Abstract
We recently reported that levels of unsaturated lysophosphatidic acid (LPA) in the small intestine significantly correlated with the extent of aortic atherosclerosis in LDL receptor-null (LDLR−/−) mice fed a Western diet (WD). Here we demonstrate that WD increases unsaturated (but not saturated) LPA levels in the small intestine of LDLR−/− mice and causes changes in small intestine gene expression. Confirmation of microarray analysis by quantitative RT-PCR showed that adding transgenic tomatoes expressing the apoA-I mimetic peptide 6F (Tg6F) to WD prevented many WD-mediated small intestine changes in gene expression. If instead of feeding WD, unsaturated LPA was added to chow and fed to the mice: i) levels of LPA in the small intestine were similar to those induced by feeding WD; ii) gene expression changes in the small intestine mimicked WD-mediated changes; and iii) changes in plasma serum amyloid A, total cholesterol, triglycerides, HDL-cholesterol levels, and the fast-performance liquid chromatography lipoprotein profile mimicked WD-mediated changes. Adding Tg6F (but not control tomatoes) to LPA-supplemented chow prevented the LPA-induced changes. We conclude that: i) WD-mediated systemic inflammation and dyslipidemia may be in part due to WD-induced increases in small intestine LPA levels; and ii) Tg6F reduces WD-mediated systemic inflammation and dyslipidemia by preventing WD-induced increases in LPA levels in the small intestine.
Keywords: 6F peptide, apolipoprotein A-I mimetic peptides, atherosclerosis, lysophosphatidic acid, genetically engineered tomato plants
Mimetics of apolipoprotein A-I (apoA-I) containing only 18 amino acids showed promise in animal models of disease (1, 2), and improved HDL function in humans when given orally at high doses despite achieving low plasma peptide levels (3). However, when high plasma levels were achieved with low doses of peptide given intravenously or by subcutaneous (SQ) injection, no improvement in HDL function was seen (4). Studies in mice surprisingly suggested that the major site of action for these peptides is in the intestine and that a high dose of peptide is required for efficacy (5, 6). The high dose requirement provides a barrier to use in humans because of the cost of chemically synthesizing these peptides. To overcome this barrier an 18 amino acid peptide was transgenically expressed in tomatoes (7).
Feeding LDL receptor-null (LDLR−/−) mice a Western diet (WD) for 13 weeks containing 2.2% by weight of freeze-dried tomato powder made from transgenic tomatoes expressing the apoA-I mimetic peptide 6F (Tg6F) reduced plasma serum amyloid A (SAA) levels, reduced plasma total cholesterol levels, reduced plasma triglyceride levels, reduced plasma unsaturated (but not saturated) lysophosphatidic acid (LPA) levels, increased plasma paraoxonase-1 activity, increased plasma HDL-cholesterol levels, and decreased the extent of aortic atherosclerosis by about 50% (7, 8). Two hours after LDLR−/− mice finished eating WD containing Tg6F, intact 6F peptide was found in the small intestine but not in the plasma (7). Plasma levels of unsaturated (but not saturated) LPA correlated with the extent of aortic atherosclerosis. The content of LPA in the tissue of the small intestine was found to decrease after feeding Tg6F and the level of LPA (but not cholesterol) in the tissue of the small intestine correlated with the extent of aortic atherosclerosis (7).
LPA is a bioactive lipid that is produced in many tissues (9), where it acts on cells via a series of cell surface G-protein-coupled receptors to generate intracellular signals that induce cell proliferation (10), lipid accumulation, and inflammation (11). Inhibiting LPA signaling in cultured primary human hepatocytes led to inhibition of apoB (but not apoA-I) secretion (12), suggesting that LPA stimulates apoB secretion and may contribute to hypercholesterolemia. It is known that plasma LPA levels are elevated in hypercholesterolemia (11) and there are increased levels of LPA in the blood of culprit coronary arteries of patients with acute coronary syndrome (13). It is known that oxidized LDL promotes activation of phospholipase D in smooth muscle cells leading to an increase in the production of LPA that stimulates smooth muscle cell proliferation (14). It is also known that oxidized LDL requires endothelial LPA receptors and autotaxin (phospholipase D) to elicit chemokine (CXC motif) ligand 1 (CXCL1)-dependent monocyte adhesion (15). In vivo, local and systemic application of unsaturated LPA 20:4 (but not saturated LPA 18:0) (2 nmol twice weekly given by intraperitoneal injection for 4 weeks) accelerated the progression of atherosclerosis in mice without altering blood lipid levels (15). Blocking the LPA receptors LPA1 and LPA3 reduced hyperlipidemia-induced arterial leukocyte arrest and atherosclerosis in the presence of functional CXCL1, suggesting that atherogenic monocyte recruitment mediated by hyperlipidemia and modified LDL crucially depends on LPA, which triggers endothelial deposition of CXCL1 (15). In vitro studies with porcine coronary artery rings and human aortic endothelial cells revealed that LPA causes endothelial dysfunction by a mechanism associated with decreased endothelial nitric oxide synthase expression and oxidative stress (16). In vitro, it was also shown that LPA inhibited the conversion of monocytes into migratory cells and favored their retention in the subendothelium (17). In vivo in LDLR−/− mice, it was shown that application of a collar to the carotid artery resulted in a time-dependent increase in artery LPA levels (18). Also in vivo, mice with selective inactivation of lipid phosphate phosphatase 3 displayed an exaggerated neointimal response to injury (19). The gene for lipid phosphate phosphatase 3 was identified as a susceptibility locus for coronary artery disease (20). In mice, LPA was shown to trigger mast cell-driven atherosclerotic plaque destabilization (21). Thus, there is increasing evidence that LPA plays an important role in vascular pathology and atherosclerosis. However, to our knowledge, there has been no previously published evidence showing that administration of LPA causes dyslipidemia in LDLR−/− mice similar to that seen on feeding WD.
In the present report, we conducted a series of experiments to: i) further delineate the effect of WD compared with chow on the small intestine of LDLR−/− mice; ii) determine the effect of feeding Tg6F and WD on phosphatidic acid (PA), a precursor to LPA; iii) determine if gene expression in the small intestine of mice fed WD was altered by feeding Tg6F; iv) determine if adding PA or LPA to normal mouse chow would mimic feeding LDLR−/− mice WD; and v) determine if feeding Tg6F would alter the changes induced by feeding LPA in mouse chow. The results presented here demonstrate that: i) WD increases the content of unsaturated (but not saturated) LPA in the small intestine and causes changes in gene expression in the small intestine; ii) Tg6F favorably alters a number of WD-mediated changes in gene expression in the small intestine; iii) adding unsaturated LPA to mouse chow increases the levels of unsaturated LPA in the small intestine to levels that are similar to those observed when the mice are fed WD; iv) adding unsaturated (but not saturated) PA or LPA to mouse chow mimics the systemic inflammation, dyslipidemia, and gene expression in the small intestine that result from feeding LDLR−/− mice WD; and v) Tg6F mitigates both WD- and LPA-induced increases in LPA levels in the small intestine and mitigates WD- and LPA-induced systemic inflammation and dyslipidemia.
MATERIALS AND METHODS
Materials
PA (purity >99%) and LPA (purity >99%) were purchased from Avanti Polar Lipids, Birmingham, AL (catalog numbers 830865X, 840885C, 840886C, 857128X, and 857125C), except for LPA 18:2 (purity >99%), which was purchased from Echelon Biosciences, Salt Lake City, UT (catalog number L0182). In experiments where PA was administered, the fatty acid was the same at both the sn-1 and sn-2 positions. In experiments where LPA was administered, the fatty acids were in the sn-1 position. All other materials were from previously described sources (7).
Mice
LDLR−/− mice, originally purchased from Jackson Laboratories on a C57BL/6J background, were obtained from the breeding colony of the Department of Laboratory and Animal Medicine at the David Geffen School of Medicine at University of California at Los Angeles. The mice used in these studies were of different ages, which are stated in each figure legend. The mice were maintained on a chow diet (Ralston Purina) before being switched to WD (Teklad, Harlan, catalog #TD88137). Tg6F or control empty vector tomatoes (EV) were freeze-dried, powdered, and added to the diet at 2.2% by weight as previously described (7). In some experiments PA and LPA were added to the chow diet. In these experiments the diet was prepared in thin sheets that were cut into blocks that were frozen and were administered to the mice each evening as previously described (6). Addition of PA and LPA was achieved, unless otherwise stated, by adding the PA and LPA to the surface of the frozen blocks of chow just before the mice were allowed to eat. This was accomplished by making a fresh stock solution of 1 mg/ml of PA or LPA in warm saline. The resulting solution was clear and uniform. The stock solution was then diluted with warm saline so that each 0.1 ml contained 4 μg of PA or LPA. Using a 1 ml syringe and a 28 gauge needle, the 0.1 ml containing 4 μg of PA or LPA was sprayed on the front and back of each 4 g block of frozen chow just before the mice were allowed to eat. For experiments in which the PA or LPA was mixed into the chow, saline containing PA or LPA sufficient to provide a final content of 1–4 μg PA or LPA per gram of chow was slowly added into chow dough. The dough was prepared using 400 g of chow pellets and 300 ml of water with thorough mixing in a heavy duty mixer to prepare 4 g chow blocks that were frozen as previously described [see “Addition of oxidized fatty acids to mouse chow” in Materials and Methods of (6)]. For experiments in which chow without added PA or LPA was fed, the chow was treated identically except that no PA or LPA was added. To determine if air oxidation of LPA added to the surface of the blocks of frozen chow might have influenced the results, LPA 18:2 or LPA 20:4 was deliberately air oxidized by transferring 100 μg in 100 μl of chloroform to a clean 16 × 125 mm glass test tube forming a thin layer along the inside wall. The solvent was evaporated under a stream of nitrogen and the lipid residue was allowed to autoxidize by exposure to air in a laminar flow hood for 24 h prior to being added to the chow as described above. The final content of PA or LPA in the frozen chow was 1 μg per gram of frozen chow unless otherwise stated. The amount of chow presented each night was 4–5 g per mouse per night and all of the chow was eaten each night. At the end of experiments, the mice were fasted overnight, blood was collected for plasma isolation and the small intestine was harvested after the mice were perfused to remove all blood as previously described (6, 7). In experiments in which small intestine tissue levels of PA or LPA were determined, the luminal contents were removed prior to analysis as previously described (6, 7).
Microarray analysis and quantitative RT-PCR
Total RNA was isolated from the small intestine of LDLR−/− mice using Qiagen RNeasy Plus kit (n = 4–8 per treatment). Illumina mouse expression arrays were used and the microarray experiments were performed at the University of California at Los Angeles Neurosciences Genomics Core. Microarray data analysis was performed and only the list of probes, which fell below the 5% false discovery rate cutoff were chosen. Significant genes were selected based on P values <0.05 and fold change ≥1.4. For enrichment analysis of biological process ontology, probe lists were analyzed in DAVID (22, 23). Microarray results were confirmed by quantitative RT-PCR (RT-qPCR), which was performed as described previously (24).
Other methods
Plasma SAA levels, plasma total cholesterol, plasma triglycerides, and plasma HDL-cholesterol were determined by previously described methods (7). Plasma separation by fast-performance liquid chromatography (FPLC) with cholesterol determination to identify lipoproteins was performed as previously described (3, 4). The levels of PA and LPA in tissue and plasma were determined by LC-ESI-MS/MS as described previously (7). PA and LPA content of the diets were determined after extraction using the protocol of Baker et al. (25) followed by LC-ESI-MS/MS analysis as previously described (7). Lipoprotein lipase (LPL) activity was determined using the STA-610 LPL activity assay kit (Cell Biolabs, Inc.).
Statistical analysis
Statistical analyses were performed by ANOVA, unpaired two-tailed t-test, or by linear regression using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA). In the case of multiple statistical comparisons, the least significant P value is shown in the figures (e.g., if one comparison yielded a value of P < 0.0001 and another yielded a value of P < 0.01, the latter value is shown in the figure because it is correct for both comparisons).
RESULTS
Feeding WD to LDLR−/− mice increases the levels of unsaturated (but not saturated) LPA in the tissue of the small intestine
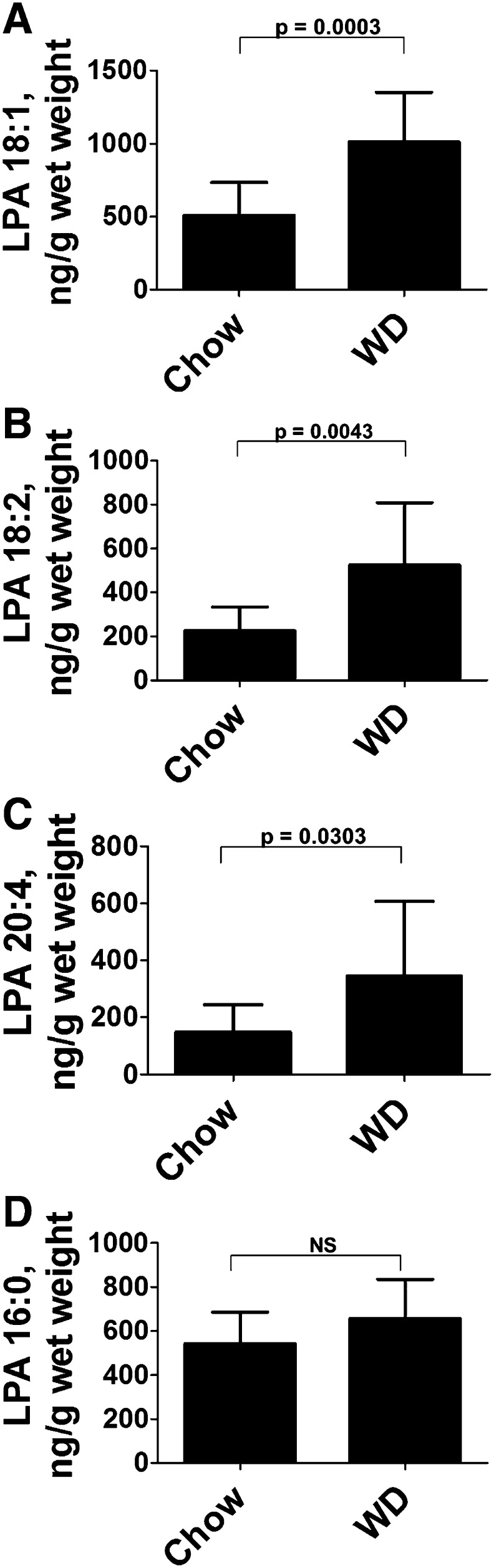
While our recent work strongly suggests that unsaturated LPA in the small intestine may play a key role in WD-mediated systemic inflammation, in that manuscript (7) we did not establish the magnitude of the changes in LPA in the small intestine induced by feeding WD compared with feeding chow. As shown in Fig. 1, feeding LDLR−/− mice WD compared with chow increased the content of unsaturated LPA in the small intestine (duodenum) about 2-fold (Fig. 1A–C), but did not alter the content of saturated LPA (Fig. 1D). The content of preformed LPA in chow was found to be consistently higher than in WD (Table 1). Because the mice were fed the same amount (i.e., equal weights) of the two diets each night, it seems very unlikely that the increase in small intestine unsaturated LPA levels after feeding WD compared with chow could be due to preformed LPA in WD.
Fig. 1.
Feeding WD increases the content of unsaturated (but not saturated) LPA in the small intestine. Female LDLR−/− mice, age 8–10 months (n = 12 per group), were fed chow or WD for 2.5 weeks. The mice were fasted overnight, and after perfusion to remove all blood, the small intestine was harvested, the luminal contents were removed, and unsaturated (A–C) or saturated (D) LPA levels in the duodenum were determined by LC-ESI-MS/MS as described in Materials and Methods. The data shown are mean ± SD.
TABLE 1.
PA and LPA content of diets
| Diet | PA 18:0 (ng/g) | PA 18:2 (ng/g) | PA 20:4 (ng/g) | LPA 18:0 (ng/g) | LPA 18:2 (ng/g) | LPA 20:4 (ng/g) |
| Chow | 42 ± 6 | 1.3 ± 0.4 | 1.7 ± 0.6 | 1.75 ± 0.1 | 9.9 ± 0.1 | 0.89 ± 1.0 |
| WD | 36 ± 8 | 1.0 ± 0.1 | 1.4 ± 0.2 | BLQ | 4.8 ± 0.3* | BLQ |
The diets were analyzed for PA and LPA content using LC-ESI-MS/MS as described in Materials and Methods. The values shown are mean ± SD. BLQ, below level of quantification. *P < 0.0001.
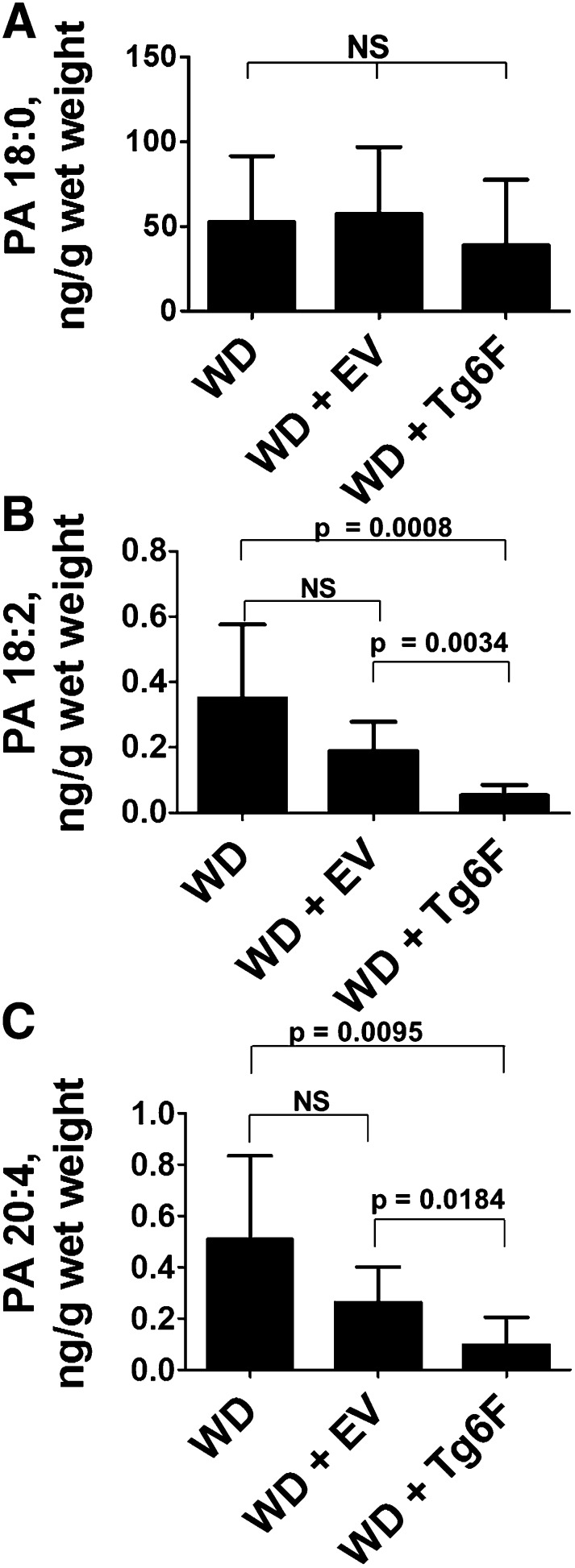
Feeding Tg6F decreases the levels of the LPA precursor PA in the tissue of the small intestine in LDLR−/− mice on WD
We previously reported that adding Tg6F (but not EV) to WD reduced the content of LPA in the tissue of the small intestine of LDLR−/− mice (7). As shown in Fig. 2, the content of saturated PA (a precursor to LPA) in the tissue of the small intestine (duodenum) of LDLR−/− mice on WD was much greater (Fig. 2A) than the content of unsaturated PA (Fig. 2B, C). Adding Tg6F (but not EV) to WD did not reduce the tissue content in the small intestine of saturated PA (Fig. 2A), but decreased the content of unsaturated PA by about 3- to 5-fold (Figs. 2B, C). Comparing Figs. 1 and 2 shows that the content of PA (even saturated PA) in the small intestine was much less than that of LPA. Both saturated and unsaturated PA contents of the chow diet were not significantly different from those of WD (Table 1). Inasmuch as the mice were fed the same amount (i.e., equal weights) of the two diets each night, it seems very unlikely that the increase in small intestine unsaturated LPA levels after feeding WD compared with chow (Fig. 1) could be due to preformed PA in WD.
Fig. 2.
Feeding Tg6F reduces levels of unsaturated PA in the tissue of the small intestine. Female LDLR−/− mice, age 6–8 months (n = 8 per group), were fed WD or WD plus 2.2% ground freeze-dried Tg6F or EV tomatoes for 3 weeks. The mice were fasted overnight, and after perfusion to remove all blood, the small intestine was harvested, the luminal contents were removed, and the levels in the tissue of the duodenum of saturated (A) or unsaturated (B, C) PA were determined as described in Materials and Methods. The data shown are mean ± SD.
Feeding Tg6F prevents WD-mediated gene expression in the small intestine of LDLR−/− mice
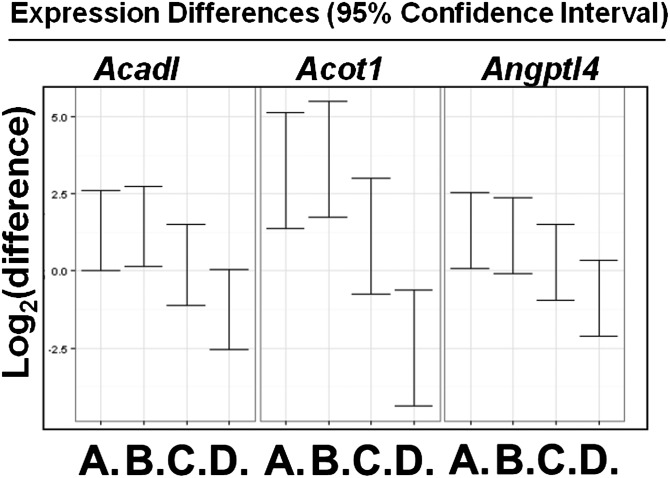
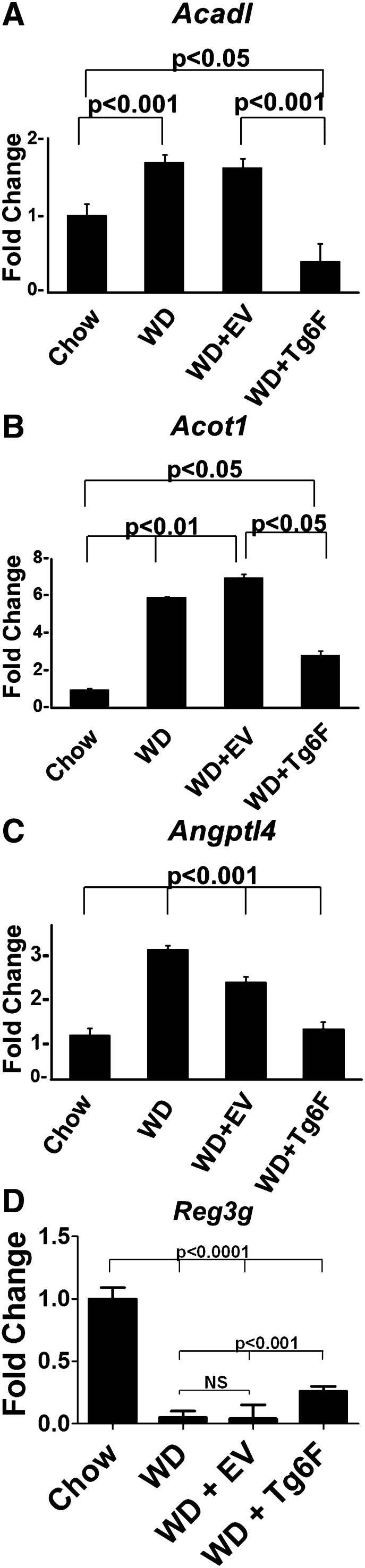
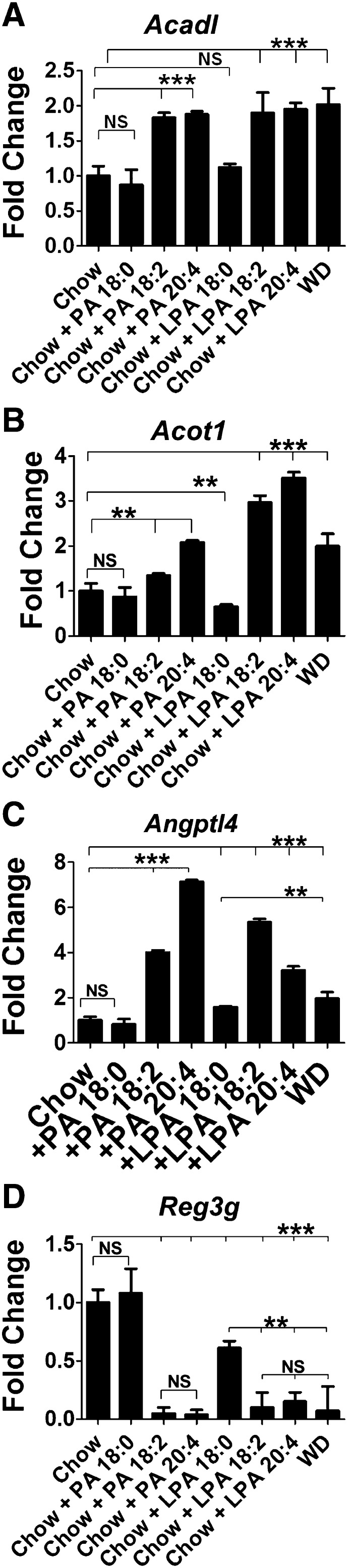
To determine if feeding Tg6F alters gene expression in the small intestine, LDLR−/− mice were fed chow or WD without or with 2.2% by weight freeze-dried ground tomatoes. After 3 weeks, RNA was isolated from the small intestine (jejunum) and subjected to microarray analysis. Only the list of probes that fell below the 5% false discovery rate cutoff were chosen. A total of 2,878 genes were identified to be significantly changed in at least one of the four comparisons: chow versus WD; chow versus EV; chow versus Tg6F; and EV versus Tg6F (supplementary Table I). Of particular interest were 198 genes whose expression changed significantly between EV- and Tg6F-fed mice (supplementary Table II). We identified 64 (out of the 198) genes that were i) significantly changed by WD compared with chow, and ii) changed by Tg6F in a direction that was opposite to the WD-induced change (Tables 2–5). The 64 genes (Tables 2, 4) are involved in several pathways including peroxisome proliferator-activated receptor (PPAR) signaling, lipid and cholesterol metabolism, mitochondrial function, and oxidative stress (Tables 3, 5). Among these 64 genes were seven genes that were identified by others as showing increased expression in the small intestine of C57BL/6J mice on a high-fat diet (26, 27), and confirmed by us on feeding WD compared with chow in LDLR−/− mice on a C567BL/6J background (Table 4). These genes were Acadl, Acot1, Angptl4, Acaa1b, Cyp4a10, Scd1, and Srebf1. A TukeyHSD plot of three of these genes is shown in Fig. 3. The microarray results were confirmed by RT-qPCR as shown in Fig. 4A–C and supplementary Fig. IA–D. These data indicate that the expression of these genes was increased on WD and was prevented by adding Tg6F to WD, but was either not prevented, or was not prevented to the same extent by EV. We also confirmed by RT-qPCR the microarray data for two genes from Table 2 which showed decreased expression on WD, which was prevented with addition of Tg6F to WD, but not EV (Fig. 4D, supplementary Fig. IE). One of these genes (Reg3g, Fig. 4D) was previously shown to have decreased expression on a high-fat diet in wild-type C57BL/6J mice (26, 27). The data in Fig. 4 are from the jejunum; similar data were obtained from the duodenum (supplementary Fig. II). These data indicate that Tg6F acts in the small intestine of LDLR−/− mice, at least in part, by preventing WD-mediated changes in gene expression.
TABLE 2.
Genes downregulated by WD, prevented by adding Tg6F (but not EV)
| Gene Symbol | Fold Change | Gene Name |
| Reg3g | 5.10 | Regenerating islet-derived protein 3γ (Reg3γ) |
| (Reg3β) or Pap | 2.52 | Regenerating islet-derived 3β |
| AA467197 | 2.16 | Expressed sequence AA467197 |
| Ptk6 | 2.15 | Protein tyrosine kinase 6 |
| Sprr2a | 1.80 | Small proline-rich protein 2A; small proline-rich protein 2A3; predicted gene 6120 |
| Gadd45g | 1.80 | Growth arrest and DNA-damage-inducible 45γ (Gadd45γ) |
| Sqle | 1.73 | Squalene epoxidase |
| 2010109I03Rik | 1.66 | Riken cDNA 2010109I03 gene |
| 1700047I17Rik | 1.65 | Riken cDNA 1700047I1 gene 2 and Riken cDNA 1700047I1 gene 1 |
| Ppp1r9a | 1.65 | Protein phosphatase 1, regulatory (inhibitor) subunit 9A |
| Ndor1 | 1.65 | NADPH-dependent diflavin oxidoreductase 1 |
| Klra7 | 1.63 | Killer cell lectin-like receptor, subfamily A, member 7 |
| Pof1b | 1.62 | Premature ovarian failure 1B |
| 1810011O10Rik | 1.62 | Riken cDNA 1810011O10 gene |
| Pcsk9 | 1.57 | Proprotein convertase subtilisin/kexin type 9 |
| Cdc42ep3 | 1.56 | CDC42 effector protein (Rho GTP-ase binding) 3 |
| Tia1 | 1.55 | Cytotoxic granule-associated RNA binding protein 1 |
| Zfp692 | 1.53 | Zinc finger protein 692 |
| Hmgb2l1 | 1.52 | HMG box domain containing 4 |
| Ndfip2 | 1.52 | Nedd4 family interacting protein 2 |
| Ptma | 1.51 | Similar to prothymosin α prothymosin α |
| Hmgcs1 | 1.50 | 3-Hydroxy-3-methylglutaryl-CoA synthase 1 |
| Pls3 | 1.49 | Plastin 3 (T-isoform) |
| E2f5 | 1.45 | E2f transcription factor 5 |
| 2410019G02Rik | 1.42 | Not annotated |
| Myom1 | 1.41 | Myomesin 1 |
| 2700055K07Rik, Tppp3 | 1.41 | Tubulin polymerization-promoting protein family member 3 |
| FAM125B, BC049806 | 1.41 | Family with sequence similarity 126, member B |
RNA was isolated from the small intestine (jejunum) of the mice described in Fig. 3 and analyzed by microarray analysis as described in Materials and Methods.
TABLE 5.
Functional information on genes listed in Table 4
| Gene Symbol | Gene Function Information |
| Scd1 | Catalyzes the insertion of a double bond into fatty acyl-CoA substrates |
| Slc6a3 | Amine transporter |
| Acot1 | Catalyzes the hydrolysis of acyl-CoAs; Active with chain-lengths of C12–C16. |
| Srebf1 | Transcription factor regulating lipid metabolism |
| Cyp4a10 | Metabolizes arachidonic acid into hydroxyeicosatetraenoic acids (HETEs) |
| Acaa1b | Lipid metabolism/fatty acid metabolism, triglyceride and fatty acid enzymes |
| Pdk4 | Activation during starvation mediated by PPARα |
| Retsat | Novel role in regulating sensitivity to oxidative stress |
| Plscr4 | Mediates ATP-independent bidirectional transbilayer migration of phospholipids |
| Pte2a Acot3 | Hydrolysis of acyl-CoAs to the free fatty acid and CoA (CoASH) |
| Gdf9 | Belongs to the TGF-β family |
| Acadl | Lipid metabolism; mitochondrial fatty acid β-oxidation |
| Ltc4s | Conjugation of leukotriene A4 with reduced glutathione to leukotriene C4 |
| Gm7049, LOC677317, ME1, Mod1 | Cytosolic or mitochondrial isoforms of malic enzyme |
| Slc27a2 | PPARα upregulates the expression of slc27a2 in the small intestine. |
| Unc93a | Function not known but is most likely an ion channel regulatory protein |
| Mfsd2a | No annotation |
| Angptl4 | Inhibits LPL activity in response to inflammatory stimuli |
| GLTPD2, C730027E14Rik | No functional information |
| Ahcy | Inhibitor of S-adenosyl-L-methionine-dependent methyl transferase reactions |
| Acaa2 | Lipid metabolism, fatty acid metabolism |
| Dhrs4 | Reduces retinal, alkyl phenyl ketones and α-dicarbonyls with aromatic rings |
| LOC192758 | Belongs to PROCA family |
| Por | Regulates retinoic acid levels and tissue distribution |
| HSD17B11 | May participate in androgen metabolism during steroidogenesis |
| PLIN2, Adfp | Regulated by PPARα, but is induced by fasting even in the absence of PPARa |
| Cyp2d26 | Cytochromes P450 are a group of heme-thiolate monooxygenases |
| Tgoln1 | Cycles between rans-Golgi and the cell surface returning via endosomes |
| Entpd5 | Promotes reglycosylation reactions involved in glycoprotein folding and quality |
| Mia2 | May play a role in the pathology of liver disease |
| N4BP2L1, B230342M21Rik | No functional information |
| Ppp2r5c | Truncated isoform of the protein phosphatase 2A B56γ regulatory subunit |
| Abcc2 | Mediates hepatobiliary excretion of numerous organic anions |
| Rnf167 | Protein modification, protein ubiquitination |
| Slc35e3 | Putative transporter |
| Pank3 | Plays a role in the physiological regulation of intracellular CoA |
TABLE 4.
Genes upregulated by WD, prevented by Tg6F (but not EV) in the experiment described in Table 2
| Gene Symbol | Fold Change | Gene Name |
| Scd1 | 6.31 | Stearoyl-CoA desaturase 1 |
| Slc6a3 | 5.89 | Solute carrier family 6 (neurotransmiter transporter, dopamine), member 3 |
| Acot1 | 4.95 | Acyl-CoA thioesterase 1 |
| Srebf1 | 3.52 | Sterol regulatory element binding transcription factor 1 |
| Cyp4a10 | 3.47 | Cyp4a10, Cyp4a31, Cyp4a32, Gm10774, Gm13015, LOC100044218, POLR2L |
| Acaa1b | 2.72 | Acetyl-CoA acyltransferase 1B |
| Pdk4 | 2.65 | Pyruvate dehydrogenase kinase, isoenzyme 4 |
| Retsat | 2.61 | Retinol saturase (all trans retinol 13,14 reductase) |
| Plscr4 | 2.41 | Phospholipid scramblase 4 |
| Pte2a or Acot3 | 2.41 | Acyl-CoA thioesterase 3 |
| Gdf9 | 2.37 | Growth differentiation factor 9 |
| Acadl | 2.36 | Acyl-CoA dehydrogenase family |
| Ltc4s | 2.24 | Leukotriene C4 synthase |
| Gm7049, LOC677317, ME1, Mod1 | 2.04 | Predicted gene 7049; similar to NADP-dependent malic enzyme (NADP-ME) |
| Slc27a2 | 1.97 | Solute carrier family 27 (fatty acid transporter), member 2 |
| Unc93a | 1.94 | Predicted gene 9992; predicted gene 8597; similar to unc-93 homolog A |
| Mfsd2a | 1.91 | Major facilitator superfamily domain containing 2 |
| Angptl4 | 1.85 | Angiopoietin-like protein 4 |
| GLTPD2, C730027E14Rik | 1.79 | Glycolipid transfer protein domain containing 2 |
| Ahcy | 1.76 | Similar to adenosylhomocysteinase (S-adenosyl-L-homocysteine hydrolase) |
| Acaa2 | 1.66 | Acetyl-CoA acyltransferase 2 (mitochondrial 3-oxoacyl-CoA thiolase) |
| Dhrs4 | 1.65 | Dehydrogenase/reductase (SDR family) member 4 |
| LOC192758 | 1.65 | Protein interacting with cyclin A1 |
| Por | 1.62 | P450 (cytochrome) oxidoreductase |
| HSD17B11 | 1.62 | Hydroxysteroid (17β) dehydrogenase 11 |
| PLIN2, Adfp | 1.61 | Adipose differentiation-related protein (ADFP)/adipophilin |
| Cyp2d26 | 1.60 | Cytochrome P450, family 2, subfamily d, polypeptide 26 |
| Tgoln1 | 1.57 | Trans-Golgi network protein 2; trans-Golgi network protein 1 |
| Entpd5 | 1.54 | Ectonucleoside triphosphate diphosphohydrolase 5 |
| Mia2 | 1.52 | Melanoma inhibitory activity 2 |
| N4BP2L1, B230342M21Rik | 1.51 | NEDD binding protein 2-like 1 |
| Ppp2r5c | 1.46 | Protein phosphatase 2, regulatory subunit B (B56), γ isoform |
| Abcc2 | 1.46 | ATP-binding casette, sub-family C (CFTR/MRP), member 2 |
| Rnf167 | 1.44 | Ring finger protein 167 |
| Slc35e3 | 1.42 | Solute carrier family 35, member E3; predicted gene 7341 |
| Pank3 | 1.41 | Pantothenate kinase |
TABLE 3.
Functional information on genes listed in Table 2
| Gene Symbol | Gene Function Information |
| Reg3g | Might be a stress protein involved in the control of bacterial proliferation in the small intestine |
| (Reg3β) or Pap | Constitutively expressed in the small intestine; controls bacterial proliferation |
| AA467197 | No functional information available |
| Ptk6 | Expressed in epithelial cells in the gastrointestinal tract |
| Sprr2a | Markedly increased in the small intestine after induction of allergic gastrointestinal inflammation |
| Gadd45g | Regulation of growth and apoptosis |
| Sqle | Catalyzes the first oxygenation step in sterol biosynthesis |
| 2010109I03Rik | Small intestine-specific glycosylphosphatidylinositol-anchored protein that accelerates apoptosis |
| 1700047I17Rik | Belongs to the FAM177 family |
| Ppp1r9a | Protein phosphatase-1 (PP1) catalytic subunit isoforms interact with diverse proteins |
| Ndor1 | Catalyzes the NADP-dependent reduction of cytochrome c and one-electron acceptors |
| Klra7 | Receptor on NK cells for class I MHC |
| Pof1b | May be involved in ovary development |
| 1810011O10Rik | May decrease apoptosis |
| Pcsk9 | PCSK9 degrades the LDL receptor |
| Cdc42ep3 | Probably involved in the organization of the actin cytoskeleton |
| Tia1 | RNA-binding protein |
| Zfp692 | Belongs to the krueppel C2H2-type zinc-finger protein family |
| Hmgb2l1 | Negatively regulates Wnt/β-catenin signaling during development |
| Ndfip2 | May be involved in NF-kappa-B and MAPK signaling pathways |
| Ptma | May mediate immune function by conferring resistance to certain opportunistic infections |
| Hmgcs1 | Mevalonic acid biosynthesis; (R)-mevalonic acid from acetyl-CoA: step 2/3 |
| Pls3 | Actin-bundling protein |
| E2f5 | Transcriptional activator that binds to E2F sites in genes involved in cell proliferation |
| 2410019G02Rik | No functional information |
| Myom1 | Binds β-integrins |
| 2700055K07Rik, Tppp3 | Onset of Tppp3 expression in joints coincides with cavitation |
| FAM125B, BC049806 | No functional information |
Fig. 3.
Microarray analysis identifies WD-induced genes whose expression was prevented by addition of Tg6F. Female LDLR−/− mice, 7–8 months of age (n = 4–6 per group), were fed chow, WD, WD plus 2.2% by weight ground freeze-dried EV tomatoes, or WD plus 2.2% by weight ground freeze-dried Tg6F. After 3 weeks the small intestine was harvested from each mouse and RNA was isolated from the jejunum and analyzed by microarray analysis as described in Materials and Methods. The data are a TukeyHSD plot for the genes Acadl, Acot1, and Angptl4. A, WD versus Chow; B, WD + EV versus Chow; C, WD + Tg6F versus Chow; D, WD + Tg6F versus WD + EV.
Fig. 4.
RT-qPCR confirms microarray analysis. The RNA isolated from the mice described in Fig. 3 was analyzed by RT-qPCR for some of the genes in Tables 2 and 4 whose expression was i) significantly changed by WD compared with chow, and ii) changed by Tg6F in a direction that was opposite to the WD-induced change. A–C: Genes whose expression was increased by WD and prevented by adding Tg6F to WD. D: A gene whose expression was decreased by WD and prevented by adding Tg6F to WD. Data shown are mean ± SD.
Adding unsaturated PA or LPA to mouse chow produces changes similar to feeding LDLR−/− mice WD
Because WD increased unsaturated LPA levels in the tissue of the small intestine (Fig. 1), and unsaturated LPA levels were previously found to be correlated with the extent of aortic atherosclerosis (7), we asked if adding PA or LPA to mouse chow would mimic feeding WD. To answer this question, we fed chow or chow to which we added 1 μg of PA or LPA (18:2 or 20:4) per gram of chow, or we fed WD to LDLR−/− mice for 18 days.
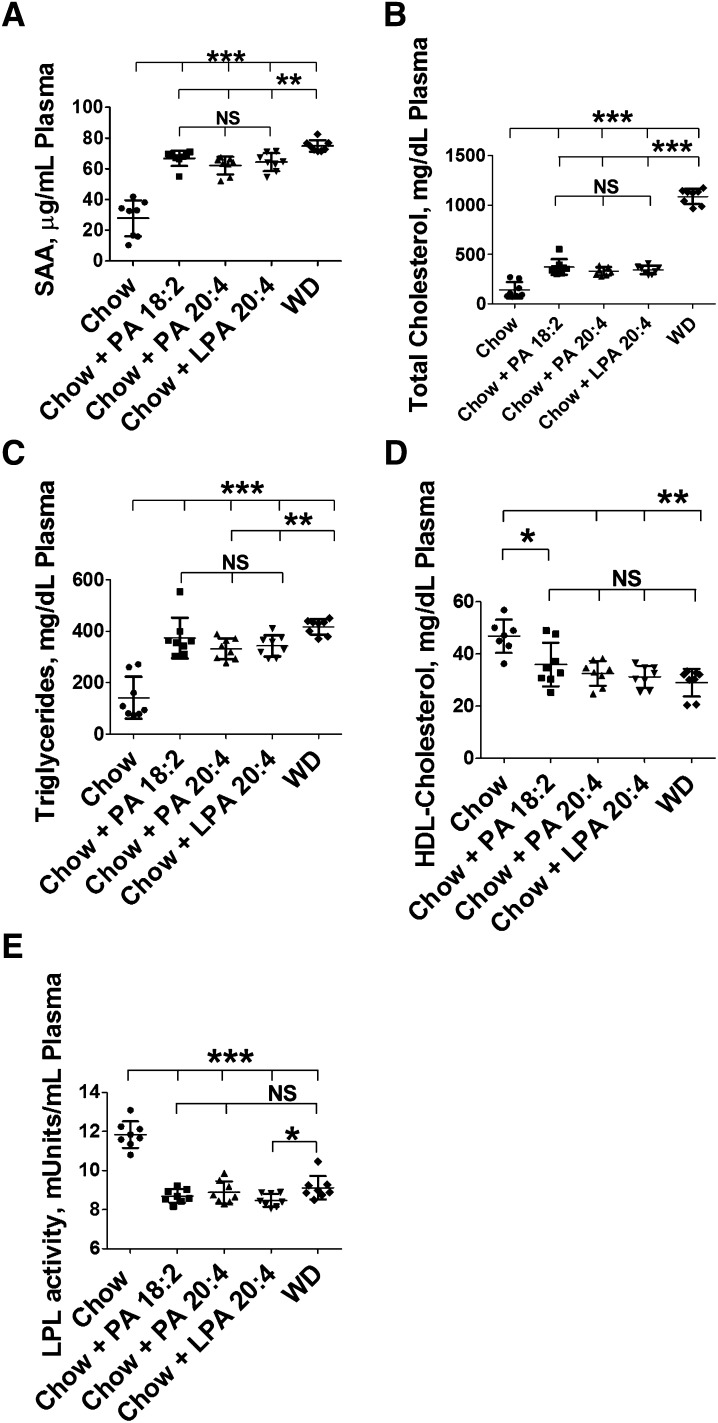
Adding this amount of unsaturated PA or LPA to mouse chow produced significant changes in plasma levels of SAA (Fig. 5A), plasma total cholesterol (Fig. 5B), plasma triglycerides (Fig. 5C), plasma HDL-cholesterol levels (Fig. 5D), and LPL activity (Fig. 5E) that were similar in direction to those observed after feeding WD. The changes in plasma cholesterol (Fig. 5B) in this experiment were highly significant, but were not quantitatively as similar to WD as was the case for the other parameters measured.
Fig. 5.
Addition of unsaturated PA or unsaturated LPA to mouse chow produces changes similar to feeding WD in LDLR−/− mice. Female LDLR−/− mice, age 8–10 months (n = 8 per group), were fed mouse chow (Chow), WD, or chow supplemented with 1 μg of PA or LPA (18:2 or 20:4) per gram chow. Each night the mice were given 20 g of chow for each cage of four mice. The mice ate all of the diet each night. After 18 days, plasma levels of SAA (A), plasma total cholesterol (B), plasma triglycerides (C), plasma HDL-cholesterol (D), and LPL activity (E) were determined as described in Materials and Methods. *P < 0.03; **P < 0.01; ***P < 0.001.
In a second experiment, adding the same amount of saturated PA or saturated LPA to mouse chow produced either no significant change (total plasma cholesterol, plasma triglycerides, and HDL-cholesterol, Figs. 6B–D, respectively) or a change that was quantitatively much less compared with adding the unsaturated species (SAA, Fig. 6A).
Fig. 6.
Addition of unsaturated (but not saturated) PA or unsaturated LPA to mouse chow produces changes similar to feeding WD in LDLR−/− mice. Female LDLR−/− mice, age 7–8 months (n = 8 per group), were fed mouse chow (Chow), WD, or chow supplemented with 1.25 μg of PA or LPA (18:0, 18:2, or 20:4) per gram chow. Each night the mice were given 16 g of chow for each cage of four mice. The mice ate all of the diet each night. After 3 weeks, plasma levels of SAA (A), plasma total cholesterol (B), plasma triglycerides (C), and plasma HDL-cholesterol (D) were determined as described in Materials and Methods. Data are mean ± SEM. *P < 0.03; **P < 0.01; ***P < 0.001.
In the experiments described in Figs. 5 and 6, the PA and LPA were added to the surface of frozen chow just before the mice were allowed to eat. Because unsaturated PA and LPA were so much more effective than saturated PA or LPA, we asked if air oxidation of the unsaturated compounds may have influenced the results. To answer this question, a third experiment was conducted as shown in supplementary Fig. III. In this experiment LPA 18:0, LPA 18:2, or LPA 20:4 were mixed into the chow (or were added to the surface as was the case in Figs. 5 and 6) or LPA 18:2 or LPA 20:4 were deliberately air oxidized prior to mixing into the chow. In the case of the air oxidized LPA, essentially all of the LPA was altered by air oxidation as determined by LC-ESI-MS/MS analysis (data not shown). We did not determine the molecular species resulting from air oxidation in this experiment; we only established that essentially all of the starting LPA was altered (data not shown). The results in supplementary Fig. III show that air oxidation of unsaturated LPA cannot explain the results obtained in Figs. 5 and 6.
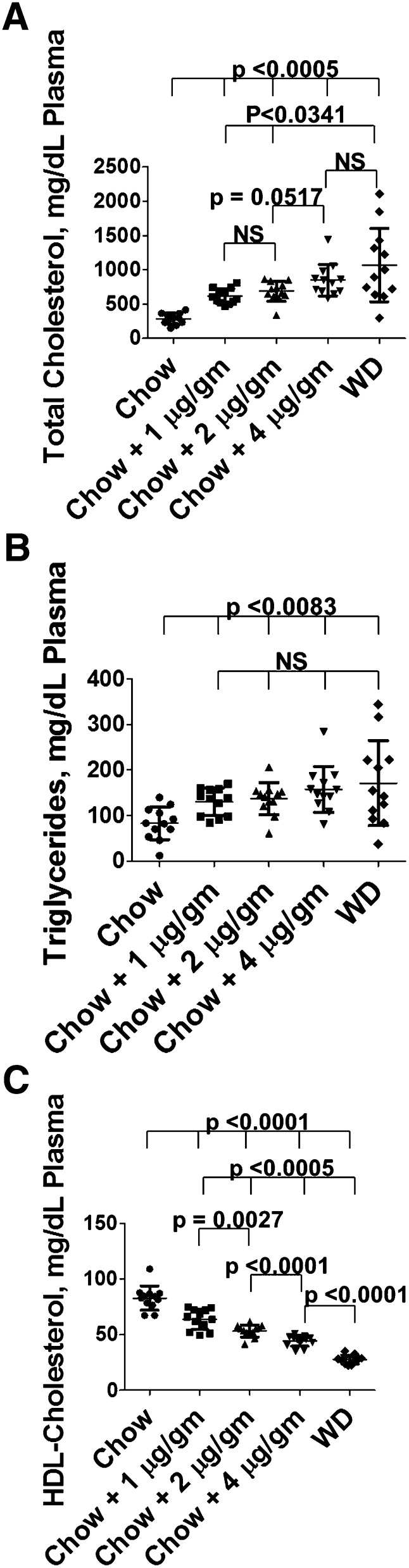
In a fourth experiment, a dose response was determined for the addition of LPA 18:2 to chow compared with WD. As shown in Fig. 7, the addition of 4 μg LPA 18:2 per gram chow produced total plasma cholesterol levels (Fig. 7A) comparable to WD. Adding as little as 1 μg LPA 18:2 per gram chow produced plasma triglyceride levels comparable to WD (Fig. 7B). There was a significant dose response resulting in lowered plasma HDL-cholesterol levels on adding from 1 to 4 μg LPA 18:2 per gram chow, almost reaching the levels achieved with WD by addition of 4 μg LPA 18:2 per gram chow (Fig. 7C).
Fig. 7.
Plasma levels of total cholesterol, triglycerides, and HDL-cholesterol after addition of increasing doses of LPA 18:2 to chow. Female LDLR−/− mice, age 6–8 months (n = 12 per group), were fed chow, chow with LPA 18:2 mixed in (at a dose of 1, 2, or 4 μg per gram chow), or WD. After 2 weeks the mice were bled and plasma levels of total cholesterol, triglycerides, and HDL-cholesterol were determined as described in Materials and Methods.
The data in Figs. 5–7 and supplementary Fig. III were obtained from female LDLR−/− mice. The data in supplementary Fig. IV show that similar results were obtained in male mice.
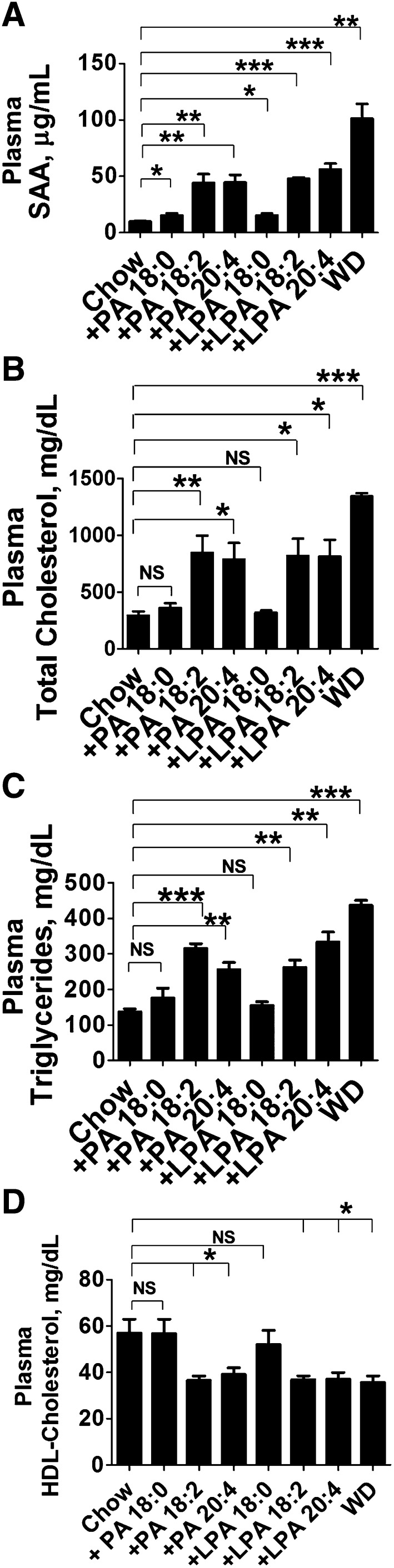
Adding unsaturated (but not saturated) PA or LPA to mouse chow produces changes in gene expression in the small intestine similar to those seen after feeding WD to LDLR−/− mice
After feeding LDLR−/− mice chow, chow with PA, chow with LPA (18:0, 18:2, or 20:4), or WD, the small intestine was harvested, RNA was isolated from the jejunum, and RT-qPCR was performed for the genes shown in Fig. 4 and supplementary Fig. I. Each of the seven genes tested in Fig. 4 and supplementary Fig. I that i) showed increased expression in the small intestine after WD and ii) was prevented when WD was supplemented with Tg6F, also showed increased expression when fed chow supplemented with unsaturated PA or LPA (Fig. 8A–C, supplementary Fig. VA–D). When saturated PA or LPA was added to chow, either the changes were not significant, or the increased expression was significantly less than when the unsaturated species were added, or gene expression actually decreased instead of increasing (Fig. 8A–C, supplementary Fig. VA–D).
Fig. 8.
Addition of unsaturated PA or unsaturated LPA to mouse chow produces changes in gene expression in the small intestine similar to those seen after feeding WD in LDLR−/− mice. Female LDLR−/− mice, age 7–8 months (n = 4–6 per group), were fed mouse chow (Chow), WD, or chow supplemented with 1 μg of PA or LPA (18:0, 18:2, or 20:4) per gram chow. After 3 weeks the small intestine was harvested from each mouse, RNA was isolated from the jejunum, and RT-qPCR for the genes shown in Fig. 4 was performed. The data shown are mean ± SD. *P < 0.05; **P < 0.01; ***P ≤ 0.001.
The expression of Reg3g and Ptk6, both of which demonstrated decreased expression on feeding WD (Fig. 4D and supplementary Fig. 1E, respectively) were found to also show decreased expression on adding unsaturated PA or LPA to chow (Fig. 8D, supplementary Fig. VE). These two genes were either not altered by saturated PA or saturated LPA, or their expression was decreased much less compared with feeding the unsaturated species (Fig. 8D, supplementary Fig. VE). The data in Fig. 8 and supplementary Fig. V were from the jejunum; similar data were obtained from the duodenum (supplementary Fig. VI).
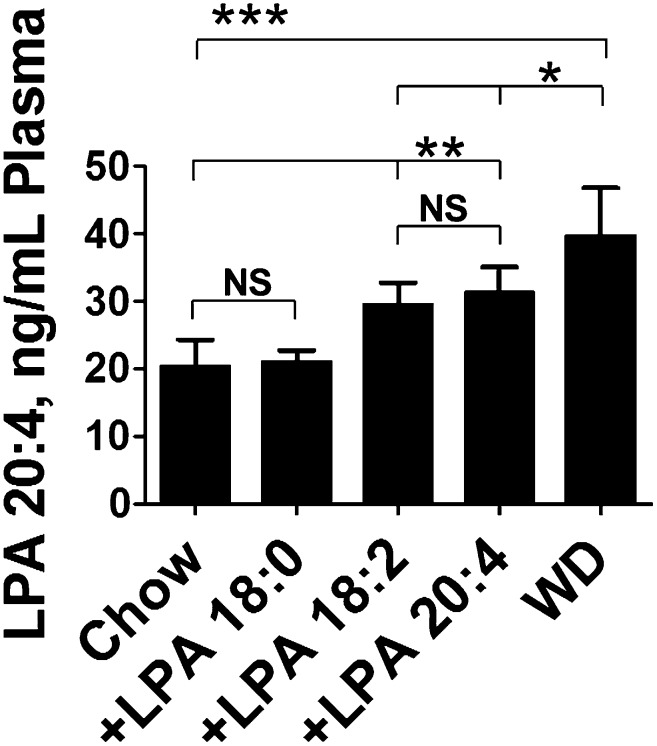
Adding 1 μg per gram of unsaturated (but not saturated) LPA to mouse chow increases plasma LPA levels to values that are between those in chow-fed and WD-fed LDLR−/− mice, and the plasma level correlates with the degree of systemic inflammation and dyslipidemia
Adding unsaturated (but not saturated) LPA to mouse chow raised plasma levels of LPA 20:4 to levels approaching those seen after feeding LDLR−/− mice WD (Fig. 9). Feeding either LPA 20:4 or LPA 18:2 at 1 μg per gram chow for 3 weeks resulted in plasma levels of LPA 20:4 that were not significantly different (Fig. 9), consistent with known processes in vivo for interconverting LPA unsaturated fatty acid species (9, 10). Interestingly, feeding the same quantity of LPA 18:0 did not change plasma LPA 20:4 levels (Fig. 9).
Fig. 9.
Addition of 1 μg/g unsaturated LPA to mouse chow produces plasma levels of LPA 20:4 in LDLR−/− mice that are between the levels seen in mice fed chow and mice fed WD. Female LDLR−/− mice, age 7–8 months (n = 8 per group), were fed mouse chow (Chow), WD, or chow supplemented with 1 μg of LPA (18:0, 18:2, or 20:4) per gram chow for 3 weeks. After fasting overnight, plasma was obtained from the mice and levels of LPA 20:4 were determined by LC-ESI-MS/MS as described in Materials and Methods. The data shown are mean ± SD. *P < 0.02; **P < 0.001; ***P < 0.0001.
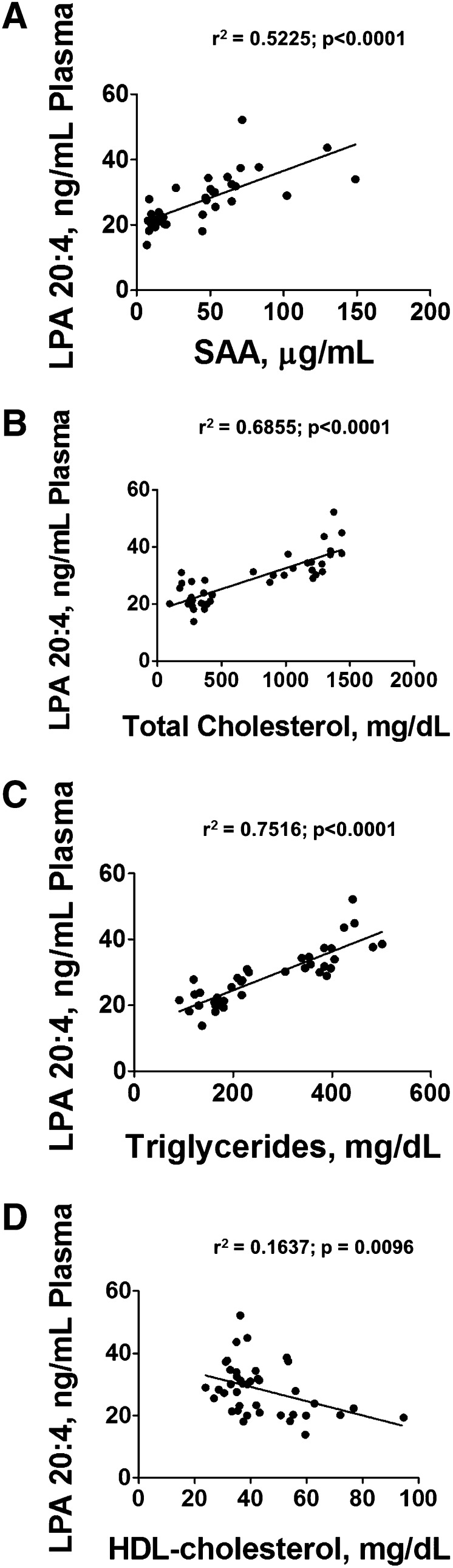
The resulting plasma levels of LPA 20:4 significantly and positively correlated with a marker of systemic inflammation, SAA (Fig. 10A). Plasma levels of LPA 20:4 also positively correlated with plasma total cholesterol (Fig. 10B) and plasma triglyceride (Fig. 10C) levels, and inversely correlated with plasma HDL-cholesterol levels (Fig. 10D).
Fig. 10.
Plasma levels of LPA 20:4 correlate with plasma SAA levels and with plasma lipid levels. SAA levels (A), total cholesterol (B), triglycerides (C), and HDL-cholesterol levels (D) were determined in the plasma from the mice described in Fig. 9 and linear regression analysis was performed as described in Materials and Methods.
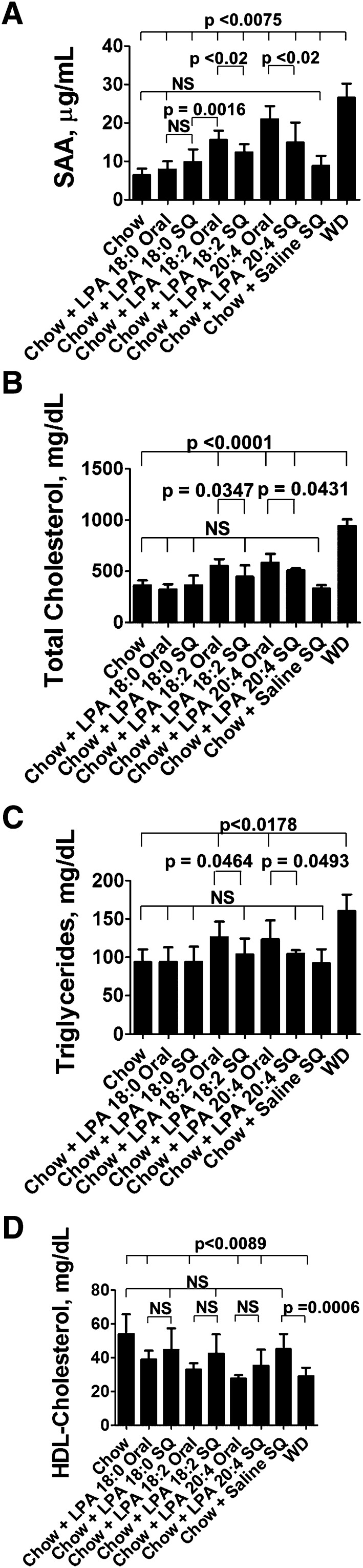
Oral administration of LPA is modestly but significantly more potent than administering the same dose by SQ injection
Adding unsaturated LPA to mouse chow modestly, but significantly, caused greater systemic inflammation and a greater increase in plasma total cholesterol and triglycerides compared with administering the same LPA dose by SQ injection (Fig. 11A–C). There was a trend for a greater reduction in plasma HDL-cholesterol after oral compared with SQ administration of unsaturated LPA, but the differences did not reach statistical significance (Fig. 11D). The results shown in Fig. 11 suggest that intestinal LPA may be particularly potent in causing systemic inflammation and dyslipidemia.
Fig. 11.
LPA administered orally to LDLR−/− mice is modestly but significantly more potent than the same dose administered by SQ injection. Female LDLR−/− mice, age 3 months (n = 8 per group), were fed mouse chow (Chow), WD, or chow supplemented with 4 μg of LPA (18:0, 18:2, or 20:4) per gram chow. Each night the mice were given 16 g of chow for each cage of four mice. The mice ate all of the diet each night. Other mice received mouse chow without LPA and received daily SQ injections on the back of 16 μg of LPA (18:0, 18:2, or 20:4) in saline, or they received saline alone. After 2 weeks, plasma levels of SAA (A), total cholesterol (B), plasma triglycerides (C), and plasma HDL-cholesterol (D) were determined as described in Materials and Methods. Data are mean ± SD.
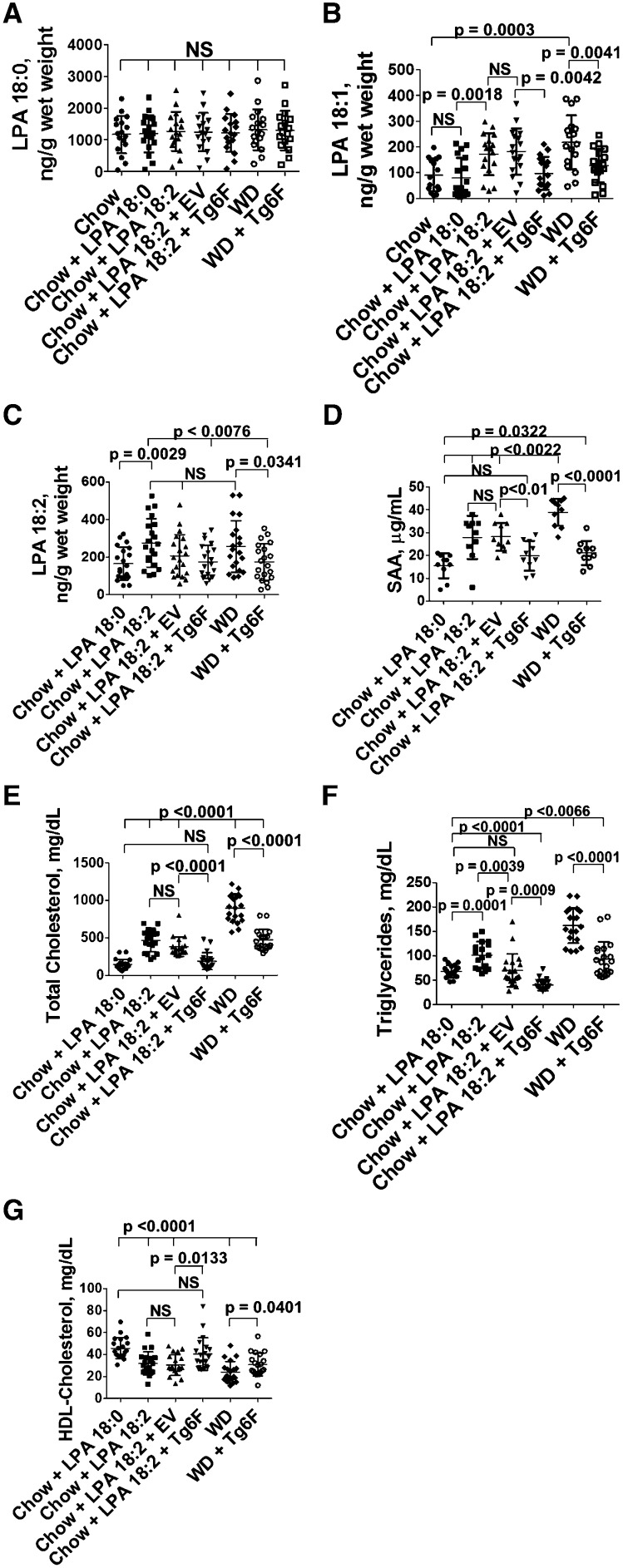
Addition of Tg6F to mouse chow supplemented with LPA prevents the increase in LPA levels in the small intestine and prevents systemic inflammation and dyslipidemia
If Tg6F was acting by reducing intestinally derived LPA, we would expect that adding Tg6F to LPA-supplemented mouse chow would prevent the increase in small intestine LPA levels, and would prevent LPA-mediated systemic inflammation and dyslipidemia better than would be the case if EV were added to the LPA-supplemented mouse chow. The data in Fig. 12A demonstrate that none of the interventions altered the content of LPA18:0 in the small intestine. The data in Fig. 12B demonstrate that adding LPA 18:0 to chow did not alter the levels of LPA 18:1 in the small intestine compared with chow only, but adding LPA 18:2 significantly increased the levels of LPA 18:1 in the small intestine and adding Tg6F (but not EV) to LPA 18:2-supplemented mouse chow or to WD significantly reduced LPA 18:1 levels in the tissue of the small intestine (duodenum). The data in Fig. 12C demonstrate that adding LPA 18:2 to chow significantly increased the levels of LPA 18:2 in the small intestine compared with adding LPA 18:0, and adding Tg6F (but not EV) to LPA 18:2-supplemented mouse chow or to WD significantly reduced LPA 18:2 levels in the tissue of the small intestine (duodenum). The data in Fig. 12D–G show that adding Tg6F (but not EV) to LPA 18:2-supplemented mouse chow or WD significantly prevented the resulting systemic inflammation and dyslipidemia. The data in supplementary Fig. VIIA–C demonstrate a highly significant correlation between plasma SAA, total cholesterol, and triglyceride levels with the content of LPA 18:2 in the tissue of the small intestine. The correlation of LPA levels in the small intestine with plasma HDL-cholesterol levels in this experiment did not reach statistical significance (data not shown).
Fig. 12.
Addition of Tg6F to chow supplemented with LPA prevents the increase in unsaturated LPA levels in the small intestine and prevents systemic inflammation and dyslipidemia. Female LDLR−/− mice, age 3–4 months (n = 10–20 per group), were fed chow only (A, B), chow supplemented with 1 μg of LPA (18:0 or 18:2) per gram chow that was mixed into normal mouse chow as described in Materials and Methods (A–G), chow supplemented with 1 μg of LPA (18:0 or 18:2) per gram chow plus 2.2% by weight freeze-dried tomato powder that was also mixed into the chow using either EV tomatoes or Tg6F tomatoes (A–G), or WD or WD plus 2.2% by weight Tg6F (A–G). After 2 weeks the mice were bled and perfused to remove all blood, the small intestine was harvested, and luminal contents were removed. LPA 18:0 levels in the tissue of the duodenum (A), LPA 18:1 levels in the tissue of the duodenum (B), and LPA 18:2 levels in the tissue of the duodenum (C) were determined as described in Materials and Methods. Plasma SAA (D), plasma total cholesterol (E), plasma triglycerides (F), and HDL-cholesterol (G) were determined as described in Materials and Methods for all groups except the chow only group.
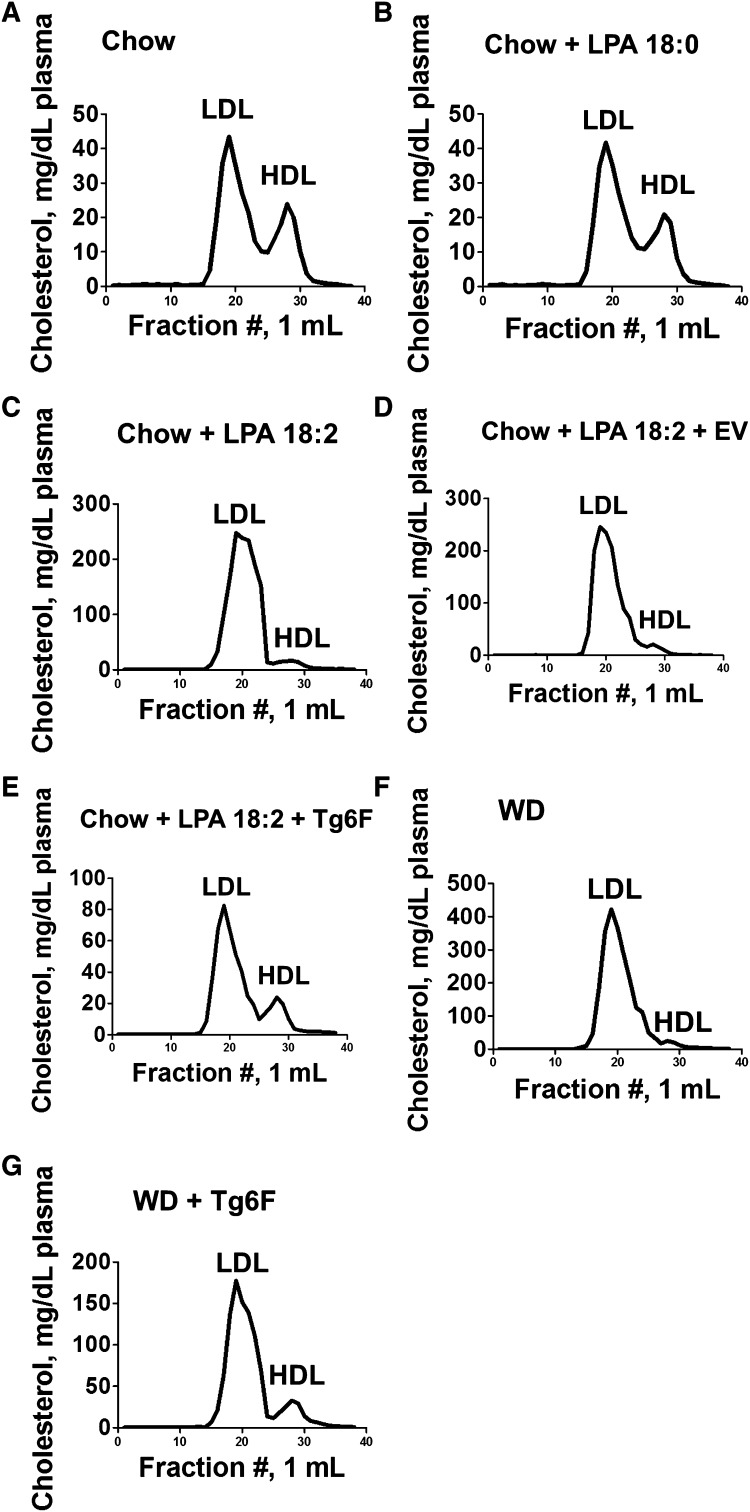
Analysis of the plasma from the mice described in Fig. 12 by FPLC demonstrated that: i) addition of LPA 18:0 to chow did not significantly change the lipoprotein profile compared with mice on chow alone; ii) addition of LPA 18:2 to chow dramatically changed the lipoprotein profile to one similar to that seen on feeding WD; and iii) adding Tg6F (but not EV) to chow supplemented with LPA 18:2 or adding Tg6F to WD largely prevented the changes in the FPLC lipoprotein profile (Fig. 13).
Fig. 13.
FPLC cholesterol profiles of plasma taken from the mice described in Fig. 12. Plasma was collected from five randomly selected mice from each of the groups described in Fig. 12. An equal volume of plasma (200 μl) from each mouse was pooled in each group and FPLC cholesterol profiles were determined as described in Materials and Methods.
DISCUSSION
It is known that various foods contain small amounts of preformed LPA (28–30). Unsaturated (but not saturated) LPA appears to be well absorbed (31). Additionally, PA, a direct precursor to LPA, is also present in small amounts in food (32). Interestingly, leafy vegetables contain more PA and LPA per gram than meat (30, 32). Consistent with these findings, we found that the chow diet contained more preformed LPA than did WD and at least as much PA (Table 1). Because the mice were fed equal amounts (i.e., equal weights) of each diet, each night, the increase in LPA levels in the small intestine on feeding WD compared with chow is not likely to be from the preformed PA and LPA in WD. The LPA content of the small intestine in our recently published studies [Figs. 11 and 12 in (7)] was inadvertently reported as “ng/mg wet weight” of small intestine. This was a typographical error; the values in that study (7) should have been shown as “ng/g wet weight” of small intestine. The data in the studies reported here (Figs. 1, 12, and supplementary Fig. VII) are correctly shown as “ng/g wet weight” of small intestine. Depending on the diet and the LPA species, the values for unsaturated LPA in the small intestine ranged between 100 and 1,000 ng/g wet weight of small intestine. The weight of the small intestine of the mice in the studies reported by us previously (7) and those reported here is ∼200 mg. Thus, from these studies, the unsaturated LPA content of the small intestine on WD in each mouse is in the range of 20–200 ng LPA depending on the diet and the LPA species. Our mice ate 4–5 g of diet per day (i.e., they were given 4–5 g of each diet, each night, for each mouse, and all was eaten by morning). From the data in Table 1, the chow diet might be expected to provide a significant amount of preformed PA or LPA that could have contributed to the LPA content of the small intestine. However, as noted above, the increase in the LPA content of the small intestine on WD cannot be explained by the PA and LPA content of WD. Thus, it seems likely that the increased unsaturated LPA content of the small intestine on WD is largely derived from local formation, which is stimulated by feeding WD through mechanisms that are yet to be determined.
Feeding WD compared with chow increased the levels of unsaturated LPA in the plasma (Fig. 9) as well as in the small intestine (Figs. 1, 12B). Compared with the quantities of PA and LPA in the diets (Table 1) and in the small intestine (Figs. 1, 12B), one might think that the doses of PA and LPA added to chow or administered SQ in the experiments reported here might overwhelm all of the relevant biological systems. Adding 1 μg of unsaturated LPA per gram chow produced levels of LPA in the plasma significantly greater than chow or chow supplemented with the same quantity of saturated LPA. However, adding this dose of unsaturated LPA to chow resulted in unsaturated LPA plasma levels that were less than those achieved on feeding WD (Fig. 9); the levels fell within the range between chow and WD. Similarly, changes in SAA, total cholesterol, triglycerides, and HDL-cholesterol levels that were produced by adding ∼1 μg of unsaturated LPA per gram chow also fell within the range between the chow and WD diets (Figs. 5–7). These “relatively modest” changes induced by adding these very high levels of PA and LPA to mouse chow suggest that most of the added PA and LPA was either not absorbed or was degraded prior to absorption.
As noted in the Materials and Methods, the same fatty acid was present in the sn-1 and sn-2 positions for PA added to chow; in the case of LPA added to chow, the fatty acid was located at the sn-1 position. The data in Figs. 5, 6, 8, and supplementary Fig. V suggest that the conversion of PA to LPA is not rate limiting.
The plasma levels of LPA 20:4 after feeding chow, WD, or chow plus 1 μg of unsaturated LPA per gram chow significantly and positively correlated with plasma levels of SAA, total cholesterol, and triglycerides, and inversely correlated with HDL-cholesterol levels (Fig. 10).
Feeding LDLR−/− mice chow supplemented with 4 μg LPA 18:2 per gram chow produced changes in plasma total cholesterol, triglycerides, and HDL-cholesterol levels that closely approximated those seen on feeding the mice WD (Fig. 7). The FPLC cholesterol profiles of LDLR−/− mice on chow compared with mice fed chow supplemented with LPA 18:0 were not different (compare Fig. 13A to Fig. 13B). However, supplementing chow with LPA 18:2 (Fig. 13C) produced a FPLC cholesterol profile similar (but less severe) to that achieved by feeding the LDLR−/− mice WD (Fig. 13F). Adding Tg6F to LPA 18:2-supplemented chow (Fig. 13E) largely prevented the LPA 18:2-mediated changes, as was also the case when Tg6F was added to WD (Fig. 13G). In contrast, adding EV to LPA 18:2-supplemented chow (Fig. 13D) did not significantly change the FPLC cholesterol profile. We would predict that such levels of dyslipidemia produced by feeding unsaturated LPA would promote atherosclerosis of the aorta similar to WD. Studies to determine the effects of chronic (months long) LPA-induced systemic inflammation and atherosclerosis are in progress in our laboratory and will be the topic of a future report. It appears from the data in Figs. 7 and 13C that HDL-cholesterol levels may be most sensitive to the dose of orally administered LPA.
The precise mechanism(s) by which intestinally derived LPA modulates these remarkable changes in plasma lipids is unknown and will require further research. It has been reported that LPA promotes hepatocyte secretion of apoB-containing proteins (12) and it is possible that some of the changes noted here may be due to changes in plasma LPA levels acting on the liver. Regardless of the mechanism(s) the data presented here further add to the growing body of evidence suggesting that LPA plays a major role in lipid metabolism, inflammation, and atherosclerosis (7, 9–21).
The studies reported here do not establish the precise signaling pathways by which intestinally derived LPA mediates systemic inflammation and dyslipidemia. The 64 genes shown in Tables 2 and 4 are involved in several pathways including PPAR signaling, lipid and cholesterol metabolism, mitochondrial function, and oxidative stress (Tables 3, 5). The expression of each of the nine genes chosen for study by RT-qPCR (Fig. 4) from the 64 genes in Tables 2 and 4 was changed in a direction similar to that induced by WD when unsaturated LPA (but not saturated LPA) was added to normal mouse chow. The fact that the expression of nine out of nine genes changed in the same direction when either LPA or WD was fed is very likely not due to chance. Microarray studies of mice fed chow or chow supplemented with LPA compared with mice fed WD are now underway in our laboratory and hopefully will help to clarify the signaling pathways by which LPA induces systemic inflammation and dyslipidemia.
Microarray analysis by others (26, 27) revealed that feeding a high-fat diet to wild-type C57BL/6 mice increased expression in the small intestine of the three genes shown in Fig. 4 and the four genes shown in supplementary Fig. I; all of which increased with WD in LDLR−/− mice. The increased expression of these seven genes in the small intestine was prevented by adding Tg6F to WD. Adding EV to WD did not prevent the expression of these genes to the same extent as adding Tg6F to WD.
Others also reported (26, 27) that feeding a high-fat diet to wild-type C57BL/6J mice decreased expression in the small intestine of Reg3g. As shown in Fig. 4D, expression of this gene was decreased in LDLR−/− mice on WD and was prevented by adding Tg6F (but not EV) to WD. Expression of another gene (Ptk6) was also decreased on feeding WD to LDLR−/− mice and was prevented by Tg6F (but not EV) (supplementary Fig. IE). Thus, the expression of nine out of nine genes changed by WD in the small intestine was prevented by Tg6F, regardless of the direction of change.
The WD-induced increase in small intestine unsaturated LPA levels (Figs. 1A–C, 12B) and the concordance in the direction of change in small intestine gene expression on feeding unsaturated LPA (Fig. 8, supplementary Fig. V) compared with WD in these studies, together with our previous publication (7), suggest the possibility that the WD-induced changes in gene expression in the small intestine are at least partially mediated by WD-induced increases in the levels of unsaturated LPA in the small intestine (Figs. 1, 12), which is prevented by Tg6F (Fig. 4, supplementary Fig. I).
Interestingly, neither of the previous studies (26, 27) using a high-fat diet, nor our microarray analysis of LDLR−/− mice fed WD revealed significant WD-induced changes in the expression of genes associated with LPA metabolism such as autotaxin, phospholipase A2, or cytoplasmic phospholipase A2 (supplementary Table I and data not shown). In preliminary unpublished studies, we found that WD increases the activity of phospholipase A2 and cytoplasmic phospholipase A2 in the small intestine without altering mRNA levels. Future research will be required to determine if the increased activity of these enzymes together with the increased fat content of WD accounts for the WD-induced increase in small intestine LPA levels.
The WD-mediated induction of Angptl4 may play a direct role in the plasma lipid changes seen in our LDLR−/− mice. Angptl4 is a protein that interacts with LPL, decreasing its activity (33, 34). Globally knocking out Angptl4 in apoE−/− mice dramatically reduced plasma triglyceride levels, significantly reduced plasma cholesterol levels, and reduced lesion size by 75% (35). Knocking out Angptl4 in LDLR−/− mice dramatically reduced plasma triglyceride levels and significantly reduced cholesterol levels (36). Triglyceride levels were also dramatically reduced in Angptl4 knockout mice made diabetic by treatment with streptozotocin (36). It has been reported that Angptl4 in the small intestine plays an important role in systemic LPL activity and is modulated by the gut microbiota (37, 38). In preliminary studies from our laboratory that will be reported in the future, we found that feeding Tg6F prevents some WD-induced changes in the microbiome. Given the recent work demonstrating a role of the microbiome in atherosclerosis (39–42), these preliminary findings, if confirmed, may be important in understanding the mechanism of Tg6F.
The data reported here demonstrate the variability that often occurs in in vivo studies. For example, the fold changes in gene expression on feeding WD in the experiment shown in Fig. 4 vary from those seen in Fig. 8. However, the direction of the WD-induced changes is identical in the two experiments. The lower values for some of the parameters in Figs. 11 and 12 after administering either LPA or WD compared with those seen in Figs. 5–7 or in supplementary Figs. III and IV may be due to the younger age of the mice in the former (3–4 months of age) compared with the latter (6–10 months of age).
The studies reported here and those recently published by us (5–7) suggest that the small intestine may play a role in chronic inflammation and atherosclerosis that is much greater than simply preparing, packaging, and shipping large quantities of triglycerides and cholesterol to the liver. These studies also suggest that modulating diet-induced changes in the small intestine by the use of oral apoA-I mimetic peptides may be a useful strategy for modulating chronic inflammation and atherosclerosis.
Supplementary Material
Footnotes
Abbreviations:
- CXCL1
- chemokine (CXC motif) ligand 1
- EV
- empty vector tomatoes (transgenic tomatoes constructed with the vector pBI121 containing the GUS gene
- 6F
- the peptide D-W-L-K-A-F-Y-D-K-F-F-E-K-F-K-E-F-F containing all L-amino acids
- FPLC
- fast-performance liquid chromatography
- LDLR−/−
- LDL receptor-null
- LPA
- lysophosphatidic acid
- PA
- phosphatidic acid
- PPAR
- peroxisome proliferator-activated receptor
- RT-qPCR
- quantitative RT-PCR
- SAA
- serum amyloid A
- SQ
- subcutaneous
- Tg6F
- transgenic tomatoes constructed with the vector pBI121 in which the GUS gene has been replaced with a sequence designed to express the apoA-I mimetic peptide 6F
- WD
- Western diet
This work was supported in part by US Public Health Service Research Grants HL-30568 and HL-34343; the Laubisch, Castera, and M. K. Grey Funds at University of California at Los Angeles; and a Network Grant from the Leducq Foundation. M.N., S.T.R., G.M.A., and A.M.F. are principals in Bruin Pharma. A.M.F. is an officer in Bruin Pharma.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of seven figures and two tables.
REFERENCES
- 1.Getz G. S., Reardon C. A. 2011. Apolipoprotein A-I and A-I mimetic peptides: a role in atherosclerosis. J. Inflamm. Res. 4: 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navab M., Reddy S. T., Van Lenten B. J., Buga G. M., Hough G., Wagner A. C., Fogelman A. M. 2012. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: novel hypotheses and review of literature. Arterioscler. Thromb. Vasc. Biol. 32: 2553–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloedon L. T., Dunbar R., Duffy D., Pinell-Salles P., Norris R., DeGroot B. J., Movva R., Navab M., Fogelman A. M., Rader D. J. 2008. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49: 1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson C. E., Weissbach N., Kjems L., Ayalasomayajula S., Zhang Y., Chang I., Navab M., Hama S., Hough G., Reddy S. T., et al. 2011. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 52: 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navab M., Reddy S. T., Anantharamaiah G. M., Imaizumi S., Hough G., Hama S., Fogelman A. M. 2011. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J. Lipid Res. 52: 1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navab M., Reddy S. T., Anantharamaiah G. M., Hough G., Buga G. M., Danciger J., Fogelman A. M. 2012. D-4F-mediated reduction in metabolites of arachidonic and linoleic acids in the small intestine is associated with decreased inflammation in low-density lipoprotein receptor-null mice. J. Lipid Res. 53: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chattopadhyay A., Navab M., Hough G., Gao F., Meriwether D., Grijalva V., Springstead J. R., Palgnachari M. N., Namiri-Kalantari R., Su F., et al. 2013. A novel approach to oral apoA-I mimetic therapy. J. Lipid Res. 54: 995–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Getz G. S., Reardon C. A. 2013. ApoA-I mimetics: tomatoes to the rescue. J. Lipid Res. 54: 878–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki J., Inoue A., Okudaira S. 2008. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta. 1781: 513–518 [DOI] [PubMed] [Google Scholar]
- 10.Mills G. B., Moolenaar W. H. 2003. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 3: 582–591 [DOI] [PubMed] [Google Scholar]
- 11.Schober A., Siess W. 2012. Lysophosphatidic acid in atherosclerotic diseases. Br. J. Pharmacol. 167: 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen X., Wang W., Wang L., Houde C., Wu W., Tudor M., Thompson J. R., Sisk C. M., Hubbard B., Li J. 2012. Identification of genes affecting apolipoprotein B secretion following siRNA-mediated gene knockdown in primary human hepatocytes. Atherosclerosis. 222: 154–157 [DOI] [PubMed] [Google Scholar]
- 13.Dohi T., Miyauchi K., Ohkawa R., Nakamura K., Kurano M., Kishimoto T., Yanagisawa N., Ogita M., Miyazaki T., Nishino A., et al. 2013. Increased lysophosphatidic acid levels in culprit coronary arteries of patients with acute coronary syndrome. Atherosclerosis. 229: 192–197 [DOI] [PubMed] [Google Scholar]
- 14.Natarajan V., Scribner W. M., Hart C. M., Parthasarathy S. 1995. Oxidized low density lipoprotein-mediated activation of phospholipase D in smooth muscle cells: a possible role in cell proliferation and atherogenesis. J. Lipid Res. 36: 2005–2016 [PubMed] [Google Scholar]
- 15.Zhou Z., Subramanian P., Sevilmis G., Globke B., Soehnlein O., Karshovska E., Megens R., Heyll K., Chun J., Saulnier-Blache J. S., et al. 2011. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 13: 592–600 [DOI] [PubMed] [Google Scholar]
- 16.Chen C., Ochoa L. N., Kagan A., Chai H., Liang Z., Lin P. H., Yao Q. 2012. Lysophosphatidic acid causes endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells. Atherosclerosis. 222: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llodrá J., Angeli V., Liu J., Trogan E., Fisher E. A., Randolph G. J. 2004. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. USA. 101: 11779–11784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bot M., Bot I., Lopez-Vales R., van de Lest C. H. A., Saulnier-Blache J. S., Helms J. B., David S., van Berkel T. J. C., Biessen E. A. L. 2010. Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. Am. J. Pathol. 176: 3073–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panchatcharam M., Miriyala S., Salous A., Wheeler J., Dong A., Mueller P., Sunkara M., Escalante-Alcalde D., Morris A. J., Smyth S. S. 2013. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 33: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schunkert H., König I. R., Kathiresan S., Reilly M. P., Assimes T. L., Holm H., Preuss M., Stewart A. F., Barbalic M., Gieger C., et al. 2011. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43: 333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bot M., de Jager S. C. A., MacAleese L., Lagraauw H. M., van Berkel T. J. C., Quax P. H. A., Kuiper J., Heeren R. M. A., Biessen E. A. L., Bot I. 2013. Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation. J. Lipid Res. 54: 1265–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis G., Jr, Sherman B. T., Hosack D. A., Yang J., Gao W., Lane H. C., Lempicki R. A. 2003. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 4: 3. [PubMed] [Google Scholar]
- 23.Huang D. W., Sherman B. T., Lempicki R. A. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4: 44–57 [DOI] [PubMed] [Google Scholar]
- 24.Yin F., Lawal A., Ricks J., Fox J. R., Larson T., Navab M., Fogelman A. M., Rosenfeld M. E., Araujo J. A. 2013. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 33: 1153–1161 [DOI] [PubMed] [Google Scholar]
- 25.Baker D. L., Desiderio D. M., Miller D. D., Tolley B., Tigyi G. J. 2001. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal. Biochem. 292: 287–295 [DOI] [PubMed] [Google Scholar]
- 26.de Wit N. J. W., Bosch-Vermeulen H., de Groot P. J., Hooiveld G. J. E. J., Bromhaar M. M. G., Jansen J., Müller M., van der Meer R. 2008. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med. Genomics. 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steegenga W. T., de Wit N. J. W., Boekschoten M. V., Ijssennagger N., Lute C., Keshtkar S., Bromhaar M. M., Kampman E., de Groot L. C., Muller M. 2012. Structural, functional and molecular analysis of the effects of aging in the small intestine and colon of C57BL/6J mice. BMC Med. Genomics. 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakane S., Tokumura A., Waku K., Sugiura T. 2001. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: distinct molecular species compositions. Lipids. 36: 413–419 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T., Tsutsui H., Hirano K., Koike T., Tokumura A., Satouchi K. 2004. Quantitative analysis of lysophosphatidic acid by time-of-flight mass spectrometry using a phosphate-capture molecule. J. Lipid Res. 45: 2145–2150 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T., Horiuchi G., Matsuoka M., Hirano K., Tokumura A., Koike T., Satouchi K. 2009. Formation of lysophosphatidic acid, a wound-healing lipid, during digestion of cabbage leaves. Biosci. Biotechnol. Biochem. 73: 1293–1300 [DOI] [PubMed] [Google Scholar]
- 31.Inoue M., Adachi M., Shimizu Y., Tsutsumi T., Tokumura A. 2011. Comparison of lysophospholipid levels in rat feces with those in a standard chow. J. Agric. Food Chem. 59: 7062–7067 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T., Kassai A., Ohmoto M., Morito K., Kashiwada Y., Takaishi Y., Urikura M., Morishige J-I., Satouchi K., Tokumura A. 2012. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J. Agric. Food Chem. 60: 4156–4161 [DOI] [PubMed] [Google Scholar]
- 33.Zhu P., Goh Y. Y., Chin H. F. A., Kersten S., Tan N. S. 2012. Angiopoietin-like 4: a decade of research. Biosci. Rep. 32: 211–219 [DOI] [PubMed] [Google Scholar]
- 34.Robal T., Larsson M., Martin M., Olivecrona G., Lookene A. 2012. Fatty acids bind tightly to the N-terminal domain of angiopoietin-like protein 4 and modulate its interaction with lipoprotein lipase. J. Biol. Chem. 287: 29739–29752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adachi H., Fujiwara Y., Kondo T., Nishikawa T., Ogawa R., Matsumura T., Ishii N., Nagai R., Miyata K., Tabata M., et al. 2009. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochem. Biophys. Res. Commun. 379: 806–811 [DOI] [PubMed] [Google Scholar]
- 36.Adachi H., Kondo T., Koh G. Y., Nagy A., Oike Y., Araki E. 2011. Angptl4 deficiency decreases serum triglyceride levels in low-density lipoprotein receptor knockout mice and streptozotocin-induced diabetic mice. Biochem. Biophys. Res. Commun. 409: 177–180 [DOI] [PubMed] [Google Scholar]
- 37.Bäckhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A., Semenkovich C. F., Gordon J. I. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 101: 15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleissner C. K., Huebel N., Abd El-Bary M. M., Loh G., Klaus S., Blaut M. 2010. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 104: 919–929 [DOI] [PubMed] [Google Scholar]
- 39.Wang Z., Klipfell E., Bennett B. J., Koeth R., Levison B. S., Dugar B., Feldstein A. E., Britt E. B., Fu X., Chung Y. M., et al. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 472: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett B. J., de Aguiar Vallim T. Q., Wang Z., Shih D. M., Meng Y., Gregory J., Allayee H., Lee R., Graham M., Crooke R., et al. 2013. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17: 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koeth R. A., Wang Z., Levison B. S., Buffa J. A., Org E., Sheehy B. T., Britt E. B., Fu X., Wu Y., Li L., et al. 2013. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19: 576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang W. H., Wang Z., Levison B. S., Koeth R. A., Britt E. B., Fu X., Wu Y., Hazen S. L. 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368: 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.