TO THE EDITOR
Punctate palmoplantar keratoderma (punctate PPK or PPKP) is a rare autosomal dominant disorder of keratinization. Three variants of this disease have been described; PPKP1 (OMIM ♯148600, Buschke–Fischer–Brauer type) is characterized by the progressive development of discrete areas of hyperkeratosis on the palms and soles, followed by more extensive diffuse hyperkeratosis on the pressure-bearing areas of plantar skin.
Linkage analyses of families affected by PPKP1 have previously identified a locus within 15q22–q24 (Martinez-Mir et al., 2003; Gao et al., 2005), but two Chinese pedigrees with a PPKP1 phenotype demonstrated linkage on chromosome 8q24.13–q24.21 (Zhang et al., 2004). Recently, nonsense mutations in AAGAB, the gene encoding alpha- and gamma-adaptin-binding protein p34, were reported in three PPKP1 families (Giehl et al., 2012). Simultaneously, we applied whole-exome sequencing and reported heterozygous loss-of-function mutations in AAGAB in 18 PPKP1 kindreds (Pohler et al., 2012). AAGAB is located on chromosome 15q22, within one of the previously reported linkage regions (Martinez-Mir et al., 2003; Pohler et al., 2012). We now report the AAGAB genotype in a further 12 PPKP1 patients from 6 independent kindreds of Scottish, English, and Mexican ancestry. This study was carried out in accordance with the Declaration of Helsinki Principles, and all subjects gave written informed consent.
Kindred 1
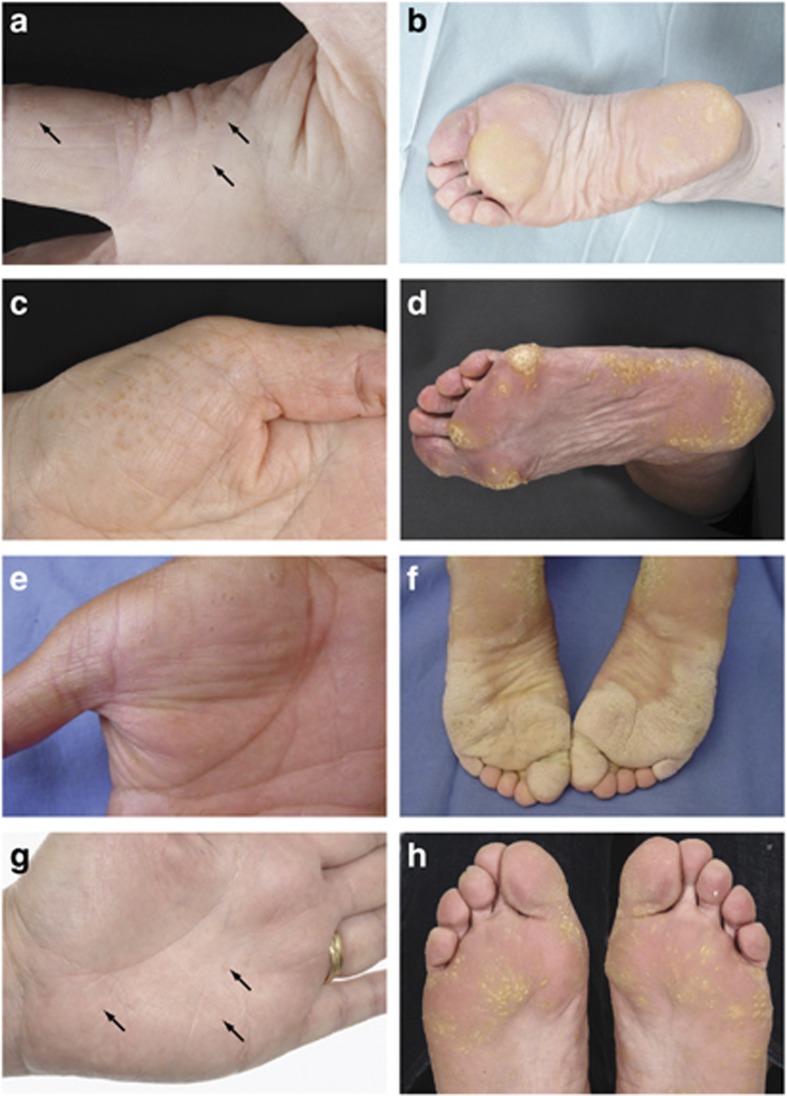
A 56-year-old Scottish woman reported a 15-year history of the progressive development of tender callosities on her soles and palms. Examination revealed multiple hyperkeratotic foci on the palmar skin of hands and fingers (Figure 1a) and diffuse hyperkeratosis on weight-bearing areas of plantar skin (Figure 1b). The proband's parents were unaffected, but one of her three offspring demonstrated a PPKP1 phenotype. Sequencing of AAGAB using reported methodology (Pohler et al., 2012) identified a heterozygous 1-bp deletion, mutation c.344del resulting in a premature termination codon (p.Asp115Valfs*7; Supplementary Figure S1 online). This mutation has previously been observed in two apparently unrelated Scottish families (Pohler et al., 2012).
Figure 1.
Clinical images of punctate palmoplantar keratoderma cases. (a) Proband from kindred 1 illustrating focal punctate callosity on palmar skin and diffuse hyperkeratosis on plantar skin (b). Palm (c) and sole (d) of a 74-year-old woman from kindred 2 showing punctate lesions coalescing to form focal areas of marked hyperkeratosis. Affected individuals from kindred 5 showing typical focal calluses on palmar skin (e) and coalescence of the lesions on plantar pressure points (f). The proband from kindred 6 is shown in g and h, demonstrating subtle discrete hyperkeratotic papules on the palmar skin with more marked hyperkeratotic foci, which coalesce in weight-bearing areas on the plantar skin. Arrows indicate punctate hyperkeratotic lesions. Clinical images from the cases in kindreds 3 and 4 are shown in Supplementary Figures 3a, b, and 4a, b online.
Kindred 2
A 74-year-old Scottish woman presented with a 25-year history of progressive hyperkeratosis of her plantar skin (Figure 1d), with lesser involvement of the palmar skin (Figure 1c). The proband's parents were unaffected, but one of her three adult children had keratoderma. DNA sequencing identified a previously unreported heterozygous nonsense mutation, c.390G>A, predicting the protein change p.Trp130*0, within AAGAB (Supplementary Figures S1 and S2b online). This mutation was not detected in ∼11,000 European and African American individuals' exome sequencing data (NHLBI Exome Sequencing Project http://evs.gs.washington.edu/EVS/ accessed 27 March 2013), nor is it reported in the 1000 Genomes Catalogue of Human Genetic Variation (http://www.1000genomes.org/ensembl-browser accessed 27 March 2013).
Kindred 3
A 79-year-old Scottish man presented with a 30-year history of hyperkeratosis of the soles and palms (Supplementary Figure S3a and b online). His medical history included a cutaneous squamous cell carcinoma on the hand and a basal cell carcinoma on the nose, but these were not at sites affected by keratoderma. The patient's parents, eight siblings, and two children were unaffected. DNA sequencing revealed a heterozygous c.870+1G>A splice-site mutation within AAGAB (Supplementary Figure S1 online), previously reported in an unrelated Scottish family (Pohler et al., 2012). The protein consequences of this mutation are unknown but is likely to represent a frameshift mutation.
Kindred 4
A 55-year-old female patient of English ancestry presented at the age of 30 years with typical features of PPKP1. She had multiple keratotic papules on palmar and plantar surfaces, which had gradually increased in number, becoming confluent and tender (Supplementary Figure S4a and b online). The patient has one affected and four unaffected siblings; her two daughters are similarly affected and her one grandson shows early signs of punctate keratoderma. DNA sequencing revealed a heterozygous frameshift mutation c.472del, leading to a premature termination codon (p.Gly158Glufs*0; Supplementary Figure S1 online), previously reported in five apparently unrelated Scottish families (Pohler et al., 2012).
Kindred 5
Thirty-one members of this Mexican family over four generations demonstrate a PPK1P phenotype with an autosomal dominant pattern of inheritance. The affected living relatives showed multiple hyperkeratotic papules on the palms and soles with no associated nail disease (representative clinical images are shown in Figure 1e and f). Linkage analysis in this family has identified the AAGAB locus at chromosome 15q22 (Martinez-Mir et al., 2003). AAGAB sequencing of DNA from five affected family members has now identified a previously unreported heterozygous 1-bp deletion in exon 3, c.275del predicting the protein change p.Leu92Leufs*18, in each affected individual (Supplementary Figures S1 and S2d online). This mutation was not detected in ∼11,000 European and African American individuals' exome sequencing data (NHLBI Exome Sequencing Project http://evs.gs.washington.edu/EVS/ accessed 27 March 2013), nor is it reported in the 1000 Genomes Catalogue of Human Genetic Variation (http://www.1000genomes.org/ensembl-browser accessed 27 March 2013).
Kindred 6
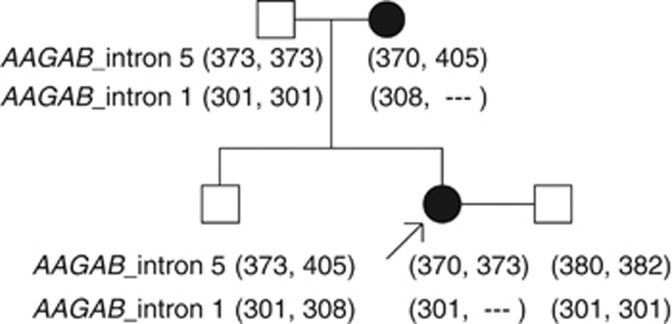
A 42-year-old Scottish woman presented with punctate callosities affecting her palms and soles, which were present since early adulthood (Figure 1g and h). The proband's mother was affected to a milder degree, and her 15-year-old son showed early signs of punctate keratoderma on the plantar surfaces. Sequencing of all exons of AAGAB in these three cases failed to identify any mutations. However, microsatellite linkage analysis in the pedigree was consistent with involvement of the AAGAB locus (Figure 2). Hemizygous inheritance of a microsatellite marker within intron 1 of AAGAB indicated a genomic deletion. A reduction in the gene dosage for AAGAB exon 1 in the proband compared with her unaffected father was confirmed using semiquantitative fluorescent PCR (Supplementary Figure S5a and b online), whereas exons 2 and 3 were amplified in equal quantities in the proband and control (Supplementary Figure S5c and d online). The precise size of this genomic deletion involving exon 1/intron 1 of AAGAB has not been defined, but it is consistent with the resultant haploinsufficiency of AAGAB.
Figure 2.
Family tree and microsatellite markers showing linkage patterns in kindred 6. Two regions of microsatellite repeats were screened: one marker within intron 5 and a second marker within intron 1 of AAGAB. Numbers indicate the length (in base pairs) of PCR products for each marker from each allele. The microsatellite marker of intron 1 is absent from one allele of AAGAB in PPKP1-affected individuals, indicating a genomic deletion resulting in hemizygosity.
It has previously been shown that knockdown of p34 in HaCaT cells in vitro leads to increased stabilization of the EGFR protein, a receptor tyrosine kinase (Pohler et al., 2012). In addition to EGFR, keratinocytes express several other receptor tyrosine kinases (RTKs). To further investigate the functional effects of AAGAB mutations, we tested the effect of gene knockdown on the transmembrane protein Axl, an RTK that is highly expressed in skin and is involved in signaling pathways to cellular proliferation (Postel-Vinay and Ashworth, 2012). We investigated the effect of p34 knockdown on Axl in HaCaT keratinocytes and found a significant increase in the amount of Axl protein in cells treated with AAGAB-specific small interfering RNA (Supplementary Figure S6 online; methods are fully described in online Supplementary Tables S1 and S2 online).
These six kindreds demonstrate replication of a newly reported genetic cause for punctate PPK1, further confirming AAGAB as a causative gene. AAGAB mutations result in hereditary deficiency of p34, leading to hyperproliferative hyperkeratosis through increased growth factor signaling, which is thought to result from impairment in the endocytosis and recycling of EGFR proteins (Pohler et al., 2012).
A total of 13 predicted loss-of-function mutations have now been reported; these are listed in Supplementary Table S3 online. We have identified recurrent mutations resulting in frameshift and deleterious splice site changes, as well as previously unreported variants. There is no apparent genotype–phenotype correlation within this small group of patients, but our clinical observation is that environmental factors and personal skin care regimes affect the degree of plantar hyperkeratosis. The PPKP1 families reported here demonstrate some diversity in the clinical appearance of plantar hyperkeratosis, whereas the punctate palmar keratoses have a more similar appearance in each kindred (Figure 1).
An association with the development of malignancy in PPKP1 patients has been reported, including breast and colonic adenocarcinoma (Bennion and Patterson, 1984; Stevens et al., 1996). It is not clear whether there is truly an over-representation of malignancy in PPKP1 cases, and malignant neoplasms were not prevalent in the six kindreds reported here.
Hyperkeratosis of the plantar skin localized to the weight-bearing areas may be explained by mechanical trauma, but the extremely focal distribution of hyperkeratosis on the palmar skin in the disorder is striking. The mechanism(s) responsible for producing this very focal disease on the background of a mutation expressed throughout the palmar skin are likely to be of clinical relevance, but they remain to be defined. It is possible that a second mutation has occurred within each focal area of hyperkeratosis. This was not identified by microdissection and sequence analysis (Pohler et al., 2012), but further investigation is warranted. A clearer understanding of the genetic and/or environmental mechanisms by which focal keratoses arise may give further insight into the role of p34 in controlling cell division. Because of the role of EGFR and Axl in various malignancies, Axl-specific inhibitors are under development (Postel-Vinay and Ashworth, 2012) and are potentially of relevance to this rare but distressing keratoderma.
The p34 protein encoded by AAGAB has been functionally implicated in the biology of intracellular transport of membrane-bound, clathrin-coated vesicles. We hypothesized that a possible link between an inherited defect in vesicle transport and epidermal hyperproliferation might involve RTKs, which are turned over by endocytosis via clathrin- and AP2-dependent processes (Ceresa, 2006; Rappoport and Simon, 2009). The upregulation of Axl protein expression after small interfering RNA knockdown of AAGAB demonstrates the involvement of p34 in RTK turnover in addition to EGFR. The identification of molecular mechanisms in palmoplantar keratoderma serves to improve our understanding of the pathogenesis of cutaneous hyperkeratosis. It may also offer insight into the control of keratinization in physiological conditions, as well as more prevalent inflammatory skin diseases characterized by hyperproliferation and hyperkeratosis.
Acknowledgments
We are grateful to the PPKP1 patients and their families for sharing clinical information and providing samples. This work was supported by the Wellcome Trust (Clinical Intermediate Fellowship WT086398MA to SJB; Programme Grant 092530/Z/10/Z to WHIM; Strategic Award 098439/Z/12/Z to WHIM) and a project grant from the Pachyonychia Congenita Project to FJDS.
Glossary
- AAGAB
gene encoding alpha- and gamma-adaptin-binding protein p34
- bp
base pair
- OMIM
Online Mendelian Inheritance in Man
- PPK(P)
(punctate) palmoplantar keratoderma
- RTK
receptor tyrosine kinase
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Bennion SD, Patterson JW. Keratosis punctate palmaris et plantaris and adenocarcinoma of the colon. J Am Acad Dermatol. 1984;10:587–591. doi: 10.1016/s0190-9622(84)80262-5. [DOI] [PubMed] [Google Scholar]
- Ceresa BP. Regulation of EGFR endocytic trafficking by rab proteins. Histol Histopathol. 2006;21:987–993. doi: 10.14670/HH-21.987. [DOI] [PubMed] [Google Scholar]
- Gao M, Yang S, Li M, et al. Refined localization of a punctate palmoplantar keratoderma gene to a 5.06-cM region at 15q22.2-15q22.31. Br J Dermatol. 2005;152:874–878. doi: 10.1111/j.1365-2133.2005.06488.x. [DOI] [PubMed] [Google Scholar]
- Giehl KA, Eckstein GN, Pasternack SM, et al. Nonsense mutations in AAGAB cause punctate palmoplantar keratoderma type Buschke-Fischer-Brauer. Am J Hum Genet. 2012;91:754–759. doi: 10.1016/j.ajhg.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Mir A, Zlotogorski A, Londono D, et al. Identification of a locus for type 1 punctate palmoplantar keratoderma on chromosome 15q22-q24. J Med Genet. 2003;40:872–878. doi: 10.1136/jmg.40.12.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohler E, Mamai O, Hirst J, et al. Haploinsufficieny for AAGAB causes clinically heterogeneous forms of punctate palmoplantar keratoderma. Nat Genet. 2012;44:1272–1276. doi: 10.1038/ng.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel-Vinay S, Ashworth A. AXL and acquired resistance to EGFR inhibitors. Nat Genet. 2012;44:835–836. doi: 10.1038/ng.2362. [DOI] [PubMed] [Google Scholar]
- Rappoport JZ, Simon SM. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J Cell Sci. 2009;122:1301–1305. doi: 10.1242/jcs.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HP, Kelsell DP, Leigh IM, et al. Punctate palmoplantar keratoderma and malignancy in a four generation family. Br J Dermatol. 1996;134:720–726. [PubMed] [Google Scholar]
- Zhang XJ, Li M, Gao TW, et al. Identification of a locus for punctate palmoplantar keratodermas at chromosome 8q24.13-8q22.21. J Invest Dermatol. 2004;122:1121–1125. doi: 10.1111/j.0022-202X.2004.22507.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.