Abstract
The objective of this study was to examine the effect of chronic dietary restriction on the physical characteristics of the intestine and gut-derived satiety hormone production. Male Wistar rats (8 weeks) were randomized to ad libitum (AL) or 35% dietary restriction (DR) for 5 months. At the end of the study, physical measurements were made on the intestine and satiety hormone secretion and mRNA expression determined. A comparison group of young, growing AL rats (5 weeks) was also examined. The adult DR rats gained less weight over 5 months and had lower fat mass than adult AL rats (p<0.05). The weight of the small intestine as a percentage of total body weight was greater in adult DR compared to adult AL but lower than young AL rats. Compared to AL, DR down-regulated proglucagon and cholecystokinin mRNA in the duodenum and ghrelin mRNA in the stomach of adult rats but was not different from young AL. Ghrelin-O-acyltransferase (GOAT) mRNA in the stomach was up-regulated 21-fold in adult AL rats compared to young AL and 14-fold compared to adult DR rats. Total and des-acyl ghrelin was approximately 50% higher in adult DR and young AL rats compared to adult AL. Plasma leptin and insulin were lower in adult DR and young AL rats compared to adult AL. Our findings suggest that long-term energy deficits continue to drive up ghrelin levels which may have profound implications for practical implementation of DR as an anti-aging or anti-obesity strategy in humans.
Keywords: Ghrelin-O-acyltransferase, Caloric restriction, Satiety, Gut hormones, Des-acyl ghrelin
1. Introduction
Long-term dietary restriction (DR) delays the onset of age-related diseases and increases longevity in several species [1,2]. The randomized, controlled CALERIE trial in overweight men and women demonstrated that 6 months of DR was associated with reduced fasting insulin and lower body temperature compared to control [3]. A recent in vitro study using sera collected from participants in the CALERIE trial showed that markers of longevity (Sirt1 protein levels and peroxisome proliferator-activated receptor coactivator-1 alpha (PGC-1α) mRNA levels) were improved following 6 months of DR [4]. While mechanisms of the anti-aging effects of DR have not been fully elucidated, they are likely manifest in part through endocrine and metabolic alterations. Insulin secretion decreases with DR in part due to enhanced insulin sensitivity [5–7]. Improvements in insulin sensitivity are likely brought about through reductions in circulating fatty acids, intramyocellular triacylglycerol and cytokine secretion from adipocytes [5]. More recently it has been suggested that hormesis, described as the beneficial effects of a treatment that at higher levels would be harmful, may play a role in the increased life span of animals subjected to DR [8]. Sub-lethal exposure to stressors may result in adaptive responses that improve stress resistance over time [8].
A complex interplay of anorexigenic (reduce food intake) and orexigenic (increase food intake) signals ultimately control energy balance in the body. The peripheral signals that regulate energy homeostasis can be categorized as long-term energy balance signals, such as leptin and insulin, or short-acting hunger and satiety signals; cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), peptide YY (PYY) and ghrelin [9,10]. We have previously demonstrated that diluting the energy density of a diet with fermentable dietary fiber increases the weight of the distal gut and enhances GLP-1 production [11–13]. Whether or not DR, which chronically suppresses delivery of food to the intestine, alters physical gut size and GLP-1 release is not known.
Ghrelin, largely produced in the stomach, is a 28-amino acid peptide that is acylated on the third amino acid serine by the enzyme GOAT (ghrelin-O-acyltransferase) to form bioactive acyl ghrelin. Acyl ghrelin is widely accepted as the active form of ghrelin based on its ability to bind and activate growth hormone secretagogue receptor-1a (GHS-R1a). In addition to triggering food intake it has been shown to affect adipogenesis, energy expenditure and gut peristalsis [14]. Injections of ghrelin (i.e. acyl ghrelin) have been shown to induce hunger and increase food intake in both lean and obese humans [15] and increase body weight when administered chronically to rats [16]. Also derived from the ghrelin gene is des-acyl ghrelin which until recently was considered inactive [17]. Des-acyl ghrelin accounts for ~90% of circulating levels of ghrelin in both rat and man [18]. Although the actions of des-acyl ghrelin on feeding behavior have not been sufficiently clarified, there is mounting evidence that it may in fact blunt the actions of circulating acyl ghrelin [19].
Given the interplay of numerous gut-derived endocrine factors in regulating energy balance and body weight, and the paucity of studies examining the integrated change in these satiety hormones with long-term DR, we investigated the physical changes in the intestine, the secretion of gut satiety hormones, and GOAT mRNA levels in male Wistar rats undergoing five months of DR. Given the age-related changes associated with gut satiety hormone production, it was also of interest to examine a group of young, growing AL-fed rats at 5 weeks of age as a separate comparison group.
2. Materials and methods
2.1. Animals
All procedures were approved by the University of Calgary Animal Care Committee and conformed to procedures set forth for the Care and Use of Laboratory Animals. At 8 weeks of age, 18 male Wister rats were randomly assigned to either the dietary-restricted (DR) treatment group (n=9) or the control AL group (n=9). AL and DR rats were paired at the beginning of the study according to body weight. One of the AL rats died from causes unrelated to the study and its pair was thereby matched to another AL rat of similar body weight. The rats were individually housed in standard rat cages and were on a 12-hour light/dark cycle (lights on at 7:00 am). For 5 months, the control rats were allowed to eat AL while treatment rats were restricted and consumed 35% less chow than their pair-fed mates. Throughout the 5 months, food intake of the AL group was weighed daily and then multiplied by 0.65 to determine the amount of food provided as a single daily amount to the DR group in the afternoon of the next day. The rat chow consisted of 24% protein, 6% fat, 5.3% fiber, 6.9% ash, 0.95% calcium, and 0.69% phosphorus (Prolab RMH 2500; PMI Nutrition International, Brentwood, MO). A separate group of male AL rats was sacrificed at 5 weeks of age to provide a young, growing comparison group. At the end of the study, blood was collected from all rats at the same time in the morning (2 h into the light cycle). The rats were over-anesthetized with Halothane; exsanguinated via cardiac bleed; and intestines and major organs removed. Once the length of intestinal segments was determined under tension from a 5 g weight and the mass of desired organs and intestinal segments measured (duodenum, jejunum, ileum and colon dissected separately), all sections were snap frozen in liquid nitrogen and stored at −80 °C.
2.2. RNA extraction and cDNA synthesis
Total RNA was extracted from the small intestine, colon and stomach using TRIzol reagent (Invitrogen, Carlsbad, USA). Total RNA (1 μg) was reverse-transcribed using Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) with oligo d(T)12–18 as primer. After reverse transcription, the same batch of diluted cDNA was subjected to real-time PCR to amplify target genes.
2.3. Real-time PCR
Primers for real-time PCR were designed to span two exon boundaries using Primer Express software (Beacon Designer, PRIMIER Biosoft International, CA, USA), thus restricting PCR amplifications to cDNA templates only. The primers for proglucagon, PYY, ghrelin, CCK and GOAT were synthesized by UC-DNA Lab (University of Calgary, Alberta, Canada). Primer sequences were: proglucagon [forward: ACCGCCCTGAGATTACTTTTCTG; reverse: AGTTCTCTTTCCAGGTTCACCAC]; ghrelin [forward: AGAGGCGCCAGCTAACAAGTAA; reverse: GCAGGAGAGTGCTGGGAGTT]; CCK [forward: GCCGCCTGCCCTCAAC; reverse: ACACACGCCGCACTTCATATC]; PYY [forward: AGCGGTATGGGAAAAGAGAAGTC; reverse: ACCACTGGTCCACACCTTCTG]; GOAT [forward: ACTCACCTACTACTCCCACTG; reverse: TTTCCAATGTCCAAATGTCCAC]; actin [forward: TATCGGCAATGAGCGGTTCC; reverse: AGCACTGTGTTGGCATAGAGG]. Real-time PCR reactions were carried out as previously described [20] and the fold change in target gene relative to beta-actin was determined using the 2−ΔΔCT method [21].
2.4. Plasma analysis
Cardiac blood was collected with the addition of diprotin-A (34 μg/ml) and aprotinin (500 kIU/ml). Plasma was stored at −80 °C until analyzed with the Rat Endocrine LINCOplex kit (Millipore, St. Charles, MO, USA). Duplicate samples of 10 μl of plasma were analyzed using antibody-immobilized beads specific for the hormones: active GLP-1, glucagon, insulin, amylin and leptin. The sensitivity of the multiplex kit is 55.6 pM for insulin and 6.2 pM for all other analytes. Des-acyl ghrelin was measured using the Rat Des-Acyl Ghrelin ELISA kit from Linco Research Inc. (Millipore, St. Charles, MO, USA). Total ghrelin was quantified using the Ghrelin (Rat, Mouse) EIA kit (Phoenix Pharmaceuticals Inc., Burlingame, CA). Blood glucose was measured immediately upon collection using an ABL 725 Blood Gas Analyzer (Radiometer, Brønshøj, Denmark).
2.5. Statistical analysis
Data and figures are presented as means±SEM. Statistical comparisons were performed using a one-way analysis of variance (ANOVA) with Tukey’s post-hoc test using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Values were considered significant when p≤0.05.
3. Results
Body weight (BW) increased in both the AL and DR rats during the 5 month study, however, DR rats gained significantly less weight and attained a final body weight that was ~37% lower than AL rats (p<0.0001, Table 1). At sacrifice, absolute weight of the heart, liver, and kidneys was lower in DR rats compared to AL rats, however, when expressed as a percentage of total body weight, only the liver remained significantly smaller in DR versus AL rats (p<0.001). Epididymal, retroperitoneal, and inguinal fat pad mass was significantly lower in the DR rats as was the combined total mass of all 3 fat depots together (Table 1). As a percentage of body weight, DR rats had nearly 50% less body fat compared to AL rats (p<0.001).
Table 1.
Body and organ weights of adult ad libitum versus dietary-restricted rats.
| AL | DR | |
|---|---|---|
| Final body weight (g) | 537.0±26.7 | 390.1±20.5† |
| Weight gain (g) | 332.0±8.5 | 186.4±6.4† |
| Organ weights | ||
| Heart (g) | 1.56±0.08 | 1.19±0.04* |
| Liver (g) | 17.12±0.75 | 9.85±0.32* |
| Kidneys (g) | 3.96±0.17 | 2.65±0.72* |
| Epididymal fat pad (g) | 7.66±0.99 | 2.91±0.17# |
| Retroperitoneal fat pad (g) | 4.52±0.68 | 0.81±0.22# |
| Inguinal fat pad (g) | 5.30±0.93 | 2.08±0.34* |
| Combined fat mass (g) | 17.49±0.95 | 6.77±0.61# |
| Organ mass as % total body weight | ||
| Heart (% BW) | 0.29±0.01 | 0.31±0.01 |
| Liver (% BW) | 3.19±0.12 | 2.52±0.05# |
| Kidneys (% BW) | 0.74±0.03 | 0.68±0.03 |
| Combined fat mass (% BW) | 3.29±0.38 | 1.73±0.15# |
Values are mean±SEM. n=8 rats in the ad libitum group and n=9 in the dietary-restricted group.
represents a significant difference between AL (ad libitum) and DR (dietary restriction) (p≤0.05).
represents a significant difference at p≤0.001.
represents a significant difference at p≤0.0001.
Absolute weight and length of the small intestine and colon of adult DR rats were lower than adult AL rats (Table 2). As expected, the young AL rats had lower absolute intestine and stomach weight compared to both adult groups (Table 2). When expressed as a proportion of overall body weight, however, the young AL rats had significantly greater intestinal weight and length than both adult groups. The adult DR rats also had proportionately greater small intestine weight and length compared to the adult AL rats. A consistent pattern of young AL>adult DR>adult AL was also seen for colon length and stomach weight when expressed relative to body weight.
Table 2.
Intestinal characteristics of ad libitum versus dietary-restricted rats.
| Young AL | Adult AL | Adult DR | |
|---|---|---|---|
| Absolute weight or length | |||
| Small intestine weight (g) | 4.4±0.3a | 8.7±0.3b | 7.0±0.2c |
| Small intestine length (cm) | 101.5±1.9a | 129.3±1.9b | 119.7±1.9c |
| Colon weight (g) | 0.7±0.1a | 1.9±0.1b | 1.3±0.1c |
| Colon length (cm) | 11.9±0.8a | 21.5±0.6b | 18.0±0.5c |
| Stomach weight (g) | 1.09±0.11a | 2.28±0.07b | 2.50±0.07b |
| Adjusted for body weight | |||
| Small intestine weight/BW (mg/g) | 30.1±0.8a | 16.3±0.5b | 17.8±0.4c |
| Small intestine length/BW (mm/g) | 7.1±0.3a | 2.4±0.05b | 3.1±0.06c |
| Colon weight/BW (mg/g) | 5.0±0.3a | 3.6±0.1b | 3.4±0.1b |
| Colon length/BW (mm/g) | 0.82±0.03a | 0.40±0.01b | 0.46±0.01c |
| Stomach weight/BW (mg/g) | 7.5±0.5a | 4.3±0.2b | 6.4±0.1c |
Values are mean±SEM. There were n=8, 9 and 6 rats respectively in the adult AL (ad libitum), adult DR (dietary-restricted), and young AL groups. Stomach weight represents empty stomach weight. Values with different letters are significantly different (p≤0.05).
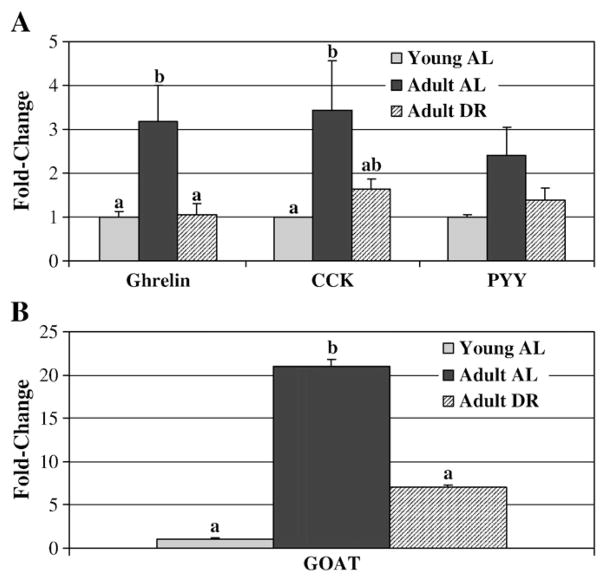
Proglucagon mRNA was down-regulated in the duodenum of adult DR rats compared to adult AL rats (Fig. 1). Young AL rats had lower proglucagon mRNA levels than both adult groups. Proglucagon mRNA in the jejunum was significantly higher in DR versus young AL rats (p<0.05). There were no differences in proglucagon (Fig. 1) or PYY (Fig. 2A) mRNA in the ileum and colon. In the stomach, ghrelin mRNA levels were significantly higher in adult AL compared to young AL and adult DR rats (Fig. 2A). CCK mRNA was significantly higher in adult AL compared to young AL rats and showed a trend towards higher expression in adult AL versus adult DR (p=0.06). GOAT mRNA in the stomach was significantly higher in adult AL compared to both adult DR and young AL rats (Fig. 2B; p<0.002).
Fig. 1.
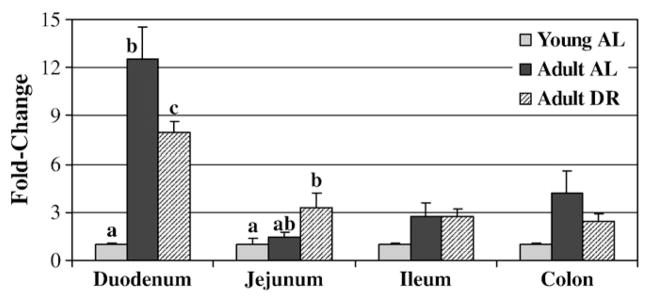
Intestinal proglucagon mRNA expression in young and adult ad libitum versus dietary-restricted rats. Results represent the mean±SEM for 8 adult AL (ad libitum), 9 adult DR (dietary restriction), and 6 young AL rats. Gene expression is expressed as a fold change from the young AL rats for which the value is set at 1.0. Using the 2−ΔΔCT calculation each gene is corrected against the housekeeping gene actin. Values with different letters represent a difference between groups within a given intestinal segment (p<0.05). Where no letters are present there are no differences between groups.
Fig. 2.
Gene expression of (A) ghrelin, cholecystokinin (CCK), peptide YY (PYY), and (B) GOAT mRNA in young and adult ad libitum versus dietary-restricted rats. Results represent the mean±SEM for 8 adult AL (ad libitum), 9 adult DR (dietary restriction), and 6 young AL rats. Gene expression is expressed as a fold change from the young AL rats for which the value is set at 1.0. Using the 2−ΔΔCT calculation each gene is corrected against the housekeeping gene actin. Values with different letters are significantly different within a given gene (p<0.05).
Plasma concentrations of leptin and insulin were significantly lower in young AL and adult DR compared to adult AL rats (p=0.001; Fig. 3). Increases in plasma des-acyl ghrelin concentrations were nearly 2.5-fold higher in the adult DR rats compared to adult AL (p<0.001). Likewise, total ghrelin was significantly higher in adult DR and young AL rats compared to adult AL rats. There were no significant differences in plasma GLP-1, amylin and glucagon between the adult DR and adult AL rats. GLP-1 and glucagon, however, were significantly higher in young AL rats compared to the two adult groups (Fig. 4). Postprandial glucose levels at sacrifice were significantly lower in adult DR rats in comparison to adult AL (5.86±0.55 versus 9.18±0.43 mmol/l respectively; p=0.003). Young AL rats were intermediate at 6.96±0.20 mmol/l.
Fig. 3.
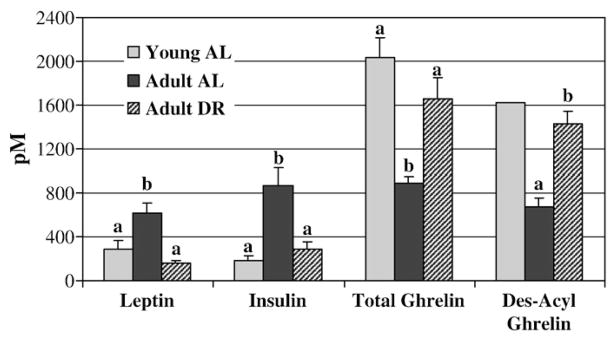
Plasma leptin, insulin, total ghrelin and des-acyl ghrelin concentrations in young and adult ad libitum versus dietary-restricted rats. Results represent the mean±SEM for 8 adult AL (ad libitum), 9 adult DR (dietary restriction), and 6 young AL rats. Blood was sampled in the morning ~2 h into the light cycle. Des-acyl ghrelin concentrations were not measured in the young AL rats due to a limited volume of plasma. The bar in the graph represents an estimated level from the known proportion of total ghrelin concentrations measured in the adult DR and AL rats. Values with different letters are significantly different within a given hormone (p<0.01).
Fig. 4.
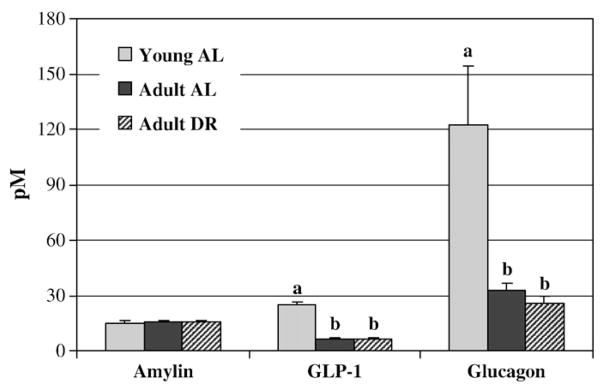
Plasma amylin, GLP-1 and glucagon concentrations in young and adult ad libitum versus adult dietary-restricted rats. Results represent the mean±SEM for 8 adult AL (ad libitum), 9 adult DR (dietary restriction), and 6 young AL rats. Blood was sampled in the morning ~2 h into the light cycle. Values with different letters are significantly different within a given hormone (p<0.01).
4. Discussion
Consistent with other studies, we demonstrate that long-term DR results in markedly reduced weight gain compared to AL-fed rats [22]. Similar to others we also found lower organ mass [23], reduced weight of epididymal, retroperitoneal and inguinal fat pads, and markedly reduced leptin and insulin levels following DR [24,25]. Our novel findings include a proportionate increase in gut size and a marked increase in both total and des-acyl ghrelin despite decreased gastric ghrelin and GOAT mRNA. Also interesting is the observation that the pattern of physical gut characteristics and the production and secretion of satiety hormones following DR resembles that seen in young, growing rats more closely than adult AL-fed rats.
The most intriguing finding of this study is the marked elevation in circulating levels of total and des-acyl ghrelin following DR without any change in the anorexigenic peptides, GLP-1 and amylin. Acyl ghrelin is a potent orexigenic hormone that plays a role in hunger and meal initiation [26]. Levels of ghrelin are increased in Sprague–Dawley rats following a 48-hour fast and fall after re-feeding [27]. Intuitively one could argue that adaptation to DR should dampen the sensation of hunger over time, yet studies examining ghrelin levels after varying lengths of restriction do not support this. Gualillo et al. [28] and Barazzoni et al. [29] observed an increase in plasma total ghrelin after 21 and 7 days of restriction respectively in rodents. Following chronic restriction, Komatsu et al. [30] showed an increase in des-acyl ghrelin and Yang et al. [31] an increase in total ghrelin in restricted versus AL-fed mice. Our study, examining both des-acyl and total ghrelin, confirms a marked increase in both forms of ghrelin in DR rats. In addition, these higher ghrelin levels resemble those seen in young, growing rats more so than adult AL rats.
Contrary to Gonzalez et al. [32], however, we observed a decrease in GOAT mRNA following DR. Gonzalez et al. [32] demonstrated that GOAT mRNA was significantly increased in the stomach mucosa of rats following 21 days of DR. We show that a period of restriction that spans months rather than weeks is associated with a marked decrease in GOAT mRNA compared to adult rats fed AL. Because Gonzalez et al. [32] did not take measurements beyond 21 days it is difficult to know whether the spike in GOAT mRNA they observe is transient or persists over time. A limitation of our study is the lack of GOAT mRNA measurements in the DR versus AL rats at earlier time points during the 5 months of restriction, presuming that levels of GOAT and other satiety factors fluctuate over the course of adaptation to the restriction. Kirchner et al. [33], however, have shown that fasting for 12, 24 or 36 h also decreases GOAT expression in mice and this is associated with an increase in plasma des-acyl but not acyl ghrelin. They go on to show that acyl ghrelin was highest at 2 h into the dark phase which represents the most intense feeding period in rodents [33]. Given that DR rats typically consume their daily food allotment within a few hours compared to the numerous smaller meals consumed by AL rats during the dark phase, it is plausible that this pattern of food consumption influenced the levels of satiety hormones we report in this study. The distinct feeding pattern of DR compared to AL has been shown to alter the circadian cycles of serum hormone levels such as corticosterone, insulin, or thyroid hormones [34] in part via regulation of the suprachiasmatic nuclei of the anterior hypothalamus [35]. Our data showing decreased GOAT mRNA with DR, measured 2 h into the light cycle, is in keeping with the suggestions of others that the addition of an acyl group to ghrelin is inhibited after a 48-hour fast in rats [36] and complete fasting for several days reduces the proportion of acyl to des-acyl ghrelin in humans [37].
It is interesting but perhaps not surprising that the expression pattern for ghrelin and proglucagon mRNA did not reflect the circulating levels of ghrelin and GLP-1. In fact, le Roux et al. [38] showed that while there was no difference in PYY mRNA in the colon of lean versus obese mice, there was a significant increase in tissue PYY levels in obese rats and a significant decrease in plasma PYY levels in obese versus lean mice. A similar report of reduced plasma GLP-1 levels together with increased tissue levels has been reported in high fat fed mice [39]. One explanation for the elevated plasma ghrelin we observed despite lower mRNA expression could be the significantly higher relative small intestine, colon and stomach weight of DR compared to AL rats. It is possible that despite lower ghrelin mRNA expression, the greater mass of the stomach in DR rats served as a larger pool of mRNA from which circulating plasma ghrelin could then be generated. Having shown similar results, Kirchner et al. [33] would suggest that it is a reflection of the nutritional status, particularly the fatty acid status of the animal that causes this discrepancy. They observed decreased GOAT mRNA and elevated plasma des-acyl ghrelin following12, 24 and 36 h of fasting. Kirchner et al. [33] suggest that GOAT mRNA is down-regulated in the fasted state due to the absence of medium chain triglycerides, a major substrate for GOAT that allows for the acylation of ghrelin.
The significance of the similarity of plasma and mRNA levels of ghrelin between the young, growing rats and adult DR rats is not entirely clear. In humans, others have described a fall in ghrelin throughout childhood and into puberty [40] as well as an age-dependent decrease in ghrelin gene expression in the adrenal cortex [41]. The growth hormone (GH) releasing effect of ghrelin also decreases with age [42]. Recent observations suggest that growth hormone (GH) secretagogues, such as ghrelin, may reverse the age-related decline in the GH/IGF-1 (insulin-like growth factor-1) axis [43]. Given the ability of DR to reverse the age-associated decrease in ghrelin, a greater examination of this temporal relationship is warranted.
The interplay between ghrelin and the long-term energy balance signals, leptin and insulin, is also noteworthy in DR given the observation that circulating leptin negatively regulates plasma ghrelin concentrations [29]. The reduced plasma concentration of leptin seen with DR or conversely the hyperleptinemia of obesity, triggers an inverse adaptive change in circulating ghrelin [28,29,44,45]. Within the central nervous system (CNS), leptin and insulin have been shown to inhibit pathways that stimulate food intake and weight gain while stimulating pathways that reduce food intake and weight gain [9] Therefore, in conditions associated with negative energy balance such as fasting or energy restriction, insulin and leptin signaling are reduced. It is via this set of integrated responses that the body attempts to replenish depleted fat stores. Ghrelin is thought to play a role in this response and has been shown to stimulate feeding via activation of orexigenic neurons [46]. Based on our observations, one may predict in humans, that maintaining prolonged voluntary energy restriction is difficult in a setting where ghrelin levels remain elevated.
Translation of animal hormonal data into behavioral changes in humans, however, is not straightforward. Reports of subjective ratings of appetite in obese adults have shown both increased [47] and decreased [48] hunger levels following energy restriction. In overweight adults enrolled in the CALERIE trial, Anton et al. [49] reported no major differences in subjective appetite between a weight stable control group and three restricted groups (25% DR; 12.5% DR plus exercise; and low calorie diet). The reason for the disparate findings is not entirely clear, but may in part be attributed to the influence of the clinical setting on subjective reporting of appetite. Anton et al. [49] suggest that collection of VAS data during feeding periods in the research center in addition to the inherent demands of participating in a study may affect how appetite is subjectively rated by participants. Perhaps equally important in explaining temporal shifts in appetite is the duration of the restriction. Anton et al. [49] generally observed changes in appetite markers in the first 2 months of their study which then stabilized from months 3 to 6. Our DR rats had not yet reached a stable body weight and this may account in part for the elevated ghrelin levels we observed.
Taken together our findings suggest that despite adaptation in many other tissues and systems, the chronic energy deficit associated with DR continues to drive up ghrelin levels in an attempt to restore energy sufficiency. This has profound implications for the practical implementation of DR as an anti-aging strategy in humans as the sensation of hunger remains heightened even months or potentially years after initiation as the body attempts to combat the ongoing energy deficit. The markedly elevated levels of ghrelin but not GLP-1 seen in DR rats in this study suggest that orexigenic peptides may be more readily influenced by long-term DR than anorexigenic peptides. Ghrelin causes robust feeding responses and this physiological drive may not diminish even after the body reaches a new lower level of energy balance in the restricted state. Indeed, it may even be speculated, but not yet proven, that the persistence of hunger is a type of low-intensity stressor that plays a role in the hormesis of caloric restriction. The persistent hunger may act as a low grade stress, caused by the sustained release of ghrelin that in turn exerts positive effects on delaying the aging process. The possibility remains to be tested.
Acknowledgments
This work was supported in part by the Natural Sciences and Engineering Research Council, Canadian Institutes of Health Research, and the Margaret Gunn Endowment for Animal Research.
References
- 1.Masoro EJ. Nutrition and aging—a current assessment. J Nutr. 1985;115:842–8. doi: 10.1093/jn/115.7.842. [DOI] [PubMed] [Google Scholar]
- 2.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 3.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allard JS, Heilbronn LK, Smith C, Hunt ND, Ingram DK, Ravussin E, et al. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS ONE. 2008;3:e3211. doi: 10.1371/journal.pone.0003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–9. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 6.Lane MA, Ball SS, Ingram DK, Cutler RG, Engel J, Read V, et al. Diet restriction in rhesus monkeys lowers fasting and glucose-stimulated glucoregulatory end points. Am J Physiol Enocrinol Metab. 1995;268:E941–8. doi: 10.1152/ajpendo.1995.268.5.E941. [DOI] [PubMed] [Google Scholar]
- 7.Cefalu WT, Wagner JD, Wang ZQ, Bell-Farrow AD, Collins J, Haskell D, et al. A study of caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): a potential model for aging research. J Gerontol A Biol Sci Med Sci. 1997;52:B10–9. doi: 10.1093/gerona/52a.1.b10. [DOI] [PubMed] [Google Scholar]
- 8.Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metabolism. 2008;7:200–3. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Carrascosa JM, Ros M, Andres A, Fernandez-Agullo T, Arribas C. Changes in the neuroendocrine control of energy homeostasis by adiposity signals during aging. Exp Gerontol. 2009;44:20–5. doi: 10.1016/j.exger.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 11.Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–56. doi: 10.1210/endo.137.9.8756571. [DOI] [PubMed] [Google Scholar]
- 12.Reimer RA, Thomson ABR, Rajotte R, Basu TK, Ooraikul B, McBurney MI. A physiological level of rhubarb fiber increases proglucagon gene expression and modulates intestinal glucose uptake in rats. J Nutr. 1997;127:1923–8. doi: 10.1093/jn/127.10.1923. [DOI] [PubMed] [Google Scholar]
- 13.Reimer RA, Russell JC. Glucose tolerance, lipids and GLP-1 secretion in JCR:LA-cp rats fed a high protein fiber diet. Obesity. 2008;16:40–6. doi: 10.1038/oby.2007.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogueiras R, Tschop M. Separation of conjoined hormones yields appetite rivals. Science. 2005;310:985–6. doi: 10.1126/science.1121214. [DOI] [PubMed] [Google Scholar]
- 15.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Int J Obes (Lond) 2005;29:1130–6. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 16.Tshop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 17.Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Soares J-B, Leite-Moreira AF. Ghrelin, des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides. 2008;29:1255–70. doi: 10.1016/j.peptides.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Qadar SS, Hakanson R, Rehfeld JF, Lundquist I, Salehi A. Proghrelin-derived peptides influence the secretion of insulin, glucagon, pancreatic polypeptide and somatostatin: a study on isolated islets from mouse and rat pancreas. Regul Pept. 2008;146:230–7. doi: 10.1016/j.regpep.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Parnell JA, Reimer RA. Differential secretion of satiety hormones with progression of obesity in JCR:LA corpulent rats. Obesity. 2008;16:736–42. doi: 10.1038/oby.2007.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 22.Merry BJ. Dietary restriction in rodents — delayed or retarded ageing? Mech Ageing Dev. 2005;126:951–9. doi: 10.1016/j.mad.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey JJ, Harper M-E, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000;29:946–68. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–49. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 25.Shimokawa I, Higami Y. A role for leptin in the antiaging action of dietary restriction: a hypothesis. Aging (Milano) 1999;11:380–2. doi: 10.1007/BF03339816. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, et al. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006;254–255:127–32. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 28.Gualillo O, Caminos JE, Nogueiras R, Seoane LM, Arvat E, Ghigo E, et al. Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obes Res. 2002;10:682–7. doi: 10.1038/oby.2002.92. [DOI] [PubMed] [Google Scholar]
- 29.Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology. 2003;124:1188–92. doi: 10.1016/s0016-5085(03)00281-6. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu T, Chiba T, Yamaza H, To K, Toyama H, Higami Y, et al. Effect of leptin on hypothalamic gene expression in calorie-restricted rats. J Gerontol A Biol Sci Med Sci. 2006;61:890–8. doi: 10.1093/gerona/61.9.890. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Youm Y-H, Nakata C, Dixit VD. Chronic caloric restriction induced forestomach hypertrophy with enhanced ghrelin levels during aging. Peptides. 2007;28:1931–6. doi: 10.1016/j.peptides.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez CR, Vazquez MJ, Lopez M, Dieguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J Mol Endocrinol. 2008;41:415–21. doi: 10.1677/JME-08-0102. [DOI] [PubMed] [Google Scholar]
- 33.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nature. 2009;15:741–5. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leakey JEA, Chen S, Manjgaladze M, Turturro A, Duffy PH, Pipkin JL, et al. Role of glucocorticoids and “caloric stress” in modulating the effects of caloric restriction in rodents. Ann NY Acad Sci. 1994;719:171–94. doi: 10.1111/j.1749-6632.1994.tb56828.x. [DOI] [PubMed] [Google Scholar]
- 35.Froy O, Chapnik N, Miskin R. The suprachiasmatic nuclei are involved in determining circadian rhythms during restricted feeding. Neuroscience. 2008;155:1152–9. doi: 10.1016/j.neuroscience.2008.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, et al. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–5. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–7. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne K, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 39.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–9. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 40.Whatmore AJ, Hall CM, Jones J, Westwood M, Clayton PE. Ghrelin concentrations in healthy children and adolescents. Clin Endocrinol (Oxf) 2003;59:649–54. doi: 10.1046/j.1365-2265.2003.01903.x. [DOI] [PubMed] [Google Scholar]
- 41.Carraro G, Albertin G, Aragona F, Forneris M, Casale V, Spinazzi R, et al. Age-dependent decrease in the ghrelin gene expression in the human adrenal cortex: a real-time PCR study. Int J Mol Med. 2006;17:319–21. [PubMed] [Google Scholar]
- 42.Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, et al. The endocrine response to ghrelin as function of gender in human young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537–42. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- 43.Frutos MG, Cacicedo L, Fernandez C, Vicent D, Velasco B, Zapatero H, et al. Insights into a role of GH secretagogues in reversing the age-related decline in the GH/IGF-1 axis. Am J Physiol Endocrinol Metab. 2007;293:E1140–52. doi: 10.1152/ajpendo.00236.2007. [DOI] [PubMed] [Google Scholar]
- 44.Hansen TK, Dall R, Hosoda H. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56:203–6. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 45.Tschop M, Weyer C, Tataranni AP, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–9. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 46.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–30. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 47.Doucet E, St-Pierre S, Almeras N, Tremblay A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite. 2003;40:137–43. doi: 10.1016/s0195-6663(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 48.Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, et al. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consul Clin Psychol. 1997;65:269–77. doi: 10.1037//0022-006x.65.2.269. [DOI] [PubMed] [Google Scholar]
- 49.Anton SD, Han Y, York E, Martin CK, Ravussin E, Williamson DA. Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet. 2009;22:141–7. doi: 10.1111/j.1365-277X.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]