Abstract
The effect of lipid headgroup structure upon the stability of lipid asymmetry was investigated. Using methyl-β-cyclodextrin -induced lipid exchange, sphingomyelin (SM) was introduced into the outer leaflets of lipid vesicles composed of phosphatidylglycerol, phosphatidylserine (PS), phosphatidylinositol, or cardiolipin, in mixtures of all of these lipids with phosphatidylethanolamine (PE), and in a phosphatidylcholine/phosphatidic acid mixture. Efficient SM exchange (>85% of that expected for complete replacement of the outer leaflet) was obtained for every lipid composition studied. Vesicles containing PE mixed with anionic lipids showed nearly complete asymmetry which did not decay after 1 day of incubation. However, vesicles containing anionic lipids without PE generally only exhibited partial asymmetry, which further decayed after 1 day of incubation. Vesicles containing the anionic lipid PS were an exception, showing nearly complete and stable asymmetry. It is likely that the combination of multiple charged groups on PE and PS inhibit transverse diffusion of these lipids across membranes relative to those lipids that only have one anionic group. Possible explanations of this behavior are discussed. The asymmetry properties of PE and PS may explain some of their functions in plasma membranes.
Keywords: cyclodextrin, lipid exchange, membrane structure
The inner and outer leaflet of many cellular membranes exhibits a difference in lipid composition. This difference is called lipid asymmetry. For example, in eukaryotic cells, the choline-containing lipids sphingomyelin (SM) and phosphatidylcholine (PC) are predominantly located in the outer leaflet of the plasma membrane, while the amine-containing phospholipids phosphatidylethanolamine (PE) and phosphatidylserine (PS) are largely or fully confined to the plasma membrane inner leaflet (1–4). Membrane asymmetry is also observed in prokaryotic cells. Although it is difficult to determine lipid distribution in the leaflets of the membrane surrounding the bacterial cytoplasm, it has been reported that phosphatidylinositol (PI) is preferentially located in the inner leaflet, phosphatidylglycerol (PG) is mainly located in the outer leaflet, and cardiolipin (CL) is distributed over both leaflets in gram-positive bacteria Micrococcus lysodeikticus (5). Studies from Bacillus megaterium suggested PE mainly resides in the inner leaflet (6). In the outer membranes of gram-negative bacteria lipopolysaccharides are predominantly localized in the outer leaflet, while phospholipids are enriched in the inner leaflet (7–9). Several important functional roles of cellular membrane are closely associated with an asymmetrical lipid distribution. For example, dissipation of membrane lipid asymmetry, resulting in PS externalization on the outer leaflet of membrane, facilitates cell recognition and phagocytosis by macrophages during apoptosis (10–18). Exposure of PS on the outer cell surface has also been known to be involved in physiologically important phenomena including blood coagulation (19, 20), cell adhesion (21–23), and myotube formation (24).
To emulate cell membranes more closely, a number of methods have been developed to try to obtain asymmetric lipid bilayers (model membranes) (25–45). However, methods to form asymmetric lipid vesicles with a wide variety of lipid compositions and highly controlled lipid distribution in each leaflet have been lacking. To tackle this problem, our laboratory has developed methods to prepare asymmetric vesicles using methyl-β-cyclodextrin (MβCD)-induced lipid exchange (43–46). Vesicles prepared by MβCD-induced exchange have great potential for various applications because the procedure is not unduly complicated, they can be prepared with a variety of lipid compositions, and they can be used to create asymmetric vesicles with various sizes as well as reconstituted vesicles with membrane-inserted peptides (43–45) and proteins. We recently extended this method by generating asymmetric vesicles containing SMs as outer leaflet lipids and a large variety of PCs with different acyl chain structures as inner leaflet lipids (46). It was found that MβCD-induced lipid exchange was always efficient. However, the stability of asymmetry in the vesicles prepared by lipid exchange was dependent on the acyl chain structure. This was found to be due to the influence of acyl chain structure upon transverse diffusion (flip-flop).
In this report, the exchange method has been extended to investigate the effect of lipid headgroup structure upon the efficiency of lipid exchange and the ability to form asymmetric vesicles. Lipid exchange was carried out by substituting SM into the outer leaflet of vesicles containing PE, PS, PG, PI, CL, or phosphatidic acid (PA). The results show that efficient exchange can always be obtained, but that headgroup structure influences whether the resulting vesicles are fully or only partially asymmetric. We propose that the effect of lipid structure upon asymmetry provides important clues as to the function of different phospholipids in membranes.
MATERIALS AND METHODS
Materials
Porcine brain SM, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DOPG), soy l-α-phosphatidylinositol (soy PI), 1′,3′-bis[1,2-dioleoyl-sn-glycero-3-phospho]sn-glycerol [tetraoleoyl cardiolipin (TOCL)], 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (POPS), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) were purchased from Avanti Polar Lipids (Alabaster, AL). 1,6-Diphenyl-1,3,5-hexatriene (DPH) and MβCD were from Sigma-Aldrich (St. Louis, MO). All lipids were dissolved in chloroform and stored at −20°C. DPH was dissolved in ethanol and stored at −20°C. Concentrations of lipids were measured by dry weight. Concentrations of DPH were determined by absorbance as described previously (45). Sepharose CL-2B and CL-4B were purchased from Amersham Biosciences (Piscataway, NJ). High-performance thin layer chromatography (HP-TLC) plates (Silica Gel 60) were purchased from VWR International (Batavia, IL). The digital thermometer was purchased from Fisher Scientific (Pittsburgh, PA).
Formation of exchange (or asymmetric) small unilamellar vesicles
Exchange (asymmetric) small unilamellar vesicles (SUVs) were prepared as described previously (46). First, donor multilamellar vesicles (MLVs), the population from which SM was to be exchanged with the outer leaflet of the acceptor SUVs, were prepared. We prepared donor MLVs composed of 16 mM SM by drying the lipid in a film under nitrogen gas stream followed by high vacuum for at least 1 h, and then this was hydrated at 70°C with 500 μl of PBS (1.8 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, and 2.7 mM KCl, pH 7.4). This sample was vortexed in a multitube vortexer (VWR International) at 55°C for 15 min and then centrifuged at 11,000 g for 5 min at room temperature to remove any remaining small vesicles. We removed the supernatant, and the pellet was resuspended using 500 μl PBS. Then 95 μl of 390 mM MβCD, which was dissolved in PBS buffer, was added to the resuspended pellet. This SM MLV/MβCD mixture was vortexed in the multitube vortexer at 55°C for 2 h.
Then, we prepared acceptor vesicles which form the inner leaflet lipids of the asymmetric vesicles. Six hundred microliters of 8 mM MLV was prepared, just like SM MLVs (see above), and vortexed for 15 min at 55°C after the lipids were hydrated. To form SUVs, the MLVs were sonicated in a bath sonicator (Special Ultrasonic Cleaner Model G1112SP1; Laboratory Supplies Co., Hicksville, NY) at room temperature for at least 15 min until the solution became transparent and then diluted to 4 mM with PBS.
In the next step, the 500 μl of 4 mM sonicated SUVs containing lipids with different headgroups were added to the final 595 μl SM MLV/MβCD mixture above and vortexed at 55°C for 30 min. The samples were cooled down to room temperature and then centrifuged at 49,000 g for 5 min using an air-driven microultracentrifuge (Beckman Airfuge). The supernatant was added to a Sepharose CL2B or CL4B column (dimensions: 25 cm length and 1 cm diameter) and eluted with PBS. (We later found that CL2B fractions were contaminated with MβCD, so we recommend using CL4B where possible. However, CL2B does minimize contamination of SUVs with MLVs.) Fractions of 1 ml each were collected, with SUVs mainly eluting into fractions 16–19 (or 12–14 for CL4B). These fractions were combined for further analysis. The lipid concentration in the combined fractions was normally ∼150 μM as assayed by HP-TLC relative to standards with known amounts of lipid. The lipid was detected by charring and quantified spot intensity (see below). In cases in which the amount of lipid was not explicitly measured, we assumed a concentration of exchange vesicles of ∼150 μM lipid.
Fluorescence measurements
Fluorescence measurements were carried out using a SPEX FluoroLog 3 spectrofluorimeter (Jobin-Yvon, Edison, NJ) with quartz semi-micro cuvettes (path length: excitation 10 mm, emission 4 mm) as previously described (45). DPH fluorescence was measured at 358 nm (excitation) and 427 nm (emission). The slit bandwidths for fluorescence measurements were set to 4.2 nm for both excitation and emission. The backgrounds from samples prepared without fluorescent probes were negligible (less than 1% of fluorescence in experimental samples), and so were not subtracted.
Steady-state fluorescence anisotropy measurements
Anisotropy measurements were carried out using a SPEX automated Glan-Thompson polarizer accessory according to previously described protocols (45). The bandwidths of slit for DPH anisotropy were set to 4.2 nm (2 mm physical size) for both excitation and emission. The steady-state DPH anisotropy within the lipid bilayer was determined using the following equation: A = {[(Ivv × Ihh)/(Ivh × Ihv)] − 1}/{[(Ivv × Ihh)/(Ivh × Ihv)] + 2}, where A represents anisotropy, I represents the fluorescence emission intensity, with the vertical (v) and horizontal (h) orientation of the excitation and emission polarization filters (47). For the temperature-dependent DPH fluorescence anisotropy measurements, DPH dissolved in ethanol was added to the cuvette, with 5 min incubation in the dark. The DPH/lipid molar ratio was 0.1:100. Samples were scanned stepwise with 4°C steps, between 16°C and 60°C. A digital thermometer was placed in the cuvette to measure the temperature and each anisotropy measurement was carried out once temperature stabilized. The DPH anisotropy was plotted as a function of temperature and the melting temperature (Tm) was defined by the midpoint of a sigmoidal fit with the curve-fitting option of SlideWrite Plus software (Advanced Graphics Software, Inc., Rancho Santa Fe, CA).
Analysis of lipid composition by HP-TLC
Lipids were extracted with chloroform/methanol (1:1 v/v) from ordinary and exchange vesicles as described in (43). The extracted lipids or pure lipid standards were applied to HP-TLC (Silica Gel 60) plates which were heated at 100°C for 30 min and cooled down to room temperature. For separating SM from PG, CL, PI, or PA, the solvent system 65:25:4 chloroform:methanol:water (v/v/v) was used. When the solvent reached the upper line of TLC plate, the plate was removed from the chamber and dried in the fume hood. The lipids loaded onto the TLC plate were visualized after spraying 3% (w/v) cupric acetate, 8% (v/v) phosphoric acid solution, followed by drying and charring at 180°C for 2–10 min. The lipids in the vesicles were quantified by measuring TLC spot intensity versus a standard curve for each lipid generated on the same TLC plate as described previously (45).
Scrambling experiments
The scrambling process, in which vesicles are destroyed and then reformed as symmetric vesicles, was carried out similarly to previously described protocols (45). Fractions 16–19 from the CL2B column were combined and then divided into four tubes (1 ml each). Two of the samples were subjected to scrambling and two were used without scrambling. The samples to be scrambled were dried by a nitrogen gas. The dried lipid film with salt was dissolved in 20 μl of ethanol, and then dispersed at 70°C with 980 μl of distilled water to create scrambled vesicles. These scrambled vesicles were cooled down to room temperature. DPH dissolved in ethanol was added to the scrambled vesicles at 0.1 mol% of total lipid concentration.
Proton NMR spectroscopy
The asymmetry of exchange SUVs with SM was studied using a Bruker Avance 700 MHz proton NMR spectrometer (Bruker Biospin, Billerica, MA). The NMR sample tubes used were Wilmad LabGlass tubes (WG-5MM Economy, 7 inch length; Wilmad Glass Co, Buena, NJ). We used the signal of the choline N-methyl groups of SM in vesicles as the indicator of lipid asymmetry. The samples were preincubated 5–10 min at 55°C and NMR measurements made at 55°C in order to keep the SM in the liquid disordered (Ld) state, in which its signal is most readily detected and linewidth minimized. [Asymmetry may be partly lost if samples are incubated for too long at elevated temperatures (45).] Two 1H choline methyl resonances were observed in SUVs at 55°C: one corresponded to the outer membrane leaflet SM at a chemical shift of 3.47–3.49 ppm, while the inner membrane leaflet choline had a chemical shift 0.02–0.04 ppm upfield. For exchange vesicles, the SUV fractions from the Sepharose column were combined, and then 500 μl were removed and transferred to the NMR tube followed by addition of 100 μl D2O. For ordinary symmetric vesicles, 1 ml of 200 μM SUVs was prepared by ethanol dilution. The desired lipid mixtures were dried, dissolved in 20 μl of ethanol, and then dispersed at 70°C in 980 μl of PBS. Ethanol was removed by dialysis using a membrane (Thomas Scientific, Swedesboro, NJ) with 6,000–8,000 Da molecular weight cut-off against PBS while constantly stirring at room temperature for 24 h. Then, 500 μl of ordinary symmetric vesicles were taken from the dialysis bag and transferred to the NMR tube. Next, 100 μl of D2O was added. The samples were incubated in the 55°C water bath for 10 min before proton NMR spectra were recorded. All spectra were acquired at 55°C. One hundred and twenty-eight scans were acquired for each sample, with an acquisition time of 2.3 s (digitized into 64 K data points) and a relaxation delay of 2 s between scans. Each acquisition required around 10 min per sample. Water suppression was achieved using a standard presaturation sequence.
Percent SM in exchange vesicles
The percent of SM in exchange vesicles was calculated from HP-TLC as described previously (46). The samples for HP-TLC were from the exchange SUV-containing fractions from the Sepharose CL-2B column. The percent of SM by TLC was evaluated after calculating the intensity of SM bands. The lipids in the vesicles were quantified by measuring TLC spot intensity versus a standard curve for each lipid generated on the same TLC plate as described previously (46).
RESULTS
Preparation of exchange (asymmetric) SUVs by MβCD-induced lipid exchange
In the lipid exchange method, the lipids in the outer leaflet from an excess of donor vesicles are transferred to the outer leaflet of acceptor SUVs using MβCD, which functions as a carrier that exchanges lipids between two different vesicle populations (43–46). After exchange and isolation of the acceptor vesicles (exchange vesicles), they should have a different lipid composition in their inner and outer leaflets if asymmetric. In this study, the method has been extended to determine whether the headgroup structure of lipids has an effect on the ability to generate an asymmetric lipid distribution and the ability of lipid to maintain lipid asymmetry. The donor lipid (desired outer leaflet lipid) was mammalian brain SM. The acceptor lipid (desired inner leaflet lipid) had various headgroup structures, including those of the most common anionic lipids: phosphatidylglycerol (POPG and DOPG), phosphatidylinositol [soy PI and 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol)], phosphatidic acid (DOPA), cardiolipin (TOCL), and phosphatidylserine (POPS), as well as mixtures of anionic lipids and the zwitterionic lipid phosphatidylethanolamine (POPE). DOPA was only studied in a mixed composition with POPC, which was found to be necessary to prepare DOPA-containing SUVs.
First, the amount of SM exchanged into acceptor vesicles was assayed as a function of acceptor lipid structure. Table 1 shows the average SM content in exchange vesicles was ∼60% of total lipids. Because the outer leaflet percent of total surface area in SUVs is ∼67%, this indicates that MβCD-induced lipid exchange is efficient, corresponding to roughly 90% exchange of outer leaflet lipids, consistent with our prior studies (46). This demonstrates that the exchange method can be successfully used with a wide variety of lipid headgroups.
TABLE 1.
Percent SM in exchange vesicles
| Sample Composition | SM Percent by TLC |
| SMo/POPG i | 57.9 ± 4.3 (4) |
| SMo/DOPG i | 57.5 ± 2.1 (2) |
| SMo/soy PI i | 59.9 ± 1.7 (5) |
| SMo/TOCL i | 67.8 ± 4.1 (2) |
| SMo/POPC:DOPA 2:1 i | 62.2 ± 2.1 (2) |
| SMo/POPS i | 59.1 ± 4.5 (2) |
| SMo/POPE:POPG 2:1 i | 63.2 ± 3.8 (2) |
| SMo/POPE:DOPG 2:1 i | 59.1 ± 2.4 (2) |
| SMo/POPE:soy PI 2:1 i | 60.5 ± 2.6 (2) |
| SMo/POPE:TOCL 2:1 i | 58.6 ± 3.5 (2) |
| SMo/POPE:POPS 2:1 i | 57.6 ± 2.6 (2) |
Percent of SM in exchange vesicles was calculated from HP-TLC analysis of Sepharose CL2B fractions (details in Materials and Methods). Average (mean) and range derived from duplicate experiments or SD when n > 2 is shown. The number of experiments is shown in parentheses. o, outer leaflet lipid; i, inner leaflet lipid.
Determination of asymmetry by comparison of ordered (gel) phase thermal stability in exchange vesicles and scrambled vesicles
To determine whether SM is asymmetrically distributed in the bilayer of the exchange vesicles (i.e., with SM in the outer leaflet and the lipids with other headgroups in the inner leaflet), the thermal stability of SM-rich ordered (gel state) domains in the exchange vesicles was compared with that in symmetric vesicles. We previously observed that when asymmetric vesicles have SM in the outer leaflet, they have a higher Tm for the gel to Ld transition than do symmetric vesicles of the same lipid composition (5°C < ΔTmasymmetric−symmetric < 10°C) (43, 45, 46). This reflects an increased thermal stability of the gel state in the asymmetric vesicles. (It should be noted that the Tm detected in the asymmetric vesicles mainly reflects the melting of the outer leaflet. The melting of the inner leaflet is generally not detected in these vesicles because it is composed of unsaturated lipids with much lower Tms.) In fact, the melting transition of the SM-rich outer leaflet in fully asymmetric vesicles occurs at a similar temperature as in pure SM vesicles, evidently because the thermal stability of the gel state of the SM-rich outer leaflet is not affected by Ld state lipids in the inner leaflet (43, 45, 46). To insure that the symmetric vesicles had identical lipid compositions as the exchange vesicles, we prepared “scrambled” vesicles in which the exchange vesicles were dissolved in organic solvents and then reconstituted to produce symmetric vesicles. When exchange vesicles are symmetric, the Tm before and after scrambling is identical (46).
Gel state thermal stability was determined from the steady-state DPH anisotropy measurements as a function of temperature (48). Anisotropy is high in tightly packed gel state and low in loosely packed Ld state (49–51). The phase transition is detected as a sigmoidal decrease in DPH anisotropy as temperature is increased, with Tm defined as the midpoint of the sigmoidal curve (52, 53).
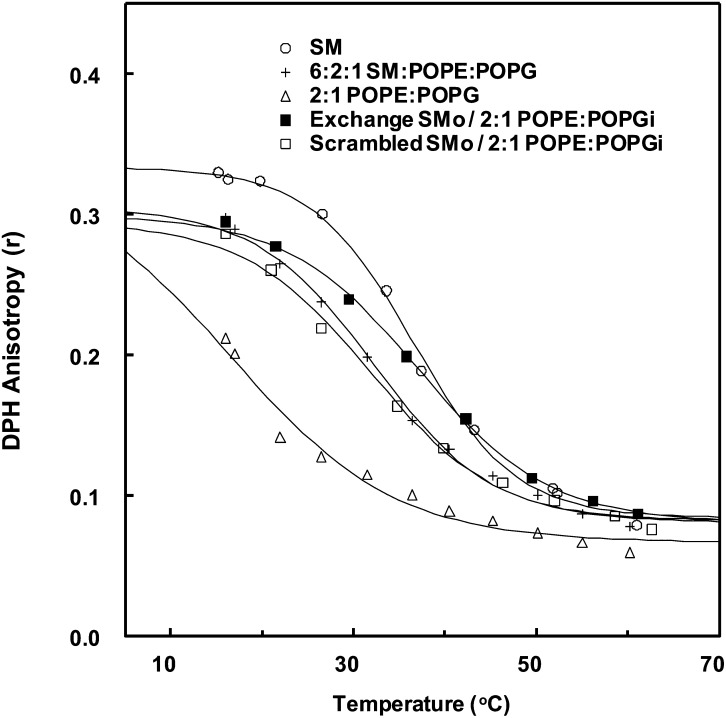
Figure 1 shows representative DPH anisotropy versus temperature curves for exchange and symmetric vesicles. In the symmetric vesicles, the highest Tm values are observed for pure SM vesicles (36.5°C), with intermediate Tm values for vesicles composed of a mixture of 6:2:1 (mol:mol:mol) SM:PE:PG (32.2°C), and lowest Tm (16.3°C) for 2:1 PE:PG. The exchange vesicles, which have an overall lipid composition (with 63.2 ± 3.8% SM) similar to that of symmetric 6:2:1 SM:PE:PG vesicles, had a Tm of 37.1°C. The observation that the Tm of the exchange vesicles was 5°C higher than in the symmetric 6:2:1 SM:PE:PG vesicles and than in the scrambled vesicles indicates that the exchange vesicles were asymmetric. The fact that the asymmetric vesicle Tm was about as high as pure SM vesicles is also indicative of asymmetry, as noted above. This result (Fig. 1) shows that the thermal stability of ordered domains in the exchange vesicles was not destabilized by the presence of unsaturated PE/PG lipids, in agreement with previous results as noted above (43, 45, 46).
Fig. 1.
Representative steady-state DPH anisotropy as a function of temperature in symmetric, scrambled, and exchange SUVs. Samples contained 0.1 mol% DPH in SM (○); 6:2:1 SM:POPE:POPG (+); 2:1 POPE:POPG (△); SMo/2:1 POPG:POPEi (■); and scrambled SMo/2:1 POPG:POPEi (□).
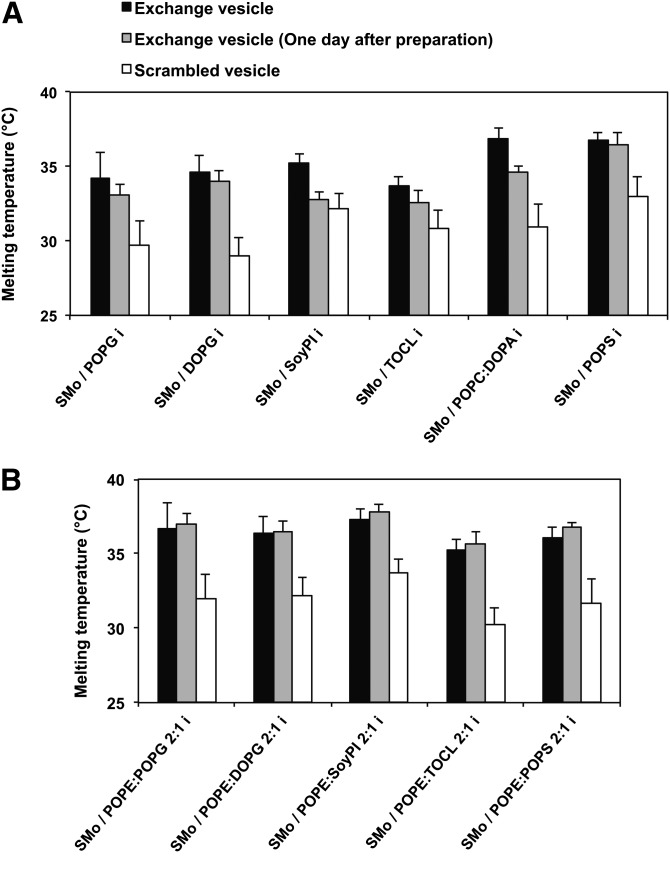
These experiments were repeated for all of the lipids studied. Figure 2 shows the Tm of exchange vesicles (black bars) and the corresponding scrambled vesicles (white bars). In most cases, Tm of the exchange vesicles before scrambling was close to that of pure SM. In addition, a substantial Tm decrease after lipid scrambling (3–6°C) was observed for all the different headgroup lipids tested. Thus, lipids with different headgroup structures form vesicles with at least some degree of lipid asymmetry. However, Tm in the asymmetric vesicles was lowest for the vesicles with inner leaflets composed of DOPG, POPG, CL, or soy PI, which might suggest a lower extent of asymmetry (see below). (Notice that we are using the nomenclature previously established for asymmetric vesicles, in this case SMo/Xi, where X is the name of the lipid(s), o is the outer leaflet lipid after exchange, and i is the inner leaflet lipid after exchange, if asymmetry is achieved. This nomenclature is used both when exchange vesicles are completely and just partly asymmetric.)
Fig. 2.
The gel to fluid melting midpoint (Tm) of exchange and scrambled SUVs. A: SMo/Xi vesicles. B: SMo/2:1 POPE:Xi vesicles. The (X) indicates a lipid other than SM. Exchange vesicles (black bars); exchange vesicles 1 day after preparation (gray bars); and exchange vesicles after scrambling (white bars). Lipid composition of exchange vesicles is shown on the x axis. The steady-state DPH anisotropy measurements as a function of temperature were made on exchange vesicles both immediately after preparation and after 1 day of incubation at room temperature. The average (mean) and range (if n = 2) or SD are shown. The number of samples is the same as shown in Table 2.
Estimating the stability and extent of asymmetry
Previously we found that the extent of lipid asymmetry in exchange vesicles was linked to the rate of spontaneous lipid movement across the bilayer (lipid transverse diffusion = flip-flop) (46), such that vesicles with lipid asymmetry were formed when flip-flop was slow. To see which exchange vesicles showed fast flip-flop, and thus had destabilized lipid asymmetry, their Tm immediately after preparation (Fig. 2, black bars) was compared with their Tm 1 day later (Fig. 2, gray bars). If asymmetry is not stable due to fast flip-flop, then Tm values after 1 day should decrease and approach that in scrambled symmetric vesicles (Fig. 2, white bars).
Figure 2 shows there was a measurable decrease of Tm 1 day after vesicle preparation for exchange vesicles in which the inner leaflet contained PG, CL, soy PI, or PA without PE. (However, the decrease in Tm was very small for PG and CL.) This suggests their asymmetry is not stable, and may have been incomplete even when measured right after preparation. This is in agreement with the low Tm values observed for PG, CL, and PI-containing vesicles right after preparation, as noted above. However, when these lipids were mixed with PE, the Tm of exchange vesicles did not decrease 1 day after vesicle preparation, and in the case of PS, the Tm of exchange vesicles was stable after 1 day, both with or without PE. This suggests these lipids have stable asymmetry, and agrees with their high Tm values right after preparation.
To see if the behavior of soy PI [which is rich in polyunsaturated (18:2) acyl chains in its 2-position] reflected a difference in acyl chain structure from the other lipids, which had either one or two oleoyl chains (18:1), we performed a few experiments using dioleoyl PI. Exchange vesicles prepared with dioleoyl PI appeared to exhibit behavior similar to that of soy PI, suggesting the behavior of soy PI does not simply reflect its acyl chains (supplementary Fig. II).
Exchange experiments were also carried out using large unilamellar vesicles (LUVs) in place of SUVs (43). These are shown in supplementary Fig. I. Exchange LUVs could not be prepared in all cases because in some cases LUVs aggregated severely after exchange, while in other cases we could not find conditions to fully purify exchange LUVs from donor vesicles. In addition, the data with LUVs also tended to be a bit less reproducible than for SUVs. Nevertheless, the data shows that asymmetric vesicles can be prepared with SM in the outer leaflet and TOCL, POPE/TOCL 2:1, or POPS in the inner leaflet. The asymmetry is stable after 1 day in the exchange LUVs containing POPE/TOCL or POPS. For the case of exchange vesicles containing TOCL in the inner leaflet, variability in Tm is too large to tell if the asymmetry is fully stable or only partly stable. We also saw evidence for asymmetry in vesicles with POPG in their inner leaflet, but vesicle aggregation reduced the reliability of the data (not shown). In addition, it should be noted that we previously found asymmetry was stable in exchange LUVs with DOPE/POPS in their inner leaflet (43). Therefore, it is possible to prepare asymmetric LUVs that maintain stable asymmetry with a wide variety of headgroups.
Apparent lack of coupling between inner and outer leaflet membrane state
Table 2 shows that, as estimated from the anisotropy of DPH at 23°C, the level of membrane order in exchange vesicles that appear to be fully asymmetric after introduction of SM is only ∼70% that of pure SM vesicles, even though Tm values were almost identical to those in pure SM vesicles. Our previous studies have shown that in SMo/PCi asymmetric vesicles in which the PC has at least one unsaturated acyl chain, 70% order reflects a relative lack of coupling between the physical state in the outer leaflet (gel state), which is nearly pure SM and composes about 67% of the total lipid, and the physical state of the inner leaflet, which remains in the Ld state (43–46). Thus, it appears that the lack of coupling between inner and outer leaflet physical states, such that the outer leaflet is in the gel state and the inner leaflet is in the Ld state, is also true for other asymmetric vesicles with SM outside and lipids with at least one unsaturated acyl chain inside.
TABLE 2.
DPH fluorescence anisotropy in symmetric and exchange vesicles at 23°C
| Sample Composition | DPH Anisotropy | Percent Ordered |
| SM | 0.311 ± 0.002 (5) | ≡100 |
| POPG | 0.115 ± 0.001 (2) | ≡0 |
| DOPG | 0.098 ± 0.002 (2) | ≡0 |
| Soy PI | 0.108 ± 0.002 (2) | ≡0 |
| TOCL | 0.099 ± 0.002 (2) | ≡0 |
| 2:1 POPC:DOPA | 0.114 ± 0.004 (2) | ≡0 |
| POPS | 0.105 ± 0.003 (2) | ≡0 |
| 2:1 POPE:POPG | 0.158 ± 0.005 (2) | ≡0a |
| 2:1 POPE:DOPG | 0.129 ± 0.003 (2) | ≡0a |
| 2:1 POPE:Soy PI | 0.144 ± 0.002 (2) | ≡0a |
| 2:1 POPE:TOCL | 0.132 ± 0.002 (2) | ≡0a |
| 2:1 POPE:POPS | 0.143 ± 0.002 (2) | ≡0a |
| SMo/POPG i | 0.239 ± 0.008 (7) | 63.3 ± 5.2 |
| SMo/DOPG i | 0.223 ± 0.012 (2) | 58.7 ± 6.4 |
| SMo/Soy PI i | 0.224 ± 0.005 (8) | 57.1 ± 3.9 |
| SMo/TOCL i | 0.210 ± 0.007 (10) | 52.4 ± 4.3 |
| SMo/POPC:DOPA 2:1 i | 0.237 ± 0.004 (6) | 62.4 ± 4.5 |
| SMo/POPS i | 0.252 ± 0.004 (6) | 71.4 ± 4.4 |
| SMo/POPE:POPG 2:1 i | 0.274 ± 0.003 (4) | 75.8 ± 6.7 |
| SMo/POPE:DOPG 2:1 i | 0.243 ± 0.004 (4) | 62.6 ± 4.5 |
| SMo/POPE:Soy PI 2:1 i | 0.281 ± 0.003 (4) | 82.0 ± 5.5 |
| SMo/POPE:TOCL 2:1 i | 0.231 ± 0.005 (4) | 55.3 ± 4.4 |
| SMo/POPE:POPS 2:1 i | 0.272 ± 0.016 (5) | 76.8 ± 10.7 |
The percent ordered state of the outer leaflet was estimated from the following equation: Percent ordered = (A − A100% Ld)/(A100% ordered − A100% Ld). A is anisotropy in the exchange vesicles, A100% ordered is anisotropy in SM, and A100% Ld is anisotropy in the appropriate unsaturated lipid. This formula assumes that the gel domain and Ld domains have A values similar to those in pure gel and Ld state vesicles, respectively. DPH was added to preformed vesicles at a concentration of 0.1 mol% of total lipid concentration. Symmetric vesicles were prepared by ethanol dilution. Average (mean) and range derived from duplicate experiments or SD when n > 2 is shown. The number of samples is shown in parentheses.
These lipid mixtures may not be totally in Ld state at room temperature.
Asymmetry of exchange vesicles assayed by proton NMR spectroscopy
The studies above provide a good indication of which vesicles are fully asymmetric and which are only partly asymmetric. To confirm these results, and to assay the asymmetry of exchange vesicles more directly, NMR was used. The difference in packing in inner and outer leaflets of SUVs influences the proton NMR chemical shift of the choline N-methyl groups of SM and PC such that the methyl signal splits into separate inside and outside methyl peaks, with the outer leaflet methyls having a more downfield chemical shift (54, 55). The relative intensity of the inside and outside choline methyl signal of SM can be used to estimate the outer/inner leaflet distribution of SM in SUVs. In order to eliminate complications due to differences in the physical state of lipids in the inner and outer leaflet, all the proton NMR experiments were carried out at 55°C, well above Tm in these vesicles.
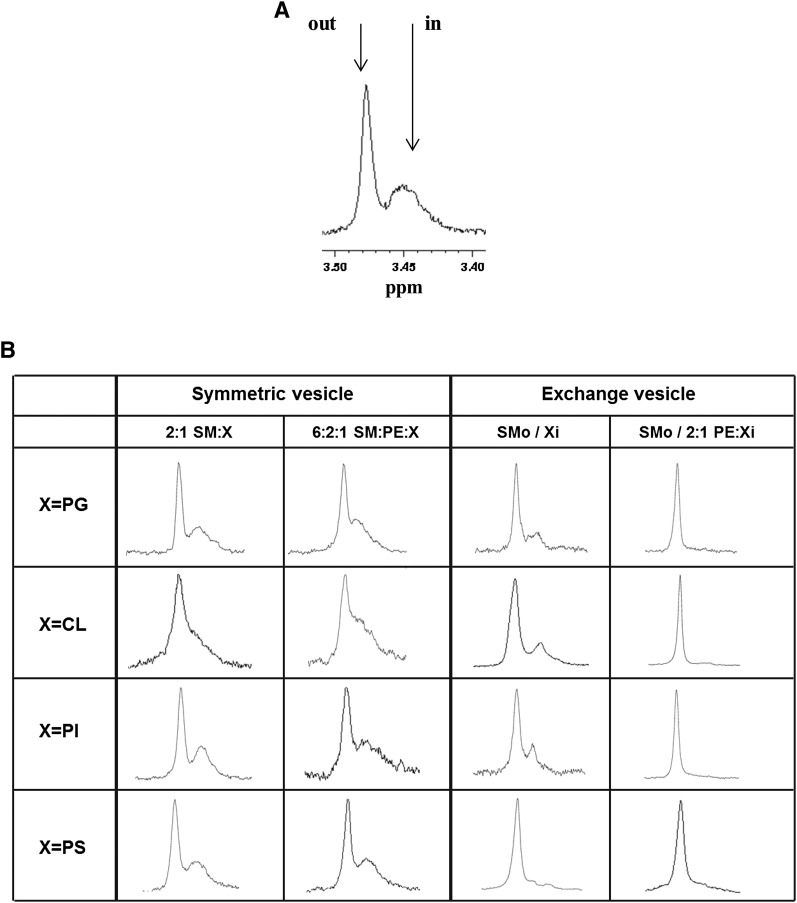
Figure 3 shows the chemical shift of the choline N-methyl groups of SM in symmetric and exchange SUVs. For symmetric SUVs composed of pure SM or a SM/different headgroup lipids mixture, two split 1H resonances were observed, as expected, corresponding to the outer membrane leaflet at a chemical shift of 3.47–3.49 ppm and the inner membrane leaflet at a chemical shift about 0.04 ppm lower (with a somewhat smaller difference in shift in some cases). This split signal confirmed SM is localized both in the outer and inner leaflet (Fig. 3). For exchange SUVs, when lipids such as PG, CL, or PI were used alone as the inner leaflet lipids, freshly prepared exchange vesicles also showed two 1H resonances of SM choline N-methyl groups. This confirmed that SM was located in both the outer leaflet and the inner leaflet, and that these vesicles were not fully asymmetric. However, the intensity of the inner leaflet peak relative to the outer leaflet peak was significantly less than in the corresponding symmetric vesicles, indicative of some degree of asymmetry. Vesicles containing PS exhibited only very weak inner leaflet SM peaks, suggesting they were more asymmetric than was the case for the other anionic lipids. Figure 3B also shows that when the various anionic lipids were mixed with PE, at most only a very small upfield choline methyl group signal was detected. This indicated that in the presence of PE the vesicles were very highly asymmetric. These results are fully in agreement with the conclusions of the Tm studies described above, and an alternative explanation for the single NMR signal, that the vesicles have fused to form LUVs, is ruled out by measurements of vesicle size (supplementary Fig. III).
Fig. 3.
Proton NMR spectra at 55°C of SM choline N-CH3. NMR spectra near the chemical shift of choline N-methyl groups of brain SM is shown with the outer leaflet signal at δ 3.47–3.49 ppm and the inner leaflet at a lower δ by 0.02–0.04 ppm. A: Spectra of symmetric pure SM vesicles. The arrows denote the SM distributed in the outer (out) and inner (in) leaflet of the membrane. B: Spectra of symmetric vesicles of 2:1 mol:mol SM:X and SM:PE:X mol:mol 6:2:1 (left columns), and spectra of exchange vesicles composed of SMo/Xi and SMo/2:1PE:Xi (right columns). The (X) indicates POPG, TOCL, soy PI, or POPS with or without POPE.
DISCUSSION
Effect of headgroup structure upon asymmetric vesicle formation and stability
This study has shown MβCD-induced lipid exchange can be used to form asymmetric vesicles having a wide variety of lipid headgroups. However, the extent and stability of asymmetry are strongly affected by lipid headgroup type. In the case of PS, or PE mixed with PS, PG, PI, or CL, almost fully asymmetric vesicles with outer leaflets composed of SM were obtained. In these cases, Tm values matched those expected for fully asymmetric vesicles based on prior studies (43, 45, 46); there was no change in Tm after 1 day, which is indicative of stable asymmetry; and NMR showed that SM was located in the outer leaflet to a much higher extent than in symmetric vesicles with the same composition. We estimate roughly that the asymmetry is on the order of 90% in these vesicles. Our prior studies with PC having the same acyl chains as the lipids used in this study (i.e., DOPC and POPC) found that stable SM/PC vesicles with a similar degree of asymmetry can be obtained (45).
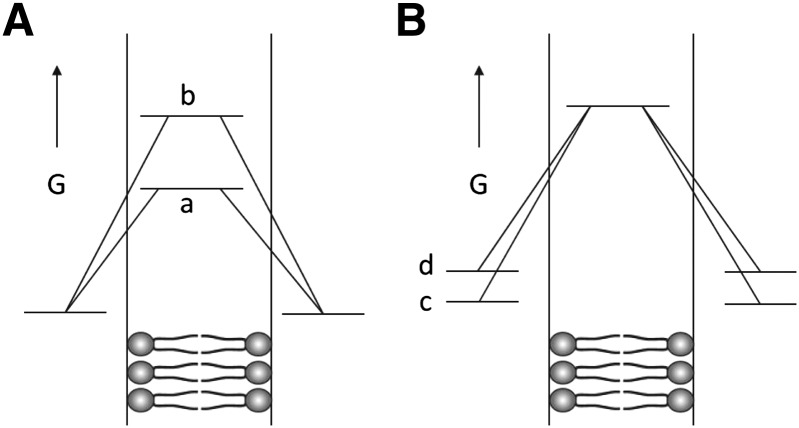
In contrast, only partial asymmetry was obtained with the other anionic lipids tested (DOPG, POPG, soy PI, DOPC, and TOCL). In these cases, Tm was lower than in fully asymmetric vesicles, and Tm was found to decrease after 1 day, indicative of further loss of asymmetry, i.e., that asymmetry is unstable. In addition, NMR showed a significant level of SM in the inner leaflet, although much less than in symmetric vesicles. These observations raise the question of why PS, PE, and PC allow/promote the formation of stable asymmetry. One likely factor is that these lipids have multiple charged groups, both anionic and cationic, and rarely (or in the case of PC never) exist in an uncharged state. In contrast, PG, PI, and CL have only one anionic charge on the phosphate (which in the case of CL means one anionic group per two acyl chains). These lipids may more readily cross the membrane in an uncharged state, either due to protonation or complexation with Na+ or K+ from the buffer, which should more readily penetrate into and cross the hydrophobic core of the bilayer (Fig. 4A). This tendency to flip would be exacerbated by repulsions between the anionic groups of neighboring anionic lipids, which would raise the free energy of the lipids in the usual nontranslocating conformation relative to the transition state expected to form when the lipids are crossing the most hydrophobic part of the bilayer (Fig. 4B). This repulsion should be lessened significantly by the presence of zwitterionic PE molecules. This may explain why asymmetry is more stable in the presence of PE. Another factor may be the small headgroup size of PE, which would lessen steric clashes between headgroups. Because the headgroup of PC is larger than PE, this factor might explain why mixtures of PC and PA did not form vesicles with very stable asymmetry. Alternatively, PA has such a small headgroup that it may cross membranes faster than other lipids.
Fig. 4.
Schematic illustration of factors affecting the stability of asymmetry for lipids with different headgroups. A: Differences in free energy in bilayer core during flipping. The (a) indicates lipids with one charged group per two acyl chains (PG, PI, PA, CL) and the (b) indicates lipids with multiple and oppositely charged groups (SM, PE, PC, and PS). B: Differences in free energy in bilayer before flipping. The (c) indicates lipids with no net electrostatic repulsions (PC, SM, PE) and the (d) indicates lipids with net electrostatic repulsions (PG, PI, PA, CL, PS). G, free energy.
It is also possible that lipids do not flip as monomers. For example, lipid dimers might form such that their headgroups interact to form H-bonds and/or salt bridges that decrease their energy when moving across the core of the bilayer. In this case, lipids that more readily form these dimers may flip more quickly.
Another question is: if only one of the two lipids in a bilayer tends to flip quickly, how can asymmetry break down? For example, PG may flip to the outer leaflet relatively quickly but loss of asymmetry also requires that SM flip back to the inner leaflet. If this later step was rate-limiting, one would predict that asymmetry would remain stable, but it does not. One possible explanation is that the balance between the number of inner and outer leaflet lipids is lost when a lipid moves from one leaflet to another. The loss of balance would increase the flipping of lipids in the opposite direction to reestablish balance.
It should be noted that most of these experiments were carried out with SUVs, which are highly curved. This allowed us to use the NMR method to assess asymmetry. However, we did find similar asymmetry for LUVs in those lipid compositions for which exchange LUVs could be prepared. In addition, previous studies have shown that lipid flip-flop, which is the process that destroys asymmetry, is similar in SUVs and LUVs (46). Furthermore, even if there is a difference in flip-flop between SUVs and LUVs, we are interested in the differences between the stability of asymmetry for different lipids, which should largely reflect differences in lipid structure when measured in the same type of vesicles. Finally, the hydrophobic core of the bilayer, which is the barrier for transverse diffusion, would be expected to be similar in SUVs and LUVs.
Nevertheless, it is possible that vesicle curvature could affect flip-flop. The curvature of SUVs is expected to increase spacing between headgroups in the outer leaflet, while inducing crowding between headgroups in the inner leaflet. If crowding increases the energy of a lipid in its normal conformation in the bilayer, it should decrease the relative height of the energy barrier for flipping in SUVs relative to that in LUVs. On the other hand, the increased spacing between headgroups in the outer leaflet and in SUVs would be expected to decrease headgroup crowding and might decrease the energy of a lipid in its normal conformation relative to that in LUVs. Because flip-flop must have equal rates in both directions in order to maintain lipid balance between the inner and outer leaflet, these effects might cancel out. Based on the considerations above, we feel that it is unlikely that the conclusions above concerning lipid asymmetry would be greatly affected by vesicle curvature, but cannot rule out an effect of curvature at this time.
Potential biological implications of the dependence of asymmetry upon lipid headgroup composition
An enduring mystery is why different lipid headgroups exist. The effect of lipid headgroup structure upon lipid asymmetry may help explain some of the functions of different lipid headgroups. The observation that mammalian membranes contain considerable amounts of PE mixed with anionic lipids in the inner leaflet may reflect the necessity to maintain lipid asymmetry without wasteful expenditure of energy from ATP-consuming lipid translocases/flippases (56). The case of PS is particularly interesting as its appearance on the outer leaflet of the plasma membrane is a signal that a cell may be undergoing programmed cell death (apoptosis), and an external signal to macrophages that the damaged apoptotic cell needs to be engulfed and consumed (10–18). We speculate that PS is a particularly good choice for this functional role relative to other anionic lipids because its tendency to spontaneously flip across the lipid bilayers is especially low, minimizing the chance that healthy cells could be accidentally targeted and destroyed.
As noted above, the differences in the stability of asymmetry for the lipids studied should reflect differences in lipid structure. Despite this, and studies suggesting that spontaneous flip-flop is similar in SUVs and LUVs, it would be interesting if the absolute values of the stability of asymmetry were affected by membrane curvature, because biological membranes in cells undergo undulations and bending events, e.g., during endocytosis. Thus, an influence of curvature on the stability of asymmetry in vivo should not yet be ruled out.
Supplementary Material
Footnotes
Abbreviations:
- CL
- cardiolipin
- DOPA
- 1,2-dioleoyl-sn-glycero-3-phosphate
- DOPG
- 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol)
- DPH
- 1,6-diphenyl-1,3,5-hexatriene
- HP-TLC
- high-performance thin layer chromatography
- Ld
- liquid disordered
- LUV
- large unilamellar vesicle
- MβCD
- methyl-β-cyclodextrin
- MLV
- multilamellar vesicle
- PA
- phosphatidic acid
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- POPE
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- POPG
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol)
- POPS
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine
- PS
- phosphatidylserine
- soy PI
- soy l-α-phosphatidylinositol
- SUV
- small unilamellar vesicle
- Tm
- melting temperature
- TOCL
- tetraoleoyl cardiolipin
This work was supported by National Science Foundation Grant DMR 1104367
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Bretscher M. S. 1972. Asymmetrical lipid bilayer structure for biological membranes. Nat. New Biol. 236: 11–12 [DOI] [PubMed] [Google Scholar]
- 2.Devaux P. F. 1991. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 30: 1163–1173 [DOI] [PubMed] [Google Scholar]
- 3.Williamson P., Schlegel R. A. 1994. Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol. Membr. Biol. 11: 199–216 [DOI] [PubMed] [Google Scholar]
- 4.Devaux P. F., Morris R. 2004. Transmembrane asymmetry and lateral domains in biological membranes. Traffic. 5: 241–246 [DOI] [PubMed] [Google Scholar]
- 5.Barsukov L. I., Kulikov V. I., Bergelson L. D. 1976. Lipid transfer proteins as a tool in the study of membrane structure. Inside-outside distribution of the phospholipids in the protoplasmic membrane of Micrococcus lysodeikticus. Biochem. Biophys. Res. Commun. 71: 704–711 [DOI] [PubMed] [Google Scholar]
- 6.Rothman J. E., Kennedy E. P. 1977. Asymmetrical distribution of phospholipids in the membrane of Bacillus megaterium. J. Mol. Biol. 110: 603–618 [DOI] [PubMed] [Google Scholar]
- 7.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67: 593–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamio Y., Nikaido H. 1976. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry. 15: 2561–2570 [DOI] [PubMed] [Google Scholar]
- 9.Nikaido H., Vaara M. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhoven B., Schlegel R. A., Williamson P. 1995. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 182: 1597–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148: 2207–2216 [PubMed] [Google Scholar]
- 12.Fadok V. A., Bratton D. L., Frasch S. C., Warner M. L., Henson P. M. 1998. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 5: 551–562 [DOI] [PubMed] [Google Scholar]
- 13.Van den Eijnde S. M., Boshart L., Reutelingsperger C. P., De Zeeuw C. I., Vermeij-Keers C. 1997. Phosphatidylserine plasma membrane asymmetry in vivo: a pancellular phenomenon which alters during apoptosis. Cell Death Differ. 4: 311–316 [DOI] [PubMed] [Google Scholar]
- 14.Krahling S., Callahan M. K., Williamson P., Schlegel R. A. 1999. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 6: 183–189 [DOI] [PubMed] [Google Scholar]
- 15.Marguet D., Luciani M. F., Moynault A., Williamson P., Chimini G. 1999. Engulfment of apoptotic cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey. Nat. Cell Biol. 1: 454–456 [DOI] [PubMed] [Google Scholar]
- 16.Fadok V. A., Bratton D. L., Rose D. M., Pearson A., Ezekewitz R. A., Henson P. M. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 405: 85–90 [DOI] [PubMed] [Google Scholar]
- 17.Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., van Oers M. H. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 84: 1415–1420 [PubMed] [Google Scholar]
- 18.McEvoy L., Williamson P., Schlegel R. A. 1986. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. USA. 83: 3311–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lentz B. R. 2003. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 42: 423–438 [DOI] [PubMed] [Google Scholar]
- 20.Jackson S. P. 2011. Arterial thrombosis–insidious, unpredictable and deadly. Nat. Med. 17: 1423–1436 [DOI] [PubMed] [Google Scholar]
- 21.Malhotra R., Taylor N. R., Bird M. I. 1996. Anionic phospholipids bind to L-selectin (but not E-selectin) at a site distinct from the carbohydrate-binding site. Biochem. J. 314: 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegel R. A., McEvoy L., Williamson P. 1985. Membrane phospholipid asymmetry and the adherence of loaded red blood cells. Bibl. Haematol. 51: 150–156 [DOI] [PubMed] [Google Scholar]
- 23.Wautier M. P., Heron E., Picot J., Colin Y., Hermine O., Wautier J. L. 2011. Red blood cell phosphatidylserine exposure is responsible for increased erythrocyte adhesion to endothelium in central retinal vein occlusion. J. Thromb. Haemost. 9: 1049–1055 [DOI] [PubMed] [Google Scholar]
- 24.van den Eijnde S. M., van den Hoff M. J., Reutelingsperger C. P., van Heerde W. L., Henfling M. E., Vermeij-Keers C., Schutte B., Borgers M., Ramaekers F. C. 2001. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J. Cell Sci. 114: 3631–3642 [DOI] [PubMed] [Google Scholar]
- 25.Low M. G., Zilversmit D. B. 1980. Phosphatidylinositol distribution and translocation in sonicated vesicles. A study with exchange protein and phospholipase C. Biochim. Biophys. Acta. 596: 223–234 [DOI] [PubMed] [Google Scholar]
- 26.Denkins Y. M., Schroit A. J. 1986. Phosphatidylserine decarboxylase: generation of asymmetric vesicles and determination of the transbilayer distribution of fluorescent phosphatidylserine in model membrane systems. Biochim. Biophys. Acta. 862: 343–351 [DOI] [PubMed] [Google Scholar]
- 27.Wacklin H. P., Thomas R. K. 2007. Spontaneous formation of asymmetric lipid bilayers by adsorption of vesicles. Langmuir. 23: 7644–7651 [DOI] [PubMed] [Google Scholar]
- 28.Kiessling V., Wan C., Tamm L. K. 2009. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta. 1788: 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiessling V., Crane J. M., Tamm L. K. 2006. Transbilayer effects of raft-like lipid domains in asymmetric planar bilayers measured by single molecule tracking. Biophys. J. 91: 3313–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin W. C., Blanchette C. D., Ratto T. V., Longo M. L. 2006. Lipid asymmetry in DLPC/DSPC-supported lipid bilayers: a combined AFM and fluorescence microscopy study. Biophys. J. 90: 228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiese A., Reiners J. O., Brandenburg K., Kawahara K., Zahringer U., Seydel U. 1996. Planar asymmetric lipid bilayers of glycosphingolipid or lipopolysaccharide on one side and phospholipids on the other: membrane potential, porin function, and complement activation. Biophys. J. 70: 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schröder G., Brandenburg K., Brade L., Seydel U. 1990. Pore formation by complement in the outer membrane of gram-negative bacteria studied with asymmetric planar lipopolysaccharide/phospholipid bilayers. J. Membr. Biol. 118: 161–170 [DOI] [PubMed] [Google Scholar]
- 33.Seydel U., Schröder G., Brandenburg K. 1989. Reconstitution of the lipid matrix of the outer membrane of gram-negative bacteria as asymmetric planar bilayer. J. Membr. Biol. 109: 95–103 [DOI] [PubMed] [Google Scholar]
- 34.Collins M. D., Keller S. L. 2008. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA. 105: 124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamada T., Miura Y., Komatsu Y., Kishimoto Y., Vestergaard M., Takagi M. 2008. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil microdroplets. J. Phys. Chem. B. 112: 14678–14681 [DOI] [PubMed] [Google Scholar]
- 36.Pautot S., Frisken B. J., Weitz D. A. 2003. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. USA. 100: 10718–10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu P. C., Li S., Malmstadt N. 2011. Microfluidic fabrication of asymmetric giant lipid vesicles. ACS Appl. Mater. Interfaces. 3: 1434–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang W. L., Chen M., Cronin B., Holden M. A., Bayley H. 2008. Asymmetric droplet interface bilayers. J. Am. Chem. Soc. 130: 5878–5879 [DOI] [PubMed] [Google Scholar]
- 39.Richmond D. L., Schmid E. M., Martens S., Stachowiak J. C., Liska N., Fletcher D. A. 2011. Forming giant vesicles with controlled membrane composition, asymmetry, and contents. Proc. Natl. Acad. Sci. USA. 108: 9431–9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagano R. E., Martin O. C., Schroit A. J., Struck D. K. 1981. Formation of asymmetric phospholipid membranes via spontaneous transfer of fluorescent lipid analogues between vesicle populations. Biochemistry. 20: 4920–4927 [DOI] [PubMed] [Google Scholar]
- 41.Hope M. J., Cullis P. R. 1987. Lipid asymmetry induced by transmembrane pH gradients in large unilamellar vesicles. J. Biol. Chem. 262: 4360–4366 [PubMed] [Google Scholar]
- 42.Everett J., Zlotnick A., Tennyson J., Holloway P. W. 1986. Fluorescence quenching of cytochrome b5 in vesicles with an asymmetric transbilayer distribution of brominated phosphatidylcholine. J. Biol. Chem. 261: 6725–6729 [PubMed] [Google Scholar]
- 43.Cheng H. T., London E. 2011. Preparation and properties of asymmetric large unilamellar vesicles: interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature. Biophys. J. 100: 2671–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiantia S., Schwille P., Klymchenko A. S., London E. 2011. Asymmetric GUVs prepared by MbetaCD-mediated lipid exchange: an FCS study. Biophys. J. 100: L1–L3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng H. T., Megha, London E. 2009. Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation. J. Biol. Chem. 284: 6079–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Son M., London E. 2013. The dependence of lipid asymmetry upon phosphatidylcholine acyl chain structure. J. Lipid Res. 54: 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lakowicz J. R. 1991. Principles of Fluorescence Spectroscopy. Plenum Press, New York [Google Scholar]
- 48.Straume M., Litman B. J. 1987. Equilibrium and dynamic structure of large, unilamellar, unsaturated acyl chain phosphatidylcholine vesicles. Higher order analysis of 1,6-diphenyl-1,3,5-hexatriene and 1-[4-(trimethylammonio)phenyl]- 6-phenyl-1,3,5-hexatriene anisotropy decay. Biochemistry. 26: 5113–5120 [DOI] [PubMed] [Google Scholar]
- 49.Lentz B. R. 1993. Use of fluorescent probes to monitor molecular order and motions within liposome bilayers. Chem. Phys. Lipids. 64: 99–116 [DOI] [PubMed] [Google Scholar]
- 50.Shinitzky M., Barenholz Y. 1978. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta. 515: 367–394 [DOI] [PubMed] [Google Scholar]
- 51.Xu X., London E. 2000. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 39: 843–849 [DOI] [PubMed] [Google Scholar]
- 52.Wenz J. J., Barrantes F. J. 2003. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry. 42: 14267–14276 [DOI] [PubMed] [Google Scholar]
- 53.Jacobson K., Papahadjopoulos D. 1975. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 14: 152–161 [DOI] [PubMed] [Google Scholar]
- 54.Brouillette C. G., Segrest J. P., Ng T. C., Jones J. L. 1982. Minimal size phosphatidylcholine vesicles: effects of radius of curvature on head group packing and conformation. Biochemistry. 21: 4569–4575 [DOI] [PubMed] [Google Scholar]
- 55.Sheetz M. P., Chan S. I. 1972. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 11: 4573–4581 [DOI] [PubMed] [Google Scholar]
- 56.Chiantia S., London E. 2012. Lipid bilayer asymmetry. In Encyclopedia of Biophysics. G. C. K. Roberts, editor. Springer-Verlag, Berlin Heidelberg. 1250–1253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.