Abstract
Aims
Circulating endothelium-derived extracellular vesicles (EV) levels are altered in pulmonary arterial hypertension (PAH) but whether they are biomarkers of cellular injury or participants in disease pathogenesis is unknown. Previously, we found that lung-derived EVs (LEVs) induce bone marrow-derived progenitor cells to express lung-specific mRNA and protein. In this study, we sought to determine whether LEV or plasma-derived EV (PEV) alter pulmonary vascular endothelial or marrow progenitor cell phenotype to induce pulmonary vascular remodelling.
Methods and results
LEV, PEV isolated from monocrotaline (MCT-EV)- or vehicle-treated mice (vehicle-EV) were injected into healthy mice. Right ventricular (RV) hypertrophy and pulmonary vascular remodelling were assessed by RV-to-body weight (RV/BW) and blood vessel wall thickness-to-diameter (WT/D) ratios. RV/BW, WT/D ratios were elevated in MCT- vs. vehicle-injected mice (1.99 ± 0.09 vs. 1.04 ± 0.09 mg/g; 0.159 ± 0.002 vs. 0.062 ± 0.009%). RV/BW, WT/D ratios were higher in mice injected with MCT-EV vs. mice injected with vehicle-EV (1.63 ± 0.09 vs. 1.08 ± 0.09 mg/g; 0.113 ± 0.02 vs. 0.056 ± 0.01%). Lineage-depleted bone marrow cells incubated with MCT-EV and marrow cells isolated from mice infused with MCT-EV had greater expression of endothelial progenitor cell mRNAs and mRNAs abnormally expressed in PAH than cells incubated with vehicle-EV or isolated from vehicle-EV infused mice. MCT-EV induced an apoptosis-resistant phenotype in murine pulmonary endothelial cells and lineage-depleted bone marrow cells incubated with MCT-EV induced pulmonary hypertension when injected into healthy mice.
Conclusions
EV from MCT-injured mice contribute to the development of MCT-induced pulmonary hypertension. This effect may be mediated directly by EV on the pulmonary vasculature or by differentiation of bone marrow cells to endothelial progenitor cells that induce pulmonary vascular remodelling.
Keywords: Pulmonary hypertension, Endothelium, Microparticles, Bone marrow stem/progenitor cells, Monocrotaline
1. Introduction
Pulmonary arterial hypertension (PAH) is a disease characterized by progressive narrowing of the small pulmonary arteries and arterioles due to abnormal cellular proliferation, fibrosis, and in situ thrombosis. Histological changes include hyperplasia and hypertrophy of vascular smooth muscle cells and abnormal proliferation of vascular endothelial cells. The remodelling of the pulmonary vasculature causes a progressive elevation of pulmonary vascular resistance and subsequent right ventricular (RV) failure.
The pathogenesis of PAH is poorly understood. Numerous abnormalities in the expression of vascular growth factors, vasoactive substances and inflammatory mediators have been described, but it is unclear if these alterations are responsible for initiating the disease process or occur in response to it. Most of the increase in pulmonary vascular resistance that is seen in PAH has been attributed to obliterative lesions in the pre-capillary arterioles that are formed by hyperplasia of endothelial cells. These endothelial cells may consist of a monoclonal population of cells that are highly resistant to apoptosis1 but why they are found throughout the pulmonary vasculature is unclear.
Vascular endothelial cells release subcellular extracellular vesicles (EVs) when injured or while proliferating or undergoing apoptosis.2 Circulating EVs are increased in PAH patients and levels correlate with pulmonary vascular resistance,3 functional impairment,4 and mortality.5 Although most of these studies suggest that plasma EV (PEV) levels are a biomarker of disease severity, others suggest that they may contribute to pulmonary vascular abnormalities by induction of endothelial dysfunction. Cultured endothelial cells exposed to PEV isolated from rats with hypoxia-induced pulmonary hypertension (PH) have decreased expression of endothelial nitric oxide synthase (eNOS) and lower nitric oxide production.6 Additionally, ex vivo exposure of aortic and pulmonary artery rings to EV obtained from pulmonary hypertensive rats have impaired endothelium-dependent relaxation.6
Many different subpopulations of subcellular vesicles have been described including microvesicles, microparticles, exosomes, ectosomes, and apoptotic vesicles.7,8 Exosomes are 30–100 nm in diameter and are derived from endocytic vesicles, whereas microvesicles are 100–1000 nm in diameter and are released from the cell surface by membrane blebbing in a calcium flux and calpain-dependent manner. As the majority of investigators use differential ultracentrifugation speeds to isolate subcellular vesicles, most preparations that have been described in the literature are inherently heterogeneous. The more-inclusive term ‘extracellular vesicles’ has been adopted by many to reflect this heterogeneity.9 EVs contain numerous proteins and RNA species and are capable of entering cells and altering protein expression. We have shown that lung tissue-derived EV (LEV) induce the expression of a profile of epithelial genes and proteins in bone marrow-derived progenitor cells (BMPCs).10 BMPCs have been implicated in the pathogenesis of PAH. Bone marrow-derived haemangioblasts give rise to cells of both endothelial and haematopoietic lineages.11 Haemangioblast-derived circulating endothelial progenitor cells (EPCs) are believed to play a central role in neoangiogenesis12 as increased numbers of these cells been observed in the peripheral blood of patients with IPAH compared with healthy controls and their levels correlate directly with pulmonary artery pressure.13 The mechanisms responsible for inducing differentiation of BMPCs into EPCs and the role of EPCs in the pulmonary vascular remodelling associated with PAH are not well understood.
In the present study, we hypothesized that LEV that are released into the circulation by damaged pulmonary vascular endothelial cells (PVECs) in PAH inhibit endothelial cell apoptosis and induce differentiation of BMPCs into EPCs that contribute to the abnormal endothelial cell phenotype throughout the pulmonary circulation. To test this hypothesis, we isolated EV from LEV and PEV of normal mice and mice with monocrotaline (MCT)-induced PH and injected them into healthy mice. We sought to determine whether LEV isolated from MCT-injured mice alter endothelial apoptotic responses and induce BMPC differentiation to EPCs that contribute to pulmonary hypertensive changes in normal mice.
2. Methods
2.1. Experimental animals
Studies were approved by the Institutional Animal Care and Use Committee at Rhode Island Hospital (CMTT# 0211-08) and conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. C57BL/6 mice were used and were euthanized by carbon dioxide inhalation followed by cervical dislocation.
2.2. MCT injury model
Cohorts of mice received weekly subcutaneous injections of MCT (600 mg/kg, Sigma) resuspended in 100 µL of saline or 100 µL of saline only (vehicle) for 4 weeks. Mice were sacrificed 1 week after the last MCT or vehicle injection. Mouse body and RV weights were recorded and blood and lungs were harvested for EV isolation.
2.3. LEV and cardiac EV isolation
Lungs and hearts were dispase-digested (Sigma) and washed with phosphate-buffered saline (PBS) by centrifugation (300 g, 10 min). Lung cells were cultured (106/mL) in Bronchial Epithelial Growth Media (Lonza) and cardiac cells were cultured (106/mL) in DMEM low glucose media (Invitrogen) at 37°C for 7 days. Cells were removed by centrifugation (300 g, 10 min, performed twice) and the supernatant (conditioned media) was used for EV isolation. Conditioned media was ultracentrifuged at 10 000 g (1 h). Pelleted material, containing cellular debris, was discarded and the supernatant was ultracentrifuged at 100 000 g, (1 h). The pelleted material containing EV was resuspended in PBS then ultracentrifuged a second time at 10 000 g (1 h). LEV and cardiac EV were then resuspended in PBS.
2.4. PEV isolation
About 750–1000 µL of blood was collected from mice, placed into sodium citrate-containing tubes and centrifuged (1 900 g, 5 min) to remove cells then platelets (5000 g, 10 min). Platelet-free plasma (PFP) was ultracentrifuged as described above for PEV isolation.
2.5. Injection of EV into healthy mice
Mice were tail vein-injected with vehicle-LEV, vehicle-PEV, MCT-LEV, MCT-PEV, or an equal volume of PBS (control). All mice received a total of 1.5 × 107 EV (given as 5 × 106 EV in 100 µL of PBS once daily for 3 days). Control mice were injected with 100 µL PBS alone once daily for 3 days. Mice were sacrificed 14, 28, or 42 days after the first injection. The dose of EV studied was selected as a way of ensuring that EV infused into one mouse was isolated from one mouse and not from a multiple donor pool. The lowest yield of EV isolated from an EV donor in this study was 1.5 × 107 EV. Therefore, all mice were infused with a total of 1.5 × 107 EV.
In separate experiments, EV isolated from cardiac tissue from vehicle- or MCT-treated mice were injected into cohorts of mice and sacrificed 42 days later. Additionally, two extra cohorts were injected with EV-depleted plasma from vehicle or MCT-injured mice (made by collecting the supernatant formed by PFP ultracentrifugation) and sacrificed 42 days later.
2.6. Isolation of whole bone marrow cells and BMPCs
Whole bone marrow (WBM) was isolated from limb bones and spines of mice. Bones were placed in PBS supplemented with 5% foetal calf serum and crushed with a mortar and pestle. Cells were strained through a 40 µm cell strainer to remove large debris and bone fragments. Cells were then centrifuged twice (300 g, 10 min) and resuspended in PBS.
Mononuclear cells were isolated from WBM by discontinuous density centrifugation (1000 g, 30 min) using OptiPrep (Accurate Chemical). Mononuclear cells were lineage-depleted by adding the following rat-anti mouse antibodies: anti-Ter119, B220, Mac-1, Gr-1, CD4, CD8 (BD Biosciences). Dynabead M450 anti-rat IgG (Dynal) was added and lineage-positive cells were removed using a magnetic column yielding a cell population enriched with BMPCs (lineage-depleted or Lin-cells).
2.7. Studies with WBM and BMPCs
Lin-cells (107/culture well) were incubated in DMEM low glucose media with 1.5 × 107 vehicle-LEV or -PEV, MCT-LEV or -PEV, or an equal volume of PBS (control) for 7 days at 37°C. 5 × 106 Lin-cells incubated with EV or PBS (as above) were infused (tail vein) into lethally irradiated mice (950 centigray, Gammacell 40 Exactor Irradiator, MDS Nordion). Mice were sacrificed 42 days later.
2.8. Endothelial cell studies
C57BL/6 mouse PVECs (Cell Biologics) were incubated for 72 h with EV or an equal volume of PBS (control). EV protein concentration was determined using the BCA protein assay kit (Pierce) and 10 or 20 µg of EV were incubated with 5 × 105 cells. Cells were labelled using the Phycoerythrin (PE) Annexin V Apoptosis Detection Kit (BD Pharmingen) and analysed using an LSR II™ flow cytometer (BD Bioscience). Apoptotic cells (annexin V-PE+, propidium iodide-) were quantified and expressed as a percentage of all endothelial cells.
2.9. Statistical analysis
Data were analysed using Student's t-test when there were fewer than six measurements within two parent groups. Alternatively, the Wilcoxon rank-sum test was performed when there were six or more measurements within two parent groups. P ≤ 0.05 represented statistical significance. Data were presented as mean ± standard error.
3. Results
3.1. PEV and LEV from MCT-treated mice induce RV hypertrophy, pulmonary vascular remodelling in normal mice
MCT-treated mice developed RV hypertrophy and pulmonary vascular remodelling as evidenced by greater RV/BW, WT/D ratios than vehicle-treated mice (Figure 1A and B). Haematoxylin and eosin staining of lung sections from MCT-treated mice revealed muscularization of pulmonary blood vessels (20–50 µm diameter) which was not seen in vehicle-treated mice (Figure 2A and B).
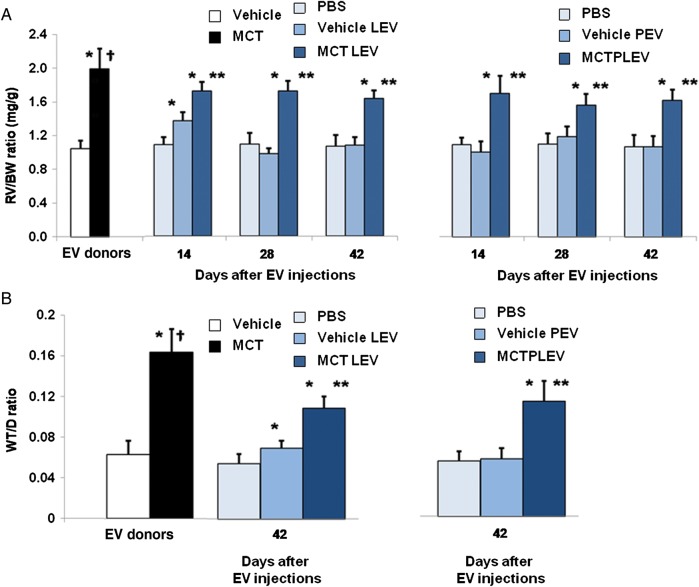
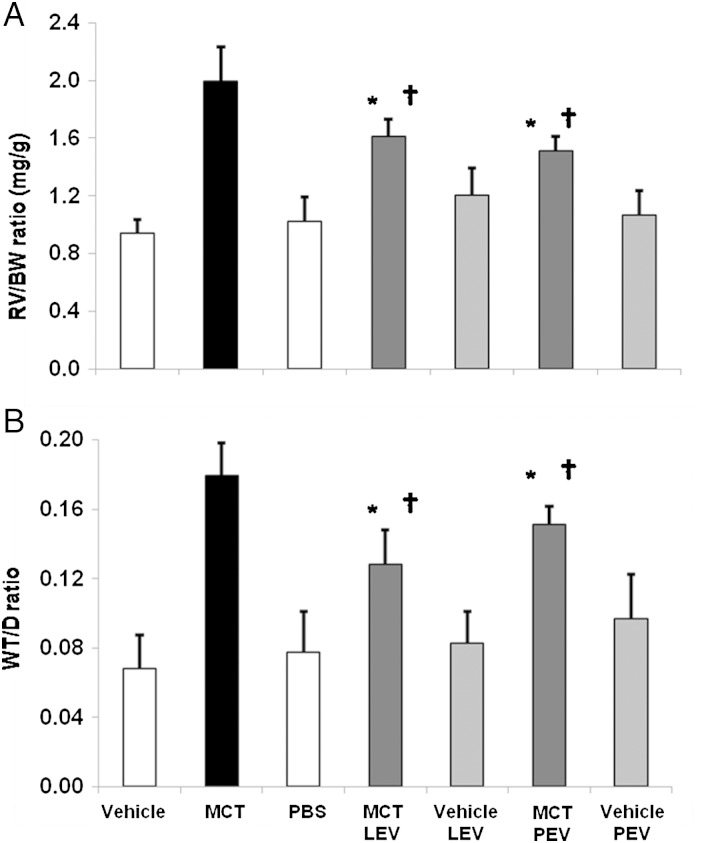
Figure 1.
RV hypertrophy, pulmonary vascular remodelling of mice infused with EV. (A) RV/BW ratios 4 weeks after vehicle, MCT injection (open, solid bars) (n = 8/cohort, P < 0.01, Wilcoxon), and 14, 28, and 42 days after PBS (light blue), LEV or PEV from vehicle- (medium blue) or MCT-injected mice (dark blue) infusion (n = 10/cohort, P < 0.01, Wilcoxon, *MCT- or vehicle-EV vs. PBS mice; **MCT-EV vs. vehicle-EV; †MCT-injected vs. all other groups). (B) WT/D ratios 42 days after infusions (n = 10/cohort, P < 0.01, Wilcoxon, *MCT- or vehicle-EV vs. PBS mice; **MCT-EV vs. vehicle-EV; †MCT-injected vs. all other groups).
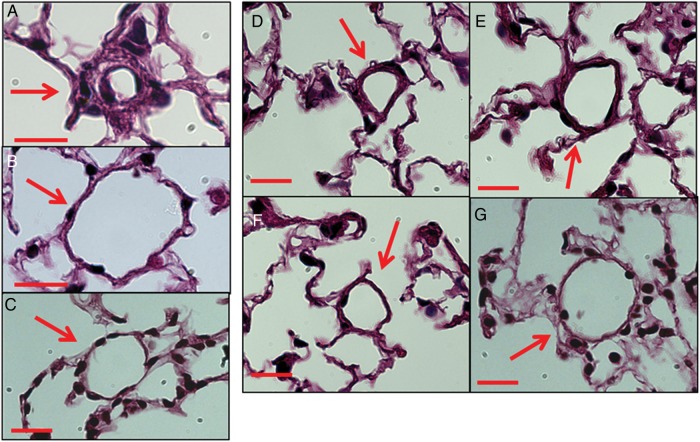
Figure 2.
Pulmonary vascular remodelling of mice infused with EV. Haematoxylin and eosin staining of lung sections 4 weeks after (A) MCT, (B) vehicle (C) PBS, (D) MCT-LEV, (E) MCT-PEV, (F) vehicle-LEV, (G) vehicle-PEV treatment. Red bars = 20 um.
Compared with LEV and PEV isolated from vehicle-treated mice, the concentration, mean size, and size range of LEV and PEV isolated from MCT-injured mice were not different (Supplementary material online, Table S1). EV in the traditional exosome (30–100 nm) and microvesicle (100–1000 nm) size ranges were identified in all EV populations studied. No MCT or MCT metabolites could be detected by liquid chromatography mass spectrometry in MCT-LEV or -PEV or in the plasma of normal mice infused with MCT-EV.
Mice given vehicle-LEV had slightly greater RV/BW, WT/D ratios than mice given PBS 14 days after injection, but no differences in RV/BW, WT/D were seen at Days 28 or 42 (Figure 1A and B). Mice given MCT-LEV had significantly higher RV/BW, WT/D ratios than mice given PBS or vehicle-LEV 14, 28, and 42 days after injection (Figure 1A and B). Muscularization of small, normally non-muscularized pulmonary blood vessels was observed in vehicle-LEV and MCT-LEV injected mice but not in PBS mice (Figure 2C–F). The per cent of muscularized pulmonary blood vessels under 35 µm in diameter was greater in mice treated with MCT-LEV than in mice given vehicle-LEV or PBS (62.2, 8.7, 0%, respectively, P < 0.05, data not shown).
Mice given MCT-PEV had significantly higher RV/BW, WT/D ratios than those given vehicle-PEV or PBS alone at 14, 28, and 42 days post-injection (Figure 1A and B). There were no differences in RV/BW, WT/D ratios between vehicle-PEV- and PBS-treated mice at any time point studied. Mice infused with MCT-PEV, but not vehicle-PEV or PBS, developed muscularization of peripheral pulmonary blood vessels (Figure 2C–G); 87.8% of pulmonary blood vessels under 35 µm in diameter in MCT-PEV recipients were at least partially muscularized compared with, none in vehicle-PEV- and PBS-treated mice (data not shown). No evidence of intravascular thrombosis or emboli was seen in EV-treated mice.
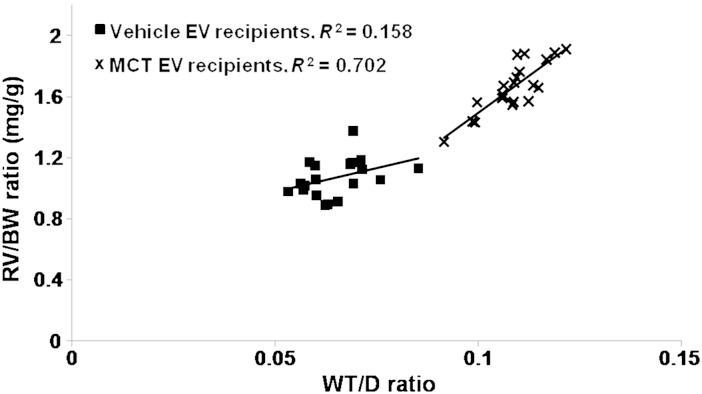
Linear regression analysis revealed a strong positive correlation between RV/BW and WT/D ratios in MCT-LEV, -PEV-treated mice, but not in vehicle-LEV, -PEV-treated mice (Figure 3) indicating that increases in the RV mass were associated with pulmonary vascular remodelling.
Figure 3.
Correlation between RV/BW, WT/D ratios. Linear regression analysis of RV/BW, WT/D ratios in vehicle-LEV, PEV-infused mice (r2 = 0.158, n = 10/cohort) and MCT-LEV, PEV-infused mice (r2 = 0.702, n = 10/cohort).
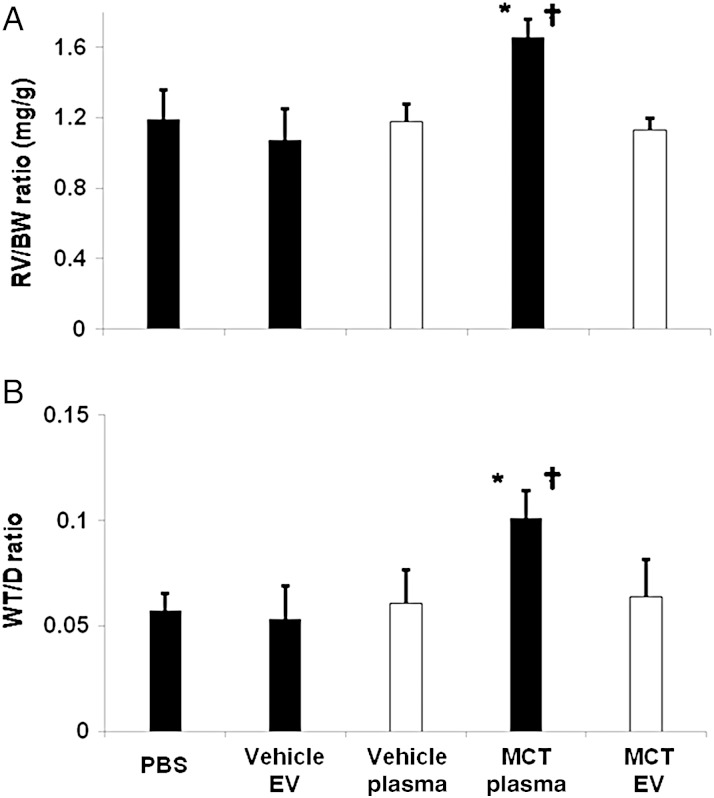
In additional experiments, healthy mice were injected with EV isolated from the hearts of vehicle-treated and MCT-injured mice or were injected with EV-depleted plasma from MCT- or vehicle-treated mice. However, neither cardiac EVs (data not shown) nor EV depleted plasma obtained from MCT-injured mice (Figure 4A and B) resulted in an increase RV/BW or WT/D ratios when injected into healthy mice.
Figure 4.
EV-depleted plasma does not induce RV hypertrophy, pulmonary vascular remodelling. (A) RV/BW, (B) WT/D ratios of mice infused with PBS, PEV from vehicle mice (Vehicle EV), EV-free plasma from vehicle mice (Vehicle Plasma), PEV from MCT-treated mice (MCT EV), and EV-free plasma from MCT-treated mice (MCT Plasma) (n = 3/cohort, P < 0.05, t-test; *MCT-EV vs. PBS; †MCT-EV vs. MCT Plasma).
3.2. mRNA content of EV and gene expression of BMPCs incubated with EV
Microarray analysis revealed higher levels of mRNA species uniquely expressed in endothelial cells (PECAM-1, e-selectin, endoglin, CD143, VE-cadherin, CD34) and higher levels of mRNA species known to be abnormal in PAH (PDGF, BMPII receptor, eNOS) in MCT-LEV compared with vehicle-LEV. The mRNA content of MCT-LEV was similar to that of mRNA isolated from whole lung tissue of MCT-injured mice (data not shown). The mRNA content of MCT-PEV did not differ from vehicle-PEV, other than higher levels of CD34 mRNA (Supplementary material online, Figure S1A).
To determine whether MCT-EV were capable of affecting the differentiation and gene expression of BMPCs in vitro, Lin-bone marrow cells, a population enriched with BMPCs, were incubated with MCT-EV and vehicle-EV. Expression of genes typically expressed by EPCs (CD133, CD34, c-kit, CXCR4) was higher in Lin-cells incubated with MCT-LEV, MCT-PEV compared with levels in cells incubated with vehicle-LEV, -PEV (Supplementary material online, Figure S1B). Expression of endothelial cell genes (e-selectin, endoglin, VE cadherin, CD143) was also higher in cells incubated with MCT-LEV, -PEV. Higher expression of genes that are typically up-regulated in PAH (IL-6, PDGF, endothelin-1, erythropoietin receptor) and lower or no expression of genes that are typically down-regulated in PAH (eNOS, BMPII receptor) were seen in cells incubated with MCT-EV compared with vehicle-EV (Supplementary material online, Figure S1B).
3.3. Proteomic and miRNA microarray analysis of EV
Several proteins typically identified in exosomes, including a variety of tetraspanins (CD9, CD81), multivesicular body formation proteins (alix, clatherin) and membrane trafficking proteins (RAS-related proteins) were present in all EV populations studied. In addition, proteins typically identified in microvesicles, including CD40 ligand, β1 integrin, and P-selectin, were also present in all EV populations studied (Supplementary material online, Table S2). A similar number of distinct proteins were found in LEV, PEV from MCT- and vehicle-treated mice (313, 302, 331, 339 for MCT-LEV, vehicle-LEV, MCT-PEV, vehicle-PEV). Forty-one LEV-based proteins were unique to MCT-treated mice and 30 were unique to vehicle-treated mice. Fifty-six PEV-based proteins were unique to MCT-treated mice and 64 were unique to vehicle-treated mice. Compared with vehicle-LEV, -PEV, 19 protein species were up-regulated at least two-fold in both MCT-LEV, -PEV, and 20 were down-regulated at least two -fold. Eighteen proteins were identified in both MCT-LEV and -PEV that were not found in vehicle-LEV or -PEV (Table 1). Compared with vehicle-PEV, MCT-PEV had a marked increase in the proportion of endothelial cell-derived proteins and a decrease in the proportion of platelet cell-derived proteins. A similar trend was seen with LEV as there were more endothelial cell proteins and fewer platelet proteins in MCT-LEV compared with vehicle-LEV (Supplementary material online, Figure S2). Endothelial proteins present in MCT-PEV but not in vehicle-PEV included CD54 (ICAM-1). Endothelial proteins present in MCT-LEV but not in vehicle-LEV included CD62e (E-selectin) and CD144 (VE-Cadherin).
Table 1.
Proteins, miRNA isolated from LEV, PEV from MCT-injured mice
| Up-regulated greater than two-fold vs. vehicle LEV, PEV | Down-regulated greater than two-fold vs. vehicle LEV, PEV | Unique to MCT-LEV, PEV |
|---|---|---|
| Proteins | ||
| Alpha-mannosidase 2 | 26S protease regulatory subunit 4 | Alanyl-tRNA synthetase, cytoplasmic |
| Barrier-to-autointegration factor | 3-Ketoacyl-CoA thiolase A, peroxisomal | Calcium-binding mitochondrial carrier protein SCaMC-1 |
| Carboxypeptidase M | 6-Phosphogluconate dehydrogenase | CDP-diacylglycerol-inositol 3-phosphatidyltransferase |
| Carnitine O-palmitoyltransferase | Acyl-coenzyme A oxidase | cGMP-specific 3′,5′-cyclic phosphodiesterase |
| Dihydrolipoyl dehydrogenase | Cadherin-1 | Complement component 1 |
| DnaJ homologue subfamily C member 18 | Calcium uniporter protein | Dermatopontin |
| EF-hand domain-containing family member A1 | Carbonic anhydrase 14 | Glutathione S-transferase omega-1 |
| Eukaryotic translation initiation factor 6 | Deoxyribonuclease-1 | Hydroxymethylglutaryl-CoA lyase, mitochondrial |
| Glycosaminoglycan xylosyl kinase | Dipeptidyl peptidase 2 | Low-density lipoprotein receptor-related protein 2 |
| Immunoglobulin superfamily member 10 | Hsp90 co-chaperone Cdc37 | Mannan-binding lectin serine protease 2 |
| MARCKS-related protein | Lymphatic vessel endothelial hyaluronic acid receptor 1 | Mitochondrial import receptor subunit TOM22 |
| Monocarboxylate transporter 10 | Prefoldin subunit 5 | Myeloid bactenecin (F1) |
| Protein QIL1 | Protein phosphatase 1 regulatory subunit 7 | Myeloperoxidase |
| Protein-cysteine N-palmitoyltransferase HHAT protein | Protein S100-A9 | NADH dehydrogenase (ubiquinone) 1 alpha |
| SH3 domain-binding glutamic acid-rich-like protein 3 | Ras-related protein Rab-27B | Phosphoenolpyruvatecarboxykinase (GTP) |
| Na+, Cl−dependent amino acid transporter B | Serine/threonine-protein kinase | Pleckstrin homology domain-containing family O |
| Solute carrier family 2, facilitated glucose transporter | T-complex protein 1 subunit epsilon | Protein disulfide-isomerase-TMX3 |
| Succinate dehydrogenase (ubiquinone) | Transmembrane protein 119 | Ras-related protein Rab-18 |
| Translocation protein SEC62 | Transmembrane protein 176B | |
| Tubulin polymerization-promoting protein | ||
| miRNA | ||
| miR-34a | miR-99b | miR-10b |
| miR-145 | miR-100 | miR-698 |
| miR-328 | miR-125a | miR-743b |
| miR-381 | miR-132 | |
| miR-451 | miR-340 | |
| miR-467c | miR-350 | |
| miR-546 | ||
MicroRNA microarray analysis revealed a similar number of miRNA species in LEV and PEV from MCT- and vehicle-treated mice (224, 215, 241, 278 for MCT-LEV, vehicle-LEV, MCT-PEV, vehicle-PEV, respectively). Of these, six were up-regulated at least two -fold in both MCT-LEV, -PEV compared with levels found in vehicle-LEV, vehicle-PEV, seven were down-regulated at least two -fold, and three were identified that were unique to both MCT-LEV and -PEV (Table 1).
3.4. Effect of EV injection on circulating EV and bone marrow cells of recipient mice
Thirty minutes after tail vein injection into normal mice, fluorescently tagged EV could be detected in small pulmonary vessels throughout the pulmonary vasculature. PKH26-labelled EVs were seen adjacent to PVECs, suggesting uptake of exogenous EV by the pulmonary microcirculation (Supplementary material online, Figure S3). There was no difference in the number of PHK26-labelled EV in the pulmonary vasculature of recipient mice after MCT-EV, vehicle-EV infusion.
Similar to PEV obtained from MCT-treated mice, PEV isolated from mice 28 days after MCT-LEV treatment contained increased levels of mRNA for e-selectin, VE-cadherin, CD143, CD31, and CD34 (endothelial cells) as well as IL-6, IL-1 receptor, endothelin-1, PDGF, and eNOS, compared with PEV isolated from vehicle-LEV-treated mice. These changes were most pronounced 28 days after MCT-EV infusion and were greater in MCT-LEV-infused mice than in MCT-PEV-infused mice (Supplementary material online, Figure S4A).
Bone marrow cells isolated from MCT-EV-infused mice had increased expression of genes typically expressed by EPCs compared with vehicle-EV-treated mice. Fourteen days after EV infusion, CD133, CD34, c-kit, and CXCR4 gene expression was higher in the bone marrow cells of MCT-PEV-, -LEV-infused mice compared with bone marrow cells of vehicle-PEV-, -LEV-infused mice. Expression of this profile of EPC-associated genes declined 28 days after EV infusion and returned to baseline by Day 42. Bone marrow cell expression of endothelial cell genes and genes believed to be important in the pathogenesis of PAH was similar in MCT-EV- and vehicle-EV-infused mice (Supplementary material online, Figure S4B).
3.5. Effect of EV incubation on endothelial cells and transplanted BMPCs
To determine whether MCT-EV had a direct effect on endothelial cell growth, MCT-EV and vehicle-EV were incubated with murine PVECs for 72 h. Significantly fewer apoptotic (annexin V+/propidium iodide-) cells were identified in PVEC incubated with MCT-EV compared with PVEC incubated with an equal amount of vehicle-EV or PBS (Figure 5A and B).
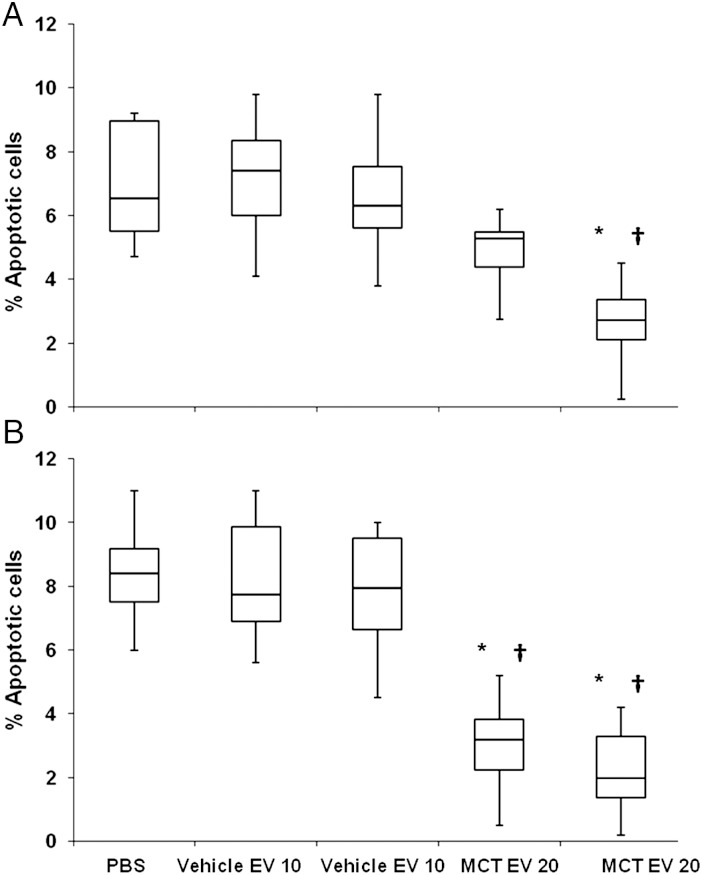
Figure 5.
Apoptotic PVECs after incubation with EV. Annexin V positive/propidium iodide negative cells expressed as a percentage of all endothelial cells, 72 h after incubation with (A) PBS, vehicle- or MCT-LEV, 10 or 20 µg (B) PBS, vehicle- or MCT-PEV, 10 or 20 µg (n = 6 incubations/group, P < 0.01, Wilcoxon, *MCT-EV vs. PBS, †P < 0.01 MCT-EV vs. vehicle-EV).
To determine whether MCT-EV could induce pulmonary vascular remodelling via their effects on BMPCs alone, BMPCs incubated with EV were injected into healthy mice. Fluorescence microscopy of BMPCs that had been incubated with PKH26-labelled EV then washed extensively with PBS revealed only EV contained within BMPC at the time of injection, indicating that EV not associated with cells were not transferred with transplanted cells (Supplementary material online, Figure S5). Mice injected with cells incubated with vehicle-EV had no evidence of pulmonary vascular remodelling 42 days after injection. In contrast, RV/BW, WT/D ratios were significantly increased in mice injected with cells incubated with MCT-EV compared with mice given cells incubated with PBS or vehicle-EV (Figure 6A and B).
Figure 6.
RV hypertrophy, pulmonary vascular remodelling of mice infused with Lin-cells incubated with EV. (A) RV/BW, (B) WT/D ratios of vehicle and MCT-injured mice (vehicle, MCT; n = 6/cohort) and mice (n = 6/cohort) 42 days after transplantation of Lin-cells incubated with MCT or vehicle-LEV, MCT or vehicle-PEV or PBS (P < 0.01, Wilcoxon rank, *MCT-LEV vs. PBS mice, †P < 0.01 MCT-EV vs. vehicle-EV).
4. Discussion
We hypothesized that EVs from MCT-injured mice play a pathogenic role in the pulmonary vascular remodelling of MCT-induced PH, in part by inducing differentiation of bone marrow cells into EPCs that home to the lung and contribute to pulmonary vascular remodelling. We found that healthy mice injected with LEV or PEV from MCT-injured mice developed PH as evidenced by increases in RV mass and pulmonary vascular wall thickness. These changes were detected at all three time points studied in mice receiving either LEV or PEV. Persistent increases in RV mass and pulmonary vascular remodelling were not observed in mice injected with LEV or PEV obtained from control mice. The greater WT/D in mice given MCT-EV compared with mice given vehicle-EV or PBS was associated with increased muscularization of peripheral pulmonary arteries. Finally, the degree of RV hypertrophy correlated with the degree of pulmonary vascular remodelling in the MCT-EV treated mice as evidenced by the close correlation between RV mass and pulmonary vascular wall thickness.
Pulmonary hypertensive changes detected in mice given MCT-EV were not attributable to residual MCT or its metabolites. Mass spectrometry failed to detect MCT or any of its metabolites in MCT-treated mice or in the plasma of normal mice infused with MCT-EV. Furthermore, injection of EV-depleted plasma obtained from mice with MCT-induced PH did not induce changes in RV mass or pulmonary vascular remodelling, suggesting that the pulmonary hypertensive changes observed were the result of the EV, not other elements in the plasma.
The mechanism by which MCT-EVs induce PH in healthy mice is unclear. EVs are increasingly regarded as important mediators of cell-to-cell communication and elevated circulating levels of EV have been observed in many disease states,14 including PAH. EVs contain numerous DNA, RNA, and protein species and are capable of inducing changes in transcription, translation and surface protein expression in cells with which they come in contact.15 Our laboratory has previously demonstrated that EVs can transfer genetic material from murine lung cells to bone marrow cells in vitro and induce expression of lung-specific mRNA and protein.10 Studies using transcriptional blocking agents suggest that the transfer of an EV-based transcription factor induces changes cellular phenotype.16 These findings are supported by the stable, long-term expression of lung-specific mRNA and protein in marrow cells cultured with LEV.17
In the present study, MCT-injured mouse EV induced increased expression of EPC and mature endothelial cell genes in a cell population enriched with BMPCs, in vitro, compared with cells incubated with vehicle-treated mouse EV. Genetic expression of numerous proteins that have been implicated in PAH-associated pulmonary vascular remodelling (IL-6, endothelin-1, erythropoietin receptor) was up-regulated in BMPCs incubated with MCT-EV, but not vehicle-EV. Furthermore, injection of MCT-EV but not vehicle-EV into healthy mice resulted in increased expression of the same endothelial cell markers in the bone marrow of the recipient mice. These findings demonstrate that circulating and LEV from mice with MCT-induced PH induce an EPC phenotype in BMPCs.
Under certain conditions, EPCs have been shown to attenuate pulmonary vascular remodelling and blunt PH development. Clinical trials of genetically programmed EPCs have been developed for PAH treatment. However, under other conditions, EPCs may contribute to the pathogenesis of PH. Recent studies have demonstrated that circulating EPCs are increased in PAH and that EPCs isolated from PAH patients have a hyperproliferative phenotype that contributes to pulmonary vascular remodelling.18,19 The mechanism(s) responsible for the generation of EPCs with a hyperproliferative phenotype and their recruitment to the lung is not understood. In the present study, EVs obtained from pulmonary hypertensive mice-induced BMPC up-regulation of endothelial markers and growth factors implicated in the pathogenesis of PAH. Injection of these cells into healthy mice caused RV hypertrophy and increased muscularization of pulmonary vessels, suggesting a potential mechanism by which EVs may induce pulmonary vascular remodelling via induction of bone marrow-derived EPCs. Such a mechanism could act to continually stimulate the release of EPCs that contribute to pulmonary vascular remodelling in PAH.
This hypothesis is further supported by additional experiments in this study in which EPC-enriched BMPCs that had been incubated with EV were transplanted into healthy mice. Cells incubated with MCT-EV, but not vehicle-EV, induced significant pulmonary vascular disease as evidenced by increases in RV/BW, WT/D ratios when transplanted into healthy mice. These findings demonstrate that circulating EV in pulmonary hypertensive mice can induce changes in EPC-enriched BMPCs that can lead to pulmonary hypertensive changes.
EVs from pulmonary hypertensive mice were also found to have a direct anti-apoptotic effect on PVECs. Incubation of PVECs with MCT-EV reduced the number of apoptotic cells to less than a third of that seen in control cells. Considering that fluorescently labelled EVs were found in the pulmonary vasculature 30 min after tail vein injection, the anti-apoptotic effect of these EV may contribute to the development of a population of apoptotic-resistant PVECs. Apoptotic-resistant endothelial cells are felt to play an important role in the development of obliterative vascular lesions in PAH1 and may contribute to the pulmonary vascular remodelling seen in EV-induced PH.
We found that EVs used in this study are heterogeneous in nature. Proteins characteristic of exosomes and microvesicles could be identified in all EV populations. Additionally, the size range of these EV was from 30 to 423 nm, further supporting the presence of both vesicle subpopulations in our preparations. EVs used in this study appear to be derived from a variety of cell sources. Microarray analysis of EV focusing on a limited panel of mRNA species supports this notion. Although MCT-LEV had higher levels of endothelial cell mRNA (PECAM-1, E-selectin, endoglin, CD143, VE-cadherin, CD34) compared with vehicle-LEV, no such differences could be seen in MCT-PEV and vehicle-PEV. However, proteomic analyses revealed more endothelial cell and fewer platelet-derived proteins in MCT-LEV and MCT-PEV compared with vehicle-LEV and vehicle-PEV. In particular, e-selectin and VE-Cadherin were found in MCT-LEV but not vehicle-LEV and ICAM-1 was found in MCT-PEV but not vehicle-PEV. The relative abundance of endothelial cell-derived proteins has been reported by others to be present in circulating EV of patients with PAH compared with healthy controls.3–5 Although the MCT-induced PH did not affect the total level of circulating EV, these data strongly suggest that MCT-induced PH increased the number of EV derived from endothelial cells.
Determination of the particular characteristics of pulmonary hypertensive EVs that are responsible for inducing pulmonary vascular remodelling in healthy mice is difficult due to the large quantities of EV-based protein and RNA. In the present study, MCT-PEV induced the same degree of PH in healthy mice as MCT-LEV, suggesting that the most important mediators of EV-induced PH were common to both types of EV. Extensive proteomic analysis revealed greater than two-fold increase or decrease in a number of proteins in both MCT-LEV and -PEV compared with vehicle-LEV and vehicle-PEV. There were also many proteins present in MCT-EV that were not found in vehicle-EV. Most of these proteins are enzymes that regulate glucose uptake, mitochondrial electron transfer, fatty acid metabolism, or other aspects of cellular respiration. Numerous metabolic derangements have been described in PAH,20 most notably a shift in cellular respiration from glucose oxidation to glycolysis that occurs despite an adequate source of oxygenation—the so-called Warberg effect.21
MCT-EV also had significantly higher levels of phosphodiesterase-5 (PDE-5), the major metabolic enzyme responsible for cGMP metabolism in pulmonary vascular smooth muscle cells. Increased pulmonary expression and activity of PDE-5 is the major rationale for the development of PDE-5 inhibitor therapy for the treatment of PAH. Considering that endothelium-dependent vasodilation is mediated by cGMP, EV-induced increases in PDE-5 expression may also contribute to previous observations of decreased endothelial-dependent vasorelaxation in pulmonary arterial rings following incubation with EV from rats with hypoxic PH.22 Furthermore, cGMP induces apoptosis in PVECs and this process is attenuated by PDE-5.23 Thus, the increase levels of PDE-5 in MCT-EV may explain their observed in vitro anti-apoptotic effects.
MCT-LEV and -PEV also showed altered expression of a variety of miRNAs that appear to be involved in pulmonary vascular remodelling. Of the six miRNA species that were found to be greater than two-fold up-regulated in both MCT-LEV and -PEV, two (miR-145, -451) have been found to be increased in hypoxia and MCT models of PH and implicated in its pathogenesis. A third, miR-328, has been shown to suppress the insulin growth factor-1 receptor, ultimately leading to apoptosis of pulmonary arterial smooth muscle cells, but is down-regulated the lungs in hypoxic PH.24 miR-10b was not seen in vehicle-EV but was present in both MCT-LEV and -PEV. miR-10b is an important modulator of EPC-mediated vascular angiogenesis in tumour cells and is up-regulated by VEGF in endothelial cells.25 VEGF-mediated pulmonary vascular angiogenesis is an important feature of the pulmonary vascular remodelling associated with PAH. Together, these findings suggest that MCT-EV carry multiple miRNAs that are capable of mediating abnormal vascular responses and have recently been found to contribute to the pathogenesis of PAH.
We chose to use MCT in the present study because it is a well-established model of PH in rodents. It is possible that the findings of the present study represent a unique feature of MCT-induced PH. However, evidence of EV-mediated pulmonary hypertensive changes have also been observed in models of hypoxic PH, and further studies are presently underway to determine whether EV from animals with other models of PH can reproduce the disease in healthy animals. Injected EV and EV-modified bone marrow cells may also affect vascular changes in the systemic as well as the pulmonary circulation. This possibility was not directly evaluated in the present studies, but we found no evidence of left ventricular hypertrophy in mice given MCT-LEV or MCT-PEV, suggesting that systemic arterial pressure did not increase significantly.
In summary, findings from the present study demonstrate for the first time that PH can be induced in healthy mice by injection of EV obtained from pulmonary hypertensive mice and that this transfer of disease is mediated by EVs. EVs from MCT-injured mice induce pulmonary vascular remodelling and RV hypertrophy similar to that caused by MCT injection. MCT-EVs contain increased levels of protein and miRNA species that have been associated with the pathogenesis of PAH and induce in BMPCs the expression of a profile of genes suggestive of EPCs. MCT-EVs inhibit apoptosis of PVECs and induce changes in EPC-enriched BMPCs that result in pulmonary vascular remodelling when transplanted into healthy mice. We hypothesize that in PH, EVs from MCT-injured mice contribute to the proliferation of apoptotic-resistant pulmonary endothelial cells and the differentiation of BMPCs into EPCs that home to the lung and induce pulmonary vascular remodelling. The ability of MCT-EV to induce PH in healthy mice strongly implicates a role for EV in the pathogenesis of MCT-induced PH and may represent a potential new target for the development of better biomarkers and therapies for pulmonary hypertensive diseases in human patients.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This project was supported by the National Institutes of Health (HL086868 to J.A., GM103468 to P.Q., HL088328 to J.K.).
Supplementary Material
References
- 1.Lee S-D, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest. 1998;101:927–934. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19:121–127. doi: 10.1097/MED.0b013e32835057e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, et al. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–1275. doi: 10.1164/rccm.200710-1458OC. [DOI] [PubMed] [Google Scholar]
- 4.Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:536–543. doi: 10.1164/rccm.200706-840OC. [DOI] [PubMed] [Google Scholar]
- 5.Amabile N, Heiss C, Chang V, Angeli FS, Damon L, Rame EJ, et al. Increased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J Heart Lung Transplant. 2009;28:1081–1086. doi: 10.1016/j.healun.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Tual-Chalot S, Guibert C, Muller B, Savineau JP, Andriantsitohaina R, Martinez MC. Circulating microparticles from pulmonary hypertensive rats induce endothelial dysfunction. Am J Respir Crit Care Med. 2010;182:261–268. doi: 10.1164/rccm.200909-1347OC. [DOI] [PubMed] [Google Scholar]
- 7.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3.22.1–3.22.29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 8.Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicle. 2013;2:1–3. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25:2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribatti D, Vacca A, Roncali L, Dammacco F. Hematopoiesis and angiogenesis: a link between two apparently independent processes. J Hematother Stem Cell Res. 2000;9:13–19. doi: 10.1089/152581600319577. [DOI] [PubMed] [Google Scholar]
- 12.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 13.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol. 2008;172:615–627. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enjeti AK, Lincz LF, Seldon M. Microparticles in health and disease. Semin Thromb Hemost. 2008;34:683–691. doi: 10.1055/s-0028-1104547. [DOI] [PubMed] [Google Scholar]
- 15.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 16.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38:233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aliotta JM, Pereira M, Amaral A, Dooner MS, Sears EH, Brilliant K, et al. Persistence of microvesicle-induced bone marrow cell phenotypic changes in long term in vitro and in vivo transplantation models. J Extracell Vesicle. 2012;1:18163. - http://dx.doi.org/10.3402/jev.v1i0.18163 . [Google Scholar]
- 18.Fadini GP, Avogaro A, Ferraccioli G, Agostini C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J. 2010;35:418–425. doi: 10.1183/09031936.00112809. [DOI] [PubMed] [Google Scholar]
- 19.Smadja DM, Mauge L, Sanchez O, Silvestre JS, Guerin C, Godier A, et al. Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J. 2010;36:1284–1293. doi: 10.1183/09031936.00130809. [DOI] [PubMed] [Google Scholar]
- 20.Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, et al. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ. 2012;2:201–213. doi: 10.4103/2045-8932.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:260–266. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tual-Chalot S, Guibert C, Muller B, Savineau JP, Andriantsitohaina R, Martinez MC. Circulating microparticles from pulmonary hypertensive rats induce endothelial dysfunction. Am J Respir Crit Care Med. 2010;182:261–268. doi: 10.1164/rccm.200909-1347OC. [DOI] [PubMed] [Google Scholar]
- 23.Zhu B, Strada S, Stevens T. Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L196–L206. doi: 10.1152/ajplung.00433.2004. [DOI] [PubMed] [Google Scholar]
- 24.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 25.Plummer PN, Freeman R, Taft RJ, Vider J, Sax M, Umer BA, et al. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013;73:341–352. doi: 10.1158/0008-5472.CAN-12-0271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.