Abstract
Background
Rodent studies show that oligofructose promotes weight loss, stimulates satiety hormone secretion, reduces energy intake, and improves lipid profiles.
Objective
Our objective was to examine the effects of oligofructose supplementation on body weight and satiety hormone concentrations in overweight and obese adults.
Design
This study was a randomized, double-blind, placebo-controlled trial. Forty-eight otherwise healthy adults with a body mass index (in kg/m2) > 25 were randomly assigned to receive 21 g oligo-fructose/d or a placebo (maltodextrin) for 12 wk. Body composition (by dual-energy X-ray absorptiometry); meal tolerance tests, including satiety hormone response; food intake; and subjective appetite ratings were determined.
Results
There was a reduction in body weight of 1.03 ±0.43 kg with oligofructose supplementation, whereas the control group experienced an increase in body weight of 0.45 ± 0.31 kg over 12 wk (P = 0.01). A lower area under the curve (AUC) for ghrelin (P = 0.004) and a higher AUC for peptide YY (PYY) with oligofructose (P = 0.03) coincided with a reduction in self-reported caloric intake (P ≤ 0.05). Glucose decreased in the oligofructose group and increased in the control group between initial and final tests (P ≤ 0.05). Insulin concentrations mirrored this pattern (P ≤ 0.05). Oligofructose supplementation did not affect plasma active glucagon-like peptide 1 secretion. According to a visual analog scale designed to assess side effects, oligofructose was well tolerated.
Conclusions
Independent of other lifestyle changes, oligofructose supplementation has the potential to promote weight loss and improve glucose regulation in overweight adults. Suppressed ghrelin and enhanced PYY may contribute in part to the reduction in energy intake. The trial was registered at clinicaltrials.gov as NCT00522353.
INTRODUCTION
Obesity is clearly linked to disturbances in energy intake; therefore, the identification of novel foods that promote satiety or dilute energy density provide a possible first line of defense in managing obesity and its associated comorbidities (1). Oligo-fructose is a potential candidate that enhances satiety and has positive organoleptic properties that would foster incorporation into a variety of foods (2). The effectiveness and mechanisms by which oligofructose may act to promote weight loss in humans warrant further investigation.
Glucagon-like peptide 1 (GLP-1) has numerous antiobesity and antidiabetic actions, including inhibition of food intake, delayed gastric emptying, weight loss, stimulation of insulin secretion, and induction of β cell proliferation (3). Colocalized with GLP-1 in the intestinal L cells is peptide YY (PYY), also considered an an-orexigenic peptide (4, 5). In human obesity, plasma concentrations of GLP-1 (6) and PYY (5) are reduced, and this impaired secretion may promote the development of obesity or hinder weight loss or both. Conversely, ghrelin stimulates food intake and weight gain as well as promotes adiposity (7, 8). Of the gut satiety hormones, those that are responsive to diet composition, including GLP-1, PYY, and ghrelin (9), are promising targets for weight management through diet modification.
Interest in oligofructose supplementation for weight management stems from rat studies that report reductions in energy intake, increased plasma GLP-1 and PYY concentrations, and decreased ghrelin (10, 11). The mechanisms by which oligofructose enhances satiety may involve fermentation by select bacterial strains and increased production of short-chain fatty acids in the gut lumen (12). Short-chain fatty acids stimulate intestinal proglucagon mRNA expression and GLP-1 secretion in rodents (13, 14) and dogs (15) and PYY secretion in the colons of rats (16). Oligofructose also improves glucose tolerance in streptozotocin-treated rats with concomitant increases in GLP-1, plasma insulin, and pancreatic insulin and β cell mass (17). Furthermore, Cani et al (18) have shown an essential role for GLP-1 receptor–related mechanisms for the effects of oligofructose because GLP-1 receptor knockout mice were insensitive to oligofructose antidiabetic actions.
Human trials specifically evaluating oligofructose supplementation for weight loss and glucose response are lacking. Cani et al (2) have shown that oligofructose enhanced subjective satiety in adults. Satiety hormone response to an oligofructose-type fiber, in humans, has only been examined by Piche et al (19) who observed increased GLP-1 concentrations after 7 d of fructooligosaccharide supplementation in subjects with gastro-esophageal reflux disease. Studies specifically designed to assess weight change and glucose tolerance in overweight individuals with long-term supplementation of oligofructose have not been performed. Our objective was to assess the effects of 12 wk oligofructose supplementation on weight loss, energy intake, plasma satiety hormone and glucose concentrations, and subjective appetite in overweight and obese adults.
SUBJECTS AND METHODS
Subjects
A total of 48 overweight or obese subjects [body mass index (BMI; in kg/m2) > 25] between the ages of 20 and 70 y were voluntarily recruited from the city of Calgary, Canada. The sample size was based on power calculations that used weight loss as the primary outcome. With an estimated weight loss of 2.0 kg and an SD of 2.0 kg (20) based on 0.8 power to detect a significant difference (P = 0.05, 2 sided), a minimum of 32 subjects were needed; 16 additional subjects were added to account for dropouts. Subjects were randomly assigned to the oligofructose or placebo group according to a computer-generated randomization list designed to control for BMI, sex, and age. All subjects had a stable body weight for ≥3 mo before the study. Subjects who had clinically significant cardiovascular abnormalities, liver or pancreatic disease, diabetes, major gastrointestinal surgeries; were pregnant or lactating; exhibited alcohol or drug dependence; were being treated with insulin; were on drugs influencing appetite; were following a diet or exercise regimen designed for weight loss; had a body mass >350 lb; or chronically used antacids or bulk laxatives were excluded from the study. All subjects completed a health and lifestyle questionnaire to determine eligibility. The Conjoint Health Research Ethics Board of the University of Calgary provided ethical approval for the study, and written informed consent was obtained from each participant.
Dietary intervention
Subjects were randomly assigned to receive either a daily supplement of 21 g oligofructose (Raftilose P95; kindly provided by Quadra Chemicals Ltd, Burlington, Canada) or an equicaloric amount of maltodextrin placebo (Grain Processing Corporation, Muscatine, IA) for 12 wk. Maltodextrin was selected as the placebo because it was used as a control in a previous study that examined oligofructose (2) and has a similar taste and appearance. The doses of oligofructose and placebo were increased over a 2-wk period to promote adaptation to the fiber and to reduce gastrointestinal symptoms. At full dose, the oligofructose group received three 7-g packets/d, providing 10.5 kcal/packet, and the placebo group received maltodextrin in three 2.63 g packets/d, providing 10.5 kcal/packet that were to be added to a 250-mL drink before meals. Both the maltodextrin placebo and the oligofructose treatment were provided to the subjects in identical opaque packages. Both the subjects and the researcher were blinded to the treatment. Subjects were instructed to return all packets to assess compliance. The blinding was evaluated through an informal interview. Subjects purchased and prepared their own meals and were not provided with any specific dietary instructions. The study was designed to examine the effects of oligofructose independent of any other diet or exercise intervention; therefore, subjects were encouraged to maintain their regular lifestyle, to eat until comfortably full, and not to consciously try to gain or lose weight throughout the study.
Exercise
Subjects recorded their physical activity daily on a calendar. These recordings were reviewed to ensure subjects maintained a consistent level of physical activity throughout the study.
Food intake
Food and beverage intakes were assessed with the use of 3-d weighed food records. Before the start of the study all subjects attended a training session where they were instructed on the use of the food scale and how to record their food intake. Subjects were then instructed to weigh and record all food consumed for 2 week days and 1 weekend day at baseline and every 3 wk thereafter. This information was analyzed with the use of Diet Analysis Plus 8.0 software (Thomson Wadsworth, Toronto, Canada).
Physical characteristics
Height was measured at the beginning of the study to confirm BMI. Every 3 wk, the investigator measured body weight with a calibrated balance beam scale. Waist circumference was measured every 6 wk. At initial and final test days, subjects underwent a dual-energy X-ray absorptiometry (DXA) scan (Hologic QDR 4500; Hologic Inc, Bedford, MA). These results were used in conjunction with Hologic QDR software to determine lean mass, bone mass, and fat mass. Percentage of fat was also calculated.
Meal tolerance test
On the initial test day after an overnight fast, subjects were given a standardized meal as part of the meal tolerance test (MTT), and blood samples were taken at 0 (fasting), 15, 30, 60, 120, 240, and 360 min postprandially. The test meal consisted of 90 g white bread, 10 g butter, 2 boiled eggs, and 200 mL unsweetened orange juice. The energy value of the meal was 520 kcal from 20 g protein, 21 g fat, and 62 g carbohydrate (21). All subjects were instructed to finish the meal in 15 min. At the end of the intervention, the same test was repeated with either a dose of oligofructose or maltodextrin mixed into the orange juice of subjects.
Blood sampling
A cannula was inserted into the antecubital vein for blood draws and kept patent with saline solution. Once drawn, blood was immediately placed in a cooled EDTA-treated tube containing diprotinin-A (0.034 mg/mL blood; MP Biomedicals, Irvine, CA); protease inhibitor (1 mg/mL blood; Sigma-Aldrich, Oakville, Canada), and Pefabloc (1 mg/mL blood; Roche, Mississauga, Canada). Additional blood was collected with sodium fluoride for glucose analysis. Within 30 min, the blood was centrifuged at 3000 rpm for 12 min at 4°C. Plasma was analyzed for ghrelin (active), glucose-dependent insulinotropic polypeptide (GIP), GLP-1 (active), insulin, leptin, and PYY. At fasting, additional blood samples were collected for lipid analysis and for human chorionic gonadotropin in women of childbearing age (used to rule out pregnancy in women before DXA). All samples were stored at −80°C.
Plasma analysis
Blood glucose concentrations were measured in triplicate with the use of a glucose Trinder assay with a sensitivity of 2 mg/dL, an intraassay CV of 1.6% and an interassay CV of 3.0% according to the manufacture (Stanbio Laboratory, Boerne, TX). Ghrelin (active), GIP, insulin, leptin, and PYY concentrations were quantified with the use of a Human Gut Hormone Panel Lin-coplex kit (Millipore, St Charles, MO). According to the manufacturer, the sensitivities are 1.8 pg/mL for active ghrelin, 157.2 pg/mL for leptin, 0.2 pg/mL for GIP, 44.5 pg/mL for insulin, and 8.4 pg/mL for PYY. The intraassay variation is <11% and the interassay variation is <19% (Millipore). Active GLP-1 was quantified with the use of a Linco enzyme-linked immuno-absorbent assay kit (Millipore). According to the manufacturer, the assay sensitivity is 2 pmol/L for a 100-μL sample size. The intraassay CV is 8% and the interassay CV is 13% at 4 pmol/L (Millipore). Calgary Laboratory Services (Calgary, Canada) quantified serum concentrations for lipid and human chorionic gonadotropin.
Hunger, satiety, adverse effects, and feasibility
Subjective hunger, satiety, and potential adverse effects were monitored with the use of 100-mm visual analog scales (VASs). Subjects were asked to complete the VAS during the MTT at each time point for which blood was collected. Scales were scored by measuring the distance (in mm) from 0 with a ruler. Similar VASs have been used previously (22).
Statistical analyses
Demographic and clinical variables are presented as mean ± SD and were compared between subjects in the control and oligofructose groups with the use of analysis of variance (ANOVA). Biochemical and experimental measures are presented as mean ± SEM. The change in plasma glucose concentration from initial to final tests within a subject was determined by subtracting the initial value from the final value. Change from baseline (fasting) for glucose and the hormones was calculated by dividing all time points by the fasting value and is reported as a percentage. Total area under the curve (tAUC) was calculated as AUC over the 6-h meal test. Hormone secretion and glucose tolerance during the MTT (curves over time) were analyzed by repeated-measures ANOVA with a Bonferroni adjustment [2-factor analysis with time (0–360 min) and diet as variables; and month (initial or final) and diet as variables]. Food record and VAS data were analyzed by 2-factor repeated-measures ANOVA with a Holm-Sidak test for multiple comparisons. The change in values between initial and final test days for DXA data were analyzed by ANOVA. Data were analyzed with STATA 8 (Stata Corp, College Station, TX) and SPSS version 16.0 software (SPSS Inc, Chicago, IL).
RESULTS
Subjects
Forty-eight subjects (39 women and 9 men) began the study. A total of 21 oligofructose subjects (17 women and 4 men) and 18 control subjects (15 women and 3 men) completed the study for a total study retention rate of 81%. No significant differences were observed in baseline characteristics of the subjects with the exception of ≈1.8-fold higher fasting GIP concentration in the oligofructose group (Table 1). An additional 2 subjects, one from each group, were deleted from the blood analyses because of excessive alcohol intake before the final test day. Reasons for dropping out of the study included medical conditions not related to the study (n = 3), time commitment (n = 5), and a report of oligofructose irritating the throat (n = 1). When an informal interview was done to assess the blinding, 47.6% and 38.9% of the oligofructose and placebo subjects, respectively, were able to correctly guess their grouping, mainly because of the presence or absence of gastrointestinal side effects, chiefly intestinal pressure and flatulence.
TABLE 1.
Demographic and clinical characteristics of the subjects1
| Characteristic | Control group | Oligofructose group |
|---|---|---|
| Females/males (n) | 15/3 | 17/4 |
| Age (y) | 38.6 ± 13.02 | 41.9 ± 12.7 |
| BW (kg) | 80.2 ± 12.8 | 83.4 ± 13.0 |
| BMI (kg/m2) | 29.8 ± 4.0 | 30.4 ± 3.4 |
| Body fat (%) | 36.5 ± 7.5 | 36.6 ± 8.0 |
| Fasting glucose (mmol/L) | 5.1 ± 0.4 | 4.9 ± 0.5 |
| Glucose tAUC (mmol · L−1 · min−1) | 1627.6 ± 138.8 | 1649.0 ± 215.8 [20]3 |
| Fasting insulin (pg/mL) | 453.0 ± 201.2 | 482.7 ± 261.5 |
| Insulin tAUC (pg · mL−1 · min−1) | 232,775.1 ± 137,934.8 | 274,830.5 ± 133,797.7 |
| GIP (pg/mL) | 11.9 ± 5.64 | 21.1 ± 13.7 |
| GIP tAUC (pg · mL−1 · min−1) | 22,456.0 ± 9657.3 [15] | 26, 754.0 ± 9483.7 |
| Fasting ghrelin (pg/mL)5 | 128.3 ± 96.6 | 160.4 ± 91.8 |
| Ghrelin tAUC (pg · mL−1 · min−1) | 38,335.2 ± 23,997.9 | 49,781.4 ± 26,500.8 |
| Fasting GLP-1 (pmol/L)5 | 4.0 ± 0.9 [16] | 3.9 ± 0.8 [19] |
| GLP-1 tAUC (pmol · L−1 · min−1) | 1662.5 ± 467.4 [14] | 1653.0 ± 404.0 [19] |
| Fasting PYY (pg/mL) | 88.6 ± 34.6 | 75.5 ± 31.3 [20] |
| PYY tAUC (pg · mL−1 · min−1) | 35,254.3 ± 11,494.9 | 33,903.5 ± 10,614.8 [19] |
| Leptin (pg/mL) | 22,132.1 ± 15,864.0 | 29,167.2 ± 22,559.7 |
| Leptin tAUC (pg · mL−1 · min−1) | 6,211,037.6 ± 4,553,887.0 | 8,802,656.8 ± 6,330,132.0 |
Descriptive characteristics for all subjects who completed the study (n = 39); total area under the curve characteristics are n = 16 control and n = 21 oligofructose supplementation. BW, body weight; tAUC, total area under the curve; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; PYY, peptide YY.
Mean ± SD and analyzed with ANOVA (all such values).
n in brackets (all such values).
Significantly different from oligofructose group, P ≤ 0.05 (ANOVA).
The active forms were measured.
Body composition
DXA analysis showed that weight loss was significantly higher with oligofructose than with placebo; the control group in fact gained weight (Figure 1). The weight loss was primarily fat mass (P = 0.005) with no change in lean mass plus bone mineral content. Regional fat assessment showed greater trunk fat loss in the oligofructose subjects than in the control subjects (P = 0.05).
FIGURE 1.
Mean (±SEM) change in physical characteristics as measured by dual-energy X-ray absorptiometry scans in the control (n = 17) and oligofructose (OFS; n = 21) groups after 12 wk. BW, body weight; BMC + Lean, bone mineral content and lean mass; FM, fat mass; TF, trunk fat. *Significantly different from control, P < 0.05 (ANOVA). Baseline values were as follows: for BW, 80.22 ± 12.8 and 83.4 ± 13.0 kg for control and OFS groups, respectively; for BMC + Lean, 50.8 ± 9.8 and 52.7 ± 9.7 kg for control and OFS groups, respectively; for FM, 29.1 ± 8.5 30.7 ± 8.5 kg for control and OFS groups, respectively; and for TF, 14.0 ± 5.3 and 15.7 ± 5.0 kg for control and OFS groups, respectively. No significant differences were found between baseline values.
Plasma satiety hormones
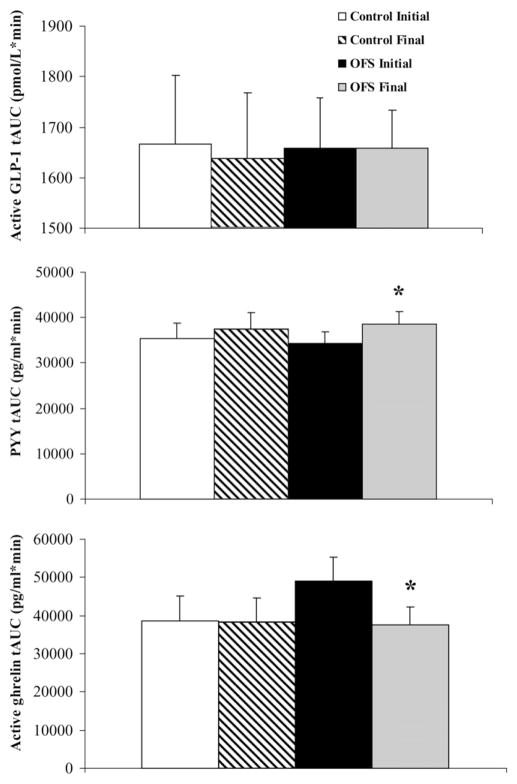
The absolute plasma concentrations and the percentage of change from baseline (fasting values) for postprandial GLP-1 (active), PYY, and ghrelin (active) are depicted in Figure 2. Oligofructose final ghrelin concentrations expressed as a percentage of baseline were lower in the oligofructose group at several time points during the MTT than in the control group final as well as the oligofructose initial values (P ≤ 0.05). At time 60 min during the MTT, GLP-1 expressed as a percentage of baseline was lower in the control subjects for the final test than for the initial test. Absolute concentrations of PYY at 120 min were increased in the oligofructose group at the final test than at the initial test, whereas ghrelin was decreased in the oligofructose group at the final test than at the initial test (Figure 2). Total AUC for ghrelin was decreased ≈23% for the final oligofructose values than for the initial oligofructose values (P = 0.004) (Figure 3). tAUC for PYY was increased ≈13% on the final test day than on the initial test day with oligofructose (P = 0.03). GLP-1 tAUC was unchanged in both the control and oligofructose groups. GIP was not affected by either placebo or oligofructose (data not shown). tAUC (mean ± SD) for leptin decreased from 9.2 ± 6.2 to 7.6 ± 4.4 mg · mL−1 · min−1 (P = 0.02), whereas leptin increased in the control group from 6.4 ±4.6 to 7.5 ±5.4 mg · mL−1 · min−1 (P = 0.08) between the initial and the final test days (month × diet interaction, P = 0.019). When expressed as change over time (subtracting baseline MTT values from final MTT values), leptin values for oligofructose were significantly lower than for the control at 60, 120, 240, and 360 min postprandially (P ≥ 0.05) (data not shown).
FIGURE 2.
Mean (±SEM) values for plasma active glucagon-like peptide 1 [GLP-1; n = 16 control, n = 19 oligofructose (OFS)] (A), total peptide YY (PYY; n = 16 control, n = 18 OFS) (B), and active ghrelin (n = 16 control, n = 20 OFS) (C) concentrations during a 6-h meal tolerance test as absolute concentrations and as a percentage of baseline. Control initial (□), OFS initial (■), control final (○), and OFS final (●). Repeated-measures ANOVA for absolute concentrations showed a time × diet effect (P = 0.04) and a month × diet effect (P = 0.05) for ghrelin and a month × diet effect for PYY (P = 0.05). Repeated-measures ANOVA showed a time × diet effect (P = 0.017) and a month × diet effect (P = 0.031) for ghrelin expressed as percentage of baseline. *Significant difference between OFS and control at the final test day, P < 0.05; †significant difference within the OFS group between initial and final tests, P < 0.05; ‡significant difference in the control group between initial and final tests, P < 0.05.
FIGURE 3.
Mean (±SEM) total area under the curve (tAUC) values for plasma active glucagon-like peptide 1 [GLP-1; n = 13 control, n = 18 oligofructose (OFS)], total peptide YY (PYY; n = 14 control, n = 18 OFS), and active ghrelin (n = 15 control, n = 20 OFS) during a 6-h meal tolerance test. Two-factor repeated-measures ANOVA showed a month × diet interaction for PYY (P = 0.007) and ghrelin (P = 0.05). *Significant difference in the OFS group between initial and final tests, P < 0.05.
Food intake, satiety, and hunger
According to the 3-d food records, the oligofructose group reported a significant reduction in energy intake. By week 6, a 29% reduction in total energy intake was reported in the oligofructose group than in the control group (P = 0.002) (Figure 4). When macronutrient intake was assessed, a similar pattern to total energy was seen. Oligofructose supplementation resulted in 27% lower carbohydrate intake than for placebo at week 6 with consumption at 259.1 ± 87.9 g/d for the control group and 189.2 ± 59.9 g/d for the oligofructose group (P = 0.01). By week 6, fat intake in the oligofructose group was 54.6 ± 19.0 g/d, which was a 40% reduction from that of the control group at 93.6 ± 44.0 g/d (P < 0.01). Protein intake in the control group was 94.6 ± 32.3 g/d, and protein intake in the oligofructose group was 71.3 ± 20.2 g/d in week 6 (P < 0.01). Protein intakes were also lower at week 3 in the oligofructose group than in the control group. Dietary fiber intakes were similar between the control and oligofructose groups at all time points. The macronutrient breakdown as a percentage of total calories for the control group at week 0 was 34.4 ± 6.1% for fat, 47.7 ± 7.9% for carbohydrates, and 17.5 ± 3.3% for protein. For the oligofructose group it was 30.7 ± 5.4% for fat, 51.7 ± 7.7% for carbohydrates, and 16.4 ± 4.6% for protein. The only notable difference in macronutrient percentage was a ≈6% higher fat intake at weeks 6 and 9 in the control group than in the oligofructose group (P = 0.03). Despite significant reductions in caloric intake in the oligofructose group, subjective appetite ratings from the VAS were not significantly changed during the MTT. For example, incremental AUC values for desire to eat were P = 0.15 for control initial (72.8 ± 5.4), control final (75.3 ± 6.5), oligofructose initial (82.0 ± 5.2), and oligofructose final (69.5 ± 6.3). Hunger ratings (not significant) were control initial (78.2 ± 4.1), control final (77.6 ± 6.4), oligofructose initial (79.2 ± 4.1), and oligofructose final (66.3 ± 6.4).
FIGURE 4.

Mean (±SEM) energy intake as determined by self-reported 3-d food records in the control (n = 18) and oligofructose (OFS; n = 21) groups over 12 wk. Two-factor repeated-measures ANOVA with a Holm-Sidak test for multiple comparisons analysis showed a significant week × diet effect (P = 0.03). *Significant difference between OFS initial and OFS final at the indicated time point, P < 0.05; †significant difference between control and OFS, P < 0.05.
Glucoregulation and serum lipids
Glucose and insulin absolute concentrations and as a percentage of baseline are depicted in Figure 5. Glucose concentrations expressed as a percentage of baseline were increased in the control group at the final test than with the initial test at 4 and 6 h in the MTT, whereas they were decreased in the oligofructose group at 6 h after eating (P = 0.05). Insulin as a percentage of baseline was higher in the control group than in the oligofructose group after the 2-h time point at the final test. Insulin was also lower in the oligofructose group at 15 and 60 min and higher in the control group at 6 h at the final test than at the initial test (P < 0.05). Absolute concentrations of glucose were lower at 6 h for the oligofructose final test than for the control final test (P = 0.001). At 4 h, glucose was increased in the control group and decreased in the oligofructose group at the final test than at the initial test (P < 0.05). Absolute concentrations of insulin were lower in the oligofructose group than in the control group at 15 min (P = 0.001), 2 h (P = 0.016), and 6 h (P = 0.04) at the final test and were decreased at the final test compared with the initial test in the oligofructose group at 60 min (P < 0.05). Both glucose and insulin were expressed as a change over time (subtracting absolute baseline MTT values from absolute final MTT values), and postprandial insulin was significantly lower in the oligofructose group than in the control group at 15, 120, and 360 min (Figure 5). tAUC for plasma glucose was reduced by 5% on the final day in the oligofructose group than on the initial day, but this was not significant (P = 0.16) (Figure 6). Insulin tAUC was reduced ≈10% in the oligofructose group at the final test day than at the initial test day (P = 0.19), whereas concentrations increased by ≈23% in the control group on the final day than on the initial day, although these changes were not significant (P = 0.09) (Figure 6). Oligofructose supplementation did not alter serum lipids (data not shown).
FIGURE 5.
Mean (±SEM) plasma glucose [n = 17 control, n = 20 oligofructose (OFS)] (A) and insulin (n = 17 control, n = 20 OFS) (B) as absolute concentrations and as a percentage of baseline during a 6-h meal tolerance test (MTT). Control initial (□), OFS initial (■), control final (○), and OFS final (●). Repeated-measures ANOVA for absolute concentrations showed a time × diet interaction for insulin (P = 0.025), a month × diet interaction for insulin (P = 0.001), and a month × diet effect for glucose (P = 0.001). Repeated-measures ANOVA for values expressed as percentage of baseline showed a time × diet effect (P = 0.067) and a month × diet effect (P = 0.003) for insulin and a month × diet effect (P = 0.068) for glucose. *Significant difference between OFS and control at final test, P < 0.05; †significant difference within the OFS group between initial and final tests, P < 0.05; ‡significant difference in the control group between initial and final tests, P < 0.05. (C) Change over time (subtracting absolute baseline MTT values from absolute final MTT values) for glucose (n = 18 control, n = 20 OFS) and insulin (n = 17 control, n = 20 OFS); control (□) and OFS (■). §Significant difference between OFS and control, P < 0.05 (repeated-measures ANOVA).
FIGURE 6.
Mean (±SEM) total area under the curve (tAUC) values for plasma glucose [n = 14 control, n = 16 oligofructose (OFS)] and insulin (n = 15 control, n = 20 OFS) during a 6-h meal tolerance test. Two-factor repeated-measures ANOVA showed a month × diet effect for insulin (P = 0.023) and glucose (P = 0.06).
Compliance and tolerance
We observed a mean return rate of 88 ± 5% and 85 ± 4% of total allotted packages for the oligofructose and placebo groups, respectively. From these packets it was determined that a minimum of 79 ± 5% of the oligofructose doses and 81 ± 5% of the placebo doses were consumed over the course of the study. Conversely, 9 ±2% and 4 ±2% of the packets were returned full, indicating missed doses. No increase in nausea, diarrhea, thirst, or constipation was observed with oligofructose. The oligofructose group reported decreased diarrhea by the final test day. Several subjects did complain of flatulence during the study, largely around the 4-wk mark. Although most symptoms subsided with adaptation to the fiber regimen, a VAS administered on the final test day indicated that 45 ± 8% of the time subjects in the oligofructose group experienced negative side effects, which would deter them from consuming the product at the current dosage compared with the placebo group (11 ± 4%; P < 0.01).
DISCUSSION
Evidence from animal studies has generally shown that oligofructose supplementation promotes weight loss and improves glucoregulation in obese and diabetic models (2, 17, 18, 23, 24). To our knowledge, this is the first randomized, double-blind, placebo-controlled trial to systematically evaluate the long-term effects of oligofructose supplementation on weight loss and gut satiety hormones in overweight and obese adults. In general, the results of this clinical trial support the findings of animal studies that showed a significant reduction in body weight because of fat loss that is independent of any other lifestyle changes (2, 18, 23). Conversely, the placebo group gained weight over the course of the intervention despite an equicaloric addition to their regular diet. Regional fat analysis showed primarily fat loss from the trunk region, a known factor critical in reducing overall disease risk, including that of type 2 diabetes (25).
The mechanism by which oligofructose triggers weight loss appears to be in part due to decreased energy intake, given our observation of lower self-reported caloric intake with oligofructose. Dietary fiber supplementation is thought to attenuate food intake through several mechanisms; however, the most widely reported mechanism with oligofructose is modification of satiety hormone response (10). Several studies report increased plasma GLP-1 and PYY and up-regulation of proglucagon mRNA with inulin-type fructans in rodents (10, 24). In the present study, PYY tAUC was higher with oligofructose supplementation, thus possibly having a role in appetite suppression. Conversely, no increase in GLP-1 plasma concentrations over a 6-h MTT occurred with 3 mo of oligofructose. This lack of change in GLP-1 is consistent with findings from studies examining cereal fiber and resistant starch in humans (26). Furthermore, the discrepancy between our results for GLP-1 in humans and those of animal studies could be due to the lower dose of oligofructose consumed by our subjects. Generally, animal studies have provided a minimum dose of 10% of total dietary intake, whereas this protocol provided ≈5% (27). Given the significant gastrointestinal side effects associated with intakes of prebiotic fibers >30 g/d, a dose roughly equivalent to the 10% used in rodent studies was not feasible (28).
Secretion of active ghrelin, an orexigenic peptide, was attenuated in the subjects consuming oligofructose. Previous studies assessing the effects of dietary fiber on ghrelin response have largely examined acute meal-related effects. Notably, blunting of postprandial ghrelin concentrations appears to be fiber specific with studies showing an effect of wheat fiber but not oat fiber (29), no effect of cereal fiber (30), and an effect of carob fiber in a liquid meal challenge but not as part of a carob fiber–enriched glucose solution (31). The mechanisms through which nutrients suppress ghrelin secretion are somewhat unclear. Ghrelin is primarily secreted from the stomach, yet this organ does not contain a nutrient-sensing mechanism (8, 32, 33). Furthermore, nutrient infusions into the stomach, duodenum, or jejunum suppressed ghrelin secretion equally, suggesting that neither gastric nor duodenal exposure to nutrients is requisite for suppression (34). One suggestion has been that the rate of nutrient absorption and intestinal lumen osmolarity could alter ghrelin secretion (34).
Rodent studies have specifically shown a reduction in ghrelin with oligofructose and a combination of oligofructose and inulin (10). Whether the lower ghrelin seen in our oligofructose group accounted for their decreased caloric intake is not definitively known. Weight loss, itself, has been shown to alter ghrelin concentrations, although the direction of that change is equivocal with some studies showing an increase after weight loss (35, 36) and others a decrease (37). Others have positively correlated ghrelin concentrations with subjective measure of appetite in humans (38). Our food records support this assumption, albeit without a corresponding decrease in subjective hunger ratings. This is not entirely unexpected, given that, in many experiments in humans, hormonal changes are not linked to subjective feelings of satiety (26). Changes in PYY and ghrelin induced by insoluble wheat fiber were not associated with acute ratings of hunger or satiety but may have potential for affecting satiety in response to a subsequent meal (29). Ghrelin is also thought to play a role in increasing adiposity. Given that administration of ghrelin reduces fat utilization (8), it is plausible that lower circulating concentrations may alleviate the downward pressure on fat oxidation seen in obesity and in turn enhance lipolysis (39). The lower leptin observed in our oligofructose group supports the reduction in adiposity measured by DXA.
Oligofructose has previously been shown to reduce post-prandial glycemia in rodents (24). Our findings suggest that, even in normoglycemic overweight or obese individuals, there may be a role for oligofructose in maintaining glucose homeostasis. Over the course of the 3-mo trial, a reduction was observed in plasma glucose response to a test meal with oligofructose, whereas the placebo group experienced a worsening of glucose control, albeit still within the normal range. It has been proposed that oligofructose may affect postprandial glycemia by stimulating incretin hormones with known ileal brake actions (18). Conversely, in our study no change in GLP-1 secretion occurred. Although these results contradict those of rodent studies (18, 24) showing marked increases in GLP-1 secretion with oligofructose, it is possible this discrepancy is due to the lower dose provided. Another factor that should be considered with respect to improvements in glucoregulation is the weight loss, because even modest weight loss is associated with improvements in plasma glucose and lipid concentrations (40). Independent of changes to GLP-1, patients with impaired glucose tolerance or type 2 diabetes may experience more pronounced glucose lowering with oligofructose than that seen in our overweight and obese population.
Consumption of oligofructose did result in some minor gastrointestinal side effects. None of these could be considered serious or harmful to health, and they subsided with adaptation over time; however, they could deter individuals from consuming the product. Also note that the long-term effects (in y) and safety of oligofructose consumption remain unknown.
The strengths of the present study are the randomized placebo-controlled design and the long-term nature of the supplementation. Although compliance was not absolute, a mean of 90% of the oligofructose doses and 94% of the placebo doses were taken. Limitations inherent in the use of self-reported diet intake are acknowledged, but having each subject participate in a training session probably reduced some reporting error. The subjects in our study were largely white and of middle to high socioeconomic status. They were otherwise healthy overweight and obese adults; therefore, the results cannot be generalized to individuals with greater metabolic dysfunction, although we speculate that, given our results, hyperlipidemia and impaired glucose tolerance may respond well to oligofructose. Future studies should target these individuals.
In conclusion, independent of any other lifestyle changes, oligofructose supplementation reduced body weight, chiefly body fat, in overweight and obese individuals over 3 mo. These results were associated with an amplified suppression of post-prandial ghrelin and stimulation of PYY and an observed significant lowering of food intake in the oligofructose group than in the placebo group. The slight increase in body weight over 3 mo in the placebo group and the worsening of their blood glucose control may be a reflection of the slow, gradual nature of the metabolic dysfunction that occurs with excess adiposity over time (41). The ability of oligofructose to attenuate this weight gain and in fact reduce body fat over 3 mo suggests it may be a promising agent in achieving or maintaining a healthy body weight.
Acknowledgments
We thank Kristine Lee for her assistance with the laboratory work. The authors’ responsibilities were as follows—JAP: designed the experiment, collected and analyzed the data, and wrote the preliminary manuscript; and RAR (principal investigator): secured funding for the project, provided advice and consultation throughout, performed statistical analysis, and edited the manuscript.
Footnotes
Supported by grants from the Canadian Institutes of Health Research and the University of Calgary and by a scholarship from the Cosmopolitan Club of Calgary (JAP). The oligofructose (Raftilose P95) was kindly provided by Quadra Chemicals Ltd (Burlington, ON, Canada).
None of the authors declared a conflict of interest.
References
- 1.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol. 2008;70:239–55. doi: 10.1146/annurev.physiol.70.113006.100506. [DOI] [PubMed] [Google Scholar]
- 2.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy humans: a pilot study. Eur J Clin Nutr. 2006;60:567–72. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Grudell AB, Camilleri M. The role of peptide YY in integrative gut physiology and potential role in obesity. Curr Opin Endocrinol Diabetes Obes. 2007;14:52–7. doi: 10.1097/MED.0b013e3280123119. [DOI] [PubMed] [Google Scholar]
- 5.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY (3–36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 6.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence. Gut. 1996;38:916–9. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 8.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 9.Neary NM, Small CJ, Bloom SR. Gut and mind. Gut. 2003;52:918–21. doi: 10.1136/gut.52.7.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr. 2005;93(suppl):S157–61. doi: 10.1079/bjn20041342. [DOI] [PubMed] [Google Scholar]
- 11.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–6. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- 12.Pylkas AM, Juneja LR, Slavin JL. Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J Med Food. 2005;8:113–6. doi: 10.1089/jmf.2005.8.113. [DOI] [PubMed] [Google Scholar]
- 13.Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–56. doi: 10.1210/endo.137.9.8756571. [DOI] [PubMed] [Google Scholar]
- 14.Tappenden KA, Thomson AB, Wild GE, McBurney MI. Short-chain fatty acids increase proglucagon and ornithine decarboxylase messenger RNAs after intestinal resection in rats. JPEN J Parenter Enteral Nutr. 1996;20:357–62. doi: 10.1177/0148607196020005357. [DOI] [PubMed] [Google Scholar]
- 15.Massimino SP, McBurney MI, Field CJ, et al. Fermentable dietary fiber increases GLP-1 secretion and improves glucose homeostasis despite intestinal glucose transport capacity in healthy dogs. J Nutr. 1998;128:1786–93. doi: 10.1093/jn/128.10.1786. [DOI] [PubMed] [Google Scholar]
- 16.Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber J-C. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused frat ileum. Endocrinology. 1998;139:3780–6. doi: 10.1210/endo.139.9.6202. [DOI] [PubMed] [Google Scholar]
- 17.Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1(7–36) amide on glycemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol. 2005;185:457–65. doi: 10.1677/joe.1.06100. [DOI] [PubMed] [Google Scholar]
- 18.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide-1 receptor. Diabetes. 2006;55:1484–90. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 19.Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003;124:894–902. doi: 10.1053/gast.2003.50159. [DOI] [PubMed] [Google Scholar]
- 20.Sloth B, Krog-Mikkelsen I, Flint A. No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am J Clin Nutr. 2004;80:337–47. doi: 10.1093/ajcn/80.2.337. [DOI] [PubMed] [Google Scholar]
- 21.Finer N, Bloom SR, Frost GS, Banks LM, Griffiths J. Sibutramine is effective for weight loss and diabetic control in obesity with type 2 diabetes: a randomised, double-blind, placebo-controlled study. Diabetes Obes Metab. 2000;2:105–12. doi: 10.1046/j.1463-1326.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 22.Eller LK, Ainslie PN, Poulin MJ, Reimer RA. Differential responses of circulating amylin to high-fat versus high-carbohydrate meal in healthy men. Clin Endocrinol (Oxf) 2008;68:890–7. doi: 10.1111/j.1365-2265.2007.03129.x. [DOI] [PubMed] [Google Scholar]
- 23.Delmee E, Cani PD, Gual G, et al. Relation between colonic proglucagon expression and metabolic response to oligofructose in high fat diet-fed mice. Life Sci. 2006;79:1007–13. doi: 10.1016/j.lfs.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Urias-Silvas JE, Cani PD, Delmee E, Neyrinck A, Lopez MG, Delzenne NM. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br J Nutr. 2008;99:254–61. doi: 10.1017/S0007114507795338. [DOI] [PubMed] [Google Scholar]
- 25.Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13. 5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–8. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 26.Weickert MO, Pfeiffer AFH. Metabolic effects of dietary fiber consumption and prevention on diabetes. J Nutr. 2008;138:439–42. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DJ, Kendall CW, Vuksan V. Inulin, oligofructose and intestinal function. J Nutr. 1999;129(suppl):1431S–3S. doi: 10.1093/jn/129.7.1431S. [DOI] [PubMed] [Google Scholar]
- 28.Kaur N, Gupta AK. Applications of inulin and oligofructose in health and nutrition. J Biosci. 2002;27:703–14. doi: 10.1007/BF02708379. [DOI] [PubMed] [Google Scholar]
- 29.Weickert MO, Spranger J, Holst JJ, et al. Wheat-fibre-induced changes of postprandial peptide YY and ghrelin responses are not associated with acute alterations of satiety. Br J Nutr. 2006;96:795–8. doi: 10.1017/bjn20061902. [DOI] [PubMed] [Google Scholar]
- 30.Weickert MO, Mohlig M, Schofl C, et al. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. 2006;29:775–80. doi: 10.2337/diacare.29.04.06.dc05-2374. [DOI] [PubMed] [Google Scholar]
- 31.Gruendel S, Otto B, Garcia AL, et al. Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. Br J Nutr. 2007;98:101–5. doi: 10.1017/S0007114507701642. [DOI] [PubMed] [Google Scholar]
- 32.Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–4. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 33.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003;144:2765–7. doi: 10.1210/en.2003-0381. [DOI] [PubMed] [Google Scholar]
- 34.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–50. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 35.Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Kocelak P, Janowska J, Semik-Grabarczyk F. The effect of weight reduction on plasma concentrations of ghrelin and insulin-like growth factor 1 in obese women. Endokrynol Pol. 2008;59:301–4. [PubMed] [Google Scholar]
- 36.Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18:1424–9. doi: 10.1007/s11695-008-9560-5. [DOI] [PubMed] [Google Scholar]
- 37.Garciade la Torre N, Rubio MA, Bordiu E, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–81. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 38.Blom WAM, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HFJ. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81:367–75. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 39.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–93. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapiro JR, Stout AL, Musante GJ. “Structure-size me”: weight and health changes in a four week residential program. Eat Behav. 2006;7:229–34. doi: 10.1016/j.eatbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]