Abstract
A systematic, efficient means of producing diverse libraries of asymmetrically branched N-glycans is needed to investigate the specificities and biology of glycan binding proteins. To that end, we describe a core pentasaccharide that at potential branching positions is modified by orthogonal protecting groups to allow selective attachment of unique saccharide moieties by chemical glycosylation. The appendages were selected in such a way that the antenna of the resulting deprotected compounds could be selectively extended by glycosyltransferases to give libraries of asymmetrical multi-antennary glycans. The power of the methodology was demonstrated by the preparation of a series of complex oligosaccharides that were printed as microarrays and screened for binding to lectins and influenza-virus hemagglutinins, which showed that recognition is modulated by presentation of minimal epitopes in the context of complex N-glycans.
Most cell surface and secreted proteins are modified by covalently-linked glycans which are essential mediators of biological processes such as protein folding, cell signaling, fertilization, embryogenesis, and the proliferation of cells and their organization into specific tissues (1). Overwhelming data support the relevance of glycosylation in pathogen recognition, inflammation, innate immune responses, and the development of autoimmune diseases and cancer (2, 3). Although the functional importance of glycoprotein glycosylation is well-established, molecular mechanisms by which these compounds exert their functions have been difficult to define. The latter is due to a lack of comprehensive libraries of well-defined complex oligosaccharides that are needed as standards to determine exact structures of glycans in complex mixtures (4, 5) and to examine specificities and biology of glycan binding proteins that occur in nature (6–8).
Naturally occurring glycans are typically isolated in small quantities as mixtures of closely related structures that are difficult to separate, and therefore do not provide a reliable source of well-defined oligosaccharides. Thus, it is widely accepted that chemical- or enzymatic approaches must be employed for the preparation of diverse glycan libraries needed for biological and structural studies (7–11). Despite ongoing progress, the chemical synthesis of complex oligosaccharides remains very time consuming, especially when highly complex structures are targeted (7). The need for more efficient approaches has stimulated the development of chemo-enzymatic methods in which a synthetic oligosaccharide precursor is modified by a range of glycosyltransferases to give more complex derivatives (10, 11). Such an approach can, however, only provide symmetrically branched oligosaccharides.
Naturally occurring branched oligosaccharides often bear unique appendages at each branching point (12). In this respect, the biosynthesis of N-linked oligosaccharides is initiated in the endoplasmic reticulum where a dolichol-linked Glc3Man9GlcNAc2 oligosaccharide precursor is transferred en bloc to an Asn-X-Ser/Thr sequon on newly synthesized polypeptides. Subsequent trimming and processing of the transferred oligosaccharide results in a GlcNAcMan3GlcNAc2 core structure, which is transported to the Golgi where additional N-acetyl glucosamine moieties (O-GlcNAc) can be added. Subsequent conversion of the O-GlcNAc stubs into N-acetyllactosamine (βGal(1,4)GlcNAc, LacNAc) provide precursors that can be elaborated by various glycosyltransferases to give rise to enormous structural diversity.
The biosynthesis of complex branched oligosaccharides generally leads to positional isomers, which are structurally difficult to assign by mass spectrometry (4, 5). Furthermore, glycan microarray technology has shown that terminal oligosaccharide motifs of complex glycans mediate biological recognition (13). However, a number of recent studies indicate a more complex picture in which the core structure can influence terminal glycan recognition (14). A synthetic technology that can give libraries of asymmetrically substituted glycans will make it possible to fabricate the next generation of glycan microarray to examine in detail glycan-protein recognition, to develop algorithms for the assignment of MS spectra and to design probes for elucidating pathways of glycoconjugate biosynthesis. Despite the urgent need for libraries of asymmetrically branched N-glycans (15), none of the currently available methods can produce collections of such compounds, and previous synthetic efforts have almost exclusively focused on the preparation of symmetrically branched compounds (16–22).
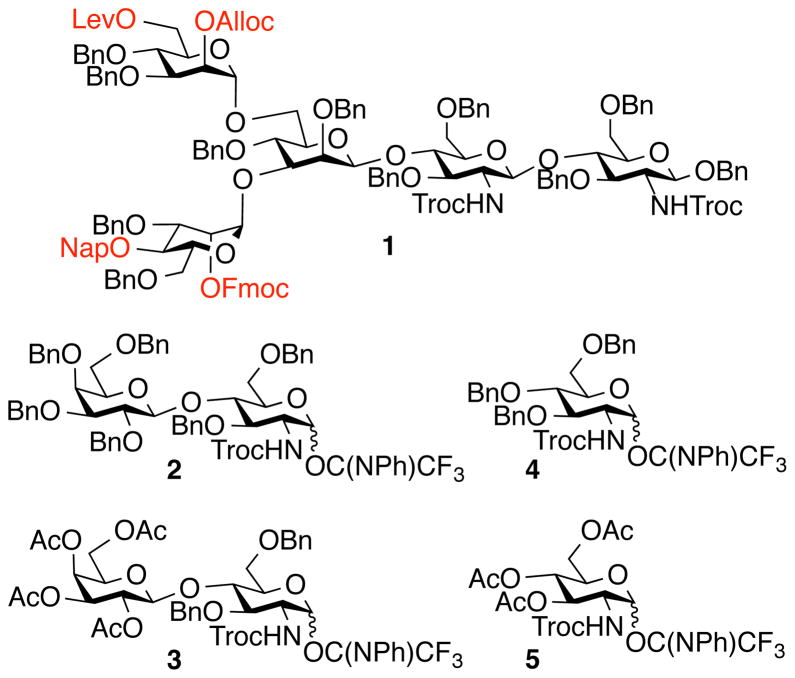
We envisaged that oligosaccharide 1 would be an attractive starting material for the preparation of libraries of asymmetrically branched N-glycans (Fig. 1). This pentasaccharide resembles the core structure common to all eukaryotic N-linked glycans (12) and is modified at positions where branching points can occur with the protecting groups levulinoyl (Lev), fluorenylmethyloxycarbonate (Fmoc), allyloxycarbonate (Alloc), and 2-naphthylmethyl (Nap). It is shown here that these protecting groups are orthogonal and therefore it was expected that libraries of complex branched bi-, tri-, and tetra-antennary structures could be generated by sequential removal of the protecting groups followed by chemical glycosylations using a diverse set of glycosyl donors. Furthermore, it was anticipated that the use of LacNAc and GlcNAc donors 2-5 followed by removal of all protecting groups except the acetyl esters, would give precursor glycans that at each antenna could be selectively extended by a panel of glycosyltransferases to rapidly give large numbers of highly complex asymmetrically substituted N-glycans. Selective extension was expected to be feasible because many relevant glycosyltransferases recognize LacNAc but not GlcNAc as a substrate (17). The latter moiety can, however, be converted into LacNAc by enzymatic galactosylation and the resulting derivative can then be elaborated by other glycosyltransferases. Furthermore, acetylation should render LacNAc and GlcNAc moieties inactive for enzymatic modification; however, the removal of these esters would give an appropriate substrate for extension by glycosyltransferases.
Fig. 1.
Orthogonally protected core pentasaccharide 1 and glycosyl donors 2-5 for extensions in a parallel combinatorial manner to give oligosaccharide precursors to enzyme substrates.
Some applications, such as the use of synthetic glycans as standards for mass spectrometry, require compounds having an unmodified reducing end. Other uses, such as the development of glycan microarrays, need compounds modified with a reactive anomeric linker. To ensure the glycans prepared by the chemo-enzymatic approach can be employed for multiple purposes, the anomeric center of compound 1 was protected as a benzyl glycoside. This protecting group will be removed during the deprotection stage to give glycans having an unmodified reducing end. The latter type of compound can, however, easily be derivatized by a reactive anomeric linker by reaction with an appropriate reagent such as 2-((methylamino)oxy)ethanamine (23).
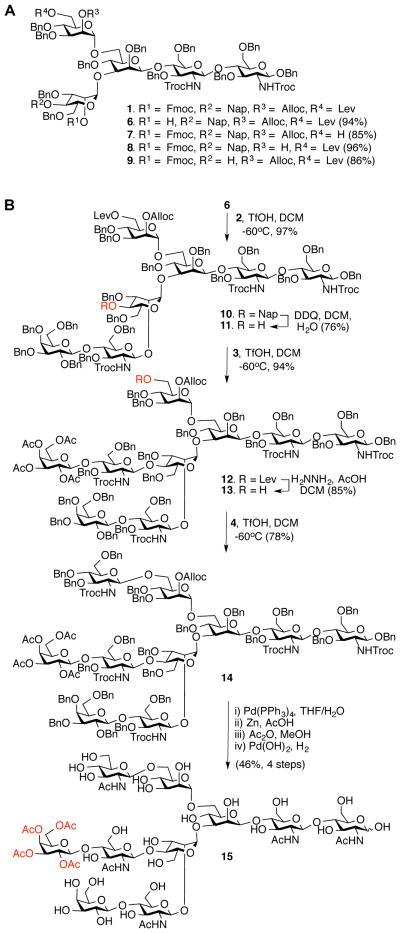
Pentasaccharide 1 was readily assembled from appropriately protected monosaccharide building blocks (Fig. S2). The Fmoc group of 1 could be selectively removed by the non-nucleophilic base triethylamine to give 6 whereas treatment with the nucleophilic base hydrazine acetate led to cleavage of the Lev ester to provide 7 without affecting the other base sensitive protecting groups (Fig. 2A). Treatment of 1 with Pd(PPh3)4 affected only the Alloc protecting group providing the corresponding hydroxyl 8 and oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) resulted in the removal of the Nap ether to give 9 in high yield.
Fig. 2.
Chemical synthesis of decasaccharide 15 for branch-specific enzymatic extensions. (A) Selective removal of temporary protecting groups. (B) Preparation of glycan precursor for enzymatic extension.
Having demonstrated the orthogonality of the temporary protecting groups, attention was focused on the preparation of tri-antennary oligosaccharide 15, which was expected to be an appropriate precursor for branch-specific enzymatic modification (Fig. 2). Glycosyl acceptor 6 was coupled with 2 using trifluoromethanesulfonic acid (TfOH) (24, 25) as the promoter to give heptasaccharide 10. The Nap ether of 10 was removed by oxidation with DDQ and the resulting acceptor 11 was glycosylated with 3 to provide nonasaccharide 12. Next, the Lev ester of 12 was cleaved with hydrazine acetate to give 13, which was coupled with 4 to give fully protected decasaccharide 14. Partial deprotection of 14 to give target compound 15 was accomplished by cleavage of the Alloc carbonate with Pd(PPh3)4 followed by removal of the 2,2,2-trichloroethyoxycarbamate (Troc) groups with Zn in acetic acid, acetylation of the resulting free amines with acetic anhydride, and catalytic hydrogenolysis of the benzyl ethers. Detailed NMR analysis of 15 showed that the acetyl esters were still intact, and thus a compound was obtained that has unique saccharide appendages at each antenna allowing selective modification by a panel of glycosyltransferases.
In addition to compound 15, pentasaccharide 1 is an appropriate starting material for the chemical synthesis of other bi-, tri-, and tetra-antennary precursor oligosaccharides by changing the number and sites of attachment of the appendages (2-5). For example, a positional isomer of 15 was readily prepared by the sequential removal of the Fmoc, Alloc, Lev groups of 1 and glycosylations with glycosyl donors 2, 3, and 4, respectively (Fig. S3).
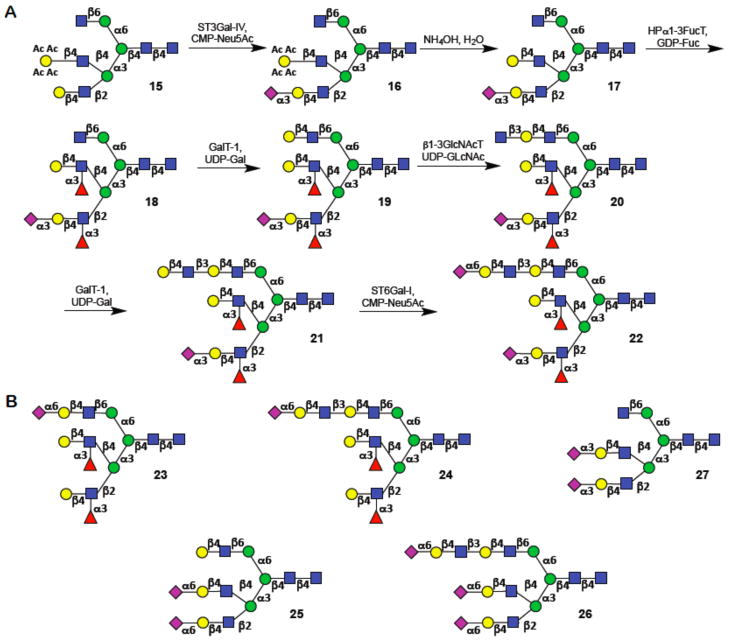
The precursor oligosaccharide 15 was further extended by glycosyltransferases to demonstrate the possibility of selective modification of each antenna to form highly complex asymmetrically branched N-glycans (Fig. 3). Many human N-glycans contain terminal sialic acids either exclusively α(2,3)- or α(2,6)-linked to N-acetyllactosamine or a combination of these two linkages (26). Furthermore, Lewis antigens such as Lewisy (Ley), Lex, and sialyl Lewisx (SLex) are found on many biologically important glycans. Therefore, attention was focused on the preparation of heptadecasacharide 22 which has SLex and Lex appendages at the C-2 and C-4 arm, respectively and a di-LacNAc moiety extended by α(2,6)-linked sialoside at the C-6 arm. A key aspect of this strategy is that relatively few glycosyltransferases are needed to elaborate these terminal glycan sequences, and enzyme expression systems that produce these and many other mammalian and bacterial glycosyltransferases useful in chemo-enzymatic synthesis have already been described (27, 28).
Fig. 3.
Chemoenzymatic synthesis of (A) asymmetrically substituted
multi-antennary glycan 22. (B) Structures of compounds
(23-26) prepared by the chemoenzymatic approach.
N-acetyl neuraminic acid (Neu5Ac,  ); D-galactose (Gal,
); D-galactose (Gal,  ); N-acetyl-D-glucosamine
(GlcNAc,
); N-acetyl-D-glucosamine
(GlcNAc,  ); D-mannose (Man,
); D-mannose (Man,
 ); L-fucose (Fuc,
); L-fucose (Fuc,
 ).
).
The LacNAc moiety of decasaccharide 15 was sialylated by α2,3-sialyltransferase (ST3Gal-IV), cytidine-5′-monophospho-N-acetylneuraminic acid (CMP-Neu5Ac), and Calf Intestine Alkaline Phosphatase (CIAP), and as expected only one of the three antennae was modified to give exclusively compound 16. Next, the acetyl esters of 16 were removed by treatment with aqueous ammonia to give compound 17, which has now an unmasked LacNAc moiety at the C-4 of the Man-α3 arm which is expected to be available for enzymatic transformations. Indeed, fucosylation of 17 with α1,3-fucosyltransferase (α3FucT) (29) resulted in the modification of the LacNAc and sialyl-LacNAc moieties to give bis-fucosylated derivative 18. The GlcNAc moiety at the C-6 antenna of 18 was converted into a LacNAc moiety by employing β1,4-galactosyltransferase (GalT-1), uridine 5′-diphosphogalactose (UDP-Gal), and CIAP to give 19. Treatment of 19 with β1,3-N-acetylglucosaminyltransferase (β1,3GlcNAcT) (30), UDP-GlcNAc, and CIAP resulted in a selective addition of a β(1,3)-linked GlcNAc moiety to the LacNAc moiety of the β1-6 branch to give 20. The Lex moiety of 19 was unaffected highlighting the feasibility of exploiting inherent substrate specificities of glycosyltransferases for the selective modification of multi-antennary glycans. The β1,6-branch was further extended by GalT-1 and α2,6-sialyltransferase (ST6Gal-1) to provide target compound 22, which has unique oligosaccharide appendages at each of the three antennae.
After each step, the product was purified by size exclusion chromatography and the resulting compound fully characterized by NMR and mass spectrometry of the permethylated derivative. If any starting material was observed, the compound was resubjected to the enzyme until a homogeneous product was obtained. In addition to target compound 22, each intermediate of the enzymatic extension (17-21) can in principle be employed for biological or biophysical studies. The precursor oligosaccharide 15 is an attractive starting material for the preparation of many other highly complex glycans. To illustrate this feature, compounds 23-27 were prepared (Figs. S14 and S15), which are asymmetrical and have varying numbers of 2,3- or 2,6-linked sialic acids at the various antennae (26). Thus, subsequent de-acetylation and bis-fucosylation of 15 to give Lex moieties at the β2 and β4 arm was followed by galactosylation to form a LacNAc moiety at the β6 arm that was capped with 2,6-Neu5Ac to form 23 or further extended with 2,6-Neu5Ac-LacNAc to provide 24. Similarly, compounds 25-27 were synthesized by either bis-α (2-3) (to give 27) or bis-α (2-6)-sialylation followed by extension of the β6 arm to provide 25 and 26 (Fig S15).
It was anticipated that compounds 22-27 would be useful for examining the activity of the various biologically relevant glycan epitopes in the context of their presence on multiantennary asymmetric structures. Thus, a glycan microarray was constructed composed of the asymmetrical tri-antennary glycans (22-27) and previously prepared linear and bi-antennary glycans having a terminal β(1-4)Gal (A–D), α(1-3)-Fuc (E–F), α(2-6)-Neu5Ac (G–L), or α(2-3)-Neu5Ac (M–Q) moiety (Table S14). Compounds 22-27 were modified with an amino-containing linker by treatment with 2-((methylamino)oxy)ethanamine (23) and the resulting derivatives were printed on NHS-activated glass slides with the reference compounds (31).
Probing the array with the Erythrina crystagalli agglutinin (ECA) specific for terminal LacNAc sequences detected the corresponding reference compounds A–D and compounds I and J; two biantennary compounds that have one branch modified with a LacNAc structure (Fig. 4). Of the synthetic triantennary compounds ECA lectin bound strongly to 25 and weakly to 22-24. The latter compounds contain LacNAc substituted with a fucoside, which is known to reduce the affinity of ECA (32). In contrast, the fucose-specific Aleuria aurantia lectin (AAL) robustly recognized the fucoside containing glycans 22-24 as well as the three reference compounds containing a Lex epitope (E, F, and M). Sambuccus nigra agglutinin (SNA) specific for terminal α(2-6)Neu5Ac recognized all structures containing this epitope (G–L and 22-26).
Fig. 4.
Glycan microarray binding analyses. Fluorescently labeled lectins (ECA, AAL, and SNA), and recombinant avian (VN/04) and human influenza A (KY07 and CA/05) HA were assessed for binding to the array. Shown is the mean signal and standard error calculated for six independent replicates on the array. Structures of each of the lettered glycans are found in Table S14.
Influenza viruses recognize sialic acids as receptors, and it is well documented that human and avian viruses exhibit differential specificity for glycans with Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal linkages, respectively. This difference in specificity represents a major barrier for transmission of avian viruses into humans (33, 34), and increasing attention is placed on glycan microarray analysis to understand the receptor requirements of avian and human virus hemagglutinins (HA) required for species tropism (35–37). To assess the potential for influenza HA to distinguish between symmetric and asymmetric glycans we evaluated the specificity of an HA from an exemplary H5N1 avian virus (VN/04), a human seasonal H1N1 virus (KY/07) and an H1N1 virus from the 2009 influenza pandemic (CA/05).
The H5 HA from VN/04 recognized compounds N–Q and 27, which contain the Neu5Acα(2-3)Gal consistent with the consensus receptor specificity of avian viruses (33, 38). Notably, this cloned HA did not recognize the Neu5Acα(2-3)Gal in the fucosylated sequence SLex in compound 22 or the reference compound M. In contrast, the HA from the two human influenza viruses exhibited binding only to glycans containing the Neu5Acα(2-6)Gal epitope (Fig. 4), but otherwise exhibited different fine specificities. The HA from the H1N1 seasonal strain A/Kentucky/07 (KY/07) recognized all the reference compounds (G–L) and all the triantennary compounds (22-26) that contained this linkage. However, relative to the linear reference compounds (G, H), the compounds that have a Neu5Acα(2–6)Gal moiety on only one branch of a biantennary glycan were bound weakly (I, J), while those that had the Neu5Acα (2–6)Gal sequence on only one branch of the triantennary glycans (23, 24) were recognized equally well. Thus, this HA distinguishes structures with a single sialic acid in the context of linear or biantennary and triantennary N-linked glycan chains. More dramatic differences are seen comparing the seasonal H1 and the pandemic HA H1 from A/California/05/09 (CA/05). The CA/05 HA recognized only reference compounds H and L and a single triantennary glycan, namely 26. These compounds have in common the Neu5Acα(2–6) epitope linked to an extended dimeric-LacNAc moiety. However, this motif is also present in triantennary glycans 22 and 24, which are not recognized by this HA. Compounds L and 26 also have in common at least two Neu5Acα(2–6) epitopes on different antennae, but so do compounds K and 25, which have a single LacNAc extension and are not recognized. These results reflect differences in the specificity of these HAs, and not simple differences in avidity, since similar array results were obtained when concentration of the HA applied to the array was titrated down in 2-fold dilutions from 100 to 6 μg/mL (Fig. S24).
These results demonstrate that glycan epitopes presented on asymmetrically branched N-linked glycans can be distinguished from the same epitopes on linear or symmetrically branched glycans. Such context-dependent recognition can be due to extended binding sites, unfavorable interactions by neighboring antennae and multivalency by proper spacing of minimal epitopes at two or more antennae. As illustrated by the selected influenza HAs, these differences are relevant to the recognition of receptors by human pathogens. A complete understanding of influenza receptor specificity and its relevance to adaptation of animal viruses to human hosts will require an extensive panel of asymmetric and symmetric glycan structures representative of those found on human and animal airway epithelia (37). Such libraries of glycans, which can be produced by the methodology presented here, will begin to define the human glycome, and provide tools to understand the biology mediated by both microbial and mammalian glycan binding proteins that mediate host pathogen interactions and innate and adaptive immune responses (13, 39).
Supplementary Material
Acknowledgments
This research was supported by NIH grants from the National Center for Research Resources, P41RR005351 (GJB, JG), the National Institute of General Medical Sciences, P41GM103390 (GJB, JG) and R01GM090269 (GJB), the Institute of Allergy and Infectious Disease, AI058113 (JCP), and a contract from the Centers for Disease Control (JCP). RPDV is a recipient of a Rubicon grant from the Netherlands Organization for Scientific Research (NWO). We thank Anna Crie and Dr. Margreet Wolfert for assistance in preparation of the manuscript, Dr. Kelley Moremen (UGA) for providing ST6Gal-1 and ST3Gal-IV, and Dr. Peng Wu (Albert Einstein College of Medicine) and Dr. Warren Wakarchuk (Ryerson University) for providing the plasmids for α1,3FucT and β1,3GlcNAcT, respectively. GJB conceived the idea, ZW performed the chemical synthesis, SGA assisted with chemical synthesis, ZSC performed the enzymatic transformations, and WP and ZW assisted with the enzymatic transformation. ZSC and JG performed the analysis of the complex glycans. WP performed the linkering of the compounds, RM performed the microarray screening, RPDV prepared the influenza HA, JCP supervised and analyzed the microarray studies. GJB and JCP wrote the paper. The data for this report are archived as supplementary materials on Science online. A patent application related to the described chemoenzymatic approach has been filed by the University of Georgia Research Foundation and lists GJB and ZW as inventors.
Footnotes
References and Notes
- 1.Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143:672. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 4.North SJ, Hitchen PG, Haslam SM, Dell A. Mass spectrometry in the analysis of N-linked and O-linked glycans. Curr Opin Struct Biol. 2009;19:498. doi: 10.1016/j.sbi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marino K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 6.Laurent N, Voglmeir J, Flitsch SL. Glycoarrays--tools for determining protein-carbohydrate interactions and glycoenzyme specificity. Chem Commun. 2008:4400. doi: 10.1039/b806983m. [DOI] [PubMed] [Google Scholar]
- 7.Boltje TJ, Buskas T, Boons GJ. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat Chem. 2009;1:611. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepenies B, Yin J, Seeberger PH. Applications of synthetic carbohydrates to chemical biology. Curr Opin Chem Biol. 2010;14:404. doi: 10.1016/j.cbpa.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Zhu XM, Schmidt RR. New principles for glycoside-bond formation. Angew Chem, Int Ed. 2009;48:1900. doi: 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]
- 10.Palcic MM. Glycosyltransferases as biocatalysts. Curr Opin Chem Biol. 2011;15:226. doi: 10.1016/j.cbpa.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Schmaltz RM, Hanson SR, Wong CH. Enzymes in the synthesis of glycoconjugates. Chem Rev. 2011;111:4259. doi: 10.1021/cr200113w. [DOI] [PubMed] [Google Scholar]
- 12.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rillahan CD, Paulson JC. Glycan microarrays for decoding the glycome. Annu Rev Biochem. 2011;80:797. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabius HJ, Andre S, Jimenez-Barbero J, Romero A, Solis D. From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem Sci. 2011;36:298. doi: 10.1016/j.tibs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Ranzinger R, Frank M, von der Lieth CW, Herget S. Glycome-DB.org: a portal for querying across the digital world of carbohydrate sequences. Glycobiology. 2009;19:1563. doi: 10.1093/glycob/cwp137. Analysis of the glycomeDB database shows that 85% of known glycan structures are asymmetrical. Almost all of the known N-linked glycans have fewer than 25 monosaccharides and such compounds should be accessible by the strategy presented here. [DOI] [PubMed] [Google Scholar]
- 16.Unverzagt C. Chemoenzymatic synthesis of a sialylated undecasaccharide-asparagine conjugate. Angew Chem, Int Ed. 1996;35:2350. [Google Scholar]
- 17.Hanashima S, Manabe S, Ito Y. Divergent synthesis of sialylated glycan chains: combined use of polymer support, resin capture-release, and chemoenzymatic strategies. Angew Chem, Int Ed. 2005;44:4218. doi: 10.1002/anie.200500777. [DOI] [PubMed] [Google Scholar]
- 18.Jonke S, Liu KG, Schmidt RR. Solid-phase oligosaccharide synthesis of a small library of N-glycans. Chem-Eur J. 2006;12:1274. doi: 10.1002/chem.200500707. [DOI] [PubMed] [Google Scholar]
- 19.Sun B, Srinivasan B, Huang XF. Pre-activation-based one-pot synthesis of an alpha-(2,3)-sialylated core-fucosylated complex type Bi-antennary N-glycan dodecasaccharide. Chem-Eur J. 2008;14:7072. doi: 10.1002/chem.200800757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unverzagt C, et al. Synthesis of multiantennary complex type N-glycans by use of modular building blocks. Chem-Eur J. 2009;15:12292. doi: 10.1002/chem.200901908. [DOI] [PubMed] [Google Scholar]
- 21.Serna S, Etxebarria J, Ruiz N, Martin-Lomas M, Reichardt NC. Construction of N-glycan microarrays by using modular synthesis and on-chip nanoscale enzymatic glycosylation. Chem-Eur J. 2010;16:13163. doi: 10.1002/chem.201001295. [DOI] [PubMed] [Google Scholar]
- 22.Walczak MA, Danishefsky SJ. Solving the convergence problem in the synthesis of triantennary N-glycan relevant to prostate-specific membrane antigen (PSMA) J Am Chem Soc. 2012;134:16430. doi: 10.1021/ja307628w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohorov O, Andersson-Sand H, Hoffmann J, Blixt O. Arraying glycomics: a novel bi-functional spacer for one-step microscale derivatization of free reducing glycans. Glycobiology. 2006;16:21C. doi: 10.1093/glycob/cwl044. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt RR, Kinzy W. Anomeric-oxygen activation for glycoside synthesis - the trichloroacetimidate method. Adv Carbohydr Chem Biochem. 1994;50:21. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Tao HC. Glycosyl trifluoroacetimidates. Part 1: Preparation and application as new glycosyl donors. Tetrahedron Lett. 2001;42:2405. [Google Scholar]
- 26.Spik G, Debruyne V, Montreuil J, van Halbeek H, Vliegenthart JF. Primary structure of two sialylated triantennary glycans from human serotransferrin. FEBS Lett. 1985;183:65. doi: 10.1016/0014-5793(85)80955-8. [DOI] [PubMed] [Google Scholar]
- 27.More then 800 expression constructs of human glycosylation enzymes in multiple expression vectors and protocols to produce the enzymes for biochemical, enzymatic synthesis, and structural studies are available at http://glycoenzymes.ccrc.uga.edu
- 28.Blixt O, Razi N. Chemoenzymatic synthesis of glycan libraries. Methods Enzymol. 2006;415:137. doi: 10.1016/S0076-6879(06)15009-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, et al. Chemoenzymatic synthesis of GDP-L-fucose and the Lewis X glycan derivatives. Proc Natl Acad Sci U S A. 2009;106:16096. doi: 10.1073/pnas.0908248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauerzapfe B, et al. Chemo-enzymatic synthesis of poly-N-acetyllactosamine (poly-LacNAc) structures and their characterization for CGL2-galectin-mediated binding of ECM glycoproteins to biomaterial surfaces. Glycoconjugate J. 2009;26:141. doi: 10.1007/s10719-008-9172-2. [DOI] [PubMed] [Google Scholar]
- 31.Blixt O, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itakura Y, et al. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and Erythrina lectins: a search by frontal affinity chromatography. J Biochem. 2007;142:459. doi: 10.1093/jb/mvm153. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekaran A, et al. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotechnol. 2008;26:107. doi: 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]
- 34.Imai M, Kawaoka Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr Opin Virol. 2012;2:160. doi: 10.1016/j.coviro.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen LM, et al. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology. 2012;422:105. doi: 10.1016/j.virol.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce MB, et al. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc Natl Acad Sci U S A. 2012;109:3944. doi: 10.1073/pnas.1119945109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walther T, et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9:e1003223. doi: 10.1371/journal.ppat.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens J, et al. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 39.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 40.Crich D, Smith M. 1-Benzenesulfinyl piperidine/trifluoromethanesulfonic anhydride: a potent combination of shelf-stable reagents for the low-temperature conversion of thioglycosides to glycosyl triflates and for the formation of diverse glycosidic linkages. J Am Chem Soc. 2001;123:9015. doi: 10.1021/ja0111481. [DOI] [PubMed] [Google Scholar]
- 41.Gaunt MJ, Yu JQ, Spencer JB. Rational design of benzyl-type protecting groups allows sequential deprotection of hydroxyl groups by catalytic hydrogenolysis. J Org Chem. 1998;63:4172. [Google Scholar]
- 42.Maiti KK, et al. Chemical synthesis and proinflammatory responses of monophosphoryl lipid A adjuvant candidates. Eur J Org Chem. 2010:80. doi: 10.1002/ejoc.200900973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boltje TJ, Li C, Boons GJ. Versatile set of orthogonal protecting groups for the preparation of highly branched oligosaccharides. Org Lett. 2010;12:4636. doi: 10.1021/ol101951u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt RR, Stumpp M. Glycosylimidates. 8. Synthesis of 1-thioglycosides. Liebigs Ann Chem. 1983:1249. [Google Scholar]
- 45.Logan SM, et al. Novel biosynthetic functions of lipopolysaccharide rfaJ homologs from Helicobacter pylori. Glycobiology. 2005;15:721. doi: 10.1093/glycob/cwi057. [DOI] [PubMed] [Google Scholar]
- 46.de Vries RP, et al. The influenza A virus hemagglutinin glycosylation state affects receptor-binding specificity. Virology. 2010;403:17. doi: 10.1016/j.virol.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 47.de Vries RP, et al. Glycan-dependent immunogenicity of recombinant soluble trimeric hemagglutinin. J Virol. 2012;86:11735. doi: 10.1128/JVI.01084-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.