Abstract
Licensed human papillomavirus (HPV) vaccines, based on virus-like particles (VLPs) self-assembled from major capsid protein L1, afford type-restricted protection against HPV types 16/18/6/11 (or 16/18 for the bivalent vaccine), which cause 70% of cervical cancers (CxCas) and 90% of genital warts. However, they do not protect against less prevalent high-risk (HR) types causing 30% of CxCa, or cutaneous HPV. In contrast, vaccination with the minor capsid protein L2 induces low-level immunity to type-common epitopes. Chimeric RG1-VLP presenting HPV16 L2 amino acids 17–36 (RG1 epitope) within the DE-surface loop of HPV16 L1 induced cross-neutralizing antisera. We hypothesized that RG1-VLP vaccination protects against a large spectrum of mucosal and cutaneous HPV infections in vivo. Immunization with RG1-VLP adjuvanted with human-applicable alum-MPL (aluminum hydroxide plus 3-O-desacyl-4′-monophosphoryl lipid A) induced robust L2 antibodies (ELISA titers 2,500–12,500), which (cross-)neutralized mucosal HR HPV16/18/45/37/33/52/58/35/39/51/59/68/73/26/69/34/70, low-risk HPV6/11/32/40, and cutaneous HPV2/27/3/76 (titers 25–1,000) using native virion- or pseudovirion (PsV)-based assays, and a vigorous cytotoxic T lymphocyte response by enzyme-linked immunospot. In vivo, mice were efficiently protected against experimental vaginal challenge with mucosal HR PsV types HPV16/18/45/31/33/52/58/35/39/51/59/68/56/73/26/53/66/34 and low-risk HPV6/43/44. Enduring protection was demonstrated 1 year after vaccination. RG1-VLP is a promising next-generation vaccine with broad efficacy against all relevant mucosal and also cutaneous HPV types.
INTRODUCTION
Human papillomaviruses (HPVs) are species-specific, epitheliotropic DNA viruses with over 120 types completely characterized today (Bernard et al., 2010). Infections are widespread and induce lesions from benign papilloma to intraepithelial neoplasia to carcinoma, the latter representing 5% (Parkin et al., 2005) of the global cancer burden. Persistent infection with a subset of mucosal HPVs causes high-grade intraepithelial neoplasias that eventually progress to invasive cancer of the cervix (CxCa) and of other anogenital and oropharyngeal sites. More than 15 high-risk (HR) types are found in almost all CxCas, causing 250,000 deaths in women worldwide every year (Munoz et al., 2004; de Sanjose et al., 2010). Low-risk (LR) mucosal HPVs cause anogenital warts in 30 million patients. Common cutaneous HPVs induce skin warts with substantial impact on high-prevalence groups such as children or immunocompromised individuals (van Haalen et al., 2009; Lally et al., 2011). Moreover, a distinct group of cutaneous (genus β) types have been implicated as subsidiary causative factor of nonmelanoma skin cancers, although this remains controversial for those other than patients with the rare genodermatosis Epidermodysplasia verruciformis (Karagas et al., 2006; Bouwes Bavinck et al., 2008). Recombinantly expressed major capsid protein L1 of papillomaviruses self-assembles into virus-like particles (L1-VLPs) (Kirnbauer et al., 1992, 1993; Hagensee et al., 1993; Rose et al., 1993; Suzich et al., 1995) that have been introduced as prophylactic vaccines. Both (bivalent Cervarix and quadrivalent Gardasil) licensed vaccines comprise VLPs of HR HPV16/18, which cause 70% of cervical disease. One (quadrivalent) vaccine additionally contains VLPs of LR HPV6/11, causing 90% of genital warts. Prophylactic vaccination confers enduring yet predominantly vaccine type–restricted protection mediated by high-titer neutralizing antibodies. To target HPVs responsible for the remaining third of CxCas (Munoz et al., 2004), a nonavalent vaccine including 7 HR types is in clinical trials (http://clinicaltrials.gov). However, the complexity of such a vaccine is unlikely to reduce the already very high costs of current HPV vaccines, which may impede delivery to the developing world with the highest CxCa burden. Furthermore, a vaccination strategy against the many types causing skin papillomas (warts) has not been established (Handisurya et al., 2009; Senger et al., 2009). Papillomavirus minor capsid protein L2–based immunogens represent an alternative strategy to multivalent L1-VLP vaccines. The amino (N) terminus of L2 contains highly conserved motifs that are buried in native virions and become exposed only shortly during the infectious process. However, immunization with L2 peptides alone can induce low-titer antibodies, which mediate cross-neutralization (Christensen and Kreider, 1991; Kawana et al., 1999; Roden et al., 2000; Pastrana et al., 2005) in vitro and cross-protection in animal models in vivo (Chandrachud et al., 1995; Gambhira et al., 2007a). An HPV16 L2 peptide comprising amino acids (aa) 17–36 is a broadly cross-neutralization B-cell epitope recognized by mAb RG1 (Gambhira et al., 2007b). Owing to its essential role for viral infectivity and its high conservation within many types, the RG1 epitope may represent an attractive target to develop a broad-spectrum HPV vaccine. We have previously introduced chimeric RG1-VLP as a possible strategy to improve immunogenicity of the RG1 epitope by its genetic insertion into the immunogenic DE-surface loop of HPV16 L1 (Slupetzky et al., 2007; Kondo et al., 2008; Schellenbacher et al., 2009; Caldeira Jdo et al., 2010). Upon expression as a recombinant fusion protein, assembly into capsids repetitively displaying RG1 epitopes on the capsid surface (RG1-VLP) is highly efficient. Vaccination induced high-titer neutralizing antibodies against HPV16 and improved L2-specific antibodies. Then, available limited in vitro assays demonstrated cross-neutralization of mucosal HR HPV18/31/45/52/58, LR HPV HPV6/11, and a single genus β-type (HPV5) (Schellenbacher et al., 2009). This study comprehensively examines RG1-VLP vaccine efficacy to cross-protect against all relevant mucosal HR HPVs in vivo and in vitro, endurance of protection (an important issue for L2-based vaccine development), and induction of cell-mediated immunity. Vaccine efficacy against natural infection was validated using authentic virion-based neutralization assays.
RESULTS
We have shown previously that vaccination of rabbits and mice with recombinant RG1-VLP plus alum-MPL (aluminum hydroxide plus 3-O-desacyl-4′-monophosphoryl lipid A) adjuvant (Schellenbacher et al., 2009) elicited high-titer neutralizing antibodies to HPV16 and cross-neutralizing antibodies to pseudovirion (PsV) of the limited number of then available mucosal HR HPV18/31/45/52/58, LR HPV6/11, and cutaneous β HPV5.
RG1-VLP vaccination induces a robust antibody response against the L2 epitope
To assess the robustness of the humoral immune responses to RG1-VLP, eight additional rabbits were vaccinated either 4 or 3 times (New Zealand White (NZW) nos. 1–6: 4 × 50 μg and nos. 7–10: 3 × 20 μg) and sera drawn 2 weeks after the last boost. Robust antibody responses to L2 (titers of 2,500–12,500) were detected for both vaccination protocols using the 16L2 N-terminal peptide (aa 11–200) as ELISA antigen (Supplementary Material online). Conversely, reactivity was absent for rabbit antiserum to HPV16 wild-type L1-VLP as expected.
Antisera to RG1-VLP neutralize distantly related mucosal HPV types in vitro
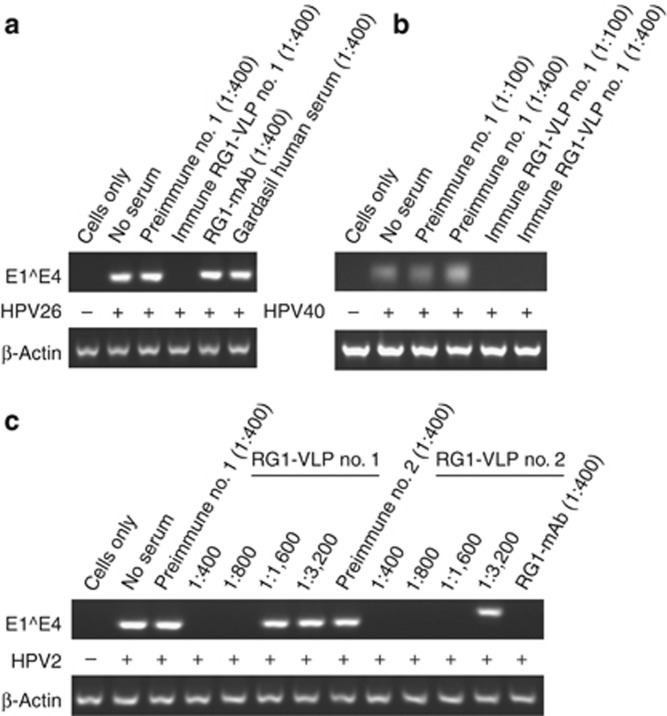
The spectrum of cross-neutralization induced by RG1-VLP vaccination was further explored by analyzing rabbit antisera (n=10) in neutralization assays for a large panel of genus-α HPV using additional PsV types (Table 1). Similar to findings reported previously for rabbits 1/2 (*), the additional eight rabbits' immune sera contained high-titer neutralizing antibodies against HPV16 (titers of 10,000–100,000). Broad-spectrum cross-neutralization was found for species α9 HPV31, 52, 58, 33, 35 (in 2, 5, 9, 6, and 9 out of 10 sera), α7 HPV18, 45, 39, 59, 68, 70 (9, 6, 5, 1, 2, 7/10), α5 HPV51, 26, 69 (3, 10, 2/10), α11 HPV73 and 34 (10 and 8/10) (titers from 25 to 10,000), but not for α6 HPV 56, 53, and 66. Cross-neutralization beyond mucosal HR types was analyzed for the most potent sera nos. 1/2 (Table 2). Apart from LR HPV6/11, α1 HPV32 (causing Heck's disease) was neutralized (titers of 50/100), whereas HPV44 (α10) sporadically found in genital warts was not neutralized. To narrow the gap between PsV-based in vitro assays and natural HPV infection, RG1-VLP-induced neutralizing antibodies were also detected using infectious native virions (Handisurya et al., 2007). As shown in Figure 1a, HaCaT cells infected with HPV26 virions (lane 2) revealed a specific band corresponding to spliced viral mRNA in nested reverse transcriptase–PCR, in contrast to uninfected control cells (lane 1). Preincubation of virions with RG1-VLP antiserum (1:400) completely abolished mRNA detection, indicating viral neutralization (lane 4), whereas preimmune serum (lane 3), mAb RG1 (Gambhira et al., 2007b) (lane 5), or serum from a Gardasil-vaccinated individual (lane 6) had no effect. RG1-VLP antiserum also cross-neutralized LR HPV40 (Figure 1b; lanes 5 and 6) and HPV6 virions (see Supplementary Material online) at dilutions of 1:100–400. Taken together, the in vitro cross-neutralization spectrum of RG1-VLP vaccination includes almost all HR HPVs causing CxCas, as well as LR mucosal types in PsV and native virion–based assays.
Table 1. Cross-neutralization of mucosal HR HPV by RG1-VLP antisera in PBNA in vitro.
|
Neutralizing titers against high-risk genital HPV pseudovirions |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
RG1-VLP plus alum-MPL (50 μg); weeks 0, 4, 6, and 8 (NZW;
n=6) |
RG1-VLP plus alum-MPL (20 μg); weeks 0, 3, and 6 (NZW;
n=4) |
|||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| HPV16 | 100,000 * | 100,000 * | 100,000 | 100,000 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 |
| HPV18 | 1,000* | 1,000 * | 100 | 100 | 1,000 | 100 | 100 | 100 | 100 | <25 |
| HPV45 | 1,000 * | 100 * | <25 | 50 | 1,000 | <25 | <25 | 50 | 100 | <25 |
| HPV31 | 10,000 * | 1,000 * | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 |
| HPV52 | 100 * | 50 * | 50 | <25 | 25 | <25 | <25 | <25 | 25 | <25 |
| HPV58 | 1,000 * | 1,000 * | 100 | 100 | 100 | 25 | 50 | 100 | 1,000 | <25 |
| HPV33 | 100 | 100 | 50 | 25 | 100 | <25 | <25 | <25 | 100 | <25 |
| HPV35 | 1,000 | 1,000 | 100 | 100 | 1,000 | 100 | 50 | 100 | 1,000 | <25 |
| HPV39 | 500 | 100 | <25 | 25 | 100 | <25 | <25 | <25 | 50 | <25 |
| HPV56 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 |
| HPV59 | 25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 |
| HPV68 | 1,000 | 100 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 |
| HPV73 | 1,000 | 1,000 | 1,000 | 100 | 100 | 100 | 50 | 25 | 100 | 25 |
| HPV51 | 25 | <25 | <25 | <25 | <25 | <25 | <25 | 50 | <25 | 50 |
| HPV26 | 100 | 100 | 100 | 100 | 100 | 50 | 25 | 1,000 | 100 | 100 |
| HPV53 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 |
| HPV69 | <25 | <25 | <25 | <25 | 100 | <25 | <25 | <25 | 50 | <25 |
| HPV66 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 | <25 |
| HPV70 | 100 | 50 | <25 | 100 | 1,000 | 50 | <25 | 50 | 100 | <25 |
| HPV34 | 1,000 | 1,000 | 100 | 100 | 1,000 | 50 | <25 | <25 | 100 | 25 |
Abbreviations: alum-MPL, aluminum hydroxide plus 3-O-desacyl-4′-monophosphoryl lipid A; HPV, human papillomavirus; HR, high risk; NZW, New Zealand White; PBNA, pseudovirion-based neutralization assay; VLP, virus-like particle.
Antisera of 10 NZW rabbits raised against RG1-VLP were analyzed for cross-neutralization of 20 mucosal HR HPV pseudovirions in duplicates using end point serial dilutions of 1:25–1:100,000. Neutralization titers were determined as described earlier (Schellenbacher et al., 2009). Data previously published are indicated by “*” (Schellenbacher et al., 2009). Boxed titers indicate sera also tested for cross-neutralization in vivo (Figure 2a–c).
Table 2. Cross-neutralization of mucosal LR and cutaneous HPV by RG1-VLP antisera in vitro.
| Pseudovirions | Neutralizing titer | |
|---|---|---|
| 1 | 2 | |
| Low-risk mucosal | ||
| HPV6 * | 100 | 50 |
| HPV11 * | 100 | <25 |
| HPV32 | 50 | 100 |
| HPV44 | <25 | <25 |
| Genus β | ||
| HPV5 * | 100 | 50 |
| HPV38 | <25 | <25 |
| Common cutaneous | ||
| HPV3 | 1,000 | 1,000 |
| HPV4 | <25 | <25 |
| HPV76 | 100 | 100 |
| Nonhuman | ||
| BPV1 | 100 | 100 |
Abbreviations: BPV1, bovine papillomavirus type 1; HPV, human papillomavirus; LR, low risk; VLP, virus-like particle.
Antisera of two rabbits (nos. 1 and 2) raised against RG1-VLP were tested for cross-neutralization of 4 LR mucosal, 2 genus β cutaneous, 3 common cutaneous HPVs, and nonhuman BPV1 as indicated. Neutralizing titers were determined as described in Table 1.
Figure 1.
In vitro reverse transcriptase–PCR (RT–PCR) neutralization assays using native cutaneous and mucosal high-risk (HR) and low-risk (LR) human papillomavirus (HPV) virions. HaCaT cells were incubated with native virions of (a) mucosal HR HPV26, (b) mucosal LR HPV40, and (c) cutaneous HPV2. Infection was detected by amplification of spliced viral mRNA. Cells were either mock treated (cells only) or infected with native virus (+) in the absence of serum (no serum), or with virions preincubated with (pre)immune sera at indicated dilutions. Neither mAb RG1 nor a human antiserum from a Gardasil-immunized individual neutralized infection with HPV26.
Antisera to RG1-VLP cross-neutralize prevalent common cutaneous and HR β HPVs
In immunocompetent individuals with palmoplantar and plane warts, the most frequently detected types are genus α HPV2/27/57 (Rubben et al., 1997), γ HPV4, μ HPV1, and α HPV3/10. Genus β HPV5/8 have been first identified in patients suffering from Epidermodysplasia verruciformis, whereas a possible role of β papillomavirus in non-melanoma skin cancer pathogenesis is still controversial. Thus, rabbit antisera nos. 1/2 were screened for cross-neutralization against available cutaneous HPVs in pseudovirion-based neutralization assay (PBNA; Table 2), showing previously reported cross-neutralization of HPV5(*), and additionally of HPV3 (α2) and HPV76 (β4) (titers of 1,000/100), but not of HPV4 (γ1) or HPV38 (β2). Furthermore, antisera nos. 1 and 2 cross-neutralized native virions of HPV2 with titers of 800 and 1,600 (Figure 1c, lanes 5 and 11), and HPV27 at dilution 1:400 (Supplementary Material online). Interestingly, antiserum to HPV2 L1-VLP neutralized HPV27, as did antiserum to HPV27 L1-VLP (1:400), indicating that closely related genotypes HPV2 and HPV27 (α4) may represent common serotypes (antisera to HPV2/27 L1-VLP were a kind gift from L Gissmann, DKFZ, Heidelberg, Germany). Neither HPV1 nor HPV4 virions (Supplementary Material online) were cross-neutralized by RG1-VLP antiserum. Sera of 7/8 prepubertal girls were neutralizing against highly prevalent cutaneous HPV1 (Supplementary Material online), indicating robust seroconversion after natural infection, whereas serum from a Gardasil-vaccinated individual did not cross-neutralize cutaneous HPV4. Moreover, the cross-neutralization spectrum of RG1-VLP vaccine even extends to bovine papillomavirus type 1 in PBNA (Table 2).
RG1-VLP vaccination induces cellular immune responses
The enzyme-linked immunospot analysis of splenocytes from mice vaccinated with RG1-VLP or similarly HPV16 L1-VLP showed IFN-γ-producing cells when stimulated with a previously described HPV16 L1 cytotoxic T lymphocyte epitope (Supplementary Material online), indicating the induction of a strong cellular immune response (Ohlschlager et al., 2003). In contrast, stimulation of cells with the RG1 peptide did not result in significant IFN-γ production.
RG1-VLP antisera efficiently protect mice against experimental vaginal challenge with mucosal HPV in vivo
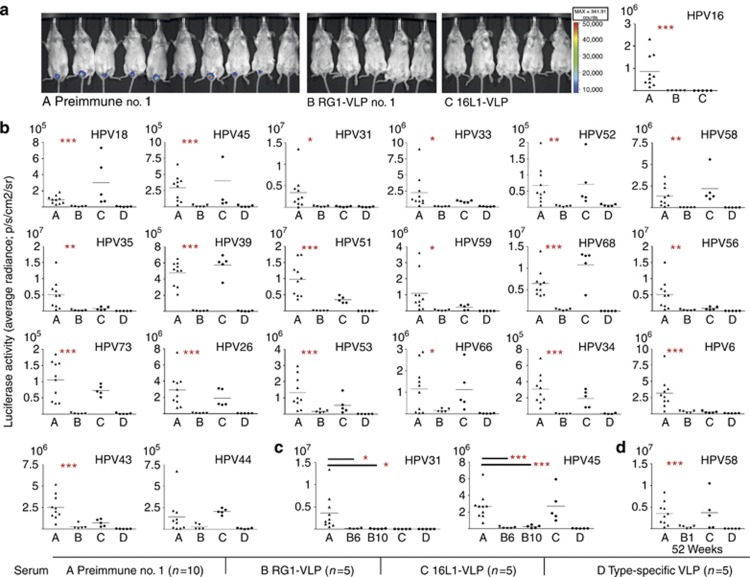
Given the broad spectrum of HR HPVs cross-neutralized in vitro, vaccine efficacy in vivo was determined using an experimental mouse model (adapted from Roberts et al., 2007). As expected, passive transfer of RG1-VLP antiserum reduced vaginal infection in mice challenged with HPV16 PsV to background levels, similar to HPV16 L1 VLP antiserum (Figure 2a). Importantly, mice were also cross-protected from infection with phylogenetically divergent types HPV31/33/35/52/58 (α9), HPV18/45/39/59/68 (α7), HPV34/73 (α11), HPV53/56/66 (α6), and HPV26/51 (α5), and LR HPV6 (α10) and HPV43 (α8) (Figure 2b and c). Indicative, yet statistically unsignificant, results were obtained for LR HPV44 (α10). The level of cross-protection by RG1-VLP antiserum was indistinguishable from that induced by type-specific antisera to the homologous L1-VLP, respectively, except for HPV53/43/44, for which protection was recognizably lower. In contrast, HPV16 L1-VLP antiserum showed predominantly type-restricted protection against homologous HPV16, with significant cross-protection against very closely related HPV31/33/35 and to a smaller extent against more divergent HPV56/59/6. Detectable in vitro cross-neutralization titers (⩾25) were always accompanied by cross-protection in vivo (Tables 1 and 2). However, RG1-VLP antisera also conferred complete (HPV56) or at least partial cross-protection (HPV53/66) in vivo (Figure 2a and b), for which types cross-neutralization was not detected in vitro, suggesting low sensitivity of current assays. To further demonstrate vaccine efficacy irrespective of subtle cross-neutralization titers in vitro, antisera nos. 6/10 were selected for in vivo assays. Antisera nos. 6/10 demonstrated a narrow cross-neutralization spectrum in vitro for only 6/19 or 3/19 mucosal HR types (Table 1). Although neither of the two sera cross-neutralized HPV31/45 in vitro, both effectively cross-protected against infection with HPV31/45 in vivo (Figure 2c).
Figure 2.
Cross-protection against vaginal challenge with mucosal high-risk (HR) and low-risk (LR) human papillomavirus (HPV) in vivo. (a–d) In vivo vaginal challenge with mucosal HPV in mice, passively transferred with RG1-VLP antiserum. Female Balb/c mice were injected intraperitoneally (i.p.) with 20 μl of rabbit (A) preimmune serum; (B) RG1-VLP immune serum (serum no. 1) (a, b); serum no. 6, no. 10 (c); (C) HPV16 L1-VLP antiserum; or (D) L1 antiserum to the respective homologous type. Mice were challenged with pseudovirion (PsV) of (a) HPV16; (b) the next 11 most prevalent HR types HPV18, 45, 31, 33, 52, 58, 35, 39, 51, 59, and 68, and less frequent (<1%) types in cervical cancer (CxCa) HPV56, 73, 26, 53, 66, 34; LR HPV6, 43, and 44; or (c) high-risk HPV31 and 45. (d) An RG1-VLP rabbit antiserum (no. 1) drawn at week 52 (10 months following the third boost with in vitro titer of <25 to HPV58) was analyzed for cross-protection against challenge with HPV58 (B no. 1, week 52). Antisera to (d, C) HPV16 L1-VLP and (d, D) HPV58 L1/L2 PsV drawn 2 weeks after the third boost were used as controls. Luciferase activity in bioluminescence imaging (Y axis: average radiance; p/s/cm2/sr) is given with the respective background luminescence (unvaccinated mice challenged with carboxymethylcellulose (CMC) only) subtracted from all data points. P-values comparing groups A and B are indicated by *P⩽0.05, **P⩽0.01, and ***P⩽0.005.
Induction of long-lasting B-cell memory and cross-protection
To examine long-term B-cell memory after RG1-VLP vaccination, rabbits 1/2 were housed for a further 10 months after the fourth immunization and boosted at week 52 with 50 μg of RG1-VLP (Supplementary Material online). When compared with sera drawn at week 10, cross-neutralization titers had declined 1–2 logs (HPV18/31) or beneath the level of detection (HPV45/52/58/6/5) in week 52. A similar 2-log decline was observed for neutralizing antibodies against HPV16, which are predominantly induced by the L1 scaffold of RG1-VLP. Importantly, boosting with RG1-VLP raised antibody titers to former levels or beyond. To determine whether cross-protection in vivo is also long lasting, antiserum drawn at week 52 was analyzed for cross-protection against in vivo challenge with HPV58. Although neutralization of HPV58 was no longer detectable in vitro at week 52, cross-protection was still conferred in vivo (Figure 2d), yet to a slightly lesser extent as compared with sera drawn at week 10 (Figure 2b).
DISCUSSION
The search for second-generation HPV vaccines is driven by the need to protect against the plurality of carcinogenic genital HPV by safe and affordable formulations. Because licensed vaccines do not target HR HPVs other than HPV16/18, causing 30% of CxCas, cytological screening programs cannot be superseded. This major limitation in particular affects developing countries, which bear >85% of the global CxCa burden. Moreover, precancerous cervical neoplasia with a substantial disease prevalence and morbidity in younger women is even more strongly associated with types other than HPV16/18 (Tjalma et al., 2012). To extend protection to the most common oncogenic HPVs, a nonavalent L1-VLP vaccine (comprising seven HR and two LR types) is in clinical trials. Although such a highly multivalent L1 vaccine is expected to significantly increase the breadth of coverage, L2-based vaccines are attractive as single-antigen formulations targeting even more mucosal and, additionally, nongenital types. The spectrum of L2-mediated cross-neutralization by chimeric RG1-VLP has been analyzed by a limited number of in vitro assays, whereas the vaccine's efficacy to confer protection in mammals against infection with a broad range of HPVs has not been substantiated so far. In a very extensive comparison of HPV16 L1 VLP versus RG1-VLP (plus alum-MPL adjuvant) provided herein, RG1-VLP vaccination (cross-)protects with high efficacy in vivo against infection with 18 divergent HR types out of 4 different species (α5/7/9/11), which cause more than 95% of CxCas worldwide, and also partially protects against the remaining four types tested (de Sanjose et al., 2010). Mice were protected from vaginal challenge with large doses of mucosal PsV despite considerable antiserum dilution (∼1:50) into the mouse circulation. Interestingly, undetectable cross-neutralization in vitro did not stringently imply incomplete protection in vivo. In line with the recently reported insufficient sensitivity of current in vitro assays to detect anti-L2 antibodies, in vivo testing has become the gold standard in detecting neutralizing HPV antibodies (Longet et al., 2011) and documenting vaccine efficacy. The results imply that the actual immunogenicity is substantially greater than that reflected by the standard in vitro neutralization assay, and suggest that this vaccine should have a significant impact not only on reducing the risk of CxCa but also on the overall incidence of genital HPV infection, thus providing a major advantage to HPV-based screening programs when compared with the current vaccines. Complementary in vitro assays were used to more extensively characterize the spectrum of cross-neutralization by RG1-VLP vaccination, which was not predicted by L1-based classification of papillomaviruses, the site of infection, or host species. In PBNA, variable (cross)-neutralization by RG1-VLP antiserum was detected for 17 mucosal HR types, whereas cross-protection in vivo was intact for the serum with least potency in vitro. Although technically demanding and limited to those few types available from productive lesions, neutralization assays using native virions may reflect viral infection more authentically than PBNA (McLaughlin-Drubin et al., 2004). Reassuringly, the RG1-VLP antisera neutralized lesion-derived virions as well. The RG1-VLP antisera also cross-neutralized LR types HPV6/11 (α10), HPV32 (α1), HPV40 (α8), and cutaneous HPV2/27 (α4), HPV3 (α1), and HPV76 (β3), but not HPV1 (μ), HPV4 (γ), and HPV38 (β). On comparing the RG1 motifs of all types cross-neutralized, sequence homology to HPV16 RG1 was at least 60% (see Supplementary Material online). Whether this lower threshold of homology necessary for inducing cross-neutralization holds out in more sensitive in vivo assays needs to be demonstrated. Therefore, RG1-VLP vaccination is expected to protect against a substantial proportion of benign papillomas, including both sexually transmitted genital and nongenital skin warts. The latter is not only of tremendous clinical importance for the growing group of immunocompromised patients (e.g., organ-transplant recipients, HIV patients), but may have expanded relevance if these types contribute to the genesis of nonmelanoma skin cancers. Because the incidence of common skin warts peaks in early childhood, this would provide a rationale for implementing the vaccine into existing childhood programs. Various approaches to enhance cross-neutralizing L2 antibodies have been presented over the past years, including conjugation of L2 peptides to a T-helper epitope and a Toll-like receptor 2 ligand (Alphs et al., 2008), concatenated multimeric L2 peptide vaccination (Jagu et al., 2009), or display on bacteriophage (Tumban et al., 2012), adeno-associated virus (Nieto et al., 2012), or HPV L1-VLP (Zamora et al., 2006; Slupetzky et al., 2007; Kondo et al., 2008; Schellenbacher et al., 2009; Caldeira Jdo et al., 2010). Favorably, RG1-VLP vaccination plus alum-MPL (as in Cervarix) not only elicits robust antibody responses against RG1 in ELISA but also retains high-level protection against the most important HPV16. Therefore, RG1-VLP vaccination should provide strong protection not just against CxCas but also against the even higher percentage of noncervical cancers attributable to HPV16. In contrast, in a comprehensive direct comparison, HPV16 L1-VLP vaccination protected against HPV16 and 31 and only to a lower extent against 35, 39, 56, and 6. Licensed vaccines provide long-term protection of at least 8.5 years, and the antibody levels required are still unknown. Importantly, sera drawn 12 months after RG1-VLP vaccination completely protected mice against vaginal challenge with HR HPV58 in vivo. RG1-VLP holds promise as next-generation vaccine with broad efficacy against the vast majority of relevant mucosal and additional cutaneous HPVs, provides a rationale for childhood vaccination, and adds economic advantage of a single antigen formulation compared with multivalent L1-VLP formulations. As post-licensure data confirm excellent safety profiles for HPV L1-VLP vaccines, we infer that RG1-VLP vaccination may prove similarly safe, offering the possibility to evaluate vaccine immunogenicity in early-phase human trials.
MATERIALS AND METHODS
Chimeric RG1-VLP
Chimeric HPV16 L1–HPV16 L2 (aa 17–36) VLP (RG1-VLP) have been generated (Schellenbacher et al., 2009) by the insertion of HPV16 L2 peptide aa 17–36 (RG1) (Gambhira et al., 2007b) into the DE-surface loop of HPV16 L1 and expressed in insect cells (Kirnbauer et al., 1992).
Immunization
NZW rabbits were immunized with 50 μg of RG1-VLP subcutaneously (weeks 0, 4, 6, and 8, n=6; NZW nos. 1–6) or 20 μg of RG1-VLP (weeks 0, 3, and 6, n=4; NZW nos. 7–10) (Charles River, Kisslegg, Germany) adjuvanted with alum-MPL (10:1) (Sigma Aldrich, St Louis, MO). Sera were drawn at weeks 10 (nos. 1–6) or 8 (nos. 7–10). Long-term antibody responses were determined for NZW nos. 1/2 kept at 10 months. Sera were drawn before and 14 days after final boost (week 52) and were stored at −20 °C. For type-specific L1 antisera, a single rabbit each was immunized with 20 μg PsV of HPV6/26/34/35/39/43/44/51/52/53/56/58/59/66/68/69, or 70, respectively (weeks 0, 3, and 6), in complete/incomplete Freund's adjuvant.
Native virion–based neutralization assays (reverse transcriptase–PCR)
Native virions HPV2/27/1/4 were extracted from plantar warts; HPV6 from genital warts; HPV26 from a highly differentiated carcinoma (Handisurya et al., 2007); and HPV40 virions were provided by Neil Christensen (Hershey, PA) (Jenkins et al., 2003). After mechanical disruption of wart tissue by high-speed homogenizer (Fastprep-24, MP Biomedicals, Eschwege, Germany) and centrifugation (14,000 r.p.m./4 °C/5 minutes), supernatants containing virions were used for neutralization assays (Smith et al., 1995; Slupetzky et al., 2007). In brief, 3 × 105 HaCaT keratinocytes were infected with virions that were either untreated or preincubated with rabbit antisera and incubated overnight. Cellular RNA was reverse transcribed, and spliced viral E1^E4 mRNA was identified by 30-cycle nested PCR (95 °C/1 minute, 60 °C/1 minute, and 72 °C/3 minutes; for primer pairs see Supplementary Material online).
Murine vaginal challenge
The intravaginal PsV challenge model (Roberts et al., 2007) was performed with slight modification (Karanam et al., 2010). Female Balb/C mice (groups of 5 or 10) were pretreated with 3 mg of progesterone subcutaneously medroxyprogesterone (Depocou, Pfizer, Vienna, Austria), and on day 3 they were passively transferred with 20 μl rabbit antiserum: RG1-VLP (pre)immune serum (rabbits 1, 6, and 10), or sera to L1-VLP of HPV16/31/33/45/6, to L1/L2-VLP of HPV18 (J Schiller, NIH, Bethesda, MD), to L1-DNA of HPV59/73, or to L1/L2-PSV of HPV26/34/35/39/51/52/53/56/58/66/68/69/ or 70, respectively. After 24 hours, vaginal microtrauma was induced by cervical cytobrush, and mice were challenged with luciferase-encoding PsV in 3% carboxymethylcellulose. After 3 days, 20 μl of luciferin (Caliper, Waltham, MA; 7.5 mg ml−1) was applied into the vagina, and infection was analyzed by bioluminescence imaging (IVIS 50, Caliper). Data are given with background signal subtracted (mice challenged with carboxymethylcellulose).
Statistical methods
Statistical analysis was performed using Microsoft Excel (heteroscedastic two-tailed unpaired t-test to evaluate P-values).
Standard techniques such as ELISA, PBNA, and enzyme-linked immunospot assays are described in Supplementary Material online.
Consent and approval
Human sera and tissue samples were collected after written informed consent of the patient or the patient's guardian in accordance with the Ethics Committee of the Medical University Vienna (ECS 1327/2012). The study was conducted according to the Declaration of Helsinki Principles.
Animal welfare
Animal studies have been approved (BMWF-66.009/0173-1I/3b/2011) and animal care was in accordance with the guidelines of the Austrian Federal Ministry for Science and Research.
Acknowledgments
We are grateful to Doug Lowy for his critical review of the manuscript and helpful suggestions. This research was supported by grants to RK from the Austrian Science Foundation (P18990B13) and the Vienna Science and Technology Fund (WWTF) (Life Science Call 2011; LS11-006), and in part by grants to RBSR from the National Institutes of Health (National Cancer Institute, SPORE in Cervical Cancer, P50 CA098252 and CA118790).
Glossary
- aa
amino acid
- alum-MPL
aluminum hydroxide plus 3-O-desacyl-4′-monophosphoryl lipid A
- CxCa
cervical cancer
- HPV
human papillomavirus
- HR
high risk
- LR
low risk
- N
amino
- PBNA
pseudovirion-based neutralization assay
- PsV
pseudovirion
- VLP
virus-like particle
RK is a co-inventor on L1 patents licensed to GlaxoSmithKline (GSK) and Merck (MSD). RBSR has received an unrestricted educational grant from GSK. RBSR is a co-inventor on L2 patents licensed to PaxVax, Shantha Biotechnics/Sanofi, and GSK, and on PsV technology licensed to GSK and MSD, and is a founder of Papivax LLC. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. The RG1-VLP vaccine is subject to a pending patent application (RK, CS, RBSR). The other authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Alphs HH, Gambhira R, Karanam B, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci USA. 2008;105:5850–5855. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard HU, Burk RD, Chen Z, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Plasmeijer EI, Feltkamp MC. beta-Papillomavirus infection and skin cancer. J Invest Dermatol. 2008;128:1355–1358. doi: 10.1038/jid.2008.123. [DOI] [PubMed] [Google Scholar]
- Caldeira Jdo C, Medford A, Kines RC, et al. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 2010;28:4384–4393. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrachud LM, Grindlay GJ, McGarvie GM, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211:204–208. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Kreider JW. Neutralization of CRPV infectivity by monoclonal antibodies that identify conformational epitopes on intact virions. Virus Res. 1991;21:169–179. doi: 10.1016/0168-1702(91)90031-p. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- Gambhira R, Jagu S, Karanam B, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007a;81:11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhira R, Karanam B, Jagu S, et al. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007b;81:13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagensee ME, Yaegashi N, Galloway DA. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handisurya A, Gambhira R, Schellenbacher C, et al. Serological relationship between cutaneous human papillomavirus types 5, 8 and 92. J Gen Virol. 2009;90:136–143. doi: 10.1099/vir.0.006189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handisurya A, Rieger A, Bankier A, et al. Human papillomavirus type 26 infection causing multiple invasive squamous cell carcinomas of the fingernails in an AIDS patient under highly active antiretroviral therapy. Br J Dermatol. 2007;157:788–794. doi: 10.1111/j.1365-2133.2007.08094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagu S, Karanam B, Gambhira R, et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009;101:782–792. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AL, Lang CM, Budgeon LR, et al. Mucosally-derived HPV-40 can infect both human genital foreskin and cutaneous hand skin tissues grafted into athymic mice. Virus Res. 2003;93:109–114. doi: 10.1016/s0168-1702(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389–395. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- Karanam B, Peng S, Li T, et al. Papillomavirus infection requires gamma secretase. J Virol. 2010;84:10661–10670. doi: 10.1128/JVI.01081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana K, Yoshikawa H, Taketani Y, et al. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol. 1999;73:6188–6190. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Booy F, Cheng N, et al. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Taub J, Greenstone H, et al. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Ochi H, Matsumoto T, et al. Modification of human papillomavirus-like particle vaccine by insertion of the cross-reactive L2-epitopes. J Med Virol. 2008;80:841–846. doi: 10.1002/jmv.21124. [DOI] [PubMed] [Google Scholar]
- Lally A, Casabonne D, Imko-Walczuk B, et al. Prevalence of benign cutaneous disease among Oxford renal transplant recipients. J Eur Acad Dermatol Venereol. 2011;25:462–470. doi: 10.1111/j.1468-3083.2010.03814.x. [DOI] [PubMed] [Google Scholar]
- Longet S, Schiller JT, Bobst M, et al. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85:13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Christensen ND, Meyers C. Propagation, infection, and neutralization of authentic HPV16 virus. Virology. 2004;322:213–219. doi: 10.1016/j.virol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Nieto K, Weghofer M, Sehr P, et al. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One. 2012;7:e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlschlager P, Osen W, Dell K, et al. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J Virol. 2003;77:4635–4645. doi: 10.1128/JVI.77.8.4635-4645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Gambhira R, Buck CB, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Roden RBS, Yutzy WH, Fallon R, et al. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270:254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- Rose RC, Bonnez W, Reichman RC, et al. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993;67:1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubben A, Kalka K, Spelten B, et al. Clinical features and age distribution of patients with HPV 2/27/57-induced common warts. Arch Dermatol Res. 1997;289:337–340. doi: 10.1007/s004030050201. [DOI] [PubMed] [Google Scholar]
- Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol. 2009;83:10085–10095. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger T, Becker MR, Schadlich L, et al. Identification of B-cell epitopes on virus-like particles of cutaneous alpha-human papillomaviruses. J Virol. 2009;83:12692–12701. doi: 10.1128/JVI.01582-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slupetzky K, Gambhira R, Culp TD, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007;25:2001–2010. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LH, Foster C, Hitchcock ME, et al. Titration of HPV-11 infectivity and antibody neutralization can be measured in vitro. J Invest Dermatol. 1995;105:438–444. doi: 10.1111/1523-1747.ep12321173. [DOI] [PubMed] [Google Scholar]
- Suzich JA, Ghim S, Palmer-Hill FJ, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalma WA, Fiander A, Reich O, et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer. 2012;132:854–867. doi: 10.1002/ijc.27713. [DOI] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Tyler M, et al. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One. 2012;7:e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haalen FM, Bruggink SC, Gussekloo J, et al. Warts in primary schoolchildren: prevalence and relation with environmental factors. Br J Dermatol. 2009;161:148–152. doi: 10.1111/j.1365-2133.2009.09160.x. [DOI] [PubMed] [Google Scholar]
- Zamora E, Handisurya A, Shafti-Keramat S, et al. Papillomavirus-like particles are an effective platform for amyloid-beta immunization in rabbits and transgenic mice. J Immunol. 2006;177:2662–2670. doi: 10.4049/jimmunol.177.4.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.