Summary
Nitric oxide (NO) and NO synthase 1 (NOS1) maintains sodium and water homeostasis. NOS1α and NOS1β splice variants are expressed in the rat inner medulla, but only NOS1β expressed in the mouse. Collecting duct NOS1 is necessary for blood pressure control. We hypothesized that NOS1 splice variant expression and NO production in the inner medullary collecting duct (IMCD) are regulated distinctly in mice and rats by high dietary sodium.
Male C57blk/J6 mice and Sprague-Dawley rats were fed a 0.4% (normal salt, NS), or 4% (high salt, HS) NaCl diet for 2 or 7 days.
Mean arterial pressure was not altered with HS, while urinary sodium excretion in mice and rats was significantly increased. Urinary NOx excretion and IMCD nitrite production was significantly greater in mice on HS compared to rats. Western blotting indicated that only NOS1β and NOS3 were expressed in the mouse IMCD and that expression was unaffected by HS diet at either time point. NOS1α and β, as well as NOS3 were detected in the IMCD of the rat. Two-day HS diet increased NOS1α and NOS1β IMCD expression in the rat, and 7-day HS further increased NOS1β expression. While NOS3 expression was unchanged by HS diet at either time point.
In conclusion, IMCD NO production in mice and rats is distinctly regulated under both NS and HS conditions including expression of NOS1 splice variants.
Keywords: Collecting duct, nitric oxide, nitric oxide synthase, salt, kidney, inner medulla, NOS1 splice variants
INTRODUCTION
Renal nitric oxide production is necessary for regulation of ion and water homeostasis and there is experimental evidence to suggest that specifically medullary nitric oxide synthase-1 (NOS1; nNOS) is involved in the regulation of blood pressure (1, 2). Renal medullary infusion of NOS1 oligonucleotides or pharmacological inhibitors in the Sprague-Dawley rat on a high salt diet (HS), lead to a significant elevation of mean arterial pressure (MAP)(1). In agreement with this, the collecting duct-specific endothelin-1 knockout mouse (CDET1KO) is hypertensive and has reduced inner medullary NOS activity (2). Thus, it appears in rats and mice that medullary NOS1 is involved in blood pressure regulation. However, recently it was reported that rats express at least three NOS1 splice variants in the kidney, NOS1α, NOS1β, and NOS1γ (3–5). NOS1α is the full length variant, with a molecular weight of 160 kDa. NOS1β and NOS1γ are N-truncated variants that lack a PDZ domain, and have a molecular weight of 130 kDa and 125 kDa, respectively (6, 7). NOS1β possesses activity comparable to NOS1α, while NOS1γ has only ~3% of the enzymatic activity (6, 7). We recently reported that the rat inner medulla expresses both NOS1α and NOS1β (3). However, in the mouse inner medullary collecting duct (IMCD) or in mIMCD-3 cells (an in vitro mouse cell line), only NOS1β is expressed (3). Given that rats and mice have differential expression of the NOS1 splice variants in the inner medulla, we hypothesized that regulation of IMCD NO production may be species specific.
Urinary excretion of NOx, an index of NO production, increases in response to high-salt diet (HS) in both rats (8–10) and mice (11–13). Although urinary NOx is classically considered a measure of total body NO, more recent studies suggest that it may represent a measure of renal NO production (14). Yet, the salt-sensitive NOS isoform and/or NOS1 splice variant that may be contributing to changes in urinary NOx is controversial and may be tubule specific. For example, in the thick ascending limb (TAL), NOS3 is the major isoform responsible for NO production in both rats and mice (15). In the rat TAL, HS for 7 days increased NOS3 expression but there was not a change in NO production, suggesting a dissociation between expression and activity (16). In the rat macula densa, 10 day HS reduced the expression of NOS1α and increased the expression of NOS1β, and lead to an increase in NO production resulting in an attenuation of tubuloglomerular feedback (TGF) (5). In agreement with this finding, NOS1αKO mice have an intact TGF mechanism (17), even when the perfusate is switched to high NaCl perfusate (18). This suggests that the NOS1β and/or NOS1γ splice variant mediates TGF, although this has not been tested directly. In the rat, IMCDs have the highest total NOS activity (19), but how high salt diet affects NO production and NOS1 splice variant expression in the rodent IMCD has not been addressed.
The aim of this study was to test the hypothesis that high salt diet induces NO production and NOS1 expression distinctly in the renal IMCD in mice and rats. To accomplish this aim, we measured nitrite production, NOS isoform expression, and NOS activity in isolated IMCDs from mice and rats on normal and high salt diet.
METHODS
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) and male C57BL/6J mice from Jackson Labs (Bar Harbor, ME) were studied in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved and monitored by the Georgia Health Sciences University Institutional Animal Care and Use Committee. Rats and mice were housed in temperature and humidity controlled, light-cycled quarters. The rats (10–12 weeks, ~250 g) were randomly assigned to either normal salt (NS, 0.4% NaCl) or high salt (HS, 4% NaCl) pellet diet groups (Teklad diets [Harlan], Indianapolis, IN). Groups of animals were maintained on the HS for either 2 days or 7 days. Rats were anesthetized using sodium pentobarbital (50 mg/kg) followed by a thoracotomy. The mice (10–12 weeks old, ~26 g) were randomly assigned to NS or HS pellet diets (Teklad diet, same as the rat diet) for 2 or 7 days, and were anesthetized using Brevital® (methohexital, 6 mg/kg, JHP Pharmaceuticals, Parsippany, NJ) followed by thoracotomy. The renal inner medullae of the mice and rats were dissected and the inner medullary collecting ducts (IMCDs) were freshly isolated using the method previously described by Hyndman et al. (3). Isolated inner medullary collecting ducts were incubated for analysis of nitrite production, or snap frozen in liquid nitrogen for NOS activity analysis, or Western blot analysis.
Mean arterial pressure
Telemetry devices (Data Sciences, PA-C10, St. Paul, MN) were implanted into the left carotid artery of isoflurane anesthetized mice. Mice were allowed 10 days to recover before collection of telemetry data. Rat telemetry devices were implanted as previously described (20).
Metabolic Cage study
Mice or rats were housed individually in metabolic cages to monitor 24 h food and water consumption and to collect urine while on a NS or 7-day HS diet. The urine was centrifuged at 1000 g for 5 min, and stored at −80°C until analysis. Urinary sodium was determined by ion selective electrode (Medica Easylyte, Bedford, MA), and urinary nitrite + nitrate (NOx) determined by High Performance Liquid Chromatography (HPLC, ENO20, Eicom, Japan). Urinary NOx and sodium excretion rates for the mice and rats were determined by multiplying the urinary NOx concentration or sodium concentration by the 24 hr urine volume and normalizing to food intake and body weight.
Western Blotting
Western blotting protocol was done as previously described (21) with minor modifications. Briefly, the frozen renal IMCD were homogenized and protein concentrations were determined by standard Bradford assay (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard. Proteins were separated by 8% SDS-PAGE and transferred to an Immobilon membrane (Millipore, Bedford, MA). Two-color immunoblots were performed using an antibody against C-terminus of NOS1 (amino acids 1095–1289, 1:1,000, BD Transduction Laboratories, NJ), and a monoclonal antibody to β-actin (1:5000, Sigma, St. Louis, MO). Additionally, two-color immunoblots were performed using a monoclonal antibody against C-terminus of NOS3 (amino acids 1025–1203, 1:1000, BD transduction Laboratories, NJ) and a polyclonal antibody to actin (1:5000, Sigma, St. Louis, MO). For mouse IMCD immunoblots, a polyclonal antibody against the C-terminus of NOS1 (R20, rat origin, Santa Cruz Biotechnology, CA) and monoclonal antibody to β-actin (1/5000) was used. The membranes were washed 5 times with tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST), then incubated in the appropriate infra-red-tagged secondary antibodies, IRD700-labeled goat anti-mouse or goat-anti-rabbit (1:1,000) and IRD800-labeled goat anti-rabbit or goat anti-mouse (1:10,000, Rockland, PA). Specific bands were detected using the Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE). Infra-red immunoreactive bands were visualized brightness and contrast modified and densitometry determined by using the Odyssey software (v.3.0.16).
Nitrite production
Freshly isolated collecting ducts were immediately incubated with Hank’s Balanced Salt Solution (HBSS, no phenol red, plus calcium and magnesium; Mediatech, Manassas, VA), 200 U/ml superoxide dismutase (Sigma) and 250 μM L-arginine (Sigma), for 1 h in a shaking, 37°C water bath. IMCDs were pelleted by centrifuging 5000 g for 5 min, and the HBSS snap frozen and stored at −80°C until analysis. Collecting ducts were lysed in 100 μl of buffer and protein concentration determined by the Bradford assay. Nitrite was measured by HPLC and nitrite production recorded as pmol nitrite/mg protein/h.
Measurement of NOS Activity
Isolated rat IMCDs were homogenized in buffer (weight-to-volume ratio,1:7): ice-cold 50 mM Tris (pH 7.4) containing 0.1 mM EDTA, 0.1 mM EGTA, 0.1% 2-mercaptoethanol, 10% glycerol, in the presence of protease inhibitors (1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μM pepstatin A, 2 μM leupeptin, and 0.1% aprotinin). Protein concentrations were determined by the Bradford assay as described above. Aliquots of homogenate were incubated with [3H]arginine (10 μM final arginine, 71 Ci/mmol; Amersham, Arlington Heights, IL) in the presence of 1 mM NADPH, 30 nM calmodulin, 3 μM tetrahydrobiopterin, 2 mM CaCl2, 1 μM FAD, and 1 μM FMN in a final volume of 50 μl for 30 minutes at room temperature as previously described (22). The non-specific NOS inhibitor Nω-nitro-L-arginine (LNNA, 1 mmol/L) was used to determine total NOS activity and the NOS 1-specific inhibitor N5-(1-imino-3-butenyl)-L-ornithine (VNIO, 1 μmol/L; Cayman Chemicals, Ann Arbor, MI) was used to assess NOS 1-specific activity. NOS activity was calculated using the following formula: Total NOS activity = (pmol NOS activity in the absence of LNNA) - (pmol NOS activity in the presence of LNNA). NOS1 specific activity was calculated the total NOS activity inhibited by VNIO using the following formula: NOS1 specific activity = [total NOS activity (pmol of NOS activity in the presence of VNIO. NOS2 specific activity was calculate as the total NOS activity inhibited by N-(3-(Aminomethyl)benzyl)acetamidine (1400W) using the formula: NOS2 specific activity = Total NOS activity- pmol of NOS activity in the presence of 1400W. NOS3 activity was calculated using the formula: NOS3 specific activity = Total NOS activity- NOS1 specific activity- NOS2 specific activity. Data is normalized to milligram of protein and reported as pmol/mg protein/30 min.
Data analysis
All data are expressed as mean ± s.e.m. The effect of dietary sodium on NOS activity and expression were analyzed by a one-way ANOVA with Dunnett’s multiple comparison post hoc test (Prism, GraphPad Software, San Diego, CA). To test for the effect of a 7-day HS diet compared to NS on sodium excretion or the effect of species on NOx excretion, a paired T-test or unpaired T-test, respectively was used. For all comparisons, P < 0.05 was considered significant.
RESULTS
Urinary sodium and NOx excretion
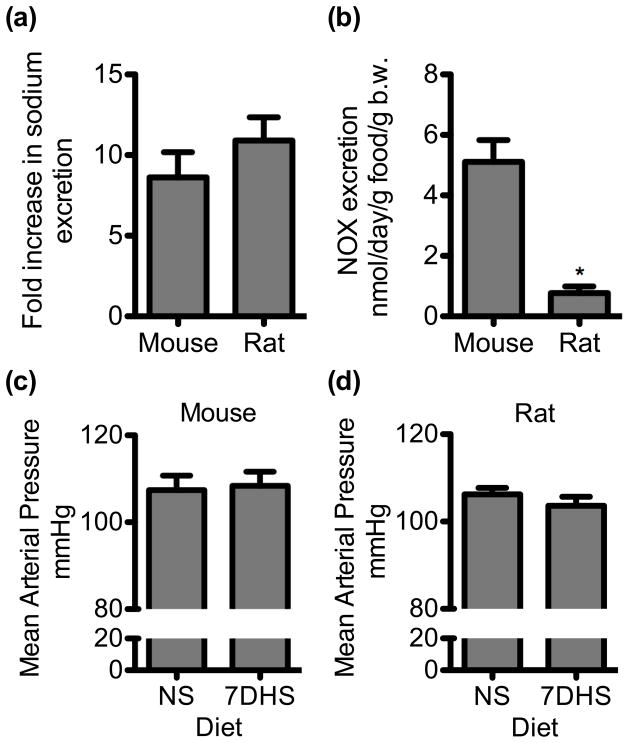
We tested whether urinary sodium and NOx excretion while on a HS diet is different between mice and rats. Mice on a NS and 7-day HS diet ate similar amounts of food (NS = 3.2 ± 0.5, HS = 3.9 ± 0.7 g, P = 0.48). Rats on a NS and 7-day HS diet ate similar amounts of food (NS = 25.3 ± 1.1 g, HS = 26.3 ± 0.8 g, P = 0.35). Urinary sodium excretion was determined from mice and rats on a NS or 7-day HS diet (Fig 1A). Given that mice and rats have significantly different body weights (b.w.) and intake significantly different amounts of food, and hence sodium, we normalized sodium excretion to b.w. and g food intake. In mice urinary sodium excretion was 1.3 ± 0.3 mmol/day/g food/g b.w. on a NS diet and significantly increased to 8.0 ± 0.6 mmol/day/g food/g b.w. on a 7-day HS diet (N = 8, P < 0.001). In rats on a NS diet, urinary sodium excretion was 0.13 ± 0.02 mmol/day/g food/g b.w. and was significantly increased compared to 7-day HS diet (1.3 ± 0.09 mmol/day/g food/g b.w., N= 6, P < 0.001). Although urinary sodium excretion rates are significantly different between mice and rats, the fold increase in sodium excretion due to 7-day HS diet is not significantly different between mice (9X) and rats (10×) (P = 0.32)(Fig 1A). Thus, mice and rats are under a similar salt stress. However, urinary NOx excretion, while on a 7-day HS diet, was significantly lower in rats compared to mice (P < 0.001) (Fig 1B). Mean arterial pressure was determined in a separate group of mice and rats under NS and 7DHS interventions. There was no significant difference in MAP between mice on a NS or 7DHS diet (N = 8, P = 0.8)(Fig 1C). Likewise, there was no significant difference in MAP between rats on a NS and 7DHS diet (N = 3, P = 0.4) (Fig 1D).
Figure 1.
Urinary sodium, NOx excretion and mean arterial pressure (MAP) from mice and rats. (a) The change in urinary sodium excretion from mice and rats on a normal salt (NS) diet compared to 7-day high salt (7DHS) diet (N = 6–8). (b) Urinary NOx excretion for mice and rats on a 7DHS. (c) MAP from mice on a NS or 7DHS diet (N = 8). (d) MAP from rats on a NS or 7dHS diet (N = 3). Mean ± s.e.m. An asterisk (*) represents a significant difference compared to mice (t-test, P < 0.001).
Inner medullary collecting duct nitrite production
NO production by freshly isolated mouse or rat IMCD was determined by measuring the NO metabolite, nitrite. Freshly isolated mouse IMCD had a significant increase in nitrite production from mice that had been fed a 2-day or 7-day HS diet (828 ± 30 and 1573 ± 278 pmol/mg protein/h, respectively; N = 8) compared to the NS mice (445 ± 75; N=8; Fig 2A) (P = 0.01). However, nitrite production from rat IMCD on a NS diet (131 ± 21 pmol/mg protein/h; N = 15) was similar to the 2-day HS (148 ± 38 pmol/mg protein/h; N=11) and 7-day HS (182 ± 54 pmol/mg protein/h; N = 8) (Fig 2B). Interestingly, isolated mouse IMCDs had a significantly higher nitrite production than rat IMCDs from either NS or HS diet (Fig 2).
Figure 2.
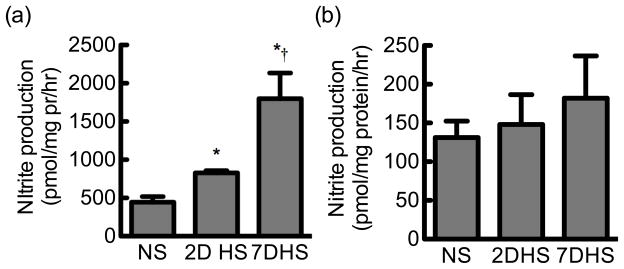
Inner medullary collecting duct nitrite production. (a) Nitrite produced by freshly isolated collecting ducts from mice on a normal salt (NS), 2-day high salt (2DHS) or 7-day high salt (7DHS) diet (N = 8–14). (b) Nitrite produced by freshly isolated collecting ducts from rats on a normal salt (NS), 2DHS, or 7DHS diet (N = 8). Mean ± s.e.m. An asterisk (*) represents a significant difference compared to NS and † represents a significant difference compared to 2DHS (ANOVA, P = 0.007).
NOS expression in the inner medullary collecting duct
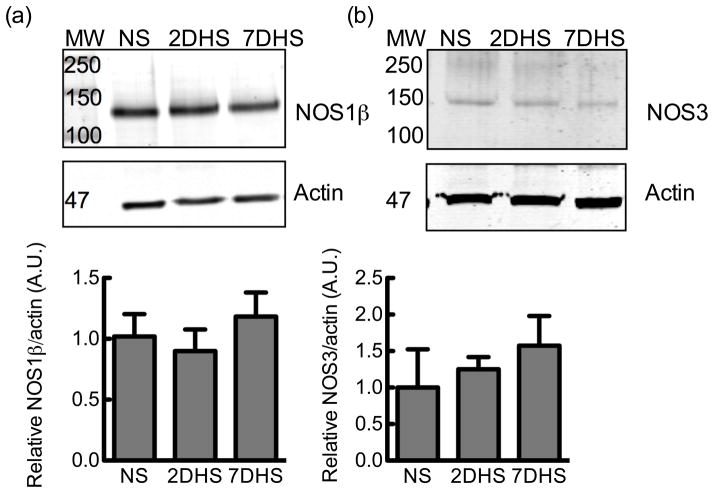
To determine if HS temporally regulates NOS1 splice variant expression in the IMCD, Western blots were analyzed with the C-terminus specific antibody of the renal IMCD from mice following NS or HS diet. The mouse IMCD express NOS1β (Fig 3A), as reported earlier (3); however 2-day or 7-day of a HS diet did not significantly affect NOS1β expression in the mouse IMCD (Fig 3A) (N = 6). The mouse collecting duct homogenate also expresses NOS3 (Fig 3B) and like NOS1β, 2-day or 7-day HS diet did not significantly affect NOS3 expression (Fig 3B) (N = 6).
Figure 3.
Mouse inner medullary collecting duct homogenate NOS expression. (a) Representative immunoblot of NOS1 splice variant expression from mice on a normal salt (NS), 2-day HS (2DHS) or 7-day high salt (7DHS) diet. The graph is the relative densitometry of the mouse collecting duct homogenate NOS1β immunoreactive band (N = 6). (b) NOS3 expression of collecting duct homogenate, and relative densitometry of the NOS3 immunoreactive band (N = 6). All values are normalized to their respective NS sample mean. Mean ± s.e.m.
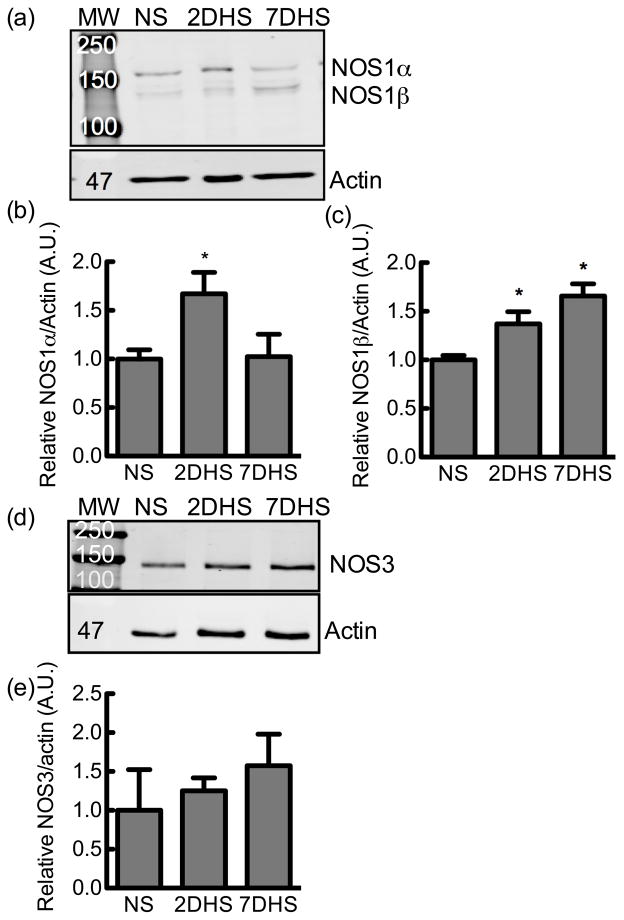
Both of the two splice variants, NOS1α and β, were detected in the rat IMCD homogenate (Fig 4). The IMCD NOS1 splice variants were temporally regulated by HS diet. Two days of HS diet significantly increased the expression of NOS1α (ANOVA P = 0.03, Dunnett’s post hoc NS vs 2-day HS P <0.05; NS vs 7-day HS P >0.05; N = 11–12) (Fig 4B), while 2-day and 7-day HS diet significantly increased NOS1β expression (ANOVA P = 0.0003, Dunnett’s post hoc NS vs 2-day HS P < 0.05; NS vs 7-day HS P <0.05; N = 11–12) (Fig 4C). High salt treatment did not significantly affect NOS3 expression in the rat IMCD (Fig 4E) (N = 5–7).
Figure 4.
Rat inner medullary collecting duct NOS1 splice variant or NOS3 expression. (a) A representative immunoblot of NOS1 splice variant expression of inner medullary homogenate from rats on a normal salt (NS), 2-day high salt (2DHS), or 7-day HS (7DHS) diet. (b) Relative densitometry of the NOS1α immunoreactive band, and (c) NOS1β immunoreactive band, N = 11–12. (d) A representative immunoblot of NOS3 expression of inner medullary collecting duct homogenate from rats on a normal salt (NS), 2-day high salt (2DHS), or 7-day HS (7DHS) diet, and relative densitometry, N = 5–7. All values are normalized to the NS sample mean. Mean ± s.e.m.
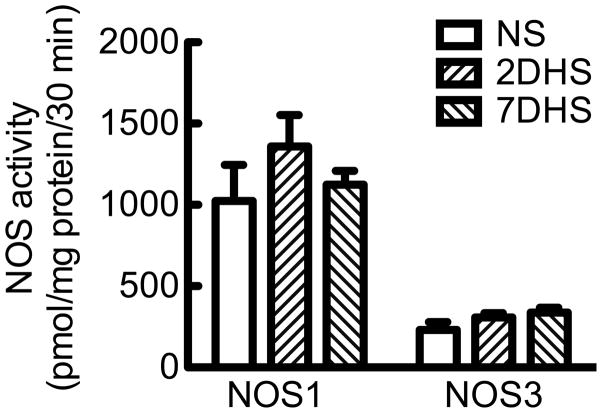
Inner medullary collecting duct NOS activity
NOS enzymatic activity was measured in the homogenate of rat renal IMCD following a NS diet and in response to HS diet for either 2 or 7 days. The rat IMCD homogenate had no detectable NOS2 activity (data not shown). The rat IMCD homogenate NOS1 activity did not change significantly among the NS (1024 ± 221 pmol/mg protein/30 min; N= 8), 2-day HS (1360 ± 192 pmol/mg protein/30 min; N=10) or 7-day HS (1124 ± 84 pmol/mg protein/30 min; N=6) (Fig 5). Likewise, rat IMCD NOS3 activity was similar among the NS (230 ± 48 pmol/mg protein/30 min; N=8), 2-day HS (307 ± 27 pmol/mg protein/30 min; N=10), or 7-day HS (337 ±31 pmol/mg protein/30 min; N= 6) (Fig 5). The rat IMCD has a ~3× higher NOS1 activity than NOS3 activity. NOS enzymatic activity measured by the conversion of radiolabeled arginine to citrulline with homogenates from mouse IMCD was below detection with the assay conditions as described and is a limitation of this study.
Figure 5.
NOS1 and NOS3 activity from rat inner medullary collecting duct homogenate. Rats were placed on a normal salt (NS), 2-day high salt (2DHS), or 7-day HS (7DHS) diet. Mean ± s.e.m., N = 6–10.
DISCUSSION
Our major finding reveals a distinct species-specific regulation of NO production and NOS1 splice variant expression in the IMCD under both normal salt and high salt conditions. NO production from mouse IMCD was over 3× greater than rat IMCD on either NS or HS diet correlating with a higher urinary NOx excretion observed in mice compared to rats. Mice exclusively express NOS1β and NOS3 in the IMCD, while the rat IMCD expresses NOS1α, NOS1β and NOS3. Although there were observed differences in mouse and rat IMCD NOS1 splice variant expression and NO production, urinary sodium excretion was increased similarly in both species. Moreover, MAP was not significantly affected by 7DHS diet in either species. These data suggest that there are multiple mechanisms involved in the regulation of fluid-electrolyte balance and blood pressure control and that these are species-specific.
There is not a consensus in the literature as to the effects of salt on NOS1 expression: NOS1 has been reported to decrease, remain unchanged, or increase depending on the conditions studied, antibodies used, and species utilized. NOS1 splice variants have been identified in the whole kidney of the rat (4, 5, 23), although functional or temporal changes were not determined. In agreement with our findings in the rat, NOS1 expression increased in the renal inner medulla following 3-weeks on 4% NaCl diet (24). Interestingly, Mattson and Higgins (24), reported that the anti-NOS1 antibody recognized a band at 160 kDa (now known as NOS1α) and a 130 kDa band that the manufacturer (Transduction Laboratories) claimed was NOS2. We speculate that this is actually NOS1β, and from re-evaluating the results presented in Mattson and Higgins (24), we found there was a significant increase in NOS1β, as well. Our results are also similar to those reported by Ni and Varzi that reported a significant increase in NOS1 after 2 days of 8.0% NaCl diet and Roczniak et al. (25), where NOS1α was significantly increased in the IMCD after 3 days of 3% NaCl diet. Interestingly, they reported an anti-NOS1 detectable band at 130 kDa, but did not show it, so it is unclear what effect their diet had on what we speculate was NOS1β. However, others report that NOS1 mRNA is unchanged in the IMCD following 7-days of 3% NaCl diet plus 0.45% NaCl in the drinking water (26) or NOS1 protein (likely NOS1α) decreased in the inner medulla after 3 weeks of a 8.0% NaCl diet (27). These studies suggest, that the NOS1 splice variants in the inner medulla are regulated by HS diet; however it may be dependent on the duration of consumption and concentration of high dietary sodium.
Our data also demonstrated that NOS1 activity was over 2-fold higher than NOS3 activity in the rat IMCD. Recently, Lu et al. (5) reported that NOS1 splice variant expression in the rat renal macula densa was regulated by HS diet. They reported that after 10-day HS diet NOS1α was significantly down-regulated and NOS1β was significantly up-regulated. As well, there was a significant increase in NOS1-dependent NO production (5). We found a similar increase in IMCD NOS1β expression in our HS fed rats, but we did not observe a change in NOS activity or nitrite production between NS and HS treatments. What remains unknown is the specific contribution of NOS1α vs NOS1β in NO production in the IMCD and other nephron segments.
The renal inner medulla is the site of the final regulation of sodium reabsorption and chronic NOS1 inhibition within the rat renal medulla increases arterial pressure in rats on a HS diet (1). Sprague Dawley rats are normotensive on NS and 7 day 4% NaCl diets (28). However, significant increases in blood pressure have been reported with 3–10 weeks of 8% NaCl diet treatment in Sprague Dawley rats (27, 29, 30). Ni and Varizi (27) found a significant ~40 mmHg increase in systolic pressure after 3 weeks of 8% NaCl diet which correlated with a significant reduction of medullary NOS1α (NOS1β was not reported and NOS3 was not significantly affected). Thus, although the Sprague Dawley rat generally isn’t categorized as having a salt-sensitive blood pressure, prolonged 8% NaCl leads to significant increases in pressure that may in part be explained by decreased NOS1 medullary expression. This suggests that Sprague Dawley rats may experience medullary NO deficiency on a prolonged 8% NaCl diet, however, on a 4% NaCl diet, these rats are capable of producing a significant natriuresis and maintaining a normotensive blood pressure, even though IMCD NO production is not significantly increased. This differs from the wild type mouse. Compared to the rat IMCD, the mouse IMCD produces significantly more NO and this is also reflected in a higher urinary NOx excretion. The mouse renal collecting duct expresses NOS1β exclusively, and 2 or 7 days on HS diet did not significantly change NOS1β expression; however it had a profound effect on NO production. During 4% NaCl dietary intervention, the mouse also displayed a significant natriuresis, while maintaining mean arterial pressure, but there was a significant increase in IMCD NO production. The commercially available NOS1 knockout mouse was targeted for deletion at exon 2 (31), thus deleting only the NOS1α splice variant. Interestingly, the NOS1α knockout mouse was found to be normotensive even on a HS diet (32). Deletion of the NOS1 splice variants from the mouse CD, in essence NOS1β as it is the variant expressed, results in a salt-sensitive blood pressure phenotype (Hyndman et al. under review). Therefore, NOS1 plays an important role in sodium and water homeostasis, and in the control of blood pressure, in both rats and mice, although the underlying mechanisms are unclear.
There are multiple potential mechanisms that may explain the differences between rat and mouse IMCD NO production. These differences in IMCD NO production may be attributed to differences in intracellular and/or extracellular superoxide production and NO bioavailability, different post-translational regulation, and/or different protein-protein interactions leading to species specific differences in NO production. The rat kidney has significantly less extracellular superoxide dismutase activity than the mouse kidney (33). This difference may lead to a decrease in NO bioavailability in the rat. Although many post-translational sites are conserved among species, the regulation of these modifications may be different. For example, phosphorylation of p53 is different between mice and rats in both normal cells and cells transformed with simian virus 40 (common models in cancer research)(34); mouse p53 phosphorylation results in a slow turnover rate for p53, while in rats p53 phosphorylation results in a fast turnover of p53. Although, the same sites in p53 are phosphorylated in these mouse and rat cells, it results in distinct protein turnover rates, and the authors speculate that this may be due to species-specific mechanisms regulating p53 phosphorylation (34). Protein-protein interactions are well established to regulate NOS1 activity (see 35). We recently reported in transfected COS7 cells, mIMCD-3 cells, and freshly isolated rat collecting ducts that dynamins regulate NOS1 specific NO production (3) and we reason that may affect the NOS1 splice variants differently, and have species-specific regulatory pathways.
In conclusion, these data indicate that during an increase in salt intake IMCD NO production is significantly elevated in mice, but not rats. However, given this distinct, species-specific regulation of high salt-mediated IMCD NO, both species presented a significant natriuresis and no significant change in blood pressure suggesting there are diverse mechanisms regulating fluid-electrolyte balance in these species.
Acknowledgments
The authors thank Mrs. Jackie Musall and Dr. David Pollock for technical help and comments on the manuscript, respectively. This work was supported in part by a National Kidney Foundation Post Doctoral Fellowship to K.A.H. and the National Institutes of Health (HL60653 to J.S.P.). This work was also supported by the NIH Program Project Grant on Endothelin Control of Renal Excretory and Hemodynamic Function (HL95499; to J.S.P.).
References
- 1.Mattson DL, Bellehumeur TG. Neural nitric oxide synthase in the renal medulla and blood pressure regulation. Hypertension. 1996;28:297–303. doi: 10.1161/01.hyp.28.2.297. [DOI] [PubMed] [Google Scholar]
- 2.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension. 2008;51:1605–10. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol. 2011;301:F118–24. doi: 10.1152/ajprenal.00534.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith C, Merchant M, Fekete A, et al. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant. 2009;24:1422–8. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu D, Fu Y, Lopez-Ruiz A, et al. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol. 2010;298:F1465–71. doi: 10.1152/ajprenal.00650.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci. 1997;19:224–31. doi: 10.1159/000111211. [DOI] [PubMed] [Google Scholar]
- 7.Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci U S A. 1997;94:3396–401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mount PF, Fraser SA, Watanabe Y, et al. Phosphorylation of neuronal and endothelial nitric oxide synthase in the kidney with high and low salt diets. Nephron Physiol. 2006;102:p36–50. doi: 10.1159/000089092. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox CS, Deng X, Welch WJ. NO generation and action during changes in salt intake: roles of nNOS and macula densa. Am J Physiol. 1998;274:R1588–93. doi: 10.1152/ajpregu.1998.274.6.R1588. [DOI] [PubMed] [Google Scholar]
- 10.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993;91:642–50. doi: 10.1172/JCI116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol. 2011;300:F657–68. doi: 10.1152/ajprenal.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiraku J, Nakamura T, Sugiyama T, et al. Dietary salt loading increases nitric oxide synthesis in transgenic mice overexpressing sodium-proton exchanger. Jpn J Pharmacol. 1999;80:181–3. doi: 10.1254/jjp.80.181. [DOI] [PubMed] [Google Scholar]
- 13.Haque MZ, Majid DS. High salt intake delayed angiotensin II-induced hypertension in mice with a genetic variant of NADPH oxidase. Am J Hypertens. 2011;24 :114–8. doi: 10.1038/ajh.2010.173. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari S, Sharma N, Gill PS, et al. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci U S A. 2008;105:6469–74. doi: 10.1073/pnas.0711283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva GB, Garvin JL. Akt1 mediates purinergic-dependent NOS3 activation in thick ascending limbs. Am J Physiol Renal Physiol. 2009;297:F646–52. doi: 10.1152/ajprenal.00270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz P, Stoos BA, Hong NJ, Boesch DM, Plato CF, Garvin JL. High-salt diet increases sensitivity to NO and eNOS expression but not NO production in THALs. Hypertension. 2003;41:682–7. doi: 10.1161/01.HYP.0000047872.07864.20. [DOI] [PubMed] [Google Scholar]
- 17.Schnermann J, Traynor T, Yang T, et al. Tubuloglomerular feedback: new concepts and developments. Kidney Int Suppl. 1998;67:S40–5. doi: 10.1046/j.1523-1755.1998.06708.x. [DOI] [PubMed] [Google Scholar]
- 18.Ren YL, Garvin JL, Ito S, Carretero OA. Role of neuronal nitric oxide synthase in the macula densa. Kidney Int. 2001;60:1676–83. doi: 10.1046/j.1523-1755.2001.00987.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol. 1999;276:F874–81. doi: 10.1152/ajprenal.1999.276.6.F874. [DOI] [PubMed] [Google Scholar]
- 20.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–50. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan JC, Pollock DM, Pollock JS. Altered nitric oxide synthase 3 distribution in mesenteric arteries of hypertensive rats. Hypertension. 2002;39:597–602. doi: 10.1161/hy0202.103286. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan JC, Giulumian AD, Pollock DM, Fuchs LC, Pollock JS. Functional NOS 1 in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol. 2002;283:H658–63. doi: 10.1152/ajpheart.00073.2002. [DOI] [PubMed] [Google Scholar]
- 23.Oberbaumer I, Moser D, Bachmann S. Nitric oxide synthase 1 mRNA: tissue-specific variants from rat with alternative first exons. Biol Chem. 1998;379:913–9. [PubMed] [Google Scholar]
- 24.Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension. 1996;27:688–92. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- 25.Roczniak A, Zimpelmann J, Burns KD. Effect of dietary salt on neuronal nitric oxide synthase in the inner medullary collecting duct. Am J Physiol. 1998;275:F46–54. doi: 10.1152/ajprenal.1998.275.1.F46. [DOI] [PubMed] [Google Scholar]
- 26.Singh I, Grams M, Wang WH, et al. Coordinate regulation of renal expression of nitric oxide synthase, renin, and angiotensinogen mRNA by dietary salt. Am J Physiol. 1996;270:F1027–37. doi: 10.1152/ajprenal.1996.270.6.F1027. [DOI] [PubMed] [Google Scholar]
- 27.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens. 2001;14:155–63. doi: 10.1016/s0895-7061(00)01234-6. [DOI] [PubMed] [Google Scholar]
- 28.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW., Jr Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266:H1918–26. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 29.Gu JW, Bailey AP, Tan W, Shparago M, Young E. Long-term High Salt Diet Causes Hypertension and Decreases Renal Expression of Vascular Endothelial Growth Factor in Sprague-Dawley Rats. J Am Soc Hypertens. 2008;2:275–85. doi: 10.1016/j.jash.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu JW, Young E, Pan ZJ, et al. Long-term high salt diet causes hypertension and alters renal cytokine gene expression profiles in Sprague-Dawley rats. Beijing Da Xue Xue Bao. 2009;41:505–15. [PubMed] [Google Scholar]
- 31.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–86. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 32.Sallstrom J, Carlstrom M, Jensen BL, Skott O, Brown RD, Persson AE. Neuronal nitric oxide synthase-deficient mice have impaired renin release but normal blood pressure. Am J Hypertens. 2008;21:111–6. doi: 10.1038/ajh.2007.16. [DOI] [PubMed] [Google Scholar]
- 33.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–55. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patschinsky T, Knippschild U, Deppert W. Species-specific phosphorylation of mouse and rat p53 in simian virus 40-transformed cells. J Virol. 1992;66:3846–59. doi: 10.1128/jvi.66.6.3846-3859.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 2006;187:433–46. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]