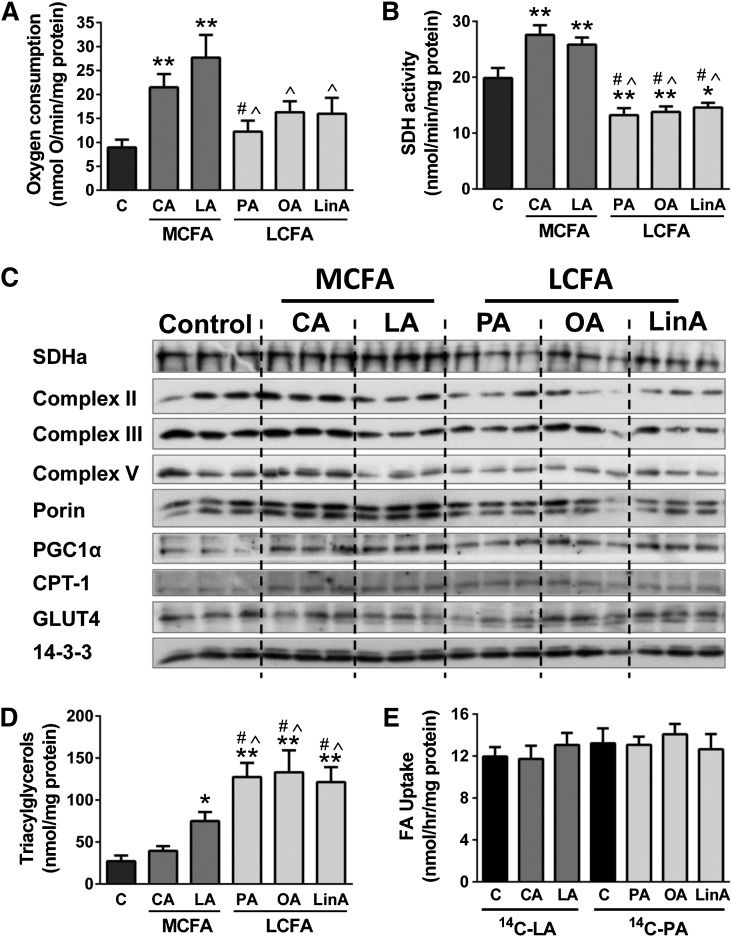
Incubation of myotubes with the MCFAs capric (C10:0) and lauric acid (C12:0) for 18 h significantly increased oxygen consumption (Fig. 1A) and SDH activity (Fig. 1B) in comparison to BSA-treated control cells, as well as protein levels of several oxidative proteins, including subunits of the mitochondrial electron transport chain (ETC), the master-regulator of mitochondrial biogenesis peroxisome proliferator-activated receptor gamma coactivator 1α, the mitochondrial content marker porin, and the mitochondrial FA transporter carnitine palmitoyl transferase 1 (CPT1) (Fig. 1C and supplementary Table I). The observed increase in SDH activity was most likely due to increased protein expression, as shown by immunoblotting for SDH subunit a (Fig. 1C). In contrast, treatment of myotubes with LCFAs, the saturated FA palmitic acid (C16:0), the mono-unsaturated FA oleic acid (C18:1n9), and the polyunsaturated FA linoleic acid (C18:2n6), did not significantly affect cellular respiration (Fig. 1A), had no effect or caused a decrease in the expression of oxidative proteins (Fig. 1C and supplementary Table I), and caused a mild decrease in SDH activity compared with control cells (Fig. 1B). Protein levels of the glucose transporter 4 were unaffected by FA treatment (Fig. 1C).
Fig. 1.
Metabolic activity of C2C12 myotubes. Oxygen consumption (A), SDH activity (B), immunoblotting for oxidative proteins (C), TAG levels (D), and FA uptake (E) were measured in muscle cells treated with various MCFAs or LCFAs (18 h incubations with 200 μM FAs). FA uptake was measured after 18 hr treatment with the respective FAs, followed by a 1 h incubation with either 14C-lauric acid (for C and MCFA-treated cells) or 14C-palmitic acid (for C and LCFA-treated cells), and was calculated as the sum of FAs that were funneled into oxidative and storage pathways (see Methods for details). Shown are means ± SEM, n = 3 with three replicates each [or two replicates in the case of panel (A)]. *P < 0.05, **P < 0.001 versus C; #P < 0.01 versus CA; ^P < 0.01 versus LA. C, control; CA, capric acid; LA, lauric acid; PA, palmitic acid; OA, oleic acid; LinA, linoleic acid; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1α GLUT4, glucose transporter 4; SDHa, SDH subunit a.
Incubation of C2C12 myotubes with LCFAs led to an average 5-fold increase in TAG accumulation in comparison to BSA-treated control cells. Interestingly, myotubes incubated with the MCFAs exhibited markedly less TAG accumulation compared with the LCFA-treated cells (Fig. 1D). The differences in TAG deposition were not due to any differences in FA uptake, as similar levels of uptake were observed for both MCFA- and LCFA-treated cells with 14C-labeled FAs (Fig. 1E). Collectively these results suggest enhanced partitioning of FAs toward oxidation with MCFAs, and the tracer experiments showed that approximately 65% of 14C-lauric acid taken up was directed to oxidative pathways (CO2 and ASMs), while this was only approximately 35% with 14C-palmitic acid (data not shown).
Effect of FA subtypes on oxidative stress in C2C12 myotubes
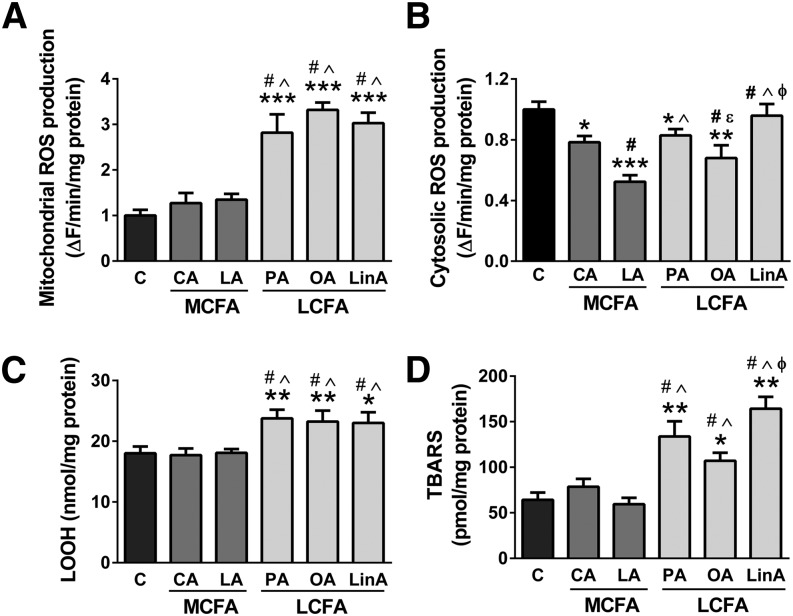
In addition to effects on lipid accumulation and mitochondrial metabolism, LCFAs have also been linked to oxidative stress and the subsequent accumulation of oxidatively damaged cellular components (9, 10). We investigated the effect of incubation of C2C12 myotubes with LCFAs and MCFAs on superoxide generation and lipid peroxidation (two markers examined: LOOHs and TBARS). Mitochondrial (Fig. 2A), but not cytosolic superoxide generation (Fig. 2B), was significantly increased with all three LCFAs, but not after MCFA treatment. Cytosolic superoxide was measured in the absence and presence of the NAD(P)H oxidase inhibitor VAS2870. Addition of VAS2870 decreased ROS generation to approximately 90–95% of the rates measured in the absence of the inhibitor, showing that NAD(P)H oxidases have only a minor contribution to cytosolic ROS production in C2C12 myotubes (data not shown). Both lipid peroxidation markers followed the same pattern as mitochondrial superoxide and were significantly increased after LCFA but not MCFA treatment (Fig. 2C, D). This highlights that MCFAs keep oxidative stress at low levels, whereas LCFAs lead to substantial increase in mitochondrial ROS production and to the accumulation of oxidative damage.
Fig. 2.
Oxidative stress markers in C2C12 myotubes. Mitochondrial (A) and cytosolic (B) superoxide generation, lipid hydroperoxide (C) and TBARS (D) levels in muscle cells treated with various MCFAs and LCFAs (18 h incubations with 200 μM FAs). Shown are means ± SEM, n = 3 with three replicates each [or n = 4 with two replicates each in the case of panel (B)]. *P < 0.05, **P < 0.01, and ***P < 0.001 versus C; #P < 0.01 versus CA; ^P < 0.01 versus LA; εP < 0.05 versus PA; φP < 0.01 versus OA. C, control; CA, capric acid; LA, lauric acid; PA, palmitic acid; OA, oleic acid; LinA, linoleic acid
Effect of FA subtypes on insulin action in C2C12 myotubes
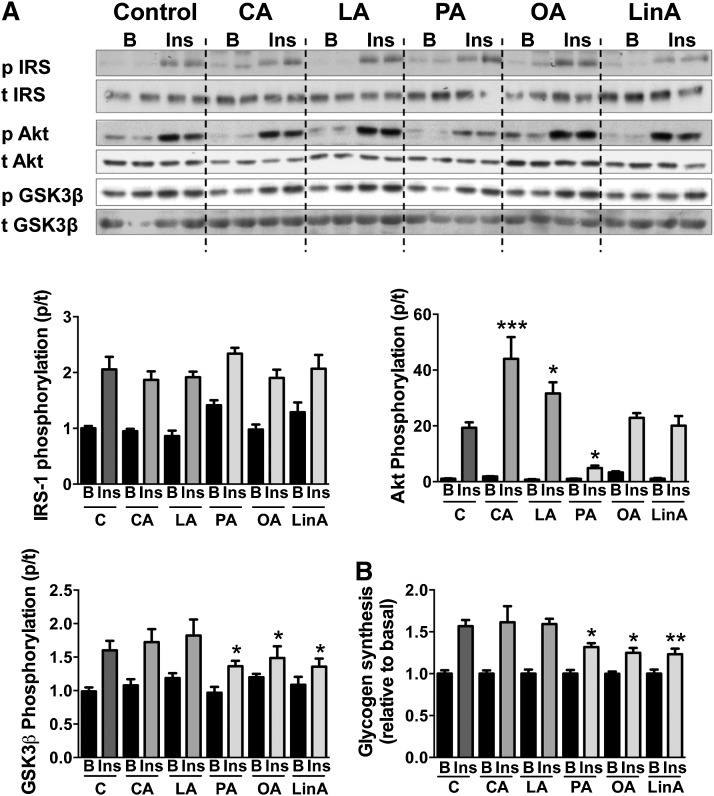
Excess lipid accumulation, mitochondrial dysfunction and oxidative stress have been associated with the development of insulin resistance (1). We next investigated how incubation of myotubes with LCFAs and MCFAs affected insulin-stimulated phosphorylation of insulin receptor substrate 1 (IRS1) (Tyr612), Akt (Ser473), and glycogen synthase kinase 3β (GSK3β) (Ser9), as well as the insulin-stimulated glycogen synthesis rate (Fig. 3). Whereas none of the FAs had an effect on phosphorylation of IRS1, incubation of muscle cells with MCFAs significantly increased Akt phosphorylation. In contrast, palmitic acid decreased insulin-stimulated Akt phosphorylation substantially, whereas no changes were observed with oleic or linoleic acid. Phosphorylation of GSK3β was decreased after LCFA but not MCFA treatment, which was accompanied by a significant decrease in insulin-stimulated glycogen synthesis rate with all three LCFAs (Fig. 3). These results suggest that MCFAs, unlike LCFAs, do not impair insulin action in muscle cells.
Fig. 3.
Insulin signaling and glycogen synthesis in C2C12 myotubes. A: Immunoblotting results in basal and insulin-stimulated muscle cells after 18 h incubation with various MCFAs and LCFAs, and 30 min stimulation with 10 nM insulin. Representative blots show n = 2 per group, but graphed changes represent n = 4–6 (two to three independent experiments). B: Glycogen synthesis rate of FA-treated cells in the absence or presence of 100 nM insulin, measured using 14C-labeled glucose. Bar graphs show means ± SEM. Statistical analysis was measured by one-way ANOVA using the ratios between basal and insulin-stimulated protein levels in each treatment group with *P < 0.05, *P < 0.01, and ***P < 0.001 versus C cells. B, basal; Ins, insulin-stimulated; C, control; CA, capric acid; LA, lauric acid; PA, palmitic acid; OA, oleic acid; LinA, linoleic acid; p, phospho; t, total
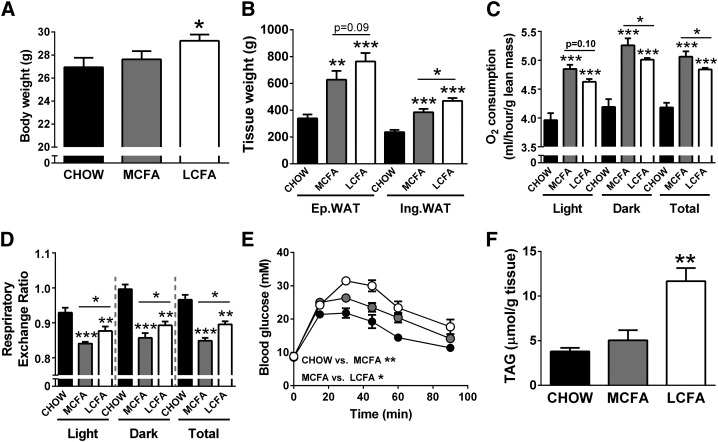
Effect of dietary MCFA and LCFA intake on adiposity, energy expenditure, and glucose tolerance
Mice fed a MCFA-rich diet were partially protected from body weight gain and increases in adiposity in comparison to LCFA-fed mice (when compared with CHOW controls) after 8 weeks of high-fat feeding (Fig. 4A, B). The differences in body composition in MCFA- and LCFA-fed mice can be to some extent explained by differences in energy intake, as cumulative calorie intake per mouse over the 8 week feeding period was increased in the MCFA and LCFA groups when compared with the CHOW-fed mice (CHOW, 11.1 ± 0.6 kcal/day; MCFAs, 12.8 ± 0.6 kcal/day; LCFAs, 12.3 ± 0.6 kcal/day). We did not assess if there were differences in lipid absorption between the two diets, however, MCFAs are more efficiently absorbed than LCFAs (23), and thus differences in energy dissipation by inefficient digestion of the dietary FAs is unlikely to underlie the differences in adiposity and body composition. Whole-body oxygen consumption (normalized to lean mass) was elevated in both fat-fed groups compared with controls, and was significantly higher in MCFA- versus LCFA-fed animals in both the light and dark phase (Fig. 4C). Furthermore, the RER showed a greater decrease in MCFA-fed mice than in LCFA-fed mice compared with control CHOW-fed mice (Fig. 4D). The increase in overall energy expenditure and the lower RER could partly explain the reduced adiposity in MCFA-fed mice. Compared with LCFA-mice, MCFA-fed animals also did not develop the same degree of high-fat diet-induced glucose intolerance (Fig. 4E) and displayed lower fasting insulin levels (Fig. 4F).
Fig. 4.
Metabolic markers in mice fed CHOW-, MCFA-, and LCFA-rich diets. Body weight (A), fat pad weight (B), oxygen consumption (C), RER (D), blood glucose levels during a glucose tolerance test (GTT) (E), and fasting plasma insulin levels (F). For the GTT, glucose (2 g/kg) was injected at the 0 time point, and blood glucose levels were monitored for 90 min after injection. Shown are means ± SEM, n = 6–8 per diet group; *P < 0.05, **P < 0.01, and ***P < 0.001. Significance values in panel (E) represent differences in the area-under-the-curve during the GTT.
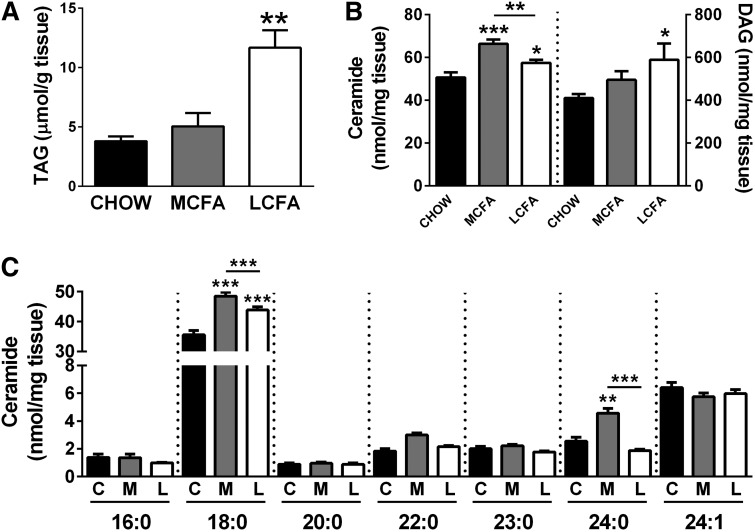
Effect of dietary MCFA and LCFA intake on muscle lipid accumulation
MCFA-fed mice displayed less muscle TAG accumulation than LCFA-fed mice (41% increase with MCFA vs. 295% increase with LCFA) (Fig. 5A). A similar proportional increase was also observed in DAG accumulation, whereas total ceramide levels were greatest in muscle from MCFA-fed mice (Fig. 5B). The increase in muscle ceramide in MCFA-fed mice was mainly due to an increase in CER18:0 and CER24:0 (Fig. 5C), while the increase in total DAG in MCFA- and LCFA-fed mice was due to an elevation in the same DAG species, mainly DAG16:0/16:0, DAG16:0/18:0, and DAG16:1/18:1 (supplementary Fig. I).
Fig. 5.
Muscle lipid accumulation in mice fed CHOW-, MCFA-, and LCFA-rich diets. Total TAG (A), ceramide and diacylglyceride levels (B), and ceramide species (C) in quadriceps muscle. Shown are means ± SEM, n = 6–8 per diet group; *P < 0.05, **P < 0.01, and *** P < 0.001. C, CHOW; M, MCFA; and L, LCFA
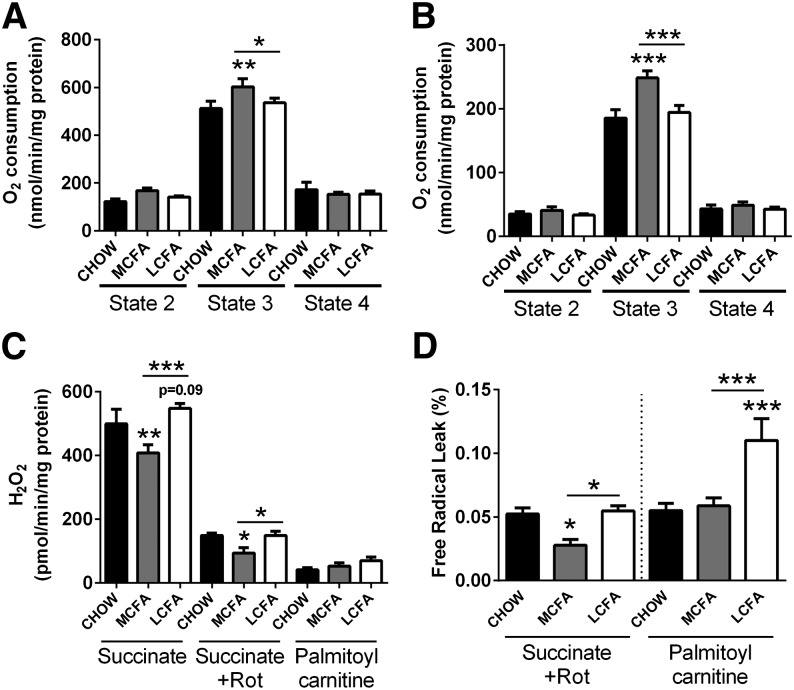
Effect of dietary MCFA and LCFA intake on mitochondrial respiration and ROS production
To determine if MCFAs altered mitochondrial function, oxygen consumption in states 2, 3, and 4 was measured in isolated muscle mitochondria using succinate and palmitoyl carnitine as substrates. Mitochondria from muscle of MCFA-fed mice showed significantly greater rates of oxygen consumption than CHOW- and LCFA-fed animals in state 3 for both succinate and palmitoyl carnitine (Fig. 6A, B). Increased mitochondrial substrate flux is often accompanied by higher ROS production, however the mitochondria from MCFA-fed mice displayed a 32% decrease in H2O2 generation in the presence of succinate (when compared with LCFA-fed mice), with no difference in ROS production detected when palmitoyl carnitine was used as a substrate for the ETC (Fig. 6C).
Fig. 6.
Mitochondrial respiration and hydrogen peroxide production in muscle from CHOW-, MCFA-, and LCFA-fed mice. Mitochondrial oxygen consumption was measured in state 2, state 3, and state 4 using succinate (A) or palmitoyl carnitine (B) as a substrate. Hydrogen peroxide production was measured in the presence of succinate, succinate plus rotenone (Rot), and palmitoyl carnitine (C). H2O2 production and state 2 oxygen consumption were used to calculate the percentage of electrons which leak out of sequence producing superoxide and subsequently hydrogen peroxide, the free radical leak (D). Shown are means ± SEM; n = 6–8 per diet group; *P < 0.05, **P < 0.01, and ***P < 0.001.
Using certain assumptions (see Methods section), it is possible to calculate the percentage of electrons that leak out of sequence during electron transfer in the ETC, and, rather than being transferred to molecular oxygen as final electron acceptor, leak out and produce superoxide. This has been called the FRL (16). With succinate (in the presence of rotenone), muscle mitochondria from MCFA-fed mice displayed a 40% decrease in FRL, with no change observed in the LCFA-fed animals in comparison to controls. In contrast, with palmitoyl carnitine the FRL was 2-fold higher in LCFA-fed mice than in MCFA- or CHOW-fed mice (Fig. 6D). Both substrates point toward a MCFA-rich diet having beneficial effects on mitochondrial electron leakage, with less superoxide molecules produced per consumed oxygen molecule.
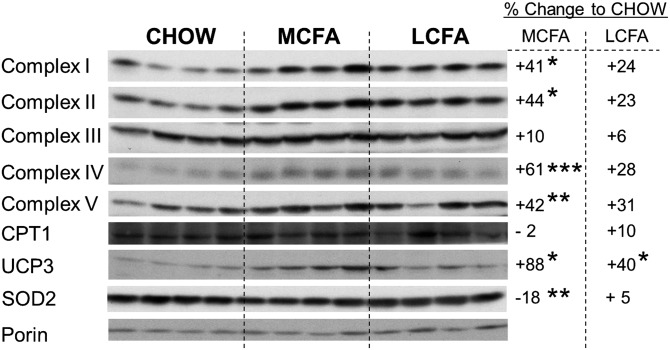
Effect of dietary MCFA and LCFA intake on protein expression of mitochondrial oxidative proteins
Immunoblotting of mitochondrial lysates was undertaken to investigate if the increased oxygen consumption in isolated mitochondria from MCFA-fed mice might be due to alterations in the expression of key oxidative proteins. Equal amounts of mitochondria from each dietary group were used for immunoblotting, as shown by quantification of the abundant mitochondrial transmembrane protein porin (Fig. 7). Protein expression of subunits of complexes I–V of the ETC, as well as uncoupling protein 3 (UCP3), were increased to a greater extent with the MCFA than the LCFA diet, when compared with mitochondria from CHOW-fed controls (Fig. 7). Superoxide dismutase 2 (SOD2) was significantly decreased in the MCFA-fed mice, whereas no difference was observed in CPT1 protein content between diet groups. These data suggest that MCFAs more effectively promote the expression of oxidative proteins than LCFAs, resulting in increased oxidative capacity per unit of mitochondria.
Fig. 7.
Markers of mitochondrial metabolism in skeletal muscle mitochondria. Representative immunoblotting results of oxidative markers in mice fed CHOW-, MCFA-, or LCFA-rich diets. Shown are n = 4 for each group, but percentage changes represent n = 6–8; *P < 0.05, **P < 0.01, and ***P < 0.001. Complexes I–V represent subunits of the complexes of the ETC. Porin was used as mitochondrial loading control and shows similar distribution in all diet groups.
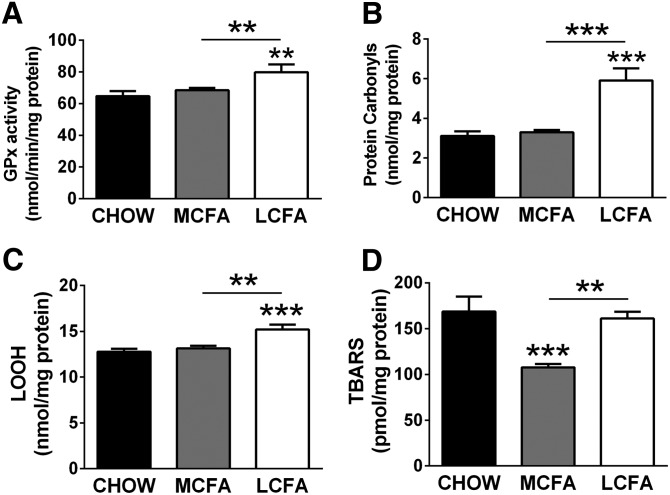
Effect of dietary MCFA and LCFA intake on oxidative stress in muscle
As described above, in both muscle cells and mitochondria from mouse muscle, MCFAs were associated with lower rates of ROS production. To determine if this was accompanied by differences in the accumulation of oxidative damage in mouse skeletal muscle (as done in C2C12 myotubes), we measured various antioxidant and oxidative damage markers. Antioxidant systems are expected to respond to the need of a tissue for protection, with greater oxidative stress levels resulting in an increase in the expression and activity of antioxidant enzymes (24). GPx, as well as the content of lipid hydroperoxides (an intermediate marker of oxidative damage to lipids) and protein carbonyls (marker for oxidative damage to proteins), were increased with the LCFA diet, but not the MCFA diet (Fig. 8A–C). TBARS, an endpoint marker of lipid peroxidation, remained unchanged in muscle of LCFA-fed mice, but were significantly decreased in MCFA-fed mice when compared with controls (Fig. 8D). Overall, these data indicate that MCFAs in contrast to LCFAs do not increase mitochondrial ROS production in vivo, with subsequently less accumulation of oxidatively damaged lipids and proteins.
Fig. 8.
Oxidative stress markers in muscle from CHOW-, MCFA-, and LCFA-fed mice. GPx activity (A), protein carbonylation (B), lipid hydroperoxide (C), and TBARS levels (D). Shown are means ± SEM; n = 6–8 per diet group; *P < 0.05, **P < 0.01 and, ***P < 0.001
DISCUSSION
Over the last 20 years, it has been repeatedly described that diets rich in LCFAs (e.g., typical Western diets) lead to the development of adiposity and insulin resistance, and increase the risk of diabetes (1, 25). In contrast, diets rich in MCFAs, when used at an equal-caloric level, do not induce the same degree of detrimental effects on metabolic health; despite a similar increase in dietary fat intake as with the LCFA diet (4–8). These favorable effects have been partially ascribed to the physical properties of MCFAs, because this class of FAs is more readily funneled into oxidative pathways, which could lead to increased energy expenditure and less fat deposition in adipose and other tissues compared with LCFAs (26). The tissue that is likely to be responsible for many of the beneficial effects of MCFAs on whole-body glucose homeostasis is skeletal muscle, where previous studies from our group have shown that a MCFA-rich diet leads to a substantially greater increase in markers of mitochondrial content than LCFAs, and a prevention of lipid accumulation and insulin resistance in this tissue (8). In the current study, we have shown that the beneficial effects of MCFAs in skeletal muscle may also be related to changes at the level of mitochondria, where we observed increased respiratory capacity and reduced ROS production.
Whereas a variety of studies demonstrated a link between muscle mitochondrial oxidative capacity and insulin action (8, 27, 28), other publications showed no such correlation [summarized in (29)]. Here we show that MCFAs induced an increase in respiration and SDH activity in muscle cells, as well as an increased protein expression of various mitochondrial proteins, suggesting an improvement in mitochondrial oxidative capacity, while no such increases were observed after LCFA treatment. Importantly, experiments in isolated mitochondria from skeletal muscle of MCFA-fed mice showed that, per unit of mitochondria, there was increased respiratory capacity, which is likely explained by an increase in the expression of oxidative proteins. Previous studies have shown that changes in the expression and activity of oxidative proteins and the capacity for substrate oxidation can occur in muscle without marked changes in total mitochondrial number, indicating improved intrinsic capacity per mitochondrion (28, 30). The findings of the current study and our previous report (8) indicate that MCFAs improve mitochondrial substrate oxidation in muscle to a greater extent than LCFAs, due to increases in both the total number of mitochondria and the oxidative capacity of individual mitochondria.
Collectively the changes in mitochondrial content and capacity could, in part, explain the enhanced whole-body energy expenditure per unit lean mass, the prevention of TAG and DAG accumulation in muscle, and the better insulin action in MCFA- versus LCFA-fed animals [current study and (8)]. We did observe a slight increase in total ceramide content in muscle from the MCFA-fed mice, and despite ceramide accumulation being previously associated with the development of insulin resistance, recent studies suggest that the FA chain length of the ceramide species is important in determining its metabolic impact (31, 32). The increase in muscle ceramide in MCFA-fed mice was mainly due to an increase in CER18:0 and CER24:0 (Fig. 5C), with CER24:0 having previously been reported to have beneficial anti-apoptotic effects (31). Importantly, we have shown that the effects of MCFAs to boost mitochondrial metabolism and avoid excess lipid deposition and insulin resistance are not uniform across tissues. In our previous work, we demonstrated that dietary MCFAs, while improving metabolic parameters in muscle, led to steatosis in the liver, likely due to upregulation of lipogenic pathways and hepatic insulin resistance (8). In the current cohort of animals, we similarly observed that hepatic lipid accumulation was increased after LCFA feeding, and to an even greater extent after MCFA feeding (data not shown). While excess hepatic lipid can have deleterious metabolic effects, it should be noted that many studies in rodents and humans have reported no adverse effects of MCFAs on liver lipid levels (4, 26, 33, 34). These disparate findings on liver lipid accumulation between studies indicates that differences in methodological factors may also be important, such as the exact composition and fat content of the MCFA-enriched diets, as well as the composition of the other macronutrients in the diet.
Another intriguing finding of the current investigation was the disparate effects of MCFAs and LCFAs on mitochondrial ROS production in muscle. Mitochondria are known to leak electrons during electron transfer along the respiratory chain. This electron leak leads to the generation of superoxide anions (an oxygen molecule with an unpaired electron), and subsequently to the production of hydrogen peroxide (H2O2), both ROS being highly damaging to any molecule they encounter (35). ROS generation is dependent upon the redox state of the mitochondria, substrate concentration, and substrate type. Compared with carbohydrates, reducing equivalents from lipid oxidation produce increased superoxide (36, 37). While still controversial, the majority of recent studies have proposed that mitochondrial ROS generation is an important early event in the induction of insulin resistance, with LCFAs increasing ROS production and promoting insulin resistance (9, 10, 38–40). Other publications support the view that cytosolic ROS, at low levels, are important for normal insulin signaling and do not induce insulin resistance (41, 42). In conjunction with both views, diets rich in LCFAs have also been reported to decrease antioxidant protection, thereby promoting oxidative damage (40, 43); whereas other reports demonstrated that antioxidants do not improve insulin action, especially in insulin-resistant humans [summarized in (44)]. Little is known about the effects of MCFAs on ROS generation and the accumulation of oxidative damage. Here we have shown that in response to MCFAs, both mitochondrial superoxide generation in C2C12 myotubes as well as mitochondrial H2O2 production in mouse skeletal muscle were similar or decreased compared with controls. This pattern can be explained by low levels of free radical leakage from the ETC in muscle mitochondria from MCFA-fed mice, as measured by percentage of electrons leaking out of sequence during mitochondrial respiration. In contrast, mitochondrial superoxide production was substantially increased in LCFA-treated cells, and in skeletal muscle of mice fed a LCFA-rich diet there was a 2-fold increase in FRL with the lipid-based substrate palmitoyl carnitine. Cytosolic superoxide production, measured in the C2C12 myotubes, was significantly reduced in response to both MCFA and LCFA treatments, indicating that mitochondrial ROS generation is likely to be a more important mediator of changes in cellular oxidative status, as recently suggested (45, 46).
In healthy systems, ROS are detoxified by various antioxidant systems. However, in diseased states and during long-term dietary LCFA intake, this “oxidant-antioxidant” balance has been shown to be tilted in favor of the reactive species, resulting in ROS-mediated damage to lipids, proteins, and DNA (47). To assess how the different FAs affected the oxidative status of muscle, we measured lipid hydroperoxides and TBARS as markers of lipid peroxidation, protein carbonylation as a marker of oxidative damage to proteins, and the antioxidant enzymes GPx (activity) and SOD2 (protein content). Consistent with previous studies (48, 49), these oxidative markers were increased in muscle of mice fed the LCFA-rich diet (with the exception of SOD2), as well as in C2C12 myotubes after overnight treatment with various LCFAs. However, MCFA-treated mice and cells displayed no evidence of oxidative stress, with oxidative damage markers and antioxidant protection remaining similar to controls (GPx, LOOH, protein carbonyls) or even being decreased (TBARS, SOD2). These results highlight that MCFAs do not promote oxidative stress in muscle and this may be an additional mechanism explaining why high-fat diets containing MCFAs do not cause the same deterioration in glucose homeostasis and insulin action as LCFAs (5). Furthermore, as antioxidant systems are expected to respond to the need of tissues for protection (24), the decrease in SOD2 protein content (and similar GPx activity) further supports the low oxidative stress levels observed with the MCFA diet.
Changes of UCP3 expression are not only used as a marker of mitochondrial oxidative metabolism (50), but also as an oxidative stress marker (51). Nègre-Salvayre et al. (52) were first to suggest a role for UCPs in regulating mitochondrial ROS production. Nowadays, several transgenic model systems suggest that UCP3 is able to curtail superoxide production by acting as a mild mitochondrial uncoupler (53, 54). MCFA-fed mice displayed an 88% increase in UCP3 content in skeletal muscle, whereas UCP3 only showed a tendency toward an increase with the LCFA diet (+40%). The elevated UCP3 content in MCFA-fed mice might also partly explain their decreased H2O2 generation and electron leakage, as well as their increased oxygen consumption.
MCFAs have been reported to improve insulin action and glucose homeostasis in various experimental models. Type 2 diabetic subjects treated acutely with MCFAs for 5 days showed an improvement in insulin-stimulated glucose disposal (55). Similarly, Han et al. (56) showed a decrease in body weight, homeostasis model of assessment-insulin resistance, and fasting insulin in type 2 diabetic subjects that consumed diets supplemented with MCFAs for 3 months when compared with subjects consuming LCFA-rich diets. Comparable beneficial effects have also been reported in various rodent models (4, 5, 8, 57) and in muscle cells (58). To test if some of the beneficial effects of MCFAs on insulin action might be related to changes in insulin signaling, we determined the phosphorylation status of the key insulin signaling intermediates IRS1, Akt, and GSK3β in muscle cells. Compared with control cells, insulin-induced Akt phosphorylation was significantly increased after MCFA treatment, but was unchanged or even decreased (in the case of palmitic acid) after LCFA treatment. Similarly, phosphorylation of GSK3β was unchanged in MCFA-treated cells but decreased after LCFA treatment. Changes in GSK3β phosphorylation were accompanied by a similar pattern in the rate of insulin-stimulated glycogen synthesis, which was significantly reduced in the LCFA-treated cells. Thus, although there is still controversy regarding the exact relationship between alterations in the activation state of insulin signaling intermediates and endpoint measures of insulin action (e.g., glucose uptake) (59, 60), our results indicate that MCFAs display enhancing effects on both the insulin signaling intermediates, as well as on glycogen synthesis, which is commonly used as an endpoint measure of insulin action in cell culture systems (61, 62).
In conclusion, our study has revealed that MCFAs increase oxidative metabolism at the level of the mitochondrion in muscle. Furthermore, in contrast to LCFAs, MCFAs prevent the induction of oxidative stress that normally arises due to excess lipid intake. Our results in myotubes indicate that capric and lauric acid (C10:0 and C12:0) are most likely responsible for the observed effects, with these two FAs comprising more than 40% of the total FA content in our MCFA diet (8). Overall these findings provide new insight into the mechanisms that contribute to the favorable effects of MCFAs on energy homeostasis and insulin action.
Supplementary Material
Acknowledgments
The authors thank the Biological Testing Facility at the Garvan Institute (Sydney, Australia) for assistance with animal care.
Footnotes
Abbreviations:
- ASM
- acid-soluble metabolite
- CPT1
- carnitine palmitoyl transferase 1
- DAG
- diacylglycerol
- DHE
- dihydroethidium
- ETC
- electron transport chain
- FRL
- free radical leak
- GPx
- glutathione peroxidase
- GSK3β
- glycogen synthase kinase 3β IRS1, insulin receptor substrate 1
- LCFA
- long-chain fatty acid
- LOOH
- lipid hydroperoxide
- MCFA
- medium-chain fatty acid
- RER
- respiratory exchange ratio
- ROS
- reactive oxygen species
- SDH
- succinate dehydrogenase
- SOD2
- superoxide dismutase 2
- TAG
- triacylglycerol
- TBARS
- thiobarbituric acid reactive substances
- UCP3
- uncoupling protein 3
This work was supported by funding from the National Health and Medical Research Council of Australia (NHMRC). B.O. was supported by a PhD scholarship and G.J.C. by a research fellowship from the NHMRC. N.T. was supported by an Australian Research Council Future Fellowship.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.Kraegen E. W., Cooney G. J. 2008. Free fatty acids and skeletal muscle insulin resistance. Curr. Opin. Lipidol. 19: 235–241 [DOI] [PubMed] [Google Scholar]
- 2.Samuel V. T., Shulman G. I. 2012. Mechanisms for insulin resistance: common threads and missing links. Cell. 148: 852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoelson S. E., Lee J., Goldfine A. B. 2006. Inflammation and insulin resistance. J. Clin. Invest. 116: 1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba N., Bracco E. F., Hashim S. A. 1982. Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with diet containing medium chain triglyceride. Am. J. Clin. Nutr. 35: 678–682 [DOI] [PubMed] [Google Scholar]
- 5.Han J., Hamilton J. A., Kirkland J. L., Corkey B. E., Guo W. 2003. Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes. Res. 11: 734–744 [DOI] [PubMed] [Google Scholar]
- 6.St-Onge M. P., Bosarge A. 2008. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am. J. Clin. Nutr. 87: 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St-Onge M. P., Ross R., Parsons W. D., Jones P. J. H. 2003. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes. Res. 11: 395–402 [DOI] [PubMed] [Google Scholar]
- 8.Turner N., Hariharan K., TidAng J., Frangioudakis G., Beale S. M., Wright L. E., Zeng X. Y., Leslie S. J., Li J. Y., Kraegen E. W., et al. 2009. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: potent tissue-specific effects of medium-chain fatty acids. Diabetes. 58: 2547–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao D., Nong S. W., Huang X. Q., Lu Y. G., Zhao H. Y., Lin Y. J., Man Y., Wang S., Yang J. F., Li J. A. 2010. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J. Biol. Chem. 285: 29965–29973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura S., Takamura T., Matsuzawa-Nagata N., Takayama H., Misu H., Noda H., Nabemoto S., Kurita S., Ota T., Ando H., et al. 2009. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 284: 14809–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner N., Bruce C. R., Beale S. M., Hoehn K. L., So T., Rolph M. S., Cooney G. J. 2007. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 56: 2085–2092 [DOI] [PubMed] [Google Scholar]
- 12.Trzcionka M., Withers K. W., Klingenspor M., Jastroch M. 2008. The effects of fasting and cold exposure on metabolic rate and mitochondrial proton leak in liver and skeletal muscle of an amphibian, the cane toad Bufo marinus. J. Exp. Biol. 211: 1911–1918 [DOI] [PubMed] [Google Scholar]
- 13.Reynafarje B., Costa L. E., Lehninger A. L. 1985. O2 solubility in aqueous media determined by a kinetic method. Anal. Biochem. 145: 406–418 [DOI] [PubMed] [Google Scholar]
- 14.Montgomery M. K., Hulbert A. J., Buttemer W. A. 2011. The long life of birds: the rat-pigeon comparison revisited. PLoS One. 6: e24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J. C. L., McClelland G. B., Faure P. A., Klaiman J. M., Staples J. F. 2009. Examining the mechanisms responsible for lower ROS release rates in liver mitochondria from the long-lived house sparrow (Passer domesticus) and big brown bat (Eptesicus fuscus) compared to the short-lived mouse (Mus musculus). Mech. Ageing Dev. 130: 467–476 [DOI] [PubMed] [Google Scholar]
- 16.Gredilla R., Sanz A., Lopez-Torres M., Barja G. 2001. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 15: 1589–1591 [DOI] [PubMed] [Google Scholar]
- 17.Ye J. M., Iglesias M. A., Watson D. G., Ellis B., Wood L., Jensen P. B., Sørensen R. V., Larsen P. J., Cooney G. J., Wassermann K., et al. 2003. PPARalpha/gamma ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am. J. Physiol. Endocrinol. Metab. 284: E531–E540 [DOI] [PubMed] [Google Scholar]
- 18.Matyash V., Liebisch G., Kurzchalia T. V., Shevchenko A., Schwudke D. 2008. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49: 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery M. K., Hallahan N. L., Brown S. H., Liu M., Mitchell T. W., Cooney G. J., Turner N. 2013. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 56: 1129–1139 [DOI] [PubMed] [Google Scholar]
- 20.Cleasby M. E., Lau Q., Polkinghorne E., Patel S. A., Leslie S. J., Turner N., Cooney G. J., Xu A., Kraegen E. W. 2011. The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signalling pathway. J. Endocrinol. 210: 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleasby M. E., Dzamko N., Hegarty B. D., Cooney G. J., Kraegen E. W., Ye J. M. 2004. Metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanisms. Diabetes. 53: 3258–3266 [DOI] [PubMed] [Google Scholar]
- 22.Bou R., Codony R., Tres A., Decker E. A., Guardiola F. 2008. Determination of hydroperoxides in foods and biological samples by the ferrous oxidation-xylenol orange method: a review of the factors that influence the method's performance. Anal. Biochem. 377: 1–15 [DOI] [PubMed] [Google Scholar]
- 23.Papamandjaris A. A., MacDougall D. E., Jones P. J. 1998. Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci. 62: 1203–1215 [DOI] [PubMed] [Google Scholar]
- 24.Papas A. M. 1996. Determinants of antioxidant status in humans. Lipids. 31(Suppl.): S77–S82 [DOI] [PubMed] [Google Scholar]
- 25.Savage D. B., Petersen K. F., Shulman G. I. 2007. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87: 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vogel-van den Bosch J., van den Berg S. A., Bijland S., Voshol P. J., Havekes L. M., Romijn H. A., Hoeks J., van Beurden D., Hesselink M. K., Schrauwen P., et al. 2011. High-fat diets rich in medium- versus long-chain fatty acids induce distinct patterns of tissue specific insulin resistance. J. Nutr. Biochem. 22: 366–371 [DOI] [PubMed] [Google Scholar]
- 27.Short K. R., Vittone J. L., Bigelow M. L., Proctor D. N., Rizza R. A., Coenen-Schimke J. M., Nair K. S. 2003. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 52: 1888–1896 [DOI] [PubMed] [Google Scholar]
- 28.Wright L. E., Brandon A. E., Hoy A. J., Forsberg G. B., Lelliott C. J., Reznick J., Löfgren L., Oscarsson J., Strömstedt M., Cooney G. J., et al. 2011. Amelioration of lipid-induced insulin resistance in rat skeletal muscle by overexpression of Pgc-1β involves reductions in long-chain acyl-CoA levels and oxidative stress. Diabetologia. 54: 1417–1426 [DOI] [PubMed] [Google Scholar]
- 29.Holloszy J. O. 2013. “Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes. 62: 1036–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacArthur D. G., Seto J. T., Chan S., Quinlan K. G. R., Raftery J. M., Turner N., Nicholson M. D., Kee A. J., Hardeman E. C., Gunning P. W., et al. 2008. An Actn3 knockout mouse provides mechanistic insights into the association between alpha-actinin-3 deficiency and human athletic performance. Hum. Mol. Genet. 17: 1076–1086 [DOI] [PubMed] [Google Scholar]
- 31.Grösch S., Schiffmann S., Geisslinger G. 2012. Chain length-specific properties of ceramides. Prog. Lipid Res. 51: 50–62 [DOI] [PubMed] [Google Scholar]
- 32.Mullen T. D., Hannun Y. A., Obeid L. M. 2012. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 441: 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldermann H., Wicklmayr M., Rett K., Banholzer P., Dietze G., Mehnert H. 1991. Changes of hepatic morphology during parenteral nutrition with lipid emulsions containing LCT or MCT/LCT quantified by ultrasound. JPEN J. Parenter. Enteral Nutr. 15: 601–603 [DOI] [PubMed] [Google Scholar]
- 34.Nosaka N., Kasai M., Nakamura M., Takahashi I., Itakura M., Takeuchi H., Aoyama T., Tsuji H., Okazaki M., Kondo K. 2002. Effects of dietary medium-chain triacylglycerols on serum lipoproteins and biochemical parameters in healthy men. Biosci. Biotechnol. Biochem. 66: 1713–1718 [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B., Gutteridge J. M. C. 2007. Free Radicals in Biology and Medicine. Oxford University Press, New York. [Google Scholar]
- 36.Lambertucci R. H., Hirabara S. M., Silveira Ldos. R., Levada-Pires A. C., Curi R., Pithon-Curi T. C. 2008. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J. Cell. Physiol. 216: 796–804 [DOI] [PubMed] [Google Scholar]
- 37.St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. 2002. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277: 44784–44790 [DOI] [PubMed] [Google Scholar]
- 38.Anderson E. J., Lustig M. E., Boyle K. E., Woodlief T. L., Kane D. A., Lin C. T., Price J. W., 3rd, Kang L., Rabinovitch P. S., Szeto H. H., et al. 2009. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119: 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoehn K. L., Salmon A. B., Hohnen-Behrens C., Turner N., Hoy A. J., Maghzal G. J., Stocker R., Van Remmen H., Kraegen E. W., Cooney G. J., et al. 2009. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA. 106: 17787–17792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuzefovych L., Wilson G., Rachek L. 2010. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 299: E1096–E1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pi J., Bai Y., Zhang Q., Wong V., Floering L. M., Daniel K., Reece J. M., Deeney J. T., Andersen M. E., Corkey B. E., et al. 2007. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 56: 1783–1791 [DOI] [PubMed] [Google Scholar]
- 42.Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S., Bruce C., Shields B. J., Skiba B., Ooms L. M., et al. 2009. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 10: 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silveira L. R., Fiamoncini J., Hirabara S. M., Procópio J., Cambiaghi T. D., Pinheiro C. H. J., Lopes L. R., Curi R. 2008. Updating the effects of fatty acids on skeletal muscle. J. Cell. Physiol. 217: 1–12 [DOI] [PubMed] [Google Scholar]
- 44.Tiganis T. 2011. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol. Sci. 32: 82–89 [DOI] [PubMed] [Google Scholar]
- 45.Brown G. C., Borutaite V. 2012. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 12: 1–4 [DOI] [PubMed] [Google Scholar]
- 46.Brand M. D. 2010. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 45: 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halliwell B. 1999. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic. Res. 31: 261–272 [DOI] [PubMed] [Google Scholar]
- 48.Bonnard C., Durand A., Peyrol S., Chanseaume E., Chauvin M. A., Morio B., Vidal H., Rieusset J. 2008. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Invest. 118: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuzawa-Nagata N., Takamura T., Ando H., Nakamura S., Kurita S., Misu H., Ota T., Yokoyama M., Honda M., Miyamoto K. I., et al. 2008. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 57: 1071–1077 [DOI] [PubMed] [Google Scholar]
- 50.Jones T. E., Baar K., Ojuka E., Chen M., Holloszy J. O. 2003. Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 284: E96–E101 [DOI] [PubMed] [Google Scholar]
- 51.Brand M. D., Esteves T. C. 2005. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2: 85–93 [DOI] [PubMed] [Google Scholar]
- 52.Nègre-Salvayre A., Hirtz C., Carrera G., Cazenave R., Troly M., Salvayre R., Pénicaud L., Casteilla L. 1997. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 11: 809–815 [PubMed] [Google Scholar]
- 53.Seifert E. L., Bézaire V., Estey C., Harper M. E. 2008. Essential role for uncoupling protein-3 in mitochondrial adaptation to fasting but not in fatty acid oxidation or fatty acid anion export. J. Biol. Chem. 283: 25124–25131 [DOI] [PubMed] [Google Scholar]
- 54.Vidal-Puig A. J., Grujic D., Zhang C. Y., Hagen T., Boss O., Ido Y., Szczepanik A., Wade J., Mootha V., Cortright R., et al. 2000. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 275: 16258–16266 [DOI] [PubMed] [Google Scholar]
- 55.Eckel R. H., Hanson A. S., Chen A. Y., Berman J. N., Yost T. J., Brass E. P. 1992. Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes. 41: 641–647 [PubMed] [Google Scholar]
- 56.Han J. R., Deng B., Sun J., Chen C. G., Corkey B. E., Kirkland J. L., Ma J., Guo W. 2007. Effects of dietary medium-chain triglyceride on weight loss and insulin sensitivity in a group of moderately overweight free-living type 2 diabetic Chinese subjects. Metabolism. 56: 985–991 [DOI] [PubMed] [Google Scholar]
- 57.Wein S., Wolffram S., Schrezenmeir J., Gasperiková D., Klimes I., Seböková E. 2009. Medium-chain fatty acids ameliorate insulin resistance caused by high-fat diets in rats. Diabetes Metab. Res. Rev. 25: 185–194 [DOI] [PubMed] [Google Scholar]
- 58.Hommelberg P. P. H., Plat J., Langen R. C. J., Schols A. M., Mensink R. P. 2009. Fatty acid-induced NF-kappaB activation and insulin resistance in skeletal muscle are chain length dependent. Am. J. Physiol. Endocrinol. Metab. 296: E114–E120 [DOI] [PubMed] [Google Scholar]
- 59.Hoehn K. L., Hohnen-Behrens C., Cederberg A., Wu L. E., Turner N., Yuasa T., Ebina Y., James D. E. 2008. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tonks K. T., Ng Y., Miller S., Coster A. C. F., Samocha-Bonet D., Iseli T. J., Xu A., Patrick E., Yang J. Y. H., Junutula J. R., et al. 2013. Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia. 56: 875–885 [DOI] [PubMed] [Google Scholar]
- 61.Hage Hassan R., Hainault I., Vilquin J. T., Samama C., Lasnier F., Ferré P., Foufelle F., Hajduch E. 2012. Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia. 55: 204–214 [DOI] [PubMed] [Google Scholar]
- 62.Mashili F., Chibalin A. V., Krook A., Zierath J. R. 2013. Constitutive STAT3 phosphorylation contributes to skeletal muscle insulin resistance in type 2 diabetes. Diabetes. 62: 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.