Abstract
We investigated the prostaglandin (PG)E2 pathway in human abdominal aortic aneurysm (AAA) and its relationship with hypervascularization. We analyzed samples from patients undergoing AAA repair in comparison with those from healthy multiorgan donors. Patients were stratified according to maximum aortic diameter: low diameter (LD) (<55 mm), moderate diameter (MD) (55–69.9 mm), and high diameter (HD) (≥70 mm). AAA was characterized by abundant microvessels in the media and adventitia with perivascular infiltration of CD45-positive cells. Like endothelial cell markers, cyclooxygenase (COX)-2 and the microsomal isoform of prostaglandin E synthase (mPGES-1) transcripts were increased in AAA (4.4- and 1.4-fold, respectively). Both enzymes were localized in vascular cells and leukocytes, with maximal expression in the LD group, whereas leukocyte markers display a maximum in the MD group, suggesting that the upregulation of COX-2/mPGES-1 precedes maximal leukocyte infiltration. Plasma and in vitro tissue secreted levels of PGE2 metabolites were higher in AAA than in controls (plasma-controls, 19.9 ± 2.2; plasma-AAA, 38.8 ± 5.5 pg/ml; secretion-normal aorta, 16.5 ± 6.4; secretion-AAA, 72.9 ± 6.4 pg/mg; mean ± SEM). E-prostanoid receptor (EP)-2 and EP-4 were overexpressed in AAA, EP-4 being the only EP substantially expressed and colocalized with mPGES-1 in the microvasculature. Additionally, EP-4 mediated PGE2-induced angiogenesis in vitro. We provide new data concerning mPGES-1 expression in human AAA. Our findings suggest the potential relevance of the COX-2/mPGES-1/EP-4 axis in the AAA-associated hypervascularization.
Keywords: cyclooxygenase pathway, prostaglandin, angiogenesis, cyclooxygenase, E prostanoid receptor, microsomal isoform of prostaglandin E synthase
Abdominal aortic aneurysm (AAA) is a late-age onset disorder that affects a high percentage of the population in industrialized countries, and rupture of AAA is associated with high mortality rates (1). The etiology of AAA is complex with a relevant contribution of genetic factors (2). Although much effort has been made to clarify the mechanism of AAA development, currently the only effective approach to prevent aneurysm rupture is surgical repair by conventional or endovascular techniques.
Evidence has been established for a relationship between atherosclerosis and AAA, both disorders being characterized by an underlying chronic inflammation. However, there are marked differences between atherosclerotic lesions and AAA. Whereas atherosclerotic plaque is characterized by leukocyte infiltration at the lumen site and hyperproliferation of vascular smooth muscle cells (VSMCs) causing neointimal hyperplasia, AAA is characterized by leukocyte infiltration into adventitia and depletion of VSMCs in the media. Other relevant features of AAA are the wall tension strength breakdown caused by proteolytic enzymes progressively destructing elastic fibers (3) and hypervascularization of aortic tissue. It has been proposed that this vascularization might contribute to the development and rupture of aneurysms (4, 5).
Prostaglandin (PG)E2 has been recognized as a relevant mediator in AAA. Biosynthesis of PGE2 begins with the formation of PGH2 through the action of cyclooxygenase (COX) (6, 7) on arachidonic acid (AAc) released by phospholipases from the membrane phosphoglycerides. PGH2 is then isomerized to PGE2 by PGE synthases. The microsomal isoform of prostaglandin E synthase (mPGES-1) is inducible by proinflammatory cytokines and seems to be the main isoenzyme involved in PGE2 biosynthesis under inflammatory conditions (8–11). COX-2/mPGES-1 is widely regarded as the major contributing enzymatic chain for PGE2 biosynthesis under pathological conditions. COX-2-derived PGE2 is involved in the pathogenesis of AAA, as data from animal models and human studies indicate (12–15). Furthermore, deletion of mPGES-1 attenuates experimental AAA in mice (16).
PGE2 exerts its cellular effects by binding to four distinct E-prostanoid receptors (EP-1–4) that belong to the family of seven transmembrane G protein-coupled receptors. Each receptor is involved in different and often opposite biological effects of PGE2. EP-2 and EP-4 are both Gs-protein coupled receptors that upregulate intracellular cAMP levels, whereas EP-3 usually counteracts EP-2- and EP-4-mediated upregulation of cAMP by preferentially coupling to Gi proteins (17). Recently, conflicting results have been reported on the role of EP-4 in AAA development in animal models (18–20). These studies have been focused on the involvement of EPs in the activation of leukocytes (mainly macrophages) and VSMCs, and in the release of proteases during the immune-inflammatory process associated to AAA.
Main data regarding COX-2-alternative targets for the development of new anti-inflammatory drugs for AAA comes from studies in animal models (16, 18–20). In these models, AAA develops fast and its etiology differs substantially from human pathology. Despite the role of PGE2 in neovascularization in cancer and other pathologies, and that the relevance of angiogenesis in AAA is widely accepted, information concerning COX-2/mPGES-1-derived PGE2 in the AAA, particularly in AAA-associated hypervascularization, is limited and restricted to COX-2-derived PGE2 from macrophages (12, 13, 21, 22). Furthermore, the beneficial effects of COX-2 inhibitors and the deletion of mPGES-1 or EP-4 in experimental AAA are not fully understood in the context of this pathology in humans. Because PGE2 modifiers have a possible therapeutic potential, the objective of the present study was to examine the elements involved in the PGE2 pathway in human AAA, particularly in microvasculature.
MATERIALS AND METHODS
Tissue samples
The study was approved by the Hospital de la Santa Creu i Sant Pau Ethics Committee, and informed consent was obtained from each patient. All procedures were reviewed by the Institutional Review Board at Hospital de la Santa Creu i Sant Pau. The aortic biopsies were obtained from patients undergoing open repair for AAA at our institution. Samples were obtained from the remaining mid-infrarenal aortic wall after exclusion and prosthetic replacement of AAA. Normal aortas (NAs) were obtained from healthy aortas of multiorgan donors and samples were also taken from the mid-portion of the infrarenal abdominal aorta at the time of organ harvest. When a luminal thrombus was present in AAA samples, it was separated before the aortic biopsy was taken and aortic tissue was washed twice with cold phosphate-buffered saline (PBS). A portion of each sample was placed in RNAlater solution (Qiagen GmbH, Hilden, Germany) and stored at 4°C for 24 h before long-term storage at −80°C until further processing for RNA isolation. Another portion was fixed in formalin solution 10% (Sigma-Aldrich, Inc., St. Louis, MO) for 24 h and included in paraffin for immunohistochemical studies. Additional portions of aorta were placed in PBS to establish VSMC cultures and to obtain tissue secretomes.
Patients were stratified by the maximum transverse aortic diameter defining three groups: low diameter (LD) (<55 mm); moderate diameter (MD) (55–69.9 mm); and high diameter (HD) (≥70 mm). To determine the maximum aortic diameter, we used a transversal measurement to the true lumen center line at infrarenal level, based on computerized tomography angiography with endovenous contrast, using Workstation AGFA IMPAX 6.4.0.4010 and OsiriX MD (US Food and Drug Administration cleared/CE IIa version) for primary diagnostic. Because surgical repair is not usually indicated in patients with an AAA maximal diameter of <55 mm, the aortic samples of the LD group were from patients with concomitant iliac artery aneurysm to be surgically repaired. Table 1 shows the characteristics of the individuals included in the study.
TABLE 1.
Clinical characteristics of individuals with AAA and NA included in the study: demographics and risk factors
| Measurements | ||||||
| mRNA levels | Plasma PGE2M | Secretiona | ||||
| Characteristic | AAA | NA | AAA | Controls | AAA | NA |
| Number | 86 | 25 | 39 | 39 | 32 | 15 |
| Aortic diameterb (mm) | 66.7 ± 13.5 | — | 63.3 ± 9.8 | — | 68.26 ± 13.1 | — |
| LD (<55 mm) | 49.7 ± 6.4 | — | 52.8 ± 1.2 | — | — | — |
| MD (55–69.9 mm) | 60.1 ± 3.2 | — | 59.9 ± 3.6 | — | — | — |
| HD (≥70 mm) | 80.8 ± 7.5 | — | 76.5 ± 6.7 | — | — | — |
| Age (years) | 70.9 ± 6.6 | 56.3 ± 16.0 | 71.2 ± 6.7 | 62.9 ± 3.0 | 71.2 ± 8.1 | 55.7 ± 16.1 |
| Male | 84 (97.7%) | 16 (64%) | 35 (89.7%) | 34 (87.2%) | 32 (100%) | 7 (46.7%) |
| Diabetes mellitus | 19 (22.1%) | 2 (8%)e | 10 (25.6%) | 1 (2.6%) | 6 (18.8%) | 2 (13.3%) |
| Hypertension | 55 (64%) | 2 (8%)e | 34 (87.2%) | 14 (35.9%) | 22 (68.8%) | 2 (13.3%) |
| Hyperlipidemia | 55 (64%) | 2 (8%)e | 30 (76.9%) | 1 (2.6%) | 21 (65.6%) | 2 (13.3%) |
| Smoking habit | ||||||
| Non-smokers | 7 (8.1%) | 21 (84%)e | 3 (7.7%) | 30 (76.9%) | 2 (6.2%) | 11 (73.3%) |
| Current smokers | 26 (30.2%) | 3 (12%)e | 9 (23.1%) | 6 (15.4%) | 10 (31.2%) | 3 (20%) |
| Ex-smokersc | 53 (61.6%) | 1 (4%)e | 27 (69.2%) | 3 (7.7%) | 20 (62.5%) | 1 (6.7%) |
| Coronary artery disease | 22 (25.6%) | 0e | 14 (35.9%) | 0 | 11 (34.4%) | 0e |
| Angor pectoris | 3 (3.5%) | 0e | 1 (2.6%) | 0 | 3 (9.4%) | 0e |
| Myocardial infarction | 6 (7%) | 0e | 6 (15.4%) | 0 | 1 (3.1%) | 0e |
| Coronary intervention/CABG | 13 (15.1%) | 0e | 7 (17.9%) | 0 | 7 (21.9%) | 0e |
| Chronic renal insufficiencyd | 36 (41.9%) | 0e | 13 (33.3%) | 0 | 10 (31.2%) | 0e |
| Dialysis | 0 (0%) | 0e | 0 (0%) | 0 | 0 | 0e |
| Peripheral vascular disease | 44 (51.1%) | 0e | 21 (53.8%) | 0 | 17 (53.2%) | 0e |
| Absence pulses | 29 (33.7%) | 0e | 14 (35.9%) | 0 | 11 (34.4%) | 0e |
| Intermitent claudication | 15 (17.4%) | 0e | 7 (17.9%) | 0 | 6 (18.8%) | 0e |
| Cerebrovascular disease | 7 (8.1%) | 1 (4%)e | 4 (10.3%) | 0 | 2 (6.2%) | 2 (13.3%) |
| Cerebral vascular attack | 5 (5.8%) | 1 (4%)e | 3 (7.7%) | 0 | 2 (6.2%) | 2 (13.3%) |
| Transient ischemic attack | 2 (2.3%) | 0e | 1 (2.6%) | 0 | 0 | 0e |
| COPD | 27 (31.4%) | 0e | 7 (17.9%) | 0 | 7 (21.9%) | 0e |
| Antiplatelet users | 39 (45.9%) | 1 (4%)e | 19 (50%) | 0 | 15 (48.4%) | 1 (6.7%) |
| Statins users | 53 (61.6%) | 0e | 30 (76.9%) | 7 (17.9%) | 19 (59.4%) | 0e |
| ACE inhibitors users | 20 (24.1%) | 0e | 13 (33.3%) | 6 (15.4%) | 8 (25.8%) | 0e |
| NSAID users | 6 (7%) | 0e | 3 (7.7%) | 0 | 3 (9.4%) | 0e |
| Corticoid users | 6 (7%) | 0e | 1 (2.6%) | 0 | 2 (6.2%) | 0e |
| Immunosuppressors users | 4 (4.7%) | 0e | 0 (0%) | 0 | 1 (3.1%) | 0e |
Nominal variables are presented as number and as percentage (%) and continuous variables as mean ± SD. eGFR, estimated glomerular filtration rate; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; PGE2M, PGE2 metabolites; ACE, angiotensin-converting enzyme; NSAID, nonsteroidal anti-inflammatory drug.
PGE2 secretion by NA and AAA samples.
Aneurysm maximum transverse diameter in mm.
Quit smoking for more than 1 year.
Estimated glomerular filtration rate <60 ml/min/1.73 m2.
In some cases due to the nature of NA samples some of the clinical characteristics are not always recorded and infra-evaluation of them is probable.
Analysis of mRNA levels in the tissues and culture cells
For total RNA extraction, tissue samples stored in RNAlater were homogenized in the FastPrep-24 homogenizer and Lysing Matrix D tubes (MP Biomedicals, Solon, OH) and RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Total RNA was extracted from cell cultures using Ultraspec (Biotecx Laboratories, Inc., Houston, TX) according to the manufacturer's instructions. cDNA was prepared by reverse transcribing 1 μg RNA with the high-capacity cDNA archive kit with random hexamers (Applied Biosystems, Foster City, CA). mRNA expression of the selected genes was studied by real-time PCR in an ABI Prism 7900HT system using predesigned validated assays (TaqMan gene expression assays; Applied Biosystems) and universal thermal cycling parameters. Relative expression was expressed as transcript/β-actin ratios.
Analysis of PGE2
To analyze plasma levels, a sample of 10 ml of peripheral blood was collected from all participants in heparin-containing tubes. In the case of AAA patients, blood was collected before anesthesia in the operating room. It was centrifuged immediately and the plasma aliquoted and frozen at −80°C until analysis. It is known that PGE2 is rapidly transformed in vivo into 13,14-dihydro-15-oxo-PGE2 that is unstable, and it undergoes a further transformation into 13,14-dihydro-15-oxo-PGA2 (23, 24). To evaluate plasma levels of PGE2, we used an enzyme immunoassay kit that converts 13,14-hihydro-15-oxo metabolites of PGE2 into a single stable derivative (Cayman Chemical, Ann Arbor, MI) following manufacturer's instructions.
Secretomes were obtained from approximately 150 mg of NA and AAA aortic fragments by incubating the tissues in 1 ml serum-free DMEM (Biological Industries, Kibbutz Beit Haemek, Israel) in a cell incubator for 48 h. Medium was then recovered and kept at −80°C until the PGE2 metabolites, 6-oxo-PGF1α, thromboxane (Tx) B2, and leukotriene (LT)B4, released were analyzed by enzyme immunoassay (Cayman Chemical) following manufacturer's instructions.
Immunohistochemistry
Immunohistochemical studies were performed using a mouse monoclonal antibody anti-COX-2 (ref 160112 clone CX229, diluted 1:50), rabbit polyclonal antibodies against EP-2 (ref 101750, diluted 1:1,000), EP-3 (ref 101760, diluted 1:400), and EP-4 (ref 101775, diluted 1:100) all from Cayman Chemical; and a rabbit polyclonal antibody against mPGES-1 (ref HPA045064 Prestige antibodies, diluted 1:50) from Sigma. Blanks were performed using the corresponding blocking peptides all from Cayman. Monoclonal antibodies (ref M0616, diluted 1:35; ref IR751, without further dilution) from Dako were used for von Willebrand factor (vWF) [endothelial cell (EC) marker] and CD45 (leukocyte marker) immunostaining. Three micrometer sections of paraffin-embedded tissue samples were stained in a Dako Autostainer Link 48 using the Dako EnVision Flex kit. Diaminobenzidine was used as chromogen. Immunostainings used for comparative purposes were processed simultaneously.
Microvascular endothelial cell culture
Microvascular endothelial cells (MVECs) were isolated from human adult foreskins using a previously described technique (25, 26). In brief, foreskins obtained from adult circumcisions were placed in PBS supplemented with penicillin (200 units/ml), streptomycin (200 μg/ml), and amphotericin B (0.5 μg/ml) (all from Biological Industries). Foreskins were cut into 3 mm squares and placed in PBS containing 0.3% trypsin and 1% EDTA at 37°C for 30 min. Segments were then washed several times with PBS, placed in a Petri dish in M199 containing 10% FBS, and individually compressed with the side of a scalpel blade to express microvascular fragments. The microvascular segments were passed through a 150 μm stainless steel mesh and collected by centrifugation at 300 g for 15 min. MVECs were seeded on a gelatin-coated cell culture flask and cultured in medium MCDB 131 with 20% FBS; l-glutamine (2 mmol/l), penicillin (200 units/ml), streptomycin (200 μg/ml), EGF (20 ng/ml), and bFGF (5 ng/ml) (all from Biological Industries). When cells reached confluence, they were purified with Dynabeads CD31 (Dynabeads; Invitrogen Dynal ASA, Oslo, Norway) following the manufacturer's instructions. Flow cytometry and positive staining for CD31 and platelet endothelial cell adhesion molecule-1 confirmed the purity of the cell population. MVECs in passage 3 were used for mRNA determinations and in passages 5–7 for angiogenesis assays.
Aortic VSMC culture
Aortic human VSMC cultures were established by an explant procedure from multi-organic donor aortas as previously described (9, 10). The artery was longitudinally split and the endothelium was removed by gently scraping. The tissue was minced and seeded onto the culture surface in a small volume of DMEM medium containing 10% FBS (Biological Industries). VSMCs were characterized by α-actin positive staining.
Immunofluorescence staining of mPGES-1 and EP-4 in MVECs
MVECs (passage 3) grown in Millicell EZslide (Millipore Corporation, Billerica, MA) were fixed in methanol:acetone (1:1) at −20°C for 20 min. For double immunostaining, cells were incubated with a mouse monoclonal antibody, anti-mPGES-1 (ref 10004350, diluted 1:100), and a rabbit polyclonal antibody, anti-EP4 (ref 101775, diluted 1:100) (both from Cayman) simultaneously for 60 min. This was followed by Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 555 donkey anti-rabbit IgG (Invitrogen) incubation for 1 h. Nuclei were counterstained with Hoechst 33342 (1 μg/ml, Sigma) in PBS for 10 min. The slips were mounted in ProLong Gold antifade reagent (Molecular Probes, Life Technologies, Eugene, OR) and photographed through an Olympus BX50 microscope.
In vitro angiogenesis assay
In vitro angiogenesis assays were performed with MVECs as described (27, 28). Briefly, 12.5 × 103 cells were seeded onto Matrigel (BD Matrigel basement membrane matrix; BD Biosciences, Bedford, MA) in 96-well plates and exposed to treatments as required. For IL-1β assays, cells were preincubated for 1 h with the assay medium with or without 50 U/ml of human recombinant IL-1β (Roche Diagnostics GmbH) before being seeded into Matrigel. After 4 h of treatment, photographs were taken with an Olympus digital camera mounted on an Olympus IMT-2 inverted microscope using a 10×/0.40 objective, and the number of closed polygons in the EC mesh was counted.
Statistical analysis
Sigma-Stat software was used for statistical analysis. When data fit a normal distribution, statistical significance between more than two groups was assessed using one-way ANOVA and the Student-Newman-Keuls test; Student's t-test was used to compare two groups. When normality failed, we used the Mann-Whitney rank sum test to compare two groups and Kruskal-Wallis one-way ANOVA on ranks for multiple comparisons (Dunn's method). To determine association between variables, data were Log10 transformed in order to normalize their distribution and the Pearson product moment correlation method was then used. A P value below 0.05 was considered significant.
RESULTS
Evaluation of hypervascularization and leukocyte infiltration in AAA samples
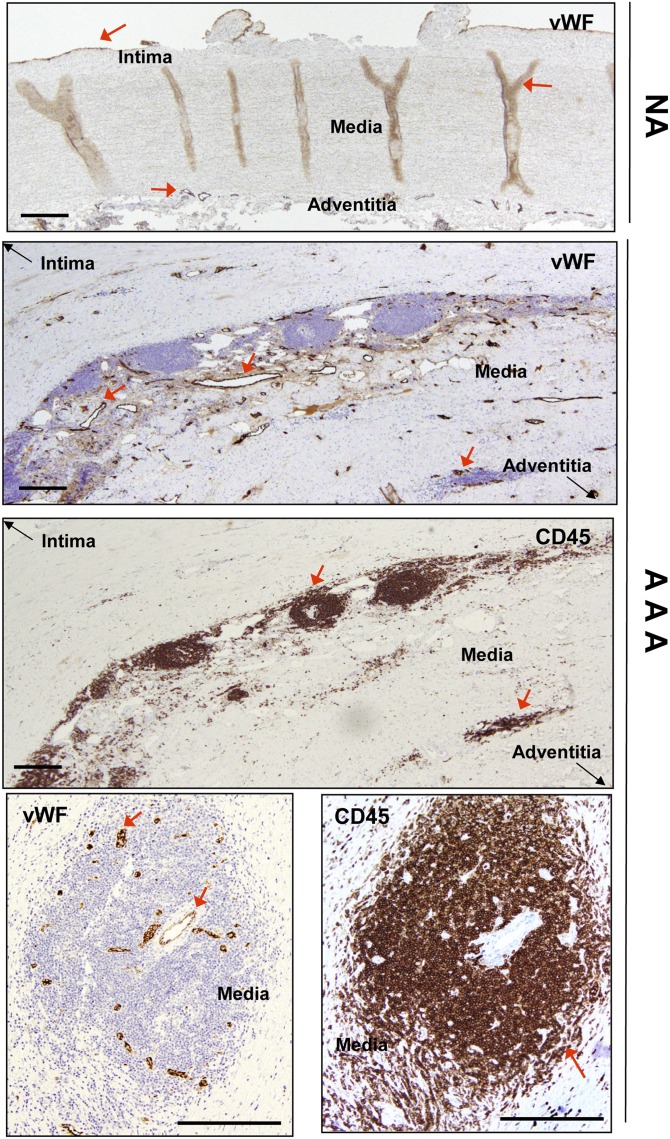
Table 1 summarizes the characteristics of patients and donors included in this study. We used vWF immunostaining to assess the distribution of microvessels in aortic samples. Microvessels in NAs originated in the adventitia, distributed regularly, traversed media, and finished in the intima (many of them after bifurcation), but round microvessels in the media were scarce (Fig. 1). Microvessels were abundant in the adventitia and in the media of AAA samples (Fig. 1), but were often absent in the intima. While infiltrated leukocytes (determined by CD45 immunostaining) were scarce in NAs, they were common in AAAs. Observation of 20 AAA samples revealed infiltrating leukocytes in the media in all of them. Adventitia was also extensively vascularized with a concomitant accumulation of leukocytes. Leukocyte infiltrate was systematically located in perivascular areas of microvessels (Fig. 1).
Fig. 1.
Representative immunohistochemical images of vWF and CD45 in aortic samples. Top panel shows NA immunostained with anti-vWF. Middle panels show serial sections of an AAA sample immunostained for vWF and CD45. Bottom panels are serial sections of an AAA sample immunostained for vWF and CD45 showing leukocyte perivascular accumulation. Red arrows highlight specific immunostaining. In the middle panels, black arrows are used to orient the position of the vessel by indicating where the intima and adventitia are. Scale bars: 500 μm.
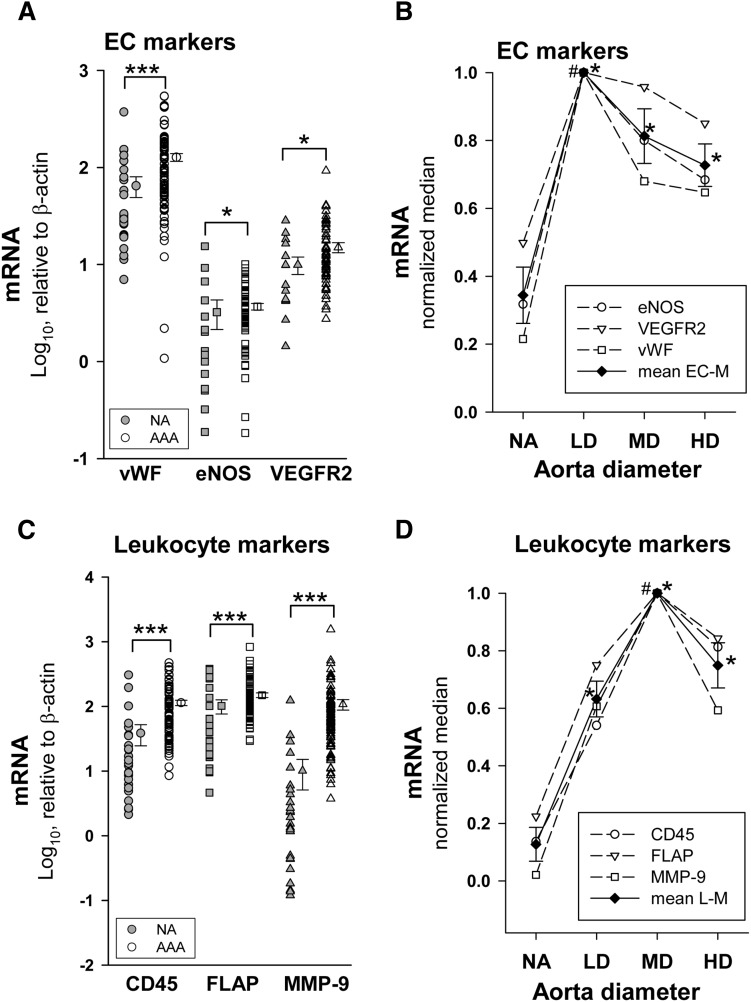
We determined mRNA levels of two EC markers, vWF and endothelial nitric oxide synthase (eNOS), as an index of vascularization. In addition, we also analyzed expression levels of the vascular endothelial growth factor receptor-2 (VEGFR2), a gene highly expressed in EC. Expression levels of these genes did not fit a normal distribution. Results in Fig. 2A show that mRNA expression levels of the three genes were significantly enhanced in AAA. To further evaluate levels of these markers at different stages of AAA development, we stratified AAA samples as a function of the maximum aortic diameter. Figure 2B shows the median of the EC marker expression for each AAA diameter group. We observed that expression of all three markers was maximal in the LD group and decreased thereafter. We also analyzed the expression levels of two leukocyte markers (undetectable in vascular cells in culture, data not shown), CD45 and FLAP. Additionally, we analyzed the expression of MMP-9, a metalloproteinase highly expressed by leukocytes. All of them were highly expressed in AAA samples (Fig. 2C) and exhibited a maximum in the MD group (Fig. 2D).
Fig. 2.
A: Expression levels of EC markers in NA (eNOS and VEGFR2, n = 17; vWF, n = 25) and AAA samples (vWF, n = 86; eNOS and VEGFR2, n = 60). Points without error bars indicate individual values and points at right are the mean ± SEM. *P < 0.05, ***P < 0.001 when compared with NA samples. B: Open points are the relative median values of mRNA levels of EC markers normalized to the highest median value in the stratified patient groups as a function the aortic diameter [NA, n = 25; LD (<55 mm), n = 14; MD (55–69.9 mm), n = 37; and HD (≥70 mm), n = 35], closed points are the mean ± SEM of the three EC markers (mean EC-M). *P < 0.05 compared with NA; #P < 0.05 when compared with the other patient groups. C: mRNA levels of leukocyte markers in NA (n = 25) and AAA (n = 86) samples; ***P < 0.001 when compared with NA samples. D: Open points are the relative median values of mRNA levels of leukocyte characteristic proteins normalized to the highest median value of the stratified patient groups; closed points are the mean ± SEM of the three leukocyte markers (mean L-M). *P < 0.05 compared with NA; #P < 0.05 when compared with the other patient groups.
Expression of the enzymes involved in PGE2 biosynthesis
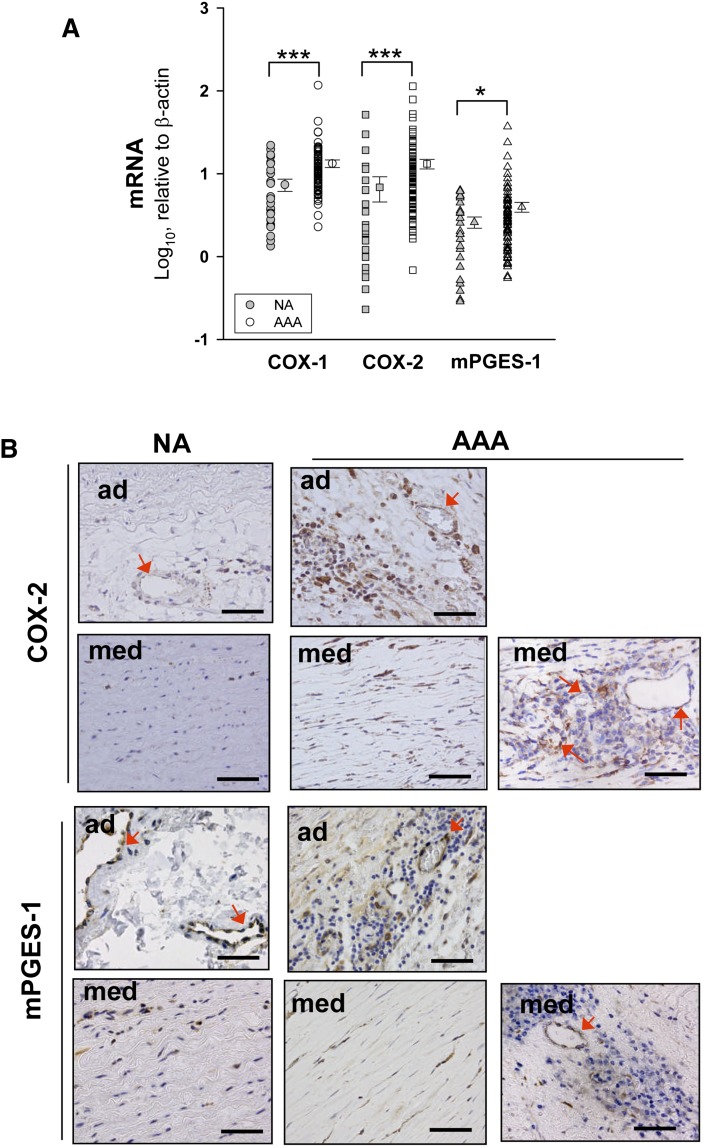
Results in Fig. 3A show that the expression of COX isoenzymes and mPGES-1 was significantly higher in AAA than in NA samples. Immunohistochemistry showed that COX-2 expression was weak but ubiquitous in NA samples, mainly in adventitial microvessels and in medial VSMCs (Fig. 3B). In AAA samples, we found COX-2 immunostained MVECs in the adventitia and media layers, VSMCs in the media, and also infiltrating leukocytes, immunostaining being markedly increased in AAA (Fig. 3B). Localization of mPGES-1 was similar to that of COX-2, NA, and AAA, displaying apparent similar immunostaining intensity (Fig. 3B).
Fig. 3.
A: Expression levels of COX isoenzymes and mPGES-1 in NA (n = 25) and AAA (n = 86) samples. Points without error bars indicate individual values and points at right are the mean ± SEM. *P < 0.05, *** P < 0.001 when compared with NA samples. B: Representative examples of immunohistochemistry for COX-2 and mPGES-1 in NA and AAA samples. Arrows indicate some immunostained cells in microvessels. ad, adventitia; med, media. Scale bars: 100 μm.
In an attempt to approach the relative contribution of the endothelium to PGE2 production in AAA, we determined the statistical association of PGE2 with other cell-characteristic eicosanoids secreted by AAA samples considered as independent variables. The best association was found between PGE2 and PGI2 (in terms of its stable metabolite 6-oxo-PGF1α; R = 0.890, P = 9.95 × 10−12, n = 32). The statistical association between PGE2 and TxA2 (in terms of TxB2) was weaker (R = 0.492, P = 0.007), and no association between PGE2 and LTB4 was observed.
Expression levels of PGE2 biosynthetic pathway enzymes in AAA stratified by aortic diameter
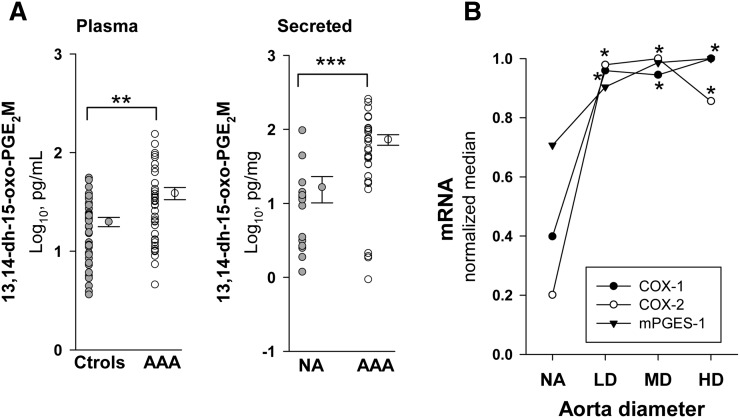
Because PGE2 is rapidly transformed in vivo, we analyzed plasma levels of its 13,14-dihydro-15-oxo-metabolites. Circulating levels of PGE2 metabolites were significantly elevated in AAA patients compared with normal controls (Fig. 4A). The characteristics of patients and controls included are shown in Table 1. In Fig. 4A, we also show production of PGE2 by NA and AAA samples after 48 h of incubation. AAA samples produced about 5-fold more PGE2 than NA samples in terms of the median. In Fig. 4B, transcript levels of COX-1, COX-2, and mPGES-1 are depicted as a function of AAA maximum diameter, showing that all of them displayed a maximum in the LD group.
Fig. 4.
A: Plasma levels of PGE2 metabolites (PGE2M) in controls (Ctrols) (n = 39) and AAA (n = 39) (left), and production of PGE2M by NA (n = 15) and AAA samples (n = 32) (right). Points without error bars indicate individual values and points at right are the mean ± SEM. B: Relative median values of transcript levels of COX-1, COX-2, and mPGES-1 [NA, n = 25; LD (<55 mm), n = 14; MD (55–69.9 mm), n = 37; and HD (≥70 mm), n = 35] normalized to the highest median value of the stratified patient groups.*P < 0.05, **P < 0.01, ***P < 0.001 when compared with NA samples.
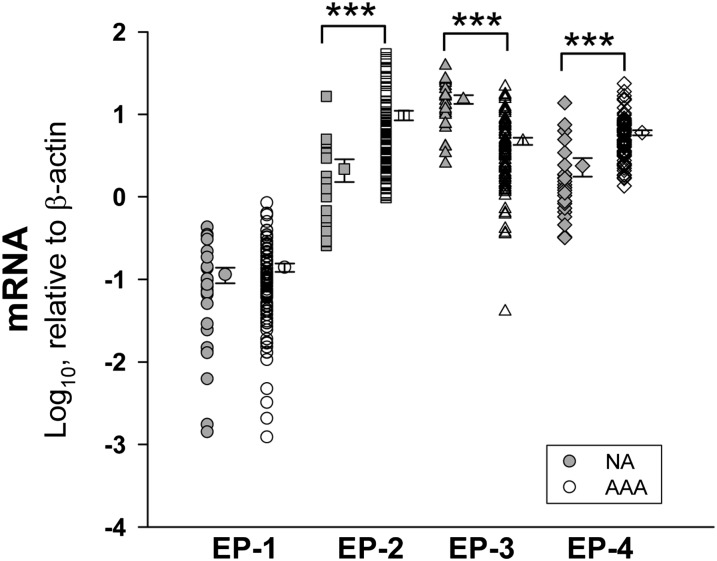
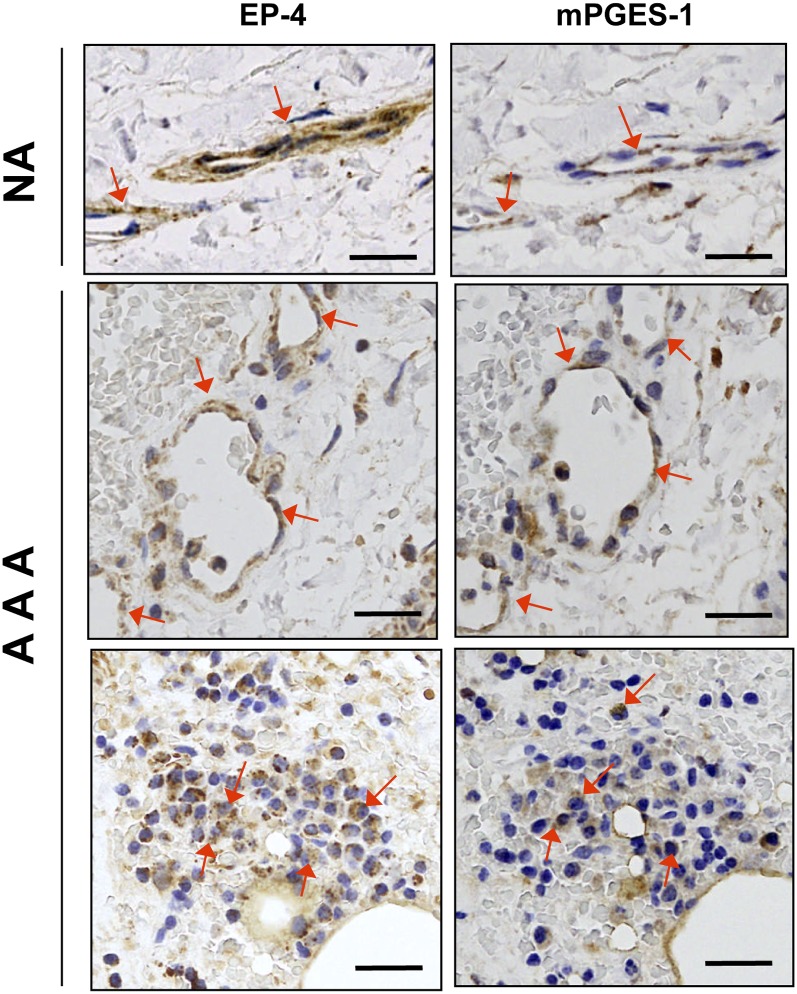
Coexpression of EP-4 and mPGES-1 in aortic microvessels
In human aortic samples, EP-1 was scarcely expressed and no significant differences were found between NA and AAA. EP-2 and EP-4 were significantly increased in AAA while EP-3 was substantially decreased (Fig. 5). EP-4 immunostaining was not detected in the VSMCs of NA or AAA samples (not shown), but in both samples it colocalized with mPGES-1 in MVECs (Fig. 6). Additionally, EP-4 and mPGES-1 were also detected in the areas of leukocyte infiltration in AAA (Fig. 6). In contrast, none of the other receptors were appreciably expressed in microvessels (not shown). We found few EP-2-positive cells scattered in the media in both NA and AAA, but it was particularly relevant in infiltrating leukocytes in AAA. EP-3-positive cells, that were abundant in the media of NA, decreased in the media of AAA samples according to the depletion of VSMCs in AAA. Nevertheless, many EP-3 immunostained cells were found in the areas of infiltrating leukocytes (not shown).
Fig. 5.
Transcript levels of PGE-receptors (EP) in NA (n = 25) and AAA (n = 86) samples. Points without error bars indicate individual values and points at right are the mean ± SEM. ***P < 0.001 when compared with NA samples.
Fig. 6.
Representative images of serial sections showing coexpression of EP-4 and mPGES-1 in microvessels of a NA and AAA samples. Arrows indicate some immunostained cells in microvessels and in leukocytes. Scale bars: 50 μm.
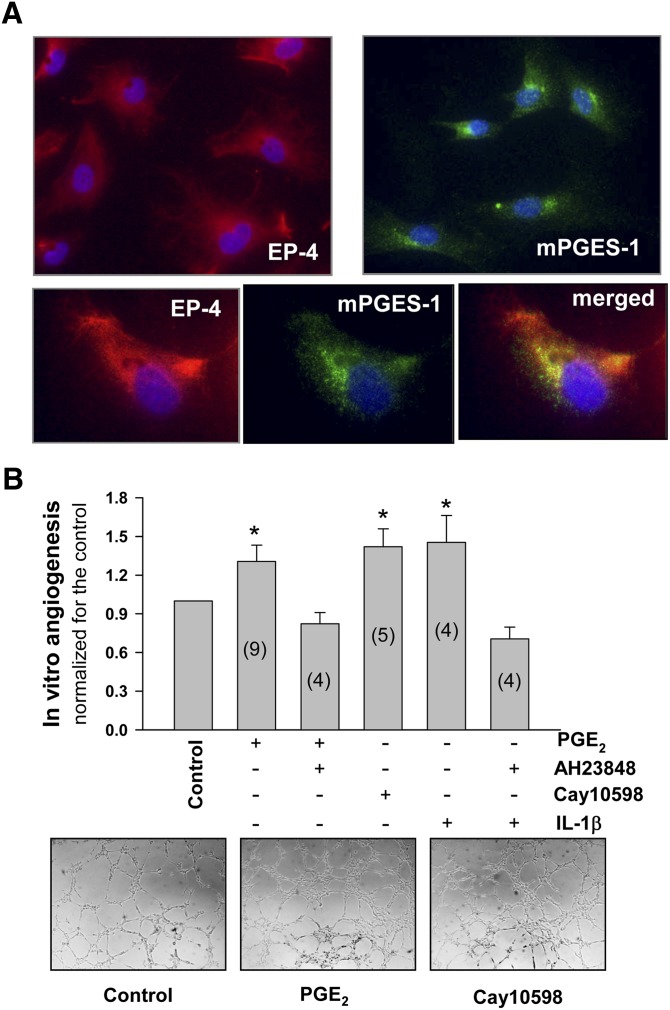
EP-4-activation-mediated in vitro angiogenesis
To test the hypothesis that PGE2-induced angiogenesis was mediated by EP-4, we performed experiments in MVECs. EP-4 was practically the only EP expressed in MVECs in culture (analyzed by RT-PCR; not shown). Both EP-4 and mPGES-1 were coexpressed in MVECs in culture (Fig. 7A). Exogenously added PGE2 and Cay10598 (a specific EP-4 receptor agonist) similarly induced angiogenesis, an effect that was abolished by the EP-4 antagonist AH23848 (Fig. 7B). It is well known that IL-1β induces AAc mobilization in addition to COX-2 and mPGES-1 expression. Therefore, to explore whether the endogenous production of PGE2 by MVECs promoted angiogenesis, we stimulated MVECs with human recombinant IL-1β. IL-1β-induced angiogenesis was also suppressed by AH23848 (Fig. 7B).
Fig. 7.
A: Immunofluorescent staining of MVECs in culture for EP-4 and mPGES-1 (upper panels), and double staining for EP-4 and mPGES-1 (bottom panels). B: Effect of EP-4 activation on in vitro angiogenesis. MVECs were seeded onto Matrigel in 96-well plates and exposed to 10 nmol/l of PGE2 or Cay10598 (an EP-4 agonist). AH23848 (200 nmol/l) was used to block EP-4. For IL-1β assays, cells were preincubated or not for 1 h with human recombinant IL-1β (50 U/ml) in the assay medium before being seeded in Matrigel. After 4 h of treatment, photographs (bottom) were taken and the number of closed polygons in the EC mesh was counted. Shaded bars are the mean ± SEM (expressed as relative to the corresponding controls). Inside the shaded bars in parentheses is the number of independent experiments performed in triplicate. Representative photographs are also shown (bottom panels). *P < 0.05, when compared with controls.
DISCUSSION
This is the first study that analyzes the expression of the PGE2 pathway as a whole in human AAA. The main objectives were: first, to investigate the expression of mPGES-1 in human AAA which has not been previously reported; second, to tentatively approach the contribution of microvascular endothelium to the bioavailability of PGE2 in AAA; and third, to highlight the potential contribution of MVEC-derived PGE2 to the AAA-associated hypervascularization.
vWF immunostaining of aortic biopsies showed notable differences between NA and AAA. NA was characterized by a regular distribution of microvessels that cross the vessel wall from the adventitia to the intima. In contrast, AAA exhibited many vessels parallel to the VSMC fibers in addition to adventitia-to-intima ones. The highest density of infiltrating leukocytes in the media was in perivascular areas of these latter microvessels. The enhanced expression of EC markers in AAA was consistent with our immunochemistry observations and confirms previous reports showing higher vascularization of AAA (4, 5). After stratification by the aortic diameter, the maximal transcript levels of EC markers were found in the LD group, whereas markers used to estimate leukocyte infiltration exhibited a maximum level in the MD group. These results are in agreement with the previous report by McMillan et al. (29), who found the highest MMP-9 expression in moderate-diameter AAA. Assuming that maximum diameter is an approach to the evolutionary stage of the aneurysmatic lesion, collectively our observations allow us to hypothesize that angiogenesis precedes the maximum inflammatory response during AAA development. A similar concept was proposed by Herron et al. (30) regarding levels of proteinases in AAA.
We found that not only the inducible COX-2 but also the constitutive COX-1 were elevated in AAA. Two main factors may influence relative levels of a particular transcript in heterogeneous cell samples; one is an effective up- or downregulation of its expression in one or more cell types present in the sample, and the other is the alteration of the proportion of cell types expressing that transcript. It is well-known that COX-1 is ubiquitously expressed (6, 7). Therefore, the increase of COX-1 in AAA was probably due to the enhanced proportion of cells with high COX-1 expression, such as macrophages. Consistent with this notion, an excellent statistical correlation was observed between COX-1 and CD68 in AAA samples (R = 0.654, P = 2.53 × 10−7). Our results regarding localization of COX-2 showed that it was highly expressed in vascular cells and infiltrating leukocytes in AAA but weakly expressed in NA. These data strongly suggest that in AAA COX-2 was effectively upregulated in vascular cells included MVECs. We found that, even though significant, the increase of mPGES-1 expression in AAA was not as high as that of COX-2 (1.4-fold vs. 4.3-fold, respectively). This suggests a dissimilar regulation of these two enzymes in AAA, as we previously described (8, 9). A factor that could explain the modest increase of mPGES-1 found in AAA is the breakdown of VSMCs in AAA. Indeed, VSMCs abundantly express mPGES-1 (9, 10), and we localized mPGES-1 immunoreactivity in all vascular cells including VSMCs in both NA and AAA. The increase in the expression of PGE2 biosynthetic machinery was also consistent with the higher in vitro production of PGE2 by the AAA tissues, and with the higher levels of PGE2 metabolites detected in the plasma of these patients. Nevertheless, the entire increase in plasma levels of PGE2 may not be coming from the AAA itself, as AAA should be considered a systemic disease of the vasculature (31) and other vascular territories may contribute to the circulating PGE2 levels.
Several reported biological activities of PGE2 account for its involvement in AAA physiopathology among others, its ability to induce the expression of MMPs (32) and to inhibit the production of extracellular matrix components such as fibronectin and collagens type I and III (33). The presence of mPGES-1 in a particular cell is necessary for PGE2 biosynthesis (8–11, 34, 35). In inflammatory diseases it is generally assumed that PGE2 comes from invading leukocytes, mainly macrophages, and from tumor cells in neoplasias. Nevertheless, we have recently shown that stroma cells present in the tumor environment could be a more relevant source of PGE2 than tumor cells themselves (28). Because COX-2 and mPGES-1 were expressed in both, vascular cells and infiltrating cells, macrophages could also be a relevant source of PGE2 in the AAA. Indeed, macrophage COX-2-derived PGE2 has been found to be relevant in the pathogenesis and rupture of AAAs (12, 13, 21, 22). We cannot rule out, however, the contribution of macrophage-associated COX-1 to the PGE2 pool, because we found that COX-1 is increased in AAA, probably due to recruited macrophages as mentioned above.
The contributions of the different cell types present in aneurysmatic tissue to the PGE2 pool is not possible to evaluate directly without seriously modifying tissue samples. As an attempt to approach the contribution of MVECs in the PGE2 produced by AAA samples, we explored the statistical association of PGE2 production with other eicosanoids typically released by vascular cells or by leukocytes, considering its production as an independent variable. A statistical association between two variables could indicate either a causal relationship between them, or that the variation of these parameters has a common cause. PGI synthase is expressed in vascular cells but not in leukocytes, TxA synthase is mainly expressed in macrophages and platelets, while 5-lipoxygenase and its counterpart FLAP are expressed in leukocytes (mainly polymorphonuclear). We observed an excellent statistical association between the secretion of PGE2 and PGI2, a modest association between PGE2 and TxA2, and a lack of association between PGE2 and LTB4. The influence of PGI2 or TxA2 on PGE2 levels is unlikely; rather a common cause should underlie these correlations. Therefore, the biosynthesis of PGE2 in AAA would be associated with cells producing PGI2 (vascular cells), and to a lesser extent with those producing TxA2 (macrophages), suggesting a relevant contribution of MVECs to PGE2 biosynthesis in these tissues. Nevertheless, this circumstantial evidence should be corroborated by further studies.
Because EP receptors are critical in the biological action of PGE2, we explored those receptors in AAA. The role of EP-3 in angiogenesis is not fully understood (36–39), but EP-2 and EP-4 have been reported to be involved in the release of angiogenic factors and/or angiogenesis in different experimental models and pathologies (40–42). We observed that EP-4 was practically the only PGE receptor detected in MVECs in culture and was mostly expressed in aortic microvessels and some infiltrating leukocytes. In addition, exogenous and endogenous PGE2 induced angiogenesis in vitro via EP-4 activation. Coexpression of COX-2, mPGES-1, and EP-4 in MVECs is consistent with a role of MVEC-derived PGE2 in AAA-associated hypervascularization and MVEC-derived PGE2 could be biologically effective in the AAA-associated angiogenesis in an autocrine manner. Hypervascularization, per se, could be a determinant factor in reducing mechanical strength, because it turns the media layer fluffy and favors leukocyte-mediated matrix degradation besides.
In summary, we provide new evidence concerning the expression of mPGES-1 and the contribution that MVECs could have in the biosynthesis of PGE2 in human AAA. Our data allow us to speculate that the COX-2/mPGES-1/EP-4 axis in MVEC is relevant for the PGE2- mediated hypervascularization of AAA from the early stages of human AAA development. Our data are also consistent with reports showing that suppression of either COX-2, mPGES-1, or EP-4 expression reduces AAA development in animal models (15, 16, 19, 20), and reinforce the potential of mPGES-1 and EP-4 as alternative targets for therapy in AAA patients.
Acknowledgments
The authors wish to thank Montserrat Gómez and Iris Rodríguez for assistance in the immunohistochemistry studies.
Footnotes
Abbreviations:
- AAA
- abdominal aortic aneurysm
- AAc
- arachidonic acid
- COX
- cyclooxygenase
- EC
- endothelial cell
- eNOS
- endothelial nitric oxide synthase
- EP
- E-prostanoid receptor
- FLAP
- 5-lipoxygenase-activating protein
- HD
- high diameter
- LD
- low diameter
- LT
- leukotriene
- MD
- moderate diameter
- MMP
- metalloproteinase
- mPGES-1
- microsomal isoform of prostaglandin E synthase
- MVEC
- microvascular endothelial cell
- NA
- normal aorta
- PG
- prostaglandin
- VEGFR2
- vascular endothelial growth factor receptor 2
- VSMC
- vascular smooth muscle cell
- vWF
- von Willebrand factor
This study was supported partially by grants SAF2010/21392, FIS PI12/01952 and Red de Investigación Cardiovascular RD12/0042/0051 and RD12/0042/0053 from the Spanish Ministerio de Economía y Competitividad (MINECO) - Instituto de Salud Carlos III (ISCIII). The authors have no conflicts of interest to declare.
REFERENCES
- 1.Weintraub N. L. 2009. Understanding abdominal aortic aneurysm. N. Engl. J. Med. 361: 1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahlgren C. M., Larsson E., Magnusson P. K. E., Hultgren R., Swadenborg J. 2010. Genetic and enviromental contributions to abdominal aortic aneurysm development in twin population. J. Vasc. Surg. 51: 3–7 [DOI] [PubMed] [Google Scholar]
- 3.Choke E., Cockerill G., Wilson W. R., Sayed S., Dawson J., Loftus I., Thompson M. M. 2005. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur. J. Vasc. Endovasc. Surg. 30: 227–244 [DOI] [PubMed] [Google Scholar]
- 4.Paik D. C., Fu C., Bhattacharya J., Tilson M. D. 2004. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp. Mol. Med. 36: 524–533 [DOI] [PubMed] [Google Scholar]
- 5.Choke E., Thompson M. M., Dawson J., Wilson W. R., Sayed S., Loftus I. M., Cockerill G. W. 2006. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler. Thromb. Vasc. Biol. 26: 2077–2082 [DOI] [PubMed] [Google Scholar]
- 6.Vila L. 2004. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: role in atherosclerosis. Med. Res. Rev. 24: 399–424 [DOI] [PubMed] [Google Scholar]
- 7.Bishop-Bailey D., Mitchell J. A., Warner T. D. 2006. COX-2 in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 26: 956–958 [DOI] [PubMed] [Google Scholar]
- 8.Solà-Villà D., Camacho M., Solà R., Soler M., Diaz J. M., Vila L. 2006. IL-1β induces VEGF, independently of PGE2 induction, mainly through the PI3-K/mTOR pathway in renal mesangial cells. Kidney Int. 70: 1935–1941 [DOI] [PubMed] [Google Scholar]
- 9.Camacho M., Gerbolés E., Escudero J. R., Anton R., García-Moll X., Vila L. 2007. Microsomal-PGE synthase-1, which is not coupled to a particular COX-isoenzyme, is essential for PGE2 biosynthesis in vascular smooth muscle cells. J. Thromb. Haemost. 5: 1411–1419 [DOI] [PubMed] [Google Scholar]
- 10.Soler M., Camacho M., Escudero J. R., Iñiguez M. A., Vila L. 2000. Human vascular smooth muscle cells but not endothelial cells express prostaglandin E synthase. Circ. Res. 87: 504–507 [DOI] [PubMed] [Google Scholar]
- 11.Salvado M. D., Alfranca A., Escolano A., Haeggström J., Redondo J. M. 2009. COX-2 limits prostanoid production in activated HUVECs and is a source of PGH2 for transcellular metabolism to PGE2 by tumor cells. Arterioscler. Thromb. Vasc. Biol. 29: 1131–1137 [DOI] [PubMed] [Google Scholar]
- 12.Holmes D. R., Wester W., Thompson R. W., Reilly J. M. 1997. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. J. Vasc. Surg. 25: 810–815 [DOI] [PubMed] [Google Scholar]
- 13.Walton L. J., Franklin I. J., Bayston T., Brown L. C., Greenhalgh R. M., Taylor G. W., Powell J. T. 1999. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms. Implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 100: 48–54 [DOI] [PubMed] [Google Scholar]
- 14.King V. L., Trivedi D. B., Gitlin J. M., Loftin C. D. 2006. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler. Thromb. Vasc. Biol. 26: 1137–1143 [DOI] [PubMed] [Google Scholar]
- 15.Gitlin J. M., Trivedi D. B., Langenbach R., Loftin C. D. 2007. Genetic deficiency of cyclooxygenase-2 attenuates abdominal aortic aneurysm formation in mice. Cardiovasc. Res. 73: 227–236 [DOI] [PubMed] [Google Scholar]
- 16.Wang M., Lee E., Song W., Ricciotti E., Rader D. J., Lawson J. A., Puré E., FitzGerald G. A. 2008. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 117: 1302–1309 [DOI] [PubMed] [Google Scholar]
- 17.Narumiya S., Sugimoto Y., Ushikubi F. 1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79: 1193–1226 [DOI] [PubMed] [Google Scholar]
- 18.Tang E. H., Shvartz E., Shimizu K., Rocha V. Z., Zheng C., Fukuda D., Shi G. P., Sukhova G., Libby P. 2011. Deletion of EP4 on bone marrow-derived cells enhances inflammation and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 31: 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao R. Y., St Amand T., Li X., Yoon S. H., Wang C. P., Song H., Maruyama T., Brown P. M., Zelt D. T., Funk C. D. 2012. Prostaglandin receptor EP4 in abdominal aortic aneurysms. Am. J. Pathol. 181: 313–321 [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama U., Ishiwata R., Jin M. H., Kato Y., Suzuki O., Jin H., Ichikawa Y., Kumagaya S., Katayama Y., Fujita T., et al. 2012. Inhibition of EP4 signaling attenuates aortic aneurysm formation. PLoS ONE. 7: e36724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapple K. S., Parry D. J., McKenzie S., MacLennan K. A., Jones P., Scott D. J. 2007. Cyclooxygenase-2 expression and its association with increased angiogenesis in human abdominal aortic aneurysms. Ann. Vasc. Surg. 21: 61–66 [DOI] [PubMed] [Google Scholar]
- 22.Cheuk B. L., Cheng S. W. 2007. Differential secretion of prostaglandin (E(2), thromboxane A(2) and interleukin-6 in intact and ruptured abdominal aortic aneurysm. Int. J. Mol. Med. 20: 391–395 [PubMed] [Google Scholar]
- 23.Granström E., Hamberg M., Hansson G., Kindahl H. 1980. Chemical instability of 15-keto-13,14-dihydro-PGE2: the reason for low assay reliability. Prostaglandins. 19: 933–957 [DOI] [PubMed] [Google Scholar]
- 24.Bothwell W., Verburg M., Wynalda M., Daniels E. G., Fitzpatrick F. A. 1982. A radioimmunoassay for the unstable pulmonary metabolites of prostaglandin E1 and E2: an indirect index of their in vivo disposition and pharmacokinetics. J. Pharmacol. Exp. Ther. 220: 229–235 [PubMed] [Google Scholar]
- 25.Brú A., Souto J. C., Alcolea S., Antón R., Remacha A., Camacho M., Soler M., Brú I., Porres A., Vila L. 2009. Tumour cell lines HT-29 and FaDu produce proinflammatory cytokines and activate neutrophils in vitro: possible applications for neutrophil-based anti-tumour treatment. Mediators Inflamm. 2009: 817498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casós K., Siguero L., Fernández-Figueras M. T., León X., Sardá M. P., Vila L., Camacho M. 2011. Tumor cells induce COX-2 and mPGES-1 expression in microvascular endothelial cells mainly by means of IL-1 receptor activation. Microvasc. Res. 81: 261–268 [DOI] [PubMed] [Google Scholar]
- 27.Alfranca A., López-Oliva J. M., Genís L., López-Maderuelo D., Mirones I., Salvado D., Quesada A. J., Arroyo A. G., Redondo J. M. 2008. PGE2 induces angiogenesis via MT1-MMP-mediated activation of the TGFbeta/Alk5 signaling pathway. Blood. 112: 1120–1128 [DOI] [PubMed] [Google Scholar]
- 28.Alcolea S., Anton R., Camacho M., Soler M., Alfranca A., Aviles-Jurado F. X., Redondo J. M., Quer M., Leon X., Vila L. 2012. Interaction between head and neck squamous cell carcinoma (HNSCC) cells and fibroblasts in the biosynthesis of PGE2. J. Lipid Res. 53: 630–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMillan W. D., Tamarina N. A., Cipollone M., Johnson D. A., Parcker M. A., Pearce W. H. 1997. The relationship between MMP-9 expression and aortic diameter. Circulation. 96: 2228–2232 [DOI] [PubMed] [Google Scholar]
- 30.Herron G. S., Unemori E., Wong M., Rapp J. H., Hibbs M. H., Stoney R. J. 1991. Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler. Thromb. 11: 1667–1677 [DOI] [PubMed] [Google Scholar]
- 31.Nordon I. M., Hinchliffe R. J., Loftus I. M., Thompson M. M. 2011. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 8: 92–102 [DOI] [PubMed] [Google Scholar]
- 32.Corcoran M. L., Stetler-Stevenson W. G., Brown P. D., Wahl L. M. 1992. Interleukin 4 inhibition of prostaglandin E2 synthesis blocks interstitial collagenase and 92-kDa type IV collagenase/gelatinase production by human monocytes. J. Biol. Chem. 267: 515–519 [PubMed] [Google Scholar]
- 33.Varga J., Diaz-Pérez A., Rosenbloom J., Jimenez S. A. 1987. PGE2 causes a coordinate decrease in the steady state levels of fibronectin and types I and II procollagen mRNAs in normal dermal fibroblasts. Biochem. Biophys. Res. Commun. 147: 1282–1288 [DOI] [PubMed] [Google Scholar]
- 34.Camacho M., López-Belmonte J., Vila L. 1998. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and PGI-synthase activity. Circ. Res. 83: 353–365 [DOI] [PubMed] [Google Scholar]
- 35.Camacho M., Vila L. 2000. Transcellular formation of thromboxane A2 in mixed incubations of endothelial cells and aspirin-treated platelets strongly depends on the prostaglandin I-synthase activity. Thromb. Res. 99: 155–164 [DOI] [PubMed] [Google Scholar]
- 36.Cattaneo M. G., Pola S., Dehò V., Sanguini A. M., Vicentini L. M. 2003. Alprostadil suppresses angiogenesis in vitro and in vivo in the murine Matrigel plug assay. Br. J. Pharmacol. 138: 377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sennlaub F., Valamanesh F., Vazquez-Tello A., El-Asrar A. M., Checchin D., Brault S., Gobeil F., Beauchamp M. H., Mwaikambo B., Courtois Y., et al. 2003. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 108: 198–204 [DOI] [PubMed] [Google Scholar]
- 38.Shoji Y., Takahashi M., Kitamura T., Watanabe K., Kawamori T., Maruyama T., Sugimoto Y., Negishi M., Narumiya S., Sugimura T., et al. 2004. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut. 53: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh H., Hosono K., Ito Y., Suzuki T., Ogawa Y., Kubo H., Kamata H., Mishima T., Tamaki H., Sakagami H., et al. 2010. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am. J. Pathol. 176: 1469–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seno H., Oshima M., Ishikawa T. O., Oshima H., Takaku K., Chiba T., Narumiya S., Taketo M. M. 2002. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 62: 506–511 [PubMed] [Google Scholar]
- 41.Mutoh M., Watanabe K., Kitamura T., Shoji Y., Takahashi M., Kawamori T., Tani K., Kobayashi M., Maruyama T., Kobayashi K., et al. 2002. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res. 62: 28–32 [PubMed] [Google Scholar]
- 42.Rao R., Redha R., Macias-Perez I., Su Y., Hao C., Zent R., Breyer M. D., Pozzi A. 2007. Prostaglandin E2–EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J. Biol. Chem. 282: 16959–16968 [DOI] [PubMed] [Google Scholar]