Summary
Preeclampsia is a life-threatening pregnancy disorder. However, its pathogenesis remains unclear. We tested the hypothesis that gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling. Time-dated pregnant and non-pregnant rats were divided into normoxic and hypoxic (10.5% O2 from the gestational day 6 to 21) groups. Chronic hypoxia had no significant effect on blood pressure or proteinuria in non-pregnant rats but significantly increased blood pressure in day 12 (systolic blood pressure: 111.7 ± 6.1 versus 138.5 ± 3.5 mmHg, P = 0.004) and day 20 (systolic blood pressure: 103.4 ± 4.6 versus 125.1 ± 6.1 mmHg, P = 0.02) in pregnant rats, as well as urine protein (μg/μL)/creatinine (nmol/μL) ratio in day 20 (0.10 ± 0.01 versus 0.20 ± 0.04, P = 0.04), as compared with the normoxic control group. This was accompanied with asymmetrical fetal growth restriction. Hypoxia resulted in impaired trophoblast invasion and uteroplacental vascular remodeling. In addition, plasma endothelin-1 levels, as well as the abundance of prepro-endothelin-1 mRNA, endothelin-1 type A receptor and angiotensin II type 1 receptor protein in the kidney and placenta were significantly increased in the chronic hypoxic group, as compared with the control animals. Treatment with the endothelin-1 type A receptor antagonist, BQ123 during the course of hypoxia exposure significantly attenuated the hypoxia-induced hypertension and other preeclampsia-like features. The results demonstrate that chronic hypoxia during gestation induces preeclamptic symptoms in pregnant rats via heightened endothelin-1 and endothelin-1 type A receptor-mediated signaling, providing a molecular mechanism linking gestational hypoxia and increased risk of preeclampsia.
Keywords: preeclampsia, hypoxia, hypertension, endothelin-1, ETAR, AT1R
Introduction
Preeclampsia is a pregnancy specific disorder that affects 2% to 8% of pregnant women, and is a leading cause of maternal and neonatal morbidity and mortality.1,2 Preeclampsia is defined by the onset of hypertension and proteinuria after 20 weeks of gestation and is often associated with fetal growth restriction (FGR). The pregnancy complications with abnormal fetal development not only significantly increase maternal and infant mortality and morbidity rates,3–6 but also have long-term adverse effects on adult health, predisposing to cardiovascular and metabolic diseases.7–10 The hallmark of preeclampsia is a shallow trophoblast invasion and insufficient spiral artery remodeling, leading to persistent placental hypoxia and the release of various mediators into the maternal circulation resulting in preeclamptic symptoms.1
Although the etiology of preeclampsia has not been clearly defined, a growing body of evidence supports the notion that hypoxia may be an important factor in the pathophysiology of preeclampsia, and plays a pivotal role in the origins of preeclampsia.11–22 Previous studies have shown that the incidence of preeclampsia is significantly increased in women who are residing at high altitudes.12–15 However, whether gestational hypoxia causes the development of preeclampsia is controversial. Previous studies have demonstrated that exposure to 11% O2 from days 6.5 to 13.5 gestation increased vascularity and potentiated trophoblast invasion in pregnant rats,23 but exposure to 9.5% O2 from day 7.5 to day 17 induced hypertension and preeclampsia-like symptoms in both wild type and IL-10−/− pregnant mice.24 These studies suggest that timing and severity of hypoxia during the course of gestation may be important in the development of preeclampsia. In the early placental development, hypoxia may promote trophoblast proliferation rather than differentiation.25,26 However, the persistence of low oxygen tension and the presence of hypoxia inducible factor-1 alpha (HIF-1α) may cause a proliferative, non-invasive phenotype of the trophoblast and the development of preeclampsia.27–30 Furthermore, the molecular mechanisms underlying hypoxia-mediated preeclampsia remain largely unclear.
Endothelin-1 (ET-1) is a peptide hormone with a potent vasoconstriction effect, and has been implicated in the pathogenesis of preeclampsia.31 Plasma ET-1 levels were increased in preeclamptic patients and were correlated with anti-angiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng) levels in these patients.32,33 In addition, both renal and placental ET-1 expressions were increased in the animal model of preeclampsia induced by sFlt-1 or angiotensin II (Ang II) type I receptor-agonistic autoantibodies (AT1R-AA).34,35 These studies suggest that increased ET-1 may be a key molecular linking adverse stimuli and the development of preeclampsia.
Hypoxia is one of the most potent inducers of ET-1 gene expression in endothelial cells and is a primary cause of heightened ET-1 signaling during cardiovascular ischemia.36,37 Therefore, in present study we investigated whether gestational hypoxia induces hypertension and preeclamptic symptoms in pregnant rats, and tested the hypothesis that heightened ET-1 signaling is a key molecular mechanism underlying chronic hypoxia-induced preeclampsia.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement.
Experimental animals
Six groups of female Sprague-Dawley rats were used: 1) normoxic control non-pregnant group; 2) hypoxic treatment non-pregnant group; 3) normoxic control time-dated pregnant group; 4) hypoxic treatment time-dated pregnant group, continuous exposure to 10.5% O2 from day 6 through day 21 of gestation; 5) normoxic pregnant rats treated with BQ123, an antagonist of ET-A receptor (ETAR), via osmotic minipumps (100 nmol/kg/d) from day 4 through day 21 of gestation; 6) hypoxic pregnant rats treated with BQ123. The BQ123 treatment was started two days prior to the initiation of 10.5% O2 treatment to allow the recovery of animals from the surgical implantation of osmotic pumps before the hypoxia treatment. The BQ123 treatment sustained the course of hypoxia treatment. Rats were euthanized under isoflurane anesthesia on gestational day 21, pups, placentas and kidneys were isolated. All procedures and protocols used in the present study were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of arterial blood pressure
Rats were implanted with catheters in femoral arteries for recording of arterial blood pressure (BP) on gestational day 4. Arterial systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were measured on day 12 and day 20 of gestation, as described previously.38,39
Measurement of proteinuria
Protein and creatinine levels in 12-hour (from 07:00 pm to 07:00 am) urine samples were measured before hypoxia treatment on day 3 and after hypoxia treatment on day 20 of gestation.
Determination of plasma ET-1 and renin activity
Plasma ET-1 was measured by ELISA kit. Renin activity was measured by a fluorometric method.
Real-time RT-PCR
Placental and renal preproET-1 mRNA abundance was determined by real-time RT-PCR.
Western immunoblotting analysis
AT1R, AT2R, ETAR and ETBR protein abundance was measured in the placenta and kidney by Western blot analysis, and HIF-1α protein abundance was measured in the placenta.
Histology and Immunohistochemistry
Kidney slices were stained with Hematoxylin and Eosin (H&E) and periodic acid Schiff (PAS) by standard techniques. Placentas with the associated mesometrial triangle (MT) were paraffin fixed and sections were cut step-serially from each implantation site parallel to the mesometrial-fetal axis. For each implantation site, one set of sections containing a central maternal arterial channel were selected for staining PAS as a fibrinoid tissue marker, cytokeratin (CK) as a trophoblast marker, α-actin as a vascular smooth muscle cells (VSMC) marker, as described previously.40–42 The degree of trophoblast invasion and spiral artery (SA) remodeling were assessed using Image J analysis system. Briefly, the lumen of each SA cross-section in the whole MT was manually delineated and stretches of trophoblast, fibrinoid and VSMC were traced separately over the lumen contour tracing, then the percentages of cytokeratin staining, fibrinoid staining and α-actin staining of the corresponding spiral artery contour were calculated.40–42 The expression of AT1R, AT2R, ETAR, ETBR and HIF-1α in the placenta and kidney was also determined by using corresponding antibodies.
Data analysis
Results were expressed as means ± SEM. The differences were evaluated for statistical significance by ANOVA or Student’s t-test, where appropriate. A two-tailed P-value of less than 0.05 was considered significant.
Results
Gestational hypoxia increased ET-1 expression
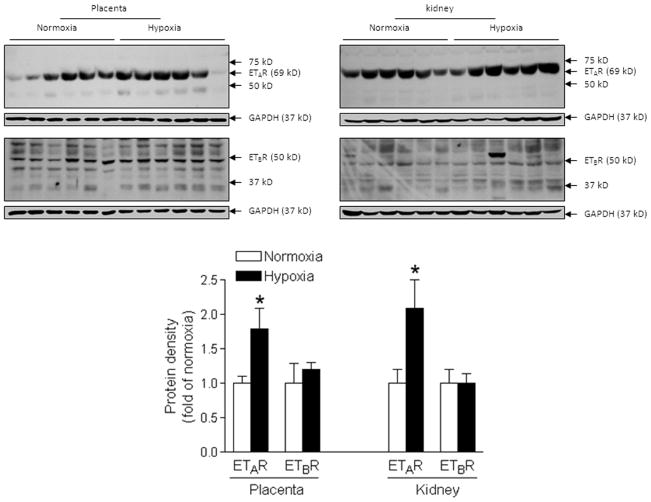
As shown in Figure 1A, plasma ET-1 levels were significantly increased in the hypoxic group at mid-gestation (day 12) and near-term (day 20) pregnant rats, as compared with the normoxic control group. Although there was a tendency of increasing plasma ET-1 levels from day 12 to day 20 of gestation, they were not significantly different. In addition, tissue levels of ET-1 production measured as preproET-1 mRNA in the kidney and placenta of hypoxic animals were significantly higher than those in the control animals (Figure 1B). Furthermore, chronic hypoxia significantly enhanced the protein expression of ETAR but not ETBR in both kidney and placental tissues (Figure 2). Immunochemistry study indicated that ETAR and ETBR were expressed throughout the placental tissue and mainly expressed in tubular but not in glomerulus cells of the kidney (Online Figure S1).
Figure 1. Chronic hypoxia increased ET-1 expression.
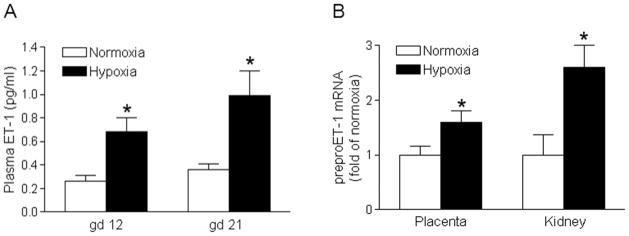
A, Plasma ET-1 levels were measured in hypoxic and normoxic control pregnant rats at day 12 (gd 12) and day 21 (gd 21) of gestation. B, mRNA abundance of preproET-1 was determined in the kidney and placenta in hypoxic and normoxic control pregnant rats at day 21 (gd 21) of gestation. Data are means ± SEM, n = 6. * P < 0.05 versus normoxia.
Figure 2. Chronic hypoxia up-regulated ETAR expression.
Protein abundance of ETAR and ETBR was determined in the kidney and placenta in hypoxic and normoxic control pregnant rats at day 21 of gestation. Data are means ± SEM, n = 6. * P < 0.05 versus normoxia.
Gestational hypoxia increased AT1R expression
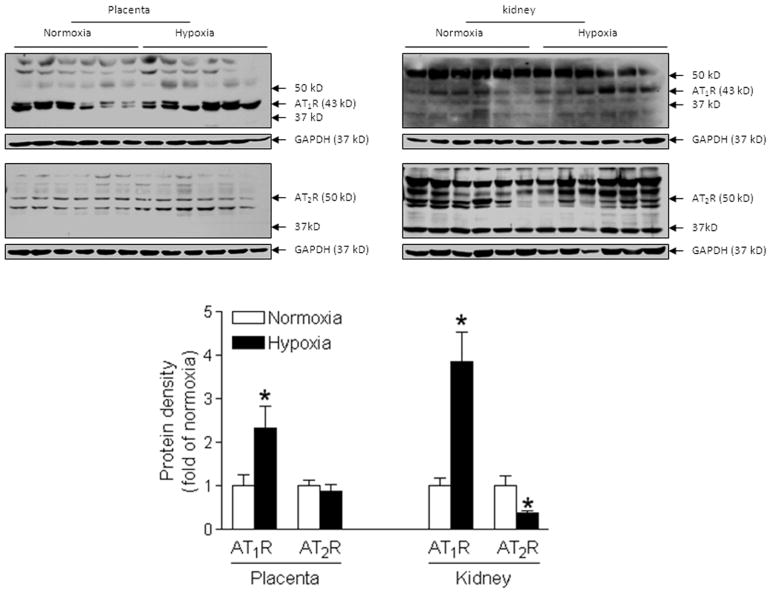
Chronic hypoxia had no significant effect on maternal plasma renin activity (Online Figure S2). However, the protein abundance of AT1R in both placenta and kidney was significantly increased in the hypoxic group, as compared with the normoxic group (Figure 3). In contrast, AT2R protein abundance was significantly decreased in the kidney, but not in the placenta in the hypoxic animals, as compared with the normoxic control (Figure 3). Immunochemistry study indicated that AT1R and AT2R were expressed throughout the placental tissue (Online Figure S1). In the kidney, AT1R was expressed mainly in tubular but not in glomerulus cells, whereas AT2R appeared expression in both tubular and glomerulus cells (Online Figure S1).
Figure 3. Chronic hypoxia enhanced AT1R expression.
Protein abundance of AT1R and AT2R was determined in the kidney and placenta in hypoxic and normoxic control pregnant rats at day 21 of gestation. Data are means ± SEM, n = 6. * P < 0.05 versus normoxia.
Gestational hypoxia increased HIF-1α expression in the placenta
As shown in Online Figure S3, HIF-1α protein abundance in the placenta was significantly increased in the hypoxic group, as compared with the normoxic group.
BQ123 abrogated the hypoxia-induced increase in blood pressure
As shown in Figure 4, chronic hypoxia significantly increased SBP, DBP and MAP in mid-gestation (day 12) and near-term (day 20) pregnant rats, as compared with normoxic control animals. Inhibition of ETAR with BQ123 abrogated the hypoxia-mediated increase in blood pressure in mid-gestation as well as near-term pregnant rats (Figure 4). Chronic hypoxia for the same duration had no significant effect on blood pressure in non-pregnant rats (Online Figure S4).
Figure 4. BQ123 abrogated chronic hypoxia-induced increase in blood pressure.
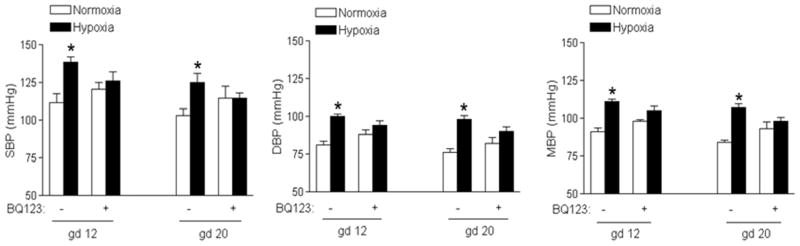
Systolic (SBP), diastolic (DBP) and mean (MBP) arterial blood pressure were measured in hypoxic and normoxic control pregnant rats at day 12 (gd 12) and day 20 (gd 20) of gestation, in the absence or presence of BQ123. Data are means ± SEM, n = 5–6. * P < 0.05 versus normoxia.
BQ123 blocked the hypoxia-induced renal damage and proteinuria
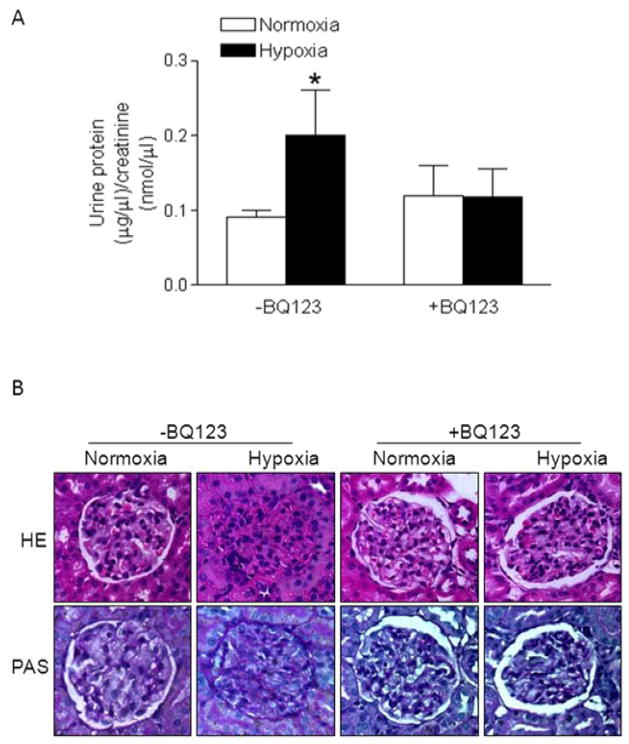
As shown in Figure 5A, chronic gestational hypoxia significantly increased urine protein levels in near-term (day 20) pregnant rats, which was blocked by BQ123. In contrast, the hypoxia treatment of non-pregnant rats had not effect on urinary protein levels (Online Figure S5). Histological examining of the kidney indicated that chronic hypoxia caused extensive endothelial swelling, narrowing, and occlusion of capillary lumen in glomeruli (Figure 5B). PAS stain of hypoxia-treated animals showed PAS-negative swollen cytoplasm of endocapillary cells. BQ123 treatment blocked the hypoxia-induced renal damage (Figure 5B).
Figure 5. BQ123 blocked chronic hypoxia-induced renal damage and proteinuria.
A, Protein and creatinine levels were determined in 12-hour urine samples in hypoxic and normoxic control pregnant rats at day 20 of gestation, in the absence or presence of BQ123. Data are means ± SEM, n = 5–6. * P < 0.05 versus normoxia. B, The kidney histology with H&E (×200) and Periodic acid Schiff (PAS) (×200) staining was examined in hypoxic and normoxic control pregnant rats at day 21 of gestation, in the absence or presence of BQ123.
BQ123 reversed the hypoxia-induced impairment of trophoblast invasion
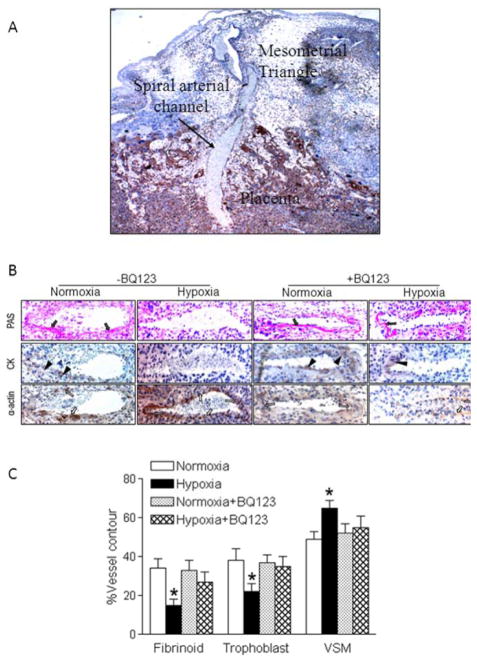
Trophoblast-associated vascular remodeling and trophoblast invasion were evaluated in the mesometrial triangle. Figure 6A shows the representative implantation site including the placenta and its associated mesometrial triangle staining with cytokerain, showing a maternal spiral arterial channel crossing the placenta. The maternal spiral arterial channel was used as a marker for each slide that was used in the subsequent quantification. Figure 6B shows representative PAS staining (solid arrows), CK staining (solid arrowheads) and α-actin staining (hollow arrows) in spiral arteries in the mesometrial triangle. PAS staining revealed the deposition of fibrinoid in the spiral artery of normoxic pregnant rats but not hypoxic animals. BQ123 restored the fibrinoid deposition in the spiral artery of the hypoxic animals. CK staining revealed the trophoblast invasion of the spiral artery in normoxic control rats, which was absent in the hypoxic animals. BQ123 treatment reversed the hypoxic effect and rescued trophoblast invasion of the spiral artery. α-Actin staining revealed that in normoxic pregnant rats vascular smooth muscle was partially disrupted underneath the trophoblast, whereas in the hypoxic animals the lumen was completely surrounded by α-actin positive vascular smooth muscle. BQ123 inhibited the hypoxic effect and recovered the vascular remodeling. As shown in Figure 6C, when expressed as percentages of the corresponding total spiral artery contour length, there were significantly fewer trophoblasts in the hypoxic group than that in the normoxic control. The amount of fibrinoid wall, expressed as a percentage of the total lumen contour, was also significantly decreased in the hypoxic group as compared with the normoxic control. In contrast, the length of vascular smooth muscle cells, expressed as a percentage of the total lumen contour, was significantly greater in the hypoxic group than that in the normoxic group. These hypoxia-mediated impairments of trophoblast invasion and vascular remodeling of spiral arteries were abrogated by BQ123 (Figure 6C).
Figure 6. BQ123 reversed chronic hypoxia-induced impairment of trophoblast invasion and spiral artery remodeling.
Placentas were obtained in hypoxic and normoxic control pregnant rats at day 21 of gestation, in the absence or presence of BQ123. A, The representative implantation site including the placenta and its associated mesometrial triangle stained with cytokeratin (×40). A spiral arterial channel is crossing the placenta. B, Representative Periodic acid Schiff (PAS) staining of fibrinoid (solid arrows), cytokeratin (CK) staining of trophoblast invasion (solid arrowheads), and α-actin staining of vascular smooth muscle (hollow arrows) in spiral arteries in the mesometrial triangle (×200). C, The percentage of fibrinoid, trophoblast, and vascular smooth muscle (VSM) of total spiral artery contour length was determined. Data are means ± SEM, n = 49–53. * P < 0.05 versus normoxia.
BQ123 inhibited the hypoxia-induced fetal growth restriction
As shown in Figure 7, chronic hypoxia resulted in fetal asymmetrical growth restriction by decreasing the fetal body weight and increasing the brain to body weight ratio and heart to body weight ratio. BQ123 significantly attenuated hypoxia-mediated effects and partially restored the fetal body weight and the brain to body weight ratio, and recovered the heart to body weight ratio (Figure 7). Chronic hypoxia did not affect the placental weight (0.56 ± 0.02 versus 0.52 ± 0.02 g, P > 0.05), but significantly increased the placenta to fetal weight ratio (0.21 ±0.01 vs. 0.13 ± 0.01, P < 0.05), which was attenuated by BQ123 (Online Figure S6). In addition, although chronic hypoxia did not affect the litter size (Online Figure S7), it significantly increased the number of resorbed fetuses, which was blocked by BQ123 (Online Figure S8).
Figure 7. BQ123 inhibited chronic hypoxia-induced asymmetrical fetal growth restriction.
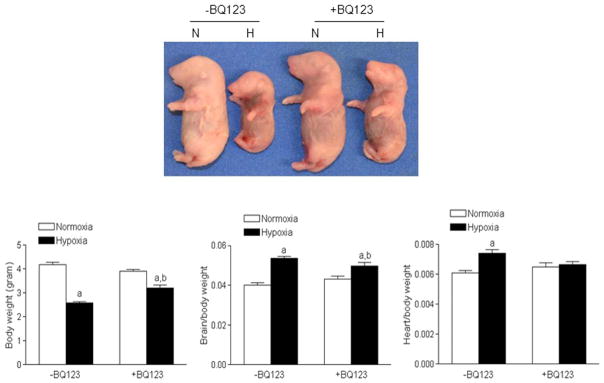
Fetal body weight, brain to body weight ratio, heart to body weight ratio were determined in hypoxic (H) and normoxic (N) control pregnant rats at day 21 of gestation, in the absence or presence of BQ123. Data are means ± SEM, n = 29–53. a P < 0.05 versus normoxia; b P < 0.05 versus -BQ123.
Discussion
The present study demonstrates that exposure to 10.5% O2 during day 6 through day 21 of gestation causes the development of preeclamptic symptoms including hypertension and proteinuria in a model of pregnant rats. Of importance, the findings provide new evidence that the heightened ET-1 signaling is a key molecular mechanism in the pathogenesis of chronic hypoxia-induced preeclampsia.
The previous studies have shown that chronic hypoxia impairs normal adaptation of the uteroplacental circulation and enhances uterine vascular tone in pregnancy,43–44 which may contribute to the pathogenesis of preeclampsia and fetal growth restriction. The present findings that chronic hypoxia significantly increased arterial blood pressure in pregnant rats, suggest that gestational hypoxia enhances arterial vascular resistance and induces hypertension in pregnancy. In addition to hypertension, maternal hypoxia also increased proteinuria in pregnant rats. This is likely resulting from hypoxia-induced renal damage as the kidney of hypoxia-treated animals showed extensive endothelial swelling, narrowing, and occlusion of capillary lumen. The finding of asymmetrical fetal growth restriction in hypoxic animals is in agreement with the previous findings in humans showing fetal growth restriction with preeclamptic pregnancy.
Growing evidence shows a key role of hypoxia in the pathophysiology of preeclampsia.11–22 Preeclampsia is associated with shallow trophoblast invasion and inadequate spiral artery remodeling, which are widely believed to lead to placental hypoxia and the local hypoxia ultimately results in the maternal manifestations of the disease. The present finding that chronic hypoxia increased HIF-1α protein abundance in the placenta provides evidence of placental hypoxia. However, whether maternal hypoxia exposure during gestation is a major factor and a key pathogenesis of preeclampsia remains unclear and controversial. Although previous studies have shown that the incidence of preeclampsia is significantly increased in women who are residing at high altitudes,12–15 not all pregnant women at high altitudes develop preeclampsia. The mechanisms behind this are not fully understood but may be due in part to different abilities of the compensatory adaptation to chronic hypoxia among different individuals. A previous study has demonstrated that maternal hypoxia (11% O2) between days 6.5 and 13.5 of gestation significantly increases the vascularity and trophoblast invasion in pregnant rats, and have suggested that maternal hypoxia during early stages of placentation may activate the invasive endovascular trophoblast cell lineage and promotes uterine vascular remodeling.23 Although it is known that local physiological “hypoxia” during normal pregnancy is important in the normal placentation and spiral artery remodeling, it is questionable that exaggerated hypoxia further induced by maternal hypoxia may improve the physiology of the placentation and vascular remodeling. Because blood pressure, kidney function and urine protein levels were not measured in this study,23 the functional significance of these findings were not clear. Indeed, a more recent study has demonstrated that maternal exposure to 9.5% O2 from gestational day 7.5 to day 17 induces clear preeclampsia-like symptoms with hypertension and proteinuria in both wild type and IL-10−/− pregnant mice.24 The present study provides additional support that gestational hypoxia decreases trophoblast invasion and impairs uterine arterial remodeling, resulting in the development of preeclampsia-like symptoms in pregnant rats.
The question arises as to how chronic hypoxia during gestation provokes these preeclamptic symptoms. Recent studies showed that ET-1 was a key pathological factor in preeclampsia.31,32 ET-1 has been implicated in a diverse range of signaling events in a wide variety of target tissues. ET-1 was first identified as a potent endothelium-derived vasoconstrictor, the most potent vasoconstrictor known. ET-1 is derived from a longer 203-amino acid precursor known as preproET-1, the active peptide proteolytically cleaved into its final 21-amino acid form. The important role of ET-1 signaling in the pathogenesis of preeclampsia has been widely demonstrated in clinic and different animal studies.31–34 Previous studies have demonstrated that an ETAR antagonist blocks hypertension induced by purified AT1R-AA from a transgenic rat model of preeclampsia or preeclamptic patients,35,45 In addition, ET-1 was increased in reduced uterine perfusion pressure (RUPP) model of preeclampsia, and the administration of an ETAR antagonist blocked hypertension.46 In the present study, we found that chronic hypoxia increased maternal plasma ET-1 levels in mid-gestation (day 12) and near-term (day 21) pregnant rats. In addition, propreET-1 mRNA levels in both placenta and kidney were also elevated with the hypoxia treatment. Hypoxia is a well-known inducer of ET-1 expression and a HIF-1α binding site has been identified on the 5′-promoter region of the preproET-1 gene.47–52 These findings suggest that the heightened ET-1 signaling contributes to the pathogenesis of chronic hypoxia-induced preeclampsia.
ET-1 mediates its physiological effects mainly through two seven-transmembrane G protein-coupled endothelin receptors, the endothelin A receptor (ETAR) and endothelin B receptor (ETBR).31 Both receptor types are widely distributed in non-cardiovascular and cardiovascular tissues. Activation of ETAR results in sustained vasoconstriction. In contrast, activation of ETBR induces vasodilatation.53 The finding that gestational hypoxia selectively up-regulated ETAR expression in both kidney and placenta reinforces the notion that ET-1 may contribute to the pathogenesis of hypoxia-induced preeclampsia through ETAR signaling. Indeed, the present finding that the sustained infusion of an ETAR selective antagonist BQ123 during the course of hypoxia treatment abrogated hypoxia-induced preeclampsia–like symptoms in pregnant rats, indicates a causative role of the heightened ET-1/ETAR-mediated signaling in the pathogenesis of hypoxia-induced preeclampsia. Future studies of blocking ETAR at different time points may provide more information detailing the role of ET-1 in the maternal hypoxia-induced preeclampsia symptoms.
The renin angiotensin system is known to stimulate ET-1 production.54 Previous studies have demonstrated that AT1R-AA increases ET-1 by activation of AT1R.45 In the present study, we found that plasma renin activity was not significantly altered by gestational hypoxia. This suggests that circulating angiotensin II may not contribute to the increased ET-1 in this animal model. However, the present findings that chronic hypoxia significantly increased AT1R protein expressions in both kidney and placenta but decreased AT2R protein expression in the kidney in pregnant rats, suggest that the increased AT1R/AT2R ratio may contribute to the ET-1 elevation and preeclampsia-like symptoms in pregnant rats.
Perspectives
Growing evidence indicates a role of gestational hypoxia in preeclampsia. Yet whether hypoxia is a causal factor and involves in the pathogenesis of preeclampsia remains unclear. The present study demonstrates that chronic hypoxia during gestation induces preeclampsia-like symptoms in pregnant rats via heightened ET-1 and ETAR-mediated signaling, providing a molecular mechanism linking gestational hypoxia and increased risk of preeclampsia. Whereas caution should be always observed in extrapolating the findings of animal studies directly to the humans, the present finding has a translational potential, and provides a mechanistic understanding worthy of investigation in humans. This is because hypoxia is a common insult during pregnancy, and preeclampsia is one of the most common complications of pregnancy.
Supplementary Material
Novelty and Significance.
What Is New?
Chronic hypoxia during gestation induces preeclampsia-like symptoms in an animal model of pregnant rats.
Gestational hypoxia impairs the trophoblast invasion and uteroplacental vascular remodeling.
Inhibition of the ET-1/ETAR signaling abrogates hypoxia-induced preeclampsia-like features.
What Is Relevant?
Chronic hypoxia is a common insult during pregnancy.
Preeclampsia is one of the most common complications of pregnancy.
Heightened ET-1 signaling has been implicated in the development of hypertension in pregnancy.
Summary
The present study provides new evidence in an animal model linking gestational hypoxia and the increased risk of preeclampsia, and reveals a mechanistic understanding of the heightened ET-1/ETAR signaling in the pathogenesis of preeclampsia.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health Grants HL110125 (LZ), HL089012 (LZ), HD031226 (LZ), DA032510 (DX), Chinese National Clinical Key Subject Construction Project (YLH) and Chinese National Natural Science Foundation 81200450 (JJZ).
Footnotes
Disclosures: None.
References
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Zhao X, Wang Z, Hu Y. Combination of lipids and uric acid in mid-second trimester can be used to predict adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;25:2633–2638. doi: 10.3109/14767058.2012.704447. [DOI] [PubMed] [Google Scholar]
- 3.Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001;184:946–953. doi: 10.1067/mob.2001.111719. [DOI] [PubMed] [Google Scholar]
- 4.Cnattingius S, Haglund B, Kramer MS. Differences in late fetal death rates in association with determinants of small for gestational age fetuses: population based cohort study. BMJ. 1998;316:1483–1487. doi: 10.1136/bmj.316.7143.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mongelli M, Gardosi J. Fetal growth. Curr Opin Obstet Gynecol. 2000;12:111–115. doi: 10.1097/00001703-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6 (Suppl 3):332–336. [PubMed] [Google Scholar]
- 7.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 10.Camm EJ, Martin-Gronert MS, Wright NL, Hansell JA, Ozanne SE, Giussani DA. Prenatal hypoxia independent of undernutrition promotes molecular markers of insulin resistance in adult offspring. FASEB J. 2011;25:420–427. doi: 10.1096/fj.10-158188. [DOI] [PubMed] [Google Scholar]
- 11.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol. 2008;295:R906–R915. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54:20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 13.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 14.White M, Zhang L. Effects of chronic hypoxia on maternal vascular changes during pregnancy. invited review. High Alt Med Biol. 2003;4:157–169. doi: 10.1089/152702903322022776. [DOI] [PubMed] [Google Scholar]
- 15.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol. 1995;79:15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effects of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol. 1995;79:7–14. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]
- 17.Yip R. Altitude and birth weight. J Pediatr. 1987;111:869–876. doi: 10.1016/s0022-3476(87)80209-3. [DOI] [PubMed] [Google Scholar]
- 18.Julian CG. High altitude during pregnancy. Clin Chest Med. 2011;32:21–31. doi: 10.1016/j.ccm.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, Wapner RJ, Thorp JM, Jr, Mercer BM, Grobman WA, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Tolosa JE, Saade G, Sorokin Y, Anderson GD Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU) The utility of uterine artery Doppler velocimetry in prediction of preeclampsia in a low-risk population. Obstet Gynecol. 2012;120:815–822. doi: 10.1097/AOG.0b013e31826af7fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol. 2012;303:H1–H8. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Norris WE, Kalkunte S. Beyond the threshold: an etiological bridge between hypoxia and immunity in preeclampsia. J Reprod Immunol. 2010;85:112–116. doi: 10.1016/j.jri.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SB, Wong AP, Kanasaki K, Xu Y, Shenoy VK, McElrath TF, Whitesides GM, Kalluri R. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176:710–720. doi: 10.2353/ajpath.2010.090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arimoto-Ishida E, Sakata M, Sawada K, Nakayama M, Nishimoto F, Mabuchi S, Takeda T, Yamamoto T, Isobe A, Okamoto Y, Lengyel E, Suehara N, Morishige K, Kimura T. Up-regulation of alpha5-integrin by E-cadherin loss in hypoxia and its key role in the migration of extravillous trophoblast cells during early implantation. Endocrinology. 2009;150:4306–4315. doi: 10.1210/en.2008-1662. [DOI] [PubMed] [Google Scholar]
- 27.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onogi A, Naruse K, Sado T, Tsunemi T, Shigetomi H, Noguchi T, Yamada Y, Akasaki M, Oi H, Kobayashi H. Hypoxia inhibits invasion of extravillous trophoblast cells through reduction of matrix metalloproteinase (MMP)-2 activation in the early first trimester of human pregnancy. Placenta. 2011;32:665–670. doi: 10.1016/j.placenta.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–769. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21 (Suppl A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 31.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24:964–969. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol. 1991;165:724–727. doi: 10.1016/0002-9378(91)90317-k. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26:236–241. doi: 10.1038/jhh.2011.29. [DOI] [PubMed] [Google Scholar]
- 34.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55:394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe T, Suzuki N, Shimamoto N, Fujino M, Imada A. Endothelin in myocardial infarction. Nature. 1990;344:114. doi: 10.1038/344114a0. [DOI] [PubMed] [Google Scholar]
- 37.Tonnessen T, nnessen T, Giaid A, Saleh D, Naess PA, Yanagisawa M, Christensen G. Increased in vivo expression and production of endothelin-1 by porcine cardiomyocytes subjected to ischemia. Circ Res. 1995;76:767–772. doi: 10.1161/01.res.76.5.767. [DOI] [PubMed] [Google Scholar]
- 38.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao D, Huang X, Xu Z, Yang S, Zhang L. Prenatal cocaine exposure differentially causes vascular dysfunction in adult offspring. Hypertension. 2009;53:937–943. doi: 10.1161/HYPERTENSIONAHA.108.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R, Dechend R. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension. 2010;56:304–310. doi: 10.1161/HYPERTENSIONAHA.110.153163. [DOI] [PubMed] [Google Scholar]
- 41.Geusens N, Hering L, Verlohren S, Luyten C, Drijkoningen K, Taube M, Vercruysse L, Hanssens M, Dechend R, Pijnenborg R. Changes in endovascular trophoblast invasion and spiral artery remodelling at term in a transgenic preeclamptic rat model. Placenta. 2010;31:320–326. doi: 10.1016/j.placenta.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Geusens N, Verlohren S, Luyten C, Taube M, Hering L, Vercruysse L, Hanssens M, Dudenhausen JW, Dechend R, Pijnenborg R. Endovascular trophoblast invasion, spiral artery remodelling and uteroplacental haemodynamics in a transgenic rat model of pre-eclampsia. Placenta. 2008;29:614–623. doi: 10.1016/j.placenta.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson SM, Zhang L. Chronic hypoxia suppresses pregnancy-induced upregulation of large-conductance Ca2+-activated K+ channel activity in uterine arteries. Hypertension. 2012;60:214–222. doi: 10.1161/HYPERTENSIONAHA.112.196097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension. 2010;56:750–757. doi: 10.1161/HYPERTENSIONAHA.110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol. 2011;186:6024–6034. doi: 10.4049/jimmunol.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- 48.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 49.Minchenko A, Caro J. Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: role of hypoxia responsive element. Mol Cell Biochem. 2000;208:53–62. doi: 10.1023/a:1007042729486. [DOI] [PubMed] [Google Scholar]
- 50.Kakinuma Y, Miyauchi T, Yuki K, Murakoshi N, Goto K, Yamaguchi I. Novel molecular mechanism of increased myocardial endothelin-1 expression in the failing heart involving the transcriptional factor hypoxia-inducible factor-1alpha induced for impaired myocardial energy metabolism. Circulation. 2001;103:2387–2394. doi: 10.1161/01.cir.103.19.2387. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, Elton TS. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol. 1994;77:1451–1459. doi: 10.1152/jappl.1994.77.3.1451. [DOI] [PubMed] [Google Scholar]
- 52.Thaete LG, Jilling T, Synowiec S, Khan S, Neerhof MG. Expression of endothelin 1 and its receptors in the hypoxic pregnant rat. Biol Reprod. 2007;77:526–532. doi: 10.1095/biolreprod.107.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rautureau Y, Schiffrin EL. Endothelin in hypertension: an update. Curr Opin Nephrol Hypertens. 2012;21:128–136. doi: 10.1097/MNH.0b013e32834f0092. [DOI] [PubMed] [Google Scholar]
- 54.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. doi: 10.1161/01.hyp.30.1.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.