Abstract
Multiple MRI modalities including Diffusion Tensor Imaging (DTI), perfusion MRI, in vivo MR Spectroscopy (MRS), volumetric MRI, contrast-enhanced MRI, and functional MRI have demonstrated abnormalities of the structural and functional integrity as well as neurochemical alterations of the HIV-infected central nervous system (CNS). MRI has been proposed as a robust imaging approach for the characterization of the stage of progression in HIV infection. However, the interpretation of the MRI findings of HIV patients is complicated by the fact that these clinical studies cannot readily be controlled. Simian immunodeficiency virus (SIV) infected macaques exhibit neuropathological symptoms similar to those of HIV patients, and are an important model for studying the course of CNS infection, cognitive impairment, and neuropathology of HIV disease as well as treatment efficacy. MRI of non-human primates (NHPs) is of limited benefit on most clinical scanners operating at or below 1.5 Tesla because this low field strength does not produce high-quality images of the relatively small NHP brain. Contemporary high field MRI (3T or more) for clinical use provides impressive sensitivity for magnetic resonance signal detection and is now accessible in many imaging centers and hospitals, facilitating the use of various MRI techniques in NHP studies. In this article, several high field MRI techniques and applications in macaque models of neuroAIDS are reviewed and the relation between quantitative MRI measures and blood T-cell alterations is discussed.
Keywords: HIV, AIDS, SIV, NeuroAIDS, CD4+, CD8+, Cerebral blood flow (CBF), Diffusion tensor imaging (DTI), In vivo MRS, Nonhuman primate
Introduction
HIV-associated central nervous system (CNS) disorders have been investigated widely with diffusion tension imaging (DTI), perfusion MRI, in vivo Magnetic Resonance Spectroscopy (MRS), functional MRI, magnetization transfer imaging, and contrast-enhanced MRI [1-7]. These studies demonstrate that MRI is a robust non-invasive approach in studies of HIV/AIDS patients. Abnormalities of MRI measures are more frequent in demented patients than in non-demented ones. The interpretation of these MRI measures as surrogates for stage of the disease progression is complicated, however, because these clinical studies cannot readily be controlled for route of infection, viral strain, or severity of disease. Simian immunodeficiency virus (SIV) infected macaque model exhibits neuropathological symptoms similar to those of HIV+ patients, and provides a valuable platform for studying the course of infection, cognitive impairment, and neuropathological sequelae of HIV disease in AIDS vaccine development [8-16]. In addition, the macaque model allows the MRI examination to be carried out with much higher image resolution and with multiple modalities in a single session. Dedicated monkey research scanners with high-strength gradient inserts are preferred for macaque neuroimaging [17], but such high-field scanners are available in only a few research centers worldwide.
Whole-body clinical scanners are accessible in most medical centers or hospitals and most offer adequate space to accommodate any special requirements of a scan setup. Such clinical scanners have been employed in many macaque studies. However, most of them operate at or below 1.5 Tesla and thus provide a level of signal sensitivity that is suboptimal for macaque neuroimaging studies. In recent years, high field clinical MRI scanners (3T or greater) has become available in many centers. The increased field strength provides significantly improved signal sensitivity. The advanced MRI technologies facilitate nonhuman primate (NHP) neuroimaging investigation, resulting in more informative neuroanatomical studies which provide new insights into the brain’s structure and function.
One of the hallmarks of the progression of AIDS is the extensive depletion of CD4+ T cell subsets, especially in untreated HIV patients [18]. A massive loss of memory-phenotype CD4+ T cells was observed in the intestine in early stages of infection [18,19]. CD4+ T cell count indicates the stage of HIV disease and is the most significant predictor of the disease progression. CD8+ T-cells provide a major immunological defense against HIV infection and their proliferation is driven by the virus load and associated with the state of inflammation in HIV infection [20-22], and neurological dysfunctions is correlated with the expansion of CD8+ T cells in the SIV infected brain [21]. Also, the CD4/CD8 ratio has been proposed as a potential biomarker for the stage of HIV infection [23]. A significant correlation between the MRI measures in CBF and DTI and the blood T-cell alterations has been demonstrated in a longitudinal study of SIV-infected macaques [24].
In the present article, the use of several MRI techniques in SIV-infected macaque neuroimaging at high field is reviewed and the relationship between these quantitative imaging measures and the CD4+ and CD8+ alterations in SIV Infection is discussed.
Equipment and Animal Handling
MRI scanner
Current whole body clinical MRI scanners have 60-cm or larger bore size and provide ample space to accommodate animals and equipments for a variety of experimental settings in NHP studies. This relatively large bore size facilitates animal handling and monitoring. Animals can be placed in either a supine position, or be placed in the scanner on the abdomen, in a “sphinx” position during scanning. Some commercial knee coils (such as Siemens CP extremity) can fit varying macaque head sizes very well for general imaging and MRS scans. Custom-built coils are also often employed to provide optimal performance for specialized studies. Because the MRI signal to noise ratio (SNR) increases with the magnet strength, high field or even ultra-high field MRI scanners are preferred for acquisition of the best possible images.
The average macaque brain volume is about 100 cm3, less than one-tenth that of a human brain. Since most clinical MRI pulse sequences and protocols are designed and hardcoded expressly for human brain imaging purposes, they must be reprogrammed and optimized to accommodate the smaller FOV and higher spatial resolution needed in macaque brain imaging.
Behavior tests and blood tests for T-cell counts
The neurological, behavioral, and cognitive similarity of the macaques to humans [25] can be exploited to evaluate possible cognitive sequalae to SIV infection. Toward this end, computer-based cognitive behavioral tests can be used to characterize the change of cognitive function in monkeys. Among the tests that can be performed are cued and uncued attention [26,27], Delayed Non-Matching-to-Sample (DNMS) (both acquisition and memory performance with delays) [28,29]. Delayed Recognition Span-spatial condition (DRST-spatial), and Spatial Reversal (SR) [30,31]. These tests are recognized measures of attention [26,27] and memory [30,31]. In order to monitor disease progression and the correlation with behavior and MRI measures, blood samples can be collected on the day before the scan. These can then be analyzed for counts of CD4+ and CD8+ T-cell subsets, by flow cytometry [12,32].
Animal immobilization, anesthesia, and physiology monitoring
Even if subjects are deeply anesthetized, the physiological motions can often produce motion artifacts on MRI images. This can be prevented by the use of a head holder to immobilize the animal. The holder in use at our facility has plastic ear bars and a tooth bar, and is designed to permit the anesthetized animal to breathe freely during the scan.
Also, the head restraint is able to provide space for the introduction of an endotrachael tube for the administration of isoflurane, an inhalated agent that produces rapid induction of and recovery from anesthesia. Monkeys are usually kept at ~1% isoflurane during scanning. Physiological parameters such as Et-CO2, inhaled CO2, O2 saturation, blood pressure, heart rate, respiration rate, and body temperature, must be monitored continuously and maintained in normal ranges during the entire scanning session.
T1 and T2 Weighted Structural MRI
T1 and T2 weighted MRI provides excellent image quality and tissue contrast for structure segmentation to examine possible volumetric abnormalities of various brain structures. Cerebral atrophy is often observed in HIV+ patients with neurological symptoms [4,7,33,34]. An inverse correlation between ventricular size and neuropsychological function was found in previous CT study of AIDS patients [35].
In macaque models, such structural volume changes can be accessed before and after SIV inoculation from T1 and T2 weighted structural images. High resolution T1 and T2 weighted images of macaques are usually acquired with the 3D MP-Rage gradient-echo sequence and the fast spin-echo sequence respectively. The structural volumes can be estimated by manual tracing or by automatic image segmentation via FSL (www.fmrib.ox.ac.uk) for voxel-based morphometry (VBM) analysis or other atlas-guided specific segmentation software. Also, the standardized planimetry to measure the ventricle-brain ratio (VBR) and the bifrontal (BFR) and bicaudate (BCR) ratios can be used for estimation of cerebral atrophy [33,34]. Briefly, The VBR is defined as the ratio of the area of the lateral ventricles over the whole brain in the slice where the brain perimeter is maximal. The BCR is the ratio of the minimum inter-caudate distance over the corresponding whole brain width in the same slice. The BFR is the ratio of the distance between the lateral tips of the frontal horns over the corresponding whole brain width in the same slice.
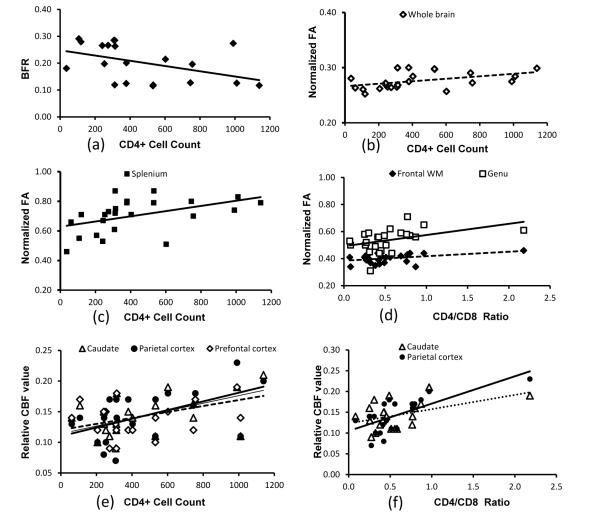
Li et al. used this method to estimate the cerebral atrophy of the SIV-infected macaques [24]. No significant difference between any two different time points was observed during the study period, in agreement with those seen in asymptomatic HIV-1-infected patients [4,36]. However, the BFR, an index of cerebral atrophy, increased progressively with CD4+ depletion as indicated by a significant correlation with CD4+ T cell count during infection (Figure 1a). This finding is consistent with the result in HIV patients in which the frontopolar cortical thinning is significantly associated with lower CD4+ counts [37]. This result suggests that the cerebral atrophy may be still occurring during the asymptomatic stage, even though no obvious volume changes are observed after the SIV infection in comparison with that of the pre-inoculation baseline.
Figure 1.
Multiple MRI measures correlate with CD4+ counts and/or CD4/CD8 ratio with P-values <0.05 confidence in a SIV-infected macaque study (Li et al. 2011). (a) brain atrophy index bifrontal ratio (BFR) correlates with CD4+ T cell counts (R2=0.20); (b) Fractional Anisotropy (FA) of whole brain correlates with CD4+ T cell counts (R2=0.21); (c) FA of splenium correlates with CD4+ counts (R2=0.22); (d) FA in frontal white matter and genu correlates with CD4/CD8 ratio (R2=0.17 and 0.18); (e) Cerebral Blood Flow (CBF) in caudate, parietal and prefrontal cortex correlates with CD4+ counts (R2=0.21, 0.29, and 0.25) (f) CBF in caudate and parietal cortex correlates with CD4/CD8 ratio(R2=0.21 and 0.50).
DTI
DTI measures the magnitude and directionality of tissue water mobility and provides a non-invasive approach to access the microstructural features of the brain white matter tissue [38,39]. However, the DTI data acquisition is very vulnerable to motion, echo time, and field inhomogeneity (susceptibility artifacts). High magnetic field provides the requisite high SNR, but produces longer T1 and shortening of T2 and T2*. Therefore, strong gradients are required to achieve acceptable echo time (TE). In comparison with the gradient strength of up to 400mT/m in animal research scanners, the gradient insert in a clinical scanner typically provides only 40 mT/m per axis. Thus, the echo time in the single-shot EPI sequence can become too long and cause severe signal drop and image distortion in high-resolution DTI of macaque brains. However, since the animal is usually anesthetized and immobilized during scanning, the multi-shot EPI pulse sequence can be utilized to reduce the echo time significantly in the high resolution DTI data acquisition of macaque brains.
DTI has been extensively employed for studying CNS anatomy and pathology in AIDS and HIV patients [40-45]. Fractional Anisotropy(FA) is more sensitive than mean diffusivity (MD) and decreases significantly in regions such as splenium, genu, internal capsule, frontal lobes, parietal lobes, temporal lobes, and occipital lobes in both symptomatic and asymptomatic HIV patients [41,44,46-50].
In comparison with the numerous DTI studies in HIV patients over a decade, DTI was just explored recently in one study of SIV macaques [24], in which, a two-segment double spin-echo EPI sequence was used for DTI data acquisition at high spatial resolution (voxel size=1.5×1.5×1.5 mm3). The DTI results of SIV macaques indicate that the whole brain FA is reduced significantly after SIV infection, in agreement with the previous results in HIV patients. In addition, longitudinal FA changes of the whole brain, splenium, genu, and frontal white matter in SIV macaques, are correlated significantly with the CD4+ T cell counts and/or CD4:CD8 ratio during infection, as illustrated also in figure 1b, 1c and 1d, suggesting that the longitudinal changes in FA are associated with the immune dysfunction during the acute and chronic SIV infection. Also, these results suggest that DTI is a robust and sensitive technique to evaluate neuroanatomical changes in both HIV+ patients and SIV macaques.
Perfusion MRI
Cerebral Blood Flow (CBF) can be measured with several neuroimaging techniques including PET, SPECT, and MRI. Of these, Arterial Spin-labeling (ASL) in the context of MRI uses endogenous arterial blood water as a freely diffusible tracer and is a non-invasive approach for the quantitative measurement of CBF [51-53]. ASL with a separate labeling coil can achieve high SNR CBF maps of macaques with reduced RF exposure but requires additional hardware [54]. In contrast, the amplitude-modulated continuous ASL (CASL) technique does not need additional hardware setting and can be readily used for CBF data acquisition of humans [55]. The CASL technique has been explored and optimized for CBF mapping of SIV macaques [24]. The resting CBF maps of SIV macaques with the CASL perfusion measurement at high spatial resolution (voxel size=1.5×1.5×1.5 mm3) have been demonstrated.
CBF has been utilized sparsely in HIV/AIDS researches. Abnormal CBF has been observed in a few studies of HIV patients and proposed as a noninvasive biomarker for HIV-associated CNS damage, perhaps with the potential for classifying or predicting the degree of neurocognitive impairment [56-58]. The relation between regional CBF abnormality and pathological and neurological alteration remains unknown.
The progressive CBF changes during SIV infection have been explored in one recent study of SIV macaques [24]. It has been demonstrated that CBF in caudate and inferior medial parietal cortex of SIV-infected macaques was reduced significantly. Reduction of CBF in prefrontal cortex was nearly statistically significant, probably due to the small sample size. In addition, the progressive change of CBF in caudate and parietal cortex correlated significantly with CD4+ counts and with the CD4/CD8 ratio during infection (Figure 1e and 1f), suggesting that regional CBF in the specific structures is associated with the immune dysfunction during the SIV infection. Also, the CBF results in SIV monkeys are consistent with previous reports in HIV patients [58-63].
In vivo Proton MRS
MRS provides a non-invasive approach to measure neurochemical alterations associated with the inflammatory process during HIV infection and has been used extensively in studies of HIV or AIDS patients [59,64-75]. Metabolite abnormality was usually observed in demented and/or symptomic HIV patients. The degree of cerebral metabolite disturbance in HIV patients is strongly associated with reduced cortical and subcortical volumes [76].
Cerebral metabolic abnormalities in SIV-infected monkeys has been examined by using localized point-resolved spectroscopy (PRESS) sequence with CHESS water suppression [77-79] or ex vivo MRS [80-83]. During acute SIV infection, significant reduction of NAA/Cr and Cho/Cr was observed in about 13 days and 27 days after inoculation, respectively, and the change of Cho/Cr was correlated with plasma viral load [84]. Cho/Cr in frontal lobe or NAA/Cr in basal ganglia was found correlated with plasma viral load [85]. The findings are similar to those noted in HIV-infected human brains. The NAA/Cr ratio is negatively correlated with the SIV CNS disease severity in the SIV-infected macaque model of encephalitis [78]. In particular, rapid decline in NAA/Cr ratios has been demonstrated in SIV macaques with CD8+ depleted [86]. The effect of the chronic morphine administration on SIV macaques has been investigated in the SIV macaque model, and the ex vivo MRS findings indicate the protection of chronic morphine against the neurotoxic effect of AIDS [82]. Also, the neuroprotection by oral minocycline was demonstrated in recent MRS study of the accelerated SIV macaque model [87].
Those metabolite abnormalities are not usually seen in the patients who are neurologically asymptomatic or with mild cognitive impairment [88]. In recent MRS study of SIV macaques, significant cerebral metabolite alteration was observed in a longitudinal MRS study of neurologically asymptomatic SIV macaques [89]. Also, the progressive change of NAA and glutamate/glutmine (Glx) in basal ganglia correlated with the CD8+ T cell percentage during the SIV infection. It is suggested that the unknown infection history and/or medication treatment may complicate the examination of in vivo MRS in HIV patients with no or mild cognitive impairment. In sum, in vivo MRS in asymptomatic macaque models may be of particular value in investigating early nervous system involvement in HIV patients with no or mild cognitive impairment.
High Field and Ultra-High Field MRI and Parallel Imaging Techniques
MRI is a non-invasive and sensitive imaging modality and being increasingly used in preclinical examination and clinical diagnosis. Two revolutionary advances in the MRI techniques emerged in recent years. These are (1) the development and application of high field and ultra-high field MRI, and (2) the advent of parallel imaging techniques.
High field (3T or more) and ultra-high field (7T or more) MRI offers increased SNR which benefits many applications using conventional and quantitative MRI methods. These include high resolution T1 and T2 weighted structural imaging, in vivo MR spectroscopy, DTI, functional MRI, ASL-based perfusion, and susceptibility weighted imaging (SWI). The SNR of ASL-based perfusion-weighted images are further improved because of the elongated blood water T1 at high field. However, technical challenges on magnetic field in homogeneities, RF coils, RF exposure, and increased T1 and shortening T2 of tissue, etc, still remain to be solved in ultra-high field MRI.
Parallel imaging technique combines multiple receiving coils in a phased array with unique imaging reconstruction algorism to reduce the scan duration significantly. Most imaging modalities such as regular anatomical T1 and T2 weighted images benefit substantially from this technique in terms of improved image quality and increased scanning speed. In particular, DTI measurement is greatly improved by using the novel parallel imaging technique [90]. In our experience, a four- or eight-channel phase-array volume coil can meet general needs of macaque brain imaging. The combination of high field and parallel imaging techniques could facilitate the use of various MRI measurements of macaque brain imaging with excellent image quality and sensitivity.
Discussion and Conclusions
HIV preferentially infects the sub-cortical structures. Although it perhaps enters the CNS as early as the initial systemic infection [91], symptomatic cognitive impairment typically occurs in late stages of HIV disease when most abnormalities in quantitative MRI measures are observed. However, the specific symptoms vary from person to person, and most MRI measures are not sensitive in the examination of HIV patients with no or mild cognitive impairment, except for DTI whose robustness has been demonstrated in some studies of non-demented HIV patients [41,46,48].
SIV-infected macaques offer an ideal model for using clinical MRI scanners to characterize the CNS injury during SIV infection and the response to treatment under controlled condition. Also, a particular advantage of the macaque model is that it permits the use of high quality, multi-parameter MRI measurements in a single session to examine the CNS injury non-invasively. DTI, CBF, and MRS measurements of SIV macaques have demonstrated their robustness and efficacy to access and evaluate the CNS injury during SIV infection in previous studies. The CBF and metabolite abnormalities in basal ganglia of SIV macaques suggest basal ganglia may be more vulnerable in SIV and HIV infection.
HIV attacks and damages the human body’s immune system in which the CD4+ T-cells play a critical role. Experimental and clinical evidence has demonstrated that CD4+ T cell depletion and accumulating CD8+ T lymphocytes are the most significant predictor of the disease severity [92]. CD4+ and CD8+ counts are typically used to access the degree of immune impairment in HIV patients. Even though clinical studies have found that CD4+ levels were associated with abnormalities on perfusion MRI, DTI, brain volumetric measurement in HIV patients, the relations between the CD4+ and/or CD8+ T cells and neuroimaging findings have not been conclusively identified.
The MRS results of SIV macaques indicate the cerebral metabolites are altered evidently in two weeks after SIV inoculation. Similarly, abnormal changes in FA and MD are observed also during acute SIV infection. Meanwhile, the correlation between the longitudinal DTI, CBF, and MRS measures of CNS and altered CD4+ and/or CD8 T cell counts during the course of SIV infection suggests that the MRI measures are sensitive to characterize the CNS injury associated with the immune dysfunction. In contrast, brain volume atrophy is not readily observed in SIV macaques and more advanced volumetric analysis may be needed to extract the subtle changes. Further investigation with these different modalities in SIV macaques may provide better understanding of the MRI findings, neurological impairment, and immune dysfunction in HIV patients.
In conclusion, high field MRI provides much improved spatial and contrast resolution and allows more accurate measurement and evaluation of macaque models of neuroAIDS. Quantitative measures in diffusion and perfusion MRI, in vivo MRS, and other modalities can be employed readily to characterize the CNS injury associated with the immune dysfunction during the course of SIV infection and relevant treatment responses. Also, with the technological advances in MRI, the methodology and application in SIV-infected macaque models are continuing to evolve and be redefined.
Acknowledgments
The authors would like to thank Dr. James Herndon for helpful comments. This project was funded by the National Center for Research Resources P51RR000165 and is currently supported by the Office of Research Infrastructure Programs / OD P51OD011132.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Lentz MR, Kim WK, Kim H, Soulas C, Lee V, et al. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol. 2011;17:220–229. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du H, Wu Y, Ochs R, Edelman RR, Epstein LG, et al. A comparative evaluation of quantitative neuroimaging measurements of brain status in HIV infection. Psychiatry Res. 2012;203:95–99. doi: 10.1016/j.pscychresns.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, et al. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge Y, Kolson DL, Babb JS, Mannon LJ, Grossman RI. Whole brain imaging of HIV-infected patients: quantitative analysis of magnetization transfer ratio histogram and fractional brain volume. AJNR Am J Neuroradiol. 2003;24:82–87. [PMC free article] [PubMed] [Google Scholar]
- 5.Arendt G. Imaging methods as a diagnostic tool in neuro-AIDS. A review. Bildgebung. 1995;62:310–319. [PubMed] [Google Scholar]
- 6.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, et al. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 7.Thurnher MM, Donovan Post MJ. Neuroimaging in the brain in HIV-1-infected patients. Neuroimaging Clin N Am. 2008;18:93–117. doi: 10.1016/j.nic.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Haigwood NL. Predictive value of primate models for AIDS. AIDS Rev. 2004;6:187–198. [PubMed] [Google Scholar]
- 9.Morgan C, Marthas M, Miller C, Duerr A, Cheng-Mayer C, et al. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 2008;5:e173. doi: 10.1371/journal.pmed.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staprans SI, Feinberg MB. The roles of nonhuman primates in the preclinical evaluation of candidate AIDS vaccines. Expert Rev Vaccines. 2004;3:S5–S32. doi: 10.1586/14760584.3.4.s5. [DOI] [PubMed] [Google Scholar]
- 11.Sopper S, Koutsilieri E, Scheller C, Czub S, Riederer P, et al. Macaque animal model for HIV-induced neurological disease. J Neural Transm. 2002;109:747–766. doi: 10.1007/s007020200062. [DOI] [PubMed] [Google Scholar]
- 12.O’Neil SP, Suwyn C, Anderson DC, Niedziela G, Bradley J, et al. Correlation of acute humoral response with brain virus burden and survival time in pig-tailed macaques infected with the neurovirulent simian immunodeficiency virus SIVsmmFGb. Am J Pathol. 2004;164:1157–1172. doi: 10.1016/S0002-9440(10)63204-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baba TW, Koch J, Mittler ES, Greene M, Wyand M, et al. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS Res Hum Retroviruses. 1994;10:351–357. doi: 10.1089/aid.1994.10.351. [DOI] [PubMed] [Google Scholar]
- 14.Villinger F, Mayne AE, Bostik P, Mori K, Jensen PE, et al. Evidence for antibody-mediated enhancement of simian immunodeficiency virus (SIV) Gag antigen processing and cross presentation in SIV-infected rhesus macaques. J Virol. 2003;77:10–24. doi: 10.1128/JVI.77.1.10-24.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed RK, Biberfeld G, Thorstensson R. Innate immunity in experimental SIV infection and vaccination. Mol Immunol. 2005;42:251–258. doi: 10.1016/j.molimm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat Neurosci. 1999;2:555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- 18.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 19.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 20.Aranda-Anzaldo A. A role for CD8+ T lymphocytes in the pathogenesis of AIDS. Res Immunol. 1991;142:541–550. doi: 10.1016/0923-2494(91)90099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, et al. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- 22.von Geldern G, Cepok S, Nolting T, Du Y, Grummel V, et al. CD8 T-cell subsets and viral load in the cerebrospinal fluid of therapy-naive HIV-infected individuals. AIDS. 2007;21:250–253. doi: 10.1097/QAD.0b013e328011ec76. [DOI] [PubMed] [Google Scholar]
- 23.Shearer WT, Pahwa S, Read JS, Chen J, Wijayawardana SR, et al. CD4/CD8 T-cell ratio predicts HIV infection in infants: the National Heart, Lung, and Blood Institute P2C2 Study. J Allergy Clin Immunol. 2007;120:1449–1456. doi: 10.1016/j.jaci.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Zhang X, Komery A, Li Y, Novembre FJ, et al. Longitudinal diffusion tensor imaging and perfusion MRI investigation in a macaque model of neuro-AIDS: a preliminary study. Neuroimage. 2011;58:286–292. doi: 10.1016/j.neuroimage.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- 26.Grant I, Heaton RK, Atkinson JH. Neurocognitive disorders in HIV-1 infection. HNRC Group. HIV Neurobehavioral Research Center. Curr Top Microbiol Immunol. 1995;202:11–32. [PubMed] [Google Scholar]
- 27.Gold LH, Fox HS, Henriksen SJ, Buchmeier MJ, Weed MR, et al. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J Med Primatol. 1998;27:104–112. doi: 10.1111/j.1600-0684.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 28.Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur J Neurosci. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 29.Comparet P, Darriet D, Jaffard R. Demonstration of dissociation between frontal and temporal lesions in man on two versions of delayed non-matching recognition tests used in monkeys. C R Acad Sci III. 1992;314:515–518. [PubMed] [Google Scholar]
- 30.Martin EM, Pitrak DL, Pursell KJ, Mullane KM, Novak RM. Delayed recognition memory span in HIV-1 infection. J Int Neuropsychol Soc. 1995;1:575–580. doi: 10.1017/s1355617700000710. [DOI] [PubMed] [Google Scholar]
- 31.Sahakian BJ, Elliott R, Low N, Mehta M, Clark RT, et al. Neuropsychological deficits in tests of executive function in asymptomatic and symptomatic HIV-1 seropositive men. Psychol Med. 1995;25:1233–1246. doi: 10.1017/s0033291700033201. [DOI] [PubMed] [Google Scholar]
- 32.Novembre FJ, De Rosayro J, O’Neil SP, Anderson DC, Klumpp SA, et al. Isolation and characterization of a neuropathogenic simian immunodeficiency virus derived from a sooty mangabey. J Virol. 1998;72:8841–8851. doi: 10.1128/jvi.72.11.8841-8851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hestad K, McArthur JH, Dal Pan GJ, Selnes OA, Nance-Sproson TE, et al. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta Neurol Scand. 1993;88:112–118. doi: 10.1111/j.1600-0404.1993.tb04201.x. [DOI] [PubMed] [Google Scholar]
- 34.Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, et al. Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology. 1992;42:2125–2130. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- 35.Jakobsen J, Gyldensted C, Brun B, Bruhn P, Helweg-Larsen S, et al. Cerebral ventricular enlargement relates to neuropsychological measures in unselected AIDS patients. Acta Neurol Scand. 1989;79:59–62. doi: 10.1111/j.1600-0404.1989.tb03710.x. [DOI] [PubMed] [Google Scholar]
- 36.Hall M, Whaley R, Robertson K, Hamby S, Wilkins J, et al. The correlation between neuropsychological and neuroanatomic changes over time in asymptomatic and symptomatic HIV-1-infected individuals. Neurology. 1996;46:1697–1702. doi: 10.1212/wnl.46.6.1697. [DOI] [PubMed] [Google Scholar]
- 37.Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bihan D. Diffusion, confusion and functional MRI. Neuroimage. 2012;62:1131–1136. doi: 10.1016/j.neuroimage.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 39.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tucker KA, Robertson KR, Lin W, Smith JK, An H, et al. Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol. 2004;157:153–162. doi: 10.1016/j.jneuroim.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, An H, Zhu H, Stone T, Smith JK, et al. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage. 2009;47:1154–1162. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang L, Wong V, Nakama H, Watters M, Ramones D, et al. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, et al. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stubbe-Dräger B, Deppe M, Mohammadia S, Keller SS, Kugel H, et al. Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol. 2012;12:23. doi: 10.1186/1471-2377-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, et al. Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- 48.Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- 49.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, et al. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoare J, Fouche JP, Spottiswoode B, Sorsdahl K, Combrinck M, et al. White-Matter damage in Clade C HIV-positive subjects: a diffusion tensor imaging study. J Neuropsychiatry Clin Neurosci. 2011;23:308–315. doi: 10.1176/jnp.23.3.jnp308. [DOI] [PubMed] [Google Scholar]
- 51.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 52.Detre JA, Zhang W, Roberts DA, Silva AC, Williams DS, et al. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed. 1994;7:75–82. doi: 10.1002/nbm.1940070112. [DOI] [PubMed] [Google Scholar]
- 53.Paiva FF, Tannús A, Talagala SL, Silva AC. Arterial spin labeling of cerebral perfusion territories using a separate labeling coil. J Magn Reson Imaging. 2008;27:970–977. doi: 10.1002/jmri.21320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Nagaoka T, Auerbach EJ, Champion R, Zhou L, et al. Quantitative basal CBF and CBF fMRI of rhesus monkeys using three-coil continuous arterial spin labeling. Neuroimage. 2007;34:1074–1083. doi: 10.1016/j.neuroimage.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, et al. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 56.Dunlop O, Rootwelt K, Rklund R, Bruun JN, Russell D, et al. Reduced Global Cerebral Blood Flow in Non-Demented HIV Positive Patients. J NeuroAIDS. 1996;1:71–78. doi: 10.1300/j128v01n04_07. Bj 0248. [DOI] [PubMed] [Google Scholar]
- 57.Ances BM, Vaida F, Cherner M, Yeh MJ, Liang CL, et al. HIV and chronic methamphetamine dependence affect cerebral blood flow. J Neuroimmune Pharmacol. 2011;6:409–419. doi: 10.1007/s11481-011-9270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maini CL, Pigorini F, Pau FM, Volpini V, Galgani S, et al. Cortical cerebral blood flow in HIV-1-related dementia complex. Nucl Med Commun. 1990;11:639–648. doi: 10.1097/00006231-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Ernst T, Itti E, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: A coregistered (1)H MRS and SPECT study. J Magn Reson Imaging. 2000;12:859–865. doi: 10.1002/1522-2586(200012)12:6<859::aid-jmri8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 60.Chang L, Ernst T, Leonido-Yee M, Speck O. Perfusion MRI detects rCBF abnormalities in early stages of HIV-cognitive motor complex. Neurology. 2000;54:389–396. doi: 10.1212/wnl.54.2.389. [DOI] [PubMed] [Google Scholar]
- 61.Modi G, Modi M, Martinus I, Vangu M. New onset seizures in HIV-infected patients without intracranial mass lesions or meningitis--a clinical, radiological and SPECT scan study. J Neurol Sci. 2002;202:29–34. doi: 10.1016/s0022-510x(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 62.Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, et al. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66:862–866. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- 63.Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73:702–708. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang L. In vivo magnetic resonance spectroscopy in HIV and HIV-related brain diseases. Rev Neurosci. 1995;6:365–378. doi: 10.1515/revneuro.1995.6.4.365. [DOI] [PubMed] [Google Scholar]
- 65.Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 66.Descamps M, Hyare H, Stebbing J, Winston A. Magnetic resonance imaging and spectroscopy of the brain in HIV disease. J HIV Ther. 2008;13:55–58. [PubMed] [Google Scholar]
- 67.Jarvik JG, Lenkinski RE, Saykin AJ, Jaans A, Frank I. Proton spectroscopy in asymptomatic HIV-infected adults: initial results in a prospective cohort study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:247–253. doi: 10.1097/00042560-199611010-00006. [DOI] [PubMed] [Google Scholar]
- 68.Keller MA, Venkatraman TN, Thomas A, Deveikis A, LoPresti C, et al. Altered neurometabolite development in HIV-infected children: correlation with neuropsychological tests. Neurology. 2004;62:1810–1817. doi: 10.1212/01.wnl.0000125492.57419.25. [DOI] [PubMed] [Google Scholar]
- 69.Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 70.Stankoff B, Tourbah A, Suarez S, Turell E, Stievenart JL, et al. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology. 2001;56:112–115. doi: 10.1212/wnl.56.1.112. [DOI] [PubMed] [Google Scholar]
- 71.Suwanwelaa N, Phanuphak P, Phanthumchinda K, Suwanwela NC, Tantivatana J, et al. Magnetic resonance spectroscopy of the brain in neurologically asymptomatic HIV-infected patients. Magn Reson Imaging. 2000;18:859–865. doi: 10.1016/s0730-725x(00)00173-9. [DOI] [PubMed] [Google Scholar]
- 72.Vion-Dury J, Confort-Gouny S, Nicoli F, Dhiver C, Gastaut JA, et al. Localized brain proton MRS metabolic patterns in HIV-related encephalopathies. C R Acad Sci III. 1994;317:833–840. [PubMed] [Google Scholar]
- 73.Wilkinson ID, Lunn S, Miszkiel KA, Miller RF, Paley MN, et al. Proton MRS and quantitative MRI assessment of the short term neurological response to antiretroviral therapy in AIDS. J Neurol Neurosurg Psychiatry. 1997;63:477–482. doi: 10.1136/jnnp.63.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McConnell JR, Swindells S, Ong CS, Gmeiner WH, Chu WK, et al. Prospective utility of cerebral proton magnetic resonance spectroscopy in monitoring HIV infection and its associated neurological impairment. AIDS Res Hum Retroviruses. 1994;10:977–982. doi: 10.1089/aid.1994.10.977. [DOI] [PubMed] [Google Scholar]
- 76.Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, et al. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol. 2010;16:435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ratai EM, Pilkenton SJ, Greco JB, Lentz MR, Bombardier JP, et al. In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci. 2009;10:63. doi: 10.1186/1471-2202-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lentz MR, Westmoreland SV, Lee V, Ratai EM, Halpern EF, et al. Metabolic markers of neuronal injury correlate with SIV CNS disease severity and inoculum in the macaque model of neuroAIDS. Magn Reson Med. 2008;59:475–484. doi: 10.1002/mrm.21556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lentz MR, Lee V, Westmoreland SV, Ratai EM, Halpern EF, et al. Factor analysis reveals differences in brain metabolism in macaques with SIV/ AIDS and those with SIV-induced encephalitis. NMR Biomed. 2008;21:878–887. doi: 10.1002/nbm.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tracey I, Lane J, Chang I, Navia B, Lackner A, et al. 1H magnetic resonance spectroscopy reveals neuronal injury in a simian immunodeficiency virus macaque model. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:21–27. doi: 10.1097/00042560-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 81.Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, et al. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- 82.Cloak CC, Chang L, O’Neil SP, Ernst TM, Anderson DC, et al. Neurometabolite abnormalities in simian immunodeficiency virus-infected macaques with chronic morphine administration. J Neuroimmune Pharmacol. 2011;6:371–380. doi: 10.1007/s11481-010-9246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.González RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, et al. Early brain injury in the SIV-macaque model of AIDS. AIDS. 2000;14:2841–2849. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- 84.Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, et al. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51:1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- 85.Fuller RA, Westmoreland SV, Ratai E, Greco JB, Kim JP, et al. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004;5:10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ratai EM, Annamalai L, Burdo T, Joo CG, Bombardier JP, et al. Brain creatine elevation and N-Acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of neuroAIDS. Magn Reson Med. 2011;66:625–634. doi: 10.1002/mrm.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ratai EM, Bombardier JP, Joo CG, Annamalai L, Burdo TH, et al. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. Plos One. 2010;5:e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52:100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- 89.Li C, Zhang X, Komery A, Li Y, Mao H, et al. Longitudinal Cerebral Metabolic Alternations in a Novel Macaque Model of Neuro-AIDS. Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM).2012. p. 1796. [Google Scholar]
- 90.Liu X, Zhu T, Gu T, Zhong J. Optimization of in vivo high-resolution DTI of non-human primates on a 3T human scanner. Methods. 2010;50:205–213. doi: 10.1016/j.ymeth.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 91.Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- 92.Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther. 2007;4:11. doi: 10.1186/1742-6405-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]