Abstract
Wolbachia are maternally inherited endosymbiotic bacteria of arthropods and nematodes. In arthropods, they manipulate the reproduction of their hosts to facilitate their own spread in host populations, causing cytoplasmic incompatibility, parthenogenesis induction, feminization of genetic males and male-killing. In this study, we investigated Wolbachia infection and studied wsp (Wolbachia surface protein) sequences in three wasp species associated with the unisexual galls of A. mukaigawae with the aim of determining the transmission mode and the reason for multiple infections of Wolbachia. Frequency of Wolbachia infected populations for A. mukaigawae, Synergus japonicus (inquiline), and Torymus sp. (parasitoid) was 75%, 100%, and 100%, respectively. Multiple Wolbachia infections were detected in A. mukaigawae and S. japonicus, with 5 and 8 Wolbachia strains, respectively. The two host species shared 5 Wolbachia strains and were infected by identical strains in several locations, indicating horizontal transmission of Wolbachia. The transmission potentially takes place through gall tissues, which the larvae of both wasps feed on. Furthermore, three recombination events of Wolbachia were observed: the strains W8, W2 and W6 apparently have derived from W3 and W5a, W6 and W7, W4 and W9, respectively. W8 and W2 and their respective parental strains were detected in S. japonicus. W6 was detected with only one parent (W4) in S. japonicus; W9 was detected in Torymus sp., suggesting horizontal transmission between hosts and parasitoids. In conclusion, our research supports earlier studies that horizontal transmission of Wolbachia, a symbiont of the Rickettsiales order, may be plant-mediated or take place between hosts and parasitoids. Our research provides novel molecular evidence for multiple recombination events of Wolbachia in gall wasp communities. We suggest that genomic recombination and potential plant-mediated horizontal transmission may be attributable to the high levels of multiple Wolbachia infections observed in A. mukaigawae and S. japonicus.
Introduction
Wolbachia are maternally inherited endosymbiotic bacteria of the family of Anaplasmataceae and infect filarial nematodes and arthropods, including insects, mites, spiders and isopods [1], [2]. In arthropods, these intracellular bacteria can manipulate host reproduction to enhance their own spread throughout host populations, causing cytoplasmic incompatibility, parthenogenesis induction, feminization of genetic males and male-killing [3]. Recent survey of Wolbachia incidence estimated that 66% of insect species are infected with Wolbachia [4]. Furthermore, a number of studies have shown that multiple strains of Wolbachia can infect a host species in various insect groups, e.g., parasitic wasps [5], fruit flies [6], ants [7], [8], gall wasps [9], beetles [10], [11], and butterflies [12]. It is difficult to explain multiple Wolbachia infection by vertical transmission alone, and obviously some species can acquire new infection through horizontal transmission and recombination [7].
Vertical transmission from parents to offspring through the egg cytoplasm has previously been considered to be the main transmission mode of Wolbachia [2]. However, phylogenetic incongruence between hosts and their Wolbachia suggests Wolbachia horizontal transmission among arthropods [5], [13]. Laboratory studies demonstrated that Wolbachia were transmitted artificially from infected to uninfected individuals by microinjection [14]–[17]. One particular also reported frequent observation of Wolbachia being naturally transmitted horizontally from infected to uninfected wasp larvae sharing a common butterfly egg [18].
The four known mechanisms of horizontal transmission of Wolbachia include host-parasitoid association, blood contact, feeding relationship, and common usage of same plant tissues. Transmission through host-parasitoid relationship is probably the main form of horizontal transmission. Several studies have found the host and the parasitoid to be infected by the same Wolbachia strain in Drosophila-parasitoid communities [13], [19], [20]. Brief contact of the blood between infected and wounded uninfected woodlice had obviously caused horizontal transmission [21]. Infection through feeding relationship was suggested by the observation that closely related Wolbachia strains in hoppers and mirid bugs feeding on the same hopper eggs [22]. Plant tissue mediated horizontal transmission of Wolbachia was supported by several studies. One study reported that individuals of two leafhopper species feeding on the same mulberry leaf substrate were infected with the same Wolbachia strain [23]. Another found that four taxonomically diverse insect species feeding on the same pumpkin leaf substrate harbored the same Wolbachia strain [24]. In addition, it has been demonstrated that insect symbiotic Rickettsia could be transferred from whiteflies Bemisia tabaci to the phloem of the leaves of host plants, and be subsequently acquired by other B. tabaci individuals [25].
Recombination in Wolbachia was considered to be rare or nonexistent [2], but recent studies have indicated that recombination in Wolbachia is by no means as rare as previously thought. The first case of recombination was reported between two Wolbachia strains in a fly host and its parasitoid wasps [26]. Additional recombination events of Wolbachia were later observed in Armadillidium vulgare [27], Byturus tomentosus [11], Formica exsecta [7], and Rhagoletis cerasi [28]. Moreover, recombination may occur between Wolbachia strains belonging to different supergroups [11], [29], [30].
Cynipidae (Hymenoptera, Cynipoidea) is a phytophagous insect family consisting of six tribes, i.e., Aylacini, Eschatocerin, Diplolepidini, Pediaspidili, Cynipini, and Synergini [31]. Species of Aylacini, Eschatocerini, Diplolepidini, Pediaspidili, and Cynipini are all gall makers and induce structurally complex and morphologically diverse galls on the host plants. In contrast, species of Synergini are mostly inquiline and are unable to induce gall, but live as obligate guest inhabitant of the gall induced by the gall-inducing host, deriving all nutrients from the gall tissue initiated by the host [32]. Wolbachia are known to infect quite a number of species in the gall inducing tribes of Aylacini [33], Diplolepidini [34], [35], and Cynipini [36]–[38], as well as in Synergini [9].
Cynipid galls are modified plant structures consisting of live tissues and are fed on by both the gall inducer and the inquiline wasps inhabiting the same galls, and thus can potentially serve as media for horizontal transmission of Wolbachia between the two. Frequently a large number of parasitic wasps, often belonging to multiple species, were reared from cynipid galls [39], [40]. These parasitic wasps may parasitize either one or both kinds of the gall inhabitants, potentially allowing for horizontal transmission of Wolbachia between the parasitoids and their hosts. A combination of the two horizontal transmission mechanisms, host-parasitoid association and common usage of the same plant tissues, may result in the infection of same Wolbachia strains across all three trophic levels and multiple infections of the same Wolbachia strains in some individuals of any of the three species, allowing for genomic recombination between strains. Therefore, cynipid galls and their associated insect communities form ideal systems for studying horizontal transmission of Wolbachia between involved species [9] and genomic recombination between Wolbachia strains.
Andricus mukaigawae of the tribe Cynipini is widely known from Japan, Korea, Russia and India [41], [42]. Like many species of Cynipini, A. mukaigawae exhibits heterogony, with alternating bisexual and unisexual generations, which induce galls on host leaves and buds, respectively [41]. Very high Wolbachia infection rates were reported for the unisexual generation in two populations of A. mukaigawae in Hinoharu and Nose, Japan [36]. The unisexual galls of A. mukaigawae are also inhabited and fed on by the inquiline species Synergus japonicus [43]. In addition, a parasitic species, Torymus sp. (Torymidae, Chacidoidea, Hymenoptera), was reared from the unisexual galls, although it is not clear whether it attacks the gall inducer or the inquiline, or both. Nonetheless, S. japonicus and Torymus sp. were not previously known to be infected by Wolbachia, and hence horizontal transmission was unheard of in the system.
In this study, we investigate the possible horizontal transmission of Wolbachia between A. mukaigawae (gall inducer), S. japonicus (inquiline) and Torymus sp. (parasitoid) associated with the unisexual galls of A. mukaigawae. We employed a PCR-based method with Wolbachia specific gene markers [44] for detection of Wolbachia infection in the adults of all three species reared from galls collected from eight locations in southern China. We subsequently sequenced the detected Wolbachia isolates and compared the sequences to study the possible relationships among them. We found Wolbachia infection in all three species, and identified nine distinct Wolbachia strains in the various populations of the sampled wasp communities. We then discussed the implications of our findings for the high levels of multiple Wolbachia infection observed in the A. mukaigawae communities.
Materials and Methods
Ethics Statement
The sampling of living material involved in our experiments included three species of wasps, i.e., A. mukaigawae, S. japonicus, and Torymus sp., associated with galls on the oak species Quercus fabri Hance. All sampling locations are not privately owned or protected. Neither the oak nor the wasp species are endangered or otherwise protected, and therefore no specific permits were required for these locations/activities.
Collection and Rearing of Galls
Unisexual galls of A. mukaigawae on Quercus fabri Hance were collected from eight locations in southern China through September–December in 2010 and 2011 (Table 1 and Figure 1). The galls were reared in a climate chamber set at room temperature. Adults of A. mukaigawae emerged in December 2010 through February 2011 and in December 2011 through February 2012. S. japonicus and Torymus sp. emerged in mid-March through early May, 2012. All adults were killed live within 2 days after emergence and preserved in 100% ethanol at −40°C.
Table 1. Sample location, gall types, sex ratio and infection frequency of Wolbachia in three wasp species associated with the unisexual galls of A. mukaigawae.
| Location (code) | Latitude/Longitude | No. of gall(Type I/II/III †) | Species | No. offemale/male | Infectionfrequency of Wolbachia (%) | No. ofindividuals tested |
| Anqing, Anhui (AQ) | 30. 42°N/116. 27°E | 148(70/29/49) | A. mukaigawae ‡ | 52/0 | 30 | 30 |
| S. japonicus | 118/32 | 60 | 30 | |||
| Torymus sp. | 18/6 | 100 | 10 | |||
| Changde, Hunan (CD) | 29. 58°N/111.38°E | 7(5/0/2) | A. mukaigawae | 5/0 | 0 | 5 |
| S. japonicus | –‡ | – | – | |||
| Yueyang, Hunan (YY) | 29. 15°N/113. 12°E | 77(30/24/23) | A. mukaigawae | 27/0 | 40 | 15 |
| S. japonicus | 49/25 | 50 | 30 | |||
| Changsha, Hunan (CS) | 28. 06°N/112. 52°E | 29(18/9/2) | A. mukaigawae | 18/0 | 40 | 15 |
| S. japonicus | 74/18 | 60 | 25 | |||
| Loudi, Hunan (LD) | 27. 45°N/112. 20°E | 93(30/43/20) | A. mukaigawae | 29/0 | 33 | 15 |
| S. japonicus | 70/29 | 60 | 20 | |||
| Shaoyang, Hunan (SY) | 26. 37°N/110. 32°E | 118(68/29/21) | A. mukaigawae | 62/0 | 40 | 25 |
| S. japonicus | 73/40 | 70 | 20 | |||
| Torymus sp. | 26/8 | 100 | 10 | |||
| Jian, Jiangxi (JA) | 26. 34°N/114. 10°E | 50(17/19/14) | A. mukaigawae | 12/0 | 0 | 10 |
| S. japonicus | 60/20 | 60 | 25 | |||
| Shaoguang, Guangdong(SG) | 25. 13°N/113. 35°E | 48(35/8/5) | A. mukaigawae | 29/0 | 40 | 20 |
| S. japonicus | 33/7 | 60 | 25 |
Gall types, I: A. mukaigawae galls; II: S. japonicus galls; III: other galls.
indicates no wasp was reared.
A. mukaigawae is the gall-inducer and the host, S. japonicas is an inquiline, or gall parasite, and Torymus sp. is a parasitoid species, whose exact host is not clear, may be either of A. mukaigawae or S. japonicas, or both.
Figure 1. The galls of A. mukaigawae on Quercus fabri Hance.
A: Young gall. B: Monolocular unisexual gall of A. mukaigawae; arrow points to the larva of A. mukaigawae. C: Multilocular gall of S. japonicus; arrow points to the adults of S. japonicus. D: Gall destroyed by various insects.
DNA Extraction and Wolbachia Screening
5–30 female adults of each of the three wasp species were picked randomly for each local gall associated community for detection of Wolbachia infection (Table 1). The insects were washed in sterile water to avoid cross-contamination before DNA extraction. Total DNA was extracted from each individual using SDS/proteinase K digestion and phenol-chloroform extraction method as previously described [37].
We screened for Wolbachia by polymerase chain reaction (PCR) with the Wolbachia-specific primers wsp-81F and wsp-691R that amplify a 575–625 bp fragment of wsp gene encoding Wolbachia surface protein [44]. The amplification was conducted using a MJ thermal cycler in a 50 µl reaction volume: 5 µl 10× PCR buffer, 1 µl Taq polymerase (2 U/µl), 2 µl dNTPs (2.5 mM each), 2 µl forward and reverse primer (10 µM), 2 µl DNA template and 36 µl sterile water. The wsp-PCR cycling conditions were: 5 min at 95°C, followed by 35 cycles of 30 s at 95°C, 45 s at 52°C, 1 min at 72°C, and a final elongation step of 15 min at 72°C. All PCR screenings were performed with positive and negative controls to check for contamination. Positive samples were prepared from known Wolbachia-infected strains of Dryocosmus kuriphilus [37]. Negative samples were Wolbachia-free strains of D. kuriphilus and ddH2O. 5 µl of PCR products were run on 1% agarose gel and electrophoresis was performed in 0.5× TBE buffer. Gels were stained with Ethidium Bromide and visualized under UV transilluminator.
Sequencing and Phylogenetic Analyses
Three individuals were picked from each Wolbachia-positive population to sequence the wsp fragment directly from purified PCR products using PCR primers. The appearance of multiple peaks in a sample through initial sequencing was taken as indication of multiple Wolbachia infection, and the wsp fragments were then sequenced for 10 different clones of the same sample to confirm multiple Wolbachia infection. To do this, PCR products of the wsp gene were purified using the V gene gel extraction kit and ligated directly into the pMD18-T cloning vector (BGI tech., Beijing, China) following the manufacturer’s protocols. For each sample, 3–5 independent positive colonies were isolated and cultured in Lysogeny broth (LB) medium fortified with ampicillin. Plasmids were extracted and partially sequenced in both directions using an ABI 377 DNA sequencer with M13F/R and BigDye Terminator sequencing ready kit (BGI tech, Beijing, China).
The final sequence dataset consisted of 180, 210, and 60 wsp sequences from A. mukaigawae, S. japonicas, and Torymus sp., respectively. All wsp sequences were aligned together using the CLUSTAL X with default settings [45]. The third hypervariable region (518–581 bp) of the sequences was removed from the analysis because it could not be confidently aligned [44], [46]. Modeltest version 2.1 was used to find the most appropriate model of molecular evolution via the Akaike Information Criterion [47]. The GTR+G model was selected as the best fit model for the dataset. The dataset was then analyzed using maximum likelihood (ML) method implemented in PAUP 4.0 b [48]. A neighbor-joining (NJ) tree was also generated in MEGA 4.0 [49] using Kimura 2-parameter distance model and gamma shape parameters chosen by Modeltest. Both ML and NJ trees were subjected to 1000 bootstrap replicates to assess branch support.
Typing and Genetic Distances of Wolbachia Strains
Genetic distances between all sequence pairs were calculated using Kimura 2-parameter distance model with Complete Deletion option in MEGA 4.0. Wolbachia strains with genetic distances smaller than 1.5% were defined as identical strains according to previously described method [24], [44]. The 9 distinct wsp sequences identified were designated as W1–W9, and were deposited in GenBank under accession number KC130968–KC130977.
Recombination Analysis of Wolbachia Strains
Six recombination detection methods implemented in the RDP3 program [50] were for identification of recombinant sequences and breakpoints: RDP, GENECONV, BootScan, Maxchi, Chimaera, and 3Seq. The default settings were used for all methods, and the highest acceptable P value cut-off was set to 0.05. The third hyper variable region of the wsp sequences was included in all analyses.
Results
Wolbachia Infection in A. mukaigawae Communities
A total of 570 unisexual galls of A. mukaigawae were collected from eight locations during September–December 2010 and 2011. The galls were classified into three types: (I) normal A. mukaigawae galls with single larval chamber; (II) S. japonicus modified galls with multiple larval chambers; (III) undeveloped small galls and galls destroyed by various insects (Table 1 and Figure 1).
A total of 234 A. mukaigawae females were acquired from eight populations. Six out of the eight sampled populations (75%) of A. mukaigawae were infected with Wolbachia, with infection frequencies varying between 30% and 40%; the two uninfected populations were from Changde and Jian. A total of 477 females and 171 males of S. japonicus were reared from seven of the eight locations. All seven populations of S. japonicus (100%) were infected with Wolbachia, with infection frequencies varying between 50% and 70%. 44 females and 14 males of Torymus sp. were reared from galls collected in Anqing and Shaoyang, with 100% Wolbachia infection frequency.
Typing and Multiple Infections of Wolbachia in A. mukaigawae Communities
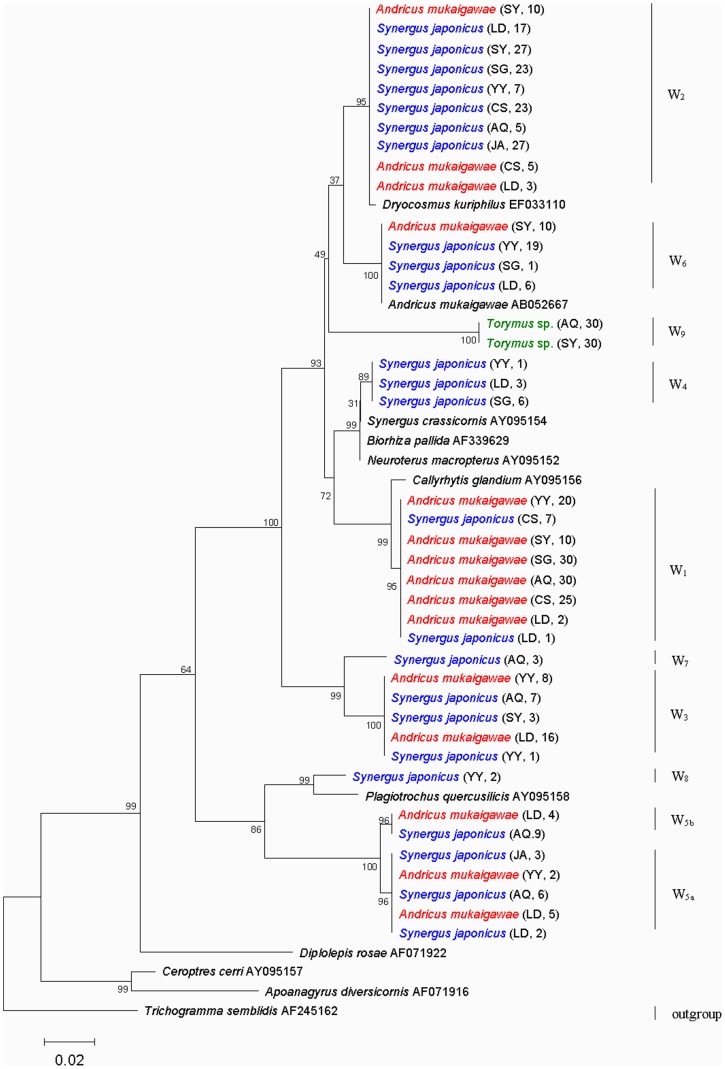
We acquired a total of 450 wsp sequences, including 180 from A. mukaigawae, 210 from S. japonicas, and 60 from Torymus sp. The phylogenetic relationship among all Wolbachia wsp sequences in A. mukaigawae communities were analyzed using neighbor-joining (NJ) algorithm in MEGA and maximum likelihood algorithm (ML) in PAUP. The resulting NJ tree and the ML tree were very similar overall (Figure 2 and Figure S1), and so we only presented the NJ trees here. As shown in the tree, the A. mukaigawae wsp sequences formed five distinct clusters, and those of S. japonicus formed eight distinct clusters (Figure 2). Each cluster represented a distinct strain, and the result clearly revealed multiple Wolbachia infections in both species (Figure 2).
Figure 2. Neighbor-joining tree for Wolbachia strains of A. mukaigawae, S. japonicus and Torymus sp. based on wsp sequence.
A. mukaigawae, S. japonicus and Torymus sp. are shown in red, blue and green, respectively. W1–W9 represent Wolbachia strains in A. mukaigawae communities. The abbreviations AQ, YY, CS, LD, SY, JA and SG in parentheses indicate the sampled populations shown in Table 1. The number following AQ, YY, CS, LD, SY, JA and SG indicate the amount of Wolbachia strains per population. Numbers above branches are bootstrap values computed from 1000 replications. Wolbachia from Trichogramma semblidis was used as outgroup.
Nine strains based on genetic distance, designated herein as W1 to W9, were identified in the wasp communities (Table 2 and Figure 2). As described above, the genetic distances between all sequence pairs were calculated in MEGA using Kimura 2-parameter model with Complete Deletion option, and pairs of Wolbachia strains with wsp genetic distances of 1.5% or greater were considered as distinct strains. The mean pairwise genetic distance of wsp sequences is 13.2%, with highest between W8 and W9 (21.1%) and the lowest between W4 and W6 (3.0%). Although W5a and W5b did not qualify as distinct strains under the 1.5% genetic distance criterion, W5b differed from W5a by having a distinct 24 bp insertion between positions 418 and 442. In addition, W6 was identical to the strain found from A. mukaigawae in the two Japanese populations [36].
Table 2. Pairwise distances between wsp consensus sequences calculated on nucleotide level†.
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | |||
| W5a | W5b | ||||||||||
| W1 | |||||||||||
| W2 | 0.082 | ||||||||||
| W3 | 0.113 | 0.103 | |||||||||
| W4 | 0.050 | 0.059 | 0.104 | ||||||||
| W5 | W5a | 0.190 | 0.182 | 0.197 | 0.188 | ||||||
| W5b | 0.182 | 0.182 | 0.197 | 0.188 | 0.011 | ||||||
| W6 | 0.054 | 0.054 | 0.123 | 0.030 | 0.180 | 0.182 | |||||
| W7 | 0.111 | 0.066 | 0.047 | 0.106 | 0.207 | 0.207 | 0.126 | ||||
| W8 | 0.158 | 0.153 | 0.072 | 0.164 | 0.121 | 0.128 | 0.175 | 0.110 | |||
| W9 | 0.135 | 0.098 | 0.159 | 0.123 | 0.196 | 0.196 | 0.118 | 0.154 | 0.211 | ||
All gaps were deleted and homogenous patterns among lineages and uniform rates among sites assumed. Kimura 2-parameter model was applied for nucleotides and distance for nucleotides was given below the diagonal.
Distribution of Wolbachia Strains in Different Geographic Populations of A. mukaigawae Communities
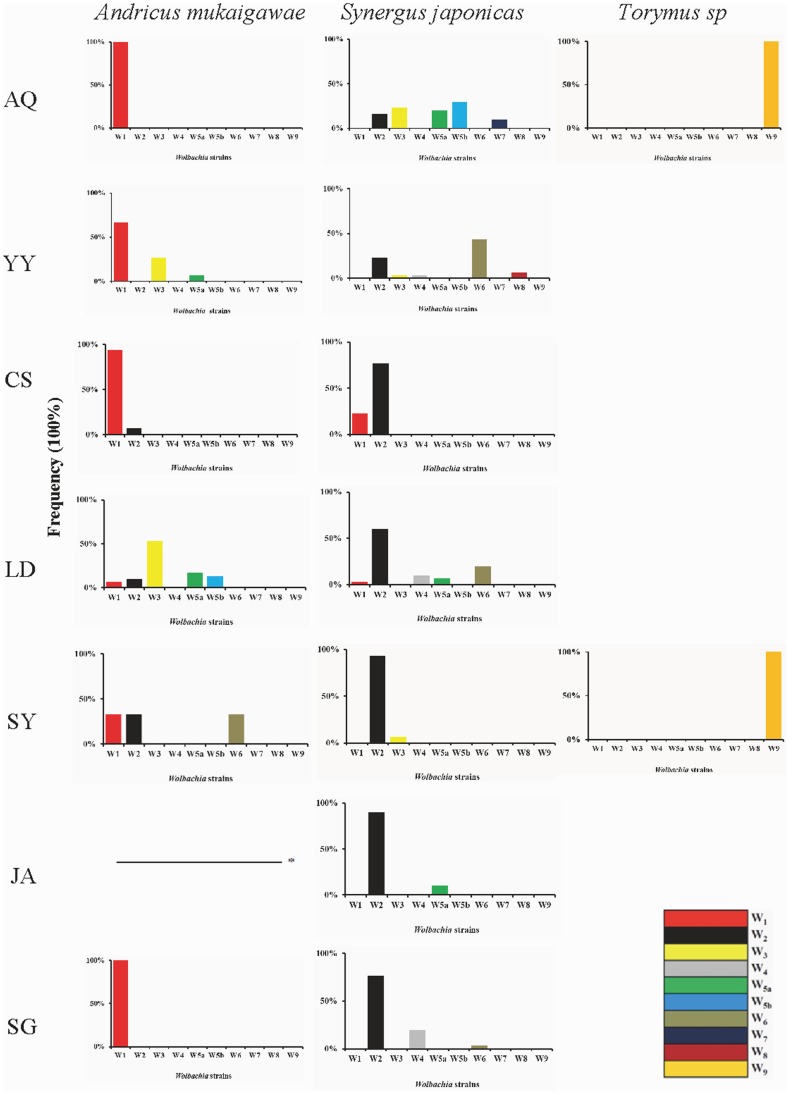
The distribution of the nine Wolbachia strains in the seven geographic populations of A. mukaigawae communities is shown in Figure 3. A. mukaigawae was infected with strains W1, W2, W3, W5a, W5b and W6, of which W1 was the most common, infecting all the six populations of A. mukaigawae that had Wolbachia infection at all. The Anqing and Shaoguang populations had only single infection (W1), whereas multiple infections were found in all other Wolbachia infected populations - 3 strains (W1, W3, and W5a) in Yueyang population, 2 (W1, W2) in Changsha, 5 (W1, W2, W3 W5a, W5b) in Loudi, and 3 (W1, W2, W6) in Shaoyang (Table S1). The inquiline species S. japonicus was infected with the Wolbachia strains W1, W2, W3, W4, W5a, W5b, W6, W7 and W8, of which W2 was the most common, being present in all seven Wolbachia infected populations, and W7 and W8 were the rarest, infecting only the Anqing and Yueyang populations, respectively (Table S2). All Wolbachia infected populations of S. japonicus had multiple infections - Anqing population was infected with 5 strains (W2, W3, W5a, W5b, W7), Yueyang with 5 (W2, W3, W4, W6, W8), Changsha with 2 (W1, W2), Loudi with 5 (W1, W2, W4, W5a, W6), Shaoyang with 2 (W2, W3), Jian with 2 (W2, W5a), and Shaoguang with 3 (W2, W4, W6). In contrast, Wolbachia in Torymus sp. populations were not nearly as diverse - the only detected strain was W9, in the Anqing and Shaoyang populations.
Figure 3. Diversity and distribution pattern of Wolbachia strains in three wasp species in different locations.
The abbreviations AQ, YY, CS, LD, SY, JA and SG indicate sampled populations shown in Table 1. Dash line (–) indicates no Wolbachia infection detected. On the horizontal axis are Wolbachia strains and the vertical axis represents the frequency of Wolbachia strains in different locations in three wasp species.
Noticeably several Wolbachia strains, including W1, W2, W3, W5a, W5b and W6, were shared by A. mukaigawae and S. japonicas, being W1 and W2 in Changsha, W1, W2 and W5a in Loudi, and W3 in Yueyang.
Recombination of wsp Gene between Wolbachia Strains in A. mukaigawae Communities
Recombination analysis of the aligned wsp sequences suggests three recombination events of Wolbachia strains in A. mukaigawae communities (Table 3, Figure S2–S4). For each recombination event, the breakpoints and probabilities varied depending on the method used, and we only present the result of a method with the highest probability (Figure S2–S4).
Table 3. Recombination analysis of Wolbachia wsp gene using 6 methods implemented RDP package.
| Recombinant | Major parent† | Minor parent | Beginning/Ending breakpoint(in alignment) | Method | P-value |
| W8 | W5a | W3 | – | RDP | – |
| – | GENECONV | – | |||
| 42/339 | BootScan | 1.260E-10 | |||
| 42/339 | Maxchi | 1.100E-13 | |||
| – | Chimaera | – | |||
| – | 3Seq | ||||
| W6 | W9 | W4 | 7/254 | RDP | 3.525E-10 |
| – | GENECONV | – | |||
| – | BootScan | – | |||
| 57/245 | Maxchi | 4.379E-10 | |||
| 57/253 | Chimaera | 2.113E-09 | |||
| 14/253 | 3Seq | 1.221E-15 | |||
| W2 | W6 | W7 | – | RDP | – |
| 120/575 | GENECONV | 1.060E-08 | |||
| 120/575 | BootScan | 7.443E-10 | |||
| 120/575 | MaxChi | 9.871E-10 | |||
| 120/560 | Chimaera | 4.636E-10 | |||
| 120/577 | 3Seq | 2.777E-13 |
Major parent: parent contributing the larger fraction of sequence; Minor parent: parent contributing the smaller fraction of sequence.
Strain W8 was detected as a recombinant by 2 of the 6 used methods: BootScan (P<10−9), Machi (P<10−12) (Table 3). Its major and minor parents were W5a and W3, respectively. BootScan analysis showed it to be more closely related to W5a at the region positions 1–42 (100% identity) and 340–603 (99.6% identity) and more closely related to W3 in the recombinant region (positions 43–339) (99.3% identity) (Figure S2).
Strain W6 was detected as a recombinant by four methods: RDP (P<10−9), Maxchi (P<10−9), Chimaera (P<10−8), 3Seq (P<10−14) (Table 3). The major and minor parents are W9 and W4, respectively. Chimaera analysis showed it to be more closely related to W9 at positions 1–57 (93.0% identity) and 254–564 (100.0% identity) while being more closely related to W4 in the recombinant region (positions 58–253) (98.0% identity) (Figure S3).
Strain W2 was detected as a recombinant by five methods: GENECONV (P<10−7), BootScan (P<10−9), Machi (P<10−9), Chimaera (10−9), 3Seq (P<10−12) (Table 3). Its major and minor parents are W6 and W7, respectively. Results of the GENECONV analysis showed it to be more closely related to W7 at the region positions 1–120 (98.3% identity) and 576–585 (100% identity) while being more closely related to W6 in the recombinant region (positions 121–575) (99.3% identity) (Figure S4).
Discussion
The Cynipidae comprises about 1400 described species and is the second most species-rich group of gall inducers after the gall midge family Cecidomyiidae (Diptera) [39], [51]. Recent studies have revealed Wolbachia infection in diverse cynipid species, including 4 species in Aylacini, 8 in Cynipini, 11 in Diplolepidini and 6 in Synergini [9], [33]–[38]. From 8 locations in southern China where we collected the unisexual galls, high levels of Wolbachia infection were revealed in the insect communities associated with these galls, occurring in 6 out of 8, 7 out of 7, and 2 out of 2 populations of A. mukaigawae, S. japonicas, and Torymus sp, respectively.
Multiple Infections of Wolbachia in A. mukaigawae and S. japonicus
The high levels of multiple Wolbachia infections found in A. mukaigawae and its associated inquiline S. japonicus, with 5 and 8 Wolbachia strains, respectively, are rather noticeable and unheard of in cynipid wasps [34]. In fact, the high levels of multiple infections comparable to what we found in the A. mukaigawae associated communities are infrequent among insects in general, although it is relatively common for host insects to be infected with two or three Wolbachia strains [5], [52]. The only other species known to us with similar high levels of multiple infections of Wolbachia include 3 species of ants, i.e., Doronomyrmex pacis (infected with 6 strains) [53], Acromyrmex octospinosus (4 strains) [8] and Formica exsecta (5 strains) [7], and one species of tephritid fruitfly Bactrocera ascita (5 strains) [54]. This phenomenon may be attributable to the increased possibility of horizontal transmission and gene recombination due to the special niche relationship between the gall inducer and the inquiline.
Frequent Recombination Events of Wolbachia in the A. mukaigawae Communities
The three recombinant strains identified in our study and their respective presumed parent strains (in parenthesis) are W8 (W3 & W5a), W2 (W6 & W7), and W6 (W4 & W9) (Figure S2–S4) (Table 3). For each of W8 (W3 & W5a) and W2 (W6 & W7) families, the recombinant strain and both parent strains were all found in some populations of S. japonicus, while no single population of S. japonicus were found to harbor all three of either of the two recombinant families. This may be due to potential historical losses and relatively low frequency of the recombinants and/or the parent strains in natural populations. For W6 (W4 & W9) recombinant family, only the recombinant strain W6 and the parent strain W4 were detected in S. japonicus; the other parental strain W9 was only detected in the parasitic wasps Torymus sp. Several studies have showed it is possible for Wolbachia to be horizontally transferred between hosts and their associated parasitoids [5], [13], [55]. Since parasitoid attack is normally fatal [56], the direction of Wolbachia horizontal transmission should be unidirectional from host to parasitoid. In our system, W9 may have historically infected S. japonicus, and subsequently been transferred from S. japonicus to Torymus sp. after a recombination event with W4. We did not detect W9 in S. japonicus, either because of potentially historical loss. Overall, our study for the first time provides molecular evidence supporting wsp gene recombination of Wolbachia in cynipid gall wasps, and is in line with findings of recent studies on Wolbachia infecting other arthropod groups as diverse as ants [7], Fig wasps [57] and spiders [58].
Recombination plays an important role in the evolution of bacteria in general and Wolbachia in particular, in ways similar to sexual reproduction of the majority of higher animals and plants [59]. Vertical transmission, the main transmission mode of Wolbachia, results in the accumulation of deleterious mutations while providing a stable and effective way of transmission. Recombination, on the other hand, can create new recombinants, resulting in strains without the deleterious mutations and increased genetic diversity of Wolbachia strains, allowing them to utilize diverse hosts, a phenomenon well documented in pathogenic bacteria [60], [61].
Plant-mediated Horizontal Transmission of Wolbachia between A. mukaigawae and S. japonicus
Some host species with multiple Wolbachia strains may acquire additional infections through horizontal transmission [13], [54]. In the wasp communities associated with the unisexual galls of A. mukaigawae, two mechanisms are probably involved with the horizontal transmission of Wolbachia: host-parasitoid interactions and contact with same plant tissues. Evidences for horizontal transmission of Wolbachia between A. mukaigawae and S. japonicus come from the fact that several Wolbachia strains, i.e., W1, W2, W3, W5a, W5b and W6, were found in both species, and in some communities, multiple identical strains were detected in both A. mukaigawae and S. japonicus, e.g., W1 and W2 in Changsha, W1, W2 and W5a in Loudi, and W3 in Yueyang (Figure 2 and Figure 3). Our results are consistent with that of a previous study, showing possible horizontal transmission of Wolbachia between gall-inducers and their associated inquilines [9].
Plant-mediated horizontal transmission of Wolbachia between host insect species was reported by several studies [23], [24]. Caspi-Fluger et al [25] found that insect symbiotic Rickettsia were transferred from whiteflies to the phloem of the host plants, and could be acquired by other whiteflies. Both the gall-inducer and its associated inquiline are phytophagous in the larval stages of their development, feeding on galls, which are modified live plant structure [31]. In our system, larvae of A. mukaigawae feed on the nutritive tissue lining the interior of its larval chamber in the center of the gall while larvae of the inquilinous S. japonicus feed in their own larval chambers made in gall tissue surrounding the central larval chamber of the A. mukaigawae [43]. During gall induction and development, plant tissues regularly come in contact with the insect secretions including surface secretions from eggs or young larvae, ovipositional fluid, frass, and/or saliva [62]. At the same time, Wolbachia are distributed in insect genital tissues, somatic tissues [63], [64], salivary glands [65], and hemolymph [66]. Therefore, it is highly likely that Wolbachia are transferred between gall wasps and inquiline wasps by way of gall tissues.
We are aware that the larvae of the S. japonicus grow faster than that of the A. mukaigawae and eventually the larvae of A. mukaigawae cease to develop [67]. Consistent with the previously studies [67], we did not find a single gall where larvae chambers of A. mukaigawae and S. japonicus coexisted in our dissection of galls (Table 1). In this case, the direction of plant-mediated horizontal transmission should be unidirectional from A. mukaigawae to S. japonicus.
Inquiline gall wasps do not always kill their hosts and an extensive parenchyma peripheral to the larval chamber in some host galls may provide extra space for the inquiline cells [31]. We suggested that if some host galls provide enough space for larvae of both S. japonicus and A. mukaigawae, inquilines (S. japonicus) and gall inducers (A. mukaigawae) may emerge from a single gall. When this happens, plant-mediated horizontal transmission between the inquiline and the gall inducer should be limited bidirectional.
This may explain the high levels multiple Wolbachia infections of A. mukaigawae and S. japonicus, especially the fact that A. mukaigawae was found to be infected with as many as 5 strains of Wolbachia.
Horizontal Transmission of Wolbachia by Host-parasitoid Association
Although we do not have direct evidence to show whether Torymus sp. parasitizes A. mukaigawae or S. japonicus, it seems more likely that the parasitoid species attacks the inquiline species because the two share a same Wolbachia strain, W9. As discussed above, the strain may have historically infected S. japonicus and subsequently been transferred to Torymus sp. through host-parasitoid interactions. Since parasitoid attack is normally fatal, horizontal transmission between S. japonicus (host) and Torymus sp. (parasitoid) is likely to be unidirectional. Nonetheless, we only had limited success in rearing the parasitoid species and further work in the future is certainly needed to elucidate a possibly much complex relationship among Torymus sp. and its host species S. japonicus, and possibly A. mukaigawae as well, and the horizontal transmission of Wolbachia between them.
Conclusions
In conclusion, the present study found high levels of multiple Wolbachia infections in A. mukaigawae and S. japonicus associated with the unisexual galls of A. mukaigawae. Our results suggested the potentialities that the horizontal transmission of Wolbachia was plant-mediated, and revealed multiple recombination events for Wolbachia in gall associated wasp communities. We further suggest that recombination events and potential plant-mediated horizontal transmission play an important role in the high levels of multiple Wolbachia infection of A. mukaigawae and S. japonicus.
Supporting Information
Maximum Likelihood tree for Wolbachia strains of A. mukaigawae , S. japonicus and Torymus sp. based on wsp sequence. W1–W9 indicate Wolbachia strains in A. mukaigawae communities. The abbreviations AQ, YY, CS, LD, SY, JA and SG in parentheses indicate the populations shown in Table 1. The number following AQ, YY, CS, LD, SY, JA and SG indicate the amount of Wolbachia strains per population. Numbers above branches were bootstrap values computed from 1000 replications. Wolbachia from Trichogramma semblidis was used as outgroup.
(TIFF)
Recombination events of wsp gene between Wolbachia strains W5a and W3 by BootScan method. Percentages around the sequence alignments show the similarities of the daughter sequence to its major or minor parent sequences (marked with the same background color). Major parent: parent contributing the larger fraction of sequence; minor parent: parent contributing the smaller fraction of sequence.
(TIF)
Recombination events of wsp gene between Wolbachia strains W9 and W4 by Chimaera method.
(TIF)
Recombination events of wsp gene between Wolbachia strains W6 and W7 by GENECONV method.
(TIF)
The distribution of Wolbachia strains in each individual from different geographic populations of A. mukaigawae. †The abbreviations AQ, YY, CS, LD, SY and SG indicate the populations shown in Table 1. The number following AQ, YY, CS, LD, SY and SG indicate different individuals from the same population. ‡W1–W6 indicate Wolbachia strains in A. mukaigawae. §The number indicate the amount of Wolbachia strains in each individual of A. mukaigawae.
(DOC)
The distribution of Wolbachia strains in each individual in geographic population of S. japonicus. †The abbreviations AQ, YY, CS, LD, SY, JA and SG indicate the populations shown in Table 1. The number following AQ, YY, CS, LD, SY, JA and SG indicate different individuals from the same population. ‡W1–W8 indicate Wolbachia strains in S. japonicus. §The number indicate the amount of Wolbachia strains in each individual of S. japonicus.
(DOC)
Acknowledgments
We thank Prof. Xun-Lin Yu of Central South University of Forestry and Technology for the identification of host plant. The parasitoid wasp was kindly identified by Dr. Hui Xiao of Institute of Zoology, Chinese Academy of Sciences.
Funding Statement
This study was supported by the National Natural Science Foundation of China (NSFC grant no. 30872036) (http://www.nsfc.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stouthamer R, Breeuwer JAJ, Hurst GDD (1999) Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annual Review of Microbiology 53: 71–102. [DOI] [PubMed] [Google Scholar]
- 2. Werren JH (1997) Biology of Wolbachia . Annual Review of Entomology 42: 587–609. [DOI] [PubMed] [Google Scholar]
- 3. Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology 6: 741–751. [DOI] [PubMed] [Google Scholar]
- 4. Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia? a statistical analysis of current data. Fems Microbiology Letters 281: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Werren JH, Zhang W, Guo LR (1995) Evolution and Phylogeny of Wolbachia - Reproductive Parasites of Arthropods. Proceedings of the Royal Society of London Series B-Biological Sciences 261: 55–63. [DOI] [PubMed] [Google Scholar]
- 6. Schuler H, Arthofer W, Riegler M, Bertheau C, Krumbock S, et al. (2011) Multiple Wolbachia infections in Rhagoletis pomonella . Entomologia Experimentalis Et Applicata 139: 138–144. [Google Scholar]
- 7. Reuter M, Keller L (2003) High levels of multiple Wolbachia infection and recombination in the ant Formica exsecta . Molecular Biology and Evolution 20: 748–753. [DOI] [PubMed] [Google Scholar]
- 8. Van Borm S, Wenseleers T, Billen J, Boomsma JJ (2001) Wolbachia in leafcutter ants: a widespread symbiont that may induce male killing or incompatible matings. Journal of Evolutionary Biology 14: 805–814. [Google Scholar]
- 9. Rokas A, Atkinson RJ, Nieves-Aldrey JL, West SA, Stone GN (2002) The incidence and diversity of Wolbachia in gallwasps (Hymenoptera; Cynipidae) on oak. Molecular Ecology 11: 1815–1829. [DOI] [PubMed] [Google Scholar]
- 10. Malloch G, Fenton B, Butcher RDJ (2000) Molecular evidence for multiple infections of a new subgroup of Wolbachia in the European raspberry beetle Byturus tomentosus . Molecular Ecology 9: 77–90. [DOI] [PubMed] [Google Scholar]
- 11. Malloch G, Fenton B (2005) Super-infections of Wolbachia in byturid beetles and evidence for genetic transfer between A and B super-groups of Wolbachia . Molecular Ecology 14: 627–637. [DOI] [PubMed] [Google Scholar]
- 12. Hiroki M, Tagami Y, Miura K, Kato Y (2004) Multiple infection with Wolbachia inducing different reproductive manipulations in the butterfly Eurema hecabe . Proceedings of the Royal Society of London Series B-Biological Sciences 271: 1751–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vavre F, Fleury F, Lepetit D, Fouillet P, Bouletreau M (1999) Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Molecular Biology and Evolution 16: 1711–1723. [DOI] [PubMed] [Google Scholar]
- 14. Boyle L, Oneill SL, Robertson HM, Karr TL (1993) Interspecific and Intraspecific Horizontal Transfer of Wolbachia in Drosophila . Science 260: 1796–1799. [DOI] [PubMed] [Google Scholar]
- 15. Braig HR, Guzman H, Tesh RB, Oneill SL (1994) Replacement of the Natural Wolbachia Symbiont of Drosophila Simulans with a Mosquito Counterpart. Nature 367: 453–455. [DOI] [PubMed] [Google Scholar]
- 16. Rasgon JL, Gamston CE, Ren XX (2006) Survival of Wolbachia pipientis in cell-free medium. Applied and environmental microbiology 72: 6934–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frydman HM, Li JM, Robson DN, Wieschaus E (2006) Somatic stem cell niche tropism in Wolbachia . Nature 441: 509–512. [DOI] [PubMed] [Google Scholar]
- 18. Huigens ME, Luck RF, Klaassen RHG, Maas M, Timmermans M, et al. (2000) Infectious parthenogenesis. Nature 405: 178–179. [DOI] [PubMed] [Google Scholar]
- 19. Haine ER, Pickup NJ, Cook JM (2005) Horizontal transmission of Wolbachia in a Drosophila community. Ecological Entomology 30: 464–472. [Google Scholar]
- 20. Heath BD, Butcher RDJ, Whitfield WGF, Hubbard SF (1999) Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Current Biology 9: 313–316. [DOI] [PubMed] [Google Scholar]
- 21. Rigaud T, Juchault P (1995) Success and failure of horizontal transfers of feminizing Wolbachia endosymbionts in woodlice. Journal of Evolutionary Biology 8: 249–255. [Google Scholar]
- 22. Kittayapong P, Jamnongluk W, Thipaksorn A, Milne JR, Sindhusake C (2003) Wolbachia infection complexity among insects in the tropical rice-field community. Molecular Ecology 12: 1049–1060. [DOI] [PubMed] [Google Scholar]
- 23. Mitsuhashi W, Saiki T, Wei W, Kawakita H, Sato M (2002) Two novel strains of Wolbachia coexisting in both species of mulberry leafhoppers. Insect Molecular Biology 11: 577–584. [DOI] [PubMed] [Google Scholar]
- 24. Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P (2006) Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microbial Ecology 51: 294–301. [DOI] [PubMed] [Google Scholar]
- 25. Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, et al. (2012) Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proceedings of the Royal Society of London Series B-Biological Sciences 279: 1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Werren JH, Bartos JD (2001) Recombination in Wolbachia . Current Biology 11: 431–435. [DOI] [PubMed] [Google Scholar]
- 27. Verne S, Johnson M, Bouchon D, Grandjean F (2007) Evidence for recombination between feminizing Wolbachia in the isopod genus Armadillidium . Gene 397: 58–66. [DOI] [PubMed] [Google Scholar]
- 28. Arthofer W, Riegler M, Schneider D, Krammer M, Miller WJ, et al. (2009) Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae). Molecular Ecology 18: 3816–3830. [DOI] [PubMed] [Google Scholar]
- 29. Baldo L, Bordenstein S, Wernegreen JJ, Werren JH (2006) Widespread recombination throughout Wolbachia genomes. Molecular Biology and Evolution 23: 437–449. [DOI] [PubMed] [Google Scholar]
- 30. Jiggins FM (2002) The rate of recombination in Wolbachia bacteria. Molecular Biology and Evolution 19: 1640–1643. [DOI] [PubMed] [Google Scholar]
- 31.Askew R (1984) The biology of gall wasps. In: Ananthakrishnan TN, editor. Biology of Galling Insects. New Delhi, India: Oxford & IBH. 223–271.
- 32. Ronquist F (1999) Phylogeny, classification and evolution of the Cynipoidea. Zoologica Scripta 28: 139–164. [Google Scholar]
- 33. Plantard O, Rasplus JY, Mondor G, Le Clainche I, Solignac M (1999) Distribution and phylogeny of Wolbachia inducing thelytoky in Rhoditini and ‘Aylacini’ (Hymenoptera: Cynipidae). Insect Molecular Biology 8: 185–191. [DOI] [PubMed] [Google Scholar]
- 34. Plantard O, Rasplus JY, Mondor G, Le Clainche I, Solignac M (1998) Wolbachia-induced thelytoky in the rose gallwasp Diplolepis spinosissimae (Giraud) (Hymenoptera: Cynipidae), and its consequences on the genetic structure of its host. Proceedings of the Royal Society of London Series B-Biological Sciences 265: 1075–1080. [Google Scholar]
- 35. Schilthuizen M, Stouthamer R (1998) Distribution of Wolbachia among the guild associated with the parthenogenetic gall wasp Diplolepis rosae . Heredity 81: 270–274. [Google Scholar]
- 36. Abe Y, Miura K (2002) Doses Wolbachia induce unisexuality in oak gall wasps? (Hymenoptera: Cynipidae). Annals of the Entomological Society of America 95: 583–586. [Google Scholar]
- 37. Zhu DH, He YY, Fan YS, Ma MY, Peng DL (2007) Negative evidence of parthenogenesis induction by Wolbachia in a gallwasp species, Dryocosmus kuriphilus . Entomologia Experimentalis Et Applicata 124: 279–284. [Google Scholar]
- 38. Yang X-H, Zhu D-H, Liu Z-W, Zhao L (2012) Sequencing and phylogenetic analysis of the wsp gene of Wolbachia in three geographic populations of an oak gall wasp, Andricus mairei(Hymenoptera: Cynipidae), From Hunan, South China. Acta Entomologica Sinica 55: 247–254. [Google Scholar]
- 39. Stone GN, Schonrogge K, Atkinson RJ, Bellido D, Pujade-Villar J (2002) The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annual Review of Entomology 47: 633–668. [DOI] [PubMed] [Google Scholar]
- 40. Chust G, Garbin L, Pujade-Villar J (2007) Gall wasps and their parasitoids in cork oak fragmented forests. Ecological Entomology 32: 82–91. [Google Scholar]
- 41. Abe Y (1986) Taxonomic status of the Andricus mukaigawae complex and its speciation with geographic parthenogenesis (Hymenoptera: Cynipidae). Applied Entomology and Zoology 21: 436–447. [Google Scholar]
- 42. Abe Y, Bhuyan M, Mech J, Bhattacharyya PR, Ide T, et al. (2012) Discovery of an oak gall wasp (Hymenoptera: Cynipidae) inducing galls on deciduous oak trees in India. Entomological Science 15: 340–342. [Google Scholar]
- 43.Abe Y (1990) Life cycles of two species of the Synergus japonicus complex (Hymenoptera: Cynipidae). Akitu: 1–7.
- 44. Zhou WG, Rousset F, O’Neill S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society of London Series B-Biological Sciences 265: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Meer MMM, Witteveldt J, Stouthamer R (1999) Phylogeny of the arthropod endosymbiont Wolbachia based on the wsp gene. Insect Molecular Biology 8: 399–408. [DOI] [PubMed] [Google Scholar]
- 47. Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- 48. Swofford DL (1993) PAUP - A computer-program for phylogenetic inference using maximum parsimony. Journal of General Physiology 102: A9–A9. [Google Scholar]
- 49. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 50. Martin D, Rybicki E (2000) RDP: detection of recombination amongst aligned sequences. Bioinformatics 16: 562–563. [DOI] [PubMed] [Google Scholar]
- 51. Csoka G, Stone GN, Melika G (2005) Biology, ecology, and evolution of gall-inducing Cynipidae. In: Biology, ecology and evolution of gall-inducing arthropods: volume Raman A, Schaefer CW, Withers TM, editors. 2: 573–642. [Google Scholar]
- 52. Kondo N, Ijichi N, Shimada M, Fukatsu T (2002) Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Molecular Ecology 11: 167–180. [DOI] [PubMed] [Google Scholar]
- 53.Wenseleers T (2001) Conflict from cell to colony. Belgium: University of Leuven.
- 54. Jamnongluk W, Kittayapong P, Baimai V, O’Neill SL (2002) Wolbachia infections of tephritid fruit flies: Molecular evidence for five distinct strains in a single host species. Current Microbiology 45: 255–260. [DOI] [PubMed] [Google Scholar]
- 55. Pattabhiramaiah M, Brückner D, Reddy MS (2010) Horizontal transmission of Wolbachia in the honeybee subspecies Apis mellifera carnica and its ectoparasite Varroa destructor . International Journal of Environmental Sciences 2: 526–535. [Google Scholar]
- 56. West SA, Cook JM, Werren JH, Godfray HCJ (1998) Wolbachia in two insect host-parasitoid communities. Molecular Ecology 7: 1457–1465. [DOI] [PubMed] [Google Scholar]
- 57. Yang C-Y, Xiao J-H, Niu L-M, Ma G-C, Cook JM, et al. (2012) Chaos of Wolbachia Sequences Inside the Compact Fig Syconia of Ficus benjamina (Ficus: Moraceae). PloS one 7: e48882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ros VID, Fleming VM, Feil EJ, Breeuwer JAJ (2012) Diversity and recombination in Wolbachia and Cardinium from Bryobia spider mites. Bmc Microbiology 12 (Suppl 1)S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freeman S, Herron JC (2007) The Adaptive Significance of Sex. Evolutionary analysis. Upper Saddle River: Pearson Prentice Hall. 302–313.
- 60. Awadalla P (2003) The evolutionary genomics of pathogen recombination. Nature Reviews Genetics 4: 50–60. [DOI] [PubMed] [Google Scholar]
- 61. Xu Z, Chen H, Zhou R (2011) Genome-wide evidence for positive selection and recombination in Actinobacillus pleuropneumoniae . Bmc Evolutionary Biology 11: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Witiak S (2009) Hormonal and Molecular Investigations of Phylloxera Leaf Gall Development. Pennsylvania, USA: Pennsylvania State University.
- 63. Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou WG, et al. (1999) Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochemistry and Molecular Biology 29: 153–160. [DOI] [PubMed] [Google Scholar]
- 64. Zouache K, Voronin D, Tran-Van V, Mousson L, Failloux AB, et al. (2009) Persistent Wolbachia and Cultivable Bacteria Infection in the Reproductive and Somatic Tissues of the Mosquito Vector Aedes albopictus . Plos One 4: e6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tsai KH, Huang CG, Wu WJ, Chuang CK, Lin CC, et al. (2006) Parallel infection of Japanese encephalitis virus and Wolbachia within cells of mosquito salivary glands. Journal of Medical Entomology 43: 752–756. [DOI] [PubMed] [Google Scholar]
- 66. Rigaud T, Soutygrosset C, Raimond R, Mocquard JP, Juchault P (1991) Feminizing endocytobiosis in the terrestrial crustacean Armadillidium vulgare latr (isopoda) - recent acquisitions. Endocytobiosis and Cell Research 7: 259–273. [Google Scholar]
- 67. Abe Y (1997) Well-developed gall tissues protecting the gall wasp, Andricus mukaigawae (Mukaigawa) (Hymenoptera: Cynipidae) against the gall-inhabiting moth, Oedematopoda sp. (Lepidoptera: Stathmopodidae). Applied Entomology and Zoology 32: 135–141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum Likelihood tree for Wolbachia strains of A. mukaigawae , S. japonicus and Torymus sp. based on wsp sequence. W1–W9 indicate Wolbachia strains in A. mukaigawae communities. The abbreviations AQ, YY, CS, LD, SY, JA and SG in parentheses indicate the populations shown in Table 1. The number following AQ, YY, CS, LD, SY, JA and SG indicate the amount of Wolbachia strains per population. Numbers above branches were bootstrap values computed from 1000 replications. Wolbachia from Trichogramma semblidis was used as outgroup.
(TIFF)
Recombination events of wsp gene between Wolbachia strains W5a and W3 by BootScan method. Percentages around the sequence alignments show the similarities of the daughter sequence to its major or minor parent sequences (marked with the same background color). Major parent: parent contributing the larger fraction of sequence; minor parent: parent contributing the smaller fraction of sequence.
(TIF)
Recombination events of wsp gene between Wolbachia strains W9 and W4 by Chimaera method.
(TIF)
Recombination events of wsp gene between Wolbachia strains W6 and W7 by GENECONV method.
(TIF)
The distribution of Wolbachia strains in each individual from different geographic populations of A. mukaigawae. †The abbreviations AQ, YY, CS, LD, SY and SG indicate the populations shown in Table 1. The number following AQ, YY, CS, LD, SY and SG indicate different individuals from the same population. ‡W1–W6 indicate Wolbachia strains in A. mukaigawae. §The number indicate the amount of Wolbachia strains in each individual of A. mukaigawae.
(DOC)
The distribution of Wolbachia strains in each individual in geographic population of S. japonicus. †The abbreviations AQ, YY, CS, LD, SY, JA and SG indicate the populations shown in Table 1. The number following AQ, YY, CS, LD, SY, JA and SG indicate different individuals from the same population. ‡W1–W8 indicate Wolbachia strains in S. japonicus. §The number indicate the amount of Wolbachia strains in each individual of S. japonicus.
(DOC)