Abstract
Previous studies conducted in Western cultures have shown that negative emotions predict higher levels of pro-inflammatory biomarkers, specifically interleukin-6 (IL-6). This link between negative emotions and IL-6 may be specific to Western cultures where negative emotions are perceived to be problematic and thus may not extend to Eastern cultures where negative emotions are seen as acceptable and normal. Using samples of 1044 American and 382 Japanese middle-aged and older adults, we investigated whether the relationship between negative emotions and IL-6 varies by cultural context. Negative emotions predicted higher IL-6 among American adults, whereas no association was evident among Japanese adults. Furthermore, the interaction between culture and negative emotions remained even after controlling for demographic variables, psychological factors (positive emotions, neuroticism, extraversion), health behaviors (smoking status, alcohol consumption), and health status (chronic conditions, BMI). These findings highlight the role of cultural context in shaping how negative emotions affect inflammatory physiology and underscore the importance of cultural ideas and practices relevant to negative emotions for understanding of the interplay between psychology, physiology, and health.
Keywords: culture, negative emotion, inflammation, Interleukin-6
1. Introduction
Numerous studies have shown that negative emotions are associated with worse health, such as cardiovascular disease (e.g., Kubzansky and Kawachi, 2000), cancer (e.g., Penninx et al., 1998), and even mortality (e.g., Pinquart and Duberstein, 2010). One of the biological pathways believed to mediate this linkage between negative emotions and health is inflammation (Everson-Rose and Lewis, 2005; Kiecolt-Glaser et al., 2002). Prior studies have shown that stress, depressive moods, and negative emotions lead to increased levels of proinflammatory cytokines (Bower et al.,, 2007; Carroll et al., 2011; Dickerson et al., 2004; Howren et al., 2009; Kiecolt-Glaser et al., 2007; Marsland et al., 2008; Stewart et al., 2009; Suarez, 2003), and an activation of inflammatory processes is involved in the development and pathogenesis of various health problems, such as diabetes (Kristiansen and Mandrup-Poulsen, 2005) and cardiovascular disease (Ridker et al., 2000). However, most previous studies have been conducted within Western cultural contexts and thus less is known about whether these pathways extend to other populations and cultural contexts.
Cultures vary in their ideas and practices relevant to emotions (Mesquita and Leu, 2007; Tsai, 2007). Historically, Western cultures have valued and encouraged the pursuit of positive emotions, but construed negative emotions as something to be avoided, and often as signs of an inability to control one's life (Kotchemidova, 2005; Ryan and Deci, 2001). In many Asian cultures, on the other hand, there is a philosophical tradition of dialectical thinking, where reality is considered to be constantly changing and comprised of opposites (Peng and Nisbett, 1999). For example, happiness and unhappiness are assumed to coexist and complement each other (Miyamoto and Ma, 2012; Spencer-Rodgers et al., 2010). Thus, the existence of negative emotions or hardship is accepted and even recognized as necessary for self-improvement (Heine et al., 1999). Reflecting cultural differences in such beliefs, cross-cultural research has shown that Easterners are more likely than Westerners to perceive there are some desirable aspects of negative emotions (Eid and Diener, 2001). For example, compared to the case in Western cultures, in Eastern cultures, negative emotions are more likely to be a source of motivation to improve the self (Uchida and Kitayama, 2009) and to invite sympathy and social support from surrounding people (Kitayama and Markus, 2000). In contrast, Westerners are more likely than Easterners to view negative emotions as unacceptable and to be avoided (Bastian et al., 2012).
Trying to avoid or reduce negative thoughts or emotions may contribute to the detrimental effects on mental health (Hayes et al., 1996). Indeed, growing evidence suggests that acceptance and observation of negative emotions, facilitated by strategies encouraged by mindfulness training rooted in Eastern religious and philosophical tradition (Kabat-Zinn, 1990; Segal et al., 2002), leads not only to better mental health, but also to better physical health outcomes (e.g., Davidson et al., 2003; Kabat-Zinn et al., 1985). These findings suggest that in Eastern cultural contexts, where negative emotions are less likely to be perceived as unacceptable or to be avoided and are more likely to be accepted as a component of normal reality, adverse health concomitants may be less evident compared to Western cultural contexts.
Cross-cultural studies provide some supporting evidence. A balance between moderate amounts of positive and negative emotion is associated with fewer physical symptoms in Japan than in the United States (Miyamoto and Ryff, 2011), indicating that moderate amounts of negative emotion, coupled with positive emotions, are not maladaptive for health in Japanese adults. Furthermore, Curhan and colleagues (2013) found that negative emotions are more closely associated with worse self-reported physical health in the United States than in Japan, independent of the effect of positive emotions. These findings suggest that negative emotions may result in poorer health in the United States, but perhaps have more minimal association in Japan.
Despite the extant evidence, little is known about whether these conclusions extend to biomarkers of health. Many previous studies conducted in Western cultures indicate chronic life stress and depressive moods can elevate inflammatory physiology, specifically shown by focusing on the pleiotropic pro-inflammatory cytokine, Interleukin-6 (IL-6; Howren et al., 2009; Kiecolt-Glaser et al., 2003; Marsland et al., 2008; Stewart et al., 2009). Thus, a central question is whether this research conducted in Western cultures will generalize to Eastern cultural contexts. It is possible the findings will not, given Eastern views of negative emotions as accepted and inevitable parts of reality, in contrast to the Western views of negative emotions as distressing, problematic, and maladaptive. In Eastern cultural contexts, negative emotions may not be as physiologically costly and thus not be predictive of elevated inflammatory markers and physiological dysregulation.
We hypothesized that negative emotions (after taking into account demographic and health control variables) would predict higher IL-6 among American adults who perceive the experience of the negative as problematic and maladaptive. Conversely, we predicted a link between negative emotions and IL-6 would be weaker among Japanese adults, because negative emotion is more typically construed as acceptable and a natural part of reality.
2. Materials and methods
2.1. Participants
American respondents were a subset from the Midlife in the United States (MIDUS) survey, which began in 1995-1996. It is a national probability sample recruited through random digit dialing. The survey included a telephone interview and a self-administered questionnaire. Using the same assessments, a follow-up survey was conducted about 9-10 years later (MIDUS II). In addition, biological data were collected from a subset of the MIDUS II respondents, who traveled to one of three General Clinical Research Centers (GCRC) for an overnight visit. The present analyses included 1044 participants for whom IL-6 data were available (474 males, 570 females; M = 55.3 years). The parallel survey, the Midlife in Japan (MIDJA), was conducted in 2008 with participants randomly selected from the Tokyo metropolitan area and completed a self-administered questionnaire. A subset of the MIDJA respondents was recruited to participate in biological data collection (N = 382; 168 males, 214 females; M = 54.2 years). These respondents visited a medical clinic near the University of Tokyo. Serum specimens were frozen and shipped to the United States for analysis. Although American respondents were slightly better educated (M =14.58 years of education) than were the Japanese respondents (M =13.62 years of education), t(1418) = 6.61, p < .001, both samples were comparable in terms of age, t(1424) = 130, p = .19, and gender composition, χ2 (N=1426) = 023, p = .63.
2.2. Negative Emotion
The measures of emotions were collected as part of comprehensive self-administered questionnaires in both MIDUS II and MIDJA, which were completed prior to biological data collection. Participants were asked to rate how much of the time during the past 30 days they felt each emotion (for details about the sources, see Mroczek and Kolarz, 1998) using a 5-point rating scale: none of the time (1), a little of the time (2), some of the time (3), most of the time (4), and all the time (5). The negative emotions included the following 6 items: so sad nothing could cheer you up, nervous, restless or fidgety, hopeless, that everything was an effort, and worthless. Cronbach's alphas were .85 and .86, for Americans and Japanese, respectively. Participants' responses to six items were averaged to compute a negative emotion score.
Following Mroczek and Kolarz (1998), retrospective report over 30 days was used. In support of this approach, Feldman Barrett (1997) showed that the average of momentary report of negative (positive) emotions over 90 days strongly predicted the retrospective report of negative (positive) emotions. However, individual differences have been observed in the discrepancy between momentary and retrospective report of emotions (Robinson and Clore, 2002), specifically that individuals high on neuroticism tend to report experiencing more negative emotions in their retrospective report than in their momentary report, whereas individuals high on extraversion tend to report experiencing more positive emotions in their retrospective report than in their momentary report (Feldman Barrett, 1997). We thus controlled for neuroticism and extraversion to rule out the possibility that cultural differences in the health correlate of negative emotions were due to personality differences in retrospective reporting of emotions.
2.3. IL-6
Frozen blood samples were shipped on dry ice from the 3 GCRC sites and from Tokyo to a single testing laboratory. Serum IL-6 levels were determined by high-sensitivity enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems, Minneapolis, MN), with a lower sensitivity of detection at 0.16 pg/mL. All values were quantified in duplicate; any value over 10 pg/mL was re-run in diluted sera to fall on the standard reference curve.
2.4. Control Variables
2.4.1. Demographic variables
Age, gender, and years of education were included as control variables because many biomarkers, including IL-6, have been shown to vary with such demographic factors (Coe et al, 2011). They were measured as part of the larger selfadministered questionnaire at MIDUS II and MIDJA.
2.4.2. Positive emotion
Because positive emotions have also been found to predict immune and hormone functions independently from negative emotions (Lyubomirsky et al., 2005; Pressman and Cohen, 2005), we included positive emotions as another control variable when examining the link between negative emotions and inflammation. The positive emotions included the following 6 items: cheerful, in good spirits, extremely happy, calm and peaceful, satisfied, and full of life. Cronbach's alphas were .90 and .93, for Americans and Japanese, respectively. Responses to six items were averaged for each participant to compute a positive emotion score.
2.4.3. Personality traits
As discussed above, because neuroticism and extraversion have been linked to the extent to which retrospective reports of emotions are biased (Feldman Barrett, 1997), we also controlled for neuroticism and extraversion. Participants rated the extent to which each of the personality traits describe them using a 4-point rating scale: not at all (1), a little (2), some (3), and a lot (4). Neuroticism included 4 items: moody, worrying, nervous, and calm (Cronbach's alphas were .76 and .56, for Americans and Japanese, respectively). Extraversion included 5 items: outgoing, friendly, lively, active, and talkative (Cronbach's alphas were .78 and .82, for Americans and Japanese, respectively). Participants' responses were averaged to compute neuroticism and extraversion scores.
2.4.4. Health behaviors
We also controlled for health behaviors, specifically smoking status (categorized as never-smoker, former smoker, current smoker, with never-smoker as referent category) and alcohol consumption (the number of drinks consumed per week), known to be associated with the level of inflammatory markers (O'Connor et al., 2009; O'Conner and Irwin, 2010).
2.4.5. Health status
To examine the possible influence of national and cultural differences in health status, we also included several measures of health status closely related to inflammation. When participants visited a clinic, they reported whether they had received a physician's diagnosis of various chronic illness conditions. Among these chronic conditions, we considered the number of conditions specifically linked to inflammation (e.g., heart disease, high blood pressure, diabetes; maximum = 9 conditions; Friedman and Herd, 2010). In addition, the height and weight of participants were measured when participants visited the clinic, which were used to compute the Body Mass Index (BMI, wt in kg/h in m2). BMI correlates highly with IL-6 (O'Connor et al., 2009), and there are also important population differences in BMI (Coe et al., 2011).
2.5. Self-Reported Health
As part of the larger questionnaire at MIDUS II and MIDJA, self-reported health was also measured; respondents rated their current health status on an 11-point scale, ranging from the worst possible health (0) to the best possible health (10). Because self-reported health status has often been used in previous research as a measure of health status (e.g., Pressman et al., in press), we also examined the association between a self-reported health status and IL-6.
3. Results
3.1. Analyses Plan
Because distributions of IL-6 and BMI were positively skewed, both values were log-transformed. To reduce the effect of extreme outliers, a small number of IL-6 scores (29 respondents) and alcohol consumption (29 respondents) were winsorized at three standard deviations from the mean (within each culture separately). That is, scores more than three standard deviations from the mean within each culture were replaced with values at the three standard deviation point from the mean. We first examined the validity of emotion measures and descriptive statistics. Then, we tested our primary hypotheses by running hierarchical multiple regression analyses.
3.2. Validity of Emotion Measures Across Cultures
To examine possible cultural differences in the meaning of emotion measures, we computed the correlation between negative and positive emotion measures and personality traits (Table 1). Consistent with previous theorizing and empirical findings (Costa and McCrae, 1980; Larsen and Ketellaar, 1991; Watson and Clark, 1992), negative emotion was strongly associated with neuroticism in both cultures, and positive emotion was strongly associated with extraversion in both cultures.
Table 1. Correlation between emotion measures and personality traits.
| Zero-Order Correlation | Partial Correlation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Negative emotion | Positive emotion | Negative emotion | Positive emotion | |||||||||
|
|
||||||||||||
| US | JP | sig. | US | JP | sig. | US | JP | sig. | US | JP | sig. | |
| Neuroticism | .59 | .46 | * | -.52 | -.32 | * | .39 | .37 | n.s. | -.22 | -.13 | n.s. |
| Extraversion | -.25 | -.22 | n.s. | .41 | .40 | n.s. | .02 | -.04 | n.s. | .34 | .34 | n.s. |
| Openness | -.15 | -.01 | * | .24 | .15 | n.s. | .00 | .08 | n.s. | .19 | .17 | n.s. |
| Conscientiousness | -.23 | -.08 | * | .23 | .09 | * | -.10 | -.04 | n.s. | .12 | .06 | n.s. |
| Agreeableness | -.12 | -.14 | n.s. | .24 | .31 | n.s. | .06 | .01 | n.s. | .22 | .27 | n.s. |
Note. * indicates significant cultural differences in correlation coefficients. Partial correlations between negative emotions and personality variables control for positive emotions, whereas partial correlations between positive emotions and personality variables control for negative emotions.
At the same time, there were some cultural differences in the strength of the associations between emotion measures and personality variables. These may be accounted for by previous demonstrations that the association between negative and positive emotion differs across cultures (Bagozzi et al., 1999; Kitayama et al., 2000; Miyamoto and Ryff, 2011). Specifically, the inverse correlation between negative and positive emotion tends to be weaker in East Asian cultures than in Western cultures, due to differences in the way positive and negative emotions are viewed and experienced. To examine the latent construct being measured by the emotion measures independently from the association between negative and positive emotion, we controlled for positive and negative emotion in separate analyses (Table 1). When positive or negative emotions were controlled, there were no cultural differences in the link between emotion measures and any of the personality variables. In both cultures, negative emotion was most strongly correlated with neuroticism, and positive emotion was most strongly correlated with extraversion. These findings suggest that emotion measures are capturing similar constructs across cultures.
3.3. Descriptive Analyses
Descriptive statistics for all the variables are presented separately for Americans and Japanese in Table 2. Consistent with our view that experiences of negative emotions are more accepted in Japanese cultural contexts than in American cultural contexts, Japanese participants reported experiencing negative emotions more frequently than American participants did, t(1419) = 6.10, p < .001, though the frequency was low in both the U.S. (M = 1.48, S.D. = 0.55) and Japan (M = 1.70, S.D. = 0.65). At the same time, individual differences were evident within each culture with some respondents experiencing negative emotions quite frequently (the maximum value was 4.83 in the U.S. and 4.33 in Japan). On the other hand, American participants (M = 3.44, S.D. = 0.70) reported experiencing positive emotions more frequently than Japanese participants did (M = 3.29, S.D. = 0.75), t(1418) = 3.54, p < .001.
Table 2. Descriptive statistics for the biological, psychological and demographic variables from the American and Japanese participants (Total N=1426).
| Variable | MIDUS | MIDJA | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| N | M(SD) | IL6 r |
NE r |
N | M(SD) | IL6 r |
NE r |
|
| Demographic | ||||||||
| Age | 1044 | 55.21(11.79) | .23* | -.20* | 382 | 54.24(14.11) | .44* | -.18* |
| Gender | 1044 | 1.55(0.50) | .02 | .08* | 382 | 1.56(0.50) | -.20* | .09 |
| Years of education | 1042 | 14.58(2.42) | -.09* | -.10* | 378 | 13.62(2.40) | -.20* | .03 |
| Emotion | ||||||||
| Positive emotions | 1040 | 3.44(0.70) | -.01 | -.66* | 381 | 3.29(0.75) | -.02 | -.47* |
| Negative emotions | 1039 | 1.48(0.55) | .08* | -- | 381 | 1.70(0.65) | -.08 | -- |
| Personality | ||||||||
| Neuroticism | 1040 | 2.03(0.63) | -.06 | .59* | 381 | 2.13(0.58) | -.14* | .46* |
| Extraversion | 1040 | 3.13(0.57) | .01 | -.25* | 381 | 2.46(0.66) | -.03 | -.22* |
| Health Behavior | ||||||||
| Smoking status | 1044 | 356 | ||||||
| Never smoked | 57.1% | -- | -- | 48.4% | -- | -- | ||
| Former smoker | 32.4% | -- | -- | 23.3% | -- | -- | ||
| Current smoker | 10.5% | -- | -- | 21.5% | -- | -- | ||
| Missing | 0% | 6.8% | -- | -- | ||||
| Alcohol consumption(drinks/week) | 1042 | 3.16(5.55) | -.04 | -.01 | 379 | 7.24(11.75) | 14* | .02 |
| Health Status | ||||||||
| BMI | 1044 | 29.15(6.02) | -- | -- | 382 | 22.58(2.96) | -- | -- |
| Log BMI | 1044 | 1.46(0.09) | .33* | .07* | 382 | 1.35(0.06) | .27* | -.14* |
| Chronic conditions | 1044 | 1.27(1.26) | .28* | .08* | 382 | 0.52(0.78) | .17* | -.02 |
| Biomarker | ||||||||
| IL6 | 1044 | 2.79(2.79) | -- | -- | 382 | 1.64(2.11) | -- | -- |
| Log IL6 | 1044 | 0.31(0.31) | -- | .08* | 382 | 0.04(0.36) | -- | -.08 |
| Self-reported health | 1043 | 7.58(1.45) | -.18* | -.39* | 382 | 6.43(1.82) | -.05 | -.35* |
Note. Gender (male = 1, female = 2). NE = Negative Emotions. Correlations are based on logtransformed IL-6 and log-transformed BMI. Means and standard deviations are based on original data before winsorizing.
indicates a significant correlation.
In addition, as reported previously (Coe et al., 2011), compared to Japanese participants, American participants had significantly higher levels of IL-6, t(1424) = 14.38, p < .001. Interestingly, although Japanese participants were twice as likely as Americans participants to be current smokers, χ2(N=1400) = 35.04, p < .001, and consumed twice as many drink as American participants, t(1419) = 8.82, p.< 001, Japanese participants had lower BMI (t(1424) = 10.86, p < .001) and fewer chronic illness conditions than American participants (t(1424) = 22.59, p < .001).
Furthermore, even though Japanese participants had better health status (i.e., lower BMI and fewer chronic illness conditions) than American participants, Japanese participants reported worse health than American participants on the self-reported measure of health, t(1423) = 12.33, p < .001. The self-reported health measure correlated with IL-6 in the United States (r = -.18, p < .001) but not in Japan (r = -.05, p = .33), and the correlation was significantly stronger in the United States than in Japan, Z = 2.20, p =.03, suggesting that objective and subjective measures of health may be more dissociated in Japan.
3.4. Cultural Differences in the Link between Negative Emotions and Inflammatory Markers
To test the primary hypotheses, we conducted several hierarchical multiple regression analyses. In the first model, culture (represented as a binary variable, U.S. or Japan), negative emotions, and the interaction between the two were entered. To determine whether the interaction remained after controlling for other covariates, the demographic variables were entered into the second model; psychological factors (i.e., positive emotions and personality variables) were entered into the third model; and health behaviors and health status measures were entered into the fourth model.
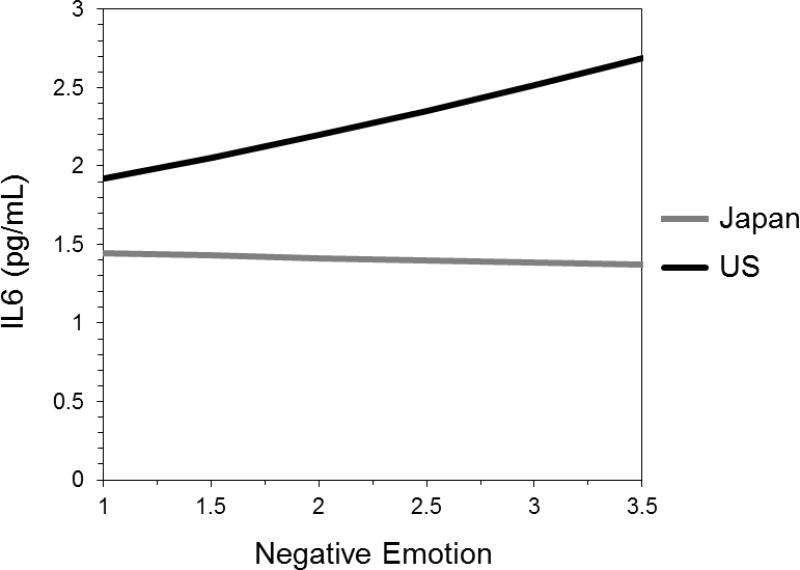
Summaries of hierarchical multiple regression analyses for IL-6 are shown in Table 3. As hypothesized, the interaction between culture and negative emotions was statistically significant, Model 1, b = 0.10, S.E. = 0.03, t(1374) = 3.06, p = .002. The interaction remained significant after controlling for demographic factors, Model 2, b = 0.09, S.E. = 0.03, t(1371) = 2.89, p = .004, psychological factors, Model 3, b = 0.10, S.E. = 0.03, t(1368) = 3.09, p = .002, and health behaviors and health status, Model 4, b = 0.07, S.E. = 0.03, t(1363) = 2.27, p = .023. As shown in Figure 1, negative emotions predicted higher IL-6 among Americans, simple slope b = 0.06, S.E. = 0.02, t(1363) = 2.68, p = .001, but were not associated with the level of IL-6 among Japanese participants, simple slope b = -0.01, S.E. = 0.03, t(1363) = 0.35, p = .73.
Table 3. Hierarchical multiple regression predicting interleukin-6 among Americans and Japanese.
| Model 1 without covariates | Model 2 +demographics | Model 3 + psychological factors | Model 4 + health behaviors & health status | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| b(S.E.) | p | b(S.E.) | p | b (S.E.) | p | b(S.E.) | p | |
|
|
||||||||
| Culture | .12 (.06) | .033 | .14 (.05) | .010 | .13 (.14) | .020 | .06 (.05) | .29 |
| Negative emotions | -.17 (.06) | .004 | -.12 (.05) | .033 | -.11 (.05) | .038 | -.08 (.05) | .14 |
| Culture × Negative emotions | .10 (.03) | .002 | .09 (.03) | .004 | .10 (.03) | .002 | .07 (.03) | .023 |
| Age | .01 (.00) | .001 | .01 (.00) | .001 | .01 (.00) | .001 | ||
| Gender | -.02 (.02) | .14 | -.02 (.02) | .16 | .02 (.02) | .20 | ||
| Years of education | -.01 (.00) | .014 | -.01 (.00) | .011 | -.00 (.00) | .20 | ||
| Positive emotions | -.01 (.02) | .41 | .00 (.02) | .89 | ||||
| Neuroticism | -.05 (.02) | .006 | -.04 (.02) | .012 | ||||
| Extraversion | -.00 (.01) | .91 | -.01 (.02) | .34 | ||||
| Smoking status (former vs. never) | .03 (.02) | .18 | ||||||
| Smoking status (current vs. never) | .07 (.03) | .007 | ||||||
| Alcohol consumption | .00 (.00) | .036 | ||||||
| Log BMI | 1.16 (.10) | .001 | ||||||
| Chronic conditions | .02 (.01) | .001 | ||||||
|
| ||||||||
| R-square | .133 | .207 | .212 | .300 | ||||
Note. Culture (1 = Americans, 2 = Japanese), Gender (male = 1, female = 2).
Fig. 1.
Cultural moderation of the association between negative emotions and IL-6 after controlling for gender, age, and years of education, positive emotions, neuroticism, extraversion, smoking status, alcohol consumption, the number of chronic conditions linked to inflammation, and log-transformed BMI (Model 5). Negative emotions predicted IL-6 in the United States, b = 0.06, S.E. = 0.02, t(1363) = 2.68, p = .001, but not in Japan, b = 0.01, S.E. = 0.03, t(1363) = 0.35, p = .73.
4. Discussion
Previous research based on American and European populations indicates that negative emotions can aggravate inflammatory processes (Kiecolt-Glaser et al., 2002; Marsland et al., 2008). The present findings provide evidence for a cultural specificity to this conclusion. Replicating prior reports, the present study found that negative emotions are linked to increased levels of the pro-inflammatory cytokine IL-6 among a large national sample of middle-age and older adults across the United States. Such effects were evident after controlling for multiple factors known to influence inflammatory markers, including demographic factors, psychological factors, health status, and life style behaviors. In contrast, in a comparable sample of middle-age and older adults in Japan, this link between negative emotions and cytokine biology was not evident. This difference is hypothesized to reflect cultural differences in how negative emotions are construed – namely, that they are viewed as more unacceptable and problematic in Western cultures than in Eastern cultures (Bastian et al., 2012). These findings highlight the role of cultural context shaping how negative emotions are associated with inflammatory physiology and convey the importance of taking cultural ideas and practices about the value of negative emotions into consideration.
The broader implications pertain to many studies in Western countries and cultures showing that negative emotions are linked with poor health (e.g., Penninx et al., 1998; Pinquart and Duberstein, 2010). The present research also indicates that negative emotions may not be universally experienced as adverse in the same way. That is, if negative emotions are not construed as problematic and maladaptive, how they feed into the pathways to illness and disease likely needs to be reconfigured with greater awareness about the importance of cultural context. More research is needed to extend the current analysis to other inflammatory markers, including acute phase reactants, as well as with other cardiovascular and neuroendocrine risk factors, in addition to morbidity and mortality, all of which have been previously linked with emotional states (Carney et al., 2002; Everson-Rose and Lewis, 2005; Kubzansky and Kawachi, 2000). However, in support of the current results, some papers have already noted that the association between work and life stress and cardiovascular disease is not as prominent in Japan as in the US and Europe (Martikainen et al., 2001).
At the same time, a recent study did conclude that both positive and negative emotions are linked to self-reported health across 142 countries, including both the United States and Japan (Pressman et al., in press). At first glance, such a finding may seem to contradict the current findings. We suspect that differences in the measures of health are at least partly responsible for the appearance of a discrepancy. Whereas Pressman et al. focused on self-reported health, the present research examined an objective, biological measure, specifically IL-6. Although self-reported health measures are known to correlate with objective health measures, including even mortality (McGee et al., 1999), the rating of one's health can also be influenced by one's emotional states (Watson and Pennebaker, 1989). Thus, the observed association between emotions and self-reported health across cultures could be partly due to shared emotional components that underlie the measures of self-reported health and emotions. In fact, in the current surveys (i.e., MIDUS and MIDJA), consistent with the conclusions of Pressman et al., the subjective health measure did significantly correlate with negative emotions in both the United States (r = -39, p < .001) and in Japan (r = -34, p < .001). At the same time, the subjective health measure correlated with IL-6 in the United States but not in Japan, pointing out a possibility that self-reported health status and objective health status may be dissociated in Japan. These findings highlight the importance of objectively confirming health status in addition to self-reported health status.
We theorized that negative emotions are less physiologically costly in Japan because negative emotions are viewed as acceptable and inevitable parts of reality. This is consistent with the growing evidence on mindfulness training, which shows that practicing skills to accept and observe negative emotions leads to better mental and physical health (Davidson et al., 2003; Kabat-Zinn et al., 1985). More acceptant views of negative emotions in Japan may serve as a buffer against the harmful effects of negative emotions on health. Because the present study did not directly measure cultural views of negative emotions, it is important for the future research to examine whether such views serve as a buffer in Japan. 1, 2
Because the present findings are based on cross-sectional data, causal directionality cannot be readily discerned, in terms of whether national differences in overall health influenced the differences found for IL-6. However, the analyses did establish that negative emotions predicted the level of IL-6 differently across cultures even after controlling for preexisting health conditions. This finding, which was maintained after controlling for the age of participants, suggests that variation in the observed link between negative emotions and IL-6 is not due to preexisting population level differences in health status. Future research is needed to examine the temporal precedence of emotions, including perhaps by studies of similar biomarkers in children and young adults in both countries. Some caution is also needed because the IL-6 values were based on only a single determination for each participant. This limitation of relying on a single blood sample is common in most surveys, and there is some statistical compensation gained by the use of large subject numbers. In addition, some papers have reported that a single assessment can be representative of IL-6 levels over an extended period of time in both Americans and Chinese samples (Hofmann et al., 2011; Lee et al., 2007; Rao et al., 1994) as well as to predict myocardial infarction and diabetes even years later (Pradhan et al., 2001, Harris et al.,1999; Ridker et al., 2000). At the same time, some have found some variability in IL-6 levels over time (Navarro et al., 2012). It is thus important for future research to obtain repeated measurements of IL-6 to verify that the population variation in the links between IL-6 and negative emotion remain stable across years and in different circumstances for any given individual.
Across cultures, people may generally prefer to feel positive emotions and avoid negative emotions (Larsen, 2000). However, cross-cultural studies have shown that cultures differ in the extent to which people believe they should suppress and even deny negative emotions (Bastian et al., 2012). By showing the cultural dependency of the link between negative emotions and inflammatory markers, our research demonstrates that cultural contexts not only shape the subjective experience of negative emotions, but also influence whether negative emotions exert a physiological toll that contributes to age-related illness.
Highlights.
Reflecting cultural variation in their values, negative emotions predicted increased IL-6 in a national sample of U.S. adults, but not in a comparable sample of Japanese.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (R01 AG027343) to conduct the study on Midlife in Japan (MIDJA) for comparative analysis with MIDUS (Midlife in the U.S., P01 AG020166). The original MIDUS study was supported by the MacArthur Foundation Research Network on Successful Midlife Development. The specimen collection was also facilitated by the General Clinical Research Centers program (M01-RR023942 [Georgetown], M01-RR00865 [UCLA]), and at UW from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (1UL1RR025011). The contributions of Ms. D. Brar in specimen processing and cytokine assays are gratefully acknowledged.
Footnotes
We also examined other potential variables that may account for a weaker association between negative emotions and IL-6 in Japan. Given that social support is more strongly associated with well-being and health in Japan than in the U.S. (Uchida et al., 2008), it is possible that social support serves as a buffer for harmful effects of negative emotions in Japan. We thus examined whether support received from spouse and friends moderates the association between negative emotions and IL-6 in Japan. The interaction between negative emotions and social support was not significant, b = -.01, S.E. = .04, t(256) = 0.19, p = .85, and b = -.01, S.E. = .05, t(332) = 0.31, p = .76, for support from spouse and friends, respectively. In addition, consumption of green tea has been associated with health benefits, including anti-inflammatory effects (Donà et al., 2003). We examined whether green tea consumption moderates the association between negative emotions and IL-6 in Japan. The interaction between negative emotions and green tea consumption was not significant, b = .01, S.E. = .02, t(319) = 0.24, p = .81. These findings suggest that neither social support nor green tea consumption serves as a buffer against a maladaptive influence of negative emotions on inflammation in Japan.
Although national and racial differences in the absolute levels of IL-6 did not account for the differential influence of psychosocial factors on IL-6 in Japan and the US, it is important to acknowledge that several allele and single nucleotide polymorphisms can affect cytokines and inflammatory responses (Berger, 2004). Thus, there could be a genetic contribution to the current results. The prevalence of these polymorphisms affecting IL-6 and TNF-α do vary across populations and geographic regions (Lim et al., 2002; Delaney et al., 2004; Gadelha et al., 2005). The polymorphisms have been associated with individual variation in inflammatory responses during urinary tract infections, and after cardiac infarction and surgical procedures (e.g., Fishman et al., 1998; Liu et al., 2006; Yamada et al., 2003), but in general the polymorphisms have less influence on basal secretion of IL-6, the biomarker we utilized in the present study. In fact, a recent analysis of monozygotic and dizygotic twin participants in MIDUS indicated that IL-6 levels are more affected by life style and contemporaneous variables, especially related to diet and obesity, than by innate constraints (Wellington et al., in prep). Cultural differences in how negative emotions are perceived are among the many social and emotional processes that significantly modulate the day-to-day levels of IL-6 found in the blood stream. Because IL-6 is secreted by many different tissues and cell types, serving myriad physiological functions (Kishimoto, 2005), it makes sense that its release would be flexible and responsive to the emotional state of the individual (Cole et al., 2010).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuri Miyamoto, University of Wisconsin, Madison.
Jennifer Morozink Boylan, University of Wisconsin, Madison.
Christopher L. Coe, University of Wisconsin, Madison
Katherine B. Curhan, Stanford University
Cynthia S. Levine, Stanford University
Hazel Rose Markus, Stanford University.
Jiyoung Park, University of Michigan.
Shinobu Kitayama, University of Michigan.
Norito Kawakami, University of Tokyo.
Mayumi Karasawa, Tokyo Woman's Christian University.
Gayle D. Love, University of Wisconsin, Madison
Carol D. Ryff, University of Wisconsin, Madison
References
- Bagozzi RP, Wong N, Yi Y. The role of culture and gender in the relationship between positive and negative affect. Cogn Emot. 1999;13:641–672. [Google Scholar]
- Bastian B, Kuppens P, Hornsey MJ, Park J, Koval P, Uchida Y. Feeling bad about being sad: The role of social expectancies in amplifying negative mood. Emotion. 2012;12:69–80. doi: 10.1037/a0024755. [DOI] [PubMed] [Google Scholar]
- Berger FG. The interleukin-6 gene: a susceptibility factor that may contribute to racial and ethnic disparities in breast cancer mortality. Breast Cancer Res Treat. 2004;88:281–285. doi: 10.1007/s10549-004-0726-0. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun. 2011;25:232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Love GD, Karasawa M, Kawakami N, Kitayama S, Markus HR, Tracy RP, Ryff CD. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav Immun. 2011;25:494–502. doi: 10.1016/j.bbi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene–social environment interaction at the human IL6 locus. Proc Natl Acad Sci. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Influence of extraversion and neuroticism on subjective well-being: happy and unhappy people. J Pers Soc Psychol. 1980;38:668–678. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- Curhan KB, Markus HR, Sim T, Kitayama S, Karasawa M, Kawakami N, Love GD, Coe CL, Miyamoto Y, Ryff CD. Unpublished manuscript. Stanford University; 2013. Negative affect is a more powerful predictor of health among U.S. adults compared to Japanese adults. [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS. TNF-α, TGF-β, IL-10, IL-6, and INF-γ alleles among African Americans and Cuban Americans. Report of the ASHI minority workshops: Part IV. Hum Immunol. 2004;65:1413–1419. doi: 10.1016/j.humimm.2004.07.240. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Donà M, Dell'Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170:4335–4341. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- Eid M, Diener E. Norms for experiencing emotions in different cultures: Inter- and intranational differences. J Pers Soc Psychol. 2001;81:869–885. doi: 10.1037//0022-3514.81.5.869. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev of Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Feldman Barrett L. The relationships among momentary emotion experiences, personality descriptions, and retrospective ratings of emotion. Pers Soc Psychol Bull. 1997;23:1100–1110. [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: Differential associations in a national probability sample (The MIDUS study) Psychosom Med. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadelha SR, Alcantara LCJ, Costa GCS, Rios DL, Galvao-Castro B. Ethnic differences in the distribution of interleukin-6 polymorphisms among three Brazilian ethnic groups. Hum Biol. 2005;77:509–514. doi: 10.1353/hub.2005.0061. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experiential avoidance and behavioral disorders: a functional dimensional approach to diagnosis and treatment. J Consult Clin Psychol. 1996;64:1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- Heine SJ, Lehman DR, Markus H, Kitayama S. Is there a universal need for positive self-regard? Psychol Rev. 1999;106:766–794. doi: 10.1037/0033-295X.106.4.766. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Yu K, Bagni RK, Lan Q, Rothman N, Purdue MP. Intra-individual variability over time in serum cytokine levels among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cytokine. 2011;56:145–148. doi: 10.1016/j.cyto.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Delacorte. New York, NY: 1990. Full catastrophe living: Using the wisdom of your mind and body to face stress, pain, and illness. [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, omega-6: omega-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69:217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Markus HR. The pursuit of happiness and the realization of sympathy: Cultural patterns of self, social relations, and well-being. In: Diener E, Suh EM, editors. Culture and subjective well-being. MIT Press; Cambridge, MA: 2000. pp. 113–161. [Google Scholar]
- Kitayama S, Markus HR, Kurokawa M. Culture, emotion, and well-being: Good feelings in Japan and the United States. Cogn Emot. 2000;14:93–124. [Google Scholar]
- Kotchemidova C. From good cheer to “drive-by smiling”: A social history of cheerfulness. J Soc Hist. 2005;39:5–37. [Google Scholar]
- Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: The good, the bad, or the indifferent? Diabetes. 2005;54:S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I. Going to the heart of the matter: do negative emotions cause coronary heart disease? J Psychosom Res. 2000;48:323–337. doi: 10.1016/s0022-3999(99)00091-4. [DOI] [PubMed] [Google Scholar]
- Larsen R. Toward a science of mood regulation. Psychol Inq. 2000;11:129–141. [Google Scholar]
- Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. J Pers Soc Psychol. 1991;61:132–141. doi: 10.1037//0022-3514.61.1.132. [DOI] [PubMed] [Google Scholar]
- Lee SA, Kallianpur A, Xiang YB, Wen W, Cai Q, Liu D, Fazio S, Linton MF, Zheng W, Shu XO. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer Epidemiol Biomarkers Prev. 2007;16:2464–2470. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]
- Lim CS, Zheng S, Kim YS, Ahn C, Han JS, Kim S, Lee JS, Chae DW. The–174 G to C polymorphism of interleukin-6 gene is very rare in Koreans. Cytokine. 2002;19:52–54. doi: 10.1006/cyto.2002.1951. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berthier-Schaad Y, Fallin MD, Fink NE, Tracy RP, Klag MJ, Smith MW, Coresh J. IL-6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. IL-6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. J Am Soc Nephrol. 2006;17:863–870. doi: 10.1681/ASN.2005050465. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: Does happiness lead to success? Psychol Bull. 2005;131:803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22:753–761. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen P, Ishizaki M, Marmot MG, Nakagawa H, Kagamimori S. Socioeconomic differences in behavioural and biological risk factors: a comparison of a Japanese and an English cohort of employed men. Int J Epidemiol. 2001;30:833–838. doi: 10.1093/ije/30.4.833. [DOI] [PubMed] [Google Scholar]
- McGee DL, Liao Y, Cao G, Cooper RS. Self-reported health status and mortality in a multiethnic US cohort. Am J Epidemiol. 1999;149:41–46. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- Mesquita B, Leu J. The cultural psychology of emotions. In: Kitayama S, Cohen D, editors. Handbook for cultural psychology. Guilford Press; New York, NY: 2007. [Google Scholar]
- Miyamoto Y, Ma X. Dampening or savoring positive emotions: A dialectical cultural script guides emotion regulation. Emotion. 2011;11:1346–1357. doi: 10.1037/a0025135. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Ryff C. Cultural differences in the dialectical and non-dialectical emotional styles and their implications for health. Cogn Emot. 2011;25:22–30. doi: 10.1080/02699931003612114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. J Pers Soc Psychol. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Navarro SL, Brasky TM, Schwarz Y, Song X, Wang CY, Kristal AR, Kratz M, White E, Lampe JW. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer Epidemiol Biomarkers Prev. 2012;21:1167–1170. doi: 10.1158/1055-9965.EPI-12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M, Irwin MR. Links between behavioral factors and inflammation. Clin Pharmacol Ther. 2010;87:479–482. doi: 10.1038/clpt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Nisbett R. Culture, dialectics, and reasoning about contradiction. Am Psychol. 1999;54:741–754. [Google Scholar]
- Penninx BWJH, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, Havlik RJ. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1979–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Gallagher MW, Lopez SJ. Is the emotion-health connection a “First-World problem”?”. Psychol Sci. doi: 10.1177/0956797612457382. in press. [DOI] [PubMed] [Google Scholar]
- Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychol Bull. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. On happiness and human potentials: A review of research on hedonic and eudaimonic well-being. Ann Rev Psychol. 2001;52:141–166. doi: 10.1146/annurev.psych.52.1.141. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: A new approach for preventing relapse. Guilford Press; New York, NY: 2002. [Google Scholar]
- Spencer-Rodgers J, Williams MJ, Peng K. Cultural differences in expectations of change and tolerance for contradiction: A decade of empirical research. Pers Soc Psychol Rev. 2010;14:296–312. doi: 10.1177/1088868310362982. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression–inflammation relationship. Brain Behav Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC. Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosom Med. 2003;65:523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- Tsai J. Ideal affect: Cultural causes and behavioral consequences. Perspectives on Psychol Sci. 2007;2:242–259. doi: 10.1111/j.1745-6916.2007.00043.x. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Kitayama S. Happiness and unhappiness in east and west: Themes and variations. Emotion. 2009;9:441–456. doi: 10.1037/a0015634. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Kitayama S, Mesquita B, Reyes JAS, Morling B. Is perceived emotional support beneficial? Well-being and health in independent and interdependent cultures Pers. Soc Psychol Bull. 2008;34:741–754. doi: 10.1177/0146167208315157. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. On traits and temperament: General and specific factors of emotional experience and their relation to the five-factor model. J Pers. 1992;60:441–476. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central v role of negative affectivity. Psychol Rev. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of interleukin-6, osteocalcin, and vitamin D receptor genes, alone or in combination, with bone mineral density in community-dwelling Japanese women and men. J Clin Endocrinol Metab. 2003;88:3372–3378. doi: 10.1210/jc.2002-021449. [DOI] [PubMed] [Google Scholar]