Abstract
Induced pluripotent stem cells (iPSc) are a scientific and medical frontier. Application of reprogrammed somatic cells for clinical trials is in its dawn period; advances in research with animal and human iPSc are paving the way for retinal therapies with the ongoing development of safe animal cell transplantation studies and characterization of patient-specific and disease-specific human iPSc. The retina is an optimal model for investigation of neural regeneration; amongst other advantageous attributes, it is the most accessible part of the CNS for surgery and outcome monitoring. A recent clinical trial showing a degree of visual restoration via a subretinal electronic prosthesis implies that even a severely degenerate retina may have the capacity for repair after cell replacement through potential plasticity of the visual system. Successful differentiation of neural retina from iPSc and the recent generation of an optic cup from human ESc in-vitro increase the feasibility of generating an expandable and clinically suitable source of cells for human clinical trials. In this review we shall present recent studies that have propelled the field forward and discuss challenges in utilizing iPS cell derived retinal cells as reliable models for clinical therapies and as a source for clinical cell transplantation treatment for patients suffering from genetic retinal disease.
Keywords: Clinical trial, disease modeling, photoreceptor, reprogramming, retinal degeneration, stem cell, induced pluripotent stem cell, transplantation
INTRODUCTION
The capacity for stem cells to proliferate and differentiate into the diverse cell types of the body has caused much interest both in developmental research and regenerative medicine. The possibility of subverting the unidirectional course of differentiation by cell-reprogramming, and thus reinstitute the capacity for a differentiated cell to adopt a new fate is conceptually intriguing and entails a prospective paradigm shift in medicine and research. Induced pluripotent stem cells (iPSc) are hence on the cutting edge of scientific investigation and hold great potential for clinical application, both for investigating disease mechanisms and for cell replacement therapies. Perhaps not surprisingly, this year the Nobel Prize for Medicine recognized two leading researchers who made seminal advances in cell reprogramming in lower vertebrates (John Gurdon) [1] and mammals (Shinya Yamanaka) [2].
The retina has emerged as an increasingly promising frontier for investigation of central nervous system (CNS) regeneration and stem cell transplantation, owing to its unique features as a surgically accessible, immune privileged organ that can be non-invasively imaged. Grafted cells can be observed directly through the clear ocular media and functional assessment of the transplantation can be provided through a vast array of visual testing. Moreover, retinal degenerations are commonly bilateral so clinical trials in the eye may be applied to only one eye, while making use of the fellow eye as a control. Finally, unlike long tracts of the brain and cerebellum, transplanted photoreceptors need only to make short synaptic connections to their immediate neighboring cells (bipolar) across non-myelinated tissues in order to restore function, which facilitates assessment of the cells physiologically [3].
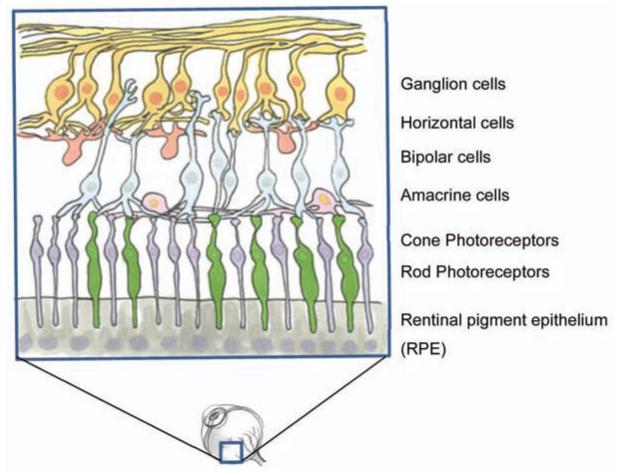
Perception of visual stimuli is dependent on light signals passing through a visual stream from the eye to the brain. The first order neurons in this pathway are the light sensitive rod and cone photoreceptors in the outer nuclear layer of the retina. Photoreceptors instigate the visual stream by converting light signals into electric impulses that are then transmitted to bipolar cells in the inner nuclear layer [4] (See Fig. 1) and travel through the optic nerve and lateral geniculate nucleus (LGN) to be processed by the visual cortex in the occipital lobe of the brain. Retinal neurons, like other neurons of the CNS, degenerate progressively throughout life and show limited ability for regeneration and repair after injury. But while single retinal neurons do not regenerate (except in specific circumstances such as during development [5]) the visual system as a whole does exhibit quite encouraging signs of neuroplasticity and preservation, even after severe retinal degeneration.
Fig. (1).
The layered structure of the retina. The first order sensory neurons of the visual system, cone and rod photoreceptors, reside in the outer nuclear layer bordering on RPE cells. Light signals are transferred to the bipolar cells of the inner nuclear layer and pass through ganglion cells and the optic nerve to reach the brain.
One reason for optimism in restoring vision by photoreceptor transplantation comes from observations in patients with advanced retinal degeneration due to retinitis pigmentosa (RP). Here post-mortem studies in humans revealed that although only 4% of photoreceptors were still present in the severely degenerate eyes, 66% of the normal bipolar cell population still remained in the degenerate tissue [6]. Furthermore, the observations that ganglion cells in rodent mammals can regenerate to restore function through peripheral nerve grafts demonstrates the plastic potential of the central targets of the visual pathway [7, 8]. But perhaps most compelling evidence for the regenerative capacities of the human visual system was heralded by the subretinal transplantation of electronic photoreceptor arrays, after which patients with visual impairments regained a degree of vision, to the extent that one patient could even read large print after such transplant [9]. Thus implying that the electronic retinal implant relays signals to a preserved and functioning visual pathway downstream to the lost photoreceptors or alternatively that there is possibility of neuronal regeneration and reorganization in the visual pathway following treatment.
Accordingly, photoreceptor cell-replacement therapies may hold the possibility of repair in a degenerating or completely degenerate retina, by reinstating light sensitive cells to project and form connections with downstream retinal cells and finally the visual cortex.
Embryonic stem cells (ESc) and photoreceptor progenitors have been widely researched in the area of ophthalmology and visual neuroscience as candidates for cell replacement. In 2006, Nrl+ rod photoreceptor progenitors were successfully transplanted and integrated into an adult degenerate retina in a mouse model of Retinitis pigmentosa (RP) [10], demonstrating that the degenerate retina is receptive to integration of transplanted tissue and establishing postmitoticphotoreceptors as better candidates for transplantation than early progenitors. In a recent study, Singh et al. [11] have shown that end-stage retinal degeneration may be reversed by reconstitution of a light-sensitive photoreceptor layer. In this study, behavioural, cortical and pupil visual responses were restored in a murine model of severe human RP after transplantation of rod photoreceptor precursors; thus highlighting cell replacement therapies as a potential tool for vision repair in even after complete degeneration of the outer retinal layer.
Photoreceptors have been successfully derived from mouse ESc (mESc) [12] and reported to integrate into the host retina and improve vision in adult blind mice. Furthermore, retinal pigment epithelium (RPE) derived from human ESc (hESc) have been shown to preserve vision in an animal model of RPE dystrophy, where photoreceptor loss is occurring secondary to a genetic defect in the RPE [13]. These studies provide proof of concept for application of in-vitro generated retinal cells in clinical rescue of vision.
Phase I/II trials using stem cells have been initiated for treatment of disease and injury in other regions of the CNS (for detailed review see [14]). Clinical trials using ESc-derived cells to treat retinal degeneration are not yet prevalent, although this year a prospective trial has been initiated [15], focused on transplanting RPE derived from hESc to patients with macular degeneration. While this study presents a good case for the initial safety of ocular delivery of RPE derived from hESc, it does not yet provide the desired evidence of vision rescue or therapeutic effect of such transplants.
It has been recognized that photoreceptor precursors ideally integrate in a host retina when obtained from donor mice around postnatal day 3 [10, 11, 16, 17], a period which is developmentally comparable with the second trimester of pregnancy in humans; hence greatly restricting the use of such human primary cells [18]. ESc are an important research avenue for in-vitro derivation of photoreceptor precursors, however their use entails ethical obstacles and thus a challenge of using ESc derived donor cells for transplantation studies or clinical trials. Additionally, the use of Esc derived retinal precursor cells in clinical trials entails a risk of immune rejection, although the eyes are protected by the blood retina barrier, the surgical manipulation to transplant cells will in itself compromise this barrier to some extent, and introduce circulating immune cells, such as T-cells into the subretinal space and foreign transplanted tissue would stand higher risk of rejection and would require constant immune suppression post transplant, which is itself associated with significant morbidity. A need therefore arises for a readily expandable, immunologically attuned source of cells for basic and clinical research.
These barriers for cell replacement may be addressed through use of induced pluripotent stem cells. First developed in mammalian vertebrates in 2006 [2], contingent on breakthroughs in cell reprogramming in lower vertebrates in 1962 [1], iPSc technology allows the reprogramming of adult somatic cells by chemically altering extrinsic signaling pathways. This consequently reinstitutes the redifferentiation of the adult somatic cell into embryonic cell lineages of the three germ layers.
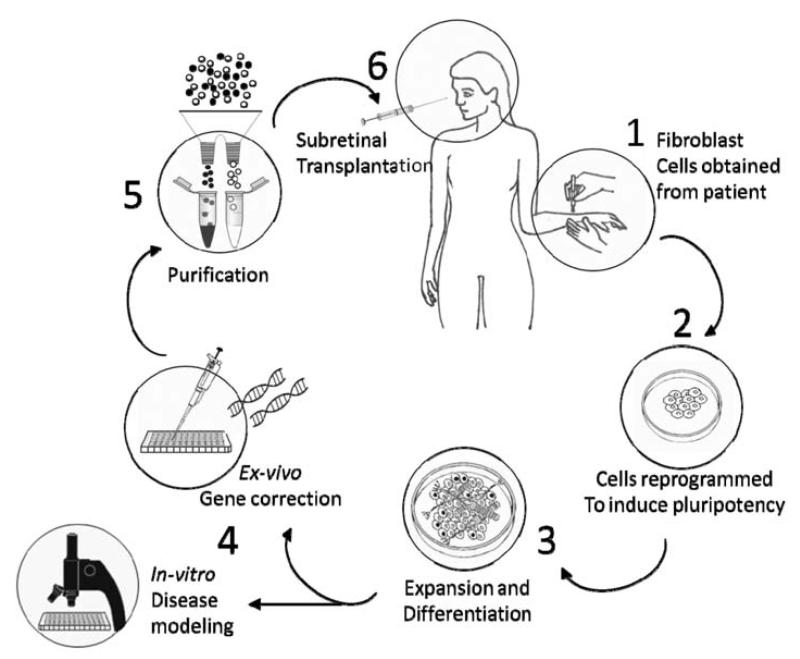
In order to resolve not only the ethical issues arising from the use of Esc, but also the need for continual immune suppression; which may in itself present a health risk to the patient, disease-specific and patient-specific iPSc would be most attractive and relevant for both research and clinic. In a model scenario, tissue would be obtained from somatic cells of a single patient afflicted with inherited retinal degeneration, reprogrammed to a pluripotent state, expanded in-vitro and then differentiated to reach an appropriate developmental state for transplantation or research. These iPSc-derived differentiated cells could subsequently be used either as in-vitro models of genetic diseases and therapy development [19] (‘disease in a dish’) or subjected to ex-vivo gene correction in order to correct the genetic mutation causing blindness. Subsequently, corrected autologous cells would be transplanted back into the patient. Fig. (2) presents a schematic representation of this process.
Fig. (2).
1. Somatic cells, such as skin fibroblasts are obtained from a patient with a genetic retinal degeneration. 2. Cells are reprogrammed to an ES-like pluripotent state. 3. Cells are expanded in-vitro and differentiated to reach an appropriate developmental state. 4. Developing cells may provide an in-vitro model of disease development and treatment or may be subjected to gene therapy with the aim of correcting the genetic source of degeneration ex-vivo. 5. In order to prevent adverse consequences of therapy, cell-colonies are to be screened and purified of proliferative and potentially malignant cells. 6. Patient receives a subretinal injection to replace degenerate cells with reprogrammed, suitably differentiated, corrected and purified autologous cells.
In considering a complex and multi-staged therapy method, it is helpful to start from the clinical need and gain perspective on advances and clinical implications of proceedings and challenges facing scientists in the field. We shall first discuss the clinical need for iPSc in treatment of retinal disease, types of retinal cells generated from iPSc and methods used for their generation. Then we will present current research advances in the use of retinal cells generated from iPSc in the forefront of scientific and clinical research and discuss translation of these studies from bench to bedside. Subsequently, with this frame of reference, we shall discuss again the scientific methods of reprogramming somatic cells, genomic integrity and epigenetic memory of the derived pluripotent cells in the context of retinal therapies. Finally, we shall raise probable practical impediments to producing iPSc derived retinal cells for human clinical trials and offer solutions arising from current research.
GENERATION OF RETINAL CELLS FROM iPSc
Retinitis pigmentosa (RP), and age-related macular degeneration (AMD) are two primary causes of inherited retinal blindness [20] affecting over 15 million people world-wide [21], and are therefore not surprisingly the focus of most retinal cell transplantation research. Both diseases cause retinal degeneration leading to eventual irreversible loss of the light sensing photoreceptors of the neural retina and vision impairment. However the underlying cellular mechanisms are divergent; In RP, an initial loss of (usually) rod photoreceptors leads to a cellular cascade that promotes the secondary degeneration of cone photoreceptors [22]. In AMD, the majority of cases are characterized by an initial degeneration believed to arise in Bruch’s membrane of the retinal pigment epithelium (RPE) which is essential for photoreceptor metabolic activity, thus leading to a secondary loss of photoreceptors [23]. Accordingly, in vitro differentiation of rod photoreceptors and RPE are in the spotlight of current retinal cell generation research. As will later be discussed, these iPSc generated retinal cells, have the potential to advance many steps on the continuum from basic science to clinical therapy; from models of disease, to in vitro gene therapy and clinical cell replacement therapies for these currently incurable and inadequately understood conditions.
In vitro retinal differentiation from ESc or iPSc largely imitates developmental landmarks of normal in-vivo differentiation [19]. In the developing human embryo at 4-5 weeks, the optic vesicle evaginates from the diencephalon of the neural tube and folds to form the optic cup. After disconnection of the lens vesicle, two layers of tissue form separately; the RPE progenitor outer layer, expressing Pax6 and the basic-helix-loop-helix-zipper transcription factor gene Mitf [24], and the neural retina progenitor layer which expresses Pax6 and the transcription factor gene Rx [25]. Later in development, photoreceptor precursors express the homeobox gene Crx [26] and eventually mature to express the pan-photoreceptor marker Recoverin. The neural retina progenitor layer differentiates into the seven principal neural retinal cell subtypes, namely ganglion cells, horizontal cells, amacrine cells, cone photoreceptors (opsin+), bipolar cells, rod photoreceptors (Rhodopsin+/Nrl+) and Müller glia, in a sequential fashion [Detailed review in [27].
In order to mimic natural development, in vitro differentiation of retinal cells from iPSc would require orchestration of sequential induction steps, amongst which are the inhibition of BMP and Wnt signaling [28-32], the initiation of IGF-1 in eye field development [33] and Notch inhibition in photoreceptor development [34]. Accordingly, a stepwise differentiation protocol was established for animal as well as human ESc, employing extrinsic chemical signaling pathways to direct cell fate into retinal progenitor cells (Pax6+/Rx+) by Wnt and Nodal inhibition [35] or direct differentiation towards photoreceptors by addition of retinoic acid and taurine to formerly established culture media [36, 37]. Additional protocols emerged inducing photoreceptor-precursor differentiation by recombinant noggin (a BMP inhibitor), IGF-1 and Dickkopf-1 (Dkk-1) (an antagonist of the Wnt/β-catenin signaling pathway) [38].
Subsequently, these protocols were directed to differentiate retinal cells from human iPSc, generating both photoreceptor and RPE phenotypes. In one of the first such studies [39], human iPS cell colonies were cultured in serum free media for 18 days, supplemented by nodal signaling inhibitor, Lefty-A and Dkk-1 to facilitate Wnt signaling inhibition [reviewed in [40]. Approximately 30 days after cell colony re-plating, Pax6+/ Mitf+ RPE progenitor cells and Pax6+/Rx+ retinal progenitors could be detected. At day 40, RPE cells were observed. After 90 days of in vitro induction Crx+ retinal precursors emerged and finally by day 120, researchers could report induction of rhodopsin+ or opsin+ photoreceptor cells.
Successful generation of retinal cells in-vitro is largely assessed in studies by expression of distinct cell markers; however it is important to consider that markers used to identify retinal progenitors are in numerous cases expressed in other developing cells of the CNS. As a consequence, characterization of cells as retinal lineage precursors cannot be viably assigned based only on appearance of singular developmental markers, but must be examined within the frame of chronological developmental landmarks [27]. Functional tests of photosensitivity may provide support for ascertaining cell fate, but photosensitivity cannot be taken as a sole measure either, as it could be indicated in any neuronal cell that expresses melanopsin [41]. Ascertaining identity of cells prior to transplantation is imperative, the types of cells being transplanted and their proliferative state have implications not only for the functional success of cell replacement treatment, but may determine the overall safety of the transplant, as transplantation of proliferative cells may induce tumor formation post treatment. Accordingly, immunological characterization of derived cells should ideally be accompanied by assessment of morphology, cell membrane function and capacity to integrate successfully and safely into the retina before assessment of suitability for use in a clinical setting.
iPSc derived retinal cells have been generated by a number of groups [e.g. 27, 42-48], using methods similar to ones used in differentiation of ESc into retinal cells and Masayo Takahashi from the RIKEN Center for Developmental Biology in Kobe in Japan is currently setting up a clinical trial with iPSc derived RPE (reported in Science Now, by Dennis Normile on 18 June 2012).
In the coming sections, we shall focus on recent studies which have generated neural retina, and applied the derived cells to provide proof of concept for clinical application, i.e. transplantation or in vitro modeling of development and disease. (Table 1) provides a summary of recent published advances in applied research of retinal cells generated from iPSc for photoreceptor replacement. These animal and human studies represent an exciting step forward in the understanding and treatment of retinal degeneration and provide proof of concept for clinical trials. We shall hence discuss current advances in iPSc cell replacement studies, modeling of retinal development and retinal-degeneration, and present a new perspective on advantages and challenges that stand before researchers and clinicians using iPSc.
Table 1.
Recent Advances in Research of iPSc Application for Retinal Degeneration
| Application | iPSc source |
Reprogramming method |
Key Cell type generated | Purification and Tumor formation |
|---|---|---|---|---|
| Purification and Transplantation of differentiated cells into a retinal degeneration mouse model (rho−/−) [44] | Mouse | Retroviral transduction OCT-4, KLF4, SOX2, and c-MYC |
Photoreceptor Precursors Pax6 +/CRX+/ recov erin+/rho+ |
Two cycles of magnetic bead depletion of cells expressing the proliferation marker SSEA1 (98% free fromresidual SSEA1+ cell contamination) No tumor formation 16 weeks post transplantation |
| Transplantation into damaged swine retina (Intravenous injection of iodoacetic acid to induce rod damage) [45] | Swine | Lentiviral transduction OCT-4, KLF4, SOX2, and c-MYC |
Rod Photoreceptors | Cells fluorescently labeled via IRBP.GFP lentiviral infection. No purifification (44% IRBP.GFP+) Tumor assessment not reported |
| Transplantation of human iPSc into a normal mouse retina [46] | Human | Lentiviral transduction OCT4, NANOG, LIN28 and SOX2 |
Rod photoreceptors | Cells fluorescently labeled via IRBP.GFP lentiviral infection. FACS Purification of IRBP.GFP+ (90% IRBP.GFP+) Insufficient cell number for post-FACS integration. Tumor assessment not reported. |
| Retinal Development modeling [27] | Human | IMR90-4 human iPS cell line |
Optic Vesicle, Optic Cup, RPE, photoreceptor progenitors | |
| Patient specific retinal degeneration modeling [19] | Human | Retroviral transduction OCT-3/4, KLF4, SOX2, and c-MYC |
Retinal progenitor, photoreceptor precursor, RPE and rod photoreceptors | |
| Patient specific retinal degeneration modeling [42] | Human | Sendai Virus OCT-4, KLF4, SOX2, and c-MYC |
Retinal progenitor, photoreceptor precursor, RPEand rod photoreceptors |
RPE, Retinal Pigment Epithelium; FACS, Fluorescence-activated cell sorting; GFP, green fluorescence protein; Rho, Rhodopsin
PURIFICATION AND TRANSPLANTATION OF iPSc DERIVED PHOTORECEPTORS
Studies aimed at generating iPSc derived photoreceptor-precursors for transplantation, must take into consideration not only the method of reprogramming and generation of the desired cells, but also a reliable method of purifying these cells before transplantation, to increase efficiency and reduce risk of teratoma formation. It is not sufficient that cell colonies used for transplantation in a clinical setting show expression of retinal cell progenitor markers, but it is also required that undifferentiated cells are not present in transplanted cohort, as even a small amount of proliferating cells would be enough to render the transplant unsafe and thus undesirable for patients and clinicians. For regulatory body approval, demonstration of safety must precede demonstration of treatment efficacy.
Patient specific iPSc overcomes the need for immune suppression after cell transplantation, as the autologous cells are a near perfect immunological match for the patient. However, it is important to note that this advantage that iPSc hold becomes a drawback in case of tumorigenesis. In the scenario of teratoma formation after transplantation of ESc, immune suppression can simply be removed, thus permitting immune cells of the host retina effectively to attack and reject the foreign ESc and tumors. However, this safety measure may not pertain if malignancy occurs after transplanting patient-specific iPSc, as transplanted proliferating cells cannot easy be identified and therefore removed via circulating immune cells, making tumor formation a greater risk for patients who would potentially undergo such treatments. Hence differentiation of ES, but in particular iPS, cells must be followed by rigorous scanning and purification steps before use in a clinical setting. In vivo transplantation studies in animals provide an opportunity to assess the clinical-safety of using these cells as well as to assess functional changes in vision following transplantation. Although it should be noted that the immune systems of rodent models and primates differ considerably.
In 2010, Lamba and colleagues [46] reprogrammed and differentiated human iPSc to generate photoreceptors in-vitro. The differentiation protocol in this study relied on previous work achieved in the same group differentiating hESc into photoreceptors [12]. Notably, in this study, human photoreceptors were identified and fluorescently tagged in culture by lentiviral infection of GFP driven from interphotoreceptor retinoid-binding protein (IRBP), a photoreceptor specific promoter. Florescent labeling of photoreceptors allowed the researchers to purify the culture using Fluorescence-activated cell sorting (FACS), thus separating photoreceptors from undifferentiated cells and other cell-types present in culture. FACS yielded a cell suspension in which 90% of cells could subsequently be histologically labeled as rod or cone photoreceptors. Purified cells were transplanted into the subretinal space of adult mice, showing signs of integration into the outer nuclear layer comparable to integration found after differentiation and transplantation of hESc into adult mice. However, these FACS purified iPSc-derived photoreceptors did not survive well; only a small number of viable cells were integrated and no measures of visual rescue were reported.
In a recent succeeding study, Tucker and colleges [44] overcame the problem of cell viability after purification by using Magnetic-activated cell sorting (MACS) to deplete the culture of proliferative cells expressing stage-specific embryonic antigen 1 (SSEA1). Regardless of differences in viability of cells post FACS or MACS sorting, purification did not affect transplanted cells in this study because the labeled cells were the ones contaminating the culture rather that the population to be enriched. In this protocol mouse somatic cells were programmed using the Yamanaka transcription factors Oct4, Sox2, KLF4 and c-Myc [2], and differentiated into retinal precursors. Researchers found a significant increase in expression of the photoreceptor markers CRX and recoverin as well as the rod marker, rhodopsin in D33 differentiated cells, when compared to undifferentiated embryoid bodies. They additionally found markers of various other retinal cell types. However, though differentiated cells were found in culture, an order of 30% of cells remained undifferentiated at this stage and expressed the pluripotency marker SSEA1. Cells were transplanted subretinally into the degenerate retinas of rho−/− host mice, either with no purification steps, with one round of SSEA1 depletion or after two consecutive rounds of SSEA1+ cell depletion. When no purification was applied, cell transplantation induced tumorigenesis in approximately 60% of host animals within 21 days of injection. Purifying cells with a single MACS procedure of SSEA1expressing cell depletion decreased the percentage of animals developing tumors to approximately 20%. After a second consecutive round of SSEA1+ cell depletion, researchers did not find tumor formation in any of the transplant recipients after 21 days and up to 16 weeks of transplantation. Transplanted iPSc were included into the outer nuclear layer of the host degenerate retina and possibly led to a small increase in the scotopic ERG response. In one case, the proliferation marker Ki67 was detected within the animals vitreal space 21 days post SSEA1-depleted cell injection, thus although no malignancy occurred, further optimization would be required in order to translate this protocol into a clinical setting, where the injection of proliferative patient specific cells would be entirely undesired. It is also noteworthy to point out, that due to the nature of depleting-purification rather than rod enrichment, and the presence of other retinal cell types in the differentiated culture, the changes observed in this study may not have occurred specifically as a result of rod cell replacement and may have been caused by a number of other cellular mechanisms.
GENERATION OF RETINAL MATRICES USING iPSc
When generating photoreceptors for therapeutic application, it is important to consider physical challenges in their transplantation into the retina. In the current standard method of retinal cell transplantation, and thus in most studies presented above, a single-cell suspension of in vitro differentiated photoreceptors is transplanted subretinally of intravitreally. While photoreceptor precursors have been shown to integrate and form connections with host retina when transplanted as a single-cell suspension [e.g. 10, 11], the percentage of synapse-forming grafted precursor cells is extremely low (in the region of 0.1% of transplanted cells) [49] and the integration of photoreceptors in the correct orientation within the outer nuclear layer is infrequently accomplished [50]. In the healthy intact human retina photoreceptors are positioned in the outer nuclear layer of the outer retina in a polarized manner, with outer segments projecting discs towards the RPE and inner segments adjoin to a layer of Müller cells at the outer limiting membrane. Orientation and integration of these cells is important for their function and survival, as contact with RPE is essential for foveal photoreceptor maturation [51], and correspondingly, loss of connection in the adherens junctions between photoreceptors and Müller cells is linked to photoreceptor degeneration [52] (reviewed in [53] & [16]). The isolated cells differentiated from ES or iPSc in vitro have not yet been shown to develop cilium or outer segment discs [37], although even had these features of photoreceptors been achieved in culture, their applicability would be relatively low, as cells are optimally transplanted during the precursor stage [10, 11, 54, 55], when these features are not yet developed. Hence, a method is required to ensure that these cells are polarized and can attach outer segment disks to The RPE and inner segments to Müller cells.
Taking these challenges into account, transplantation of single cells into the retina may be questioned as effective therapy for retinal degeneration. In order to promote integration of the transplanted photoreceptors into the retina, a method that would insure their polarized integration post transplant would be ideal. Biodegradable polymer scaffolds have accordingly been suggested to promote cell integration and survival in areas of the damaged retina. Dissociated mouse progenitor cells were differentiated in vitro and expanded on a polymer scaffold to form artificial retinal tissue in culture, prior to localized subretinal delivery in mouse models of retinal degeneration (Rho −/−) [56]. This innovative method employed injection of the engineered retinal tissue on a scrollable scaffold, reducing trauma during injection. This method may provide an important step forward in cell replacement transplantations and may indeed prove to be applicable to the expansion and transplantation of differentiated iPSc-derived retinal cells.
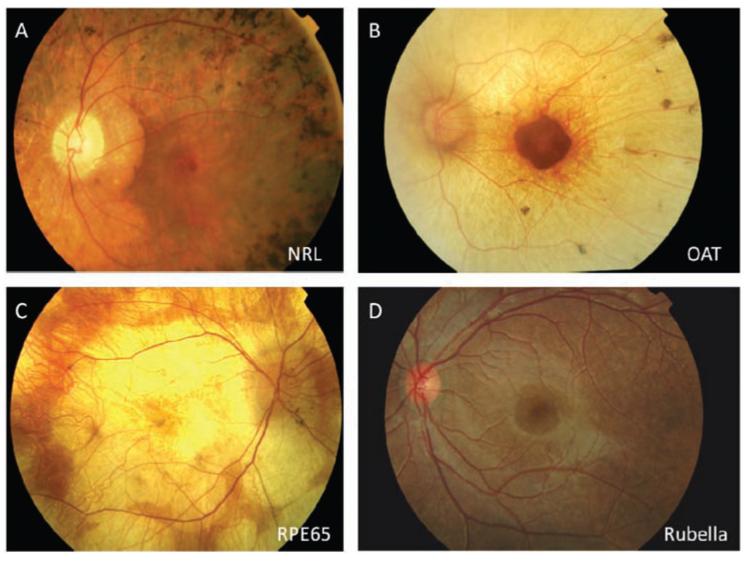
In an interesting recent study [57], human ESc were used to produce a self forming optic cup in a 3D in vitro culture system, previously successfully achieved by the same group using mESc [58]. The ES derived human optic cup is a multi layered tissue, containing rod and cone photoreceptor progenitors, inter-neurons and ganglion cells. This research unlocks the exciting possibility of similarly generating a human optic cup from iPSc and harvesting photoreceptor progenitors within a retinal matrix with RPE bound to the outer segment discs, thus transplanting retinal sheets in place of single cells. An additional consideration supporting this strategy is the fact that RP does not have a constant disease-trajectory of degeneration, it manifests differently in different patients and in some cases loss of photoreceptors may be accompanied by secondary retinal degeneration or RPE loss (Fig. 3), thus in such cases transplanting photoreceptors with RPE cells may have additional therapeutic effect.
Fig. (3).
There is extensive genetic heterogeneity and variability in the phenotypes of outer retinal disease. The four examples of photoreceptor degeneration shown here represent different degeneration mechanisms with divergent phenotypes. A. Retinal degeneration due to rod specific gene- neural retina leucine zipper (NRL). B. Retinal degeneration due to a gene expressed outside the eye- ornithine amino-transferase (OAT). C. Retinal degeneration due to an RPE specific gene- RPE65. D. Retinal degeneration due to acquired infection - rubella. These different phenotypes would require different treatments based on the underlying genetic background and cell types needing to be replaced.
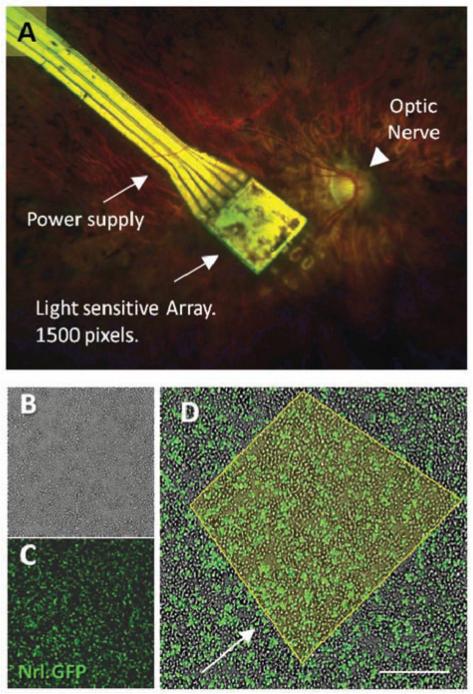
The clinical relevance of the question whether to transplant single cells or a retinal matrix is central not only for integration but also when considering the visual resolution expected after cell replacement. Clinical trials carried out with electronic retinal implants may offer important transferable lessons for the design of cell replacement therapies. In recent clinical trial [9], an electronic microchip was transplanted subretinally under a human retina, recovering a degree of vision to a blind patient. The electronic prosthesis was implanted subretinally to replace the degenerate photoreceptor of the outer nuclear layer, similar to the prospective site of integration for cells in cell replacement therapies. Visual rescue in this trial implies that downstream processing of vision can be achieved - the electronic microchip transfers light signals to adjacent bipolar cells remaining in the retina to transfer the signal through intact pathways to the visual cortex. This can be compared to transplantation of single cells into the retina; in both cases the upper limit of spatial resolution obtained by treatment would be limited by the density of bipolar cells remaining in the patient’s retina (Fig. 4). When transplanting a retinal matrix, however, where photoreceptors are transplanted accompanied by their ocular environment, developing bipolar cells could form connections with photoreceptors pre-transplantation through ribbon synapses that include horizontal cells and therefore include lateral inhibition. Hence visual resolution would depend on the density of transplanted cells and not relay on the remaining structure of bipolar cells in the degenerate retina. Indeed this may also be possible with electronic devices, if a form of lateral inhibition can be programmed into the light sensing diodes.
Fig. (4).
A. Subretinal electronic implant in a human degenerate retina. The retinal implant device (Retina Implant AG, Reutlingen, Germany) is a light-sensitive electronic chip with 1,500 pixel-generating elements located under the retina in contact with bipolar cells and powered via a subretinal cable. B. Dissociated retinal cells from early postnatal (PN3) Nrl.GFP mouse retina in culture. C. florescence microscopy image of retinal dissociation, florescent cells are rod photoreceptor precursors expressing Nrl. D. Merged image; Nrl.GFP cells in the in vitro cultured retina. Yellow outline represents an area of light sensitive cells, corresponding to the 1,500 pixels transmitted by the electronic chip. Scale bar 30μm. Observations of restored vision in patients with the electronic retinal implant are proof of concept for how an iPS cell-generated photoreceptor monolayer might similarly reverse blindness in retinitis pigmentosa (RP). The cell-therapy approach however has the advantage of generating energy from the oxidation of glucose and would not therefore require an external power supply.
Animal studies of retinal sheet engraftment may also provide proof of principle and provide perspective; In studies conducted in rodents and rabbits, after subretinal transplantation of embryonic or early neonatal whole retinal sheets into a host retina, retinal grafts were shown to differentiate and survive long-term [59-61] and reported to form connections with neurons, restoring a degree of visual response in the degenerate host retina [62]. The development of a human optic cup from iPSc mentioned above, may allow researchers to consider iPSc derived retinal grafts for cell replacement therapies of hereditary retinal diseases in place of single cell transplants.
MODELING RETINAL DEVELOPMENT AND DEGENERATION USING iPSc: “DISEASE IN A DISH”
Underlying genetic causes for retinal degenerations are extremely heterogeneous. In RP alone, mutations have been discovered in close to 200 genes (see database in RetNet https://sph.uth.tmc.edu/retnet/sum-dis.htm) [63]. While the biological defects and severity of RP are extremely varied, they are encompassed under the same title as they share overlapping phenotypic expression and are characterized by the degeneration of rod photoreceptors and the potential subsequent secondary degeneration of cone photoreceptors, loss of lamination of the inner retina and invasion of RPE cells into the neural retina [64] eventually leading to loss of visual field properties and night blindness due to loss of rods or in some cases complete blindness following secondary loss of cone photoreceptors. Genetic heterogeneity implies that diverse mechanisms are at the base of the different subtypes of the disease; making drug treatment extremely difficult to fit to a patient whose underlying genetic causes for the disease are unknown, as patients with different underlying mutations may require different drugs [19]. Furthermore, the vast number of genetic causes and expression patterns implies that animal models of RP will unavoidably lack in translational characteristics for the different subtypes of the disease.
iPSc have been successfully generated from patients in a number of diseases, including Parkinson’s disease [65], sickle cell anemia [66], amyotrophic lateral sclerosis (ALS) [67], catecholaminergic polymorphic ventricular tachycardia (CPVT) [68], muscular dystrophy and Huntingdon’s disease [69]. Patient-specific iPSc are likely to be extremely beneficial in the retinal line of research as they allow in vitro recapitulation of the patient’s disease phenotype for drug discovery, drug screening or gene-therapy optimization.
In 2011, Jin et al. [19] reprogrammed fibroblasts from five patients with identified mutations in the RP1, RP9, PRPH2 or RHO genes, and proceeded to differentiate these patient-specific iPSc into mature rod photoreceptors (Rho+/Nrl+) to model retinal degeneration in vitro. In this study, rods were generated in-vitro by day 120, in accordance with differentiation of human iPSc in previous studies (e.g [40]), but unlike photoreceptors derived from healthy human iPSc, in these patient-specific iPSc, a significant reduction of cells expressing Rho+ was evident by in vitro day 150. iPSc derived rods exhibited oxidation or endoplasmic reticulum stress in culture after differentiation and interestingly, photoreceptor cells derived from patients with different mutations responded differently to antioxidant drugs, sustaining the notion that drug treatment for RP must be considered in the context of causative genetic mutation. Later, the same group achieved a model of RP, using a non integrating Sendai-virus vector [42], demonstrating for the first time the possibility of disease modeling with integration free patient specific iPSc.
Pluripotent cells derived from skin cells of patients with RP have also been used recently to conduct genetic analysis after exome sequencing and lead to identification of the cilia-related gene male germcell-associated kinase (MAK) as a cause RP [70]. The ability to recapitulate disease phenotype and screen drugs in vitro may be a step in the direction of personalized medicine for retinal disorders and indeed be a model for other CNS diseases. The ability to manipulate patient specific cells in culture conditions would benefit not only drug development and screening, but could be used as an ex-vivo model in which patient-specific iPSc would be differentiated, treated and transplanted back into the patient.
EX VIVO GENE CORRECTION
Modeling of disease-specific and patient-specific retinal degeneration provides evidence to show that genetic mechanisms at play in vivo in inherited retinal degeneration are mimicked in vitro after cell reprogramming. This provides empirical evidence of the basic defining barrier to patient specific cell replacement - that cells derived from patients afflicted with retinal disease will inevitably express the same genetic phenotype. However, modeling retinal degeneration can be a step towards crossing this barrier, as patient specific autologous cells could undergo ex vivo gene correction in culture to produce healthy tissue for cell replacement therapy. IPSc generated from patients with identified genetic mutations or whose cellular degeneration has been studied in vitro, could be corrected and used to produce healthy cells for retinal transplantation. Nonetheless, the varied inherited genetic mutations involved in retinal diseases will undoubtedly present a challenge in the development of gene correction for retinal disease. Furthermore, transplantation of iPSc derived cells that have also undergone gene therapy will require overcoming additional regulatory restrictions when seeking approval for clinical trials.
Retinal degenerations that result in sight loss in late adulthood may still be amenable to iPS cell treatments in which the gene has not been corrected. Whilst transplanting non-corrected cells would not ideal, these cells may still survive for years post transplantation. This might be the case for instance in late onset Stargardt disease or in mild rhodopsin mutations, where the degeneration is relatively slow and new cells may possibly temporarily recover a degree of vision, while harboring the genetic mutation.
Interestingly, there may be an exception to the need for gene correction of iPSc in a small population of RP patients. A number of patients suffering from RP have been reported to have another genetic condition- somatic mosaicism [71, 72], a condition in which a single individual may carry different genotypes in various cell populations. So potentially, healthy photoreceptors could be derived from iPSc generated from somatic cells which are not afflicted with the mutation causing RP [20]. While this would pose an intriguing alternative for a few patients, certainly this condition is not the norm and the majority of individuals suffering from RP and other forms of hereditary visual loss would require gene correction before transplantation of autologous iPSc. Similarly in mitochondrial disease, heteroplasmy may allow the generation of cells with a larger proportion of non-defective mitochondria which are resistant to degeneration, although purifying these cells is likely to be challenging.
Ex vivo gene correction has been achieved in a mouse model of sickle cell anemia [73], where mice were rescued after transplantation of corrected autologous iPSc derived hematopoietic progenitors. Gene specific targeting of the sickle hemoglobin allele was carried out by use of retrovirus, providing notable proof of concept for such a procedure in a clinical setting. This pioneering study sets the stage for development of autologous corrected iPSc for transplantation in humans afflicted with genetic diseases, although the method of gene correction would need to be reconsidered for a clinical setting. Retroviral vectors introduce external DNA into the cells, therefore presenting the hazard of inserting novel variation and mutagenesis into the genome while attempting to correct the target gene. Development of genetic therapies for patient-specific or disease-specific iPSc should attempt to use techniques that preserve genomic integrity and do not introduce oncogenic or foreign material into cells. In order to obtain a full picture of the genomic structure and integrity of iPSc, we should consider the induction steps cells go through prior to being differentiated and the advances and challenges associated with cell reprogramming.
PROCEEDINGS AND CHALLENGES IN REPROGRAMMING TECHNOLOGY
In the early embryo, pluripotent stem cells can differentiate into all types of somatic cells. Every nucleated cell in the body (excluding gametes and some immunological cells) contains the same genomic DNA, and different cells types gain their unique characteristics by sequential regulation of distinct genes during development. These epigenetic changes to chromosomal DNA regulate access of transcription factors to specific genes, providing appropriate molecular switches required for cell division, growth and finally specific cell function [53]. Adult somatic cells can thus be reprogrammed to regain stem-cell like pluripotency through over-expression of defined transcription factors.
In a milestone study by Takahashi and Yamanaka in 2006 [2], mouse somatic cells were first reprogrammed by over-expression of four transcription factors, namely oc-tamer-binding transcription factor-3/4 (OCT3/4), SRY-related high-mobility-group (HMG)-box protein-2 (SOX2), c-MYC and Kruppel-like factor-4 (KLF4), followed by generation of iPSCs from human adult fibroblast cells using the same four transcription factors [74]. Although the original protocol produces the most robust reprogramming, the omission of the potentially oncogenic factor c-Myc from reprogramming strategies reduced the risk of tumor formation [75], potentially facilitating use of these cells in a clinical setting [53]. Reprogramming of immature fibroblasts can be successfully achieved with only the transcription factors OCT4 and Sox2 [76], although the clinical relevance of this is low, since most retinal degenerative diseases occur in an older age and iPSc should ideally be optimized to be produces from adult skin. A recent study [77], suggests that retroviral transduction of these four factors increases levels of reactive oxygen species (ROS), leading to DNA damage and subsequent activation of p53, a tumor suppressor that is in charge of stimulation of aging and apoptosis in the cell. Indeed in this study, these cell cycle phases were overrepresented. These results align with the well established outcome that downregulation of p53 enhances reprogramming of both mouse and human somatic cells [60, 78-84]. While the most efficient method of reprogramming adult differentiated cells into pluripotent stem cells is sought, it is important, especially for clinical purposes, to consider a delicate balance of the genome. As p53 is a DNA damage response gene [85], by its downregulation, enhanced reprogramming may result in DNA damage and genomic instability [86]. Accordingly, variations in cell reprogramming protocols have been largely explored, mostly with the aim of reducing the viral induction of potentially tumorogenic factors, namely c-Myc and KLF4 into the genome [87-94].
Retro- and lentiviruses have largely been the main delivery method chosen to overexpress reprogramming factors to induce pluripotency efficiently in somatic cells, but as previously mentioned; these methods have a drawback of integrating foreign DNA into the reprogrammed cell genome, thus presenting a threat of unpredictable effects of random DNA integration. Therefore, methods to reduce or avoid viral integration have been sought. Replacing viral vectors with non integrating plasmids [95, 96], episomal vectors [97, 98], excisional techniques [99, 100], direct delivery of reprogramming protein [101], miRNA [102], and minicircle DNA [103] to name a few. A single leading robust non-integrating reprogramming technique has yet to ascend, but accomplishments in cell reprogramming by non-integrating delivery methods are imperative stepping stones in the path to obtain safe and efficient iPSc for research and therapy.
Recent research has indicated that autologous iPSc produce an immune response, and transplanted undifferentiated autologous iPSc were rejected by matched host mice even when pluripotency was induced using a non-integrating episomal vector, although to a lesser degree [104] this important observation should be considered in generation of iPSc derived cells for cell replacement research, yet more research is required in order to determine whether the same immune response would be evident in iPSc derived photoreceptor precursor cells. Generation and potential use of iPSc for clinical trials will be largely influenced by the genomic state of differentiated iPSc, as genomic instability or threat of tumor formation may render cells inappropriate for both clinical-research and transplantation in patients. Genomic integrity of iPSc has been questioned and widely reviewed [for recent review see [76] & [105] for its high relevance to clinical trial initiation. While chromosomal abnormalities [106] and mitochondrial genome mutations [107] have been observed in iPSc, these aberrations have yet to be linked with practical deficiencies or compromise cellular function [105].
EPIGENETIC MEMORY
A further interesting avenue to consider is the somatic source from which iPSc should be obtained. The notion of ‘epigenetic memory’ questions whether the origin of reprogrammed cells imprints the differentiation pattern and efficacy of resulting iPSc. This phenomenon has been suggested to occur in iPSc, regardless of whether integrating or non-integrating vectors were used [108] as lingering fibroblast genes have been detected in iPSc [109] and neural genes were found in reprogrammed neural stem cells when reprogrammed without use of integrating vectors [110]. Importantly, iPSc have been reported to differentiate back to the original lineage from which it had been derived [111]; this property of iPSc could be utilized for development of specific iPSc from favorable somatic tissue.
Hu et al. [112], studied the epigenetic imprint of iPSc derived from RPE by testing these cells tendency to re-differentiate into RPE without being prompted to do so. In their report, some reprogrammed cells did indeed retain memory of their previous state and spontaneously differentiated to form pigmented RPE cells. Such differentiation preference may be put to use in the future to create human disease-specific iPSc from different somatic lineages. However, the idea of epigenetic memory remains a matter for debate. It has been suggested that there is no intrinsic difference in the pluripotency state or iPSc as appose to ESc, but rather that different laboratories are more attune to certain results according to the cells that they are accustomed to work with [113]. Conversely, Ruiz et al. [114] studied seventeen human iPSc lines, derived from six different cell types and found epigenetic aberrations in all cell lines, regardless of cell source. Studying the existence of a genetic imprint in reprogrammed cells could be clinically relevant and also help understand the genomic structure of these cells and how they compare to ESc.
MAINTAINING iPSc DERIVED RETINAL CELLS FOR CLINICAL USE
A central question in the translation of iPSc to clinical trials is the feasibility of manufacturing cells in a safe and stable manner for clinical trials. Human rod photoreceptor differentiation requires long in vitro culture periods, and may take roughly 80 days to differentiate photoreceptorprogenitors from iPSc [27, 46] and in the order of 100-120 days to produce mature photoreceptors [19, 40, 57]. This is a costly, time consuming and delicate procedure that must be closely monitored throughout all stages to ensure viability and safety of the final cell product. As an alternative, after initial culture, large-stocks could be cryopreserved and delivered in frozen aliquots for short term additional culture and generation of newly differentiated cells to produce storable neural retina generated from human iPSc [55] for use in research and clinical trials.
The potential of safely storing iPSc would provide the possibility of maintaining personalized human leukocyte antigen (HLA)-haplotype matched cell lines in iPSc banks [115, 116] similar to banks formerly suggested for Esc [117]. Such banks could reduce the financial resources and time required to produce clinically suitable patient-specific or immune-matched cells, by maintaining differentiated and purified tissue for immunologically-personalized transplantation. However, several hurdles still need to be crossed in order to produce clinical-grade preserved iPSc. Recovery of human iPSc after cryopreservation is still underway and thawed cells have been reported to demonstrate compromised cloning efficacy and reduced cell survival [118]. A number of groups have been working to improve cell survival and efficacy after cryopreservation using Rho-kinase (ROCK) inhibitors [115, 119, 120] but there is still need for improvement of cryopreservation protocols before this strategy can be applied [78] and regulatory approval is required before crayopreserved human iPSc are used in clinical settings.
CONCLUSION
In the short time since the development of iPSc, a great wealth of research has been generated in the field. The potential to produce and expand and renewable source of human cells is revolutionary for both medicine and basic research. Scientific researchers are making commendable progress in developments and characterization of iPSc derived cells; however, these advances have not yet been translated into iPSc clinical trials.
The key barrier standing before iPS cell translation into the clinic is the genomic state and broad safety of these cells. Risk of proliferative cells dividing after transplantation and inducing malignant tumors limits their use in human clinical trials, at least until genomic structure of iPSc is better understood and methods of reprogramming and gene correction which are both viable and safe are accomplished. Furthermore, cell purification techniques could not only be used as a tool for depletion of target cells from tumorogenic factors, but indeed be used to enrich the transplanted or investigated cell population. In the interim, much may be learned from research and clinical trials carried out with embryonic stem cells, where post-transplant immune suppression may be removed to impede tumor formation. While transplantation studies are a stimulating and central avenue for the use of reprogrammed iPSc, their potential use in scientific studies is no less remarkable; disease-specific and patient-specific iPSc may revolutionize our understanding of development and disease and may provide imperative insight into treatment of retinal degeneration.
ACKNOWLEDGEMENTS
This research was supported by the NIHR Biomedical Research Centres at the Oxford Radcliffe Trust and Moor-fields Eye Hospital, the Medical Research Council, the Wellcome Trust, the Health Foundation, Fight for Sight (UK), the Lanvern Foundation, the Royal College of Surgeons of Edinburgh and the University of Oxford Clarendon Fund.
LIST OF ABBREVIATIONS
- AMD
Age-related Macular Degeneration
- CNS
Central Nervous System
- ESc
Embryonic Stem cells
- FACS
Fluorescence-Activated Cell Sorting
- hESc
human Embryonic Stem cells
- iPSc
Induced Pluripotent Stem cells
- MACS
Magnetic-Activated Cell Sorting
- mESc
Mouse Embryonic Stem cells
- Rho
Rhodopsin
- RP
Retinitis Pigmentosa
- RPE
Retinal Pigment Epithelium
Footnotes
CONFLICT OF INTEREST The author(s) confirm that this article content has no conflict of interest.
PATIENT CONSENT Declared none.
REFERENCES
- [1].Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962;4:256–73. doi: 10.1016/0012-1606(62)90043-x. [DOI] [PubMed] [Google Scholar]
- [2].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [3].MacLaren RE. Re-establishment of visual circuitry after optic nerve regeneration. Eye. 1999;13:277–284. doi: 10.1038/eye.1999.77. [DOI] [PubMed] [Google Scholar]
- [4].Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010;190:953–63. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].MacLaren RE, Taylor JS. Regeneration in the developing optic nerve: correlating observations in the opossum to other mammalian systems. Prog Neurobiol. 1997;53(3):381–98. doi: 10.1016/s0301-0082(97)00041-5. [DOI] [PubMed] [Google Scholar]
- [6].Stone JL, Barlow WE, Humayun MS, de Juan E, Jr, Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110:1634. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- [7].Vidal-Sanz M, Bray GM, Villegas-Pérez MP, et al. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987;7:2894–909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whiteley SJ, Sauvé Y, Avilés-Trigueros M, et al. Extent and duration of recovered pupillary light reflex following retinal ganglion cell axon regeneration through peripheral nerve grafts directed to the pretectum in adult rats. Exp Neurol. 1998;154:560–72. doi: 10.1006/exnr.1998.6959. [DOI] [PubMed] [Google Scholar]
- [9].Zrenner E, Bartz-Schmidt KU, Benav H, et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci. 2011;278(1711):1489–97. doi: 10.1098/rspb.2010.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- [11].Singh MS, Charbel Issa P, Butler R, et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. PNAS; 2013. Published online before print, January 3. http://www.pnas.org/content/110/3/1101.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx- deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embry- onic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–35. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- [14].Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta Stone. Neuron. 2011;70(4):597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- [15].Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–20. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- [16].Singh MS, MacLaren RE. Stem cells as a therapeutic tool for the blind: biology and future prospects. Proc Biol Sci. 2011;278(1721):3009–16. doi: 10.1098/rspb.2011.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Michalakis S, Muhlfriedel R, Tanimoto N, et al. Restoration of Cone Vision in the CNGA3-/- Mouse Model of Congenital Complete Lack of Cone Photoreceptor Function. Mol Ther. 2010;18:2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [19].Jin ZB, Okamoto S, Osakada F, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PloS one. 2011;6(2):e17084. doi: 10.1371/journal.pone.0017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jin ZB, Okamoto S, Mandai M, Takahashi M. Induced pluripotent stem cells for retinal degenerative diseases: a new perspective on the challenges. J Genet. 2009;88(4):417–24. doi: 10.1007/s12041-009-0063-5. [DOI] [PubMed] [Google Scholar]
- [21].Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- [22].Sahel JA, Mohand-Said S, Léveillard T, et al. Rod-cone interdependence: implications for therapy of photoreceptor cell diseases. Prog Brain Res. 2001;131:649–61. doi: 10.1016/s0079-6123(01)31051-8. [DOI] [PubMed] [Google Scholar]
- [23].Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest. Ophthalmol Vis Sci. 1996;37:1236–49. [PubMed] [Google Scholar]
- [24].Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–91. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- [25].Furukawa T, Kozak CA, Cepko CL. Rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci U.S.A. 1997;94:3088–93. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–41. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- [27].Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009;106:16698–703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, et al. Dickkopf1 is required for embryonic head induction and limb morpho-genesis in the mouse. Dev Cell. 2001;1:423–34. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- [29].Bachiller D, Klingensmith J, Kemp C, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–61. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- [30].Lamb TM, Knecht AK, Smith WC, et al. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–18. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- [31].Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–9. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- [32].Hemmati-Brivanlou A, Kelly OG, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus laevis. Cell. 1994;77:283–95. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- [33].Pera EM, Wessely O, Li SY, De Robertis EM. Neural and head induction by insulin-like growth factor signals. Dev Cell. 2001;1:655–65. doi: 10.1016/s1534-5807(01)00069-7. [DOI] [PubMed] [Google Scholar]
- [34].Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–23. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- [35].Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:11331–6. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–24. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- [37].Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–24. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- [38].Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769–74. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–31. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- [40].Osakada F, Sasai Y, Takahashi M. Control of neural differentiation from pluripotent stem cells. Inflamm Regen. 2008;28:166–73. [Google Scholar]
- [41].Melyan Z, Tarttelin EE, Bellingham J, et al. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–5. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- [42].Jin ZB, Okamoto S, Xiang P, Takahashi M. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Transl Med. 2012;1(6):503–9. doi: 10.5966/sctm.2012-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27(10):2427–34. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- [44].Tucker BA, Park IH, Qi SD, et al. Transplantation of Adult Mouse iPS Cell-Derived Photoreceptor Precursors Restores Retinal Structure and Function in Degenerative Mice. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhou L, Wang W, Liu Y, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem cells. 2011;29(6):972–80. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PloS one. 2010;5(1) doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Carr A-J, Vugler AA, Hikita ST, et al. Protective Effects of Human iPS-Derived Retinal Pigment Epithelium Cell Transplantation in the Retinal Dystrophic Rat. PLoS ON. 2009;4(12) doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Westenskow PD, Moreno SK, Krohne TU, et al. Using flow cytometry to compare the dynamics of photoreceptor outer segment phagocytosis in iPS-derived RPE cells. Invest Ophthalmol Vis Sci. 2012;53(10):6282–90. doi: 10.1167/iovs.12-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pearson RA, Barber AC, West EL, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19:487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yao J, Tao SL, Young MJ. Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering. Polymers. 2011;3(2):899–914. [Google Scholar]
- [51].Jeffery G. The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci. 1997;20:165–9. doi: 10.1016/s0166-2236(96)10080-1. [DOI] [PubMed] [Google Scholar]
- [52].Gosens I, den Hollander AI, Cremers FP, Roepman R. Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res. 2008;86:713–26. doi: 10.1016/j.exer.2008.02.005. [DOI] [PubMed] [Google Scholar]
- [53].Comyn O, Lee E, MacLaren RE. Induced pluripotent stem cell therapies forretinal disease. Curr Opin Neurol. 2010;23(1):4–9. doi: 10.1097/WCO.0b013e3283352f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bartsch U, Oriyakhel W, Kenna PF, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86:691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- [55].Eberle D, Kurth T, Santos-Ferreira T, Wilson J, Corbeil D, Ader M. Outer segment formation of transplanted photoreceptor precursor cells. PloS one. 2012;7(9) doi: 10.1371/journal.pone.0046305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Redenti S, Neeley WL, Rompani S, et al. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30(20):3405–14. doi: 10.1016/j.biomaterials.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nakano T, Ando S, Takata N, et al. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell. 2012;10(6):771–85. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- [58].Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- [59].Seiler MJ, Aramant RB. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest. Ophthalmol Vis Sci. 1998;39:2121–31. [PubMed] [Google Scholar]
- [60].Ghosh F, Johansson K, Ehinger B. Long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999;40:133–42. [PubMed] [Google Scholar]
- [61].Zhang Y, Arner K, Ehinger B, Perez MT. Limitation of anatomical integration between subretinal transplants and the host retina. Invest Ophthalmol Vis Sci. 2003;44:324–31. doi: 10.1167/iovs.02-0132. [DOI] [PubMed] [Google Scholar]
- [62].Seiler MJ, Thomas BB, Chen Z, Wu R, Sadda SR, Aramant RB. Retinal transplants restore visual responses: trans-synaptic tracing from visually responsive sites labels transplant neurons. Eur J Neurosci. 2008;28:208–20. doi: 10.1111/j.1460-9568.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- [63].Kaplan HJ, Fernandez de Castro JP. Retinal regeneration and stem cell therapy in retinitis pigmentosa. Taiwan J Ophthalmol. 2012;2(2):41–4. [Google Scholar]
- [64].Li ZY, Possin DE, Milam AH. Histopathology of bone spicule pigmentation in retinitis pigmentosa. Ophthalmology. 1995;102:805–16. doi: 10.1016/s0161-6420(95)30953-0. [DOI] [PubMed] [Google Scholar]
- [65].Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ye L, Chang JC, Lin C, Sun X, Yu J, Kan YW. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc Natl Acad Sci USA. 2009;106:9826–30. doi: 10.1073/pnas.0904689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- [68].Itzhaki I, Maizels L, Huber I, et al. Modeling of Catecholaminergic Polymorphic Ventricular Tachycardia With Patient-Specific Human-Induced Pluripotent Stem Cells. J Am Coll Cardiol. 2012;60(11):990–1000. doi: 10.1016/j.jacc.2012.02.066. [DOI] [PubMed] [Google Scholar]
- [69].Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tucker BA, Scheetz TE, Mullins RF, et al. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germcell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2011;108:E569–76. doi: 10.1073/pnas.1108918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Schwartz SB, Aleman TS, Cideciyan AV, Swaroop A, Ja- cobson SG, Stone EM. De novo mutation in the RP1 gene (Arg677ter) associated with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:3593–7. doi: 10.1167/iovs.03-0155. [DOI] [PubMed] [Google Scholar]
- [72].Jin ZB, Gu F, Matsuda H, Yukawa N, Ma X, Nao-i N. Somatic and gonadalmosaicism in X-linked retinitis pigmentosa. Am J Med Genet A. 2007;143:2544–2548. doi: 10.1002/ajmg.a.31984. [DOI] [PubMed] [Google Scholar]
- [73].Hanna J, Wernig M, Markoulaki S, et al. Treatment of mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- [74].Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- [75].Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- [76].Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–75. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- [77].Mah N, Wang Y, Liao MC, et al. Molecular insights into reprogramming-initiation events mediated by the OSKM gene regulatory network. PLoS One. 2011;6:e24351. doi: 10.1371/journal.pone.0024351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Drews K, Jozefczuk J, Prigione A, Adjaye J. Human induced pluripotent stem cells-from mechanisms to clinical applications. J Mol Med. 2012;90(7):735–45. doi: 10.1007/s00109-012-0913-0. [DOI] [PubMed] [Google Scholar]
- [79].Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kawamura T, Suzuki J, Wang YV, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–4. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li H, Collado M, Villasante A, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–9. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Marion RM, Strati K, Li H, et al. A p53- mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–53. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Utikal J, Polo JM, Stadtfeld M, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–8. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhao Y, Yin X, Qin H, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–9. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [85].Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA. 1992;89:7491–5. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pasi CE, Dereli-Öz A, Negrini S, et al. Genomic instability in induced stem cells. Cell Death Differ. 2011;18(5):745–53. doi: 10.1038/cdd.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Carey BW, Markoulaki S, Hanna J, et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci U S A. 2009;106:157–62. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human- induced pluripotent stem cells. Nat Protoc. 2008;3:1180–6. doi: 10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]
- [89].Welstead GG, Brambrink T, Jaenisch R. Generating iPS cells from MEFS through forced expression of Sox-2, Oct-4, c-Myc, and Klf4. J Vis Exp. 2008;14:734. doi: 10.3791/734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- [91].Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- [92].Sommer CA, Stadtfeld M, Murphy GJ, et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–9. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shao L, Feng W, Sun Y, et al. Generation of iPS cells using defined factors linked via the self-cleaving 2A sequences in a single open reading frame. Cell Res. 2009;19:296–306. doi: 10.1038/cr.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gonzalez F, Barragan Monasterio M, Tiscornia G, et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci U S A. 2009;106:8918–22. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- [96].Cheng L, Hansen NF, Zhao L, et al. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10(3):337–44. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- [98].Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–5. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, Wu JC. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc. 2011;6(1):78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- [105].Pasi CE, Dereli-Öz A, Negrini S, et al. Genomic instability in induced stem cells. Cell Death Differ. 2011;18(5):745–53. doi: 10.1038/cdd.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–31. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- [107].Prigione A, Hossini AM, Lichtner B, et al. Mitochondrial-associated cell death mechanisms are reset to an embryonic-like state in aged donor-derived iPS cells harboring chromosomal aberrations. PLoS One. 2011;6:e27352. doi: 10.1371/journal.pone.0027352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wolfrum K, Wang Y, Prigione A, Sperling K, Lehrach H, Adjaye J. The LARGE principle of cellular reprogramming: lost, acquired and retained gene expression in foreskin and amniotic fluid-derived human iPS cells. PLoS One. 2010;5:e13703. doi: 10.1371/journal.pone.0013703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Chin MH, Mason MJ, Xie W, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–23. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional signature and memory retention of human induced pluripotent stem cells. PLoS One. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ohi Y, Qin H, Hong C, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol. 2011;13:541–9. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hu Q, Friedrich AM, Johnson LV, Clegg DO. Memory in induced pluripotent stem cells: reprogrammed human retinal- pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells. 2010;28:1981–91. doi: 10.1002/stem.531. [DOI] [PubMed] [Google Scholar]
- [113].Guenther MG, Frampton GM, Soldner F, Hockemeyer D, Mitalipova M, Jaenisch R, Young RA. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell. 2010;7:249–57. doi: 10.1016/j.stem.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ruiz S, Diep D, Gore A, et al. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109(40):16196–201. doi: 10.1073/pnas.1202352109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nat Biotechnol. 2008;26:739–40. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- [116].Gourraud PA, Gilson L, Girard M, Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells. 2012;30(2):180–6. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- [117].Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2008;366(9502):2019–25. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- [118].Rizzino A. Stimulating progress in regenerative medicine: improving the cloning and recovery of cryopreserved human pluripotent stem cells with ROCK inhibitors. Regen Med. 2010;5:799–807. doi: 10.2217/rme.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ichikawa H, Nakata N, Abo Y, et al. Gene pathway analysis of the mechanism by which the Rho-associated kinase inhibitor Y-27632 inhibits apoptosis in isolated thawed human embryonic stem cells. Cryobiology. 2012;64:12–22. doi: 10.1016/j.cryobiol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- [120].Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580–9. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]