Abstract
The development of a new vaccine as a substitute for Bacillus Calmette–Guerin or to improve its efficacy is one of the many World Health Organization goals to control tuberculosis. Mycobacterial vectors have been used successfully in the development of vaccines against tuberculosis. To enhance the potential utility of Mycobacterium smegmatis as a vaccine, it was transformed with a recombinant plasmid containing the partial sequences of the genes Ag85c, MPT51, and HspX (CMX) from M. tuberculosis. The newly generated recombinant strain mc2-CMX was tested in a murine model of infection. The recombinant vaccine induced specific IgG1 or IgG2a responses to CMX. CD4+ and CD8+ T cells from the lungs and spleen responded ex vivo to CMX, producing IFN-γ, IL17, TNF-α, and IL2. The vaccine thus induced a significant immune response in mice. Mice vaccinated with mc2-CMX and challenged with M. tuberculosis showed better protection than mice immunized with wild-type M. smegmatis or BCG. To increase the safety and immunogenicity of the CMX antigens, we used a recombinant strain of M. smegmatis, IKE (immune killing evasion), to express CMX. The recombinant vaccine IKE-CMX induced a better protective response than mc2-CMX. The data presented here suggest that the expression of CMX antigens improves the immune response and the protection induced in mice when M. smegmatis is used as vaccine against tuberculosis.
Introduction
Tuberculosis (TB) was the eighth most frequent cause of death worldwide in 2008 according to a World Health Organization (WHO) report, although since the Millennium Development Goals and Stop TB Strategy were established by WHO, more than 51 million people have been successfully treated and cured of TB [1]. Although these data are encouraging, the incidence of the disease is persistently high in several countries, explaining the continual spread of TB.
Early diagnostic and prophylactic strategies are still required to control TB. Bacillus Calmette–Guerin (BCG), the currently available vaccine for TB, although safe, does not protect against TB in adults [2]. To date, several TB vaccines have been tested in preclinical and clinical trials and in 2012, a review article described 12 promising vaccines. Those vaccines differ in their vaccination schemes and their use as primary vaccines or boosters for the BCG vaccine. The only vaccine being tested in a phase 3 trial to date is an attenuated Mycobacterium indicus pranii, to be used in immunotherapy [3].
To select molecules or agents that can be tested as TB vaccines, it is important to understand the immune response induced during TB infection. TB has several clinical forms, and approximately one third of the world population is infected with M. tuberculosis (Mtb), comprising a massive population of individuals with latent disease. It has been shown by our group and others that the immune response of individuals infected with Mtb specifically recognizes a set of proteins, including HspX, produced by Mtb bacilli under stress conditions that mimic in vivo circumstances [4]–[7]. Patients that present with active pulmonary TB, for instance, preferentially recognize and respond to other Mtb antigens, i.e., the GLc-B, MPT51, Ag85 complex [8]–[12]. Some responses in patients with extrapulmonary forms of TB are the same as those seen with the active pulmonary TB form [12], [13]. These features support the hypothesis that a good candidate vaccine should induce immune responses to antigens common to more than one form of TB and should include more than one protein in a single formulation. Antigens recognized by both the humoral and cellular immune responses might also improve the protection profile of a vaccine [14], [15].
Several proteins from Mtb have been tested as subunit vaccines or in recombinant vectors, and those vaccines composed of more than one protein/antigen showed sufficient potential to proceed to clinical assays [3]. Recently, we showed that a fusion protein construct (CMX) containing the immune epitopes of antigens 85 c and MPT51 and the entire HspX antigen from Mtb was immunogenic in mice and antigenic in individuals with active TB, confirming its potential utility as a vaccine for TB [15]. Because M. smegmatis is attenuated, grows rapidly, can express Mtb antigens, and has been shown to induce enhanced protection against TB in a murine model of infection, we used M. smegmatis expressing CMX as a recombinant vaccine.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Committee of Sociedade Brasileira de Animais de Laboratório (SBAL). The protocol for this work was approved by the Committee on the Ethics of Animal Experiments of the University Federal de Goiás (permit number: 229/11).
Bacterial Strains, Plasmids, and Vaccine Construction
The M. smegmatis (mc2) strains used in this study were derived from laboratory strain mc2155. During the study, the strains were grown in 7H9 liquid medium containing 10% Oleic acid, dextrose and catalase (OADC), 0.5% glycerol, and 0.05% Tween 80 for maintenance, transformation, and transduction.
Recombinant CMX–M. smegmatis (mc2-CMX) construction. A DNA sequence encoding the Ag85c and MPT51 immunodominant epitopes and the entire HspX gene, described in a previous study [15], was used as the template for PCR amplification using a set of primers that allowed the creation of flanking restriction enzyme sites and facilitated posterior cloning. The product of this amplification was cloned into the pGEM-T Easy vector (Promega, USA). The recombinant pGEM-T Easy vector containing the fused gene sequence (CMX) was digested with KpnI and NotI to remove the CMX fragment. The digested fragment was then ligated into the Mycobacterium/Escherichia coli shuttle vector pLA71 [16], previously digested with the same enzymes, and resolved on agarose gel to remove the PhoA gene. The recombinant pLA71/CMX vector was sequenced to check for mutations. The pLA71/CMX and pLA71 vectors were transformed into mc2 cells and screened on medium containing kanamycin, to generate the recombinant mc2-CMX strain and the mc2 control vaccine, respectively.
Immune killing evasion (IKE) M. smegmatis mutant construction. The M. smegmatis mutant, mc26462 or IKE (mc2155Δ 0615–0626) was constructed by homologous recombination using a specialized transducing phage [17], [18]. The deletion phagemid for the mutant strain mc26462 was constructed by PCR amplification of the 5′-flanking and 3′-flanking regions of the genes to be knocked out, and then cloned into p0004s and packaged into the temperature-sensitive phage ph159. This phage was used to generate the knockout strain using specialized transduction, as described previously [19]. The clones obtained were confirmed by PCR and Southern analysis. Then mc26463, an unmarked strain, was generated from mc26462 by transducing the latter with phage phAE280 containing resolvase, to remove the selection and counterselection markers, hygromycin and SacB, after plating on 7H10 plates containing 5% sucrose. The medium was supplemented with 50 µg/mL hygromycin, 20 µg/mL apramycin, or 20 µg/mL kanamycin when required.
SDS–PAGE and Western Blotting
Western blotting was performed to detect recombinant CMX protein expression. The SDS-PAGE-separated proteins were electrotransferred to a nitrocellulose membrane. A 1∶10,000 dilution of polyclonal antiserum, obtained from BALB/c mice after three vaccinations with rCMX and complete Freund’s adjuvant were preadsorbed with E. coli proteins, and then incubated with the nitrocellulose membrane. The membrane was then incubated with horseradish-peroxidase-conjugated anti-mouse IgG (Sigma-Aldrich®). The presence of the CMX protein was detected by incubation with the substrate diaminobenzidine (Roche, Germany).
Animals
Specific-pathogen-free female BALB/c mice (4–8 weeks old), purchased from CEMIB-Unicamp, were maintained in ABSL-2 racks adapted with an HEPA air filter, with water and food provided ad libitum, at the animal-care facility of the Institute of Tropical Pathology and Public Health at University Federal of Goiás. The temperature was maintained at 20–24°C with a relative humidity of 40%–70%, and 12 h light/dark cycles. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Committee of Sociedade Brasileira de Animais de Laboratório (SBAL) and approved by the Research Ethical Committee of Federal University of Goiás (Permit Number: 229/11).
Immunization
Frozen stocks of recombinant mc2-CMX or mc2 containing empty pLA71 were thawed and the mice were inoculated subcutaneously with 107 colony-forming units (CFU). The control mice were inoculated with sterile saline. The mice were immunized twice with 100 µL volumes, with an interval of 15 days between immunizations. For protection experiments a group of BALB/c mice were vaccinated subcutaneously with 106 CFU of BCG-Moreau, forty-five days previous to the Mtb infection. All experiments were performed thrice.
Mycobacterium Tuberculosis Challenge and Bacterial Load Determination
Mycobacterium tuberculosis strain H37Rv was grown to mid-log phase in 7H9 medium containing OADC and 0.05% Tween 80 and then frozen in aliquots in 20% glycerol at –80°C until required. Mice were infected intravenously with 100 µL of 107 CFU/mL. The bacterial loads were determined by plating whole or partial organ homogenates onto nutrient 7H11 agar supplemented with OADC. The colonies were counted after incubation for 3–4 weeks at 37°C.
Serum Collection
Serum samples were collected from the immunized mice 30 and 45 days after the second immunization. The samples were incubated for 1 h at 37°C, centrifuged at 1,200g at 4°C for 15 min to separate the serum, and stored at –20°C.
Enzyme-linked Immunosorbent Assay (ELISA)
The ELISA was performed as optimized by Sousa et al. (2012) [15]. Briefly, 96-well polystyrene plates (Nunc®) were coated with 10 µg/mL CMX recombinant fusion protein diluted in 0.05 M sodium carbonate/bicarbonate buffer, and incubated at 4°C for 16 h. The wells were blocked with PBS containing 1% skim milk. The serum samples were diluted 1∶800, added to the wells, and incubated for 2 h at 37°C. Biotin-conjugated antibodies (anti-IgG1 or anti-mouse IgG2a; Pharmingen®) diluted 1∶5,000 were added to the plates, which were then incubated for 1 h at 37°C. Streptavidin peroxidase, diluted 1∶1,000, was added and the plates were incubated again for 1 h at 37°C. After incubation with the substrate solution, the absorbance at 492 nm was read on an ELISA reader (Labsystems Multiskan Thermo®).
CMX-specific Responses in Lung and Spleen Cells
Thirty days after the final immunization and 30 days after the Mtb challenge, the mice were killed and their lungs and spleens removed aseptically. The cells were separated with a Potter tissue homogenizer (Corning, USA). The erythrocytes were lysed with Gey’s solution. The cell concentration was adjusted to 1×106 cells/mL and plated in a 24-well plate. The splenocyte and lung homogenates were stimulated with ConA (10 µg/mL) or CMX (10 µg/mL) or not stimulated. After incubation in a 5% CO2 incubator for 2 h at 37°C, monensin solution (eBioscience) was added to the cells, which were then incubated for a further 4 h. The cells were then stained for CD4+, CD8+, IFN-γ, IL2, IL17, and TNF-α using the BD Cytofix/Cytoperm Kit according to the manufacturer’s instructions. The cells were acquired with a BD Biosciences FACSCanto II flow cytometer (Albert Einstein College of Medicine and Laboratório de Nanotecnologia Farmacêutica e Sistema de Liberação de Fármacos, UFG), and the data were analyzed using the FlowJo 8.7 software. Thirty thousand lymphocytes were acquired from each sample.
Statistical Analysis
The results were tabulated with Excel (version 14.3.4, 2011 for Mac) and the Prism software (version 5.0a, GraphPad). The differences between groups were assessed with a two-tailed Student’s t test after a nonparametric (Mann–Whitney U) test. The results were considered significantly different when p<0.05.
Results
mc2-CMX Vaccine is Stable in vivo
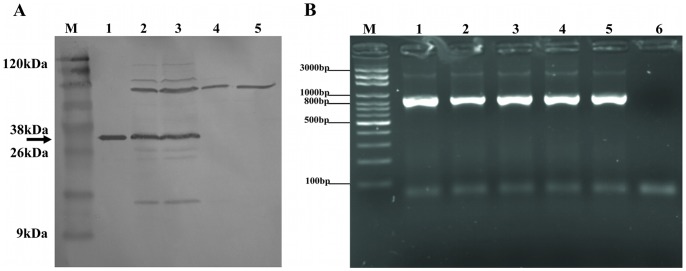
The recombinant M. smegmatis vaccine transformed with pLA71 containing CMX-encoding DNA expressed the CMX fusion protein (Figure 1). The expected size of the recombinant CMX protein (∼36 kDa) was determined with a polyclonal anti-CMX antibody when the protein extracted from mc2-CMX was analyzed. The Ag85, MPT51, and HspX bands were also detected with the polyclonal antibody directed against CMX (Figure 1A).
Figure 1. Recombinant mc2-CMX vaccine containing pLA71/CMX DNA expressed the recombinant protein and persisted in mice.
(A) The recombinant CMX protein (38 kDa) expressed from the mc2-CMX vaccine was only detected when analyzed with an anti-CMX polyclonal antibody. Cross-reactivity with Ag85 and MPT-51 proteins was also observed. Lane M: BlueStep™ Broad Range Protein Marker (Amresco); lane 1: purified recombinant CMX protein; lanes 2 and 3: mc2-CMX; lanes 4 and 5: mc2. (B) To check that the live vaccine survived in vivo and retained the plasmid, spleen homogenates from vaccinated mice were plated on antibiotic-containing medium and the retrieved colonies were tested by PCR for the presence of the CMX fusion gene. All the isolated colonies showed a positive PCR. Lane M, Axygen 100 bp DNA Ladder (Biosciences); lanes 1–5: colonies of bacteria recovered and tested by PCR; lane 6, negative PCR control.
To check whether the live vaccine persisted in vivo without antibiotic selection and retained the plasmid, bacteria were recovered from the spleens of the vaccinated animals and grown in medium with or without kanamycin. The retrieved colonies were then tested with PCR for the presence of the plasmid containing the insert. We recovered recombinant vaccine for up to eight days after vaccination. All the colonies isolated were PCR-positive for the CMX fusion gene (Figure 1B).
mc2-CMX Induces Both Humoral and Cellular Immune Responses
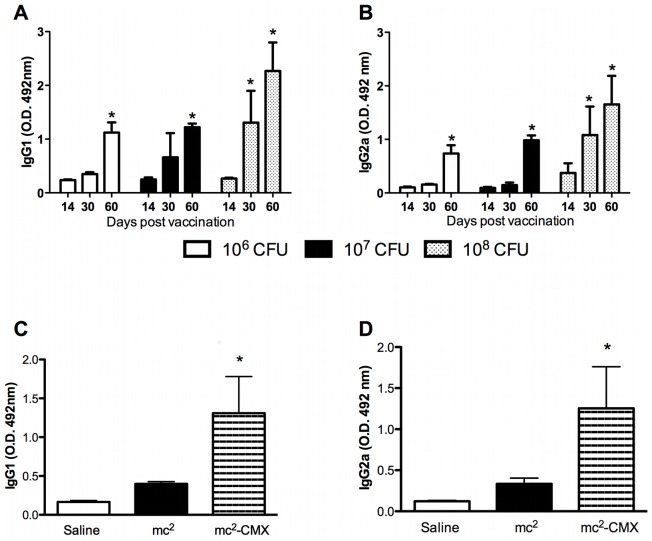
To determine the optimal dose of mc2-CMX for use as a vaccine, groups of mice were vaccinated with a single dose containing 106, 107, or 108 CFU of the mc2-CMX vaccine, and the specific humoral immune response was assessed. As shown in Figure 2 (A and B), all vaccine doses induced specific IgG1 or IgG2a responses to CMX. Therefore, we selected the intermediate dose (107 CFU) for the prime-boost strategy. To check the immunogenicity of this strategy, the sera and peripheral blood mononuclear cells from immunized mice were analyzed. IgG1 and IgG2a levels were measured and showed that a specific humoral immune response to CMX was induced for both IgG isotypes: IgG1 and IgG2a (Figure 2C and D, respectively; p<0.05).
Figure 2. Induction of CMX-specific IgG1 and IgG2a by the mc2-CMX vaccine.
(A and B) Animals were immunized with a single dose of different concentrations of the mc2-CMX vaccine and their sera were tested with an indirect ELISA at the specified time points (n = 4; *p<0.05: differences were compared on day 14 after vaccination). (C and D) Groups of 4–6 mice were vaccinated twice with 107 CFU of mc2, mc2-CMX, or saline. Thirty days after vaccination, their blood was collected and their sera tested for CMX-specific IgG1 and IgG2a. *p<0.05. The results are representative of three independent experiments.
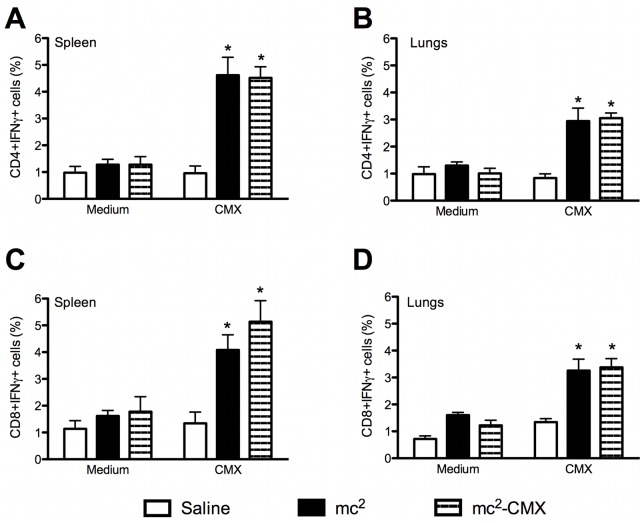
Because the Th1 immune response is associated with protection, we investigated whether the subcutaneous live vaccine could induce a specific Th1 response in the spleens and lungs of vaccinated mice. Gated CD4+ T cells, selected from cells with a live lymphocyte phenotype, were evaluated for the expression of IFN-γ after ex vivo restimulation with CMX (Figure 3A and B). Both the mc2 and mc2-CMX vaccines induced IFN-γ-positive CD4+ T cells in the lungs and spleens of the vaccinated animals (p<0.05).
Figure 3. Induction of specific cellular responses to ex vivo stimulation with CMX.
Lung and spleen single-cell suspensions from vaccinated and control mice were restimulated with CMX or medium, and the CD4+ and CD8+ IFN-γ-positive T cells were analyzed by flow cytometry. Lymphocytes were selected by their size and granularity and the CD4+ cells were gated and analyzed for IFN expression. A and B show CD4+ IFN-γ-positive cells from the spleen and lungs, respectively. C and D show CD8+ IFN-γ-positive cells (n = 4; *p<0.05) from the spleen and lungs, respectively. The results are representative of two independent experiments.
It has been shown that CD8+ T cells also contribute to the protective cytokine profile. As shown in Figure 3C and D, CD8+ splenocytes showed specific responses to CMX when mc2 and mc2-CMX were used as vaccines.
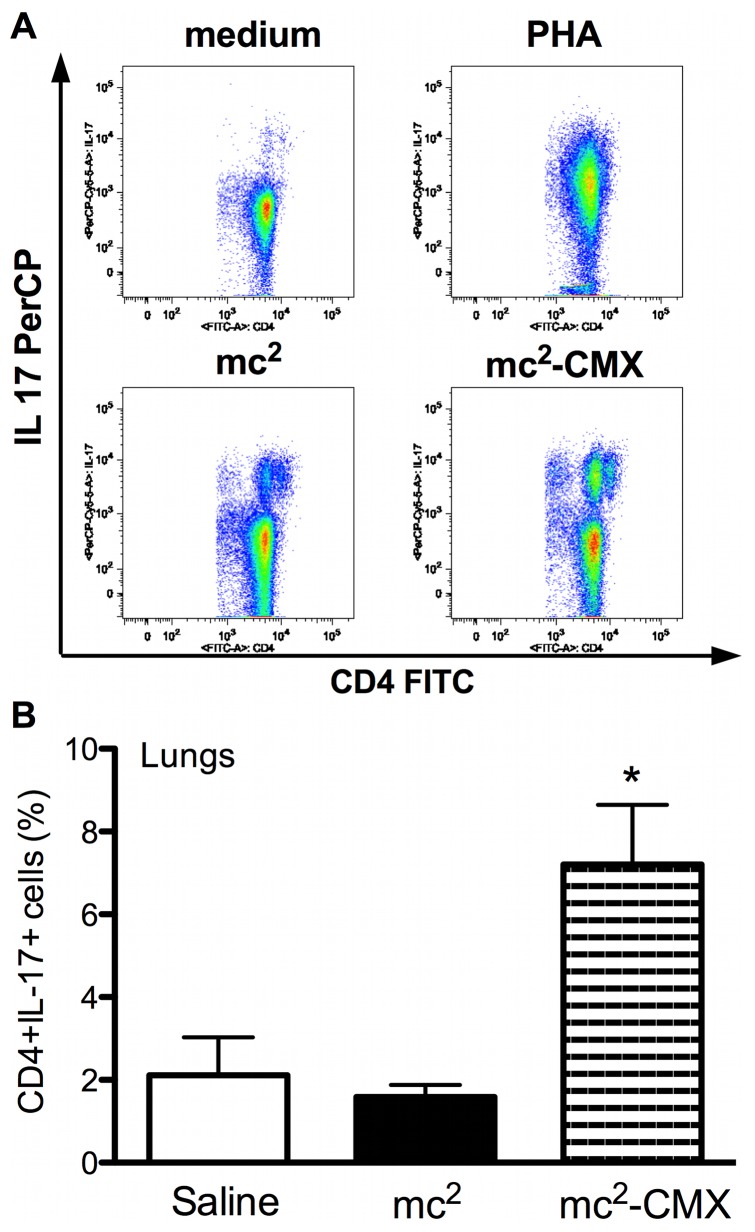
Th17 cells have been associated with a persistent memory phenotype in a murine model of TB vaccination [20]. We tested this cell population in both spleen and lung homogenates. Although we saw no difference in the induction of CMX-specific CD4+IL17+ responses in the spleen (Figure 4A), there was a distinct and specific response to CMX cells in the lungs of the mc2-CMX-vaccinated animals (Figure 4B).
Figure 4. Th17 induction after vaccination with mc2-CMX.
(A) Representative dot plots of spleen CD4+ T cells expressing IL17. Spleens from mice vaccinated with saline, mc2, or mc2-CMX were evaluated after ex vivo stimulation with medium, phytohemagglutinin (PHA), or recombinant CMX. No differences were found between the mc2 and mc2-CMX groups. (B) Lung cells from saline-, mc2-, and mc2-CMX-treated animals were restimulated ex vivo with recombinant CMX, and the CD4+ T cells were analyzed for IL-17 expression (n = 4; *p<0.05, different from the saline-vaccinated group). The results are representative of two independent experiments.
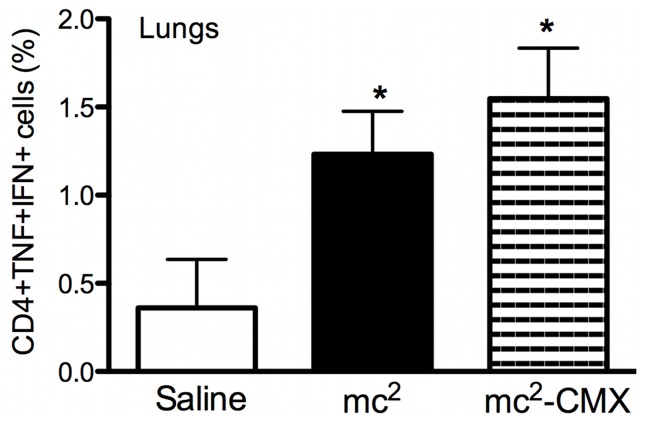
Polyfunctional T lymphocytes expressing protective cytokines are also associated with protection against TB. We assessed the induction of lung CD4+ T cells positive for both TNF-α and IFN-γ after this vaccination schema. Again, the animals vaccinated with mc2 or mc2-CMX showed the induction of specific double-positive CD4+TNF-α+/IFN-γ+ lymphocytes (Figure 5), although the greatest induction occurred in the mc2-CMX-immunized group (Figures S1 and 5).
Figure 5. Induction of high levels of CD4+ T cells doubly positive for TNF-α and IFN-γ.
Lung cells from saline-, mc2-, and mc2-CMX-treated animals were restimulated ex vivo with recombinant CMX, and the CD4+ T cells were analyzed for TNF-α and IFN-γ expression (n = 4; *p<0.05, different from the saline-vaccinated group). The results are representative of two independent experiments.
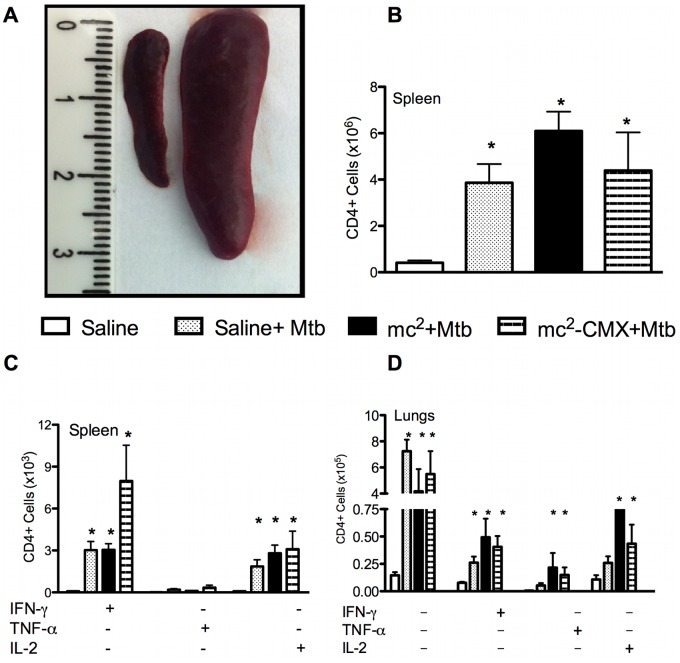
We then investigated whether challenging the animals with Mtb affected the immune response elicited by vaccination. After intravenous infection with Mtb, splenomegaly was observed in all groups, with an almost fivefold increase in the total number of CD4+ T cells (Figures 6A and B). Whereas the total numbers of these cells increased in all the groups tested, the greatest increase in CD4+IFN-γ+ cells was observed predominantly in animals vaccinated with mc2-CMX (Figure 6C). The total numbers of CD4+ T cells and CD4+IFN-γ+ T cells also increased in the lungs of the challenged animals (Figure 6D), but similar increases in the double-positive CD4+TNF-α+ and CD4+IL2+ cells were only observed in the vaccinated and challenged groups.
Figure 6. Challenge with Mtb induced massive splenomegaly and increases in the CD4+ T-cell populations.
(A) Representative photographs of the spleens from control and Mtb-infected animals. Mice were challenged with Mtb and 45 days after infection, their spleens were collected for flow-cytometric analysis. (B) Total CD4+ T cells from mice vaccinated with saline, mc2, or mc2-CMX and infected with Mtb. (C) Spleen CD4+ T-cell response to ex vivo stimulation with CMX. CD4+ T cells selected with lymphocyte gating for IFN-γ, TNF-α, and IL2 were quantified. (D) Lung CD4+ T-cell response to ex vivo stimulation with CMX (n = 4; *p<0.05, different from the saline-vaccinated group). The results are representative of two independent experiments.
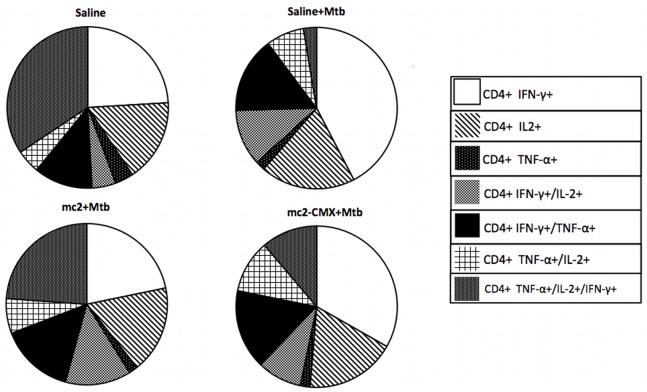
Triple-positive polyfunctional CD4+ T cells were evaluated in the spleen, and although Mtb induced increases in spleen CD4+IFN-γ+ and CD4+IL2+ cells, the infection significantly reduced the frequencies of triple-positive CD4+TNF-α+IFN-γ+ cells. Previous vaccination with mc2-CMX prevented this dramatic reduction in triple-positive cells, and doubled the frequency of CD4+TNF-α+IL2+ cells (Figure 7).
Figure 7. Reduction in splenic polyfunctional CD4+ T cells after M. tuberculosis infection prevented by mc2-CMX vaccination.
Mice were challenged with Mtb and 45 days after the infection, their spleens were collected for flow-cytometric analysis. CD4+ T cells selected with lymphocyte gating for IFN-γ, TNF-α, and IL-2 were quantified. Populations were defined as singly, doubly, or triply positive for the cytokines.
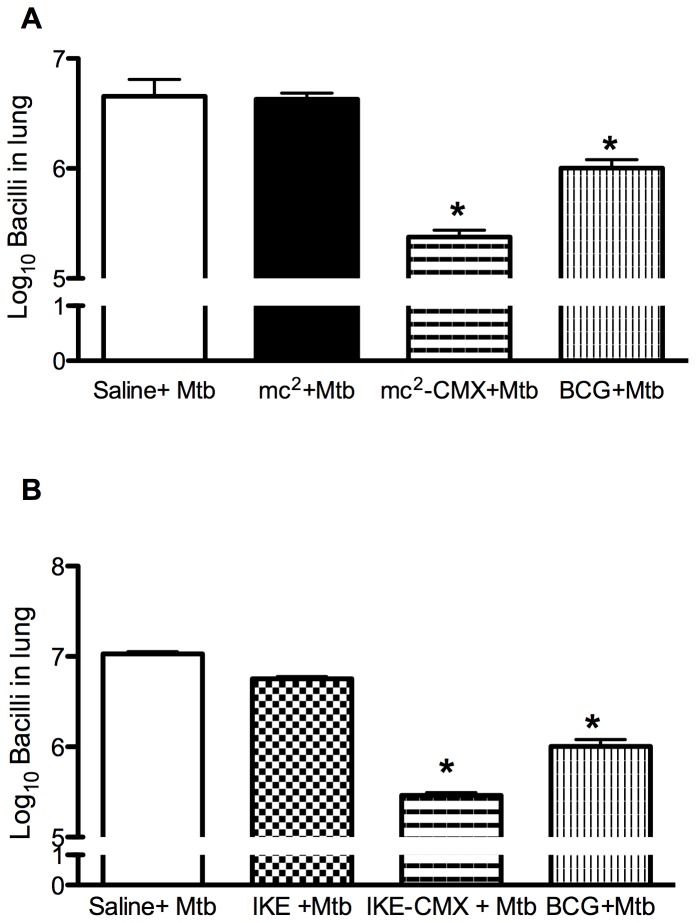
We then assessed whether vaccination with mc2-CMX protected the mice against Mtb challenge. The mc2-CMX vaccine reduced the number of bacteria recovered from the lungs by approximately 1 log unit, compared with both the nonvaccinated (saline control) and mc2-vaccinated groups. Surprisingly mc2-CMX vaccinated group showed 0.5 Log of CFU reduction when compared to BCG vaccinated group (Figure 8A).
Figure 8. Vaccination with mc2-CMX or IKE-CMX reduced the bacterial load in Mtb-challenged mice.
Groups of mice vaccinated with saline, mc2, mc2-CMX, or BCG (A), and then vaccinated with saline, IKE, IKE-CMX, or BCG (B) were challenged with Mtb 45 days after immunization. Thirty days after infection, their lungs were collected and CFU counted. *p<0.05 when compared with the saline+Mtb group.
We also investigated whether the recombinant fusion protein could induce protection against Mtb in the context of the safer M. smegmatis background in the same way as was shown here. IKE M. smegmatis was transformed with pLA71 containing CMX (IKE-CMX) and after mice were subjected to the same vaccination scheme, they were challenged with Mtb. IKE alone reduced the bacterial load compared with that in the infection-only control group, as shown previously [19], whereas the IKE-CMX vaccine reduced the bacterial load even further when compared to BCG vaccination (Figure 8B; p<0.05).
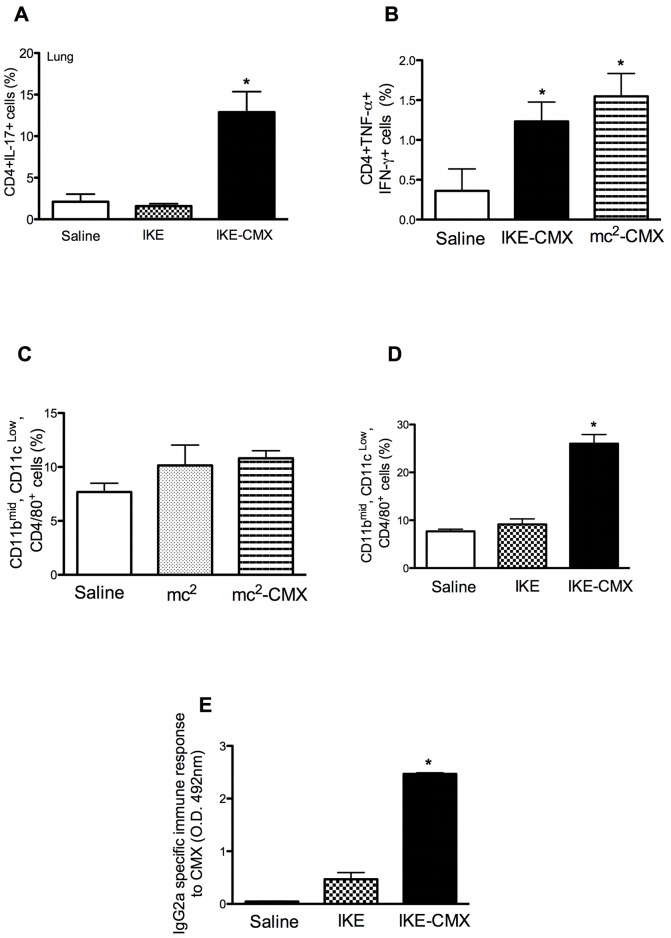
Once we had established that both vaccines reduced the bacterial load in the lung and spleen, and that the protection induced by IKE-CMX was similar to that induced by mc2-CMX, we analyzed the specific immune response to CMX and macrophage activation. As shown in Figure 9, IKE-CMX induced the same frequencies of both CD4+IL17+ and CD4+TNF-α+IFN-γ+ cells as mc2-CMX (A and B) and similar results were observed for all polyfunctional and single-positive CD4+ T cells (data not shown). However, although there was no difference in the proportion of spleen macrophages (CD11bmid, CD11clow, CD4+/80+ cells) after mc2-CMX vaccination (Figure 9C), IKE-CMX induced a robust increase in the frequency of this cell population (Figure 9D). Similar to the results obtained with mc2-CMX, the IKE-CMX vaccine induced a specific humoral immune response to CMX (Figure 9E).
Figure 9. IKE-CMX vaccination activates CD4+ T cells, macrophages, and the IgG2a immune response.
Groups of mice vaccinated with saline, IKE, or IKE-CMX (A) and then vaccinated with saline, IKE-CMX, or mc2-CMX (B) were challenged with Mtb 45 days after immunization. Thirty days after infection, their lungs were collected. CD4+ T cells selected with lymphocyte gating for IL-17 (A), or IFN-γ and TNF-α (B) were quantified. Lung macrophages, defined as CD11bmid, CD11clow, and F4/80+, were quantified after mc2-CMX (C) or IKE-CMX (D). ELISA was used to detect CMX-specific serum IgG2a after the IKE-CMX vaccination scheme (E).
Discussion
In this study, a vaccine composed of a nonpathogenic mycobacterium expressing a recombinant fusion protein composed of epitopes from Mtb antigens (CMX) induced a significant immune response in mice, affording them protection against Mtb challenge.
Mycobacterium smegmatis is a nonpathogenic member of the genus Mycobacterium that grows rapidly and is easily manipulated to produce recombinant bacteria. Consequently, it is a valuable host for use as a live vaccine against TB [19], [21]. Mycobacterium smegmatis does not survive long within the cellular host environment because it is readily eliminated by phagosomal proteases [22]. Therefore, unlike pathogenic mycobacteria, these bacteria are processed upon infection and presented very rapidly by antigen-presenting cells [23]. Our results show that M. smegmatis expressing CMX survived for up to 8 days after vaccination, indicating that this period of time was sufficient to induce a specific immune response to CMX. This result corroborates the notion that nonpathogenic mycobacteria induce macrophages that produce higher levels of innate related cytokines than those induced by pathogenic mycobacteria [24]. They also activate dendritic cells by upregulating class I major histocompatibility complex and costimulatory molecules [25].
Another interesting characteristic of this bacterium, making it a suitable vaccine vector, is its nontoxicity in immunodeficient animal models lacking NK or T cells, as seen in the studies by Young et al. This suggests that this vehicle is safe for use as a vaccine in immunocompromised individuals [26], [27]. Consequently, M. smegmatis has been used to express recombinant proteins in several studies. A particular M. smegmatis recombinant vaccine expressing a fusion protein containing ESAT-6 and CFP10 induced higher humoral and cellular immunity than M. bovis BCG vaccination in a mouse model [21]. Similarly, Yi et al. demonstrated that a recombinant M. smegmatis vaccine that produced a fusion of murine IL12 and human granulysin in BALB/c mice induced an efficient protective Th1 response, including high levels of IFN-γ and IL12, similar to that induced by M. bovis BCG [28]. Here we showed that recombinant M. smegmatis vaccines expressing CMX in two different backgrounds (mc2 and IKE) were able to reduce the bacterial load of lungs from Mtb infected animals, when compared to M. bovis BCG vaccinated BALB/c mice.
We have shown that our recombinant vaccine mc2-CMX is capable of inducing a specific humoral response to CMX in three different vaccination schemes. This response probably reflects the immunogenicity of the recombinant fusion protein when used in this vaccine vector. The results of our group and others have confirmed that Ag85C, MPT51, and HspX are Mtb antigens with immunogenic and antigenic properties represented by the epitopes expressed in the recombinant fusion protein [6], [12], [14], [15], [29]–[31]. Because this protein specifically induced both IgG2a and IgG1, we can infer that both Th1 (IFN-γ) and Th2 (IL4) subpopulations of cells were induced [32], [33].
The CMX-specific response of CD4+ and CD8+ T cells ex vivo showed that both vaccines, with or without CMX expression, induced IFN-γ-positive CD4+ and CD8+ T cells. The ability of mc2 alone to induce a response to CMX might seem unexpected, but it can be explained by the fact that M. smegmatis contains genes that encode Ag85C, MPB-51, and a hypothetical protein MSMEG_3932, with high similarities (up to 86%) to the partial sequences of Mtb Ag85C, MPT51, and HspX, respectively, which were used to construct the recombinant fusion protein [15]. Further evidence that mc2 expresses those individual proteins and that they cross-react with polyclonal antibodies directed against CMX is presented in Fig. 1A. Despite these CD4 and CD8 T cells responses in both vaccinated groups (mc2 and mc2-CMX), only mice vaccinated with mc2-CMX showed better protection. This raise the question addressed currently if interferon gamma alone is responsible for protection and could be used as surrogate marker for protection once individuals with active TB presents specific interferon producers T cells [34], [37].
Here, we have shown that vaccination with mc2 induced high levels of CD4+ T cells in spleens expressing IL17 that were not specifically generated by the presence of CMX. Nevertheless, lung Th17 CD4+ T cells were generated and responded specifically to CMX. Although it has recently been shown that IL17 and IL22 do not contribute to Mtb control in mice [34], several studies have suggested that Mtb vaccines generate long-lasting memory Th17 cells [20], [35]. However, to our knowledge, no previous work has investigated whether the expansion of lung specific Th17 cells before challenge with Mtb contributes to a protective effect. Additionally in this work, we showed that higher levels of Th17 cells in the spleens of vaccinated mice were not associated with protection because mice vaccinated with mc2 did not reduced the bacterial load. Prompting us to hypothesized that maybe only specific lung Th17 cells were important for protection [20], [35].
Vaccination with mc2-CMX induced a huge increase in CD4+IFN-γ+ cells specific to CMX in both the spleen and lungs. We speculate that the mobilization of specific Th1 and Th17 cells to the lungs before challenge with Mtb may contribute to the protective effect by increasing the activities of phagocytes.
IFN-γ and TNF-α act synergistically in mediating the killing of intracellular pathogens, increasing the bactericidal activity of macrophages, and preventing the spread of bacilli [36]–[38]. IL2 is necessary for the secondary expansion of memory T cells and therefore in the generation of persistent vaccine-induced immunity [39], [40]. The differences between the types of cytokines produced by individual cells have profound implications for their ability to mediate memory or effector functions. Therefore, the ability to induce persistent Mtb-specific T-cell responses is an important factor in the development new vaccines [41]. Double-positive CD4+ T cells have been shown to express CCR7 in their membranes and can proliferate in the presence of the antigen, and are therefore characterized as central memory cells [42]–[44]. Although the results presented here do not show this cell-surface marker, we speculate that the CD4+TNF-α+IL-2+ cells were memory cells.
Polyfunctional specific CD4+ T cells were detected in animals vaccinated with mc2 and this response increased after immunization with mc2-CMX, which induced the highest numbers of CD4+TNF-α+IFN-γ+ cells. Several studies have shown that the induction of polyfunctional T cells correlates directly with protection from intracellular pathogens, such as viral infections, parasites, and chronic bacterial infections [44]–[48]. It also appears that the highest frequency of polyfunctional specific CD4+ T cells co-expressing IL2, IFN-γ, and TNF-α is associated with the best outcomes of different vaccine formulations directed against Mtb [47], [48]. It is very important to note that in this study, Mtb infection reduced the numbers of polyfunctional CD4+ T cells and that this phenomenon was prevented by the mc2-CMX vaccine. Although our results are consistent with those of previous studies, some controversial findings regarding polyfunctional CD4+ T cells clearly indicate the need of further research to understand their role in TB [47], [49]–[51].
We could not determine whether the immune responses to Ag85C, MPT51, and HspX were directly responsible for the reduced bacterial load observed in our results. Those three antigens are very important for Mtb survival, both in vivo and in vitro. Ag85C is a member of the family of proteins responsible for mycolic acid synthesis, a critical cell wall component [52], MPT51 is an α-hydrolase and has been shown to bind to host fibronectin [53], and HspX is a secreted protein produced by bacteria under stress conditions [54]. Therefore, the specific immune response induced to those antigens and others expressed by mc2 and other vaccines may have impaired bacterial growth in the organs examined. It is important to notice that both recombinant vaccines expressing CMX presented better protective performance than BCG, favoring our conclusions.
The expression of CMX in an IKE background increased the efficacy of our recombinant vaccine. The development a vaccine in a genetic background that has already been shown to be safe for immunocompromised mice or patients is essential (19). IKE-CMX induced almost double the number of CD4+IL17+ cells, which are associated with the memory response (20). There was also a huge increase in spleen macrophages (CD11bmid, CD11clow, CD4+/80+), suggesting that this recombinant strain of M. smegmatis is handled by the innate immune response, resulting in the generation of improved effector and protective memory immune responses.
In conclusion, the expression of CMX improved the immune responses and the protection induced when M. smegmatis mc2 or IKE were used as a vaccine against TB.
Supporting Information
Flow cytometry quadrant sets to quantify CD4+IFN-γ+TNF-α+ T spleen cells. Representative dot plots of spleen CD4+ T cells expressing IFN-γ and TNF-α are shown. Lymphocytes were gated based upon size and granulocity. Then CD4+ were further gated and analyzed for cytokines expression. Quadrant sets were set using panels with anti- CD4 FITC and rat IgG1-APC and rat IgG1- PE and then compared to the panels using anti- CD4 -FITC, anti-IFN-γ -APC and anti-TNF-α- PE.
(TIFF)
Acknowledgments
The authors thank the Flow Cytometry Core Facility of Albert Einstein College of Medicine.
Funding Statement
This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grants numbers: 301976/2011-2, 472906/2011-9, 301198/2009-8, 472909/2011-8); Fundação de Amparo a Pesquisa do Estado de Goiás: FAPEG-PRONEX. APJK was awarded with a Science without borders senior training fellowship from CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2012) Global tuberculosis report 2012.
- 2. Andersen P, Doherty TM (2005) The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol 3: 656–662. [DOI] [PubMed] [Google Scholar]
- 3. Raviglione M, Marais B, Floyd K, Lonnroth K, Getahun H, et al. (2012) Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 379: 1902–1913. [DOI] [PubMed] [Google Scholar]
- 4. Rabahi MF, Junqueira-Kipnis AP, Dos Reis MC, Oelemann W, Conde MB (2007) Humoral response to HspX and GlcB to previous and recent infection by Mycobacterium tuberculosis . BMC Infect Dis 7: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reis MC, Rabahi MF, Kipnis A, Junqueira-Kipnis AP (2009) Health care workers humoral immune response against GLcB, MPT51 and HSPX from Mycobacterium tuberculosis . Braz J Infect Dis 13: 417–421. [DOI] [PubMed] [Google Scholar]
- 6. Reis MC, Silva BD, Sousa EM, Junqueira-Kipnis AP (2011) Role of antibodies reactive to HspX in discriminating pulmonary tuberculosis contacts with high risk of developing active disease. Braz J Infect Dis 15: 617–618. [DOI] [PubMed] [Google Scholar]
- 7. Bretl DJ, He H, Demetriadou C, White MJ, Penoske RM, et al. (2012) MprA and DosR coregulate a Mycobacterium tuberculosis virulence operon encoding Rv1813c and Rv1812c. Infect Immun 80: 3018–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Araujo-Filho JA, Vasconcelos AC Jr, de Sousa EM, Kipnis A, Ribeiro E, et al. (2008) Cellular responses to MPT51, GlcB and ESAT-6 among MDR-TB and active tuberculosis patients in Brazil. Tuberculosis (Edinb) 88: 474–481. [DOI] [PubMed] [Google Scholar]
- 9. Melo CCA, Vasconcelos AC Jr, Kipnis A, Andrade AL, Junqueira-Kipnis AP (2008) Humoral immune responses of tuberculosis patients in Brazil indicate recognition of Mycobacterium tuberculosis MPT-51 and GlcB. Clin Vaccine Immunol 15: 579–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Achkar JM, Jenny-Avital E, Yu X, Burger S, Leibert E, et al. (2010) Antibodies against immunodominant antigens of Mycobacterium tuberculosis in subjects with suspected tuberculosis in the United States compared by HIV status. Clin Vaccine Immunol 17: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa ACR, Guerreiro MCG; Silva, BDS, Trentini, MM, Junqueira-Kipnis, AP (2011.) Resposta Imune Humoral ao antígeno rGroEs do Mycobacterium tuberculosis em pacientes com tuberculose e seus contatos domiciliares. Revista de Patologia Tropical (Impresso) 40: p.23–34.
- 12. Kashyap RS, Shekhawat SD, Nayak AR, Purohit HJ, Taori GM, et al. (2013) Diagnosis of tuberculosis infection based on synthetic peptides from Mycobacterium tuberculosis antigen 85 complex. Clin Neurol Neurosurg 115: 678–683. [DOI] [PubMed] [Google Scholar]
- 13. Limongi LC, Olival L, Conde MB, Junqueira-Kipnis AP (2011) Determination of levels of specific IgA to the HspX recombinant antigen of Mycobacterium tuberculosis for the diagnosis of pleural tuberculosis. J Bras Pneumol 37: 302–307. [DOI] [PubMed] [Google Scholar]
- 14. Silva BD, da Silva EB, do Nascimento IP, Dos Reis MC, Kipnis A, et al. (2009) MPT-51/CpG DNA vaccine protects mice against Mycobacterium tuberculosis . Vaccine 27: 4402–4407. [DOI] [PubMed] [Google Scholar]
- 15. de Sousa EM, da Costa AC, Trentini MM, de Araujo Filho JA, Kipnis A, et al. (2012) Immunogenicity of a fusion protein containing immunodominant epitopes of Ag85C, MPT51, and HspX from Mycobacterium tuberculosis in mice and active TB infection. PLoS One 7: e47781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim EM, Rauzier J, Timm J, Torrea G, Murray A, et al. (1995) Identification of mycobacterium tuberculosis DNA sequences encoding exported proteins by using phoA gene fusions. J Bacteriol 177: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pavelka MS Jr, Jacobs WR Jr (1999) Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J Bacteriol 181: 4780–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bardarov S, Bardarov Jr S Jr, Pavelka Jr MS Jr, Sambandamurthy V, Larsen M, et al. (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis . Microbiology 148: 3007–3017. [DOI] [PubMed] [Google Scholar]
- 19. Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, et al. (2011) A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis . Nat Med 17: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindenstrom T, Woodworth J, Dietrich J, Aagaard C, Andersen P, et al. (2012) Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun 80: 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H, Peng P, Miao S, Zhao Y, Mao F, et al. (2010) Recombinant Mycobacterium smegmatis expressing an ESAT6-CFP10 fusion protein induces anti-mycobacterial immune responses and protects against Mycobacterium tuberculosis challenge in mice. Scand J Immunol 72: 349–357. [DOI] [PubMed] [Google Scholar]
- 22. Kuehnel MP, Goethe R, Habermann A, Mueller E, Rohde M, et al. (2001) Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell Microbiol 3: 551–566. [DOI] [PubMed] [Google Scholar]
- 23. Cheadle EJ, O’Donnell D, Selby PJ, Jackson AM (2005) Closely related mycobacterial strains demonstrate contrasting levels of efficacy as antitumor vaccines and are processed for major histocompatibility complex class I presentation by multiple routes in dendritic cells. Infect Immun 73: 784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bohsali A, Abdalla H, Velmurugan K, Briken V (2010) The non-pathogenic mycobacteria M. smegmatis and M. fortuitum induce rapid host cell apoptosis via a caspase-3 and TNF dependent pathway. BMC Microbiol 10: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cayabyab MJ, Hovav AH, Hsu T, Krivulka GR, Lifton MA, et al. (2006) Generation of CD8+ T-cell responses by a recombinant nonpathogenic Mycobacterium smegmatis vaccine vector expressing human immunodeficiency virus type 1 Env. J Virol 80: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young SL, Murphy M, Zhu XW, Harnden P, O’Donnell MA, et al. (2004) Cytokine-modified Mycobacterium smegmatis as a novel anticancer immunotherapy. Int J Cancer 112: 653–660. [DOI] [PubMed] [Google Scholar]
- 27. Yang C, He YL, Zhang L, Xu L, Yi Z, et al. (2009) GLS/IL-12-modified Mycobacterium smegmatis as a novel anti-tuberculosis immunotherapeutic vaccine. Int J Tuberc Lung Dis 13: 1360–1366. [PubMed] [Google Scholar]
- 28. Yi Z, Fu Y, Yang C, Li J, Luo X, et al. (2007) Recombinant M. smegmatis vaccine targeted delivering IL-12/GLS into macrophages can induce specific cellular immunity against M. tuberculosis in BALB/c mice. Vaccine 25: 638–648. [DOI] [PubMed] [Google Scholar]
- 29. da Silva EB, Silva BD, Leon JR, Kipnis A, Santos IK, et al. (2011) Using BCG, MPT-51 and Ag85 as antigens in an indirect ELISA for the diagnosis of bovine tuberculosis. Vet J 187: 276–278. [DOI] [PubMed] [Google Scholar]
- 30. Taylor JL, Wieczorek A, Keyser AR, Grover A, Flinkstrom R, et al. (2012) HspX-mediated protection against tuberculosis depends on its chaperoning of a mycobacterial molecule. Immunol Cell Biol 90: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geluk A, van den Eeden SJ, van Meijgaarden KE, Dijkman K, Franken KL, et al. (2012) A multistage-polyepitope vaccine protects against Mycobacterium tuberculosis infection in HLA-DR3 transgenic mice. Vaccine 30: 7513–7521. [DOI] [PubMed] [Google Scholar]
- 32. Flynn JL, Chan J (2001) Immunology of tuberculosis. Annu Rev Immunol 19: 93–129. [DOI] [PubMed] [Google Scholar]
- 33. Maassen CB, Boersma WJ, van Holten-Neelen C, Claassen E, Laman JD (2003) Growth phase of orally administered Lactobacillus strains differentially affects IgG1/IgG2a ratio for soluble antigens: implications for vaccine development. Vaccine 21: 2751–2757. [DOI] [PubMed] [Google Scholar]
- 34. Torrado E, Cooper AM (2010) IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev 21: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chatterjee S, Dwivedi VP, Singh Y, Siddiqui I, Sharma P, et al. (2011) Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog 7: e1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bogdan C, Moll H, Solbach W, Rollinghoff M (1990) Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol 20: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 37. Flynn JL (2006) Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect 8: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 38. Ulrichs T, Kaufmann SH (2006) New insights into the function of granulomas in human tuberculosis. J Pathol 208: 261–269. [DOI] [PubMed] [Google Scholar]
- 39. Williams MA, Tyznik AJ, Bevan MJ (2006) Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, et al. (2010) Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol 40: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tenbusch M, Kuate S, Tippler B, Gerlach N, Schimmer S, et al. (2008) Coexpression of GM-CSF and antigen in DNA prime-adenoviral vector boost immunization enhances polyfunctional CD8+ T cell responses, whereas expression of GM-CSF antigen fusion protein induces autoimmunity. BMC Immunol 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, et al. (2002) Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol 3: 852–858. [DOI] [PubMed] [Google Scholar]
- 43. Zaph C, Uzonna J, Beverley SM, Scott P (2004) Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med 10: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 44. Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, et al. (2007) Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major . Nat Med 13: 843–850. [DOI] [PubMed] [Google Scholar]
- 45. Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, et al. (2007) Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol 81: 12071–12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ciuffreda D, Comte D, Cavassini M, Giostra E, Buhler L, et al. (2008) Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol 38: 2665–2677. [DOI] [PubMed] [Google Scholar]
- 47. Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, et al. (2008) Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 181: 4955–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, et al. (2009) Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 4: e5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, et al. (2009) HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med 180: 1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, et al. (2010) Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol 40: 2211–2220. [DOI] [PubMed] [Google Scholar]
- 51. Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, et al. (2011) Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187: 2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kremer L, Maughan WN, Wilson RA, Dover LG, Besra GS (2002) The M. tuberculosis antigen 85 complex and mycolyltransferase activity. Lett Appl Microbiol 34: 233–237. [DOI] [PubMed] [Google Scholar]
- 53. Wilson RA, Maughan WN, Kremer L, Besra GS, Futterer K (2004) The structure of Mycobacterium tuberculosis MPT51 (FbpC1) defines a new family of non-catalytic alpha/beta hydrolases. J Mol Biol 335: 519–530. [DOI] [PubMed] [Google Scholar]
- 54. Siddiqui KF, Amir M, Agrewala JN (2011) Understanding the biology of 16 kDa antigen of Mycobacterium tuberculosis: scope in diagnosis, vaccine design and therapy. Crit Rev Microbiol 37: 349–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometry quadrant sets to quantify CD4+IFN-γ+TNF-α+ T spleen cells. Representative dot plots of spleen CD4+ T cells expressing IFN-γ and TNF-α are shown. Lymphocytes were gated based upon size and granulocity. Then CD4+ were further gated and analyzed for cytokines expression. Quadrant sets were set using panels with anti- CD4 FITC and rat IgG1-APC and rat IgG1- PE and then compared to the panels using anti- CD4 -FITC, anti-IFN-γ -APC and anti-TNF-α- PE.
(TIFF)