Abstract
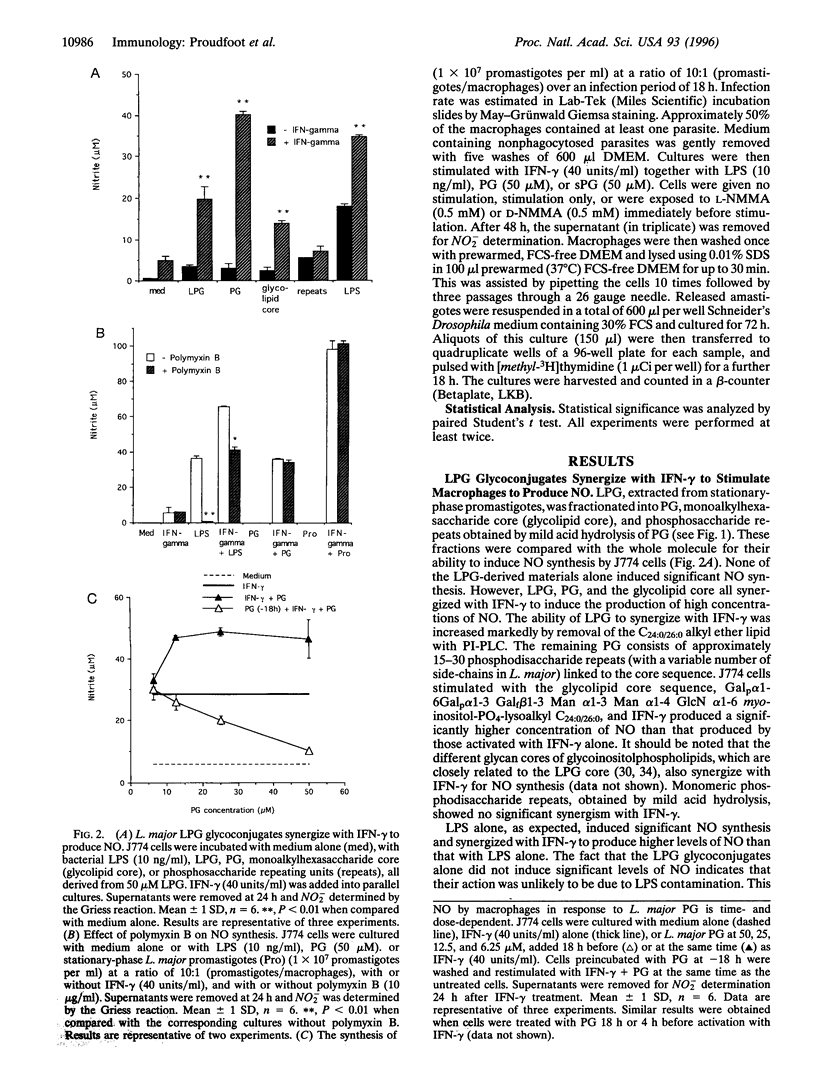
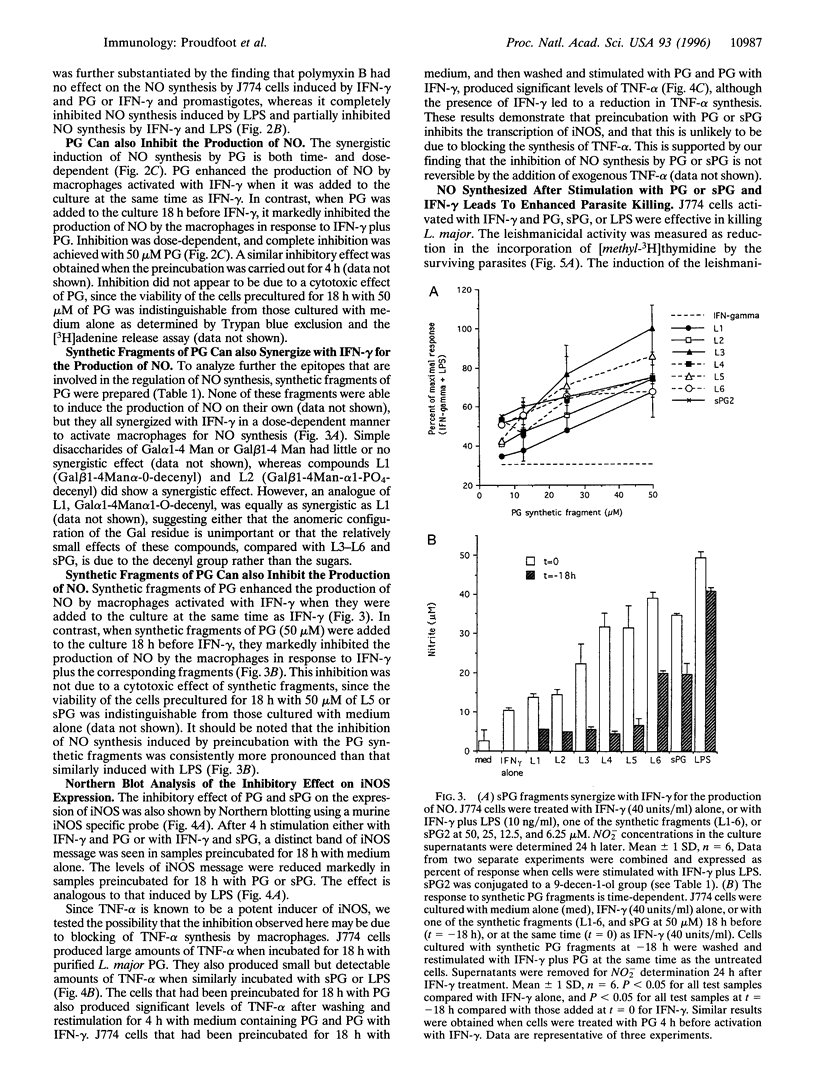
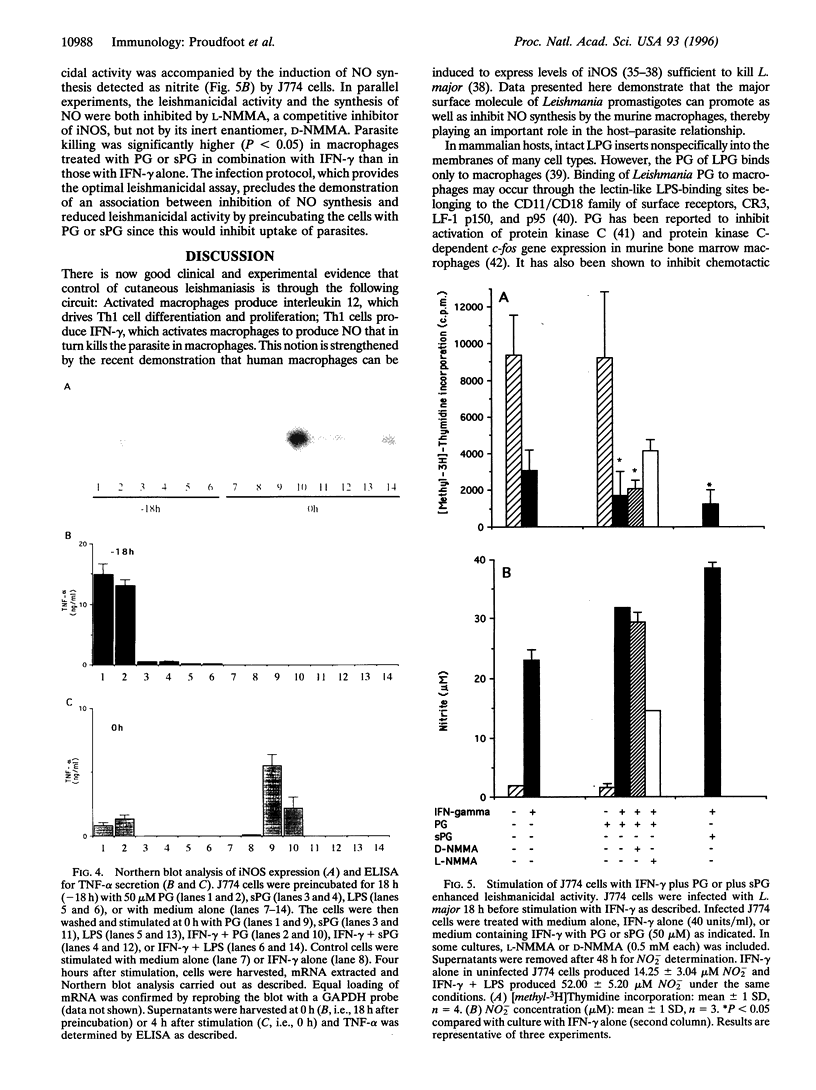
Lipophosphoglycan (LPG) glycoconjugates from promastigotes of Leishmania were not able to induce the expression of the cytokine-inducible nitric oxide synthase (iNOS) by the murine macrophage cell line, J774. However, they synergize with interferon gamma to stimulate the macrophages to express high levels of iNOS. This synergistic effect was critically time-dependent. Preincubation of J774 cells with the LPG glycans 4-18 h before stimulation with interferon gamma resulted in a significant reduction in the expression of iNOS mRNA and of NO synthesis, compared with cells preincubated with culture medium alone. The regulatory effect on the induction of iNOS by LPG is located in the LPG phosphoglycan disaccharide backbone. Synthetic fragments of this backbone had a similar regulatory effect on NO synthesis. Further, the production of NO by activated macrophages in the present system was correlated directly with the leishmanicidal capacity of the cells. These data therefore demonstrate that LPG glycoconjugates have a profound effect on the survival of Leishmania parasites through their ability to regulate the expression of iNOS by macrophages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Russell D. G. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- Andreoli S. P., Baehner R. L., Bergstein J. M. In vitro detection of endothelial cell damage using 2-deoxy-D-3H-glucose: comparison with chromium 51, 3H-leucine, 3H-adenine, and lactate dehydrogenase. J Lab Clin Med. 1985 Sep;106(3):253–261. [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. I., Nottet H. S., Schmidtmayerova H., Dubrovsky L., Flanagan C. R., Mullins M. E., Lipton S. A., Gendelman H. E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995 Feb 1;181(2):735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva R. P., Hall B. F., Joiner K. A., Sacks D. L. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989 Jul 15;143(2):617–622. [PubMed] [Google Scholar]
- De Maria R., Cifone M. G., Trotta R., Rippo M. R., Festuccia C., Santoni A., Testi R. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994 Nov 1;180(5):1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux A., Turco S. J., Sacks D. L., Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J Immunol. 1991 Apr 15;146(8):2747–2753. [PubMed] [Google Scholar]
- Frankenburg S., Leibovici V., Mansbach N., Turco S. J., Rosen G. Effect of glycolipids of Leishmania parasites on human monocyte activity. Inhibition by lipophosphoglycan. J Immunol. 1990 Dec 15;145(12):4284–4289. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Schnur L. F., Spithill T. W., Mitchell G. F. Passive transfer of Leishmania lipopolysaccharide confers parasite survival in macrophages. J Immunol. 1986 Dec 1;137(11):3608–3613. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Ilg T., Stierhof Y. D., Wiese M., McConville M. J., Overath P. Characterization of phosphoglycan-containing secretory products of Leishmania. Parasitology. 1994;108 (Suppl):S63–S71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- James S. L., Hibbs J. B., Jr The role of nitrogen oxides as effector molecules of parasite killing. Parasitol Today. 1990 Sep;6(9):303–305. doi: 10.1016/0169-4758(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Kelleher M., Bacic A., Handman E. Identification of a macrophage-binding determinant on lipophosphoglycan from Leishmania major promastigotes. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):6–10. doi: 10.1073/pnas.89.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb J. P., Paul-Eugene N., Damais C., Yamaoka K., Drapier J. C., Dugas B. Interleukin-4 stimulates cGMP production by IFN-gamma-activated human monocytes. Involvement of the nitric oxide synthase pathway. J Biol Chem. 1994 Apr 1;269(13):9811–9816. [PubMed] [Google Scholar]
- Liew F. Y., Cox F. E. Nonspecific defence mechanism: the role of nitric oxide. Immunol Today. 1991 Mar;12(3):A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Severn A., Millott S., Schmidt J., Salter M., Moncada S. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991 Oct;21(10):2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Liew F. Y., O'Donnell C. A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- Lorsbach R. B., Russell S. W. A specific sequence of stimulation is required to induce synthesis of the antimicrobial molecule nitric oxide by mouse macrophages. Infect Immun. 1992 May;60(5):2133–2135. doi: 10.1128/iai.60.5.2133-2135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauël J., Ransijn A., Buchmüller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol. 1991 Jan;49(1):73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Thomas-Oates J. E., Ferguson M. A., Homans S. W. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990 Nov 15;265(32):19611–19623. [PubMed] [Google Scholar]
- McNeely T. B., Rosen G., Londner M. V., Turco S. J. Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidylinositol antigens of the protozoan parasite Leishmania. Biochem J. 1989 Apr 15;259(2):601–604. doi: 10.1042/bj2590601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mossalayi M. D., Paul-Eugène N., Ouaaz F., Arock M., Kolb J. P., Kilchherr E., Debré P., Dugas B. Involvement of Fc epsilon RII/CD23 and L-arginine-dependent pathway in IgE-mediated stimulation of human monocyte functions. Int Immunol. 1994 Jul;6(7):931–934. doi: 10.1093/intimm/6.7.931. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Handman E. Treatment of murine macrophages with interferon-gamma inhibits their ability to bind leishmania promastigotes. J Leukoc Biol. 1992 Oct;52(4):369–376. doi: 10.1002/jlb.52.4.369. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Vlassara H., Edelson P. J., Cerami A. Leishmania promastigotes are recognized by the macrophage receptor for advanced glycosylation endproducts. J Exp Med. 1987 Jan 1;165(1):140–145. doi: 10.1084/jem.165.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994 May 13;269(19):13725–13728. [PubMed] [Google Scholar]
- Nikolaev A. V., Chudek J. A., Ferguson M. A. The chemical synthesis of Leishmania donovani phosphoglycan via polycondensation of a glycobiosyl hydrogenphosphonate monomer. Carbohydr Res. 1995 Aug 11;272(2):179–189. doi: 10.1016/0008-6215(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Proudfoot L., O'Donnell C. A., Liew F. Y. Glycoinositolphospholipids of Leishmania major inhibit nitric oxide synthesis and reduce leishmanicidal activity in murine macrophages. Eur J Immunol. 1995 Mar;25(3):745–750. doi: 10.1002/eji.1830250318. [DOI] [PubMed] [Google Scholar]
- Proudfoot L., Schneider P., Ferguson M. A., McConville M. J. Biosynthesis of the glycolipid anchor of lipophosphoglycan and the structurally related glycoinositolphospholipids from Leishmania major. Biochem J. 1995 May 15;308(Pt 1):45–55. doi: 10.1042/bj3080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puentes S. M., Sacks D. L., da Silva R. P., Joiner K. A. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J Exp Med. 1988 Mar 1;167(3):887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P., Pattabiraman T. N. Reevaluation of the phenol-sulfuric acid reaction for the estimation of hexoses and pentoses. Anal Biochem. 1989 Aug 15;181(1):18–22. doi: 10.1016/0003-2697(89)90387-4. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Locksley R. M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Roach T. I., Kiderlen A. F., Blackwell J. M. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991 Nov;59(11):3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Novakovic S., Gerold P., Schwarz R. T., McConville M. J., Tachado S. D. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol. 1996 Mar 1;156(5):1886–1896. [PubMed] [Google Scholar]
- Severn A., Xu D., Doyle J., Leal L. M., O'Donnell C. A., Brett S. J., Moss D. W., Liew F. Y. Pre-exposure of murine macrophages to lipopolysaccharide inhibits the induction of nitric oxide synthase and reduces leishmanicidal activity. Eur J Immunol. 1993 Jul;23(7):1711–1714. doi: 10.1002/eji.1830230747. [DOI] [PubMed] [Google Scholar]
- Stenger S., Thüring H., Röllinghoff M., Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994 Sep 1;180(3):783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Turco S. J., Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y., Kwon N. S., Pospischil M., Manning J., Paik J., Nathan C. Inactivation of nitric oxide synthase after prolonged incubation of mouse macrophages with IFN-gamma and bacterial lipopolysaccharide. J Immunol. 1994 Apr 15;152(8):4110–4118. [PubMed] [Google Scholar]
- Vouldoukis I., Riveros-Moreno V., Dugas B., Ouaaz F., Bécherel P., Debré P., Moncada S., Mossalayi M. D. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988 Feb;56(2):363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Jong M. T. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986 Dec 1;164(6):1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]




