Abstract
Endotoxin tolerance allows macrophages to produce large quantities of proinflammatory cytokines immediately after their contact with lipopolysaccharides (LPSs), but prevents their further production after repeated exposure to LPSs. While this response is known to prevent overproduction of proinflammatory cytokines, the mechanism through which endotoxin tolerance is established has not been identified. In the current study, we demonstrate that sufficient production of geranylgeraniol (GGOH) in macrophages is required to maintain endotoxin tolerance. We show that increased synthesis of 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) protein following LPS treatment is required to produce enough GGOH to inhibit expression of Malt1, a protein known to stimulate expression of proinflammatory cytokines, in macrophages repeatedly exposed to LPSs. Depletion of GGOH caused by inhibition of HMGCR led to increased Malt1 expression in macrophages subjected to repeated exposure to LPSs. Consequently, endotoxin tolerance was impaired, and production of interleukin 1-β and other proinflammatory cytokines was markedly elevated in these cells. These results suggest that insufficient production of GGOH in macrophages may cause autoinflammatory diseases.
Keywords: Malt1, HMG-CoA reductase, interleukin 1-β
Inflammatory reactions initiated by macrophages following detection of microbial infection are critical for innate immune responses. However, these reactions have to be regulated tightly in order to prevent tissue damage caused by uncontrolled inflammatory responses. A classical example of this type of regulation is endotoxin tolerance, which allows macrophages to produce large quantities of proinflammatory cytokines immediately after their contact with bacterial endotoxins such as lipopolysaccharides (LPSs), but prevents their further production after repeated exposure to LPSs (1). While the clinical significance of endotoxin tolerance has been well established (2), its underlying mechanism has yet to be identified.
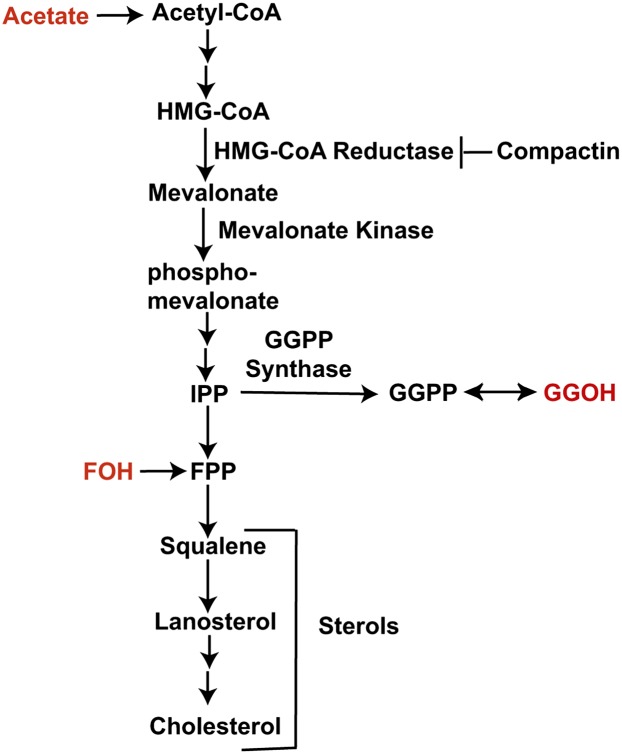
Recent observations suggest that the mevalonate pathway, a biosynthetic pathway through which sterols and nonsterol isoprenoids are synthesized (Fig. 1) (3), is closely related to inflammatory reactions elicited by macrophages. It was reported that sterol regulatory element binding protein 1a, a transcription factor activating almost all genes encoding enzyme catalyzing reactions in the mevalonate pathway, also stimulated transcription of Nlrp1a, a component of inflammasome that is required for processing and secretion of interleukin 1-β (IL-1β), a major proinflammatory cytokine (4). The amount of several sterols, particularly lanosterol, was elevated in macrophages treated with endotoxin (5, 6). The products of the mevalonate pathway also affect macrophage-mediated inflammatory responses. Desmosterol and some other sterols were shown to inhibit macrophage-induced proinflammatory responses through activation of the liver X receptor (7, 8). Geranylgeraniol (GGOH), a nonsterol isoprenoid produced through the mevalonate pathway (Fig. 1), also inhibits macrophage-mediated inflammatory reactions following LPS treatment (9–12), but the underlying mechanism has not been identified.
Fig. 1.
The mevalonate pathway. Sterols and nonsterol products of the mevalonate pathway as well as key enzymes catalyzing their synthesis are indicated. Compounds indicated in red can feed into the mevalonate pathway when added into culture medium.
In the current study we determined that LPS treatment enhanced expression of 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR), the rate-limiting enzyme in the mevalonate pathway (3), by activating translation of the protein. The increased expression of HMGCR allowed macrophages to produce GGOH at a level that was high enough to maintain endotoxin tolerance. Inhibition of HMGCR did not affect the initial production of proinflammatory cytokines by macrophages following LPS treatment, but such inhibition completely eliminated endotoxin tolerance so that macrophages continued to produce large amounts of proinflammatory cytokines, such as IL-1β, even after repeated treatment with LPSs. These results raise the possibility that insufficient production of GGOH may lead to autoinflammatory diseases.
EXPERIMENTAL PROCEDURES
Materials
We obtained LPSs from List Biological Laboratories, Inc.; GGOH, farnesol (FOH), squalene, and lanosterol from Sigma; fluid thioglycollate medium from BD Diagnostic Systems; polyclonal anti-calnexin from Novus Biologicals; polyclonal anti-p52, RelB, and lysine-specific demethylase 1 from Cell Signaling Inc.; monoclonal anti-ubiquitin Lys63-specific from Millipore Inc.; horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit IgGs from Jackson ImmunoResearch Laboratories; [3H]acetate from American Radiolabeled Chemicals; and silica gel TLC plates from Macherey-Nagel. Monoclonal anti-HMGCR was described previously (13). Sodium compactin and sodium mevalonate were prepared as previously described (14).
Cell culture
Murine peritoneal macrophages were isolated as previously described (15). Briefly, C57BL/6 mice (6–8 weeks old) were injected intraperitoneally with 3% thioglycollate medium, and macrophages were collected from the intraperitoneal cavity 3 days after the injection. The cells were then treated with RBC lysis buffer (eBioscience) and cultured in medium A (RPMI 1640 medium containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate) at 37°C in 5% CO2.
RAW264.7 cells were maintained in medium B (DMEM low glucose medium containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate) at 37°C in 5% CO2.
Immunoblot analysis
Aliquots of the lysate were subjected to SDS-PAGE followed by immunoblot analysis. Antibodies used in the current study were monoclonal anti-HMGCR (5 μg/ml) and anti-K63-specific polyubiquitin chain (1:1,000 dilution), polyclonal anti-actin (1:3,000 dilution), anti-p52 (1:1,000 dilution), RelB (1:1,000 dilution), and lysine-specific demethylase 1 (1:1,000 dilution). Horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit IgGs (0.2 μg/ml) were used as the secondary antibody in all immunoblot analyses. Bound antibodies were visualized by chemiluminescence using the SuperSignal substrate system (Pierce) according to the manufacturer's instructions.
RT-QPCR
Real-time quantitative PCR (RT-QPCR) was performed as previously described (16). Each measurement was made in triplicate from cell extracts pooled from duplicate dishes. The relative amount of RNA was calculated through the comparative cycle threshold method by using mouse cyclophilin mRNA as the invariant control.
Measurement of synthesis of lanosterol and GGOH
Synthesis of lanosterol and GGOH measured by radiolabeled analysis was performed by labeling cells with [3H]acetate. For measurement of lanosterol synthesis, cells pulled from three dishes were lysed in 1 ml 0.1 M NaOH. After addition of 40 μg of nonradioactive lanosterol standard and saponification in 15% KOH and 40% ethanol at 80°C for 1 h, lipids were extracted twice with 4 ml petroleum ether. For measurement of GGOH synthesis, cells pulled from three dishes were homogenized in buffer A [50 mM Tris-HCl (pH 7.5), 250 mM sucrose, and 100 mM KCl]. Lipids in the homogenate were extracted by 0.5 ml of chloroform/methanol (2:1; v/v), and mixed with 40 μg of nonradioactive GGOH standard. For measurement of synthesis of both lipids, lipid extracts were dried under nitrogen gas with heat, resuspended in 50 μl heptane, and analyzed by thin-layer chromatography (TLC) on plastic-backed silica gel TLC plates in a solvent system of benzene/ethyl acetate (4:1; v/v) for separation of the lipids. Following visualization by exposing the TLC plates to I2 vapor, bands containing the lipid were excised, and the amount of radioactivity in them was determined by scintillation counting. The activity of synthesis of the lipids was determined by radioactivity found in the band normalized by the amount of cellular protein.
Pulse-chase analysis
Pulse-chase analysis of HMGCR was performed exactly as previously described (17). ImageJ was used for quantification of the result.
Polysome fractionation analysis
Cells pooled from five 60 mm dishes were used in polysome fractionation analysis. Cells were treated with 0.1 mg/ml cycloheximide for 5 min, washed with PBS containing 0.1 mg/ml cycloheximide, and lysed with 1 ml of buffer B [50 mM HEPES (pH 7.5), 250 mM KCl, 5 mM MgCl2, 2 mM DTT, and 150 μg/ml cycloheximide] supplemented with 1 mg/ml heparin, 1% (v/v) TX-100, and 1 mM PMSF. The lysate was centrifuged for 10 min at 10,000 g. The supernatant was layered onto a 10 ml sucrose gradient consisting of 2 ml of layers extending from 10% (w/v) sucrose to 50% (w/v) sucrose in 10% increments. The sucrose was dissolved in buffer B supplemented with 0.5 mg/ml heparin. After centrifugation for 3 h at 150,000 g at 4°C, fractions of 1 ml were collected. Total RNAs were extracted from each fraction for quantification of HMGCR mRNA by RT-QPCR. The fractions containing polysomes were determined by measurement of light absorption at 254 nm in each fraction as previously described (18). The statistical analysis was performed with a one-tailed unpaired t-test.
ELISA
The amount of IL-1β protein secreted into culture medium was measured through ELISA using Quantikine for mouse IL-1β (R&D Systems) according to the instructions of the manufacturer.
RNA interference
Duplexes of siRNA were synthesized by Dharmacon Research. The two small interfering RNA (siRNA) duplexes siMalt1-A and siMalt1-B target nucleotide positions 1753-1769 and 999-1014 (relative to the codon for the initiating methionine) for mouse Malt1 (National Center for Biotechnology Information accession number NM_172833), respectively. Control siRNA targeting green fluorescent protein was reported previously (19). Peritoneal macrophages in suspension were transfected with 0.1 μM siRNA using Lipofectamine TM RNAiMAX reagent (Invitrogen) according to the manufacturer's instructions, after which the cells were used for experiments as described in the figure legends.
Microarray analysis
Macrophages were treated with compactin and LPSs in the absence or presence of GGOH two times, as described in Fig. 5C. Microarray analysis was performed exactly as previously described (20) to determine the effect of GGOH on gene expression. The result was deposited in Gene Expression Omnibus with the accession number GSE 48646.
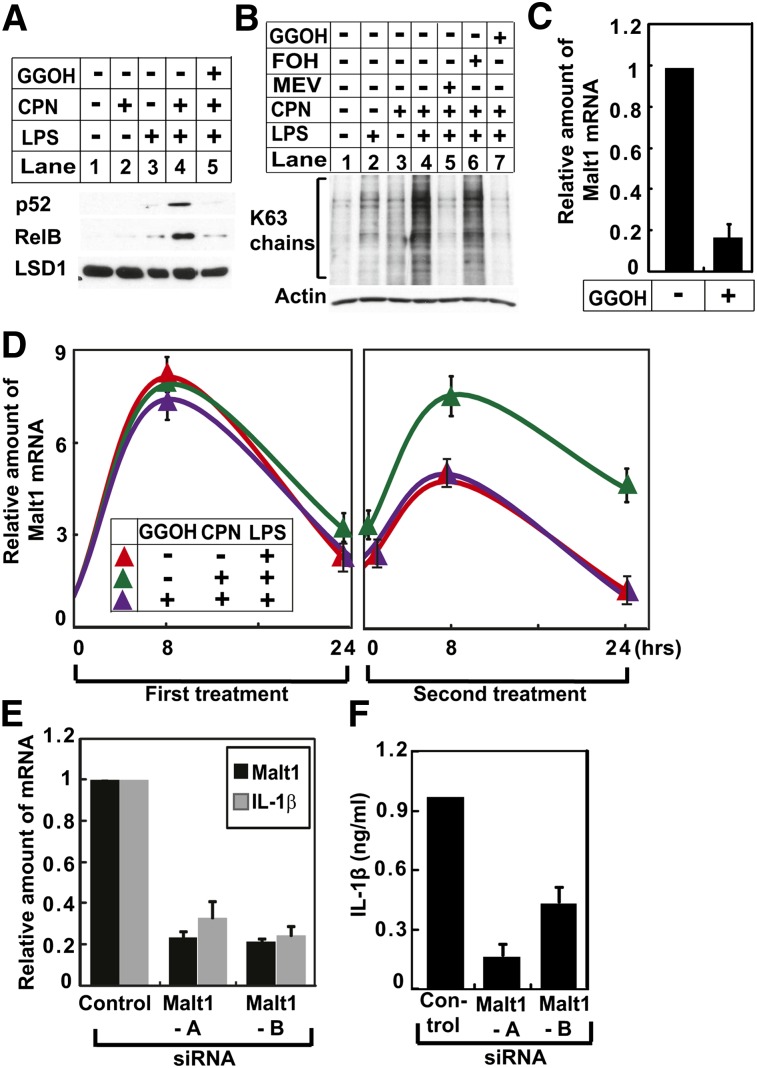
Fig. 5.
GGOH maintains endotoxin tolerance by inhibiting expression of Malt1. A, B: Peritoneal macrophages were set up and treated with LPSs, compactin (CPN), and GGOH as indicated, two times as described in Fig. 4A. Twenty-four hours after the second treatment, nuclear extracts were subjected to immunoblot analysis with antibodies against the NF-κB family of transcription factors. The immunoblot of lysine-specific demethylase 1 (LSD1) was used as a loading control (A). Cell lysates were subjected to immunoblot analysis with an antibody against K63-linked polyubiquitin chains (B). C: Peritoneal macrophages were set up and treated with 200 ng/ml LPSs and 25 μM compactin with or without 10 μM GGOH as indicated, two times as described in Fig. 4A. Twenty-four hours after the second treatment, the amount of Malt1 mRNA was quantified by RT-QPCR as described in Fig. 3A. D: The amount of Malt1 mRNA in peritoneal macrophages subjected to the indicated treatment for the indicated time was quantified as described in Fig. 3A. E, F: On day 0, peritoneal macrophages were transfected with 0.5 μM of indicated siRNA, and seeded at a density of 1.25 × 106 cells per well in a 12-well plate. Twenty-four hours later on day 1, cells were treated with LPSs and compactin two times as described in Fig. 4A. On day 3, 24 h after the second treatment, cells were harvested for quantification of indicated mRNA through RT-QPCR (E), and culture medium was collected to determine the amount of IL-1β secreted into medium through ELISA (F).
RESULTS
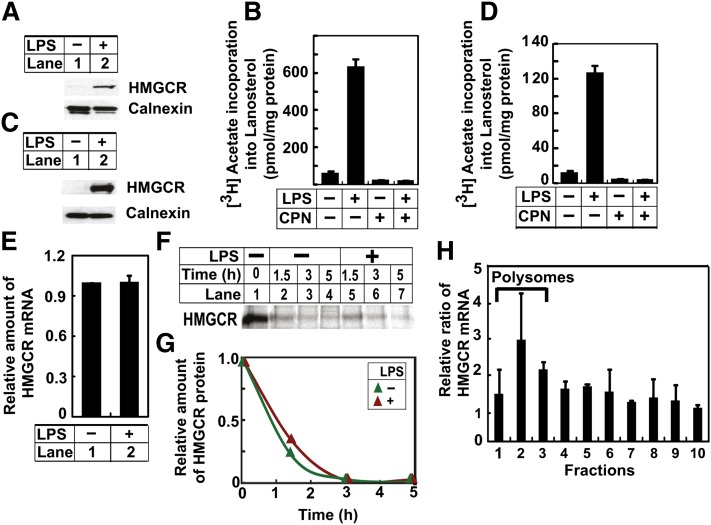
Inasmuch as endotoxin treatment increased the amount of lanosterol in macrophages (5, 6), we decided to determine the effect of such treatment on expression of HMGCR that catalyzes the rate-limiting step in the mevalonate pathway (3). Treatment of mouse peritoneal macrophages with LPSs increased the amount of HMGCR protein (Fig. 2A). Elevated expression of HMGCR led to increased synthesis of lanosterol in LPS-treated cells, as inhibition of HMGCR by compactin completely abolished LPS-induced synthesis of lanosterol (Fig. 2B). These results were reproduced in RAW264.7 cells, a transformed line of mouse macrophages (Fig. 2C, D). We thus used RAW264.7 cells to further identify the mechanism through which LPSs increase the expression of HMGCR. LPS treatment did not increase HMGCR mRNA (Fig. 2E), nor did it retard the degradation rate of HMGCR protein as determined by pulse-chase analysis (Fig. 2F, G). To determine the effect of LPSs on translation of HMGCR protein, we performed polysome fractionation analysis that was used previously to analyze translation of the protein (21). Actively translated mRNAs attach to multiple ribosomes referred to as polysomes, so they can be separated from untranslated mRNAs free of ribosomes through density centrifugation analysis (18). Compared with untreated cells, LPS treatment increased the amount of HMGCR mRNA in the polysome fractions that contained rapidly translated mRNA (Fig. 2H). Thus, LPSs induce HMGCR expression by stimulating translation of the protein.
Fig. 2.
LPSs induce HMGCR protein expression in macrophages. A, B: On day 0, peritoneal macrophages were seeded at 5 × 106 cells per 60 mm dish. A: On day 1, cells were treated with (+) or without (−) 200 ng/ml LPSs for 8 h. The amount of HMGCR protein in the cells was then determined by immunoblot analysis. B: On day 1, cells were treated with 200 ng/ml LPSs and 25 μM compactin (CPN) as indicated for 8 h. These cells were then labeled with 10 μCi [3H]acetate in the presence of 0.5 mM unlabeled acetate for 2 h, and the amount of the radioactivity incorporated into lanosterol was determined as described in Experimental Procedures. C–H: On day 0, RAW264.7 cells were seeded at 3.5 × 105 cells per 60 mm dish. C, E: On day 3, cells were treated with 200 ng/ml LPSs. On day 4, 24 h after the treatment, the amount of HMGCR protein was determined by immunoblot analysis (C), and the amount of its mRNA was determined by RT-QPCR (result is reported as mean ± SE. from triplicate measurements of a representative experiment) (E). D: On day 3, cells were treated with 200 ng/ml LPSs and 25 μM compactin (CPN) as indicated for 16 h, and synthesis of lanosterol was determined as described in (B). F: On day 4, cells were pulse-labeled with 150 μCi/ml 35S-protein labeling mix for 1 h and then chased with unlabeled methionine (0.5 mM) and cysteine (1 mM) in the absence or presence of 200 ng/ml LPSs for the indicated period of time. HMGCR was then immunoprecipitated from the cell lysate, and detected by SDS-PAGE followed by 35S autoradiography. G: Quantification of the result shown in (F) with the intensity immediately prior to chasing set to one. H: On day 3, cells were treated with 200 ng/ml LPSs. On day 4, 24 h after the treatment, cells were harvested for polysome fractionation analysis as described in Experimental Procedures. Value in each fraction represents the amount of HMGCR mRNA in cells treated with LPSs relative to that in untreated cells. P < 0.05 for comparison of the average value from fractions 1–3 versus that in fractions 4–10 with one-tailed unpaired t-test. A–G: Unless otherwise indicated, bar graphs are presented as mean ± SE from three independent experiments.
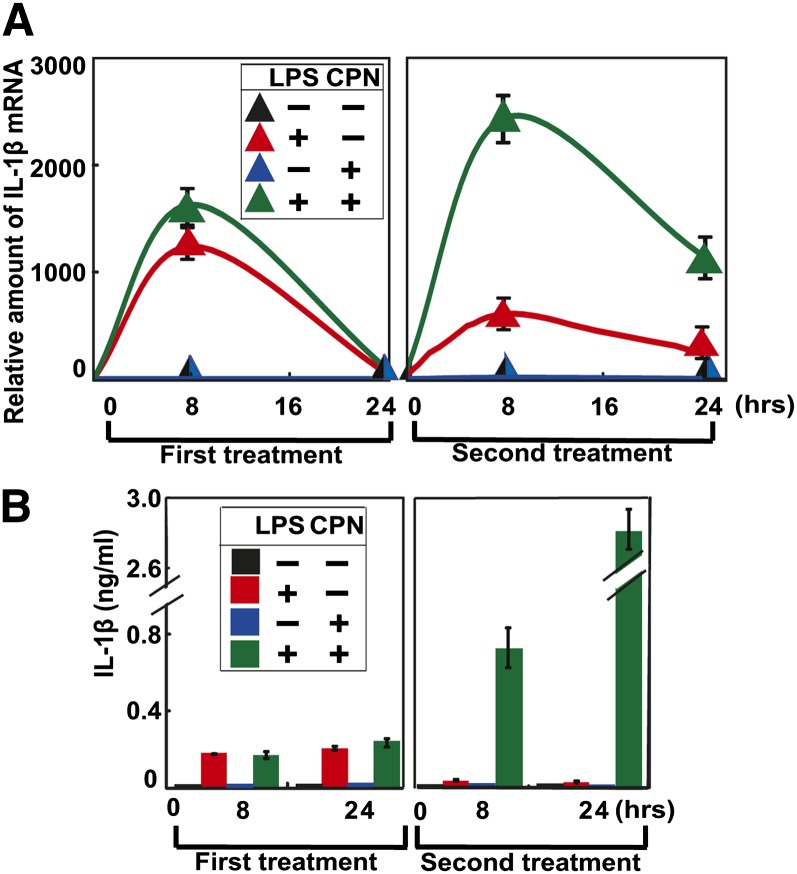
We then determined the functional significance of LPS-induced expression of HMGCR. Treatment of peritoneal macrophages with LPSs for 8 h markedly increased expression of IL-1β mRNA, and the amount of the mRNA dropped to the basal level 24 h after the treatment (Fig. 3A, first treatment, red line). These cells were then treated with fresh LPSs again. Owing to endotoxin tolerance, treating the cells with LPSs for the second time was less efficient in increasing IL-1β mRNA expression compared with the first treatment (Fig. 3A, second treatment, red line). Cotreatment with compactin, an inhibitor of HMGCR, did not affect LPS-induced IL-1β mRNA expression during the first LPS treatment (Fig. 3A, first treatment, green line). However, this treatment led to even more expression of IL-1β mRNA in cells treated with LPSs for the second time compared with those treated for the same period of time during the first treatment (Fig. 3A, second treatment, green line). Consistent with these results, compactin did not affect LPS-induced secretion of IL-1β protein from peritoneal macrophages during the first LPS treatment, but it markedly enhanced the amount of the protein secreted into culture medium during the second LPS treatment (Fig. 3B). These results suggest that endotoxin tolerance is impaired in cells treated with compactin.
Fig. 3.
Inhibition of HMGCR impairs endotoxin tolerance. A, B: On day 0, peritoneal macrophages were seeded at 5 × 106 cells per 60 mm dish. On day 1, cells were switched to medium containing (+) or not containing (−) 200 ng/ml LPSs or 25 μM compactin (CPN) as indicated (first treatment). After incubation for the indicated time, some cells were harvested for quantification of IL-1β mRNA through RT-QPCR, with the amount of the mRNA prior to the treatment set to one (A). The amount of IL-1β protein secreted into medium was determined through ELISA as well (B). On day 2, 24 h after the first treatment, the rest of the cells were switched to fresh medium containing the same concentration of LPSs and compactin as indicated (second treatment). After incubation for the indicated time, the amount of IL-1β mRNA inside the cells and that of IL-1β protein secreted into medium was determined as described above. Results are presented as mean ± SE from three independent experiments.
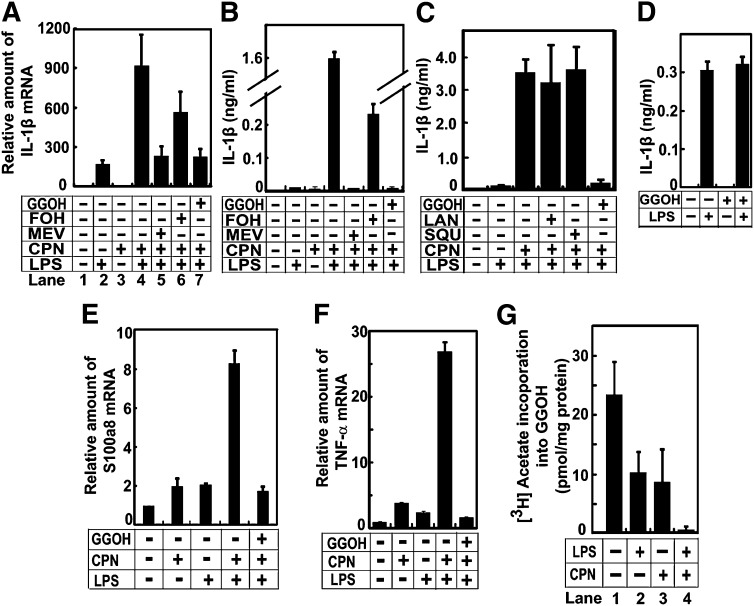
We then tried to identify the product of the mevalonate pathway required to maintain endotoxin tolerance. As shown above, compactin cotreatment significantly elevated the amount of IL-1β mRNA in peritoneal macrophages subjected to repeated treatment with LPSs (Fig. 4A, lanes 2 and 4). Supplementation of mevalonate, the direct product of HMGCR (Fig. 1), completely eliminated the effect of compactin on expression of IL-1β mRNA (Fig. 4A, lane 5). Supplementation of GGOH, a mevalonate-derived isoprenoid that is unable to be converted to sterols (Fig. 1), produced the same result (Fig. 4A, lane 7). In contrast, supplementation of FOH, another mevalonate-derived isoprenoid, was less potent in inhibiting the effect of compactin in these cells (Fig. 4A, lane 6). Consistent with these results, supplementation of mevalonate or GGOH, but not FOH, completely inhibited the effect of compactin on secretion of IL-1β protein from macrophages repeatedly exposed to LPSs (Fig. 4B). Because only FOH, but not GGOH, can be fed into synthesis of sterols (Fig. 1), these results suggest that sterols synthesized through the mevalonate pathway may not be required to inhibit expression of IL-1β in macrophages subjected to repeated exposure to LPSs. This hypothesis is supported by the observation that supplementation of squalene or lanosterol, two sterol intermediates of the mevalonate pathway (Fig. 1), failed to inhibit the effect of compactin on secretion of IL-1β protein from macrophages treated repeatedly with LPSs (Fig. 4C). Importantly, pretreatment with GGOH did not affect secretion of IL-1β from macrophages exposed to LPSs for the first time (Fig. 4D). The effect of GGOH was not restricted to IL-1β, as the isoprenoid was also required to suppress expression of other proinflammatory cytokines such as S100a8 and tumor necrosis factor-α in macrophages subjected to repeated LPS treatment (Fig. 4E, F). These results suggest that GGOH is required to establish endotoxin tolerance.
Fig. 4.
GGOH is required to maintain endotoxin tolerance. A–C, E, F: On day 0, peritoneal macrophages were seeded at 5 × 106 cells per 60 mm dish. On day 1, cells were switched to medium containing (+) or not containing (−) 200 ng/ml LPSs, 25 μM compactin (CPN), 250 μM mevalonate (MEV), 10 μM FOH, GGOH, squalene (SQU), and lanosterol (LAN) as indicated. On day 2, 24 h after the first treatment, cells were switched to fresh medium that was used in the first treatment. On day 3, 24 h after the second treatment, cells were harvested for quantification of indicated mRNA as described in Fig. 3A (A, E, F). The amount of IL-1β protein secreted into medium was determined as described in Fig. 3B (B, C). D: On day 0, peritoneal macrophages were seeded at 5 × 106 cells per 60 mm dish. On day 1, cells were treated with 10 μM GGOH as indicated for 24 h. On day 2, cells were switched to the same medium with or without the addition of 200 ng/ml LPSs as indicated. On day 3, 24 h later, the amount of IL-1β protein secreted into medium from macrophages exposed to LPSs for the first time was determined as described in Fig. 3B. G: On day 0, peritoneal macrophages were seeded at 5 × 106 cells per 60 mm dish. On day 1, cells were switched to medium containing 200 ng/ml LPSs and 25 μM compactin as indicated. Eight hours after the treatment, cells were switched to the identical medium containing 10 μCi [3H]acetate and 0.5 mM unlabeled acetate. The amount of the radioactivity incorporated into GGOH was determined as described in Experimental Procedures. A–G: Results are presented as mean ± SE from three independent experiments.
The results shown above suggest that increased HMGCR expression in LPS-treated macrophages is required to synthesize enough GGOH so that endotoxin tolerance can be established to prevent overproduction of proinflammatory cytokines in macrophages repeatedly exposed to LPSs. To test this hypothesis, we measured GGOH synthesis by labeling peritoneal macrophages with [3H]acetate, and determined the amount of radioactivity incorporated into GGOH. Treatment with LPSs alone reduced the amount of [3H]acetate incorporated into GGOH by more than 2-fold (Fig. 4G, lane 2) even though expression of HMGCR was elevated under such treatment (Fig. 2A). Compactin alone also reduced the amount of [3H]acetate incorporated into GGOH by more than 2-fold (Fig. 4G, lane 3). Cotreatment with LPSs and compactin drastically inhibited production of GGOH as the amount of [3H]acetate incorporated into GGOH was inhibited by more than 10-fold under this condition (Fig. 4G, lane 4). These results suggest that LPS treatment causes disappearance of GGOH, but this loss is partially compensated by increased synthesis of GGOH owing to elevated expression of HMGCR. This scenario explains why compactin alone only partially inhibited GGOH synthesis, but cotreatment with compactin and LPSs depleted GGOH from macrophages.
Inasmuch as GGOH suppresses expression of multiple proinflammatory cytokines in macrophages subjected to repeated exposure to LPSs, we examined the effect of the isoprenoid on activation of nuclear factor-κB (NF-κB), a family of transcription factors that play a major role in activating transcription of proinflammatory genes (22). NF-κB is retained in cytosol through interaction with NF-κB inhibitor (IκB). Proinflammatory stimulations lead to nuclear translocation of NF-κB by triggering degradation of IκB (23). The amount of nuclear p52 and RelB, both of which belong to the NF-κB family of transcription factors, was low in macrophages repeatedly exposed to LPSs owing to endotoxin tolerance (Fig. 5A, lane 3). Cotreatment with compactin markedly increased the amount of these proteins in the nucleus, and this effect was completely abolished by supplementation of the cells with GGOH (Fig. 5A, lanes 4 and 5). We then examined the effect of GGOH on formation of K63-linked polyubiquitin chains that are required for phosphorylation and subsequent proteasomal degradation of IκB (23, 24). Consistent with the result of nuclear localization of NF-κB, the amount of K63-linked polyubiquitin chains was low in macrophages subjected to repeated exposure to LPSs (Fig. 5B, lane 2). Cotreatment with compactin markedly raised the amount of the polyubiquitin chains in these cells (Fig. 5B, lane 4). This increase was abolished by supplementation of the cells with mevalonate or GGOH, but not with FOH (Fig. 5B, lanes 5–7).
We then performed a microarray analysis to identify the mechanism through which GGOH inhibits synthesis of K63-linked polyubiquitin chains. This analysis revealed that supplementation with GGOH reduced expression of Malt1, which has been reported to facilitate assembly of K63-linked polyubiquitin chains (25), in macrophages treated with compactin and subjected to repeated exposure to LPSs. RT-QPCR analysis confirmed this result (Fig. 5C). We further determined that compactin did not affect LPS-induced Malt1 expression during the initial LPS treatment but significantly increased its expression during the second LPS treatment, and this increase was completely inhibited by cosupplementation of the cells with GGOH (Fig. 5D). These results suggest that GGOH-mediated suppression of Malt1 expression may be responsible for establishment of endotoxin tolerance. To test this hypothesis, we transfected two different duplexes of siRNA targeting Malt1 into macrophages treated with compactin and subjected to repeated exposure to LPSs. Knockdown of Malt1 by siRNA significantly reduced the amount of IL-1β mRNA in these cells (Fig. 5E). Consistent with this result, the amount of IL-1β protein secreted into culture medium was also reduced by this treatment (Fig. 5F).
DISCUSSION
The current study demonstrates that sufficient production of GGOH is required to establish endotoxin tolerance in macrophages. We show that LPS treatment enhances expression of HMGCR through increasing translation of the protein so that enough GGOH can be synthesized to inhibit expression of Malt1, a protein activating NF-κB through various mechanisms including facilitating assembly of K63-linked polyubiquitin chains (25, 26), in macrophages repeatedly exposed to LPSs. Consequently, expression of proinflammatory genes driven by the NF-κB family of transcription factors is inhibited. Exactly how GGOH inhibits Malt1 expression remains unclear. Geranylgeranyl pyrophosphate, a derivative of GGOH, can serve as a lipid donor for protein geranylgeranylation (27). Mice deficient in geranylgeranyl protein transferase I, an enzyme catalyzing protein geranylgeranylation, developed severe joint inflammation as a result of overproduction of proinflammatory cytokines from macrophages (28). This result suggests that geranylgeranylation of certain proteins may play a role in inhibiting expression of Malt1.
HMGCR is subjected to feedback regulation at multiple levels including mRNA transcription, protein degradation, and protein translation (3). Our results indicate that treatment of macrophages with LPSs increased the amount of HMGCR by activating translation of the protein. Such activation led to increased synthesis of lanosterol, an observation suggesting that elevated expression of HMGCR protein also enhanced the activity of the protein. Under this circumstance, HMGCR may no longer be the rate-limiting enzyme in the mevalonate pathway. Instead, lanosterol 14α-demethylase, which is the first enzyme that converts lanosterol to cholesterol and catalyzes the rate-limiting step in the process (29), may become the rate-limiting enzyme for the entire mevalonate pathway. This scenario may explain why LPS treatment raised the amount of lanosterol more than that of cholesterol (5, 6). Exactly how LPSs activate translation of HMGCR remains unclear. It was reported previously that accumulation of an unidentified nonsterol isoprenoid inhibited translation of HMGCR, but the underlying mechanism remains unclear (30). It will be interesting to determine whether LPSs and the isoprenoid control HMGCR translation through a similar mechanism.
Results from the current study suggest that insufficient production of GGOH may result in autoinflammatory diseases caused by overproduction of proinflammatory cytokines from macrophages (31). Indeed, hyperimmunoglobulin D and periodic fever syndrome (HIDS), a class of autoinflammatory disease, is caused by mutations in mevalonate kinase that inhibit, but do not completely eliminate, the activity of the enzyme (32, 33). Mevalonate kinase is an enzyme involved in the mevalonate pathway, and it catalyzes a reaction immediately following that catalyzed by HMGCR (Fig. 1) (3). While HIDS patients suffer from recurrent inflammation as a result of deficiency in GGOH production in leukocytes, they do not appear to have gross defects in cholesterol synthesis (10, 11). This is probably because HMGCR, but not mevalonate kinase, catalyzes the rate-limiting step in the mevalonate pathway (3). Thus, mutations that inhibit the activity of the kinase may not have a significant impact on cholesterol biosynthesis in cells that have not been exposed to LPSs. The situation may be different in macrophages activated by LPSs. These cells have increased expression of HMGCR so that the enzyme may not be so rate limiting. As a result, mutations inhibiting the activity of mevalonate kinase may profoundly affect GGOH synthesis in these cells. Thus, the deficiency in endotoxin tolerance caused by insufficient production of GGOH may lead to overproduction of proinflammatory cytokines in HIDS patients. Unlike HIDS, the genetic and metabolic defects of other autoinflammatory diseases, such as systemic onset juvenile idiopathic arthritis, have not been identified (31, 34). It will be interesting to determine whether macrophages derived from these patients are defective in GGOH synthesis following activation by LPSs.
It should be emphasized that GGOH synthesis is unlikely to be inhibited by a low concentration of statins in the clinical setting. As a result, uncontrolled inflammation has not been observed as a side effect of statins for most people who have no defect in the mevalonate pathway. However, it remains unclear whether overproduction of proinflammatory cytokines is responsible for muscle and joint pains in the small percentage of the population that is statin intolerant (35). If this indeed is the case, it will be interesting to determine whether these patients have predisposed defects in GGOH synthesis, and whether supplementation of GGOH improves the outcomes of statin treatment for these patients.
Acknowledgments
The authors thank Lisa Beatty and Muleya Kapaale for help with tissue culture, and Saada Abdalla for technical assistance.
Footnotes
Abbreviations:
- FOH
- farnesol
- GGOH
- geranylgeraniol
- HIDS
- hyperimmunoglobulin D and periodic fever syndrome
- HMGCR
- 3-hydroxy-3-methyl-glutaryl-CoA reductase
- IκB
- NF-κB inhibitor
- IL-1β
- interleukin 1β LPS, lipopolysaccharide
- NF-κB
- nuclear factor-κB
- RT-QPCR
- real time quantitative PCR
- siRNA
- small interfering RNA
This work was supported by grants from the National Institutes of Health (HL-20948) and the Welch Foundation (I-1832).
REFERENCES
- 1.Biswas S. K., Lopez-Collazo E. 2009. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30: 475–487 [DOI] [PubMed] [Google Scholar]
- 2.Cavaillon J. M., Adrie C., Fitting C., Adib-Conquy M. 2003. Endotoxin tolerance: is there a clinical relevance? J. Endotoxin Res. 9: 101–107 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein J. L., Brown M. S. 1990. Regulation of the mevalonate pathway. Nature. 343: 425–430 [DOI] [PubMed] [Google Scholar]
- 4.Im S. S., Yousef L., Blaschitz C., Liu J., Edwards R., Young S., Raffatellu M., Osborne T. 2011. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 13: 540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennis E. A., Deems R. A., Harkewicz R., Quehenberger O., Brown H. A., Milne S. B., Myers D. S., Glass C. K., Hardiman G., Reichart D., et al. 2010. A mouse macrophage lipidome. J. Biol. Chem. 285: 39976–39985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreyev A. Y., Fahy E., Guan Z., Kelly S., Li X., McDonald J. G., Milne S., Myers D., Park H., Ryan A., et al. 2010. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res. 51: 2785–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spann N. J., Garmire L. X., McDonald J. G., Myers D. S., Milne S. B., Shibata N., Reichart D., Fox J. N., Shaked I., Heudobler D., et al. 2012. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 151: 138–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im S. S., Osborne T. F. 2011. Liver X receptors in atherosclerosis and inflammation. Circ. Res. 108: 996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frenkel J., Rijkers G. T., Mandey S. H. L., Buurman S. W. M., Houten S. M., Wanders R. J. A., Waterham H. R., Kuis W. 2002. Lack of isoprenoid products raises ex vivo interleukin-1β secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum. 46: 2794–2803 [DOI] [PubMed] [Google Scholar]
- 10.Houten S. M., Frenkel J., Waterham H. R. 2003. Isoprenoid biosynthesis in hereditary periodic fever syndromes and inflammation. Cell. Mol. Life Sci. 60: 1118–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandey S. H., Kuijk L. M., Frenkel J., Waterham H. R. 2006. A role for geranylgeranylation in interleukin-1β secretion. Arthritis Rheum. 54: 3690–3695 [DOI] [PubMed] [Google Scholar]
- 12.Giriwono P. E., Shirakawa H., Ohsaki Y., Hata S., Kuriyama H., Sato S., Goto T., Komai M. 2013. Dietary supplementation with geranylgeraniol suppresses lipopolysaccharide-induced inflammation via inhibition of nuclear factor-κB activation in rats. Eur. J. Nutr. 52: 1191–1199 [DOI] [PubMed] [Google Scholar]
- 13.Liscum L., Luskey K. L., Chin D. J., Ho Y. K., Goldstein J. L., Brown M. S. 1983. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase and its mRNA in rat liver as studied with a monoclonal antibody and a cDNA probe. J. Biol. Chem. 258: 8450–8455 [PubMed] [Google Scholar]
- 14.Gong Y., Lee J. N., Lee P. C. W., Goldstein J. L., Brown M. S., Ye J. 2006. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 3: 15–24 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Goncalves R., Mosser D. 2008. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 83: 14.1.1–14.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang G., Yang J., Horton J. D., Hammer R. E., Goldstein J. L., Brown M. S. 2002. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 277: 9520–9528 [DOI] [PubMed] [Google Scholar]
- 17.Sever N., Yang T., Brown M. S., Goldstein J. L., DeBose-Boyd R. A. 2003. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol. Cell. 11: 25–33 [DOI] [PubMed] [Google Scholar]
- 18.del Prete M. J., Vernal R., Dolznig H., Müllner E. W., Garcia-Sanz J. A. 2007. Isolation of polysome-bound mRNA from solid tissues amenable for RT-PCR and profiling experiments. RNA. 13: 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams C. M., Reitz J., De Brabander J. K., Feramisco J. D., Li L., Brown M. S., Goldstein J. L. 2004. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 279: 52772–52780 [DOI] [PubMed] [Google Scholar]
- 20.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam S. P., Brissette L., Ramharack R., Deeley R. G. 1991. Differences between the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and low density lipoprotein receptor in human hepatoma cells and fibroblasts reside primarily at the translational and post-translational levels. J. Biol. Chem. 266: 16764–16773 [PubMed] [Google Scholar]
- 22.Sen R. 2011. The origins of NF-κB. Nat. Immunol. 12: 686–688 [DOI] [PubMed] [Google Scholar]
- 23.Skaug B., Jiang X., Chen Z. J. 2009. The role of ubiquitin in NF-κB regulatory pathways. Annu. Rev. Biochem. 78: 769–796 [DOI] [PubMed] [Google Scholar]
- 24.Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z. J. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 103: 351–361 [DOI] [PubMed] [Google Scholar]
- 25.Sun L., Deng L., Ea C. K., Xia Z. P., Chen Z. J. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell. 14: 289–301 [DOI] [PubMed] [Google Scholar]
- 26.Thome M. 2008. Multifunctional roles for MALT1 in T-cell activation. Nat. Rev. Immunol. 8: 495–500 [DOI] [PubMed] [Google Scholar]
- 27.Zhang F. L., Casey P. J. 1996. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65: 241–269 [DOI] [PubMed] [Google Scholar]
- 28.Khan O. M., Ibrahim M. X., Jonsson I. M., Karlsson C., Liu M., Sjogren A. K., Olofsson F. J., Brisslert M., Andersson S., Ohlsson C., et al. 2011. Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice. J. Clin. Invest. 121: 628–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi M., Goldstein J. L., Brown M. S. 1988. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J. Biol. Chem. 263: 8929–8937 [PubMed] [Google Scholar]
- 30.Gaylor J. R. 1981. Formation of sterols in animals. In Biosynthesis of Isoprenoid Compounds. Vol. 1. J. W. Porter and S. L. Spurgeon, editors. John Wiley and Sons, New York. 481–543. [Google Scholar]
- 31.Kastner D. L., Aksentijevich I., Goldbach-Mansky R. 2010. Autoinflammatory disease reloaded: a clinical perspective. Cell. 140: 784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houten S. M., Kuis W., Duran M., de Koning T. J., van Royen-Kerkhof A., Romeijn G. J., Frenkel J., Dorland L., de Barse M. M. J., Huijbers W. A. R., et al. 1999. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat. Genet. 22: 175–177 [DOI] [PubMed] [Google Scholar]
- 33.Houten S. M., Wanders R. J. A., Waterham H. R. 2000. Biochemical and genetic aspects of mevalonate kinase and its deficiency. Biochim. Biophys. Acta. 1529: 19–32 [DOI] [PubMed] [Google Scholar]
- 34.Vastert S. J., Kuis W., Grom A. A. 2009. Systemic JIA: new developments in the understanding of the pathophysiology and therapy. Best Pract. Res. Clin. Rheumatol. 23: 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghatak A., Faheem O., Thompson P. D. 2010. The genetics of statin-induced myopathy. Atherosclerosis. 210: 337–343 [DOI] [PubMed] [Google Scholar]