Abstract
Objective
We aimed to investigate whether vitamin D supplementation modulates peripheral blood mononuclear cell telomerase activity in overweight African Americans.
Design
A double blind, randomized, and placebo-controlled clinical trial (#NCT01141192) was recently conducted.
Subjects and methods
African American adults were randomly assigned to either the placebo, or the vitamin D group (60,000 IU/month [equivalent to ~2,000 IU/day] oral vitamin D3 supplementation). Fresh peripheral blood mononuclear cells (PBMC) were collected from 37 subjects (18 in the placebo group and 19 in the vitamin D group) both at baseline and 16 weeks. PBMC telomerase activity was measured by the telomeric repeat amplification protocol.
Results
Serum 25 hydroxyvitamin D levels increased from 40.7±15.7 nmol/L at baseline to 48.1±17.5 nmol/L at posttest (p=0.004) in the placebo group, and from 35.4±11.3 nmol/L at baseline to 103.7±31.5 nmol/L posttests (p<0.0001) in the vitamin D group. In the vitamin D group, PBMC telomerase activity increased by 19.2% from baseline (1.56±0.29 AU) to posttest (1.86±0.42 AU, p<0.0001). The significance persisted after controlling for age, sex and body mass index (p=0.039). PBMC telomerase activity in the placebo group did not change from baseline (1.43±0.26 AU) to posttest (1.46±0.27 AU, p=0.157).
Conclusion
Vitamin D supplementation significantly increased PBMC telomerase activity in overweight African Americans. Our data suggest that vitamin D may improve telomere maintenance and prevent cell senescence and counteract obesity-induced acceleration of cellular aging.
Keywords: vitamin D supplementation, telomerase activity, 25(OH)D, obesity, African Americans
Introduction
Telomeres, specialized DNA structures located at the chromosome ends, protect chromosome integrity and stability. Telomeres naturally shorten with every cell cycle, and cells with critically short telomeres undergo replicative senescence and apoptosis. Oxidative stress and inflammation dramatically decrease telomerase activity and accelerate telomere shortening. Obesity is characterized as a state of chronic inflammation and heightened oxidative stress. Several large studies have shown that shorter telomeres are associated with obesity in adulthood suggesting obesity accelerates cellular aging.1–4 Telomerase is an essential enzyme that maintains telomere length and cellular replicate potential. Sustained telomerase activity stabilizes telomere length and delays/prevents replicative senescence of T cells.5–6 Importantly, increasing telomerase activity not only affects the telomeres and proliferative potential, but also preserves healthy cell function and long-term immune function.7–8 Moreover, evidence from mouse models suggests that telomerase deficiency is associated with increased risk of cardiovascular disease,9–12 impaired glucose metabolism and insulin secretion.13
Telomerase activity measured in peripheral blood mononuclear cells (PBMCs) samples constitutes a relatively new parameter, which has been measured only in very few studies in humans to date. For example, resting PBMC telomerase activity is elevated in major depression and predicts antidepressant responses.14 Low PBMC telomerase activity was associated with increased cardiovascular risk factors in 62 healthy women, suggesting that low leukocyte telomerase constitutes an early marker of cardiovascular risk.15
Recent studies highlight the beneficial effects of positive lifestyle changes on the cellular aging process, a process associated with telomere/telomerase decay. Farzaneh-Far et al.16 recently showed that individuals in the lowest quartile of omega-3 fatty acids (docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA]) experienced the fastest telomere shortening, whereas those in the highest quartile experienced the slowest telomere shortening over 5 years. The pioneer comprehensive life style intervention study conducted by Ornish et al. 17 showed that PBMC telomerase activity had increased by 29% by the end of a 3-month lifestyle intervention in 24 low risk prostate cancer patients.
Vitamin D is considered to play an important role in a broad range of bodily function beyond bone health, including immune function and cardiovascular health.18–20 Vitamin D deficiency, commonly present in obese and African Americans,21 is associated with various aging-related diseases including hypertension, type 2 diabetes mellitus, cardiovascular diseases and all-cause mortality.19–20,22–26 We recently demonstrated that vitamin D supplementation improved arterial stiffness27 and endothelial function28 in African Americans. A cross-sectional study suggests that higher serum 25 hydroxyvitamin D [25 (OH)D] level is associated with longer leukocyte telomere length in 2160 Caucasian women.29 Therefore, we aimed to explore the hypothesis that vitamin D supplementation increases PBMC telomerase activity in humans.
Subjects and Methods
Subjects
Seventy African American subjects (aged 19 to 50 years) reported to the Georgia Prevention Institute for onsite screening. Only apparently healthy, African American men and women, who denied a history of cardiovascular, pulmonary, or metabolic disease, were included into the study. Subjects were excluded if they 1) had evidence of diabetes (screening HbA1c ≥ 6.5) or GI/malabsorptive disorders, 2) were taking any medications known to affect calcium and/or vitamin D metabolism, 3) were taking any vasoactive medications, 4) were taking any vitamin, mineral, or herbal supplements, 5) were unable to swallow pills, and 6) were pregnant. Of the 70 subjects screened, 57 were found to be eligible and subsequently randomized into the study. All subjects provided written informed consent prior to study initiation. The protocol was approved by the Human Assurance Committee of the Georgia Health Sciences University (28).
Experimental Design
A double-blind, randomized, placebo controlled clinical trial (#NCT01141192) was conducted. Following screening, all eligible subjects were randomly assigned into either the vitamin D group or placebo group by the Georgia Health Sciences University’s Clinical Research Pharmacy. Following baseline assessments, all the subjects were given investigator supervised dosing during their visits to Georgia Prevention Institute at every 4 weeks for a total of 16 weeks. The doses were dispensed by clinical research pharmacy of Georgia Health Sciences University. For each dosing visit, the experimental group received a 60,000 IU oral vitamin D3 pill equivalent to 2,000 IU per day (Bio-Tech Pharmacal Inc, Fayetteville, AR) whereas the control group received an identical appearing placebo pill from the same manufacturer (microcrystalline cellulose, fumed silica). A dose of 2,000 IU/day vitamin D3, previously recognized as the Tolerable Upper Intake Level, was considered to have no adverse health effects.30
Testing protocol
Testings at baseline and 16 weeks included anthropometric assessments of height, weight, and body mass index (BMI). Resting blood pressure was evaluated in all subjects using established protocols.31 Total cholesterol, high density lipoproteins (HDL), low-density lipoproteins (LDL) and triglycerides were obtained using the Cardiochek device (Polymer Technology Systems, Indianapolis, IN). Glycocylated hemoglobin (HbA1c) was determined using the Bayer A1cNow+ point of care device (Bayer HealthCare LLC, Sunnyvale, CA).
Biochemical Analysis of 25-Hydroxyvitamin D, Parathyroid Hormone, and Calcium
Serum 25 hydroxyvitamin D (25(OH)D), serum intact parathyroid hormone (PTH), serum calcium (Ca2+), and urine Ca2+ were measured at baseline and 16 weeks. Serum 25(OH)D concentrations were determined using an enzyme immunoassay (Immunodiagnostic Systems, Fountain Hills, AZ) according to the manufacturer’s instructions. Analytical reliability of the 25(OH)D assays was monitored though participation in DEQAS (Vitamin D External Quality Assessment Scheme) and was deemed acceptable. The intra- and inter assay coefficient of variation (CV) for serum 25(OH)D were 5.9% and 6.6%, respectively. Bioactive intact PTH was determined in serum using an ELISA (Immutopics, Inc., San Clemente CA) according to the manufacturer’s instructions. The intra-assay CV for serum PTH was 5.7%. Both serum and urine Ca2+ were determined using the BioVision Colorimetric Calcium Assay Kit (Biovision, Mountain View, CA) according to the manufacturer’s instructions. The intra-assay CVs for serum and urine calcium were 5.9% and 6.6%, respectively.
Detection of telomerase activity by the Telomeric Repeat Amplification Protocol (TRAP)
PBMCs were isolated from whole blood by Ficoll-Pague Premium (Sigma-Aldrich, St. Louis, MO) gradient centrifugation within 1 hour after blood draw. Isolated PBMCs were stored in a cryopreservation media composed of RPMI-1640, 10% dimethyl sulfoxide and 10% fetal bovine serum at liquid nitrogen tank until further processing.
Telomerase activity was assayed by the Telo TAGGG Telomerase PCR ELISA kit (Roche Applied Science, Indianapolis, IN). PBMCs were counted with a hemocytometer (BrightLine hemocytometer, Buffalo, NY) by use of Trypan blue (Invitrogen, Carlsbad, CA). Two × 105 live cells were pelleted, suspended in 200 ul lysis reagent and incubated for 30 min on ice. The lysate mixture was centrifuged at 16,000 g for 20 min at 4 °C and the supernatant was carefully transferred to a fresh tube. Two microliters of cell extract (corresponding to 2 × 103 cell equivalents) were added to the PCR reaction. Each sample was done in duplicate and had its own separate negative control (heat-treated). The human kidney (293) cell line was used as a positive telomerase activity control and standard. Absorbances of the samples were measured at 450 nm (with a reference wavelength of 690 nm) within 30 min after addition of the Stop reagent, using a BioTek PowerWave HT Microplate Spectrophotometer (BioTek Instruments, Inc. Winooski, VT). Absorbance reading unit (AU) was reported as the A450 nm reading against reference wavelength A690 nm reading (A450 nm – A690 nm unit). Samples were regarded as telomerase positive if ∆A (AU) = A sample – A negative control is higher than 0.2 AU. Intra-assay CV was 5.9%, and inter-assay CV was 4.8%. Baseline and 16 week posttest samples of the same participants were measured on the same plate by the same lab investigator who was blinded to all information about the participants.
Statistical Analysis
Descriptive statistics are presented as mean ± SD if not stated otherwise. Differences in baseline group descriptive characteristics were compared by independent t tests if data were distributed normally. Group differences for categorical variables were tested by Fisher’s exact tests. Serum 25(OH)D and PBMC telomerase activity in response to vitamin D supplementation were analyzed using repeated measures analysis of variance (ANOVA, two groups by two time points). When indicated by significant interaction, simple main effects were performed to identify where the specific differences exist. Age, sex and BMI were considered as potential confounders. Subsequently conclusions were drawn because all independent variables consisted of only two levels and 1 degree of freedom. All tests were conducted two-sided, and a p value <0.05 was considered statistically significant. All statistics were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Of the 57 subjects randomized, 8 subjects (4 in each group) were unobtainable/unresponsive to follow up and were dropped from the study. Eight subjects had PBMCs collected only during the 1 visit. Additionally, the numbers of PMBC collected from 4 subjects were too low to run the TRAP assay. Therefore, pre and post PBMC telomerase activity testing were measured for remaining 37 subjects (19 subjects in the vitamin D group). Baseline subject characteristics are presented in Table 1. Except for the greater baseline LDL level in the placebo group compared to the vitamin D group, all other subject characteristics at baseline were similar between the two groups.
Table 1.
Baseline Subject Characteristics
| Variable | Placebo | Vitamin D | p-value |
|---|---|---|---|
| N | 18 | 19 | - |
| Men/Womena | 6/12 | 7/12 | 0.593 |
| Age (yrs) | 31.9 ± 10.3 | 31.1 ± 10.0 | 0.799 |
| Height (cm) | 1.70 ± 0.1 | 1.70± 0.1 | 0.988 |
| Weight (kg) | 87.2 ± 21.9 | 85.6 ± 25.4 | 0.846 |
| BMI (kg/m2) | 30.4 ± 7.7 | 29.9 ± 8.9 | 0.844 |
| SBP (mmHg) | 121.2 ± 13.9 | 124.9 ± 18.4 | 0.500 |
| DBP (mmHg) | 74.1 ± 10.8 | 74.8 ± 10.1 | 0.852 |
| Total cholesterol (mg/dL) | 161.7 + 56.1 | 135.5 ± 29.5 | 0.090 |
| HDL (mg/dL) | 46.4 ± 15.2 | 55.2 ± 16.9 | 0.108 |
| LDL (mg/dL) | 121.4 ± 58.0 | 67.6 ± 28.1 | 0.006 |
| Triglycerides (mg/dL) | 83.5 ± 27.4 | 72.6 ± 21.6 | 0.194 |
| HbA1c (mg/dL) | 5.5 ± 0.5 | 5.4 ± 0.5 | 0.553 |
| 25 (OH)D (nmol/L) | 40.7±15.7 | 35.4±11.3 | 0.249 |
| Telomerase activity (AU) | 1.43±0.26 | 1.56±0.29 | 0.146 |
Values are mean ± SD. All results not marked were based on independent t test.
Test of significance between groups were based on Fisher’s exact test.
Abbreviations: BMI, Body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; HDL, High density lipo-protein; LDL, Low density lipo-protein; HbA1c, Hemoglobin A1c.
Response of serum 25 (OH)D and PBMC telomerase activity to vitamin D3 supplementation
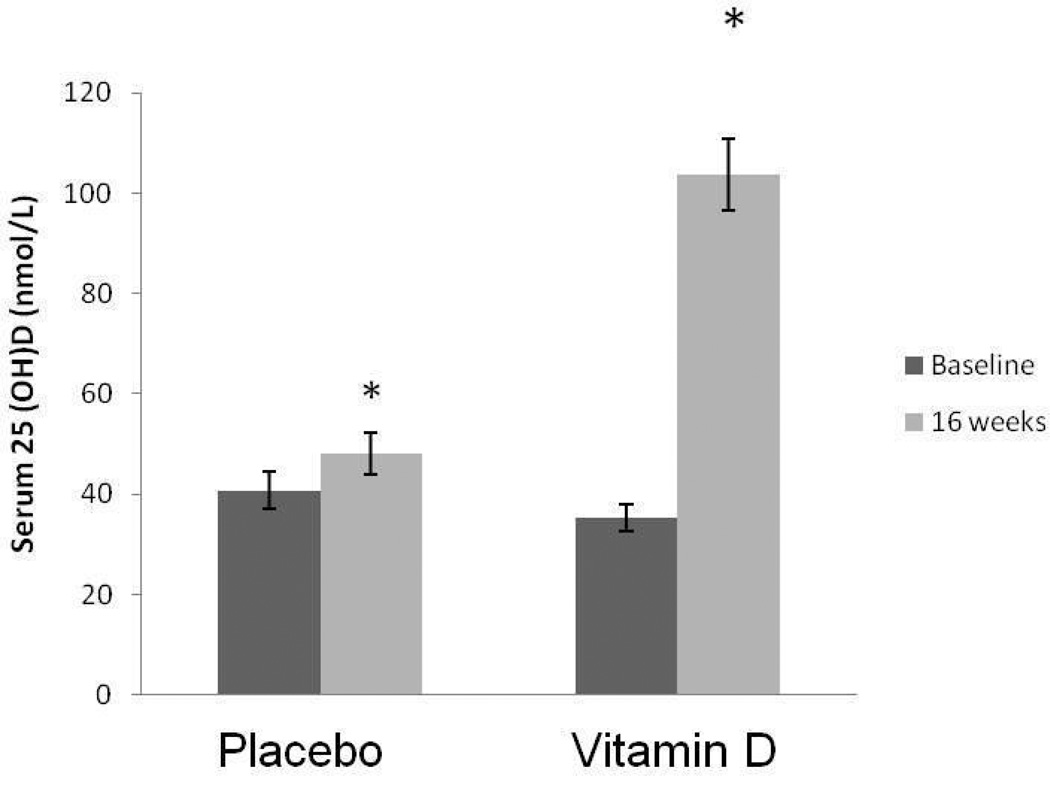
Serum 25(OH)D levels did not differ between groups at baseline. A significant group×time interaction (F1,36 = 62.8; p<0.0001) was detected. Serum 25(OH)D level increased significantly from 35.4 ± 11.3 nmol/L to 103.7 ± 31.5 nmol/L in the vitamin D group (F1,18 = 86.8; p<0.0001, Figure 1), while it only modestly increased from 40.7 ± 15.7 nmol/L to 48.1 ± 17.5 nmol/L in the placebo group (F1,17 = 11.3; p=0.004) at baseline (in February) and after 16 weeks (in May), possibly due to increased sun exposure. These findings also indicated that subjects in the placebo group remained vitamin D insufficient (25(OH)D <50 nmol/L ), even after statistically significant, although modest, increase in their serum 25(OH)D level at post-testing. No differences in PTH or urinary calcium, either between groups or within groups were observed at either baseline or at posttest (data not shown).
Figure 1.
The effect of 16 weeks of placebo or vitamin D3 supplementation on 25 hydroxyvitamin D (25(OH)D) (mean ± SE). * Significant from baseline.
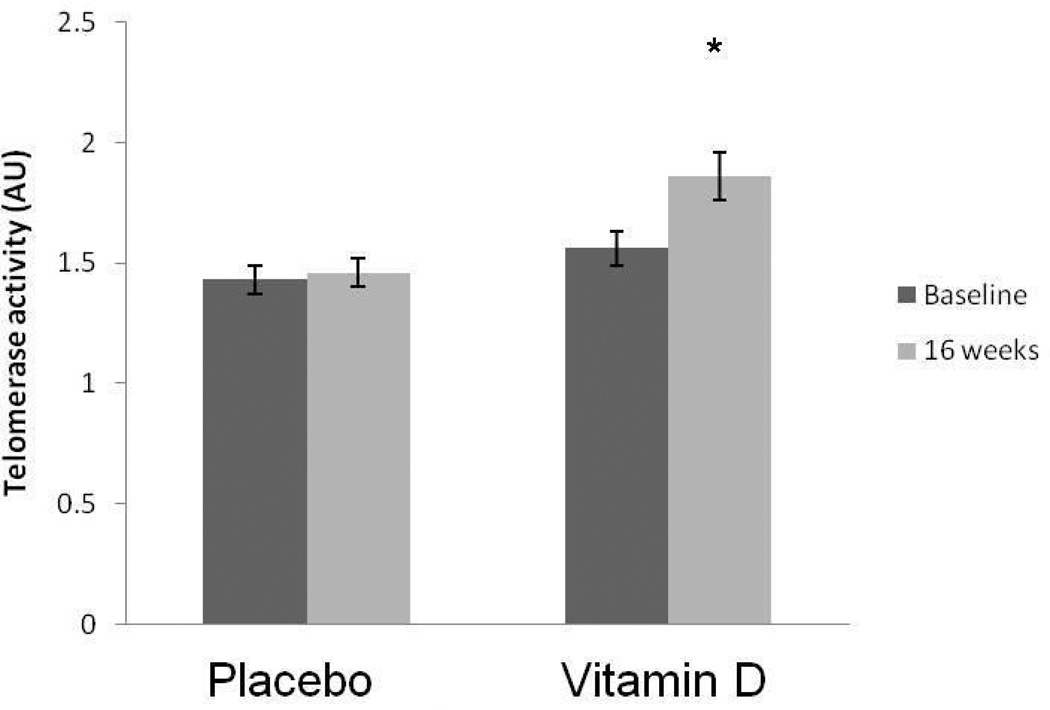
PBMC telomerase activity did not differ between the two groups at baseline. A significant group×time interaction (F1,36 = 13.3; p=0.001). PBMC telomerase activity increased significantly by 19.2% in the vitamin D group from baseline 1.56 ± 0.29 AU to posttest 1.86 ± 0.42 AU (F1,18 = 20.0; p<0.0001). The significance persisted after further adjustment for age, sex and BMI (p=0.039). In the placebo group, PBMC telomerase activity remained unchanged from baseline 1.43 ± 0.26 AU to posttest 1.46 ± 0.27 AU (F1,17 = 2.2; p=0.157) (Figure 2).
Figure 2.
The effect of 16 weeks of placebo or vitamin D3 supplementation on PBMC telomerase activity (mean ± SE). * Significant from baseline.
We also found significant correlation between the degree of increase in telomerase activity and the serum vitamin D levels after the treatment with each individual even after adjusting with age, sex, and BMI (r = 0.421, p = 0.013).
Discussion
We recently conducted a 16-week double blind, randomized clinical trial of vitamin D3 supplementation, placebo vs. 60,000 IU monthly (equivalent to 2,000 IU/day) in overweight African American adults.28 The major finding of the present study is that vitamin D3 supplementation significantly increased PBMC telomerase activity by 19.2% during the time course of 16-week intervention. Our data suggest that vitamin D may improve telomere maintenance and prevent cell senescence.
To our knowledge, our study is the first randomized placebo-controlled clinical trial to evaluate the effect of oral vitamin D3 supplementation on PBMC telomerase activity in humans, particularly in overweight African Americans who are at greater risk for both vitamin D deficiency and cardiovascular risk. The pioneer study conducted by Ornish et al.17 showed that a 3-month life style intervention, including nutrition and physical activity modification, increased PBMC telomerase activity by 29% in 24 low risk prostate cancer patients. Our randomized clinical trial demonstrated that 16-week of 60,000 IU monthly (2,000IU daily) oral vitamin D3 supplementation was able to increase PBMC telomerase activity by 19.2% in overweight African American adults. Our findings support the previously reported cross-sectional observation that higher serum 25(OH)D level is associated with longer leukocyte telomere length,29 which may be achieved through increased telomerase activity. The anti-cellular aging function of vitamin D might result from its anti-inflammation, anti-oxidative stress effects 32–34 or through nongenomic activation of a VDR/PI3K/Akt survival pathway. 35–38
PBMC cells are critical components in the immune system involving in cytotoxicity and the production of cytokines and chemokines. Telomerase activity is an important determinant of telomere length and may be an earlier predictor of long-term cellular viability or genomic stability than telomere length.17 Increasing telomerase activity not only affects the telomeres and proliferative potential, but also preserves healthy cell function and long-term immune function.7–8 Emerging evidence show that telomerase contributes to cell physiology independently of its ability to elongate telomeres. Telomerase promotes proliferation of resting stem cells,39 functions as a transcriptional co-activator in Wnt signaling,40 and regulates mitochondrial function.41 Telomerase offers cell protection especially in the context of shorter telomeres.42 Obese individuals tend to have shorter telomeres.1–4 The increase in PBMC telomerase activity shown in our study suggest that vitamin D supplementation could help to counteract obesity-induced acceleration of immune cellular aging and improve immune cell function.
Vitamin D has known immunomodulatory effects on a wide range of immune cells including CD4+ and CD8+ lymphocytes, B lymphocytes, macrophages as well as dendritic cells which are the main components of PBMCs.18,43 Several in vitro studies revealed that 1,25(OH)2D3 increases regulatory CD4+ T cell percentages,44–46 and may reduce CD8+ T cell percentage.47 Moreover, there are differences in telomerase dynamics between CD4 and CD8 T cells.7,48 Therefore, we cannot exclude the possibility that vitamin D-induced increase in PBMC telomerase activity is due to the increase in certain cell types within the PBMC with higher telomerase activity since we did not sort the subtypes of PBMC at the time of collection and telomerase measurement. Future studies to clarify the issue are warranted.
Vitamin D supplementation has been shown to have many positive health benefits including on cardiovascular health.49–50 We recently conducted two 16-week randomized clinical trials of 2,000 IU vitamin D3 supplementation daily and showed improved arterial stiffness in African American adolescents,27 and improved endothelial function in overweight African American adults.28 These results suggest that vitamin D might decelerate the vascular aging process. Recently, Jaskelioff et al. 51 showed that in aged, telomerase-deficient mice, telomerase reactivation can reverse tissue degeneration. The anti-cellular aging effect of vitamin D in overweight African Americans demonstrated in the present study adds to a growing list of beneficial effects of vitamin D.
We observed significant difference in LDL levels between placebo and vitamin D groups at the baseline. We acknowledge that high LDL might influence leukocyte telomerase activity to a lesser extent by increasing the amount of oxidized LDL in serum and thus, affecting cell growth and cytokine production. Tsirpanlis et al.52 found inverse correlation between oxidized LDL and PBMC telomerase activity in hemodialysis patients (mean age 51 years) living in Athens, Greece. However, we did not find any significant correlation between LDL and telomerase activity both at the baseline and after treatment in our young and relatively healthy African American study population. Further adjustment for LDL along with age, sex, and BMI did not change the result.
The major strengths of the present study include 1) a randomized, double blinded and placebo-controlled clinical trial can overcome the problem of confounding that exist in observational studies; 2) 100% compliance achieved by investigator supervised dosing helps to overcome the major disadvantage in clinical trials.
However, the limitations and concerns are noteworthy. First, the sample size of this study is relatively small. Therefore, our findings warrant replication in a larger study. Second, our results was observed following a 16-week supplementation, longer-term clinical trials (e.g., 12 months) are warranted to demonstrate the sustainability of the effects of vitamin D3 supplementation on telomerase activity and telomere length. Third, we did not sort subtypes of PBMC at the time of sample collection. Therefore, we cannot exclude the possibility that vitamin D-induced increase in PBMC telomerase activity is due to the increase in certain cell types within the PBMC with higher telomerase activity. Forth, whether our data obtained in African American adults are generalizable to other populations remains to be determined. Last, the underlying cellular mechanisms of our findings need to be further investigated.
In conclusion, for the first time we have demonstrated that vitamin D supplementation increased PBMC telomerase activity in overweight African Americans, suggesting that vitamin D may have an anti-cellular aging/senescence property. Large independent clinical trials with a longer-term vitamin D supplementation with different dose and across different racial/ethnic groups are warranted.
Acknowledgements
We thank our subjects for participating in the study.
Sources of Funding
This study was funded by the Georgia Health Science University Diabetes and Obesity Discovery Institute (JW) and Cardiovascular Discovery Institute (HZ). HZ and YD are also supported by National Heart, Lung, and Blood Institute HL77230 and HL69999.
Footnotes
Conflict of interest: None
References
- 1.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 2.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18:816–820. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M, Martin H, Firpo MA, Demerath EW. Inverse association between adiposity and telomere length: The Fels Longitudinal Study. Am J Hum Biol. 2011;23:100–106. doi: 10.1002/ajhb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 6.Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol. 2011;46:135–140. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng NP, Granger L, Hodes RJ. Telomere lengthening and telomerase activation during human B cell differentiation. Proc Natl Acad Sci U S A. 1997;94:10827–10832. doi: 10.1073/pnas.94.20.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Rivero G, Ruiz-Torres MP, Rivas-Elena JV, Jerkic M, Diez-Marques ML, Lopez-Novoa JM, et al. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114:309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. [DOI] [PubMed] [Google Scholar]
- 10.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 11.Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco S, Segura I, Riese HH, Blasco MA. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 2002;62:552–559. [PubMed] [Google Scholar]
- 13.Kuhlow D, Florian S, von Figura G, Weimer S, Schulz N, Petzke KJ, et al. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging. 2010;2:650–658. doi: 10.18632/aging.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2011 Jan 18; doi: 10.1038/mp.2010.133. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 18.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–348. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 21.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J Am Med Dir Assoc. 2010;11:617–628. doi: 10.1016/j.jamda.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Gouni-Berthold I, Krone W, Berthold HK. Vitamin D and cardiovascular disease. Curr Vasc Pharmacol. 2009;7:414–422. doi: 10.2174/157016109788340686. [DOI] [PubMed] [Google Scholar]
- 23.Reddy Vanga S, Good M, Howard PA, Vacek JL. Role of vitamin D in cardiovascular health. Am J Cardiol. 2010;106:798–805. doi: 10.1016/j.amjcard.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57:1595–1603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 25.Semba RD, Houston DK, Bandinelli S, Sun K, Cherubini A, Cappola AR, et al. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr. 2010;64:203–209. doi: 10.1038/ejcn.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 27.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–4591. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 28.Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, et al. Vitamin D(3) Supplementation for 16 Weeks Improves Flow-Mediated Dilation in Overweight African-American Adults. Am J Hypertens. 2011;24:557–562. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007;86:1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yates AA, Schlicker SA, Suitor CW. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. J Am Diet Assoc. 1998;98:699–706. doi: 10.1016/S0002-8223(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 31.Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;34:1026–1031. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- 32.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–897. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 33.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 34.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I, Galaktidou G. Paricalcitol reduces basal and lipopolysaccharide-induced (LPS) TNF-alpha and IL-8 production by human peripheral blood mononuclear cells. Int Urol Nephrol. 2010;42:181–185. doi: 10.1007/s11255-009-9541-1. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zanello LP. Vitamin D receptor-dependent 1 alpha,25(OH)2 vitamin D3-induced anti-apoptotic PI3K/AKT signaling in osteoblasts. J Bone Miner Res. 2008;23:1238–1248. doi: 10.1359/JBMR.080326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 37.Xia L, Wang XX, Hu XS, Guo XG, Shang YP, Chen HJ, et al. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br J Pharmacol. 2008;155:387–394. doi: 10.1038/bjp.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junhui Z, Xiaojing H, Binquan Z, Xudong X, Junzhu C, Guosheng F. Nicotine-reduced endothelial progenitor cell senescence through augmentation of telomerase activity via the PI3K/Akt pathway. Cytotherapy. 2009;11:485–491. doi: 10.1080/14653240902887267. [DOI] [PubMed] [Google Scholar]
- 39.Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovalenko OA, Caron MJ, Ulema P, Medrano C, Thomas AP, Kimura M, et al. A mutant telomerase defective in nuclear-cytoplasmic shuttling fails to immortalize cells and is associated with mitochondrial dysfunction. Aging Cell. 2010;9:203–219. doi: 10.1111/j.1474-9726.2010.00551.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhu J, Wang H, Bishop JM, Blackburn EH. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci U S A. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 44.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 45.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer MT, Lee YK, Maynard CL, Oliver JR, Bikle DD, Jetten AM, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011;286:997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lysandropoulos AP, Jaquiery E, Jilek S, Pantaleo G, Schluep M, Du Pasquier RA. Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and healthy control subjects. J Neuroimmunol. 2011;233:240–244. doi: 10.1016/j.jneuroim.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 50.Geleijnse JM. Vitamin D and the Prevention of Hypertension and Cardiovascular Diseases: A Review of the Current Evidence. Am J Hypertens. 2010;24:253–262. doi: 10.1038/ajh.2010.199. [DOI] [PubMed] [Google Scholar]
- 51.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsirpanlis G, Chatzipanagiotou S, Boufidou F, Kordinas V, Zoga M, Alevyzaki F, et al. Serum oxidized low-density lipoprotein is inversely correlated to telomerase activity in peripheral blood mononuclear cells of haemodialysis patients. Nephrology (Carlton) 2006;11:506–509. doi: 10.1111/j.1440-1797.2006.00697.x. [DOI] [PubMed] [Google Scholar]