Abstract
With the increasing prevalence of obesity among children and adolescents, it is imperative to understand the implications of early diet-induced obesity on bone health. We hypothesized that cancellous bone of skeletally immature mice is more susceptible to the detrimental effects of a high fat diet (HFD) than mature mice, and that removing excess dietary fat will reverse these adverse effects. Skeletally immature (5 weeks old) and mature (20 weeks old) male C57BL/6J mice were fed either a HFD (60% kcal fat) or low fat diet (LFD; 10% kcal fat) for 12 weeks, at which point, the trabecular bone structure in the distal femoral metaphysis and third lumbar vertebrae were evaluated by micro-computed tomography. The compressive strength of the vertebrae was also measured. In general, the HFD led to deteriorations in cancellous bone structure and compressive biomechanical properties in both age groups. The HFD-fed immature mice had a greater decrease in trabecular bone volume fraction (BVF) in the femoral metaphysis, compared to mature mice (p=0.017 by 2-way ANOVA). In the vertebrae, however, the HFD led to similar reductions in BVF and compressive strength in the two age groups. When mice on the HFD were switched to a LFD (HFD:LFD) for an additional 12 weeks, the femoral metaphyseal BVF in immature mice showed no improvements, whereas the mature mice recovered their femoral metaphyseal BVF to that of age-matched lean controls. The vertebral BVF and compressive strength of HFD:LFD mouse bones, following diet correction, were equivalent to those of LFD:LFD mice in both age groups. These data suggest that femoral cancellous metaphyseal bone is more susceptible to the detrimental effects of HFD before skeletal maturity and is less able to recover after correcting the diet. Negative effects of HFD on vertebrae are less severe and can renormalize with LFD:LFD mice after diet correction, in both skeletally immature and mature animals.
Keywords: High fat diet, obesity, trabecular bone, age dependence, diet correction
Introduction
Obesity is reaching epidemic proportions in the United States and the developed world due, in part, to Western diets and decreased physical activity. In 2010, 33.8% of the U.S. adult population was obese. Childhood obesity is a particularly troubling public health concern. An estimated 16.9% of American adolescents are obese, with an alarming 9.7% of infants and toddlers also falling into this category [1]. Obesity is associated with an increased risk for several serious illnesses including type 2 diabetes, hypertension, and heart disease [2]. Associations between obesity and bone health, however, are still unclear. Evidence suggests that obese children are at risk of decreased bone mineral density (BMD) [3–5] and have increased fracture risk [3, 6–8]. A recent study using peripheral quantitative computed tomography (pQCT) found reductions in volumetric BMD with increased fat mass in children, after correcting for lean mass, despite increased bone size [5]. The peak ages for increasing BMD and bone mineral content (BMC) are during adolescence, in the years 12–14 for girls and 13–15 for boys [9]. The lower BMD, BMC, and increased fracture risk in obese adolescents suggest that factors associated with obesity could be detrimental to the accrual of peak bone mass, a critical factor in the etiology of osteoporosis [10].
Many integral factors are associated with obesity, ranging from genetic to environmental. Excessive dietary fat, which is preventable, has become a particular concern in recent decades. It is recommended by the USDA that fats be reduced in the diet of Americans [11]. Several studies have investigated the effects of a high fat diet (HFD) on bone in animal models, with the consensus that excessive dietary fat is detrimental to bone homeostasis and has a greater effect on trabecular than cortical bone [12–17]. These studies demonstrated adverse effects of the HFD on bone health in adult as well as adolescent mice or rats. Ionova-Martin et al. examined the effects of HFD on cortical bone from adolescence to adulthood in mice and observed similar trends in bone mineral and mechanical properties between the two age groups [18]. The possible differential impact between adolescents and adults of high dietary fat on cancellous bone, to the best of our knowledge, has not been reported. Studying this question may help determine if HFDs or the associated obesity and metabolic syndrome contribute to skeletal deficits in growing individuals; and whether this may lead to unrecoverable deficits later in life, even after potential interventions and life-style changes (e.g. diet). We hypothesized that 1) Skeletally immature mice would be more susceptible to HFD-induced deterioration in cancellous bone structure, mineralization and strength compared to skeletally mature mice and 2) The HFD-associated deterioration in bone structure and strength would be alleviated after reducing dietary fat intake. These hypotheses were studied using skeletally immature (5 weeks old) and mature (20 weeks old) mice that were exposed to a HFD for 12 weeks and then transitioned to a low fat diet (LFD) for an additional 12 weeks. Mice that were maintained on the LFD throughout the experiment were used as controls.
2. Materials and Methods
2.1 Animals and Tissue Processing
Animal studies were performed in accordance with protocols approved by the University of Rochester’s Committee on Animal Resources. Male C57BL/6J mice were purchased from Jackson Research Labs (Bar Harbor, ME) at 5 and 20 weeks of age to represent skeletally immature and mature mice, respectively. These ages were chosen based on studies of bone density as well as bone tissue and mechanical properties in C57BL/6J mice peaking in the age range of 16–24 weeks [19–21]. After a brief acclimation period, mice from each age group were placed either on a high fat diet (HFD; 60% kcal fat; Research Diets, Inc., New Brunswick, NJ) or low fat diet (LFD; 10% kcal fat; Research Diets, Inc., New Brunswick, NJ) for 12 weeks. After those 12 weeks, half of the mice from each group were switched to or continued on the lean diet (HFD:LFD or LFD:LFD, respectively) for an additional 12 weeks, while the other half were sacrificed for tissue collection (n=7–8 per age group, diet, and time point). Immediately after isolation and removal of soft tissue, the right femurs were used for micro-computed tomography (micro-CT) imaging, while the third lumbar (L3) vertebrae were wrapped in saline-soaked gauze and frozen at −80°C until the day of micro-CT and biomechanical testing.
2.2 Glucose Measurements
Sixteen hours prior to sacrifice, food was removed from mouse cages to allow measurement of fasting blood glucose. Immediately before sacrifice, the mice were anesthetized under isofluorane gas, the distal tip of the tail was excised, and blood samples were collected to measure blood glucose levels using One Touch glucose meters (Lifescan, Inc.; Milpitas, CA).
2.3 Serum Leptin Measurements
At the time of sacrifice, blood samples were collected via heart puncture. Sera were frozen at −80°C until analysis. Serum leptin levels were quantitated using a mouse leptin ELISA kit (EMD Millipore, St. Charles, MO). Sera were diluted 1:4 before analysis. All procedures were according to the manufacturer’s instructions.
2.4 Micro-Computed Tomography
Femurs and L3 vertebrae were scanned by micro-CT (VivaCT 40; Scanco Medical; Bassersdorf, Switzerland), at a 10.5-micron isotropic resolution using an integration time of 300 ms, energy of 55 kVp and intensity of 145 μA. For trabecular analysis in the distal femoral metaphysis, a 200 μm region proximal to the growth plate was used for quantification. Femoral cortical bone was measured at the mid-diaphysis by averaging over a 200 μm region (19 slices). For vertebral measurements, the volume within the endosteal margin of each vertebral body was used to assess trabecular bone. Cortical thickness was measured at the mid-level of each vertebral body by averaging over a 200 μm thick region. Total cross-sectional bone area was similarly measured from the region between the caudal endplate and transverse processes. The trabecular bone morphology of the femoral metaphysis and vertebral bodies, including the bone volume fraction (BVF), connective density (Conn.D), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and structural model index (SMI) were determined using Scanco’s 3D analysis tools (direct model).
2.5 Biomechanical Testing
The whole bone strength of L3 vertebral bodies was tested under compressive loading through a modified, published method [22, 23]. Briefly, the L3 vertebrae were dissected of all soft tissue including the intervertebral discs and the pedicles were cut from the vertebral body. The vertebral body end plates were embedded in 0.5 mm of polymethylmethacrylate (PMMA) bone cement using a custom jig to ensure axial alignment of the vertebral body and even load distribution over the end plates (Fig. 4A). The vertebral bodies were then hydrated in phosphate buffered saline (PBS) for 2 hours at room temperature prior to compressive testing at a rate of 1 mm/min, with a 1.5 N preload, until failure using an Instron 8841 DynaMight™ Axial Testing System (Instron Corp.; Canton, MA) with a 50 N load cell. The compressive load data were plotted against displacement data, which were normalized by the height of each vertebral body (apparent strain), to determine the yield and maximum strength, compressive stiffness, and energy to maximum loading. The yield point was determined by a 0.2% strain offset. Apparent stresses were estimated by normalizing the loads by the total cross-sectional bone area of each vertebral body.
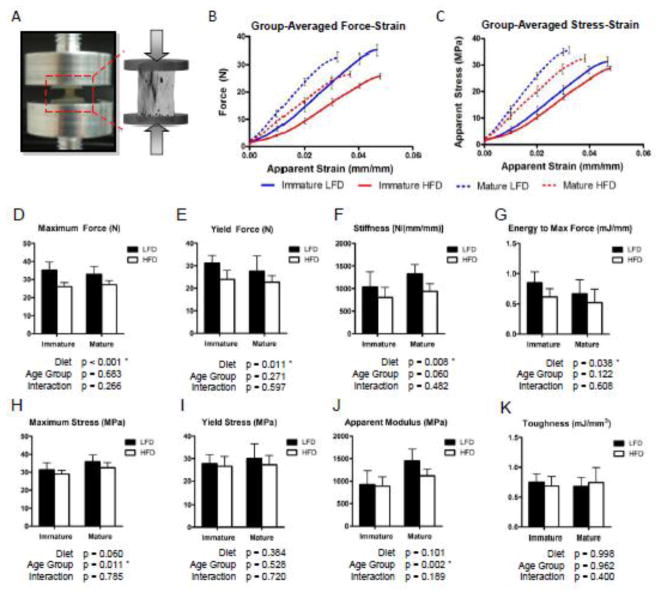
Figure 4.
Vertebral compressive strength is significantly reduced by a high fat diet similarly between age groups. A) Uniaxial compressive testing of vertebral bodies was performed with the endplates embedded in 0.5 mm PMMA to ensure axial alignment and even load distribution. Group-averaged force-strain (B) and stress-strain (C) compressive testing curves (error bars represent SEM), plotted up to the point of maximum loading, illustrate the significant reduction in strength of HFD-fed mice compared to LFD-fed mice. The HFD significantly reduced the maximum force (D), force at yielding (E), stiffness (F), and energy to maximum force (G) similarly between the age groups. However, after correcting for differences in the cross-sectional bone area, the apparent material properties (H–K) were not significantly affected by the high fat diet. Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA.
2.6 Data Analysis
To determine whether the HFD affects immature versus mature mice differently, a two-way analysis of variance (ANOVA) was used to elucidate the effects of diet, age group and their interaction (Diet×Age Group). The D’Agostino-Pearson normality test was performed on each metric, which supported that the data were consistent with a Gaussian distribution. A two-way ANOVA approach was used because the interactive effect describes whether the age groups were indeed affected differently by the HFD. Next, the persistence of any HFD-induced deficits in bone structure or strength after diet correction was assessed by comparing HFD and HFD:LFD mice across the two age groups by two-way ANOVA. Considering there is an effect of intragroup aging between the 12 and 24 week time points, the HFD-fed groups were normalized to their age-matched lean controls for this analysis (HFD/LFD vs. HFD:LFD/LFD:LFD). Therefore, in this normalized analysis, a significant diet effect indicates that there is a difference in the relationship of HFD-fed mice to lean controls from before and after diet correction. When interactions in the two-way ANOVAs were statistically significant, Bonferonni’s post-hoc test was used to determine whether the differences due to diet were significant within each age group. Differences were deemed statistically significant when p < 0.05.
3. Results
3.1 Effect of High Fat Diet on Skeletally Immature and Mature Mice
3.1.1 Body Weight, Fasting Blood Glucose and Serum Leptin
As expected, 12 weeks on the HFD induced significant weight gain (Fig. 1A) along with elevated fasting blood glucose (Fig. 1B) and serum leptin levels (Fig. 1C) in both immature and mature age groups of male C57BL/6J mice. The mature mice gained significantly more weight than the immature mice and had significantly greater increases in fasting blood glucose levels, as evidenced by the significant interactive effects and post-hoc comparisons. The insignificantly different leptin concentrations in the HFD-fed mice across the two age groups suggests that similar levels of obesity were reached while on this diet.
Figure 1.
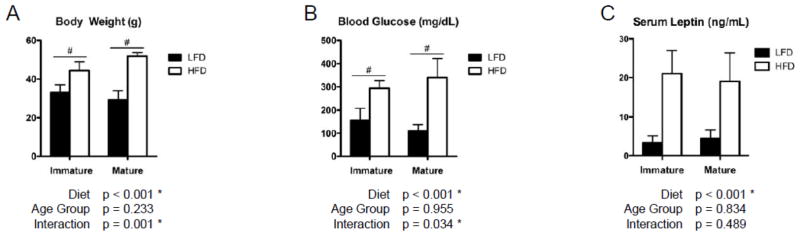
High fat diet increases body weight, blood glucose and serum leptin levels significantly. Mice on the high fat diet (HFD) were significantly heavier (A) and had significantly higher fasting blood glucose levels (B) compared to low fat diet (LFD) controls. The interactive effect (Diet×Age Group) was also significant in both body weight and blood glucose, which indicates that a synergistic effect existed between Diet and Age Group. Serum leptin levels (C) were also significantly elevated in the HFD-fed mice. Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA. # indicates p < 0.05 for intra-age group comparisons made with Bonferonni’s post-hoc test when the interaction is significant.
3.1.2 Bone Structure and Mineralization
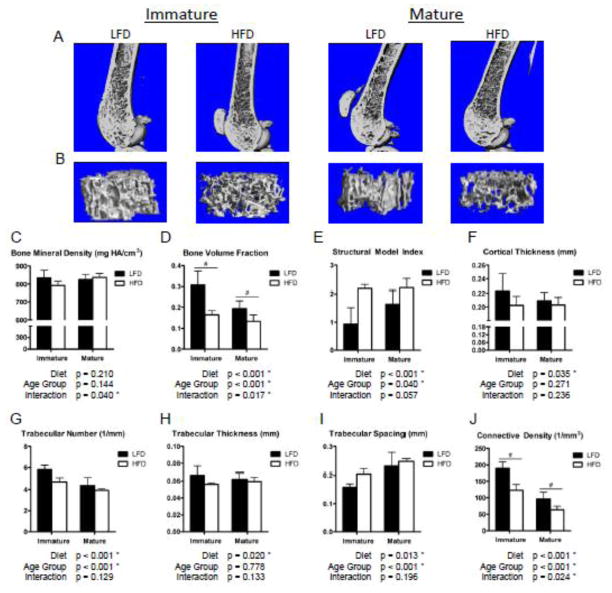
Micro-CT scans of the distal femur demonstrated a lower cancellous bone volume in the HFD mice than LFD controls (Fig. 2A, B), with significantly reduced trabecular BVF in HFD compared to LFD mice. A significantly greater reduction in BVF was observed in immature than mature mice (Fig. 2D), suggesting a greater susceptibility to HFD-induced bone deficits prior to skeletal maturity. This result is supported by additional trends and significant decreases in trabecular number, thickness and connectivity, as well as increases in trabecular spacing and structural model index (SMI) (Fig. 2E, G–J) in cancellous bone within the distal femur of immature HFD-fed mice compared to mature mice. Further, the cortical bone was significantly thinner in HFD than LFD mice (Fig. 2F). The polar moment of inertia and the moment of inertia about the medial-lateral axis of the femoral mid-shaft, however, were not significantly affected by the diet in either age group. The cancellous BMD of the distal femur (Fig. 2C) exhibited a significant interaction between Diet and Age Group, indicating that the two age groups may have been affected differently, but the Diet and Age Group main effects were not significant.
Figure 2.
High fat diet results in significantly reduced femoral trabecular bone volume fraction and structural metrics relative to low fat diet controls, with a greater decrement in immature mice. 3D renderings of sagittal cross-sections (A) and metaphyseal trabecular volumes of interest (B) from micro-CT scans of the distal femur qualitatively demonstrate the lower trabecular bone volumes and cortex thickness in the high fat diet (HFD)-fed mice compared to low fat diet (LFD) controls. The trend in BMD (C) was significantly different between immature and mature mice, with immature HFD-fed mice tending to have a lower BMD than LFD controls. Trabecular bone volume fraction (D) was significantly affected by the HFD and was affected differently between the two age groups, as indicated by the significant interaction (Diet×Age Group), with greater decrement observed in the immature mice. This result is reiterated by trends and significant effects in the trabecular structural model index (E), number (G), thickness (H), spacing (I) and connective density (J) as well as the cortical thickness (F). Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA. # indicates p < 0.05 for intra-age group comparisons made with Bonferonni’s post-hoc test when the interaction is significant.
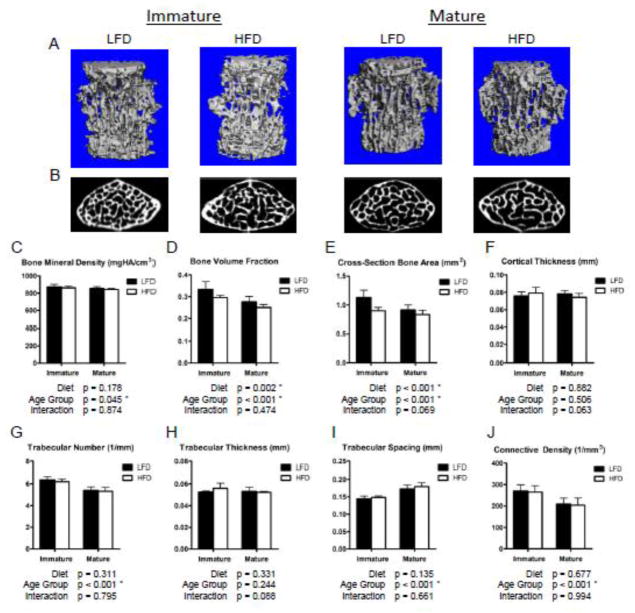
As with the distal femur, HFD decreased vertebral cancellous bone volume relative to LFD controls as demonstrated by 3D renderings of micro-CT images (Fig. 3A, B). Within the L3 vertebral bodies, the trabecular BVF was again significantly lower in the HFD compared to the LFD groups (Fig. 3D), but the decrement was not as drastic as that observed in the femur. Unlike the distal femur, this effect was equivalent across the age groups as the interactive effect was insignificant. Despite the significantly lower trabecular BVF in the HFD-fed mice, the Conn.D, Tb.N, Tb.Sp, and Tb.Th as well as the cortical shell thickness (Fig. 3F–J) were not significantly affected by the HFD. The total cross-sectional (transverse) bone area measurements had similar trends to the BVF, with significant reductions in the HFD-fed mice and trends towards a greater deficit in HFD-fed immature mice (Fig. 3E).
Figure 3.
Vertebral trabecular bone volume fraction is significantly reduced with a high fat diet compared to lean controls, affecting immature and mature mice similarly. 3D renderings of vertebral body trabecular bone (A) and transverse cross-sections of vertebral bodies, caudal to the transverse process, (B) from micro-CT scans qualitatively demonstrate the reductions in trabecular bone volume in HFD-fed relative to LFD-fed mice. While the overall bone volume fraction (D) was significantly lower in the HFD group, the trabecular bone mineral density (C) and individual structural metrics (G–J) were not significantly affected. The effect on total cross-sectional (transverse) bone area (E) is similar to the 3D bone volume fraction, considering the difference in cortical shell thickness is insignificant (F). Though the interaction was not statistically significant, the total cross-sectional bone area (E) tended to be reduced more substantially, relative to LFD controls, in immature HFD-fed mice than mature HFD-fed mice. Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA.
3.1.3 Vertebral Compressive Strength
Consistent with the lower trabecular BVF and total cross-sectional bone area of the vertebrae, we observed a significantly lower maximum compressive force, yield force, stiffness and energy to maximal loading in the HFD-fed mice (Fig. 4D–G). In accordance with the structural changes, this reduction in compressive strength was similar between the two age groups. After adjusting the compressive force by the cross-sectional bone areas to estimate the apparent stresses, the HFD did not significantly affect the maximum stress, yield stress, modulus, or toughness (Fig. 4H–K). This suggests that the bone tissue quality may not be significantly affected by the HFD after 12 weeks in either immature or mature mice.
3.2 Persistence of Initial HFD Effects After Diet Correction Relative to Lean Controls
3.2.1 Body Weight, Fasting Blood Glucose and Serum Leptin
After transitioning the HFD-fed mice to a LFD for an additional 12 weeks, the body weight in both age groups returned to that of age-matched LFD:LFD mice (Fig. 5A–B, Table S1). The increased fasting glucose and serum leptin concentration were also returned to normal levels in both age groups after the diet correction (Fig. 5C–D, Table S1). Interestingly, the fasting glucose levels of the mature LFD:LFD group were significantly higher than the mature HFD:LFD group (Table S1).
Figure 5.
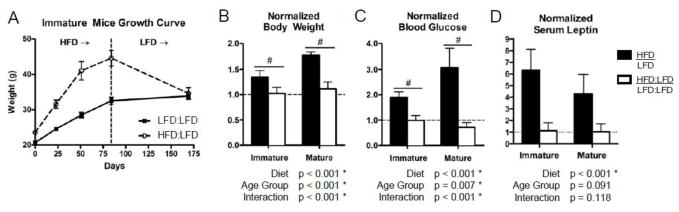
Body weight, glucose, and leptin levels of initially obese mice match those of lean mice after diet correction. As illustrated by the immature mice growth curve (A), the body weight (B) as well as the fasting blood glucose (C) and serum leptin (D) levels are significantly reduced after switching mice from a high fat diet to a low fat diet (HFD:LFD). The black bars represent mice that were fed a HFD for 12 weeks and white bars represent mice that were subsequently transitioned from the HFD to a LFD for an additional 12 weeks. Each of the groups is normalized to age-matched LFD controls to adjust for aging differences during the diet time course. Body weight and blood glucose levels equilibrate with those of age-matched, lean controls after diet correction (normalized values ≈ 1). Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA. # indicates p < 0.05 for intra-age group comparisons made with Bonferonni’s post-hoc test when the interaction is significant.
3.2.2 Bone Structure and Mineralization
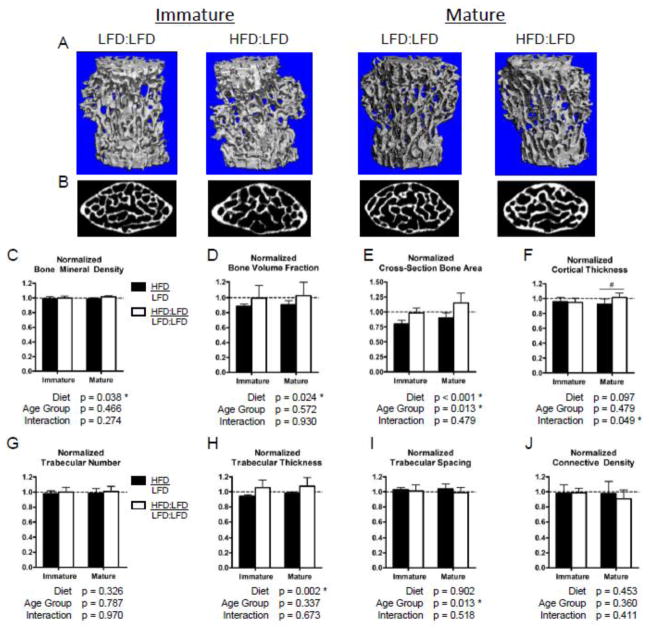
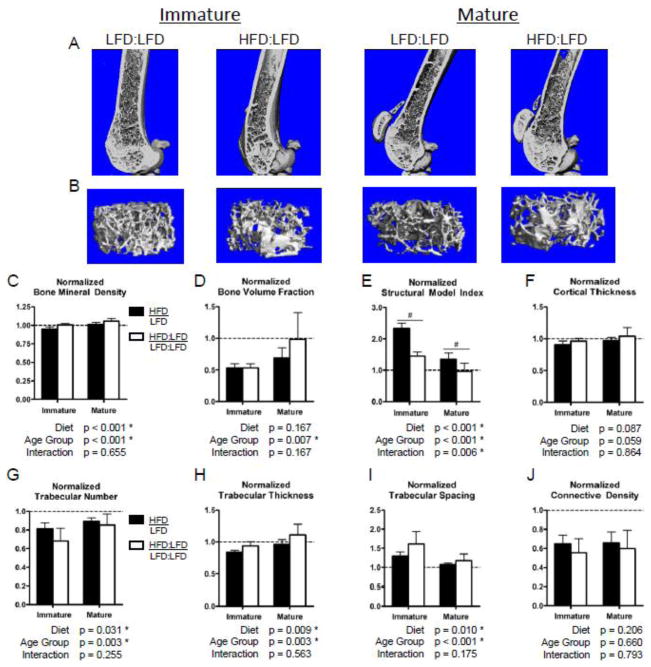
After normalizing the HFD groups to the age-matched LFD controls to correct for aging effects between time points, we observed a significant relative improvement in bone structural properties and BMD in the vertebrae (Fig. 7) but not in the distal femur (Fig. 6). The trabecular BMD of the distal femur (Fig. 6C) as well as the L3 vertebrae (Fig. 7C) were significantly improved upon diet correction in both age groups, after adjusting to lean controls. The mean trabecular BVF in the distal femur of mature HFD:LFD mice was equivalent to the age-matched LFD:LFD controls; however, a relative deficit with no improvement persisted in the normalized BVF of immature mice (Fig. 6D). A trend towards improved cortical thickness (Fig. 6F) and significant relative improvements in SMI (Fig. 6E) as well as Tb.Th (Fig. 6H) were observed in the femurs of both age groups after diet correction (HFD:LFD). However, all other trabecular structure metrics remained inferior to age-matched lean controls in the distal femur (Table S2). In the L3 vertebrae, relative improvements were observed with diet correction in the trabecular BVF, total cross-sectional bone area, and Tb.Th in both age groups (Fig. 7D, E, H). Interestingly, the vertebral Tb.Th of HFD-fed mice significantly exceeds that of age-matched LFD:LFD controls in both age groups after diet correction (Table S3). Further, the cortical shell thickness of the vertebral bodies is significantly improved after diet correction in the mature, but not immature, mice (Fig. 7F). In accordance with the recovered BVF and cortical thickness, as well as the increasing Tb.Th, the total cross-sectional bone area was significantly improved with diet correction in both age groups (Fig. 7E). The vertebral bone area was equivalent to age-matched LFD:LFD controls in the immature group and tended to exceed those of LFD:LFD controls in the mature group (Table S3).
Figure 7.
After diet correction, trabecular bone volume fraction in L3 vertebral bodies returns to that of age-matched lean controls in both age groups. 3D renderings of vertebral body trabecular bone (A) and transverse cross-sections of vertebral bodies caudal to the transverse processes (B) from micro-CT scans provide qualitative comparisons of trabecular bone volumes in mice that were returned to a low fat diet from a high fat diet (HFD:LFD) compared to continuous low fat diet (LFD:LFD)-fed mice. After normalizing values from HFD-fed mice and diet corrected (HFD:LFD) mice to those of age-matched lean controls, there was significant improvement in the bone mineral density (C), bone volume fraction (D), and total cross-sectional bone area (E) with diet correction across both age groups. There is a significant relative increase in the cortical thickness (F) with diet correction in the mature group only. Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA. # indicates p < 0.05 for intra-age group comparisons made with Bonferonni’s post-hoc test when the interaction is significant.
Figure 6.
Diet correction tends to normalize the bone volume fraction of mature mice, but not immature mice, with that of age-matched lean controls. 3D renderings of sagittal cross-sections (A) and metaphyseal trabecular volumes of interest (B) from micro-CT scans of the distal femur provide qualitative comparisons of trabecular bone volumes in the mice that were returned to a low fat diet from a high fat diet (HFD:LFD) compared to continuous low fat diet (LFD:LFD)-fed mice. The immature HFD:LFD group does not improve in trabecular bone volume fraction relative to lean controls after diet correction, but mature HFD:LFD mice tend to renormalize with lean, age-matched control mice (D). The trabecular number (G), spacing (I), and connective density (J) of HFD:LFD mice remain inferior to that of age-matched LFD controls. Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA. # indicates p < 0.05 for intra-age group comparisons made with Bonferonni’s post-hoc test when the interaction is significant.
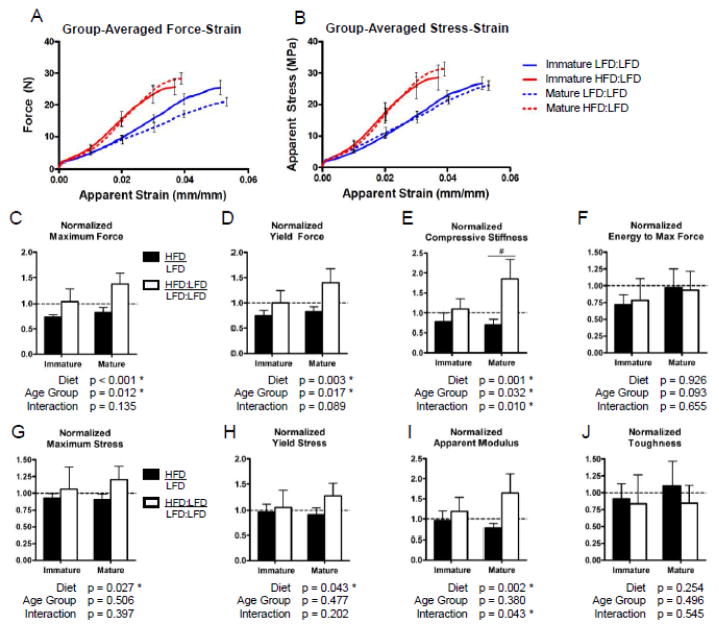
3.2.3 Vertebral Compressive Strength
The compressive strength of the L3 vertebral bodies followed the relative improvements of bone structure after transitioning the HFD-fed mice to a lean diet. The maximum force, yield force and stiffness were significantly increased with the diet correction (HFD:LFD), after normalizing to age-matched LFD controls, in both age groups (Fig. 8C–E). Interestingly, while the strength of immature HFD:LFD mice vertebrae were equivalent to that of lean controls, the strength of mature HFD:LFD mice vertebrae tended to exceed that of their respective lean controls (Table S4). The effect of diet correction and trends in improvement remain significant after normalizing the compressive loads by the total cross-sectional bone areas (Fig. 8G–I). This result suggests that apparent bone tissue quality may be improved with diet correction, in relation to that of lean controls, particularly in mature mice.
Figure 8.
Vertebral compressive strength is equivalent to or exceeds that of lean, age-matched controls after diet correction in both age groups. Group-averaged force-strain (A) and stress-strain (B) compressive testing curves (error bars represent SEM) illustrating the significant differences in vertebral strength between mice that were fed a HFD for 12 weeks followed by a LFD for 12 weeks (HFD:LFD) and age-matched mice that were continuously on a LFD (LFD:LFD). After adjusting values from HFD-fed mice and HFD:LFD mice to those of age-matched lean controls, there was a significant improvement with diet correction in the maximum force (C), force at yielding (D) and stiffness (E), but not in the energy to maximum loading (F) in relation to the lean controls. Further, after adjusting for differences in cross-sectional bone area, the apparent material strength had significant relative improvements with diet-correction in the maximum stress (G), stress at yielding (H), and apparent modulus (I). Bars represent means and error bars represent SD. * indicates p < 0.05 by two-way ANOVA. # indicates p < 0.05 for intra-age group comparisons made with Bonferonni’s post-hoc test when the interaction is significant.
4. Discussion
The effects of excessive dietary fat and the associated obesity and metabolic syndrome on bone during skeletal growth periods may have substantive implications for decreased BMD and increased fracture risk in young, obese individuals. Similar to previous studies, we observed significant deficits at cancellous bone sites in mice that were exposed to excessive dietary fat, which reiterates the negative effect of HFD on bone in mice [13–15]. The simultaneous examination of skeletally immature and mature mice in this study reveals that the HFD-associated effects on the femoral trabecular BVF are more pronounced in the younger age group. Further, the HFD tended to reduce the volumetric BMD in the distal femur of only the immature animals. Taken together, these disparate effects on bone volume and volumetric BMD between the immature and mature age groups support the controversial hypothesis of increased fracture risk in obese adolescents, but not in obese adults. Similar to this study, Ionova-Martin et al. [18] examined the effects of HFD (60% kcal fat) for 16 weeks on male C57BL/6J mice beginning from 3 or 15 weeks of age, but focused on the cortical rather than cancellous bone. The whole body BMC appeared to have a greater reduction in the young mice, but the spinal areal BMD and cortical thickness of the femur seemed to be affected more in the older mice. The HFD apparently affected all other cortical bone properties similarly across the two age groups. Considering that Ionova-Martin et al did not test comparisons across age groups, our observations regarding potential age-dependent effects in that study can only be based on trends.
The age-dependence of HFD effects on trabecular bone that were observed in the current study may depend on anatomic site. While age and diet synergistically affected the trabecular BVF in the distal femoral metaphysis, the effects of HFD on lumbar vertebrae were equivalent in the two age groups and were less substantial than those observed in the femur. A similar observation of anatomic site difference was observed in genetic, leptin-related mouse models of obesity [24]. Specifically, the femoral BMC, BMD and strength were affected significantly compared to lean controls, but lumbar vertebral bone was unaffected. Considering that significant differences in the vertebrae were not observed with genetically-induced obesity, but were with HFD-induced obesity, suggests that the effects observed in the current study may be independent of body mass and are more directly associated with the excessive dietary fat and resulting metabolic syndrome. Consistent with this interpretation, more dramatic decrements in femoral bone properties were observed in the HFD-fed immature mice than in the HFD-fed mature mice despite smaller increases in body mass and hyperglycemia.
In addition to the variations by anatomic site, the HFD effects on cortical bone are less pronounced than those on cancellous bone in this study and in previous studies [13, 15]. Cao et al. [13] observed a 23% decrement in trabecular BVF, but no effect on cortical bone, in the proximal tibia of male C57BL/6J mice that were fed a HFD (45% kcal fat) from 6 to 20 weeks of age. Examining older female C57BL/6J mice (12 months of age), Halade et al. [15] found that the trabecular BVF in the distal femur was 66% lower in mice that were fed a corn oil diet for 6 months without any significant effect on cortical bone. Similar deficits in BVF were observed in the current study, but we also found that the femoral cortical thickness was significantly reduced by the HFD when both age groups are considered in a 2-way ANOVA. This reduction, however, was far less substantial than the change in cancellous bone BVF. As pointed out by Cao et al. [13], this varying response between trabecular and cortical bone with HFD is expected, as the turnover rate of cancellous bone generally exceeds that of cortical bone [25]. Keeping with this concept, the surfaces of trabeculae would be the first sites to improve if the diet correction alleviated the imbalance in bone homeostasis. Indeed, the trabecular thickness of HFD:LFD mice tended to be increased in the femur of mature, but not immature, mice and was significantly increased in the vertebrae of both age groups compared to lean controls.
Obesity and glucose-related metabolic disorders that were induced by the initial HFD did not persist in the HFD:LFD groups. However, the relative deficit in femoral trabecular bone did remain after diet correction in the immature age group, but tended to normalize with that of lean controls in the mature group. Importantly, the femoral BVF of immature mice remained at approximately 50% of lean controls after diet correction, which suggests that the detrimental effects on cancellous bone during growth may increase the risk of osteoporosis later in life. In contrast, the normalized vertebral bone structure and strength equaled or exceeded that of age-matched lean controls. Therefore, the degree of initial bone deficit or the anatomic site may partially dictate the relative recovery after diet correction in this study.
Unexpectedly, the mature HFD:LFD group was improved relative to the mature LFD:LFD-fed mice in the vertebral trabecular thickness, cross-sectional bone area, and compressive stiffness. These results suggest an attenuation of aging-related bone deterioration in the mature mice that were obese prior to diet correction. This study focused on the effects of high dietary fat during adolescence (immature group) or early adulthood (mature mice) and the persistence of its effects later in life. Therefore, the HFD was limited to the first half of this study to focus only on differences in the relationship of HFD-fed mice to age-matched lean controls. Future studies may be designed to investigate the effects of sustained HFD from adolescence into adulthood (HFD:HFD), which could better elucidate the relative improvements associated with diet correction over continued obesity.
In conclusion, this study has demonstrated that immature mice have a significantly greater reduction in femoral metaphyseal trabecular BVF than mature mice when fed a HFD for 12 weeks, but the adverse effects of HFD on the vertebrae is equivalent in immature and mature animals. After 12 weeks of diet correction, the HFD-fed immature mice show no relative improvement in femoral BVF or other trabecular parameters, while the femoral BVF of mature mice tends to recover to that of the lean controls. The results of this study demonstrate a complex interplay between growth, aging, anatomic site and excessive dietary fat on cancellous bone homeostasis in male mice and require further study to elucidate the biological mechanisms underpinning these effects.
Highlights.
High fat-fed immature mice had a greater decrease in femoral trabecular bone volume fraction (BVF) compared to mature mice.
Switching from high to low fat diet, femoral BVF of immature mice didn’t improve but mature mice fully recovered.
Negative effects of HFD on vertebrae were less severe and renormalized after diet correction in immature and mature mice.
Acknowledgments
The Authors would like to thank Mr. Michael Thullen for his excellent technical assistance with micro-CT and Robert Maynard for his assistance with histology and serum assays. The study was supported by NIAMS/NIH grant P30AR061307 and the AO Trauma Research Fund. Jason Inzana is supported by NSF graduate research fellowship 2012116002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation, National Institutes of Health, or AO Foundation.
Footnotes
Disclosure
The authors have no conflicts of interest and nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason A. Inzana, Email: jason_inzana@urmc.rochester.edu.
Ming Kung, Email: ming_kung@urmc.rochester.edu.
Lei Shu, Email: lei_shu@urmc.rochester.edu.
Daisuke Hamada, Email: daisuke_hamada@urmc.rochester.edu.
Lian Ping Xing, Email: lianping_xing@urmc.rochester.edu.
Michael J. Zuscik, Email: michael_zuscik@urmc.rochester.edu.
Hani A. Awad, Email: hani_awad@urmc.rochester.edu.
Robert A. Mooney, Email: robert_mooney@urmc.rochester.edu.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA : the journal of the American Medical Association. 1999;282:1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. The Journal of pediatrics. 2001;139:509–15. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 4.Dimitri P, Wales JK, Bishop N. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:527–36. doi: 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- 5.Cole ZA, Harvey NC, Kim M, Ntani G, Robinson SM, Inskip HM, Godfrey KM, Cooper C, Dennison EM. Increased fat mass is associated with increased bone size but reduced volumetric density in pre pubertal children. Bone. 2012;50:562–7. doi: 10.1016/j.bone.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:2090–6. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 7.Dimitri P, Bishop N, Walsh JS, Eastell R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone. 2012;50:457–66. doi: 10.1016/j.bone.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, Brady S, Reynolds JC, Calis KA, Yanovski JA. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117:2167–74. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM. Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Archives of disease in childhood. 2002;87:341–7. doi: 10.1136/adc.87.4.341. discussion 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. The Journal of clinical investigation. 1994;93:799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. Washington, DC: U.S. Government Printing Office; Dec, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JR, Lazarenko OP, Wu X, Tong Y, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Obesity reduces bone density associated with activation of PPARgamma and suppression of Wnt/beta-catenin in rapidly growing male rats. PloS one. 2010;5:e13704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44:1097–104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. The Journal of nutritional biochemistry. 2010;21:1162–9. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Experimental gerontology. 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyung TW, Lee JE, Phan TV, Yu R, Choi HS. Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. The Journal of nutrition. 2009;139:502–6. doi: 10.3945/jn.108.100032. [DOI] [PubMed] [Google Scholar]
- 17.Lac G, Cavalie H, Ebal E, Michaux O. Effects of a high fat diet on bone of growing rats. Correlations between visceral fat, adiponectin and bone mass density Lipids in health and disease. 2008;7:16. doi: 10.1186/1476-511X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ionova-Martin SS, Wade JM, Tang S, Shahnazari M, Ager JW, 3rd, Lane NE, Yao W, Alliston T, Vaisse C, Ritchie RO. Changes in cortical bone response to high-fat diet from adolescence to adulthood in mice. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22:2283–93. doi: 10.1007/s00198-010-1432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 20.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999;14:2159–66. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 21.Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcified tissue international. 2004;74:469–75. doi: 10.1007/s00223-003-0101-x. [DOI] [PubMed] [Google Scholar]
- 22.Silva MJ, Brodt MD, Uthgenannt BA. Morphological and mechanical properties of caudal vertebrae in the SAMP6 mouse model of senile osteoporosis. Bone. 2004;35:425–31. doi: 10.1016/j.bone.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 24.Ealey KN, Fonseca D, Archer MC, Ward WE. Bone abnormalities in adolescent leptin-deficient mice. Regulatory peptides. 2006;136:9–13. doi: 10.1016/j.regpep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Morgan EF, Barnes GL, Einhorn TA. The bone organ system: form and function. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. 3. Burlington, MA: Elsevier Academic Press; –2008.pp. 3–25. [Google Scholar]